Introduction
Cholangiocarcinoma (CCA) has been reported to be the
second most common hepatic malignancy after hepatocellular
carcinoma (HCC), and its incidence has gradually increased
worldwide (1–2). CCA is a relatively infrequent
malignancy arising from epithelial cells lining the biliary tree,
and is classified anatomically as intrahepatic (iCCA), perihilar
(pCCA) or distal (3). The
incidence of iCCA has increased globally over the past few decades.
According to the mortality rates reported in the World Health
Organization database, the age-standardized mortality rates for
iCCA have increased in almost all countries across all continents
(4). Surgery is the preferred
treatment option for a minority of patients with early-stage CCA
(~35%). However, the currently available systemic therapies are of
limited effectiveness for patients with advanced-stage or
unresectable CCA, due to its absence of clinical symptoms and
anatomical location of difficult access (5). Therefore, it is urgent to explore
novel markers and molecules for CCA therapy.
Epithelial-mesenchymal transition (EMT) is
associated with tumor invasion and migration, and is defined as a
reversible dynamic process in which epithelial cells lose their
phenotypic characteristics and adopt mesenchymal cell structural
and functional features (6,7).
These changes involve downregulation of E-cadherin and β-catenin,
which are two main phenotypic characteristic components of
epithelial cells, as well as upregulation of proteins involved in
the mesenchymal phenotype (N-cadherin, α-smooth muscle actin,
vimentin, fibronectin and MMPs), which occur via EMT-inducing
transcription factors. The EMT-inducing transcription factor
comprise three families: Snail, zinc finger E-box-binding homeobox
and Twist-related proteins (TWIST) (8).
EMT is involved in human physiological and
pathological processes, including embryonic and organ development,
organ fibrosis, wound healing, and cancer progression (9,10),
and is accompanied by marked changes in cellular morphology,
enhanced migratory and invasive capabilities, as well as loss and
remodeling of cell-cell interactions and cell-matrix adhesion
(11). Thus, EMT may be an
important mechanism involved in the progression of CCA, and EMT
biomarkers may provide new insights into the progression and
migration of CCA. In addition, the activities of cellular (c)-SRC
kinase, and PI3K and AKT, which are potentially located downstream
of c-SRC, are hallmarks for malignant transformation and
progression.
Carcinoembryonic antigen-related cell adhesion
molecule 6 (CEACAM6), a member of the CEACAM family, is normally
expressed on the surface of myeloid and epithelial cells, while
aberrant CEACAM6 expression leads to the development of human
malignancies (12–14). Previous studies have reported that
CEACAM6 is overexpressed in several epithelial carcinomas,
including colon, breast and non-small cell lung cancer, as well as
iCCA (15,16). CEACAM6 mediates cell-cell adhesion,
which is required for guiding cells to their correct location
during embryonic development, and for the integration of single
cells into functional tissues and organs (17). However, overexpression of CEACAM6
has been shown to alter tissue architecture in several carcinoma
cell lines (18). In addition,
overexpression of CEACAM6 reduces cell apoptosis, indicating that
CEACAM6 plays a key role in promoting the aberrant growth of
adherent cells (19). Previous
studies have shown that CEACAM6 induces EMT, and mediates invasion
and migration in pancreatic and gastric cancer, and its clinical
importance in colorectal cancer has also been reported (20,21).
However, the physiological function of CEACAM6, and its potential
involvement in tumor formation and progression in CCA have not been
fully elucidated to date.
The present study demonstrated that CEACAM6 is an
important regulator of CCA EMT, as well as of cell migration and
invasion in vitro, which suggests that CEACAM6 may be an
important target for the treatment of human CCA.
Materials and methods
Tissues and cell line
Primary CCA tissues and matched adjacent
paracancerous tissues (distance, 1 cm) were surgically obtained
from 27 patients with CCA 20 males and 7 females with an age range
of 56–78 years (mean age, 66.4 years), including 9 cases of highly
differentiated CCA and 18 cases of less differentiated CCA) at The
Second Hospital of Hebei Medical University (Shijiazhuang, China)
from January 2016 to December 2017. Ethics approval (approval no.
2014018) was obtained from The Second Hospital of Hebei Medical
University and all samples were collected with written informed
consent from the patients.
RBE cells (Qiao Xinzhou Biotechnology Co., Ltd.)
were cultured at 37°C with 5% CO2 in DMEM (Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS;
Biological Industries; Sartorius AG) and 1% penicillin and
streptomycin (Sigma-Aldrich; Merck KGaA). Cells in the logarithmic
growth phase were used for experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from CCA and paracancerous
tissue and RBE cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), and first-strand cDNA was
synthesized using PrimeScript™ RT Reagent kit (Takara Biotechnology
Co., Ltd.). qPCR was performed in triplicate with SYBRII qPCR
Master Mix (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol using GAPDH as a control. RT was performed
at 37°C for 15 min and 85°C for 5 min. Thermocycling conditions
were as follows: Initial denaturation at 95°C for 30 sec, followed
by 40 cycles of 95°C for 5 sec and 60°C for 31 sec. The relative
mRNA levels of the target genes were calculated with the
2−ΔΔCq method (22).
The primer sequences used in qPCR are listed in Table I.
 | Table I.Primer sequences for quantitative
PCR. |
Table I.
Primer sequences for quantitative
PCR.
| Gene | Forward (5′-3′) | Reverse (3′-5′) |
|---|
| MMP-2 |
CCAACTACAACTTCTTCCCTCG |
TCACATCGCTCCAGACTTG |
| MMP-9 |
ACGCAGACATCGTCATCCA |
AGGGACCACAACTCGTCATC |
| ICAM-1 |
GTCATCATCACTGTGGTAGCAG |
GGCTTGTGTGTTCGGTTTC |
| E-cadherin |
GTGGTCAAAGAGCCCTTACTG |
CGTTACGAGTCACTTCAGGC |
| N-cadherin |
TCATTGCCATCCTGCTCTG |
CATCCATACCACAAACATCAGC |
| Vimentin |
AAATGGCTCGTCACCTTCG |
AGAAATCCTGCTCTCCTCGC |
| TWIST1 |
GGAGTCCGCAGTCTTACGA |
CTTGAGGGTCTGAATCTTGCT |
| Bcl-2 |
TGTGTGGAGAGCGTCAACC |
TGGATCCAGGTGTGCAGGT |
| Bax |
TTTCTGACGGCAACTTCAAC |
AGTCCAATGTCCAGCCCAT |
| Caspase 3 |
ACTGGACTGTGGCATTGAGAC |
TTGTCGGCATACTGTTTCAGC |
| Caspase 8 |
TGTTGGAGGAAAGCAATCTG |
CTGCCTGGTGTCTGAAGTTC |
| Caspase 9 |
GCAGATTTGGCTTACATCCTG |
ACGGCAGAAGTTCACATTGT |
| CEACAM6 |
CACTATTGAATCCACGCCG |
TTGCCATCCACTCTTTCG |
| VEGFA |
CTTGCCTTGCTGCTCTACCT |
TGATGATTCTGCCCTCCTCCT |
| c-SRC |
GAGGAGCCCATTTACATCG |
CTTGAGAAAGTCCAGCAAACTC |
| PI3K |
GCCTGCTCTGTAGTGGTAGATG |
GGAGGTGTGTTGGTAATGTAGC |
| AKT |
TACGAGATGATGTGCGGTC |
TCTTGAGCAGCCCTGAAAG |
Western blotting
Western blotting was performed as previously
described (18). Briefly, total
protein was extracted from RBE (5×106) cells, CCA
tissues and paracancerous tissues with cold RIPA buffer containing
protease and phosphatase inhibitors (all Beijing Solarbio Science
and Technology Co., Ltd.), followed by quantification of protein
concentration using the BCA assay. Protein samples (30 µg/lane)
were heated to 100°C for 10 min in SDS-PAGE loading buffer (Thermo
Fisher Scientific, Inc.) and separated on 10% SDS-PAGE. The
separated proteins were transferred at 200 mA to a PVDF membrane
(MilliporeSigma) for 2 h at 4°C. After blocking the membrane with
TBS containing 5% skimmed milk for 1.5 h at room temperature, the
membranes were incubated with primary antibodies at 4°C overnight.
The primary antibodies used were as follows: β-actin (rabbit
monoclonal; 1:2,000; Abcam), Bax (rabbit monoclonal; 1:500; Abcam),
Bcl2 (rabbit monoclonal antibody, dilution: 1:500; Abcam), cleaved
caspase-3 (rabbit monoclonal antibody dilution: 1:500; Abcam),
cleaved caspase-8 (rabbit polyclonal antibody, dilution: 1:500;
Abcam), cleaved caspase-9 (rabbit polyclonal; 1:500; Abcam), MMP2
(rabbit polyclonal antibody, dilution: 1:500; Abcam), MMP9 (rabbit
polyclonal antibody, dilution: 1:500; Abcam), ICAM-1 (rabbit
polyclonal antibody, dilution: 1:500; Abcam); VEGFA (rabbit
polyclonal; 1:500; Abcam); E-cadherin (rabbit polyclonal; 1:500;
Abcam); N-cadherin (rabbit polyclonal antibody, dilution: 1:500;
Abcam); TWIST (mouse monoclonal antibody, dilution: 1:500, Santa
Cruz Biotechnology, Inc.); Vimentin (mouse monoclonal antibody,
dilution: 1:500; Santa Cruz Biotechnology, Inc.); CSRC (rabbit
monoclonal; 1:500; Abcam); p-CSRC (rabbit monoclonal antibody,
dilution: 1:500; Abcam); PI3K, p-PI3K, AKT, p-AKT (rabbit
monoclonal antibody, dilution: 1:500; Cell Signaling Technology,
Inc.). Appropriate horseradish peroxidase-conjugated secondary
antibody (goat anti-rabbit IgG, dilution: 1:5,000 and goat
anti-mouse IgG, dilution: 1:3,000) at 25°C for 1 h. The bands were
detected using SuperECL Plus detection reagents (LI-COR
Biosciences) and quantified with a Bio-Rad ChemiDoc imaging system
(Bio-Rad Laboratories, Inc.) using β-actin as an internal
control.
Cell transfection
RBE cells (30–40% confluence) were transfected with
Lipofectamine® RNAiMAX Reagent (Thermo Fisher
Scientific, Inc.) and 20 nM small interfering RNA (siRNA)
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
instructions for 4 h at 37°C. The sequences of all specific and
control siRNAs are as follows: siRNA-1 CCUCUACAAAGAGGUGGACAGAGAATT;
siRNA-2 CCAUGGUGAGAAAUUGACGACUUCATT; siRNA-3
CACCACUGCCAAGCUCACUAUUGAATT and control,
CCUCUUACCUCAGUUACAAUUUAUATT. Three separate CEACAM6-specific siRNA
sequences were used, and an unrelated control siRNA sequence was
transfected using the same protocol, which had no effect on CEACAM6
expression. Non-transfected cells were also used as a negative
control; these were treated with serum-free DMEM for 4 h at 37°C.
Knockdown of CEACAM6 expression was confirmed at 48 h by western
blotting.
Cell viability determination
The viability of RBE cells was determined using a
Cell Counting Kit (CCK)-8 assay (Abcam) according to the
manufacturer's instructions. Briefly, transfected cells
(2×103) in 96-well plates were incubated for 24 h at
37°C and 5% CO2. CCK-8 reagent (10 µl) was added for 1 h
at 37°C and optical density was measured at 460 nm.
Cell apoptosis analysis
RBE cells (80–90% confluence) transfected with
siRNAs for 48 h were washed twice with PBS (both 30 sec at room
temperature) and then resuspended in staining buffer containing
0.025 mg/ml Annexin V-FITC and 1 mg/ml propidium iodide (Shanghai
Yisheng Biotechnology Co., Ltd.). Double staining was performed for
10 min at room temperature in the dark, and the number of apoptotic
cells was then determined by flow cytometry using a BD FACSCanto™
ІІ flow cytometer (BD Biosciences). The software used for data
analysis is FlowJo (7.6.1; FlowJo, LLC.).
In vitro invasion assay
Cell invasion assays were performed using Transwell
inserts (Corning, Inc.) coated with Matrigel (37°C for 1 h) were
performed as previously described (23). Briefly, RBE cells
(1×105) in 0.2 ml serum-free DMEM were placed in the
upper chamber, and the lower chamber was loaded with 0.5 ml medium
containing 15% FBS. Cells that migrated to the lower surface of the
filters were stained with 0.005% crystal violet solution for 40 min
at room temperature, and cells in five fields of view were counted
after 24 h of incubation at 37°C and 5% CO2 using light
microscope. Three wells were examined for each cell type and
condition, and the experiments were conducted in triplicate.
Wound healing assay
RBE cells (6×105) were seeded in 6-well
plates. When cells reached 90% confluency, a 100-µl pipette tip was
used to scratch the serum-starved cell monolayer (time 0 h). Images
of migrating cells during the closure of the wounded region were
captured at 0 and 24 h using light microscope (magnification,
×10).
Statistical analysis
All statistical analyses were conducted using SPSS
version 19.0 (IBM, Corp.). Data are presented as the mean ±
standard error of the mean of three independent repeats.
Comparisons of the means of different groups were performed by
using two-way mixed ANOVA or one-way ANOVA. Pairwise comparisons
with homogeneity of variance were performed using the post hoc
Tukey's honestly significant difference and Student-Newman-Keuls
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
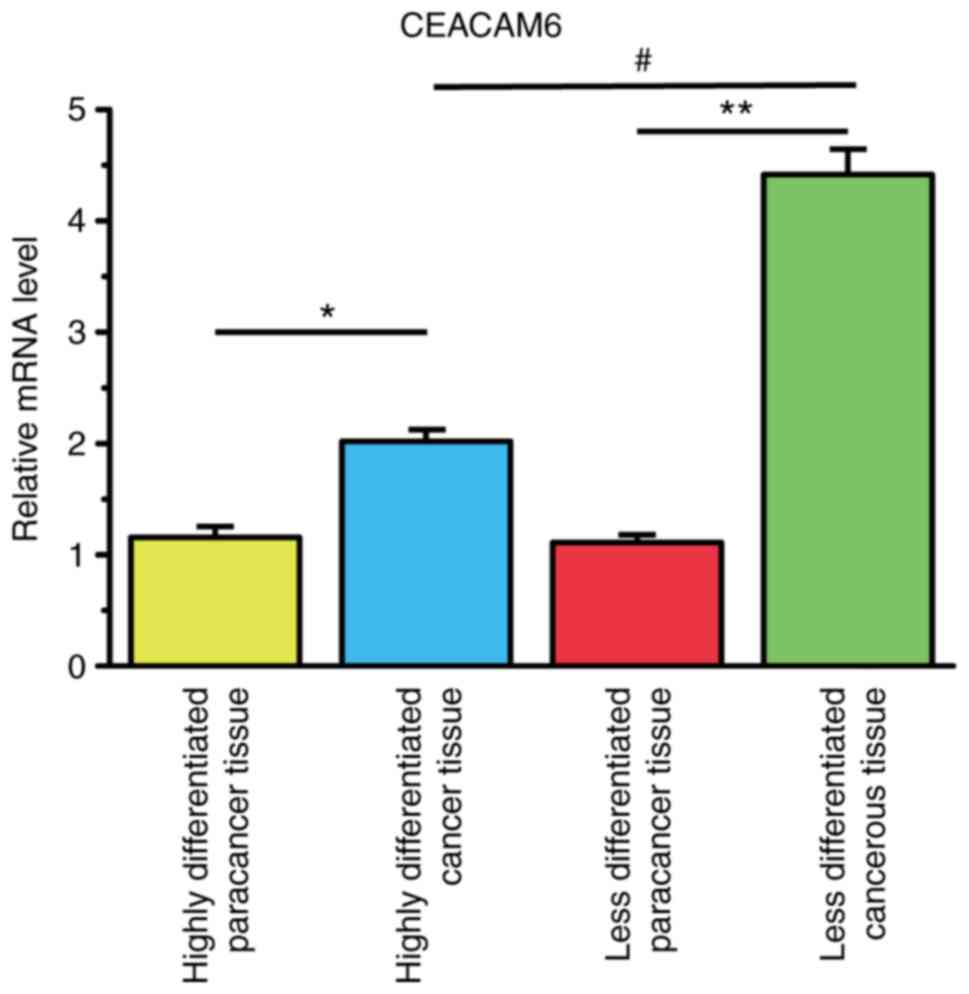
CEACAM6 is overexpressed in CCA
tissues, and its expression level is negatively associated with
tumor differentiation degree
As shown in Fig. 1,
CEACAM6 was overexpressed in highly differentiated and less
differentiated CCA tissues compared with its expression levels in
highly differentiated and less differentiated paracancerous tissues
(P<0.05). Furthermore, lesser differentiation degrees of CCA
tissues resulted in higher CEACAM6 expression levels
(P<0.05).
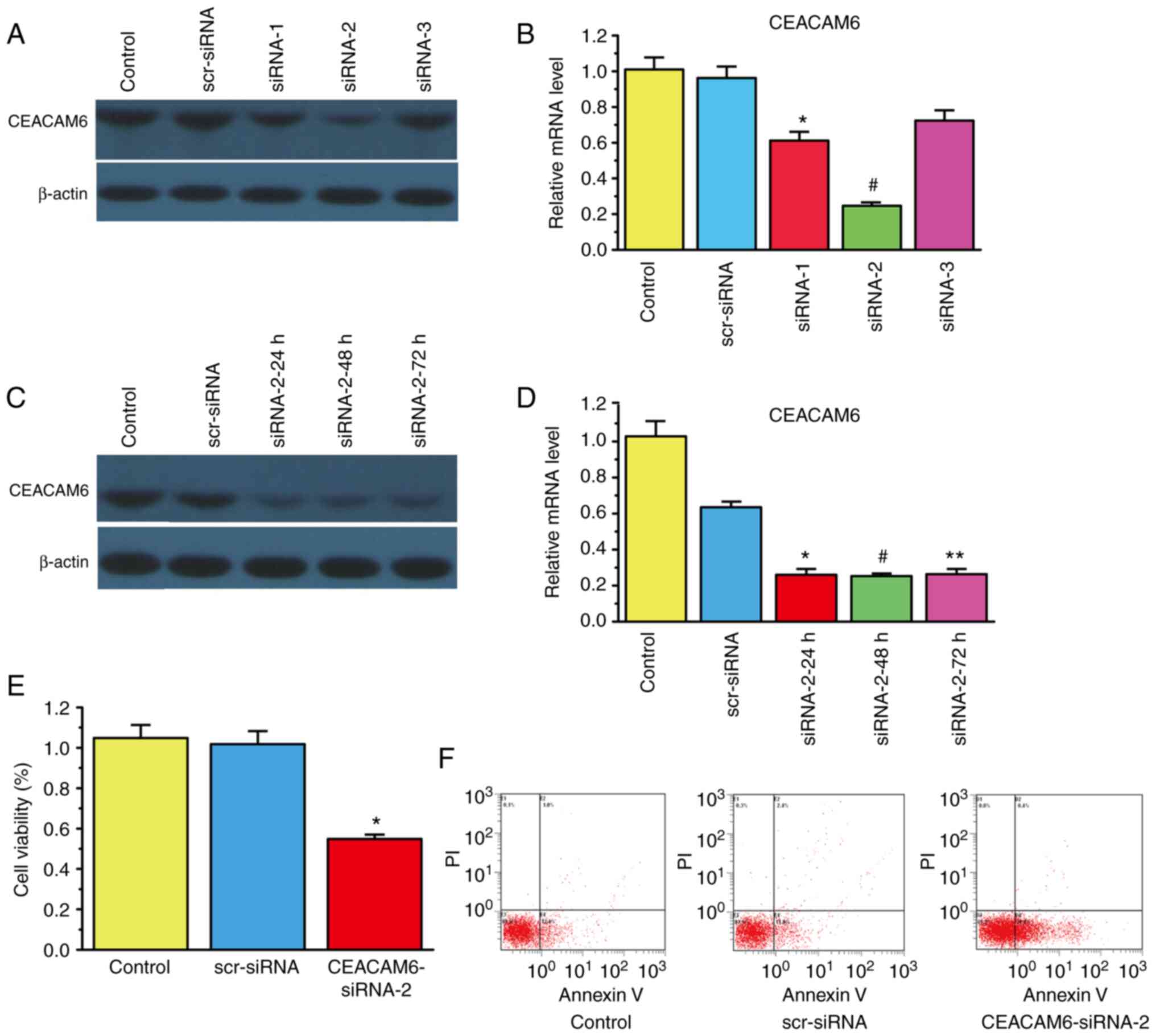
Silencing CEACAM6 inhibits cell and
promotes cell apoptosis in RBE cells
After determining that CEACAM6 was highly expressed
in CCA tissues, the role of CEACAM6 in the invasion and migration
of RBE cells was next verified. siRNA transfection was used to
silence the expression of CEACAM6. The results of Fig. 2A-D show that CEACAM6-siRNA-2
exhibited the best knockdown efficiency after 48 h. Thus,
CEACAM6-siRNA-2 was utilized in subsequent experiments. As shown in
Fig. 2E, CEACAM6-siRNA-2
significantly decreased RBE cell viability by ~50 compared with
that of the control group (P<0.05). In addition, Fig. 2F shows that the percentage of
apoptotic cells increased after transfection of CEACAM6-siRNA-2
(34.4%) compared with that of the scramble (scr)-siRNA group
(13.8%; P<0.05).
CEACAM6 knockdown inhibits the
invasion and migration of RBE cells
As shown in Fig. 3A and
C, the invasion ability of RBE cells was decreased after
transfection with CEACAM6-siRNA-2. Similar results were obtained
regarding the migration ability of RBE cells, which was also
declined upon CEACA-siRNA-2 transfection, as shown in Fig. 3B and D (P<0.05).
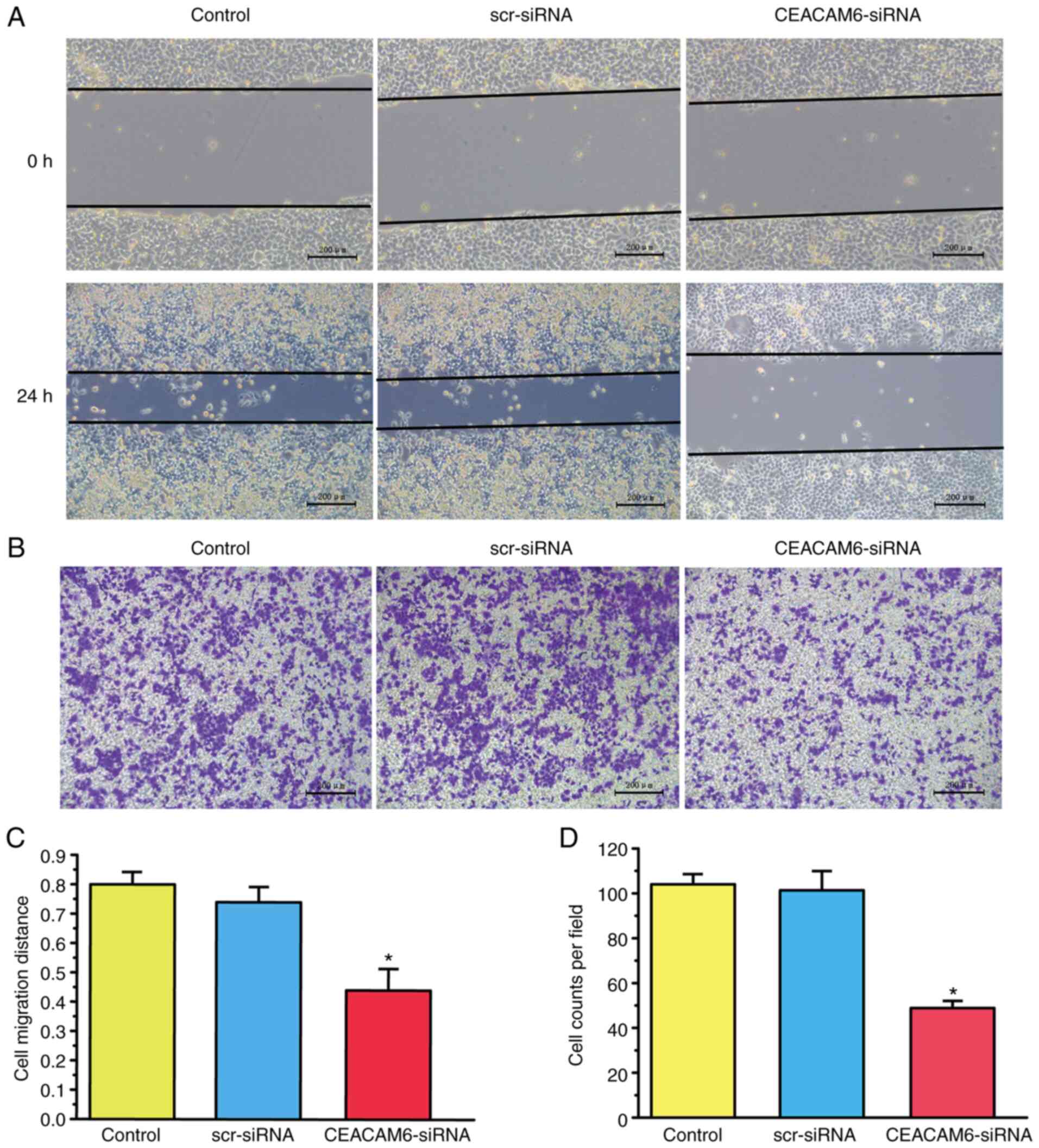 | Figure 3.Effects CEACAM6 silencing on the
invasion and migration of RBE cells in vitro. (A and C)
Effects of silencing CEACAM6 via transfection of CEACAM6-siRNA-2
for 48 h on the wound healing and invasion abilities of RBE cells
(magnification, ×100; scale bar, 200 µm). (B and D) Effects of
silencing CEACAM6 due to transfection of CEACAM6-siRNA-2 for 48 h
on the invasion of RBE cells (magnification, ×100; scale bar, 200
µm). Data are shown as the mean ± standard error of the mean (n=8),
and were analyzed with one-way ANOVA followed by
Student-Newman-Keuls post hoc test, *P<0.05, scr-siRNA vs.
CEACAM6-siRNA. CEACAM6, carcinoembryonic antigen-related cell
adhesion molecule 6; siRNA, small interfering RNA; scr,
scramble. |
CEACAM6 silencing increases
anti-apoptotic protein expression and decreases pro-apoptotic
protein expression
Since the percentage of apoptotic cells increased
after transfection with CEACAM6-siRNA-2, the present study next
investigated whether the mRNA and protein expression levels of
apoptosis-related proteins were consistent with the aforementioned
findings. The results showed that the mRNA and protein levels of
anti-apoptotic Bcl-2 decreased, while those of pro-apoptotic Bax
increased after transfection with CEACAM6-siRNA-2.
The caspase family is composed of a class of
proteolytic enzymes that mediate apoptosis and are activated during
cell apoptosis. Caspases-3, −8 and −9 are major members of the
caspase family. The results showed that the protein of cleaved
caspases-3, −8 and −9 and mRNA levels of caspases-3, −8 and −9 were
significantly upregulated after transfection with CEACAM6-siRNA-2
(Fig. 4A-G).
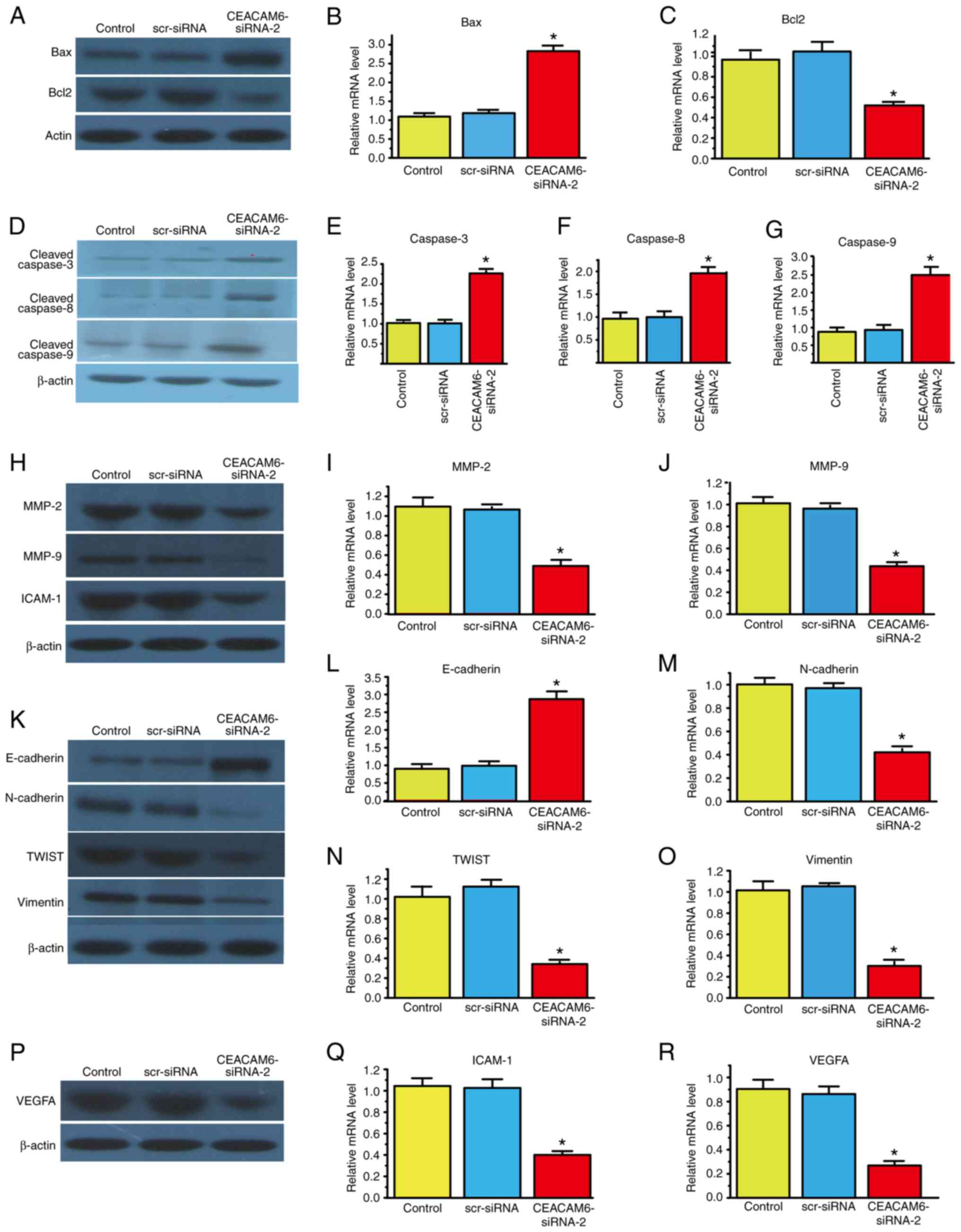 | Figure 4.Effects of silencing CEACAM6 with
CEACAM6-siRNA-2 after 48 h of transfection on the expression of
apoptosis-related molecules and epithelial-mesenchymal transition
markers. (A-G) Western blot and reverse transcription-quantitative
PCR analyses were employed to detect the effects of silencing
CEACAM6 on the expression of the pro-apoptotic protein Bax, cleaved
caspases-3, −8 and −9, and the anti-apoptotic protein Bcl-2. (H-R)
Western blot and reverse transcription-quantitative PCR analyses
were used to detect the effects of silencing CEACAM6 on the
expression of the extracellular matrix-related proteins MMP-2 and
MMP-9, the epithelial cell marker E-cadherin, the interstitial cell
marker N-cadherin, the intermediate filament protein vimentin, the
transcription factor TWIST, the tumor nutrient vascular
formation-related molecule VEGFA, and the tumorigenesis-promoting
factor ICAM-1. Data are shown as the mean ± standard error of the
mean (n=8), and were analyzed with one-way ANOVA followed by
Student-Newman-Keuls post hoc test. *P<0.05 scr-siRNA vs.
CEACAM6-siRNA. CEACAM6, carcinoembryonic antigen-related cell
adhesion molecule 6; siRNA, small interfering RNA; scr, scramble;
ICAM-1, intercellular cell adhesion molecule-1; TWIST,
Twist-related protein. |
CEACAM6 silencing decreases EMT marker
expression in RBE cells
There are four types of EMT markers (epithelial and
interstitial cell, transcription factor and cytoskeleton); of them,
the epithelial cell marker E-cadherin and the interstitial cell
marker N-cadherin are the most studied. In the present study,
E-cadherin expression was significantly increased, while N-cadherin
expression was significantly decreased after CEACAM6-siRNA-2
transfection.
MMPs are a group of zinc ion
(Zn2+)-dependent endopeptidases that degrade
extracellular matrix (ECM) and then induce pathological processes,
such as tumor cell invasion and migration. MMP-2 and MMP-9 are
important MMPs, and the protein and mRNA expression levels of these
endopeptidases were reduced after siRNA transfection.
The TWIST transcription factor enhances EMT by
inhibiting E-cadherin expression and enhancing tumor cell migration
and invasion. The protein and mRNA expression of TWIST decreased
after transfection with CEACAM6-siRNA-2.
Vimentin is an intermediate filament protein in
mesenchymal cells that regulates protein-protein interactions, such
as those involving cytoskeletal proteins and cell adhesion
molecules, which may participate in cell invasion, migration and
signal transduction. After transfection with CEACAM6-siRNA-2, the
expression level of this tumor-promoting molecule decreased.
Furthermore, tumor angiogenesis is associated with
VEGFA overexpression in human iCCA and pCCA (24). In the present study, the protein
and mRNA levels of VEGFA and tumorigenesis-promoting ICAM-1
decreased when CEACAM6-siRNA-2 was transfected into RBE cells. The
aforementioned results are shown in Fig. 4H-R.
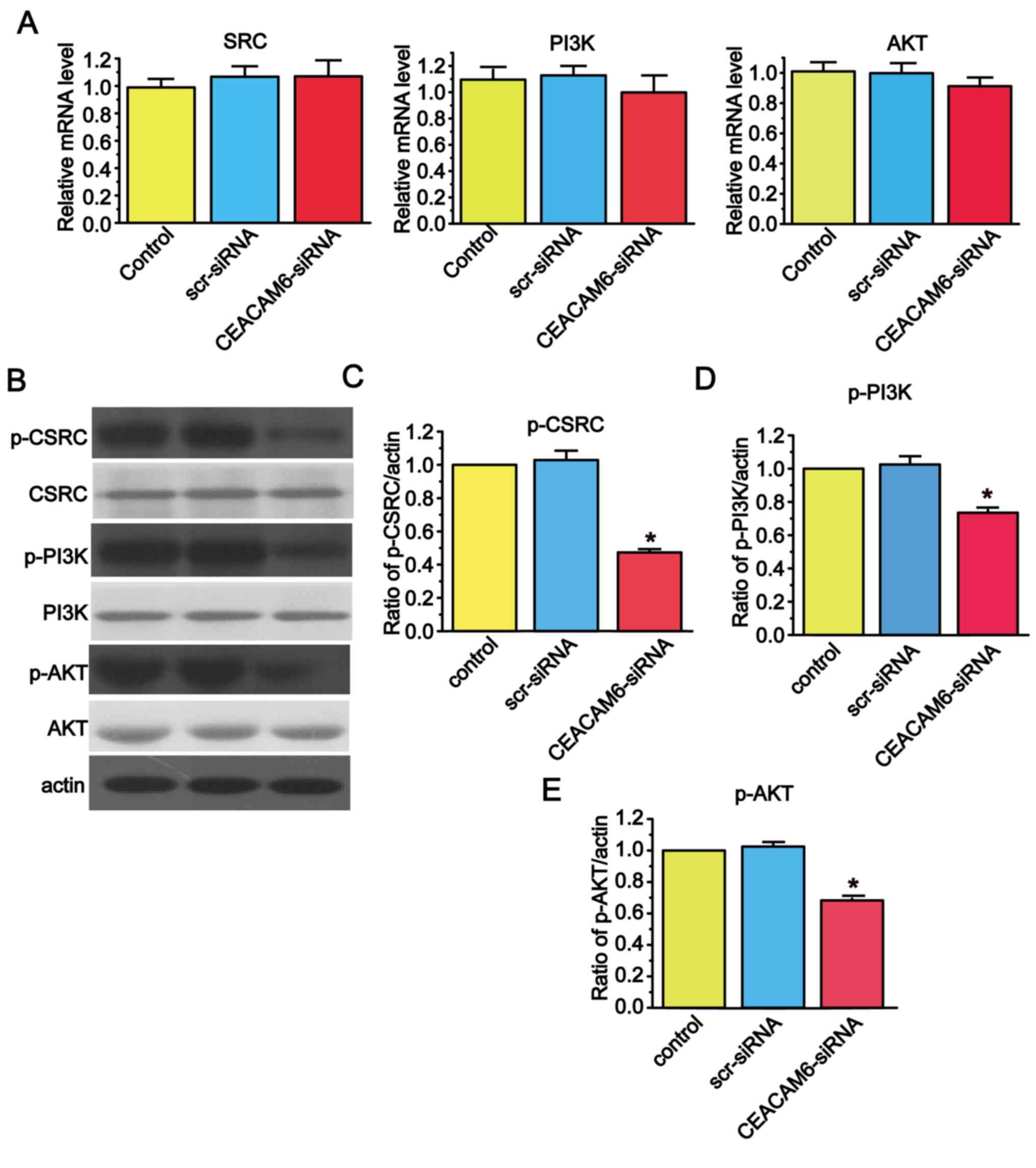
CEACAM6 silencing decreases the
expression of members of the SRC/PI3K/AKT signal transduction
pathway in RBE cells
The present study demonstrated that there was no
significant difference in the relative mRNA levels of molecules
involved in the SRC/PI3K/AKT signal transduction pathway when
CEACAM6 was knocked down (Fig.
5A). However, when CEACAM6-siRNA-2 was added to the cells, the
levels of phosphorylated molecules involved in the SRC/PI3K/AKT
signal transduction pathway was reduced (Fig. 5B-E).
Discussion
CCA is the second most common type of primary liver
cancer in human patients after HCC (5). The incidence and mortality rates of
iCCA are increasing worldwide. Despite advances in the detection
and treatment of metastatic CCA, mortality from this disease
remains high due to its limited early-stage cytological and
pathological diagnoses as well as limited effective treatments
(1). Therefore, it is important to
explore treatment methods based on specific markers and
targets.
CEACAM6 is a member of the immunoglobulin
superfamily, which is overexpressed in several human cancer types,
including colorectal, pancreatic, lung and breast cancer (20,25,26).
A previous report has revealed that CEACAM6 expression is
associated with adverse pathological features and prognosis in
pancreatic cancer (14). In
agreement with those findings, the present study demonstrated that
CEACAM6 was highly expressed in CCA tissues and was negatively
associated with the differentiation degree of CCA, suggesting that
CEACAM6 may be involved in the development and progression of CCA.
The present study demonstrated that CEACAM6 was highly expressed in
CCA tissue and was negatively associated with degree of
differentiation.
CEACAM6 is oncogenic, as it inhibits cell
differentiation, causes loss of cell polarity, and promotes cell
adhesion, invasion and metastasis (27). These oncogenic properties are
inhibited by an anti-CEACAM6 antibody in breast, pancreatic and
colorectal cancer (28).
Similarly, the present in vitro results revealed that the
invasion and migration of RBE cells decreased after CEACAM6-siRNA-2
transfection.
Apoptosis, or programmed cell death, is an essential
physiological process that plays a critical role in development and
tissue homeostasis (29). The
caspase cascade plays vital roles in the induction, transduction
and amplification of intracellular apoptotic signals. The present
results showed that the protein and mRNA expression levels of
cleaved caspases-3, −8 and −9 increased after transfection with
CEACAM6-siRNA-2.
EMT has gained increased attention in the past years
regarding metastatic dissemination (9). The present study demonstrated that
CEACAM6 was an important regulator of EMT biomarkers in CCA.
However, there may be other mechanisms involved in CCA progression,
such as the role played by circulating tumor cells or that of
exosomes released by different tumor cell types (30,31),
which suggests the need for further studies. Furthermore, a
previous study has shown that the function of CEACAM6 is dependent
on the c-SRC signaling pathway in pancreatic cancer (32).
In conclusion, the present study has demonstrated
that CEACAM6 is significantly overexpressed in CCA tissues, which
indicates that CEACAM6 could be utilized as a potential tumor EMT
biomarker. Understanding the clinical significance of CEACAM6
expression and its oncogenic mechanism may eventually lead to the
identification of a novel therapeutic target for human CCA
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hebei Science and
Technology Plan 2015 (grant no. 152377768D).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL performed the experiments and acquired data. MW
analyzed and processed data, and wrote and revised the manuscript.
HL, BL, XY and WZ analyzed and interpretated data. HL, BL, XY and
WZ confirm the authenticity of all the raw data. WW designed the
experiments and gave final approval of the version to be published.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethics approval was from The Second Hospital of
Hebei Medical University. Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saha SK, Zhu AX, Fuchs CS and Brooks GA:
Forty-year trends in cholangiocarcinoma incidence in the U.S.:
Intrahepatic disease on the rise. Oncologist. 21:594–599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kayhanian H, Smyth EC and Braconi C:
Emerging molecular targets and therapy for cholangiocarcinoma.
World J Gastrointest Oncol. 9:268–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Taylor-Robinson SD, Toledano MB,
Beck A, Elliott P and Thomas HC: Changing international trends in
mortality rates for liver, biliary and pancreatic tumours. J
Hepatol. 37:806–813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Battaglia S, Benzoubir N, Nobilet S,
Charneau P, Samuel D, Zignego AL, Atfi A, Brechot C and Bourgeade
MF: Liver cancer-derived hepatitis C virus core proteins shift
TGF-beta responses from tumor suppression to epithelial-mesenchymal
transition. PLoS One. 4:e43552009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaquero J, Guedj N, Claperon A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu JY, Zhou F, Yu L and Zhang J:
Epithelial-mesenchymal transition of small airway epithelium in
patients receiving lung tumor surgery with normal lung function and
chronic obstructive pulmonary disease. Zhonghua Yi Xue Za Zhi.
99:2681–2686. 2019.(In Chinese). PubMed/NCBI
|
|
10
|
Baum B, Settleman J and Quinlan MP:
Transitions between epithelial and mesenchymal states in
development and disease. Semin Cell Dev Biol. 19:294–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizeq B, Zakaria Z and Ouhtit A: Towards
understanding the mechanisms of actions of carcinoembryonic
antigen-related cell adhesion molecule 6 in cancer progression.
Cancer Sci. 109:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamanka T, Kuroki M, Matsuo Y and Matsuoka
Y: Analysis of heterophilic cell adhesion mediated by CD66b and
CD66c using their soluble recombinant proteins. Biochem Biophys Res
Commun. 219:842–847. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ieta K, Tanaka F, Utsunomiya T, Kuwano H
and Mori M: CEACAM6 gene expression in intrahepatic
cholangiocarcinoma. Br J Cancer. 95:532–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rose JB, Correa-Gallego C, Li Y, Nelson J,
Alseidi A, Helton WS, Allen PJ, D'Angelica MI, DeMatteo RP, Fong Y,
et al: The role of biliary carcinoembryonic antigen-related
cellular adhesion molecule 6 (CEACAM6) as a biomarker in
cholangiocarcinoma. PLoS One. 11:e01501952016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farina A, Dumonceau JM, Antinori P,
Annessi-Ramseyer I, Frossard JL, Hochstrasser DF, Delhaye M and
Lescuyer P: Bile carcinoembryonic cell adhesion molecule 6 (CEAM6)
as a biomarker of malignant biliary stenoses. Biochim Biophys Acta.
1844:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuespert K, Pils S and Hauck CR: CEACAMs:
Their role in physiology and pathophysiology. Curr Opin Cell Biol.
18:565–571. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ilantzis C, DeMarte L, Screaton RA and
Stanners CP: Deregulated expression of the human tumor marker CEA
and CEA family member CEACAM6 disrupts tissue architecture and
blocks colonocyte differentiation. Neoplasia. 4:151–163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ordonez C, Screaton RA, Ilantzis C and
Stanners CP: Human carcinoembryonic antigen functions as a general
inhibitor of anoikis. Cancer Res. 60:3419–3424. 2000.PubMed/NCBI
|
|
20
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: CEACAM6 gene silencing impairs anoikis resistance and
in vivo metastatic ability of pancreatic adenocarcinoma cells.
Oncogene. 23:465–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Li Q, An Y, Lv N, Xue X, Wei J,
Jiang K, Wu J, Gao W, Qian Z, et al: CEACAM6 induces
epithelial-mesenchymal transition and mediates invasion and
metastasis in pancreatic cancer. Int J Oncol. 43:877–885. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Zang M, Li J, Ji J, Zhang J, Liu
X, Qu Y, Su L, Li C, Yu Y, et al: CEACAM6 promotes tumor migration,
invasion, and metastasis in gastric cancer. Acta Biochim Biophys
Sin (Shanghai). 46:283–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mancarella S, Serino G, Dituri F, Cigliano
A, Ribback S, Wang J, Chen X, Calvisi DF and Giannelli G:
Crenigacestat, a selective NOTCH1 inhibitor, reduces intrahepatic
cholangiocarcinoma progression by blocking VEGFA/DLL4/MMP13 axis.
Cell Death Differ. 27:2330–2343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim KS, Kim JT, Lee SJ, Kang MA, Choe IS,
Kang YH, Kim SY, Yeom YI, Lee YH, Kim JH, et al: Overexpression and
clinical significance of carcinoembryonic antigen-related cell
adhesion molecule 6 in colorectal cancer. Clin Chim Acta.
415:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis-Wambi JS, Cunliffe HE, Kim HR,
Willis AL and Jordan VC: Overexpression of CEACAM6 promotes
migration and invasion of oestrogen-deprived breast cancer cells.
Eur J Cancer. 44:1770–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi M, Miki Y, Ebina M, Abe K, Mori
K, Narumi S, Suzuki T, Sato I, Maemondo M, Endo C, et al:
Carcinoembryonic antigen-related cell adhesion molecules as
surrogate markers for EGFR inhibitor sensitivity in human lung
adenocarcinoma. Br J Cancer. 107:1745–1753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zang M, Zhang B, Zhang Y, Li J, Su L, Zhu
Z, Gu Q, Liu B and Yan M: CEACAM6 promotes gastric cancer invasion
and metastasis by inducing epithelial-mesenchymal transition via
PI3K/AKT signaling pathway. PLos One. 9:e1129082014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makler A and Asghar W: Exosomal biomarkers
for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn.
20:387–400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng Z, Wu J, Xu S, Chen F, Zhang Z, Jin A
and Wang J: Exosomes-microRNAs interacted with gastric cancer and
its microenvironment: A mini literature review. Biomark Med.
14:141–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duxbury MS, Ito H, Ashley SW and Whang EE:
c-Src-dependent cross-talk between CEACAM6 and alphavbeta3 integrin
enhances pancreatic adenocarcinoma cell adhesion to extracellular
matrix components. Biochem Biophys Res Commun. 317:133–141. 2004.
View Article : Google Scholar : PubMed/NCBI
|