Introduction
Leukemia is a malignant cancer of the hemopoietic
system (1). The morbidity rate of
patients with leukemia remains high due to factors, such as the
living environment and diet (2).
Chemotherapy and bone marrow transplantation are the main treatment
strategies for leukemia (3);
however, patients with different types of leukemia may receive
different treatments (4). Acute
B-lymphocytic leukemia (B-ALL) has a high mortality rate (5) and no effective treatment strategies
(6,7). Thus, identification of diagnostic and
prognostic biomarkers of B-ALL can contribute to the development of
novel therapeutic methods and drugs, which can improve the survival
outcomes of patients with B-ALL.
Circular RNAs (circRNAs) are non-coding RNA
molecules, with a closed ring structure that lacks the 5′ and 3′
ends of traditional linear RNA (8). circRNAs are conserved,
tissue-specific, insensitive to RNase and more stable compared with
linear RNA (9). These
characteristics suggest that circRNAs may have great potential for
cancer diagnosis, treatment and identification of novel methods to
overcome human cancer (10,11).
Previous studies have demonstrated that the expression levels of
some circRNAs are higher in cancer tissues compared with normal
tissues, and some circRNAs can serve as prognostic biomarkers
(12–14). Thus, it is important to investigate
differentially expressed circRNAs in B-ALL cells and normal human B
lymphoblasts.
The present study aimed to investigate the
expression profiles of circRNAs in B-ALL and HMy2.CIR cells via
high throughput RNA sequencing, identify downregulated circRNAs in
B-ALL cells compared with HMy2.CIR cells, and perform Gene Ontology
(GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analyses of differentially expressed circRNAs to determine
their function. In addition, the present study aimed to validate
these differentially expressed circRNAs using bone marrow samples
from patients with and without B-ALL.
Materials and methods
Bone marrow samples
Bone marrow samples were collected from six patients
treated at the Department of Hematology, Shengjing Hospital of
China Medical University, (Shenyang, China), between September 2014
and December 2016. All patients were diagnosed with B-ALL and
received the CCLG-ALL-2008 treatment plan (15). These patients included three boys
and three girls (mean age, 5.8 years; age range, 4–8 years). Bone
marrow samples were also collected from six individuals without
B-ALL (control). These patients included three boys and three girls
(mean age, 5.5 years; age range, 4–8 years). The inclusion criteria
were as follows: Initial diagnosis was according to the 2008 World
Health Organization classification standard of lymphoid and
hematopoietic tissue tumors and bone marrow cytology, immunotyping,
chromosome karyotype analysis and fusion gene analysis were
performed (16). The exclusion
criteria were as follows: i) Patients received anti-leukemia
treatment prior to admission; ii) patients with mature B-ALL and
mixed cell leukemia and iii) patients with secondary ALL or other
tumors. The present study was approved by the China Medical
University Ethics Committee (institution review board no. 2020028)
and written informed consent was provided by all participants prior
to the study start.
Cells and cell culture
The human B-ALL cell line, Ball-1, and the human B
lymphoblast cell line, HMy2.CIR, were purchased from the Type
Culture Collection of The Chinese Academy of Sciences. Cells were
maintained in RPMI-1640 medium (Hyclone; Cytiva) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), at
37°C with 5% CO2.
The HMy2.CIR cell line was used as the control
according to previous studies (17–20),
which demonstrated that HMy2.CIR cells are comparable with human B
lymphoblasts and have a lower malignancy potential than B-ALL
cells.
circRNA expression profile
analysis
Total RNA was extracted from Ball-1 and HMy2.CIR
cells with TRIzol® reagent (Beijing Solarbio Science
& Technology Co., Ltd.). A total of three samples were taken
from each cell line. circRNAs were enriched from 5 µg total RNA
using the circRNA Enrichment kit (CloudSeq, www.cloud-seq.com.cn). Subsequently, strand-specific
RNA-seq libraries were prepared using the TruSeq Stranded Total RNA
Library Prep kit (Illumina, Inc.), which were subjected to deep
sequencing using BioAnalyzer 2100 (Agilent Technologies, Inc.).
Identification of circRNAs
The RNA-seq FASTQ reads were mapped to a human
reference genome (GRCh37/hg19) using TopHat2 (21). The number of spliced reads were
used as the expression level of each circRNA. The total number of
reads was used to standardize the samples, and log2
transformation was performed to obtain the standardized number of
reads. Using the standardized number of reads and the R software
package DEGseq (22), differential
expression of circRNAs between Ball-1 and HMy2.CIR cells were
calculated. Log2 (fold change) >2.0 (or <-2.0) and
P<0.05 were considered to indicate significantly differentially
expressed circRNAs. Hierarchical clustering analysis was also
performed to generate an overview of circRNA expression profiles
between the two cell lines.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from Ball-1 cells, HMy2.CIR
cells, B-ALL bone marrow and non-B-ALL bone marrow using
TRIzol® reagent, and reverse transcribed into cDNA using
PrimeScript™ RT reagent Kit with gDNA Erase (Takara Biotechnology
Co., Ltd.), according to the manufacturer's protocol. qPCR was
subsequently performed using the SYBR-Green PCR Master Mix (Takara
Biotechnology Co., Ltd.) within the Fast-Real-Time PCR System
(CFX96™ Optics Module; Bio-Rad Co., Ltd.). The following primer
sequences were used for qPCR: GAPDH forward,
5′-GGCCTCCAAGGAGTAAGACC-3′ and reverse, 5′-AGGGGAGATTCAGTGTGGTG-3′.
Primer sequences for the circRNAs are listed in Table SI. The following thermocycling
conditions were used for qPCR: 95°C for 30 sec, followed by 40
cycles of 95°C for 3 sec and 60°C for 30 sec and a final extension
step at 60°C for 7 min. Relative expression levels were calculated
using the 2−ΔΔCq method (23) and normalized to the internal
reference gene GAPDH.
Bioinformatic analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID, http://david.ncifcrf.gov) was used to perform GO
enrichment and KEGG pathway analyses of the differentially
expressed circRNAs to determine the processes and pathways
involved.
Cell transfection
Under normal culture conditions, cells were starved
in serum-free RPMI-1640 medium (Hyclone, Cytiva) for 2 h after
reaching 60–70% confluence. OE-CIRC and OE-negative control (NC)
were respectively transfected into HMy2.CIR cells using
Lipofectamine® 3000, according to the manufacturer's
protocol. Cells were harvested for subsequent experimentation after
24–72 h. The concentration of plasmid OE-CIRC and OE-NC of
hsa_circ_0000745 purchased from jtsbio company was 100 nM/l. The
temperature and duration of transfection were 37°C and 24–72 h.
Fluorescence intensity was detected via fluorescence microscopy
(magnification, ×200; Nikon Corporation), according to the
manufacturer's instructions.
Cell proliferation
After 24 h of transfection, cell suspensions of the
experimental and control groups were inoculated into 96-well plates
at a density of 3,000 cells/well/100 µl. The plates were
precultured in the incubator at 37°C with 5% CO2. Cell
Counting Kit-8 (10 µl) reagent (Cofitt Life Science Company;
www.cofitt.com) was subsequently added to each
well and incubated for an additional 4 h. The absorbance was
measured at a wavelength of 450 nm, using a microplate reader
(BioTek Instruments, lnc.). The OD value of each well was measured
for 3 consecutive days.
Statistical analysis
Statistical analysis was performed using SPSS 23.0
software (IBM Corp.). Edge R 4.1.0 software (https://www.r-project.org) was used to establish a
linear model of negative binomial distribution according to various
factors in the experimental design, and the dispersion coefficient
of each factor was calculated (Fig.
1). The Quasi-Likelihood F test (P<0.05 and fold change
≥2.0) was used to screen the differentially expressed circRNAs. The
volcano map was constructed to compare differences between the two
groups (P<0.05 and fold change ≥2.0). The Heatmap. 2 function of
R 4.1.0 software (https://www.r-project.org) was used to cluster the
differentially expressed circRNAs. Unpaired independent sample
t-test was used to compare the expression of circRNAs in the
different cell lines and samples. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of differentially
expressed circRNAs in Ball-1 and HMy2.CIR cells
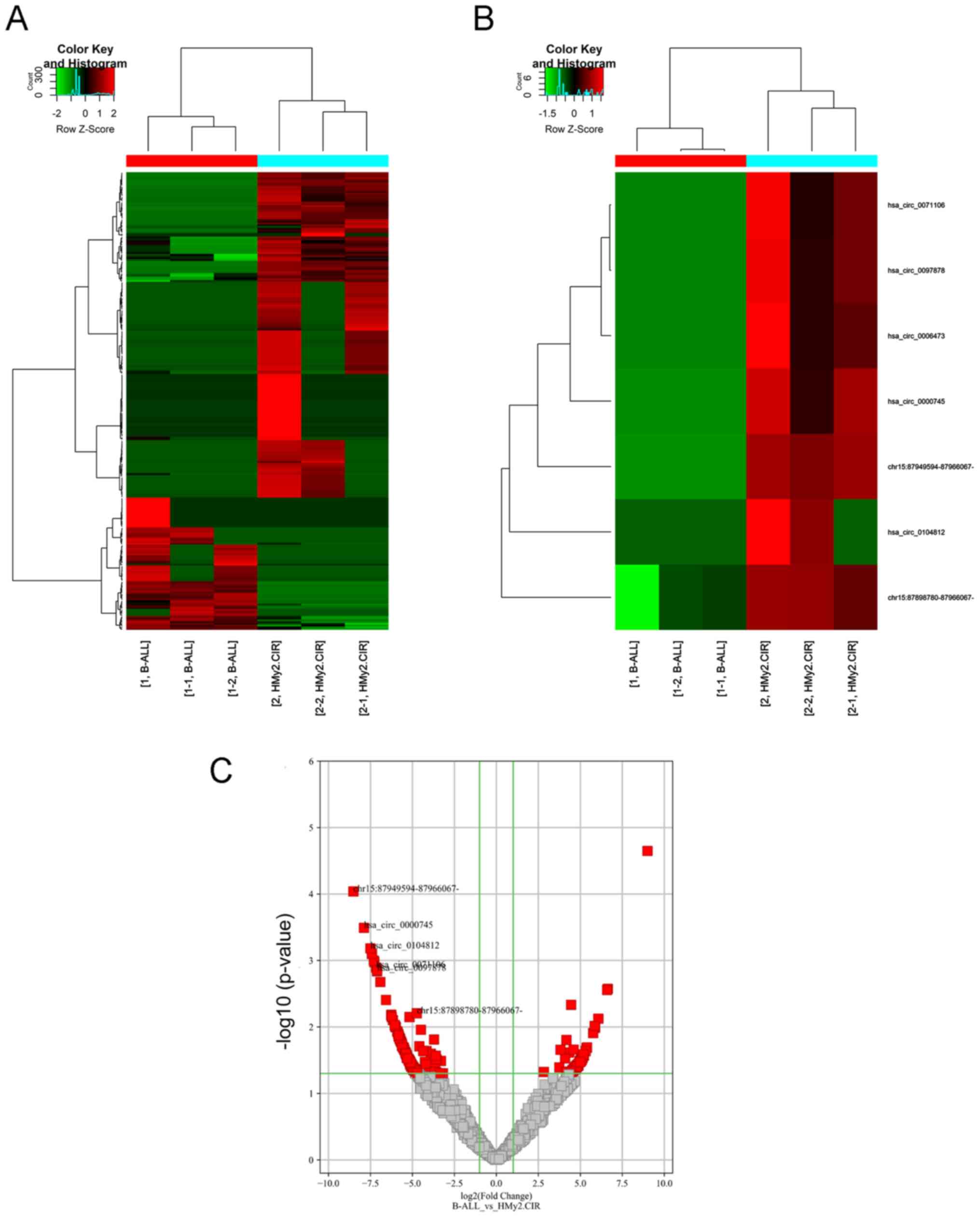
To identify the differentially expressed circRNAs in
Ball-1 and HMy2.CIR cells, the circRNA expression profiles were
analyzed via high-throughput circRNA microarray analysis. A total
of 263 differentially expressed circRNAs were identified
[log2 (fold change) >2.0 (or ≥2.0) and P<0.05], of
which 76 were upregulated and 187 were downregulated in Ball-1
cells compared with HMy2.CIR cells (Fig. 1A-C).
GO enrichment and KEGG pathway
analyses of differentially expressed circRNAs
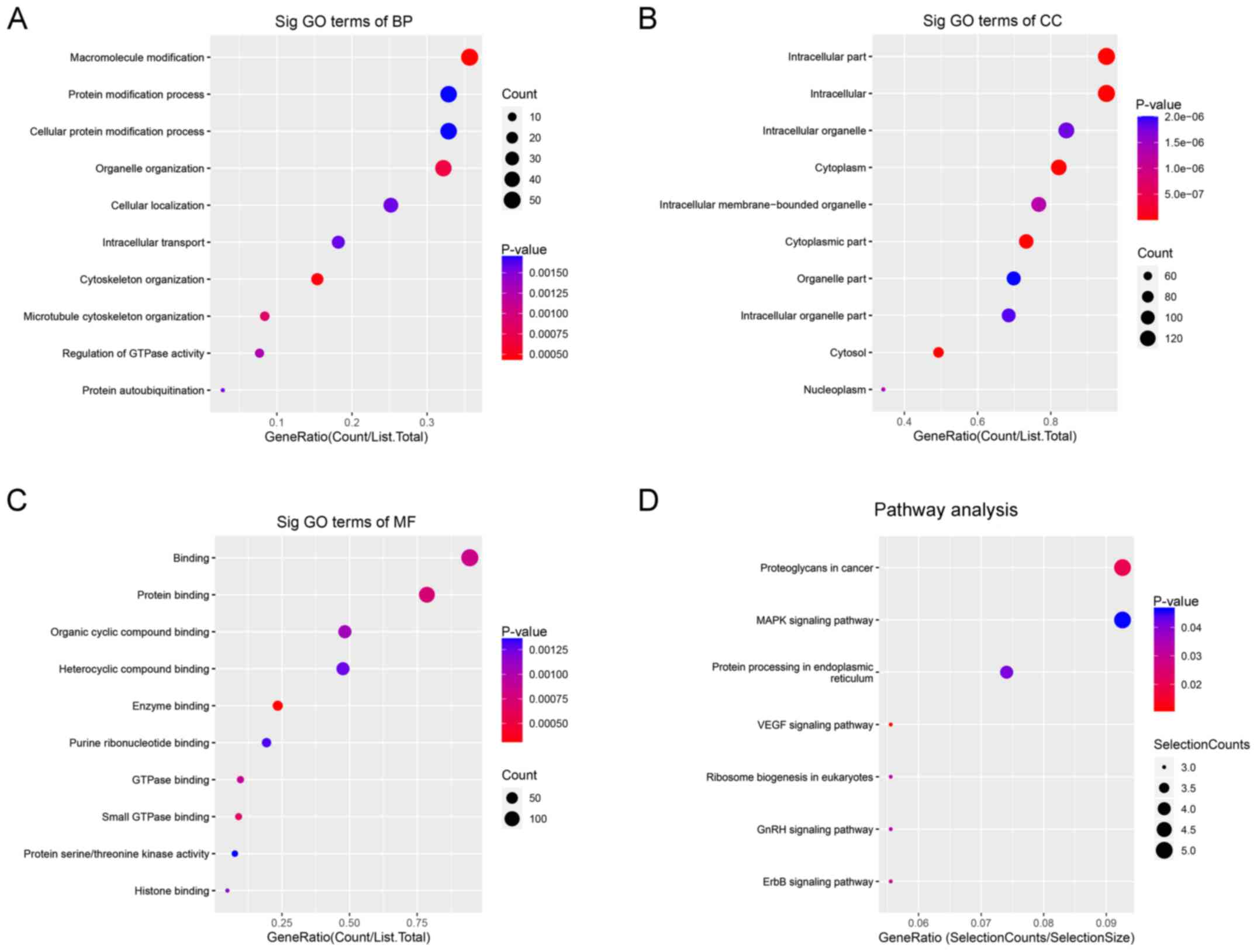
GO enrichment and KEGG pathway analyses of the
differentially expressed circRNAs were performed using DAVID. GO
enrichment analysis identified three terms, biological process
(BP), cellular component (CC) and molecular function (MF).
For BP, upregulated circRNAs were mainly enriched in
‘macromolecule modification’ (Fig.
2A). For CC, upregulated circRNAs were mainly enriched in
‘intracellular parts’ (Fig. 2B).
For MF, upregulated circRNAs were mainly enriched in ‘protein
binding’ (Fig. 2C). KEGG pathway
analysis demonstrated that upregulated circRNAs were mainly
enriched in ‘Proteoglycans in cancer’, ‘MAPK signaling pathway’ and
‘protein processing in endoplasmic reticulum’ (Fig. 2D).
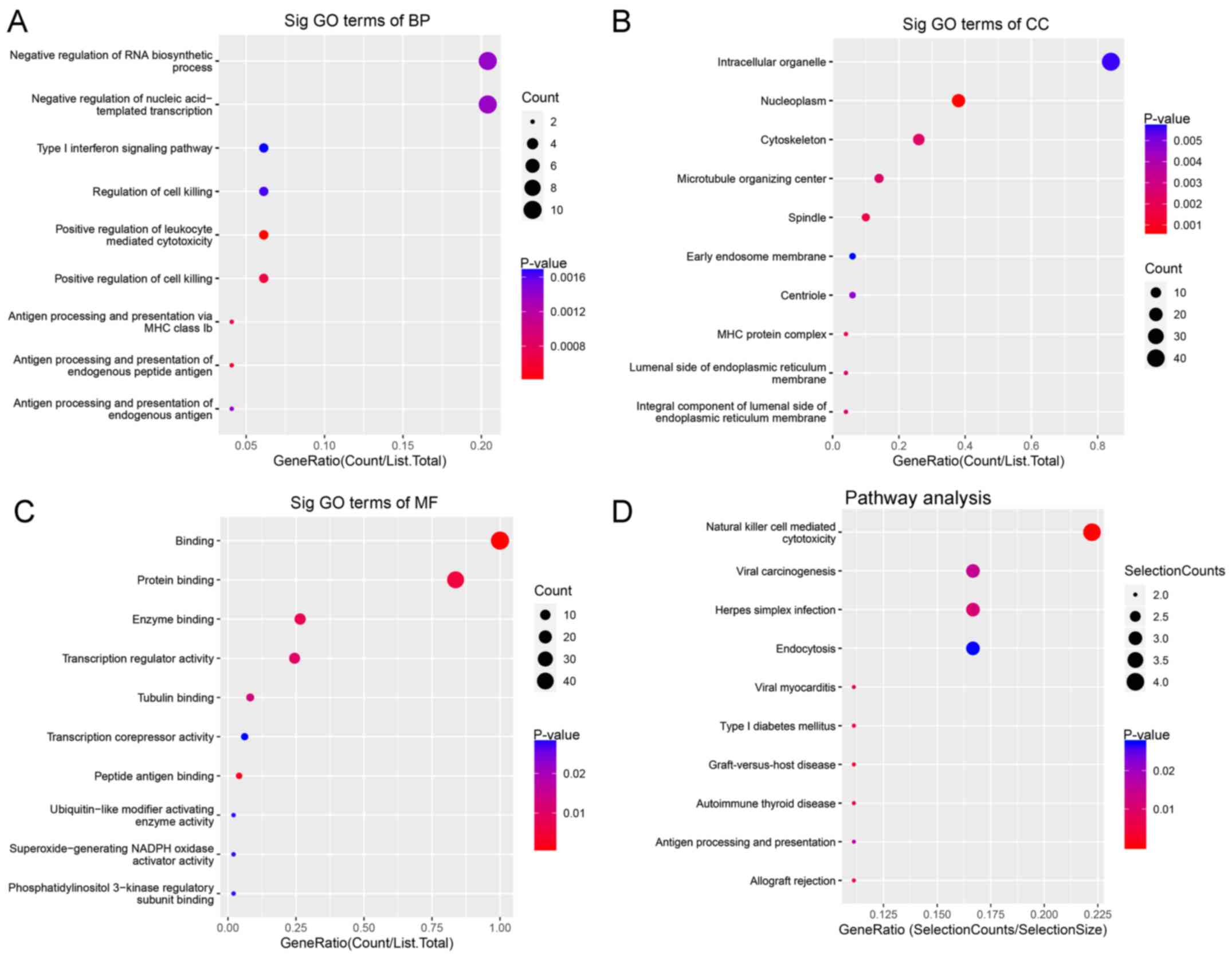
For BP, downregulated circRNAs were mainly enriched
in ‘negative regulation of RNA biosynthetic processes’ and
‘negative regulation of nucleic acid templated transcription’
(Fig. 3A). For CC, downregulated
circRNAs were mainly enriched in ‘intracellular organelles’
(Fig. 3B). For MF, downregulated
circRNAs were mainly enriched in ‘transcription regulator activity
and binding’ (Fig. 3C). KEGG
pathway analysis demonstrated that downregulated circRNAs were
mainly enriched in ‘natural killer cell-mediated cytotoxicity’ and
‘viral carcinogenesis’ (Fig.
3D).
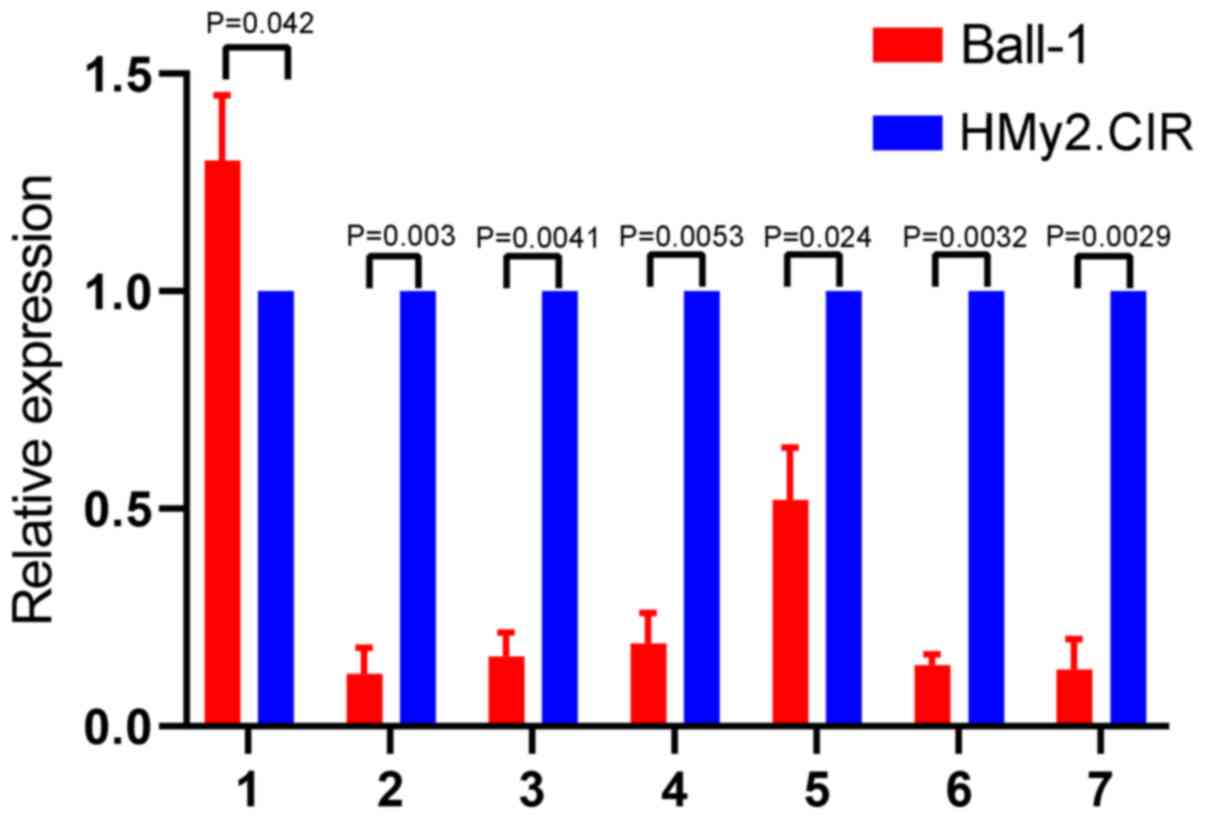
Validation of seven downregulated
circRNAs in Ball-1 cells
To validate the microarray results, seven circRNAs
downregulated in Ball-1 cells were selected and their expression
was validated via RT-qPCR analysis. The seven differentially
expressed circRNAs were selected according to the following
criteria: i) High fold-change value, ii) significant P-value and
iii) not reported in previous studies. The results are presented in
Fig. 4. A total of six circRNAs
(hsa_circ_0000745, chr15:87949594-87966067-,
chr15:87898780-87966067-, hsa_circ_0006473, hsa_circ_0071106 and
hsa_circ_0097878) were significantly downregulated in Ball-1 cells
compared with HMy2.CIR cells (P=0.003; P=0.0041; P=0.0053; P=0.024;
P=0.0032; P=0.0029), while has_circ_0104812 expression was slightly
but significantly upregulated in Ball-1 cells compared with
HMy2.CIR cells (P=0.042).
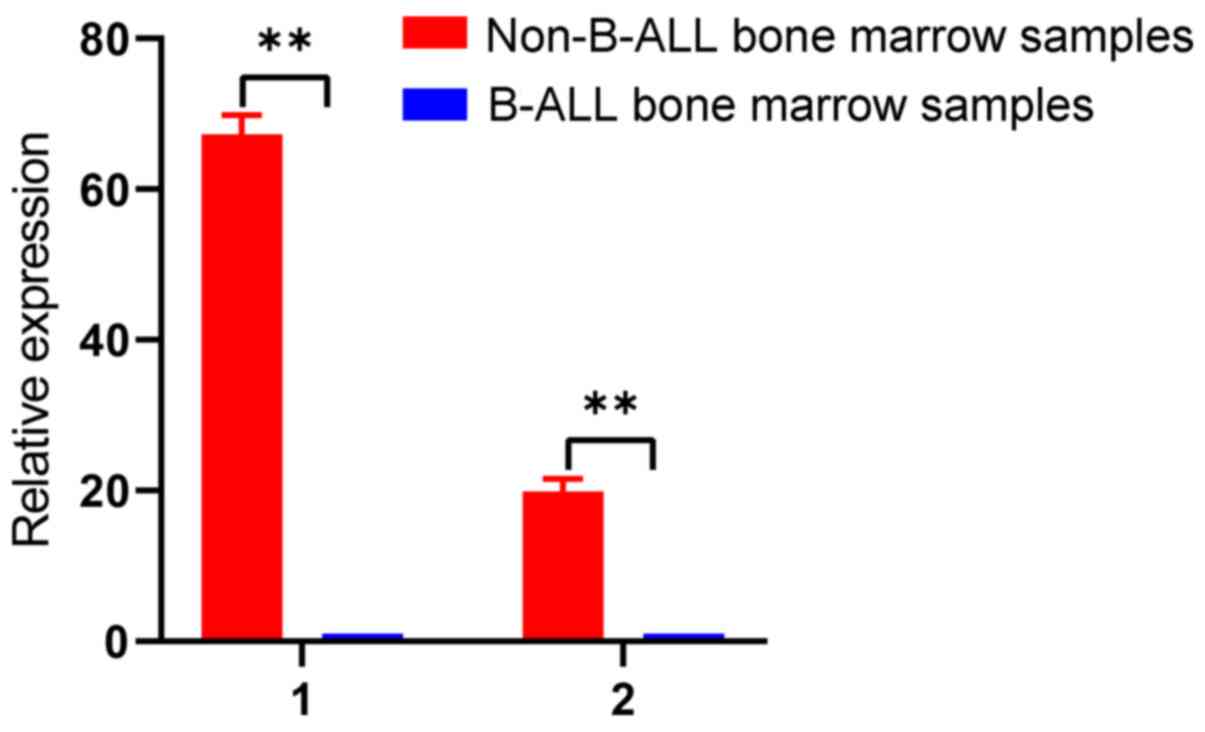
Validation of two downregulated
circRNAs in B-ALL bone marrow samples
To validate the results from the cell lines,
hsa_circ_0000745 and chr15:87949594-87966067- were selected to
validate bone marrow samples from patients with B-ALL. The results
demonstrated that these two circRNAs were significantly
downregulated in B-ALL bone marrow samples compared with non-B-ALL
bone marrow samples (P<0.01; Fig.
5). Therefore, these two circRNAs may serve as biomarkers for
B-ALL. Detailed characteristics of the patients and controls are
presented in Table SII.
Validation of the function of
hsa_circ_0000745 in HMy2.CIR cells
hsa_circ_0000745 expression was overexpressed in
HMy2.CIR cells, and the results demonstrated that high
hsa_circ_0000745 expression significantly inhibited the
proliferation of HMy2.CIR cells (P=0.034; Fig. S1). Transfection efficiency is
presented in Fig. S2.
Discussion
The development of next-generation sequencing
methods, such as RNA sequencing, has led to the discovery of
non-coding RNAs, including circRNAs, that may contribute to the
progression of cancer (24).
circRNAs can regulate proliferation, clonal selection and somatic
hypermutation, posing a risk of malignant transformation of normal
B cells (25). In addition,
deregulation of circRNAs, such as circESYT2, circFBXW4 and
circCAMSAP1, promotes the development of B-cell malignancies, such
as B-ALL (25). Further, circRNAs
can function as microRNA sponges, thereby acting as ceRNAs to
regulate the development of B-ALL (26,27).
The present study identified differentially
expressed circRNAs in Ball-1 and HMy2.CIR cells using microarray.
Downregulated circRNAs in Ball-1 cells were enriched in the
regulation of leukocyte-mediated cytotoxicity, suggesting that
these circRNAs may be involved in the progression of B-ALL. A total
of seven downregulated circRNAs in Ball-1 cells were validated. The
results demonstrated that six circRNAs (hsa_circ_0000745,
chr15:87949594-87966067-, chr15:87898780-87966067-,
hsa_circ_0006473, hsa_circ_0071106 and hsa_circ_0097878) were
significantly downregulated in Ball-1 cells compared with HMy2.CIR
cells. Similar results were obtained using B-ALL bone marrow
samples, whereby hsa_circ_0000745 and chr15:87949594-87966067- were
significantly downregulated in patients with B-ALL. These results
suggest that these circRNAs can be used as biomarkers for patients
with B-ALL.
B-ALL is the most common cancer diagnosed in
children (28,29). Although multi-drugs chemotherapy
has been widely applied in clinical practice, delayed diagnosis and
the relapse of B-ALL are major causes of mortality (30,31).
The pathogenesis and molecular mechanism of B-ALL occurrence and
progression remain largely unknown. Thus, identifying diagnostic
and prognostic biomarkers of B-ALL is important to improve the
survival rate and quality of life of those affected. The results of
the present study suggest that hsa_circ_0000745 and
chr15:87949594-87966067- may serve as biomarkers for the diagnosis,
and even treatment of B-ALL.
The present study is not without limitations. First,
the validated circRNAs require further investigation to determine
their roles in the development of B-ALL. Secondly, the prognostic
effects of these circRNAs require further investigation using
clinical samples.
In conclusion, the present study identified and
validated six differentially expressed circRNAs in Ball-1 cells and
further confirmed the downregulation of two circRNAs in bone marrow
samples from patients with B-ALL. Thus, these circRNAs may serve as
potential biomarkers in patients with B-ALL.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used analyzed during the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
BZ and LZ performed most of the experiments and
drafted the initial manuscript. LT, YZ and RW performed the
experiments, and BZ performed statistical analysis. QH and YJZ
conceived the present study, and participated in its design and
coordination. BZ analyzed and interpreted the results. BZ and YJZ
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the China Medical
University Ethics Committee (institution review board no. 2020028)
and written informed consent was provided by all participants prior
to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kantarjian HM, Keating MJ and Freireich
EJ: Toward the potential cure of leukemias in the next decade.
Cancer. 124:4301–4313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miranda-Filho A, Piñeros M, Ferlay J,
Soerjomataram I, Monnereau A and Bray F: Epidemiological patterns
of leukaemia in 184 countries: A population-based study. Lancet
Haematol. 5:e14–e24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atsuta Y, Kato S, Morishima Y, Ohashi K,
Fukuda T, Ozawa Y, Eto T, Iwato K, Uchida N, Ota S, et al HLA
Working Group of the Japan Society for Hematopoietic Cell
Transplantation, : Comparison of HLA allele mismatch and antigen
mismatch in unrelated bone marrow transplantation in patients with
leukemia. Biol Blood Marrow Transplant. 25:436–442. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung C and Ma H: Driving toward precision
medicine for acute leukemias: Are we there yet? Pharmacotherapy.
37:1052–1072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fousek K, Watanabe J, Joseph SK, George A,
An X, Byrd TT, Morris JS, Luong A, Martínez-Paniagua MA, Sanber K,
et al: CAR T-cells that target acute B-lineage leukemia
irrespective of CD19 expression. Leukemia. 35:75–89. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19–28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tambaro FP, Garcia-Manero G, O'Brien SM,
Faderl SH, Ferrajoli A, Burger JA, Pierce S, Wang X, Do KA,
Kantarjian HM, et al: Outcomes for patients with chronic
lymphocytic leukemia and acute leukemia or myelodysplastic
syndrome. Leukemia. 30:325–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patop IL, Wüst S and Kadener S: Past,
present, and future of circRNAs. EMBO J. 38:e1008362019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: circRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shuai M, Hong J, Huang D, Zhang X and Tian
Y: Upregulation of circRNA_0000285 serves as a prognostic biomarker
for nasopharyngeal carcinoma and is involved in radiosensitivity.
Oncol Lett. 16:6495–6501. 2018.PubMed/NCBI
|
|
13
|
Lin G, Sheng H, Xie H, Zheng Q, Shen Y,
Shi G and Ye D: circLPAR1 is a novel biomarker of prognosis for
muscle-invasive bladder cancer with invasion and metastasis by
miR-762. Oncol Lett. 17:3537–3547. 2019.PubMed/NCBI
|
|
14
|
Chaichian S, Shafabakhsh R, Mirhashemi SM,
Moazzami B and Asemi Z: Circular RNAs: A novel biomarker for
cervical cancer. J Cell Physiol. 235:718–724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui L, Li ZG, Chai YH, Yu J, Gao J, Zhu
XF, Jin RM, Shi XD, Zhang LP, Gao YJ, et al; Chinese Children
Leukemia Group (CCLG), . Outcome of children with newly diagnosed
acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first
nation-wide prospective multicenter study in China. Am J Hematol.
93:913–920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A, et al: The 2008 revision of the
World Health Organization (WHO) classification of myeloid neoplasms
and acute leukemia: Rationale and important changes. Blood.
114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He M, Dong C, Konishi T, Tu W, Liu W,
Shiomi N, Kobayashi A, Uchihori Y, Furusawa Y, Hei TK, et al:
Differential effects of p53 on bystander phenotypes induced by
gamma ray and high LET heavy ion radiation. Life Sci Space Res
(Amst). 1:53–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye S, Yuan D, Xie Y, Pan Y and Shao C:
Role of DNA methylation in long-term low-dose γ-rays induced
adaptive response in human B lymphoblast cells. Int J Radiat Biol.
89:898–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Tan Q, Hu B, Wu H, Wang C, Liu R
and Tang C: Somatostatin improved B cells mature in macaques during
intestinal ischemia-reperfusion. PLoS One. 10:e01336922015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye S, Yuan D, Xie Y, Pan Y and Shao C:
Role of DNA methylation in the adaptive responses induced in a
human B lymphoblast cell line by long-term low-dose exposures to
γ-rays and cadmium. Mutat Res Genet Toxicol Environ Mutagen.
773:34–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Feng Z, Wang X, Wang X and Zhang
X: DEGseq: An R package for identifying differentially expressed
genes from RNA-seq data. Bioinformatics. 26:136–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adnan M, Morton G and Hadi S: Analysis of
rpoS and bolA gene expression under various stress-induced
environments in planktonic and biofilm phase using 2(−ΔΔCT) method.
Mol Cell Biochem. 357:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao
L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, et al: The
landscape of circular RNA in cancer. Cell. 176:869–881.e13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahl M, Kristensen LS and Grønbæk K: Long
non-coding RNAs guide the fine-tuning of gene regulation in B-cell
development and malignancy. Int J Mol Sci. 19:24572018. View Article : Google Scholar
|
|
26
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Locatelli F, Schrappe M, Bernardo ME and
Rutella S: How I treat relapsed childhood acute lymphoblastic
leukemia. Blood. 120:2807–2816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhojwani D and Pui CH: Relapsed childhood
acute lymphoblastic leukaemia. Lancet Oncol. 14:e205–e217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jing W and Li J: Identification of
biomarkers for the prediction of relapse free survival in pediatric
B precursor acute lymphoblastic leukemia. Oncol Rep. 41:659–667.
2019.PubMed/NCBI
|