Introduction
Carnosine (β-alanyl-L-histidine) is a natural
dipeptide that is found in muscle and brain tissue, especially in
lean beef, fish and chicken (1).
Previous studies have shown that carnosine has a number of
physiological effects, including antiaging effects (2), cerebral protection (3), antioxidation (4), inhibiting inflammation (5), reducing Parkinson's disease (6) and inhibiting metastasis (7,8).
Carnosine has been shown to inhibit the proliferation of human
gastric carcinoma cells by regulating Akt and mammalian target of
rapamycin (mTOR) signaling (9). In
addition to the physiological effects of carnosine itself, there
are various physiological and regulatory effects that are induced
by the metabolites of carnosine. During methylation, carnosine will
be reacted to anserine and ophidine. These reactions lead to
methylhistidine formation, which is an important indicator of
muscle breakdown (10). However,
the hydrolysis of carnosine produces histidine and β-alanine.
β-alanine is involved in the synthesis of CoA, nucleic acids and
histidine, the decarboxylation of which yields histamine (11). Carnosine reduces apoptosis in
murine podocytes by reducing Bcl-2-associated X protein (Bax) and
increasing B-cell lymphoma-2 (Bcl-2) mRNA levels (12). Lee et al (13) also show that carnosine can induce
apoptosis and cell cycle arrest to lead to reduced cell viability
in human colorectal HCT-116 cells. However, the investigation of
carnosine-mediated suppression of cell proliferation and induction
of cell growth in human colorectal cells is remains an important
issue.
Worldwide, colorectal cancer (CRC) is the third most
diagnosed cancer and the fourth leading cause of mortality
(14). In addition, the incidence
of CRC was 10.2% and the mortality was 9.0% in 2018 according to
global cancer statistics (15).
Instead of clinical cancer treatments, such as surgery,
chemotherapy and radiotherapy, chemoprevention by active compounds
in food to reduce or inhibit cancer cell proliferation could be a
good strategy for reducing cancer-related damage to human health.
Between the various forms of cell death such as necrosis,
apoptosis, autophagy and necroptosis, a balance exists between
normal cell proliferation and growth, abnormal cancer cell
proliferation and programmed cell death induction (16). Wnt/β-catenin/transcription factor 4
(Tcf-4) signaling activation serves an important role in regulating
cell proliferation (17). This
transcription process can modify the cell cycle distribution and
cell proliferation (17).
Apoptosis serves a major role in the regulation of carcinogenesis
(18). Apoptosis is controlled by
a large number of genes, such as the Bcl-2 family genes and
cysteine proteases and is regulated by signaling pathways (19). Autophagy is triggered by various
types of intracellular stress, including DNA damage and low
nutrient levels (20). The
hyperactivation of autophagy can lead to autophagic cell death.
Akt/mTOR/1A/1B-light chain 3 (LC3) signaling is an important
pathway for autophagy induction (21). Recently, necroptosis was
demonstrated to be a new form of programmed cell death that differs
from apoptosis but is similar to necrosis (22). Necroptosis is involved in specific
physiological and pathological processes (22). Receptor interaction protein 3
(RIP3) and mixed lineage kinase domain-like protein (MLKL) are both
required for the activation of necroptosis (23). Excessive reactive oxygen species
(ROS) production not only acts as a signal to stimulate apoptosis
and DNA damage but is also associated with necroptosis (24). In addition, angiogenesis, which is
involved in tumorigenesis, is regulated by specific molecules, such
as vascular endothelial growth factor (VEGF), epidermal growth
factor receptor (EGFR) and hypoxia-inducible factor 1-α (HIF-α)
(25). These molecules are also
important for tumorigenesis (26).
Proliferation and cell death pathways are involved in inhibiting
tumorigenesis (26). The present
study investigated the effects of carnosine on reducing cell
proliferation and inhibiting tumorigenesis as a potential
chemoprevention strategy and aimed to investigate the effects of
carnosine on cell proliferation, apoptosis, autophagy, necroptosis
and angiogenesis in human CRC cells.
Materials and methods
Reagents
Carnosine {2ST-2-[(3-amino-1-oxopropyl)
amino]-3-(3H-imidazol-4-yl)} propanoic acid; β-alanyl-L-histidine
was purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and carnosine
treatment
The human colon carcinoma cell line HCT-116 was
purchased from the Bioresource Collection and Research Center.
HCT-116 cells (passages 43–65) were maintained in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 humidified atmosphere.
In the present study, 1×105 HCT-116 cells
per 30-mm culture plate were used for mRNA expression analysis and
5×105 HCT-116 cells per 60-mm culture plate were used to
determine cell viability, apoptosis and autophagy percentages and
ROS and ATP levels. After a series of preliminary tests, the
optimum experimental concentration of carnosine was confirmed to be
0.5–15 mM. To determine the effects of carnosine on cell viability,
various cell death-regulating molecules were analyzed in cultured
cells that were treated with 0.5, 1, 5, 10 or 15 mM carnosine for
72 or 96 h. Carnosine was dissolved in sterilized H2O
and cells that were treated with only H2O were used as a
control group.
Cell viability and morphological
analysis
The 3-(4,5-dimethyl-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT; Sigma-Aldrich; Merck KGaA) assay and morphological
examination were used to assess cell viability. The MTT assay was
performed as described by Denizot and Lang (1986) (27). HCT-116 cells were treated with 0.5,
1, 5, 10 or 15 mM carnosine for 72 or 96 h; MTT reagent (5 mg/ml)
was then added and the cells were incubated for 3 h at 37°C,
followed by extraction with 1 ml of isopropanol after the cells
were washed with cold phosphate-buffered saline (PBS; 3.2 mM
Na2HPO4, 0.5 mM KH2PO4,
1.3 mM KCl, 135 mM NaCl, pH 7.4). Cell viability was determined by
measuring the optical density at 570 nm using a Microplate
Biokinetics Reader (BioTek Instruments, Inc.). A phase-contrast
inverted fluorescence microscope was used to determine
morphological changes (Olympus IX 51; Olympus Corporation).
Analysis of apoptosis and autophagy
percentages
To determine the apoptosis percentage, an Annexin V
Assay using the NucleoCounter® NC-3000 System
(ChemoMetec Inc.) was used. HCT-116 cells were treated with 0.5, 1,
5, 10 or 15 mM carnosine for 96 h and then washed with cold PBS
twice. HCT-116 cells were suspended in 100 µl of Annexin V binding
buffer with 2 µl of Annexin V-CF488A conjugate (FITC-labeled
Annexin V; staining early stage apoptoic cells) and 2 µl of
Solution 15 (10 µg/ml Hoechst 33342 to stain the total population).
Then, the cells were incubated for 15 min at 37°C and centrifuged
at 400 × g for 5 min at 4°C. After the supernatant was removed, the
cell pellets were resuspended in 300 µl of Annexin V binding buffer
and centrifuged at 400 × g for 5 min at 4°C. The cell pellets were
resuspended in 100 µl of Annexin V binding buffer and 2 µl of
Solution 16 (500 µg/ml propidium iodide; staining late apoptotic
cells) was added. Using 8-chamber NC-Slides A8, analysis was
performed via NucleoCounter® NC-3000 fluorescence image
cytometer (ChemoMetec Inc.).
HCT-116 cell autophagy in response to carnosine
treatment was analyzed by a CYTO-ID® autophagy detection
kit (cat. no. ENZ-51031; Enzo Life Sciences, Inc.). HCT-116 cells
were treated with 0.5, 1, 5, 10 or 15 mM carnosine at 37°C in a 5%
CO2 incubator for 96 h and then washed with PBS at room
temperature twice. Then, the cells were centrifuged at 400 × g for
5 min at room temperature. After the supernatant was removed, the
cell pellets were resuspended in 200 µl PBS at room temperature for
20 min and 0.4 µl Cyto-ID Green stain solution was added for 5 min
of staining at room temperature and then 0.2 µl Hoechst 33342 stain
solution was added for 20 min of staining at room temperature. The
number of autophagic vacuoles was measured after the cells were
harvested and stained with Cyto-ID Green fluorescent dyes
(excitation, ~480 nm; emission, ~530). Using 2-chamber NC-Slides
A2, analysis was performed using a NucleoCounter®
NC-3000 fluorescence image cytometer (ChemoMetec Inc.).
Reverse transcription-quantitative
(RT-q)PCR analysis of the mRNA expression of autophagy-,
necroptosis- and angiogenesis-associated genes
A total of ~5×105 HCT-116 cells/30-mm
plate were incubated in the absence or presence of 0.5, 1, 5, 10 or
15 mM carnosine for 96 h with 5% CO2 at 37°C and total
RNA was isolated using a modified version of the method described
by Chomczynski and Sacchi (1987) (28). The cells were added to 1 ml TRIzol
reagent (Sigma-Aldrich; Merck KGaA) and mixed thoroughly, and then
200 µl chloroform was added and mixed thoroughly. Cooling on ice
for 5 min was followed by centrifugation at 16,200 × g for 15 min
at 4°C. To precipitate RNA, the aqueous phase (500 µl) was
transferred to a fresh tube and mixed with 500 µl isopropanol
cooled on ice for 5 min, followed by centrifugation at 16,200 × g
for 15 min at 4°C. The RNA pellet was washed with 75% (v/v) ethanol
and the supernatant was removed. The pellet was air-dried at room
temperature and then resuspended in 30–50 µl DEPC-treated water and
stored at −80°C until use. The total RNA purity was determined by
measuring the absorbance on an Epoch microplate spectrophotometer
(BioTek Instruments, Inc.). The absorption ratios (260/280 nm) of
all RNA samples were >2, and this indicated that the total RNA
was of high quality. The RNA samples were stored at −80°C until
use. Total RNA (3 µg) was used for RT, which was performed using
Superscript III reverse transcriptase (Thermo Fisher Scientific,
Inc.) and oligo d(T)21 as a primer, and the reaction conditions
used were as recommended by the manufacturer. Real-time PCR primers
for β-catenin, Tcf-4, Bax, Bcl-2, Caspase-3, Caspase-8,
poly(ADP-ribose) polymerase (PARP), MLKL, Beclin-1, LC3, PI3K III,
VEGF, EGFR and HIF-α were prepared and designed as previously
described (7). The thermal profile
for the real-time quantitative PCR was 95°C for 2 min and 95°C for
10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min, according to the manufacturer's protocol. Gene expression is
presented as the fold change relative to the β-actin level, which
was calculated as 2−ΔΔCq as previously described
(29) and is shown in Table I. In addition, melting curve
analysis was performed to assure the specificity of the PCR
products in this experiment. The results were obtained from three
independent experiments (n=3).
 | Table I.List of primer sequences used for
reverse transcription-quantitative PCR analysis. |
Table I.
List of primer sequences used for
reverse transcription-quantitative PCR analysis.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) | GenBank accession
no. |
|---|
| β-actin |
ATGTGCAAGGCCGGCTTC |
GAATCCTTCTGACCCATGCC | NM001101.3 |
| Tcf-4 |
ACCAGCAACCAGCACTTTCC |
GCCCAACATTCCTGCATAGC | NM001083962.2 |
| Bax |
TGTTTTCTGACGGCAACTTCA |
AGCCCATGATGGTTCTGATCA | NM001291428.1 |
| Bcl-2 |
CCTGTGGATGACTGAGTACCTGAAC |
CAGCCAGGAGAAATCAAACAGA | NM000633.2 |
| Caspase-3 |
TGGATTATCCTGAGATGGGTTTATG |
GCTGCATCGACATCTGTACCA | NM004346.3 |
| Caspase-8 |
TCCAAATGCAAACTGGATGATG |
TTTTCAGGATGTCCAACTTTCCTT | NM001080124.1 |
| PARP |
TGGTCAAGACACAGACACCCA |
ACGGAGGCGCTGGTTTCT | NM001618.4 |
| MLKL |
CCTGGGCACAGGAAGATCAG |
TTTCTAATCGTCTCAGTGAAGCTTCT | NM001142497.2 |
| Beclin-1 |
CTGGACACGAGTTTCAAGATCCT |
GTTAGTCTCTTCCTCCTGGGTCTCT | NM001313998.1 |
| LC3 |
TCCTGGACAAGACCAAGTTTTTG |
ACCATGCTGTGCTGGTTCAC | NM032514.3 |
| PI3K III |
TTGGAGACAGGCACCTGGAT |
CCATTTCTTTATTCAGCTTCATTGG | NM001308020.1 |
| VEGF |
CGAGGGCCTGGAGTGTGT |
TGGTGAGGTTTGATCCGCATA | NM_001025366.2 |
| EGFR |
GGCGTCCGCAAGTGTAAGAA |
TCGTAGCATTTATGGAGAGTGAGTCT | NM_005228.5 |
| HIF-1α |
TAACTTTGCTGGCCCCAGC |
ACTTCCTCAAGTTGCTGGTCATC | NM_001243084.1 |
Statistical analysis
All experimental data were analyzed using SPSS
statistical analysis software for Windows, version 20.0 (IBM
Corp.). One-way analysis of variance and Tukey's post hoc test were
used to evaluate the significance of differences between two mean
values. P<0.05 was considered to indicate a statistically
significant difference.
Results
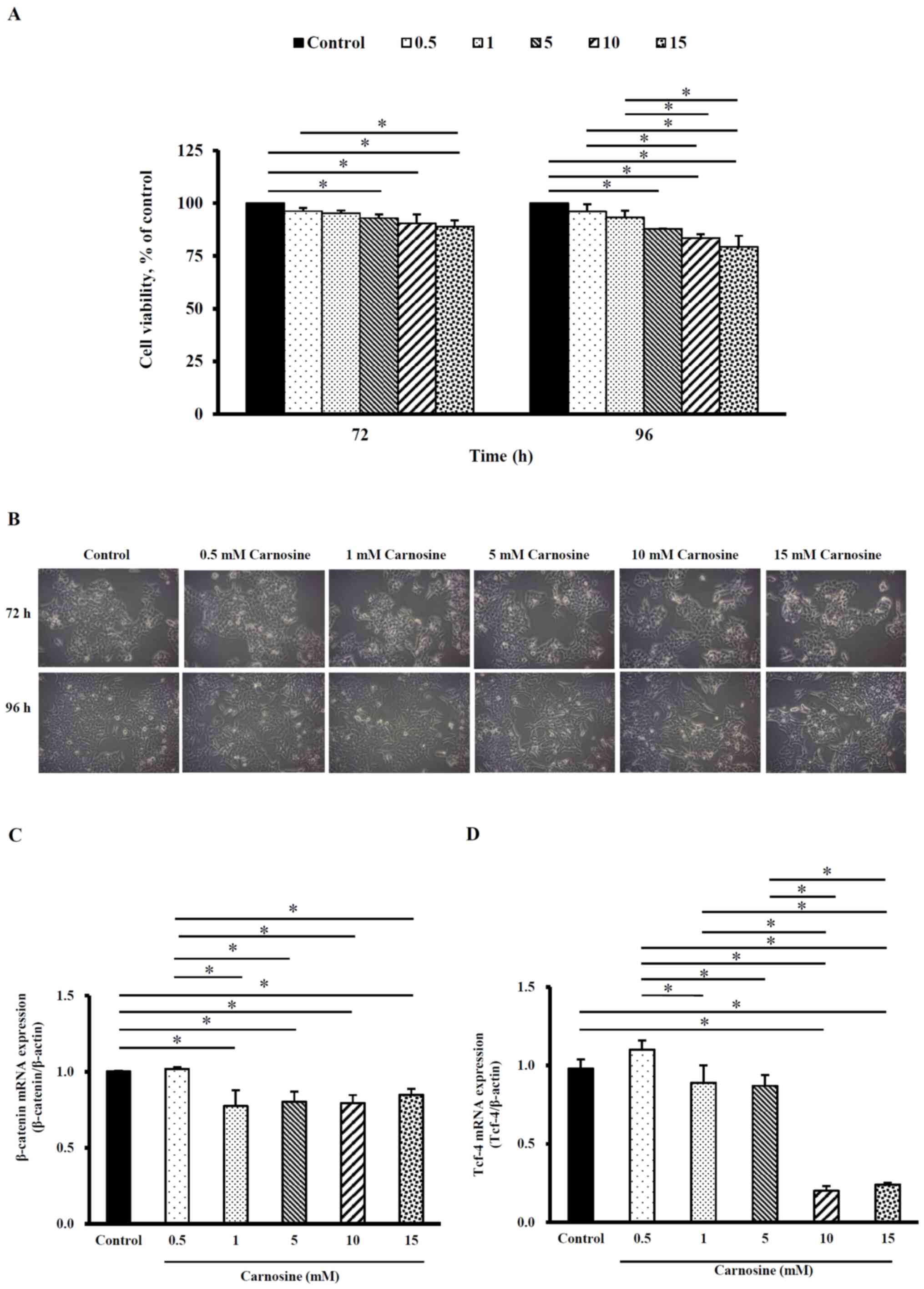
Carnosine suppresses the proliferation
of HCT-116 cells
When HCT-116 cells were treated with 5, 10 or 15 mM
carnosine for 72 h, the viability was 93.0±1.8%, 90.0±4.3% and
89.0±2.9%, respectively. The viability of HCT-116 cells was
87.9±0.2%, 83.5±1.9% and 79.4±5.2% following treatment with 5, 10
or 15 mM carnosine for 96 h, respectively. The viability was
significantly lower than that of the control group (100%)
(P<0.05) after 72 and 96 h of incubation (Fig. 1A). However, cell morphology was
examined by inverted microscopy and cell number in each of the
various carnosine treatment groups was similar to that in the
control group (Fig. 1B).
Therefore, the employed carnosine concentrations were used in all
tests in this study. Fig. 1C shows
that the β-catenin mRNA levels were significantly reduced in
HCT-116 cells treated with 1, 5, 10 or 15 mM carnosine for 96 h by
15–23% compared with those in the control group (P<0.05). When
HCT-116 cells were treated with 1, 5, 10 or 15 mM carnosine, the
Tcf-4 mRNA levels were significantly reduced by 11–76% compared
with those of the control group (P<0.05) (Fig. 1D). These results demonstrate that
carnosine can reduce cell proliferation and the carnosine-induced
reduction in β-catenin/Tcf-4 signaling activation may be involved
in HCT-116 cell proliferation.
Carnosine did not induce apoptosis in
HCT116 cells
RT-qPCR analysis showed that the treatment of
HCT-116 cells with 5, 10 or 15 mM carnosine did not affect the mRNA
expression of Bax, Bcl-2, Caspase-3, Caspase-8, or PARP (Fig. 2 A-E). In addition, after HCT-116
cells were incubated with 5, 10 or 15 mM carnosine, the apoptosis
percentage was no different from that of the control group
(Fig. 2F and G). Carnosine (1–15
mM) did not induce apoptosis in HCT-116 cells even after 96 h of
treatment.
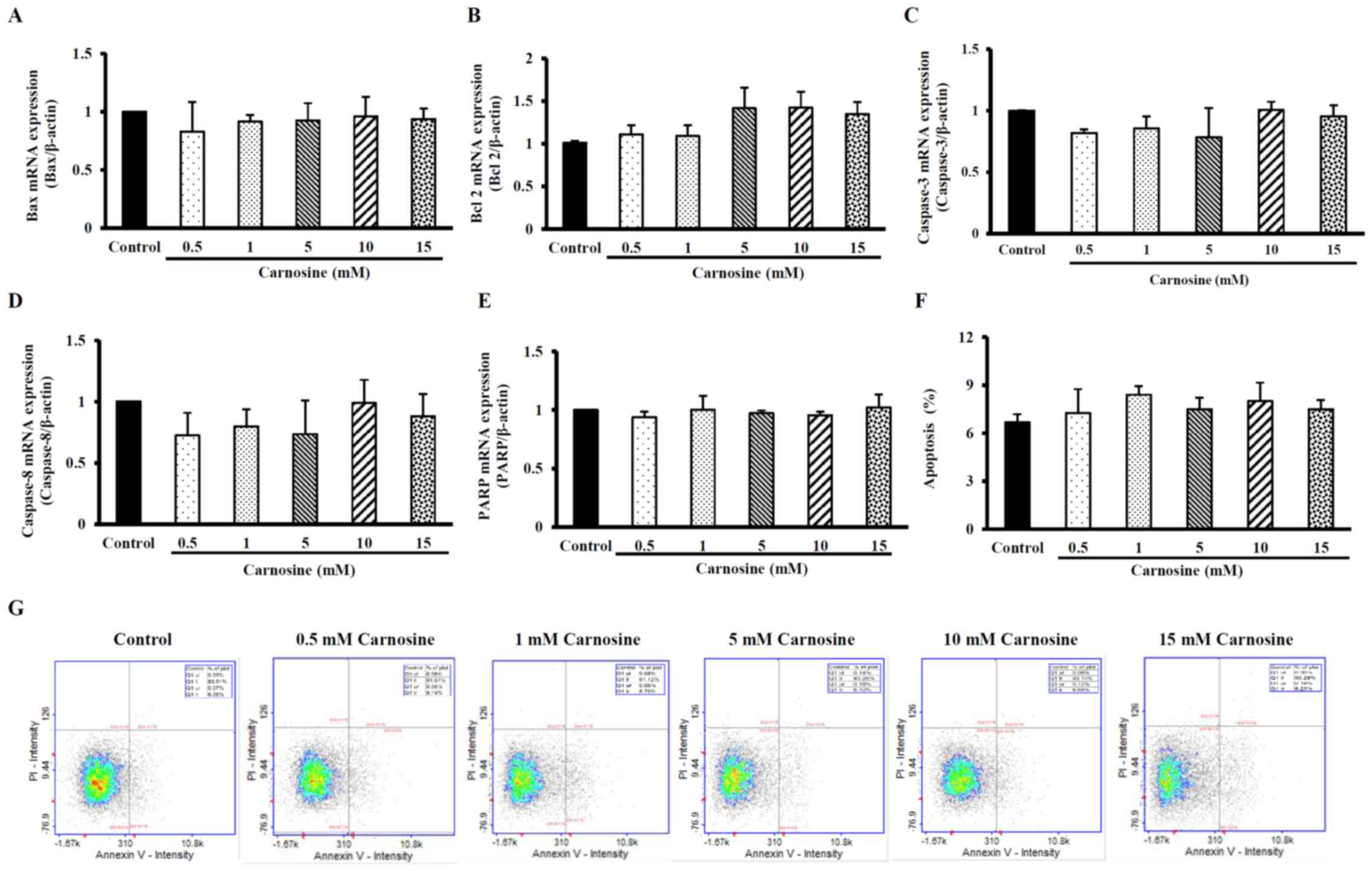 | Figure 2.Effect of carnosine on apoptosis
levels and the mRNA expression of apoptosis-related molecules in
HCT-116 cells. HCT-116 cells (1×105 cells per 30-mm
plate for the mRNA expression analysis and 5×105 cells
per 60-mm plate for the apoptosis assay) were treated with 0.5, 1,
5, 10 or 15 mM carnosine for 96 h. The control group was treated
with sterilized. The mRNA expression of (A) Bax, (B) Bcl-2, (C)
Caspase-3, (D) Caspase-8, (E) PARP, (F) the levels of apoptosis and
(G) apoptosis via FITC-labeled Annexin V staining of HCT-116 cells
were examined. The values are presented as the mean ± SD (n=3-5).
Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; PARP,
poly(ADP-ribose) polymerase. |
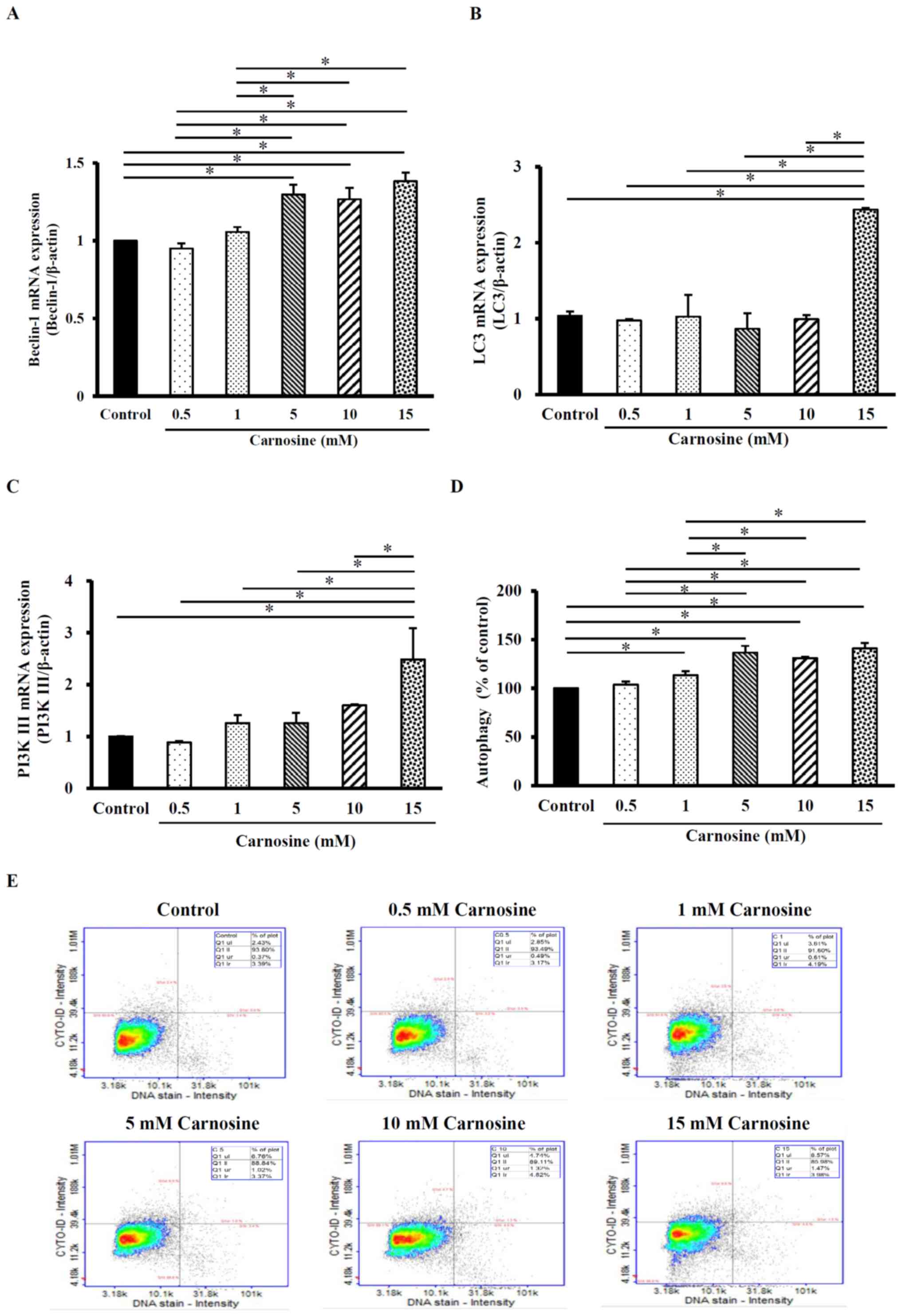
Carnosine induces autophagy in HCT116
cells
Beclin-1 mRNA expression in HCT-116 cells was
significantly increased by 6–38% after 5, 10 or 15 mM carnosine
treatment for 96 h compared with those in the control group
(P<0.05; Fig. 3A). The LC3 mRNA
expression levels were also significantly increased by 144% after
the cells were treated with 15 mM carnosine for 96 h compared with
those in the control group (Fig.
3B) and PI3K III mRNA levels were significantly increased by
149% compared with those of the control group (P<0.05; Fig. 3C). In addition, the formation of
autophagosomes (stained by LYSO-ID1 Green dye) increased after
carnosine treatment compared with the control group (Fig. 3D). Autophagy was significantly
increased by 13–41% after 1, 5, 10 or 15 mM carnosine treatment for
96 h (P<0.05; Fig. 3D and E).
These results showed that 15 mM carnosine can induce autophagy by
regulating the indicated regulatory molecules. However, 0.5 mM
carnosine did not exert any effect and was equal to control.
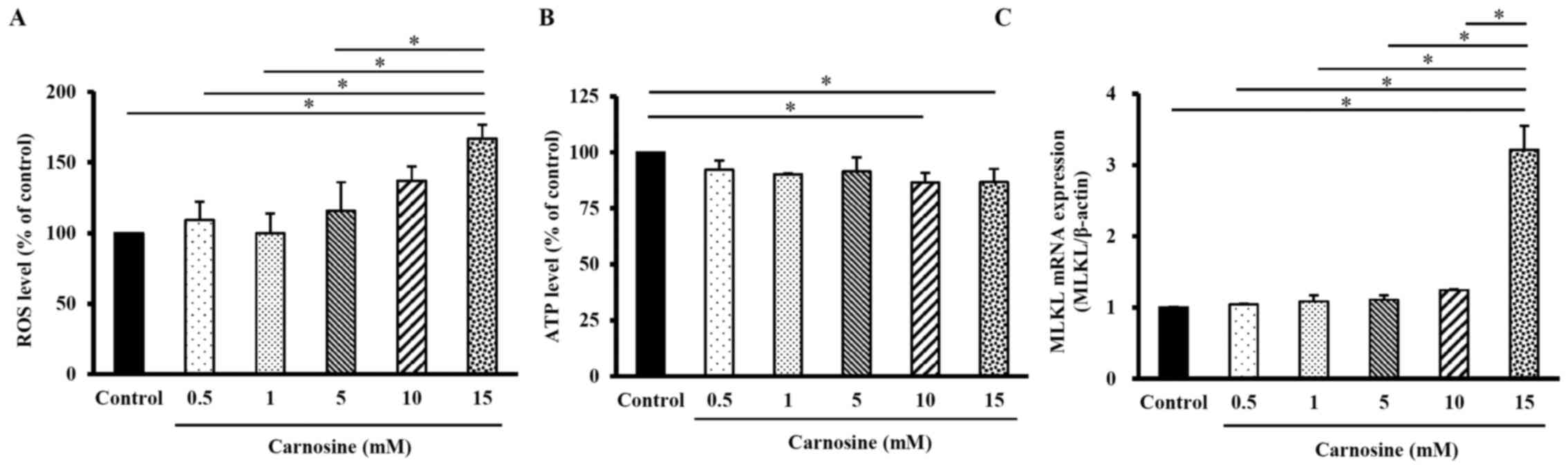
Carnosine induces necroptosis in
HCT116 cells
As shown in Fig.
4A, the levels of ROS in HCT116 cells treated with 15 mM
carnosine for 96 h were significantly increased by 67% compared
with those in the control group (100%; P<0.05). The ATP levels
in HCT-116 cells treated with 10 or 15 mM carnosine were both
significantly decreased by 13% compared with those in the control
group (100%; P<0.05; Fig. 4B).
The MLKL mRNA expression level in HCT-116 cells treated with 15 mM
carnosine was significantly increased by 221% compared with that in
the control group (P<0.05; Fig.
4C). These results showed that 10–15 mM carnosine could induce
necroptosis in HCT-116 cells.
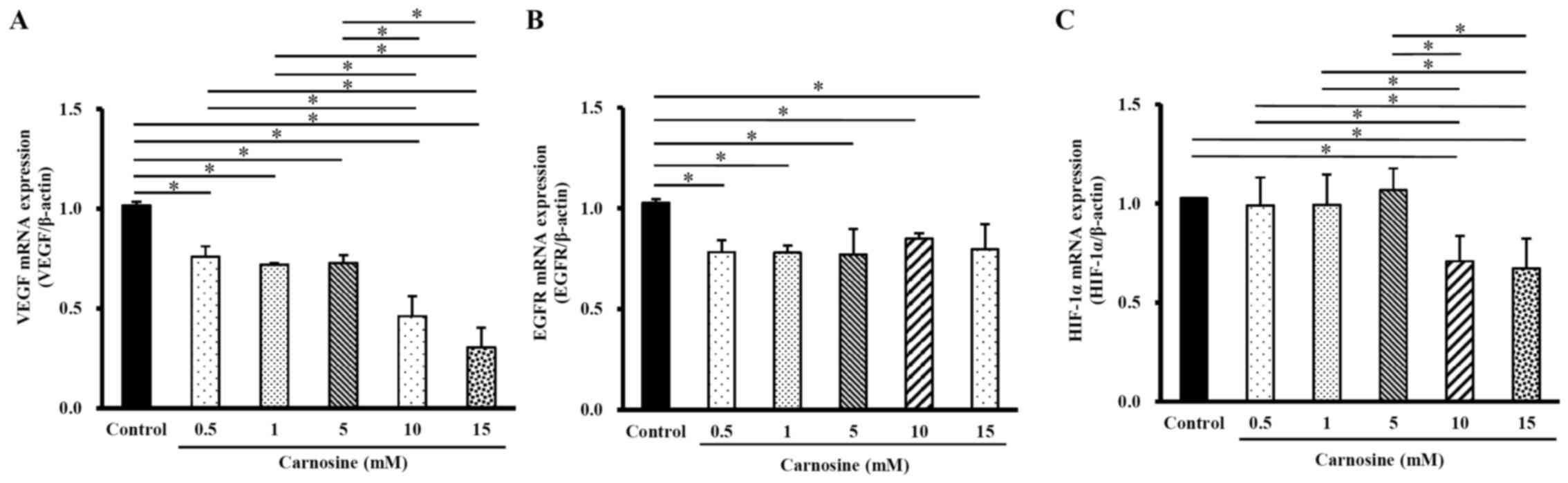
Carnosine suppresses angiogenesis in
HCT116 cells
The mRNA levels of VEGF in HCT-116 cells treated
with 10 or 15 mM carnosine for 96 h were significantly lower (24%
or 70%, respectively) than the control group (100%; P<0.05;
Fig. 5A), while EGFR mRNA
expression significantly decreased by 15–22% following treatment
with 0.5, 1, 5, 10 or 15 mM carnosine for 96 h (P<0.05; Fig. 5B). However, the mRNA levels of
HIF-α in cells exposed to 10 and 15 mM carnosine for 96 h
significantly decreased by 29% and 33%, respectively, compared with
those in the control group (Fig.
5C). Carnosine significantly reduced HCT-116 cell angiogenesis
by reducing these angiogenesis-regulating molecules.
Discussion
In the present study, carnosine significantly
suppressed the proliferation and viability of HCT-116 human CRC
cells by inducing necroptosis and autophagy and inhibiting
angiogenesis. In the current study, the mRNA expression of
β-catenin and Tcf-4, two key molecules in cell
proliferation-associated signaling, was decreased after HCT-116
cells were treated with carnosine. Carnosine could increase the
mRNA expression levels of Beclin-1 and PI3K and reduced LC-3 mRNA
expression, leading to autophagy in HCT-116 cells. When HCT-116
cells were treated with carnosine, ATP levels were significantly
decreased and ROS levels and MLKL mRNA expression were
significantly increased. Carnosine could induce necroptosis to
decrease the viability of HCT-116 cells. In addition, VEGF, EGFR
and HIF-α mRNA levels were significantly reduced after HCT-116
cells were treated with carnosine. Carnosine could reduce
angiogenesis in HCT-116 cells by reducing these
angiogenesis-regulatory molecules. These results showed that
carnosine can reduce colorectal cell proliferation and induce
cancer cell death. However, the molecular regulatory mechanism and
gene expression modifications require further study.
In the clinic, there are several chemotherapeutic
drugs that have been reported to participate in cell proliferation,
autophagy, apoptosis and angiogenesis. For example, 5-fluorouracil
can regulate cell proliferation by reducing Wnt/β-catenin signaling
(30), temozolomide and sorafenib
both can induce autophagy by inducing LC3 expression (31,32),
dabrafenib can induce necroptosis by regulating MLKL expression
(33) and lenvatinib can exhibit
anti-angiogenesis effects by reducing VEGF expression (34). In the present study, carnosine
could reduce cell proliferation, induce autophagy and necroptosis
and suppress angiogenesis. It has a marked potential for cancer
prevention and therapy.
The proliferation of normal cells includes cell
growth and division to replace lost cells (35). The cell cycle serves a central role
in cell growth and proliferation. Abnormal regulation of the cell
cycle can lead to the over proliferation of cells and an
accumulation of abnormal numbers of cells (35). However, apoptosis is usually an
important pathway for the removal of excess and impaired cells in
normal tissue and organs (36).
Investigating how to suppress abnormal cell proliferation and cell
growth by active compounds is an important issue in cancer
proliferation and cell growth (37).
The present study showed that the treatment of
HCT-116 cells with 0.5–15 mM carnosine for 96 h did not induce
apoptosis. The results showed that the mRNA levels of the
proapoptotic factor Bax and the apoptotic factor Bcl-2 in HCT-116
cells were not affected by treatment with carnosine for 96 h. In
addition, there were similar results showing that the intrinsic
apoptotic pathway marker Caspase-3, the extrinsic apoptotic pathway
marker Caspase-8 and PARP in HCT-116 cells were not affected by
carnosine treatment. However, Lee et al (13) showed that 200 mM carnosine can
induce apoptosis in HCT-116 cells after 24 h by increasing Bax,
Caspase-3 and cyclin D1 protein levels. Carnosine (200 mM) can also
induce apoptosis in SGC-7901 and MKN45 human gastric carcinoma
cells by reducing Bcl-2 and increasing Bax and PARP protein
expression (9). In addition, Shi
and Zhang (38) (2011) showed that
20 mM carnosine could protect human umbilical vein endothelial
cells (HUVECs) from high glucose-induced apoptosis. Carnosine (5–20
mM) can also protect murine podocytes from high glucose-induced
apoptosis by reducing Bax and Caspase-3 levels (12). Tiwari (36) (2011) showed that exogenous and
endogenous compounds induce, suppress, or exhibit no effects on
apoptosis and the cellular dose response and kinetics must be
considered. The dose and treatment times of compounds determine not
only the sensitivity and tolerance time but also the form of cell
death (36). These aforementioned
studies (9,12,13,38)
have shown that carnosine regulates apoptosis and the dose-response
relationship between apoptosis and the treatment agent. These
results differ from those of the present study regarding apoptosis
induction. Different experimental models, carnosine doses and
treatment times may cause differences in apoptosis induction.
Although carnosine did not induce apoptosis, it did
block the reduction in β-catenin and Tcf-4 activation by reducing
β-catenin and Tcf-4 expression in the present study.
Wnt/β-catenin/Tcf-4 signaling is a major transcriptional regulator
of c-myc, cyclin D1 and VEGF, which are important regulators of the
cell cycle, cell proliferation and angiogenesis (39,40).
In addition, Sebio et al (41) showed aberrant Wnt/β-catenin
signaling is a characteristic feature of colorectal cancer (CRC).
In the present study, carnosine reduced β-catenin and Tcf-4
expression, which may be important in reducing the proliferation of
HCT-116 cells. Previous studies (39,40,42,43)
have shown that when HCT-116 cells were treated with celecoxib, a
selective cyclooxygenase-2 inhibitor, Tcf-4 expression was
significantly inhibited and Wnt/β-catenin and Tcf-4 expression was
blocked, reducing human colon cancer cell proliferation (42). Quercetin can also reduce SW480 cell
proliferation through the downregulation of Wnt/β-catenin/Tcf-4
expression (43). In cancer
prevention and therapy, the regulation of β-catenin/Tcf4 signaling
to reduce abnormal cell proliferation with active components could
be a potential strategy. In addition, carnosine also reduced the
mRNA expression of VEGF. VEGF transcription is also regulated by
Wnt/β-catenin/Tcf-4 signaling (39,40).
Previous studies (44,45) have shown that tolfenamic acid, a
fenamic acid-derived nonsteroid anti-inflammatory drug, can
downregulate β-catenin mRNA expression in a dose- and
time-dependent manner to reduce the proliferation of human colon
cancer cell lines. Tolfenamic acid also decreases the expression of
the β-catenin target gene VEGF, leading to reduced angiogenesis in
human colon cancer cell lines (44). Ginkgo biloba exocarp
extracts (GBEE) can suppress Wnt3a and β-catenin protein expression
and VEGF mRNA levels in Lewis lung cancer (LLC) cells (45). GBEE can also inhibit the growth of
LLC-transplanted tumors in C57BL/6 mice in a dose-dependent manner
by suppressing tumor growth in the lungs by reducing β-catenin and
VEGF protein expression (45).
These studies and the present results show that carnosine can
reduce cell proliferation and that angiogenesis may inhibit the
Wnt/β-catenin/Tcf-4 signaling pathway. In the future, investigating
the cellular Wnt expression and β-catenin-DNA binding activity may
aid understanding of the effect of carnosine in cell proliferation
through regulating the Wnt/catenin signaling.
In the present study, carnosine significantly
increased Beclin-1, PI3K III and LC-3 mRNA levels and the level of
autophagy. These results showed that carnosine reduced not only
cell proliferation but also cell viability by inducing autophagy.
Beclin-1 is one of three core activated autophagic complex
proteins: Beclin-1, Vps34 and Bcl-2 (46). When Bcl-2 is phosphorylated,
Beclin-1 is activated, followed by PI3K III and LC3-II, triggering
autophagosome formation (47).
Beclin-1/PI3K III/LC3-II signaling pathways are involved in
preautophagosome formation (48).
Therefore, the above molecules are changed by carnosine, which
leads to autophagy in HCT-116 cells. A previous study shows that
quercetin can induce autophagy in human gastric cells by increasing
LC3-II and Beclin-1 expression (25). The clinical chemotherapy imatinib
induces cellular autophagy by increasing the levels of PI3K and
LC-3 and inhibiting the viability of leukemia cells (49). Previous studies (50–53)
have shown that Grias neuberthii extract can induce
autophagy in human colon cancer cells by increasing intracellular
Beclin-1 and LC-3 levels (50).
Our previous studies also showed that sedanolide and
α-phellandrene, which are active components of celery, can increase
PI3K, Beclin-1 and LC-3 protein levels, leading to autophagy
induction in human colorectal HCT-116 cancer cells and liver J-5
cancer cells (51,52). Salidroside, a natural active
ingredient extracted from Rhodiola rosea, has been shown to
decrease the expression of autophagy proteins, suggesting that
salidroside induces autophagy through the PI3K/Akt/mTOR pathway in
human gastric cancer (53). Based
on the aforementioned studies (25,49–52),
the autophagic induction mechanism of carnosine is similar to the
autophagic induction by quercetin, imatinib, Grias
neuberthii extract and α-phellandrene, and this may be
attributed to the induction of autophagy via the upregulation of
Beak-1, PI3K and LC3 expression. However, except for the
Beclin-1/PI3K III/LC3-II signaling pathways investigated in the
present study, the effects of carnosine on PI3K/Akt/mTOR signaling
pathway in autophagy need future investigation.
Necroptosis is another mode of programmed cell death
that differs from apoptosis (54).
Necroptosis is also a cell death pathway, including programmed
necroptosis, coercion, iron death and mitochondrial permeability
transition, which is regulated by intracellular molecules. Among
them, necroptosis is regulated by RIP3 and MLKL (55). Additionally, ROS production
involves the stabilization of the necrosome complex composed of
RIP1 and RIP3 (56). In the
present study, carnosine significantly increased ROS and MLKL
levels but decreased ATP levels. These are all major regulatory
molecules of necroptosis. A previous study showed that apigenin
could induce necroptosis by increasing ROS levels and reducing ATP
levels and MLKL phosphorylation (57). Liu et al (58) showed that tanshinone A, a major
compound of Salvia miltiorrhiza Bunge (Danshen), could
induce necroptosis by increasing ROS, depleting ATP and
downregulating MLKL to reduce the viability of lung NCI-H1299 and
A549 cells. The findings of the present study revealed that
carnosine could increase the expression of ROS and MLKL but
decreased ATP levels to induce necroptosis in HCT-116 cells.
HIF-1, a heterodimer that binds to
hypoxia-responsive elements, is one of the major regulatory
molecules involved in cancer cell proliferation and metastasis and
activates VEGF transcription (59). VEGF is involved in tumor cell
proliferation and the regulation of blood vessel density (60). Additionally, activated EGFR
signaling leads to the proliferation of epidermal cells to induce
tumor formation under hypoxic conditions (60). In the present study, carnosine
significantly reduced angiogenesis by decreasing VEGF, EGFR and
HIF-1α expression. Huang et al (61) showed that wogonin, a plant-derived
flavone, can reduce the angiogenesis of human breast MCF-7 cells by
degrading HIF-1α protein and reducing VEGF and EGFR expression. In
human astrocytoma U251 cells, hepatoma Hep3B cells and an HUVEC
culture experimental model, the oligomer procyanidin, which is
isolated from grape seeds, can inhibit angiogenesis by suppressing
the HIF-1α-dependent pathway (62). The results of the present study and
the above studies show that if cellular VEGF, EGFR and HIF-1α
expression is decreased, cells can reduce angiogenesis in various
cell culture models. An investigation of the suppression of
carnosine in tumorigenesis in animal models is required.
Carnosine serves an important role in inhibiting
non-enzyme protein glycosylation (63,64).
Glycosylation, a crucial post-translational process in protein
modification, is characteristic of physiological and pathological
functions (64). The tumor
microenvironment produces altered glycans by glycosylation that
contributes to cancer progression and aggressiveness (64). Glycosylation of tumor-cell-surface
glycans is involved in enhancing transient cell cycle arrest
(65), regulating autophagosome
forming leading to induce autophagy (66) and degrading the extracellular
matrix to activate the angiogenesis (67). The reactive glycosylation rate
occurs rapidly with the lysine-histidine sequence (68). Carnosine has a glycine-histidine
structure similar to the lysine-histidine sequence, but it inhibits
sugar-mediated cross-linking of a specific protein (69). The tumor microenvironment produces
altered glycans that contribute to cancer progression and
aggressiveness. Abnormal glycosylation is widely observed in tumor
angiogenesis. Hipkiss and Gaunitz (69) showed that carnosine could reduce
the glycosylation then reduce cell proliferation and migration.
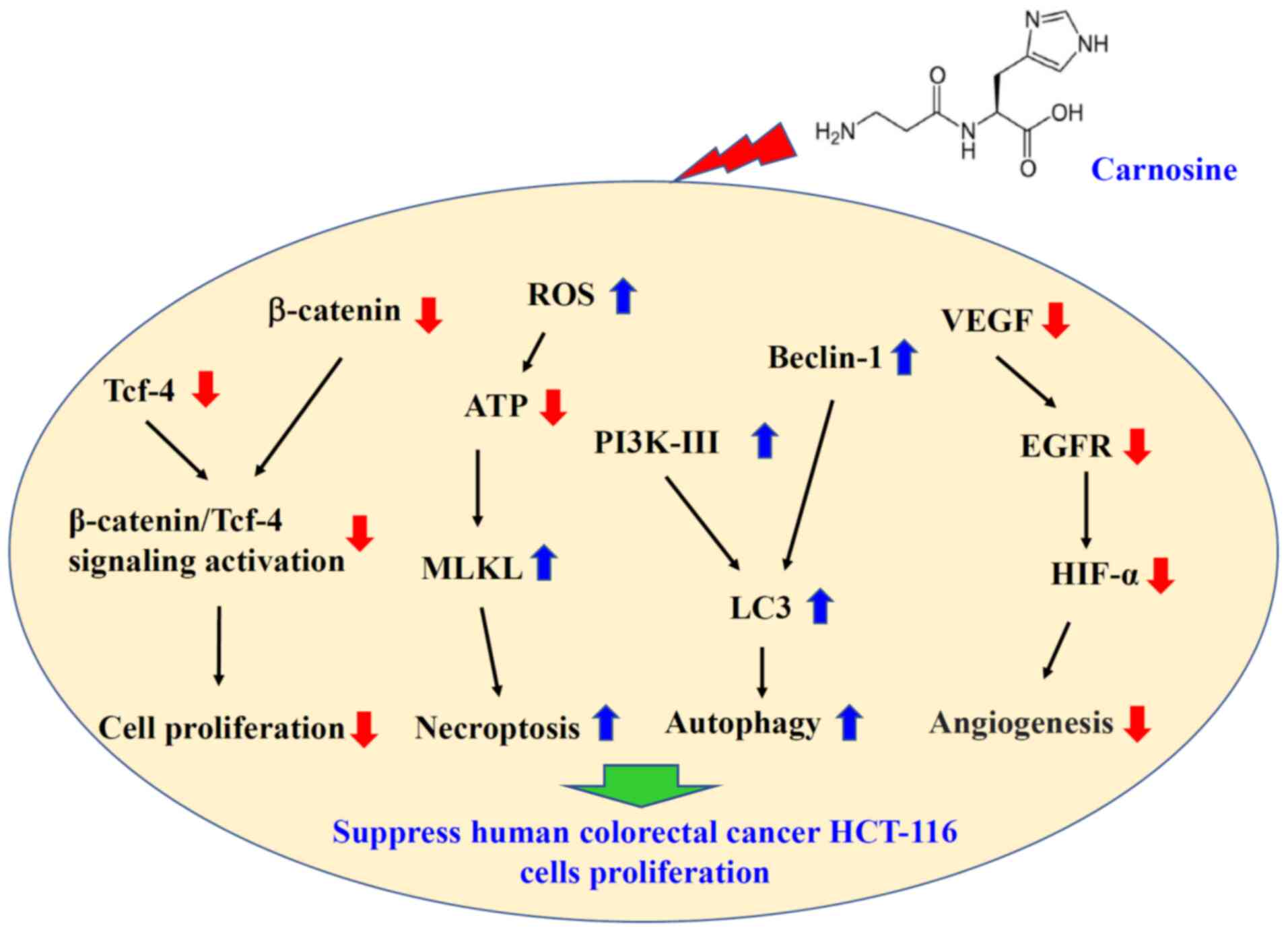
As shown in Fig. 6,
carnosine suppressed cell proliferation by reducing β-catenin/Tcf-4
signaling activation, including inhibition of the expression of
β-catenin and Tcf-4. In addition, carnosine suppressed angiogenesis
by reducing VEGF, EGRF and HIF-α expression. Carnosine induced
necroptosis, through reduced ATP levels and increased ROS and MLKL
levels and autophagy, through increasing Beclin-1 and PI3KIII
expression. In this present study, only the mRNA expression of
important protein regulators involved in cell proliferation,
apoptosis, autophagy and angiogenesis was analyzed but not the
protein levels. There are consistent results between the mRNA
expression of these molecules and the physiological functions. Li
et al (70) show that
accurate determination of mRNA levels can be used in both
laboratory and clinical studies to describe the biological,
pathological and clinical roles of genes in health and disease. For
speedy and precise analysis of the regulatory mechanism of these
regulators and cell physiological effects, mRNA analysis was used
in the present study. However, the protein contents should be
measured in the future; if the mRNA expression proves different
from the physiological functions of these molecules it may be that
some post-translation modification is involved (71).
In conclusion, the present study showed that
carnosine can reduce human CRC cell viability and proliferation.
Mechanistically, carnosine induced autophagy and necroptosis and
reduced angiogenesis in HCT-116 cells. In the context of cancer
prevention and therapy, understanding the molecular regulatory
mechanisms and animal studies are required in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Ministry of
Science and Technology of Taiwan (grant no. MOST 108-2221-E-992
−047 -MY3).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CCW and SLH conceived and designed the study and
were major contributors to writing and critically revising the
manuscript. JHL, CDD and CWC performed the experiments and analyzed
the data. CDD and CWC provided advice on the experiments and
technical assistance. CCW and SLH supervised the study. SLH, JHL
and CCW confirm the authenticity of all the raw data. All authors
agreed to be accountable for all aspects of the research and ensure
that the accuracy or integrity of any part of the work is
appropriately investigated and resolved. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
HIF-α
|
hypoxia-inducible factor 1-alpha
|
|
LC3
|
1A/1B-light chain 3
|
|
MLKL
|
mixed lineage kinase domain like
pseudokinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
RIP3
|
receptor interaction protein 3
|
|
Tcf-4
|
transcription factor 4
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Boldyrev AA, Aldini G and Derave W:
Physiology and pathophysiology of carnosine. Physiol Rev.
93:1803–1845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hipkiss AR, Baye E and de Courten B:
Carnosine and the processes of ageing. Maturitas. 93:28–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain S, Kim ES, Kim D, Burrows D, De
Felice M, Kim M, Baek SH, Ali A, Redgrave J, Doeppner TR, et al:
Comparative cerebroprotective potential of d- and l-carnosine
following ischemic stroke in mice. Int J Mol Sci. 21:30532020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prokopieva VD, Yarygina EG, Bokhan NA and
Ivanova SA: Use of carnosine for oxidative stress reduction in
different pathologies. Oxid Med Cell Longev. 2016:29390872016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caruso G, Fresta CG, Musso N, Giambirtone
M, Grasso M, Spampinato SF, Merlo S, Drago F, Lazzarino G, Sortino
MA, et al: Carnosine prevents Aβ-induced oxidative stress and
inflammation in microglial cells: A key role of TGF-β1. Cells.
8:642019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bermúdez ML, Seroogy KB and Genter MB:
Evaluation of carnosine intervention in the Thy1-aSyn mouse model
of Parkinson's disease. Neuroscience. 411:270–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh SL, Hsieh S, Lai PY, Wang JJ, Li CC
and Wu CC: Carnosine suppresses human colorectal cell migration and
intravasation by regulating EMT and mMP expression. Am J Chin Med.
47:477–494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu CC, Lai PY, Hsieh S, Cheng CC and Hsieh
SL: Suppression of carnosine on adhesion and extravasation of human
colorectal cancer cells. Anticancer Res. 39:6135–6144. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Miao L, Wu X, Liu G, Peng Y, Xin
X, Jiao B and Kong X: Carnosine inhibits the proliferation of human
gastric carcinoma cells by retarding Akt/mTOR/p70S6K signaling. J
Cancer. 5:382–389. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamaki N, Funatsuka A, Fujimoto S and Hama
T: The utilization of carnosine in rats fed on a histidine-free
diet and its effect on the levels of tissue histidine and
carnosine. J Nutr Sci Vitaminol (Tokyo). 30:541–551. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gariballa SE and Sinclair AJ: Carnosine:
Physiological properties and therapeutic potential. Age Ageing.
29:207–210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao K, Li Y, Wang Z, Han N and Wang Y:
Carnosine protects mouse podocytes from high glucose induced
apoptosis through PI3K/AKT and Nrf2 pathways. BioMed Res Int.
2019:43489732019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Park JR, Lee H, Jang S, Ryu SM, Kim
H, Kim D, Jang A and Yang SR: L-carnosine induces apoptosis/cell
cycle arrest via suppression of NF-κB/STAT1 pathway in HCT116
colorectal cancer cells. In Vitro Cell Dev Biol Anim. 54:505–512.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joshi RK, Kim WJ and Lee SA: Association
between obesity-related adipokines and colorectal cancer: A
case-control study and meta-analysis. World J Gastroenterol.
20:7941–7949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaabane W, User SD, El-Gazzah M, Jaksik
R, Sajjadi E, Rzeszowska-Wolny J and Los MJ: Autophagy, apoptosis,
mitoptosis and necrosis: Interdependence between those pathways and
effects on cancer. Arch Immunol Ther Exp (Warsz). 61:43–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martinez-Font E, Pérez-Capó M, Ramos R,
Felipe I, Garcías C, Luna P, Terrasa J, Martín-Broto J, Vögler O,
Alemany R, et al: Impact of Wnt/β-Catenin Inhibition on Cell
Proliferation through CDC25A Downregulation in Soft Tissue
Sarcomas. Cancers (Basel). 12:25562020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hikita H, Kodama T, Shimizu S, Li W,
Shigekawa M, Tanaka S, Hosui A, Miyagi T, Tatsumi T, Kanto T, et
al: Bak deficiency inhibits liver carcinogenesis: A causal link
between apoptosis and carcinogenesis. J Hepatol. 57:92–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J,
Musarrat J, Al-Khedhairy AA, AlSalhi MS and Alrokayan SA: Oxidative
stress mediated apoptosis induced by nickel ferrite nanoparticles
in cultured A549 cells. Toxicology. 283:101–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galati S, Boni C, Gerra MC, Lazzaretti M
and Buschini A: Autophagy: A player in response to oxidative stress
and DNA damage. Oxid Med Cell Longev. 2019:56929582019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu S, Yoshida T, Tsujioka M and
Arakawa S: Autophagic cell death and cancer. Int J Mol Sci.
15:3145–3153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson N, Ganesan R, Hegedűs C, Kovács
K, Kufer TA and Virág L: Programmed necrotic cell death of
macrophages: Focus on pyroptosis, necroptosis, and parthanatos.
Redox Biol. 26:1012392019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu W, Liu P and Li J: Necroptosis: An
emerging form of programmed cell death. Crit Rev Oncol Hematol.
82:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manda G, Isvoranu G, Comanescu MV, Manea
A, Debelec Butuner B and Korkmaz KS: The redox biology network in
cancer pathophysiology and therapeutics. Redox Biol. 5:347–357.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fulda S: Cell death and survival signaling
in oncogenesis. Klin Padiatr. 222:340–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang
DW, Park JC, Il Kim T, Clevers H and Choi KY: 5-FU promotes
stemness of colorectal cancer via p53-mediated WNT/β-catenin
pathway activation. Nat Commun. 11:53212020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, García-Palomo A, Muntané J, González-Gallego J and Mauriz JL:
Modulation of autophagy by sorafenib: Effects on treatment
response. Front Pharmacol. 7:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Dong G and Sheng C: Targeting
necroptosis in anticancer therapy: Mechanisms and modulators. Acta
Pharm Sin B. 10:1601–1618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capozzi M, De Divitiis C, Ottaiano A, von
Arx C, Scala S, Tatangelo F, Delrio P and Tafuto S: Lenvatinib, a
molecule with versatile application: From preclinical evidence to
future development in anti-cancer treatment. Cancer Manag Res.
11:3847–3860. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chao DL, Sanchez CA, Galipeau PC, Blount
PL, Paulson TG, Cowan DS, Ayub K, Odze RD, Rabinovitch PS and Reid
BJ: Cell proliferation, cell cycle abnormalities, and cancer
outcome in patients with Barrett's esophagus: A long-term
prospective study. Clin Cancer Res. 14:6988–6995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tiwari M: Apoptosis and survival. Indian J
Hum Genet. 17:120–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Loo G: Redox-sensitive mechanisms of
phytochemical-mediated inhibition of cancer cell proliferation
(review). J Nutr Biochem. 14:64–73. 2003.(review). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi Y and Zhang CJ: The effects of
carnosine on high glucose-induced apoptosis of human umbilical vein
endothelial cells. Adv Mat Res. 345:365–369. 2011.
|
|
39
|
Mateyak MK, Obaya AJ and Sedivy JM: c-Myc
regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle
progression at multiple independent points. Mol Cell Biol.
19:4672–4683. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian Y, Wan H and Tan G: Cell
cycle-related kinase in carcinogenesis. Oncol Lett. 4:601–606.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sebio A, Kahn M and Lenz HJ: The potential
of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther
Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sakoguchi-Okada N, Takahashi-Yanaga F,
Fukada K, Shiraishi F, Taba Y, Miwa Y, Morimoto S, Iida M and
Sasaguri T: Celecoxib inhibits the expression of survivin via the
suppression of promoter activity in human colon cancer cells.
Biochem Pharmacol. 73:1318–1329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shan BE, Wang MX and Li RQ: Quercetin
inhibit human SW480 colon cancer growth in association with
inhibition of cyclin D1 and survivin expression through
Wnt/beta-catenin signaling pathway. Cancer Invest. 27:604–612.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ha T, Lou Z, Baek SJ and Lee SH:
Tolfenamic acid downregulates β-catenin in colon cancer. Int
Immunopharmacol. 35:287–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han D, Cao C, Su Y, Wang J, Sun J, Chen H
and Xu A: Ginkgo biloba exocarp extracts inhibits
angiogenesis and its effects on Wnt/β-catenin-VEGF signaling
pathway in Lewis lung cancer. J Ethnopharmacol. 192:406–412. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mizushima N: Autophagy. FEBS Lett.
584:12792010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ertmer A, Huber V, Gilch S, Yoshimori T,
Erfle V, Duyster J, Elsässer HP and Schätzl HM: The anticancer drug
imatinib induces cellular autophagy. Leukemia. 21:936–942. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guamán-Ortiz LM, Romero-Benavides JC,
Suarez AI, Torres-Aguilar S, Castillo-Veintimilla P,
Samaniego-Romero J, Ortiz-Diaz K and Bailon-Moscoso N: Cytotoxic
property of Grias neuberthii extract on human colon cancer
cells: A crucial role of autophagy. Evid Based Complement Alternat
Med. 2020:15653062020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsieh LC, Hsieh SL, Chen CT, Chung JG,
Wang JJ and Wu CC: Induction of α-phellandrene on autophagy in
human liver tumor cells. Am J Chin Med. 43:121–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hsieh SL, Chen CT, Wang JJ, Kuo YH, Li CC,
Hsieh LC and Wu CC: Sedanolide induces autophagy through the PI3K,
p53 and NF-κB signaling pathways in human liver cancer cells. Int J
Oncol. 47:2240–2246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galluzzi L, Kepp O, Chan FK and Kroemer G:
Necroptosis: Mechanisms and relevance to disease. Annu Rev Pathol.
12:103–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dhuriya YK and Sharma D: Necroptosis: A
regulated inflammatory mode of cell death. J Neuroinflammation.
15:1992018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schenk B and Fulda S: Reactive oxygen
species regulate Smac mimetic/TNFα-induced necroptotic signaling
and cell death. Oncogene. 34:5796–5806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee YJ, Park KS, Nam HS, Cho MK and Lee
SH: Apigenin causes necroptosis by inducing ROS accumulation,
mitochondrial dysfunction, and ATP depletion in malignant
mesothelioma cells. Korean J Physiol Pharmacol. 24:493–502. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu X, Zhang Y, Gao H, Hou Y, Lu JJ, Feng
Y, Xu Q, Liu B and Chen X: Induction of an MLKL mediated
non-canonical necroptosis through reactive oxygen species by
tanshinol A in lung cancer cells. Biochem Pharmacol.
171:1136842020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jackson AL, Zhou B and Kim WY: HIF,
hypoxia and the role of angiogenesis in non-small cell lung cancer.
Expert Opin Ther Targets. 14:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lichtenberger BM, Tan PK, Niederleithner
H, Ferrara N, Petzelbauer P and Sibilia M: Autocrine VEGF signaling
synergizes with EGFR in tumor cells to promote epithelial cancer
development. Cell. 140:268–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang KF, Zhang GD, Huang YQ and Diao Y:
Wogonin induces apoptosis and down-regulates survivin in human
breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int
Immunopharmacol. 12:334–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng HL, Yang J, Hou Y, Sun B, Zhang Q,
Mou Y, Wand L and Wu C: Oligomer procyanidins (F2) isolated from
grape seeds inhibits tumor angiogenesis and cell invasion by
targeting HIF-1α in vitro. Int J Oncol. 46:708–720. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Quinn PJ, Boldyrev AA and Formazuyk VE:
Carnosine: Its properties, functions and potential therapeutic
applications. Mol Aspects Med. 13:379–444. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hipkiss AR: Carnosine, a protective,
anti-ageing peptide? Int J Biochem Cell Biol. 30:863–868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sampathkumar SG, Jones MB, Meledeo MA,
Campbell CT, Choi SS, Hida K, Gomutputra P, Sheh A, Gilmartin T,
Head SR, et al: Targeting glycosylation pathways and the cell
cycle: Sugar-dependent activity of butyrate-carbohydrate cancer
prodrugs. Chem Biol. 13:1265–1275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fahie K and Zachara NE: Molecular
functions of glycoconjugates in autophagy. J Mol Biol.
428:3305–3324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cheng WK and Oon CE: How glycosylation
aids tumor angiogenesis: An updated review. Biomed Pharmacother.
103:1246–1252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hipkiss AR, Michaelis J and Syrris P:
Non-enzymatic glycosylation of the dipeptide L-carnosine, a
potential anti-protein-cross-linking agent. FEBS Lett. 371:81–85.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hipkiss AR and Gaunitz F: Inhibition of
tumour cell growth by carnosine: some possible mechanisms. Amino
Acids. 46:327–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Wang K, Chen L, Zhu X and Zhou J:
Quantification of mRNA Levels Using Real-Time Polymerase Chain
Reaction (PCR). Methods Mol Biol. 1406:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Deribe YL, Pawson T and Dikic I:
Post-translational modifications in signal integration. Nat Struct
Mol Biol. 17:666–672. 2010. View Article : Google Scholar : PubMed/NCBI
|