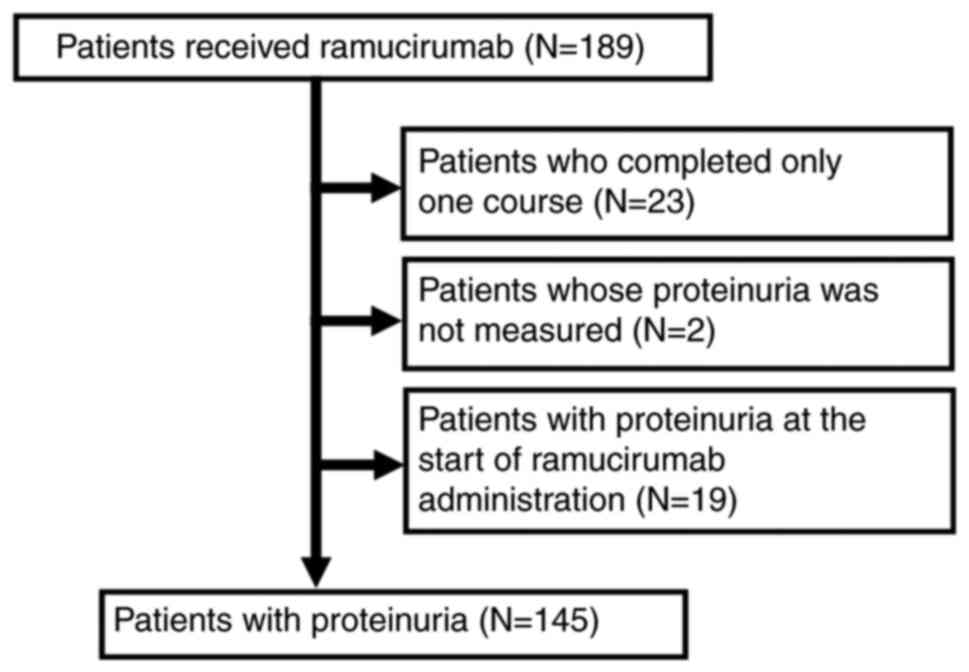
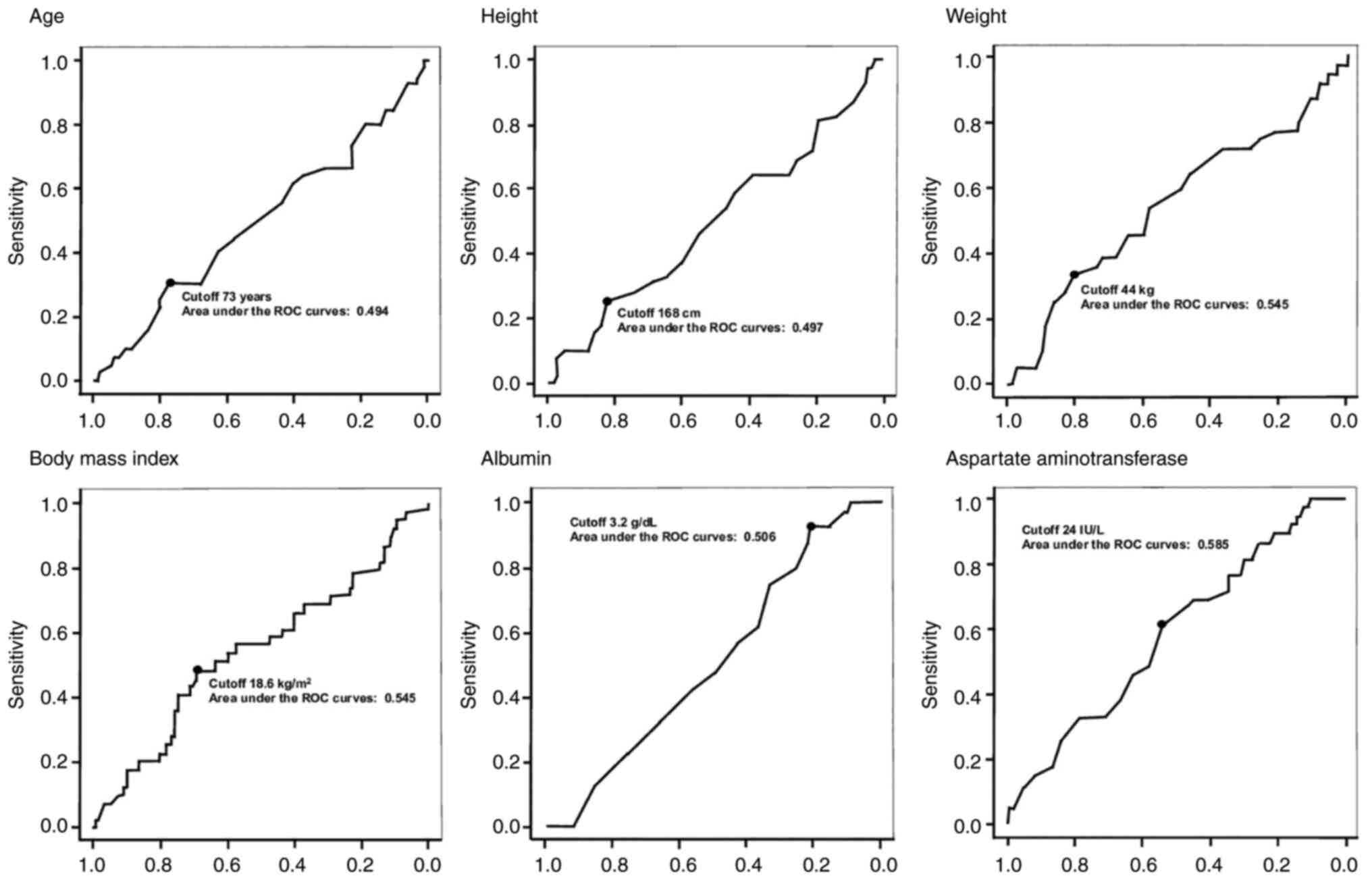
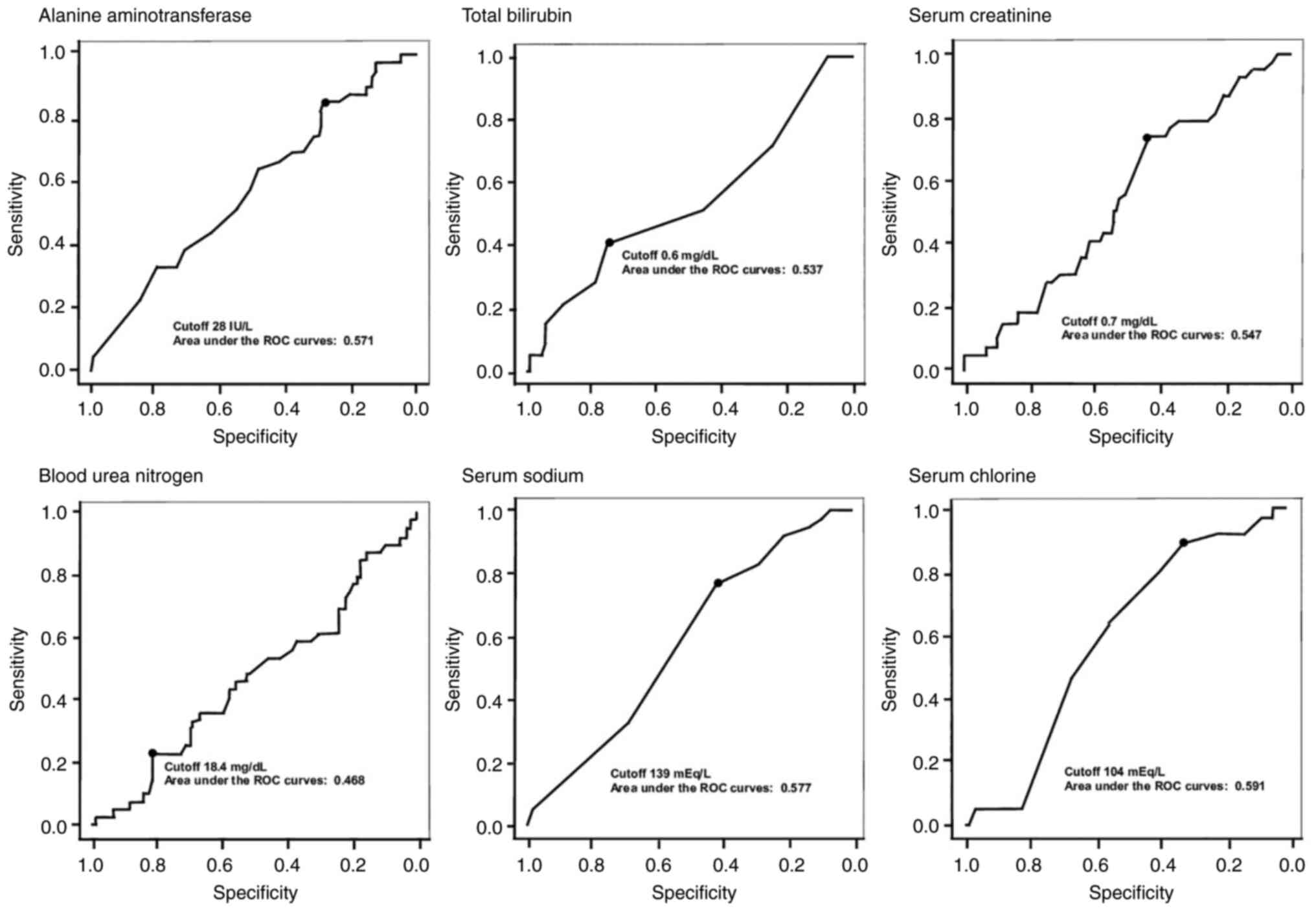
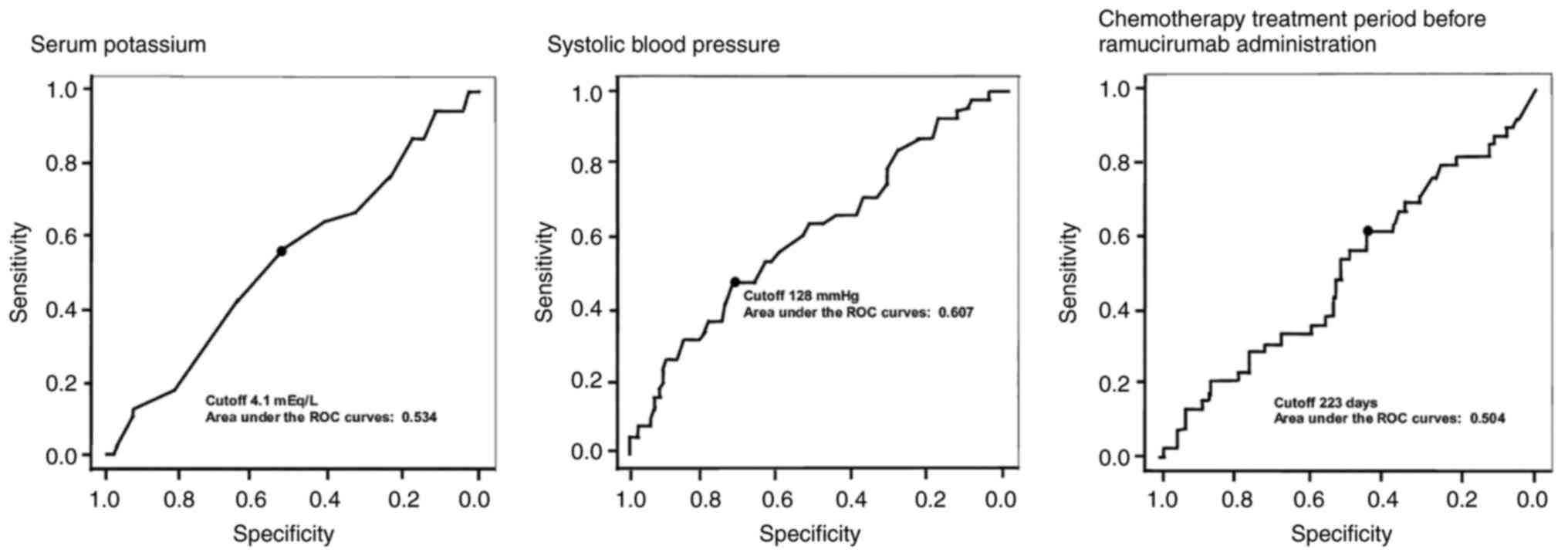
|
1
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakagawa K, Garon EB, Seto T, Nishio M,
Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et
al: Ramucirumab plus erlotinib in patients with untreated,
EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 20:1655–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdel-Rahman O and ElHalawani H:
Proteinuria in patients with solid tumors treated with ramucirumab:
A systematic review and meta-analysis. Chemotherapy. 60:325–333.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka T, Kurata Y, Takase N, Himoto M,
Shinmen T, Dan K, Kajizono M, Masaoka Y, Nakamoto A, Nawa H, et al:
Risk factor of proteinuria in patients receiving ramucirumab. Iryo
Yakugaku. 47:250–255. 2021.(In Japanese).
|
|
6
|
Sobue N, Iwai M, Usami E, Okada K, Kimura
M, Nakao T, Yoshimura T and Nishijima T: Analysis of the risk
factors for onset of proteinuria with bevacizumab administration
and the effect of renin-angiotensin system depressant drugs. Iryo
Yakugaku. 42:381–386. 2016.(In Japanese).
|
|
7
|
Teramachi H, Shiga H, Komada N, Tamura K,
Yasuda M, Umeda M, Tachi T, Goto C and Tsuchiya T: Risk factors
contributing to urinary protein expression resulting from
bevacizumab combination chemotherapy. Pharmazie. 68:217–220.
2013.PubMed/NCBI
|
|
8
|
Kobayashi T, Tanizawa Y, Nagaoka S, Chen
Y, Kitagawa N, Muto M and Piao Y: Incidence of proteinuria among
Japanese colorectal cancer patients after receiving
ramucirumab-cohort study using claim database. Gan To Kagaku Ryoho.
47:917–922. 2020.(In Japanese). PubMed/NCBI
|
|
9
|
Yen CJ, Muro K, Kim TW, Kudo M, Shih JY,
Lee KW, Chao Y, Kim SW, Yamazaki K, Sohn J, et al: Ramucirumab
safety in East Asian patients: A meta-analysis of six global,
randomized, double-blind, placebo-controlled, phase III clinical
trials. J Glob Oncol. 4:1–12. 2018. View Article : Google Scholar
|
|
10
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thigpen JT, Blessing JA, Ball H, Hummel SJ
and Barrett RJ: Phase II trial of paclitaxel in patients with
progressive ovarian carcinoma after platinum-based chemotherapy: A
gynecologic oncology group study. J Clin Oncol. 12:1748–1753. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Jongh FE, van Veen RN, Veltman SJ, de
Wit R, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ,
Stoter G and Verweij J: Weekly high-dose cisplatin is a feasible
treatment option: Analysis on prognostic factors for toxicity in
400 patients. Br J Cancer. 88:1199–1206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanbayashi Y, Ishikawa T, Tabuchi Y,
Sakaguchi K, Ouchi Y, Otsuji E, Takayama K and Taguchi T:
Predictive factors for the development of proteinuria in cancer
patients treated with bevacizumab, ramucirumab, and aflibercept: A
single-institution retrospective analysis. Sci Rep. 10:20112020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibata M, Sato KK, Uehara S, Koh H,
Kinuhata S, Oue K, Kambe H, Morimoto M and Hayashi T: Blood
pressure components and the risk for proteinuria in Japanese men:
The Kansai healthcare study. J Epidemiol. 27:505–510. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirai T, Shuji Y, Takiyama M, Hanada K and
Itoh T: Renin-angiotensin system inhibitors for countering
proteinuria induced by angiogenesis inhibitors: A retrospective
observational analysis. Cancer Chemother Pharmacol. 84:195–202.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lafayette RA, McCall B, Li N, Chu L,
Werner P, Das A and Glassock R: Incidence and relevance of
proteinuria in bevacizumab-treated patients: Pooled analysis from
randomized controlled trials. Am J Nephrol. 40:75–83. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenstock JL, Pommier M, Stofels G, Patel
S and Michelis MF: Prevalence of proteinuria and albuminuria in an
obese population and associated risk factors. Front Med (Lausanne).
5:1222018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JK, Ju YS, Moon SJ, Song YR, Kim HJ
and Kim SG: High pulse pressure and metabolic syndrome are
associated with proteinuria in young adult women. BMC Nephrol.
14:452013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Li HY, Zhou Q, Peng ZW, An X, Li
W, Xiong LP, Yu XQ, Jiang WQ and Mao HP: Renal function and
all-cause mortality risk among cancer patients. Medicine
(Baltimore). 95:e37282016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MJ, Kang YU, Kim CS, Choi JS, Bae EH,
Ma SK, Kweon SS and Kim SW: Proteinuria as a risk factor for
mortality in patients with colorectal cancer. Yonsei Med J.
54:1194–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|