Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most fatal cancers worldwide (1). The incidence of PDAC has continuously
increased during the past two decades, and its incidence has been
reported to have increased from 12.1 per 100,000 in 1997–2000 to
15.3 per 100,000 in 2013–2016 (2).
It has been reported that PDAC is the second leading cause of
cancer-associated mortality worldwide (3,4).
Currently, the therapeutic approaches used to treat patients with
PDAC are still based on surgical resection, traditional
chemotherapy and radiation (5).
Despite advancements in these treatment modalities, the survival
rate of patients with PDAC remains unsatisfactory with 3-year
survival ranging between 16.9 and 25.4% during 1997–2016 (2,6).
Along with the development of molecular science, investigators are
now focusing on the molecular genetics of PDAC to identify its
subtypes and design precision medicine strategies (7,8).
Currently, several genetic variations have been identified in PDAC,
such as mutations in the KRAS, cyclin-dependent kinase inhibitor 2A
and TP53 genes (9,10). However, the effects of these
mutations have been mostly restricted to the research level
(9,11). Thus, the application of these
findings on treating patients with PDAC is yet to be implemented
(7,8).
circular RNAs (circRNAs), characterized by the
closed coil structure resulting from a lack of Poly A tail, are a
class of small endogenous molecules (12). Despite recent advancements in
circRNA research, the origin and roles of circRNAs remain unclear.
Several biological functions of circRNAs have been established,
such as regulating the structure of protein complexes and the
expression of their original genes, and sponging microRNAs
(miRNAs/miRs) (13–15). With regards to the role of circRNAs
in oncology, microarray analyses have indicated that partial
circRNAs are aberrantly expressed in different types of cancer,
such as lung adenocarcinoma, squamous cell carcinoma and glioma,
and involved in their initiation and progression (16–18).
Notably, circRNAs exhibit a more stable structure compared with
other types of non-coding RNAs in human tissues and cells (19); therefore, several studies have been
performed to investigate the expression profiles and the potential
functions of circRNAs in patients with cancer (20,21).
Previously, two studies analyzed the circRNA expression profiles in
patients with PDAC; however, the differentially expressed circRNAs
(DEcircRNAs) identified in these studies lacked validation in large
scale clinical studies, and their associations with clinical
characteristics or survival have not yet been investigated
(22,23).
Based on the two datasets from the previous studies,
GSE69362 and GSE79634, the present study performed a secondary
analysis to determine the candidate circRNAs by identifying the
DEcircRNAs with accordant expression trends in the two datasets. A
total of nine candidate circRNAs were identified and validated in
tissue samples from 60 patients with PDAC via reverse
transcription-quantitative (RT-q)PCR analysis. The present study
also investigated the correlation of these candidate circRNAs with
clinicopathological characteristics, and their association with
survival of patients with PDAC.
Materials and methods
Microarray data collection and
analysis
To identify the DEcircRNAs in PDAC, the circRNA
expression profile datasets from the National Center of
Biotechnology Information Gene Expression Omnibus database were
searched (NCBI GEO, http://www.ncbi.nlm.nih.gov/geo). A total of two
datasets, GSE69362 and GSE79634, including the circRNA expression
profiles in patients with PDAC were screened and downloaded from
the GEO database. The GSE69362 dataset included circRNA expression
profiles from six PDAC tissues and six paired adjacent normal
tissues (24). The GSE79634
dataset included circRNA expression profiles from 20 PDAC tissues
and 20 paired adjacent normal tissues (23).
The GEOquery (http://www.bioconductor.org/packages/release/bioc/html/GEOquery.html)
and limma (http://www.bioconductor.org/packages/release/bioc/html/limma.html)
packages in R software (version 3.3.2; http://www.r-project.org) were used to process the
expression matrix and differential expression analysis, as
previously described (25).
Principal component analysis (PCA) was performed using the
Factoextra package in R software (version 1.0.7; http://cran.r-project.org/web/packages/factoextra/index.html).
Log2 [fold change (FC)]≥|1| and an adjusted P-value
(Padj)≤0.05 were set as thresholds to identify the
DEcircRNAs, which were presented as volcano plots using the ggplot2
package (version 3.3.5; http://cran.r-project.org/web/packages/ggplot2/index.html)
in R software (version 3.3.2; http://www.r-project.org).
Gene Ontology (GO; http://geneontology.org) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.kegg.jp) enrichment analyses were performed
to assess the DEcircRNAs, based on the identified genes. The
overlapping upregulated and downregulated DEcircRNAs in both
GSE69362 and GSE79634 datasets were displayed using Venn diagrams.
A total of nine DEcircRNAs were identified. The circRNA-miRNA
network, depicting the interactions between the nine candidate
circRNAs and their target miRNAs, was predicted using Miranda
software (version 3.3a; http://anaconda.org/bioconda/miranda).
Patients and samples
To further validate the correlation between the
expression profiles of the nine candidate circRNAs and the
clinicopathological characteristics of patients with PDAC, PDAC and
paired adjacent normal tissues were collected from 60 patients with
PDAC who underwent surgery between November 2016 and December 2019.
Para-cancer tissues were taken 2 cm away from the tumor margin.
There were 36 male patients and 24 female patients. The mean age of
patients with PDAC was 63.3±7.2 years (range, 43–75 years). The
surgery for patients with PDAC was performed at Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). The inclusion criteria for all patients
with PDAC were: i) Pathologically confirmed as PDAC; ii) age >18
years; iii) underwent surgical resection; iv) had snap-frozen PDAC
and paired adjacent normal tissues; and v) without neoadjuvant
therapy. The exclusion criteria were: i) Incomplete clinical data
and survival data; and ii) complicated with other malignancies. The
PDAC and paired adjacent normal tissues were snap-frozen in liquid
nitrogen immediately after surgery. The specimens were stored at
−80°C. All patients were followed up every 3–6 months by clinic
visits or telephone calls. The median follow-up duration was 16.0
months (range, 2.0-36.0 months; between November 2016 and December
2019), and the last follow-up date was December 31, 2019. The
present study was approved by the Ethics Committee of Tongji
Medical College, Huazhong University of Science and Technology
[approval no. (2014), ethics approval no. (S108)]. Written informed
consent was provided by all patients or their legal representatives
prior to the study start.
Clinical data collection
The clinical data of patients were reviewed, and the
clinical characteristics [age, sex, smoking and drinking history,
tumor location, pathological grade, tumor size, lymph node
metastasis (LNM), T stage, N stage and TNM stage] were recorded.
Tumor staging was performed according to the American Joint
Committee on Cancer (version 8) TNM staging system (26). Survival data were extracted from
the patients' follow-up documents, which were used to measure
overall survival (OS) time. Follow-up lasted from November 2016 to
December 2019. Patients were followed up every 3–6 months by clinic
visits or telephone calls.
Determination of candidate
circRNAs
The expression levels of the nine candidate circRNAs
were detected in 60 PDAC tissues and paired adjacent normal tissues
via RT-qPCR analysis. Total RNA was extracted from PDAC tissues and
normal tissues using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). The linear RNA was subsequently digested using
RNase R (Epicentre; Illumina, Inc.) and reverse transcribed into
cDNA using the PrimeScript® RT reagent kit (Takara
Biotechnology Co., Ltd.). qPCR was subsequently performed using TB
Green® Fast qPCR Mix (Takara Biotechnology Co., Ltd.).
The primer sequences used for qPCR are listed in Table SI. The specific conditions for RT
were as follows: 37°C for 15 min and 85°C for 5 sec. The
thermocycling conditions for qPCR were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 61°C for 10 sec. The relative expression was calculated
using the 2−ΔΔCq method (27).
Classification of candidate
circRNAs
The candidate circRNAs were classified into high-
and low-expression groups according to their median value in the
tumor tissue, as follows: 3.345 for circ_0000515, 2.564 for
circ_0000517, 2.114 for circ_0000520, 2.999 for circ_0000514, 4.675
for circ_0011385, 1.865 for circ_0055033, 2.395 for circ_0072088,
1.594 for circ_0003528, 1.086 for circ_0008514.
Statistical analysis
Data processing and graphs were plotted using R
software (version 3.3.2; http://www.r-project.org), SPSS 22.0 software (IBM
Corp.) or GraphPad Prism 7.02 software (GraphPad Software Inc.).
Data are presented as the mean ± SD for continuous data with normal
distribution, median with interquartile range for continuous data
with skewed distribution and number with percentage for categorical
data. Data distribution was determined using the Kolmogorov-Smirnov
test. The Wilcoxon signed-rank sum test was used to compare
differences in the expression levels of the candidate circRNAs
between PDAC tissues and adjacent normal tissues. Receiver
operating characteristic (ROC) curve analysis was performed to
analyze the performance of the candidate circRNAs in distinguishing
between PDAC tissues and adjacent normal tissues. Spearman's rank
correlation analysis was performed to assess the correlation
between the circRNAs and the clinicopathological characteristics of
patients with PDAC. The OS time was measured from the date of
surgery to mortality or the last follow-up via the Kaplan-Meier
method and log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
circRNA expression profiles and
enrichment analysis
In the GSE79634 dataset, PCA analysis revealed that
circRNAs were differentially expressed between 20 PDAC tissues and
20 paired adjacent normal tissues (Fig. 1A). The volcano plot revealed that
there were 161 upregulated and 128 downregulated DEcircRNAs in PDAC
tissues compared with paired adjacent normal tissues (Fig. 1B). Furthermore, GO enrichment
analysis demonstrated that the DEcircRNAs were predominantly
enriched in the ‘ATP binding’ in terms of molecular function, the
‘cytosol’ in terms of cellular component and the ‘G-protein coupled
receptor signaling pathway’ in terms of biological function
(Fig. 1C). In addition, the most
significantly enriched pathways in the KEGG enrichment analysis
associated with the candidate DEcircRNAs were the ‘regulation of
actin cytoskeleton’, ‘protein processing in endoplasmic reticulum’,
‘cGMP-PKG signaling’ and ‘insulin signaling’ pathways (Fig. 1D). These results suggested that the
circRNAs in the GSE79634 dataset were differently expressed between
the tumor tissue and adjacent tissue with 161 upregulated and 128
downregulated DEcircRNAs, and they may be involved in several
cellular process.
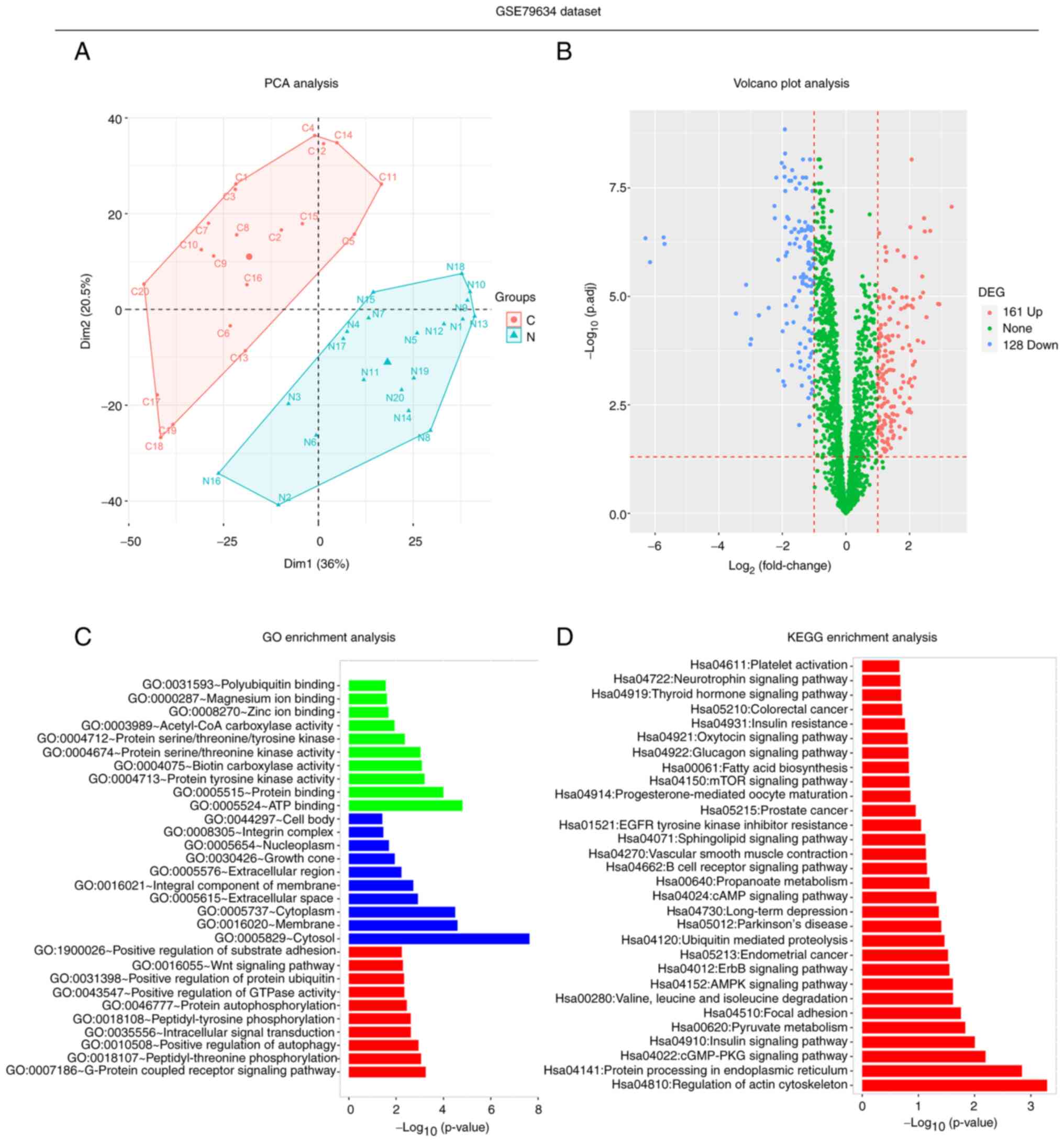 | Figure 1.Secondary analyses of the GSE79634
dataset. (A) PCA, (B) volcano plot, (C) GO enrichment (green,
molecular function; blue, cellular component; red, biological
function) and (D) KEGG enrichment analyses in the GSE79634 dataset,
which includes 20 PDAC tissues and 20 paired adjacent normal
tissues. PCA, principal component analysis; GO, Gene Ontology;
KEGG, Kyoto Encyclopedia of Genes and Genomes; PDAC, pancreatic
ductal adenocarcinoma; DEcircRNA, differentially expressed circular
RNA; C, cancer; N, normal. |
In the GSE69362 dataset, PCA analysis revealed that
circRNAs were differentially expressed between six PDAC tissues and
six paired adjacent normal tissues (Fig. 2A). The volcano plot revealed that
there were 64 upregulated and 10 downregulated DEcircRNAs in PDAC
tissues compared with normal tissues (Fig. 2B). Furthermore, GO enrichment
analysis demonstrated that the DEcircRNAs were predominantly
enriched in the ‘zinc ion binding’ in terms of molecular function,
the ‘cytoplasm’ in terms of cellular component and the ‘mitotic
nuclear envelope disassembly’ in terms of biological process
(Fig. 2C). In addition, the most
significantly enriched pathways in the KEGG enrichment analysis
associated with the candidate DEcircRNAs were the ‘RNA transport’,
‘prostate cancer’, ‘melanogenesis’ and ‘insulin resistance’
signaling pathways (Fig. 2D).
These results suggested that the circRNAs in the GSE69362 dataset
were differently expressed between the tumor tissue and adjacent
tissue with 64 upregulated and 10 downregulated DEcircRNAs, and
they might be involved in several cellular process.
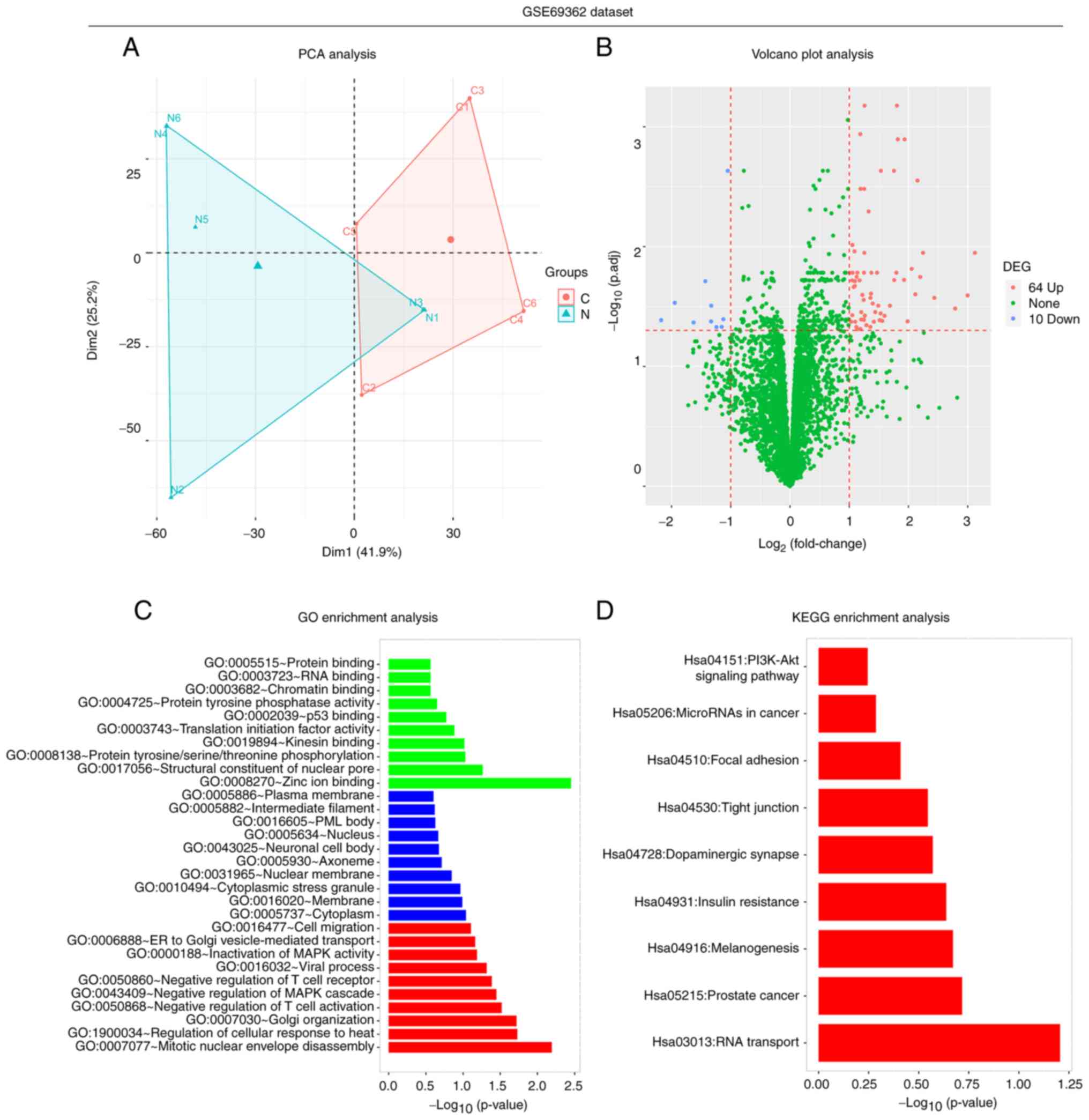 | Figure 2.Secondary analyses of the GSE69362
dataset. (A) PCA, (B) volcano plot, (C) GO enrichment and (D) KEGG
enrichment analyses in the GSE79634 dataset, which included six
PDAC tissues and six paired adjacent normal tissues. PCA, principal
component analysis; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of
Genes and Genomes; PDAC, pancreatic ductal adenocarcinoma;
DEcircRNA, differentially expressed circular RNA; C, cancer; N,
normal. |
Selection of candidate circRNAs
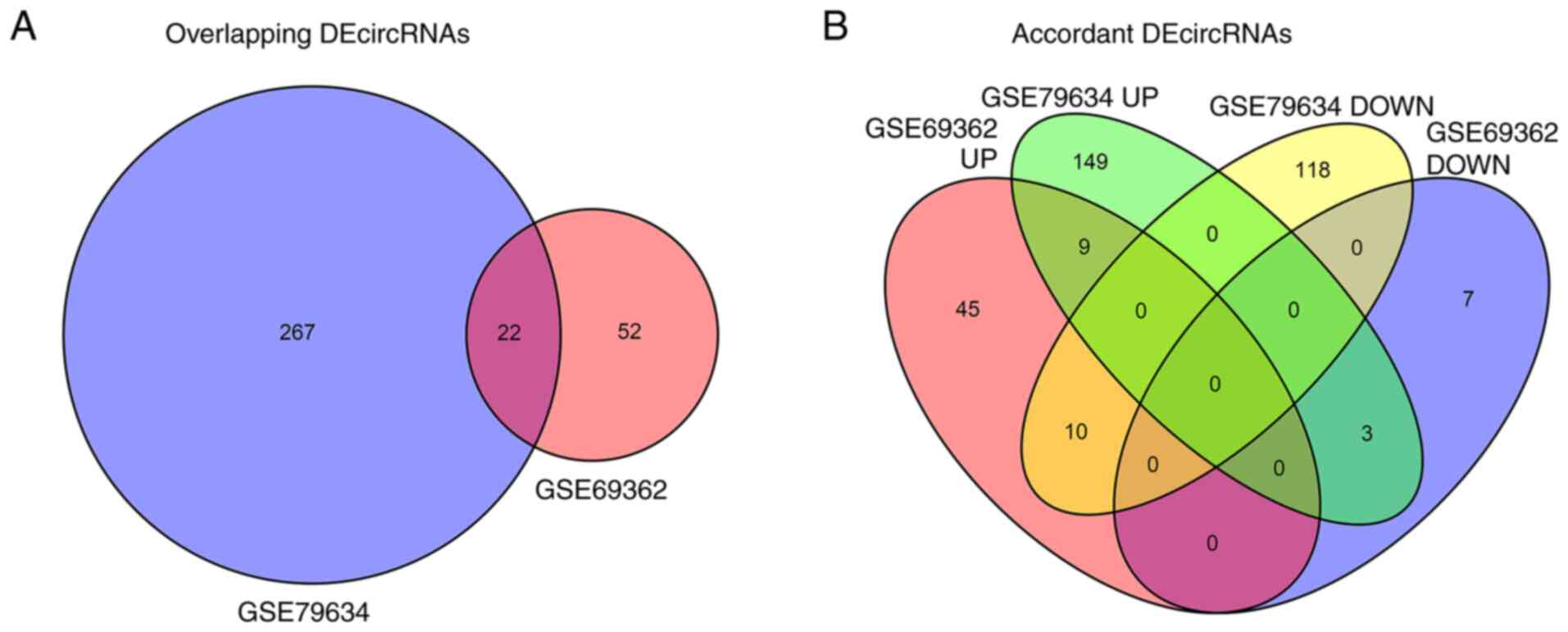
Further analysis revealed that 22 DEcircRNAs were
overlapped between the GSE79634 and GSE69362 datasets (Fig. 3A). Among the overlapping
DEcircRNAs, nine were upregulated in PDAC tissues compared with
paired adjacent normal tissues in both datasets (Fig. 3B). These nine DEcircRNAs were
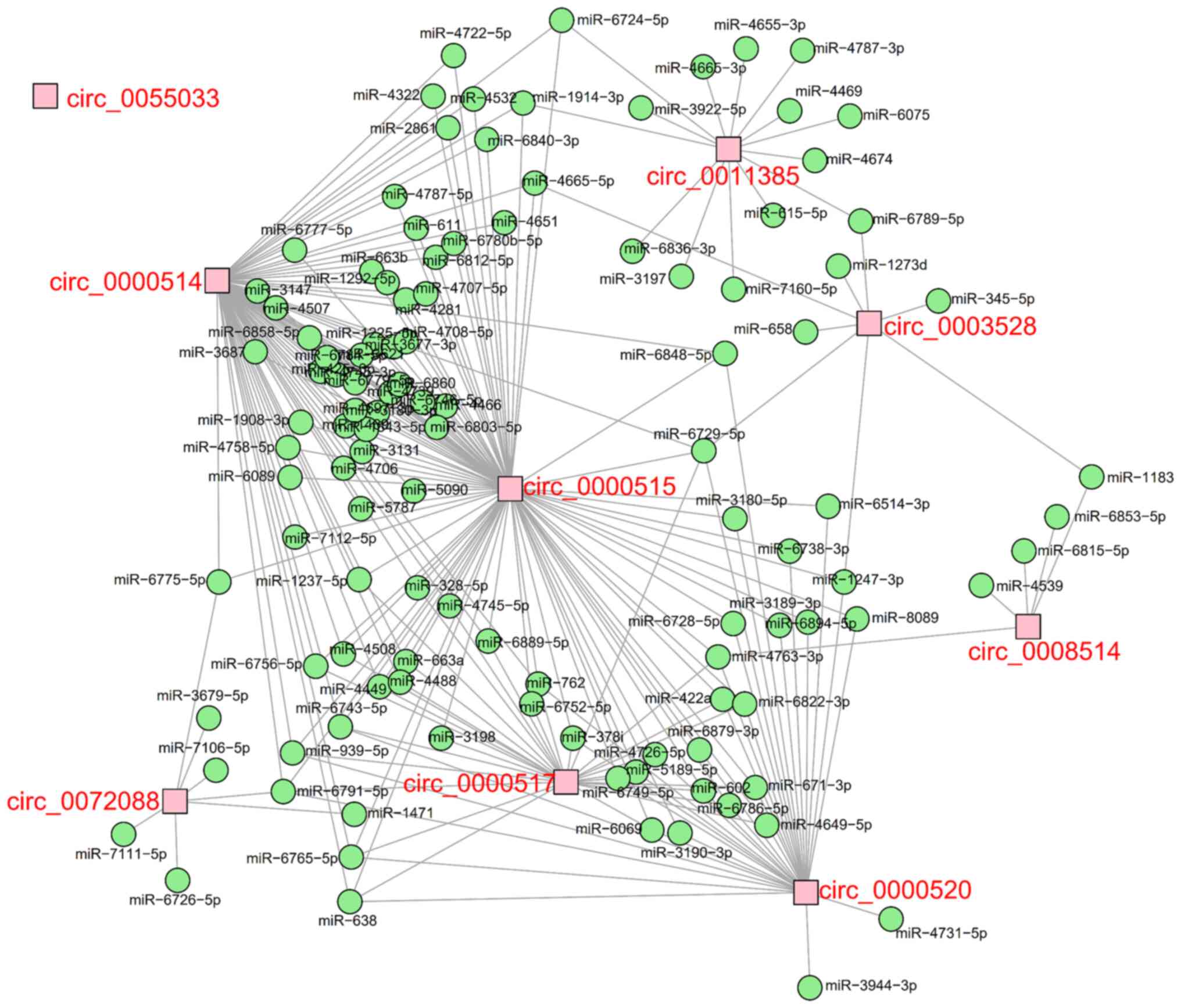
selected as candidate circRNAs and their target miRNAs were
predicted. The regulatory network of the DEcircRNAs and their
target miRNAs is presented in Fig.
4. The information of these nine candidate circRNAs is
presented in Table I, which
includes the probe name, located chromosome, start, end, type, gene
symbol, expression trend, log2(FC), P-value and
Padj-value in the GSE79634 and GSE69362 datasets. These
results suggested that 9 upregulated in tumor tissue, overlapping
DEcircRNAs between GSE79634 and GSE69362 datasets were identified
as candidate circRNAs.
 | Table I.Information on the nine candidate
circRNAs screened from the GSE79634 and GSE69362 datasets. |
Table I.
Information on the nine candidate
circRNAs screened from the GSE79634 and GSE69362 datasets.
|
|
|
|
|
|
|
|
| GSE79634
dataset | GSE69362
dataset |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| circRNA | Probe name | Chromosome | Start | End | Type | Gene symbol | Trend |
Log2(FC) | P-value |
Padj-value |
Log2(FC) | P-value |
Padj-value |
|---|
| circ_0000515 | ASCRP000071 | Chrom14 |
20811305 |
20811534 | Intragenic | RPPH1 | Up | 1.84572 |
2.74×10−6 |
1.80×10−5 | 1.25008 |
2.57×10−4 |
1.65×10−2 |
| circ_0000517 | ASCRP000315 | Chrom14 |
20811404 |
20811492 | Intragenic | RPPH1 | Up | 2.45408 |
4.03×10−9 |
1.58×10−7 | 1.05478 |
2.17×10−4 |
1.65×10−2 |
| circ_0000520 | ASCRP000343 | Chrom14 |
20811436 |
20811559 | Intragenic | RPPH1 | Up | 2.65125 |
1.16×10−8 |
3.08×10−7 | 2.24541 |
9.91×10−5 |
1.13×10−2 |
| circ_0000514 | ASCRP000389 | Chrom14 |
20811305 |
20811436 | Intragenic | RPPH1 | Up | 1.90533 |
1.34×10−6 |
1.04×10−5 | 1.82034 |
1.74×10−6 |
1.28×10−3 |
| circ_0011385 | ASCRP000535 |
Chrom1 |
32691771 |
32692131 | Exonic | EIF3I | Up | 1.64374 |
1.87×10−5 |
8.20×10−5 | 2.15259 |
9.61×10−6 |
2.81×10−3 |
| circ_0055033 | ASCRP003063 |
Chrom2 |
69304539 |
69318051 | Exonic | ANTXR1 | Up | 1.35802 |
6.24×10−6 |
3.43×10−5 | 1.17478 |
4.39×10−4 |
1.91×10−2 |
| circ_0072088 | ASCRP004099 |
Chrom5 |
32379220 |
32388780 | Exonic | ZFR | Up | 2.93726 |
2.18×10−6 |
1.51×10−5 | 3.12151 |
9.53×10−5 |
1.13×10−2 |
| circ_0003528 | ASCRP004228 |
Chrom5 | 134032815 | 134044578 | Exonic | SEC24A | Up | 1.03133 |
1.15×10−5 |
5.66×10−5 | 1.25562 |
9.67×10−5 |
1.13×10−2 |
| circ_0008514 | ASCRP004441 |
chrom6 | 107031202 | 107050797 | Exonic | RTN4IP1 | Up | 1.03306 |
1.34×10−6 |
1.04×10−5 | 2.19814 |
2.98×10−4 |
1.79×10−2 |
Characteristics of patients with
PDAC
Among the 60 patients with PDAC, 36 (60.0%) were men
and 24 (40.0%) were women, with a mean age of 63.3±7.2 years, and
the number of patients with tumor location at pancreas head,
pancreas body and pancreas tail was 27 (45.0%), 21 (35.0%) and 12
(20.0%), respectively. The number of patients with G1, G2 and G3
pathological grade was 8 (13.3%), 26 (43.3%) and 26 (43.3%),
respectively. The mean tumor size was 3.8±1.5 cm. A total of 42
patients (70.0%) had PDAC LNM. In addition, the number of patients
with IA, IB, IIA, IIB and III TNM stage was 4 (6.7%), 4 (6.7%), 8
(13.3%), 28 (46.7%) and 16 (26.7%), respectively. All
clinicopathological characteristics are presented in Table II. These results indicated the
characteristics of patients with PDAC enrolled in the present
study.
 | Table II.Clinical characteristics of patients
with pancreatic ductal adenocarcinoma (n=60). |
Table II.
Clinical characteristics of patients
with pancreatic ductal adenocarcinoma (n=60).
| Characteristic | Patient, n |
|---|
| Age, years (mean ±
SD) | 63.3±7.2 |
| Sex, n (%) |
|
|
Male | 36 (60.0) |
|
Female | 24 (40.0) |
| History of smoking,
n (%) |
|
| No | 26 (43.3) |
|
Yes | 34 (56.7) |
| History of
drinking, n (%) |
|
| No | 29 (48.3) |
|
Yes | 31 (51.7) |
| Tumor location, n
(%) |
|
|
Pancreas head | 27 (45.0) |
|
Pancreas body | 21 (35.0) |
|
Pancreas tail | 12 (20.0) |
| Pathological grade,
n (%) |
|
| G1 | 8
(13.4) |
| G2 | 26 (43.3) |
| G3 | 26 (43.3) |
| Tumor size, cm
(mean ± SD) | 3.8±1.5 |
| LNM, n (%) |
|
| No | 18 (30.0) |
|
Yes | 42 (70.0) |
| T stage, n (%) |
|
| T1 | 9
(15.0) |
| T2 | 29 (48.3) |
| T3 | 15 (25.0) |
| T4 | 7
(11.7) |
| N stage, n (%) |
|
| N0 | 18 (30.0) |
| N1 | 33 (55.0) |
| N2 | 9
(15.0) |
| TNM stage, n
(%) |
|
| IA | 4 (6.7) |
| IB | 4 (6.7) |
|
IIA | 8
(13.3) |
|
IIB | 28 (46.7) |
|
III | 16 (26.6) |
Candidate circRNA expression levels in
patients with PDAC
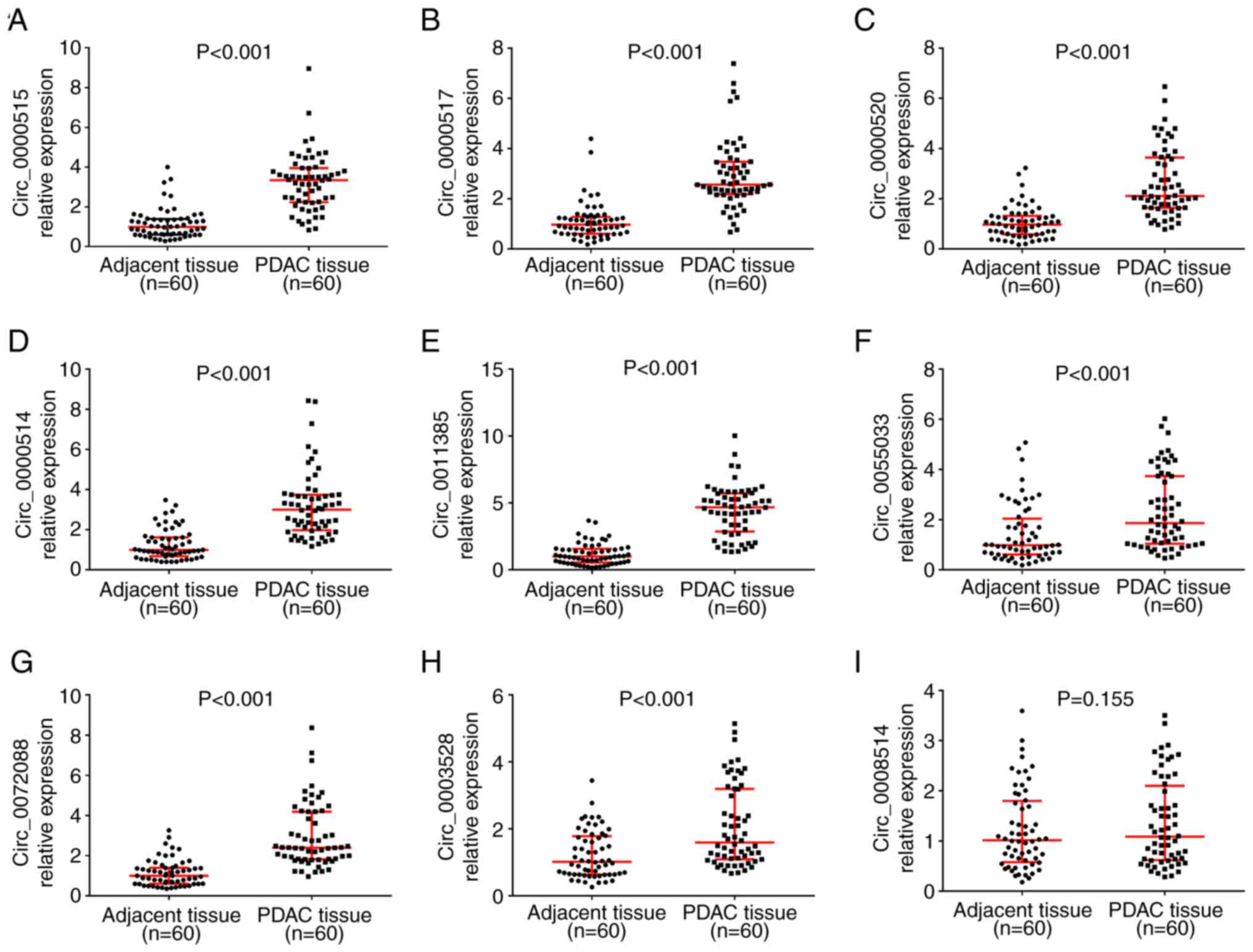
Among the nine candidate circRNAs, the expression
levels of circ_0000515 (P<0.001; Fig. 5A), circ_0000517 (P<0.001;
Fig. 5B), circ_0000520
(P<0.001; Fig. 5C),
circ-0000514 (P<0.001; Fig.
5D), circ_0011385 (P<0.001; Fig. 5E), circ_0055033 (P<0.001;
Fig. 5F), circ_0072088
(P<0.001; Fig. 5G) and
circ_0003528 (P<0.001; Fig. 5H)
were significantly upregulated in PDAC tissues compared with paired
adjacent normal tissues. However, circ_0008514 expression (P=0.155;
Fig. 5I) was not significantly
different between PDAC tissues and paired adjacent normal tissues.
Furthermore, ROC curve analysis demonstrated that circ_0000515
(Fig. 6A), circ_0000517 (Fig. 6B), circ_0000520 (Fig. 6C), circ_0000514 (Fig. 6D), circ_0011385 (Fig. 6E) and circ_0072088 (Fig. 6G) exhibited good area under the
curve (AUC) values to distinguish PDAC tissues from paired adjacent
normal tissues. The AUC values for these circRNAs were 0.920,
0.922, 0.887, 0.899, 0.953 and 0.902, respectively. Conversely,
circ-0055033 (Fig. 6F) and
circ_0003528 (Fig. 6H) exhibited
ordinary values in differentiating PDAC tissues from paired
adjacent normal tissues, with AUC values of 0.697 and 0.727,
respectively. Notably, circ_0008514 (Fig. 6I) failed to distinguish between
PDAC tissues and paired adjacent normal tissues. These findings
suggested that eight candidate circRNAs were highly expressed in
PDAC tissues compared with paired adjacent normal tissues and they
could distinguish PDAC tissues from paired adjacent normal
tissues.
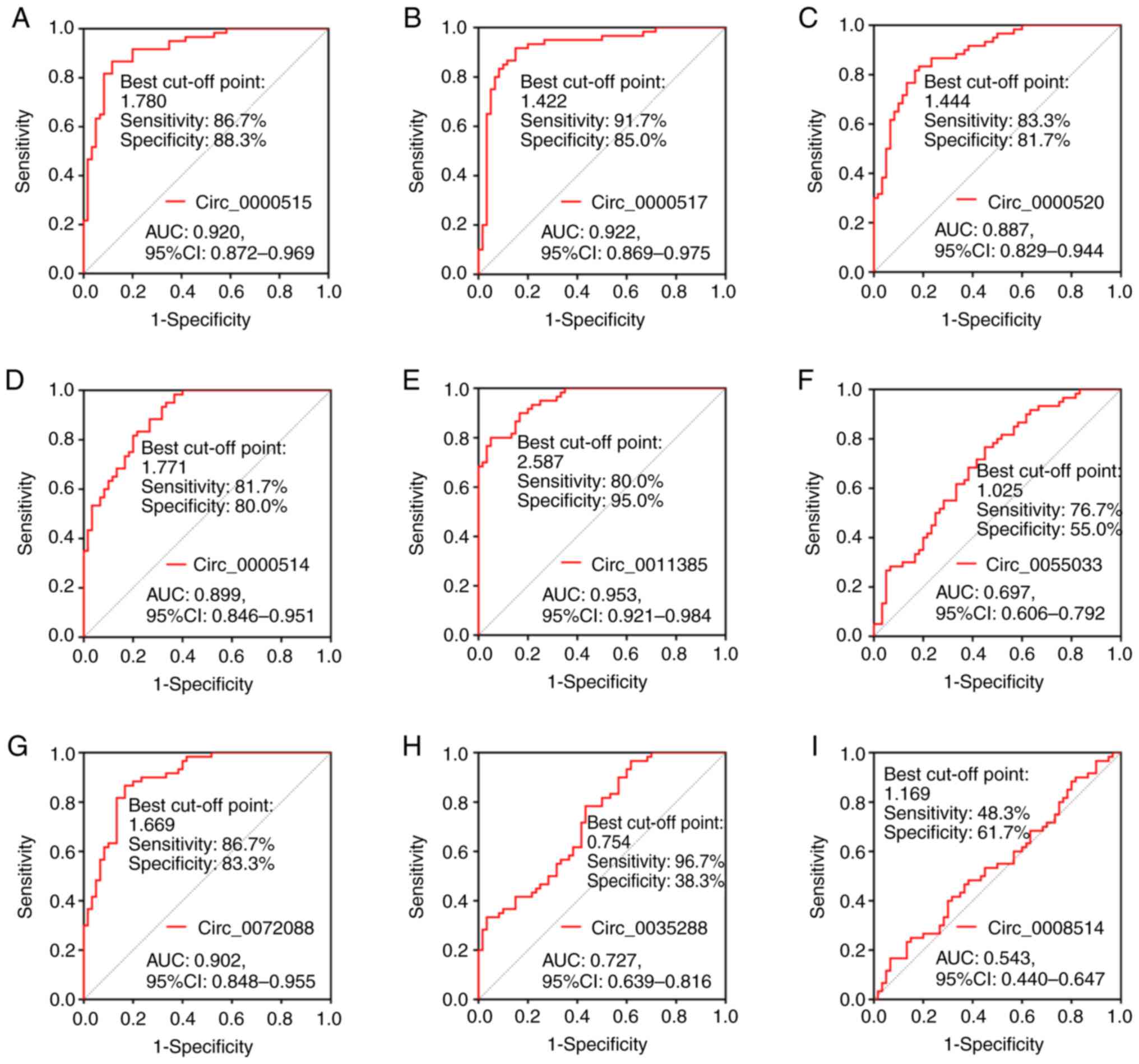 | Figure 6.ROC curve analysis of the candidate
circRNAs for distinguishing PDAC tissues from paired adjacent
normal tissues. ROC curve analysis was performed to determine the
AUC values of (A) circ_0000515, (B) circ_0000517, (C) circ_0000520,
(D) circ_0000514, (E) circ_0011385, (F) circ_0055033, (G)
circ_0072088, (H) circ_0003528 and (I) circ_0008514 for
distinguishing PDAC tissues from paired adjacent normal tissues.
ROC, receiver operating characteristic; circRNA, circular RNA;
PDAC, pancreatic ductal adenocarcinoma; AUC, area under the curve;
CI, confidence interval. |
Correlation between the expression
levels of the candidate circRNAs and the clinicopathological
characteristics of patients with PDAC
Spearman's rank correlation analysis was performed
to assess the correlation between the circRNAs and the
clinicopathological characteristics of patients with PDAC. The
results demonstrated that tumor circ_0000515 expression was
positively correlated with T stage (P=0.038) and TNM stage
(P=0.013) in patients with PDAC. In addition, tumor circ_0000514
expression was positively correlated with T stage (P=0.024), while
tumor circ_0011385 expression was positively correlated with T
stage (P=0.024), N stage (P=0.010) and TNM stage (P<0.001).
Furthermore, tumor circ_0072088 expression was positively
correlated with T stage (P=0.021) and TNM stage (P=0.011) in
patients with PDAC (Table III).
The aforementioned findings mentioned demonstrated that four
candidate circRNAs were associated with aggravating
clinicopathological characteristics of patients with PDAC.
 | Table III.Correlation between tumor circRNA
expression levels and the clinicopathological characteristics of
patients with pancreatic ductal adenocarcinoma. |
Table III.
Correlation between tumor circRNA
expression levels and the clinicopathological characteristics of
patients with pancreatic ductal adenocarcinoma.
|
| Pathological grade,
n (%) | T stage, n (%) | N stage, n (%) | TNM stage, n
(%) |
|---|
| circRNA | G1 | G2 | G3 | T1 | T2 | T3 | T4 | N0 | N1 | N2 | I | II | III |
|---|
| circ_0000515 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 5 (62.5) | 15 (57.7) | 10 (38.5) | 7 (77.8) | 15 (51.7) | 6 (40.0) | 2 (28.6) | 11 (61.1) | 16 (48.5) | 3 (33.3) | 7 (87.5) | 18 (50.0) | 5
(31.3) |
|
High | 3 (37.5) | 11 (42.3) | 16 (61.5) | 2 (22.2) | 14 (48.3) | 9 (60.0) | 5 (71.4) | 7
(38.9) | 17 (51.5) | 6 (66.7) | 1 (12.5) | 18 (50.0) | 11 (68.8) |
|
rs/P-value |
rs, 0.200; P=0.125 |
rs, 0.269;
P=0.038a |
rs, 0.177; P=0.176 |
rs, 0.317;
P=0.013a |
| circ_0000517 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 5 (62.5) | 15 (57.7) | 10 (38.5) | 6 (66.7) | 15 (51.7) | 6 (40.0) | 3 (42.9) | 12 (66.7) | 15 (45.5) | 3 (33.3) | 6 (75.0) | 18 (50.0) | 6
(37.5) |
|
High | 3 (37.5) | 11 (42.3) | 16 (61.5) | 3 (33.3) | 14 (48.3) | 9 (60.0) | 4 (57.1) | 6
(33.3) | 18 (54.5) | 6 (66.7) | 2 (25.0) | 18 (50.0) | 10 (62.5) |
|
rs/P-value |
rs, 0.200; P=0.125 |
rs, 0.161; P=0.218 |
rs, 0.232; P=0.075 |
rs, 0.212; P=0.105 |
| circ_0000520 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 7 (87.5) | 12 (46.2) | 11 (42.3) | 6 (66.7) | 15 (51.7) | 6 (40.0) | 3 (42.9) | 10 (55.6) | 17 (51.5) | 3 (33.3) | 6 (75.0) | 18 (50.0) | 6
(37.5) |
|
High | 1 (12.5) | 14 (53.8) | 15 (57.7) | 3 (33.3) | 14 (48.3) | 9 (60.0) | 4 (57.1) | 8
(44.4) | 16 (48.5) | 6 (66.7) | 2 (25.0) | 18 (50.0) | 10 (62.5) |
|
rs/P-value |
rs, 0.217; P=0.096 |
rs, 0.161; P=0.218 |
rs, 0.122; P=0.352 |
rs, 0.212; P=0.105 |
| circ_0000514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 6 (75.0) | 12 (46.2) | 12 (46.2) | 7 (77.8) | 16 (55.2) | 4 (26.7) | 3 (42.9) | 10 (55.6) | 16 (48.5) | 4 (44.4) | 6 (75.0) | 17 (47.2) | 7
(43.8) |
|
High | 2 (25.0) | 14 (53.8) | 14 (53.8) | 2 (22.2) | 13 (44.8) | 11 (73.3) | 4 (57.1) | 8
(44.4) | 17 (51.5) | 5 (55.6) | 2 (25.0) | 19 (52.8) | 9
(56.3) |
|
rs/P-value |
rs, 0.126; P=0.336 |
rs, 0.292;
P=0.024a |
rs, 0.077; P=0.557 |
rs, 0.154; P=0.239 |
| circ_0011385 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 6 (75.0) | 10 (38.5) | 14 (53.8) | 7 (77.8) | 15 (51.7) | 7 (46.7) | 1 (14.3) | 13 (72.2) | 15 (45.5) | 2 (22.2) | 8 (100.0) | 19 (52.8) | 3
(18.8) |
|
High | 2 (25.0) | 16 (61.5) | 12 (46.2) | 2 (22.2) | 14 (48.3) | 8 (53.3) | 6 (85.7) | 5
(27.8) | 18 (54.5) | 7 (77.8) | 0 (0.0) | 17 (47.2) | 13 (81.3) |
|
rs/P-value |
rs, 0.017; P=0.898 |
rs, 0.292;
P=0.024a |
rs, 0.332;
P=0.010a |
rs, 0.480;
P<0.001b |
| circ_0055033 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 5 (62.5) | 13 (50.0) | 12 (46.2) | 6 (66.7) | 15 (51.7) | 6 (40.0) | 3 (42.9) | 8
(44.4) | 19 (57.6) | 3 (33.3) | 4 (50.0) | 20 (55.6) | 6
(37.5) |
|
High | 3 (37.5) | 13 (50.0) | 14 (53.8) | 3 (33.3) | 14 (48.3) | 9 (60.0) | 4 (57.1) | 10 (55.6) | 14 (42.4) | 6 (66.7) | 4 (50.0) | 16 (44.4) | 10 (62.5) |
|
rs/P-value |
rs, 0.091; P=0.491 |
rs, 0.161; P=0.218 |
rs, 0.013; P=0.922 |
rs, 0.115; P=0.383 |
| circ_0072088 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 5 (62.5) | 14 (53.8) | 11 (42.3) | 6 (66.7) | 17 (58.6) | 6 (40.0) | 1 (14.3) | 12 (66.7) | 15 (45.5) | 3 (33.3) | 6 (75.0) | 20 (55.6) | 4
(25.0) |
|
High | 3 (37.5) | 12 (46.2) | 15 (57.7) | 3 (33.3) | 12 (41.4) | 9 (60.0) | 6 (85.7) | 6
(33.3) | 18 (54.5) | 6 (66.7) | 2 (25.0) | 16 (44.4) | 12 (75.0) |
|
rs/P-value |
rs, 0.145; P=0.268 |
rs, 0.298;
P=0.021a |
rs, 0.232; P=0.075 |
rs, 0.326;
P=0.011a |
| circ_0003528 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 2 (25.0) | 15 (57.7) | 13 (50.0) | 4 (44.4) | 13 (44.8) | 9 (60.0) | 4 (57.1) | 10 (55.6) | 17 (51.5) | 3 (33.3) | 4 (50.0) | 19 (52.8) | 7
(43.8) |
|
High | 6 (75.0) | 11 (42.3) | 13 (50.0) | 5 (55.6) | 16 (55.2) | 6 (40.0) | 3 (42.9) | 8
(44.4) | 16 (48.5) | 6 (66.7) | 4 (50.0) | 17 (47.2) | 9
(56.3) |
|
rs/P-value |
rs, −0.072; P=0.587 |
rs, −0.122; P=0.353 |
rs, 0.122; P=0.352 |
rs, 0.057; P=0.664 |
| circRNA | G1 | G2 | G3 | T1 | T2 | T3 | T4 | N0 | N1 | N2 | I | II | III |
| circ_0008514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low | 3 (37.5) | 14 (53.8) | 13 (50.0) | 6 (66.7) | 13 (44.8) | 8 (53.3) | 3 (42.9) | 10 (55.6) | 16 (48.5) | 4 (44.4) | 5 (62.5) | 18 (50.0) | 7 (43.8) |
|
High | 5 (62.5) | 12 (46.2) | 13 (50.0) | 3 (33.3) | 16 (55.2) | 7 (46.7) | 4 (57.1) | 8 (44.4) | 17 (51.5) | 5 (55.6) | 3 (37.5) | 18 (50.0) | 9 (56.3) |
|
rs/P-value |
rs, −0.036; P=0.786 |
rs, 0.070; P=0.594 |
rs, 0.077; P=0.557 |
rs, 0.106; P=0.421 |
Association between the expression
levels of the candidate circRNAs and OS time in patients with
PDAC
The OS time was significantly shorter in patients
with high circ_0000515 expression than those with low circ_0000515
expression (P=0.013; Fig. 7A).
Furthermore, OS time was significantly shorter in patients with
high circ_0011385 expression than those with low circ_0011385
expression (P=0.014; Fig. 7E).
However, the expression levels of circ_0000517 (P=0.128; Fig. 7B), circ_0000520 (P=0.163; Fig. 7C), circ_0000514 (P=0.135; Fig. 7D), circ_0055033 (P=0.184; Fig. 7F), circ_0072088 (P=0.271; Fig. 7G), circ_0003528 (P=0.667; Fig. 7H) and circ_0008514 (P=0.862;
Fig. 7I) in PDAC tissues were not
associated with OS time. As aforementioned, high levels of these
two candidate circRNAs (circ_0000515 high and circ_0011385 high)
were associated with shorter OS times.
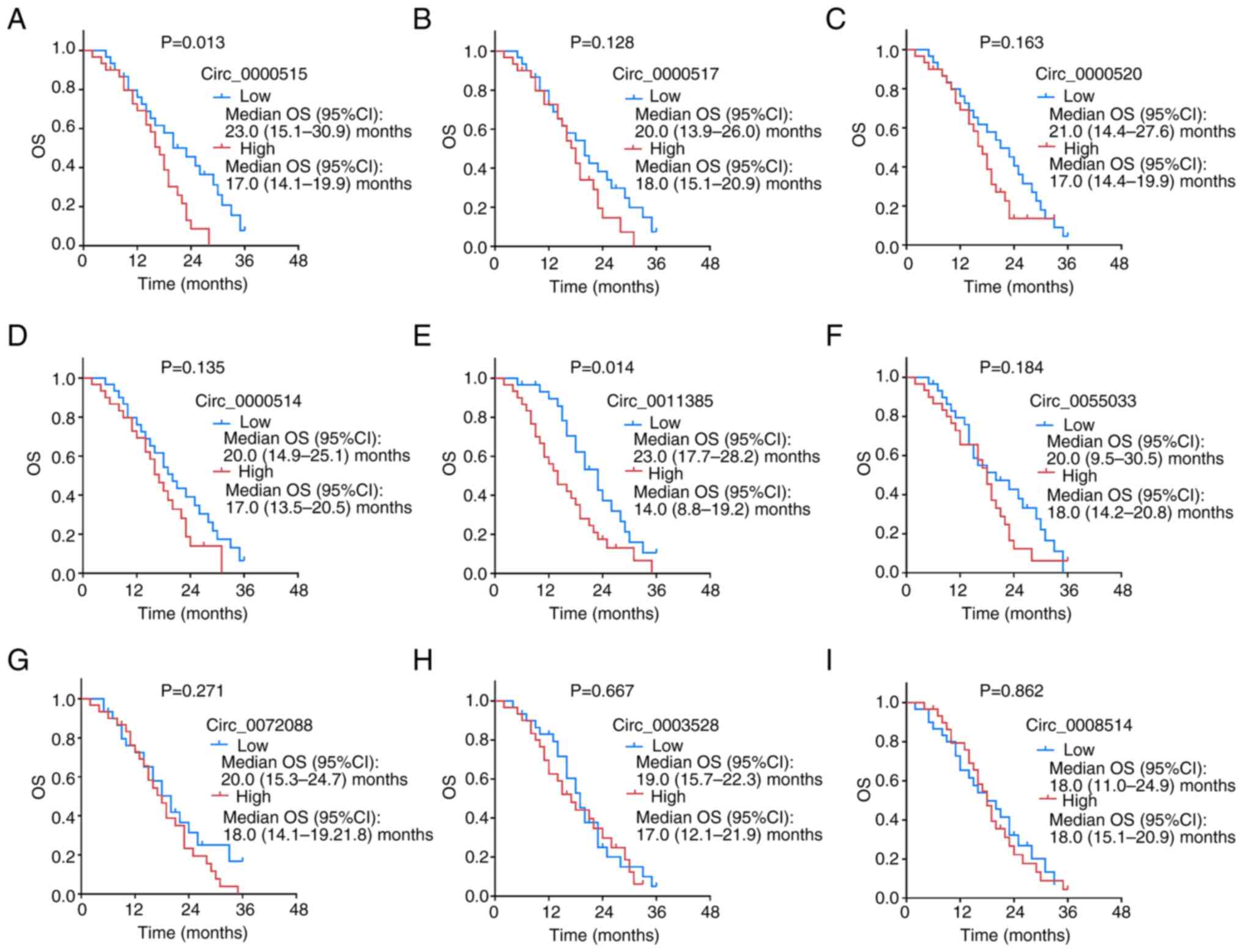 | Figure 7.OS analysis between patients with low
and high expression levels of the candidate circRNAs. The
association between the expression levels of (A) circ_0000515, (B)
circ_0000517, (C) circ_0000520, (D) circ_0000514, (E) circ_0011385,
(F) circ_0055033, (G) circ_0072088, (H) circ_0003528 and (I)
circ_0008514 and OS time in patients with pancreatic ductal
adenocarcinoma. OS, overall survival; circRNA, circular RNA; CI,
confidence interval. |
RT-qPCR analysis demonstrated that among the nine
candidate circRNAs in patients with PDAC, eight candidate circRNAs
were markedly dysregulated in PDAC tissues compared with paired
adjacent normal tissues; four were correlated with tumor stages and
two were associated with OS in patients with PDAC (Table IV). Notably, the expression levels
of circ_0000515 and circ_0011385 were upregulated in PDAC tissues
compared with paired adjacent normal tissues, and were correlated
with tumor stage, and associated with OS of patients with PDAC.
Thus, these two candidate circRNAs may be used as potential
biomarkers in the treatment of PDAC.
 | Table IV.Summary of study findings. |
Table IV.
Summary of study findings.
|
| Significant
difference | Significant
correlation |
|---|
|
|
|
|
|---|
| circRNA | PDAC vs.
adjacent | Pathological
grade | T stage | N stage | TNM stage | OS |
|---|
| circ_0000515 | Yes | No | Yes | No | Yes | Yes |
| circ_0000517 | Yes | No | No | No | No | No |
| circ_0000520 | Yes | No | No | No | No | No |
| circ_0000514 | Yes | No | Yes | No | No | No |
| circ_0011385 | Yes | No | Yes | Yes | Yes | Yes |
| circ_0055033 | Yes | No | No | No | No | No |
| circ_0072088 | Yes | No | Yes | No | Yes | No |
| circ_0003528 | Yes | No | No | No | No | No |
| circ_0008514 | No | No | No | No | No | No |
Discussion
The present study performed a secondary analysis
based on datasets from two previous studies, namely GSE79634 and
GSE69362, and candidate circRNAs were identified among the
DEcircRNAs (22,28). The expression levels of the
candidate circRNAs and their correlation with the
clinicopathological characteristics of patients with PDAC were
evaluated in a larger sample size. It was revealed that nine
circRNAs exhibited the same expression profile in both datasets.
These circRNAs were selected as candidate circRNAs and their
expression profile in tissue samples from 60 patients with PDAC was
assessed via RT-qPCR analysis. The results demonstrated that 8/9
candidate circRNAs exhibited differentiated expression profiles in
tumor tissues compared with paired adjacent normal tissues.
Furthermore, five candidate circRNAs were positively correlated
with tumor stage, and two (circ_0000515 and circ_0011385) were
negatively associated with OS in patients with PDAC. Notably,
circ_0000515 and circ_0011385 were not only highly expressed in
PDAC tissues compared with normal tissues, but were also correlated
with more advanced TNM stage and shorter survival time in patients
with PDAC.
The roles of circRNAs in PDAC remain unclear, unlike
in other solid tumors, such as gastric carcinoma and nasopharyngeal
carcinoma (29,30). A previous study reported that
circ-ASH2-like histone lysine methyltransferase complex subunit
(ASH2L) is upregulated in PDAC cells, which enhances PDAC cell
proliferation, migration and invasion capacities (31). Another study demonstrated that
circ-bifunctional apoptosis regulator (BFAR) elevates the
proliferation, migration and invasion of PDAC cells, and promotes
the growth and metastasis of tumors in PDAC animal models (32). Furthermore, circ-BFAR can
positively regulate the expression levels of
epithelial-to-mesenchymal transition markers by mediating the
miR-34b-5p/mesenchymal-to-epithelial transition factor/protein
kinase B (Akt) axis in PDAC cells (32). Only a few studies have investigated
the correlation between the expression of circRNAs and the
clinicopathological characteristics of patients with PDAC (33,34).
However, a previous study reported that decreased circ_0001649
expression in PDAC tissues is associated with low tumor stage and
increased differentiation level in patients (35). Taken together, these studies
suggest that certain circRNAs are associated with disease
progression and may serve as potential biomarkers in PDAC.
The present study identified nine circRNAs
presenting with the same expression trends in the two datasets
(GSE79634 and GSE69362), which were selected as candidate circRNAs
for RT-qPCR analyses in a cohort of 60 patients with PDAC. The
results demonstrated that 8/9 candidate circRNAs were notably
upregulated in PDAC tissues compared with paired adjacent normal
tissues, and five of them were positively associated with TNM stage
in patients with PDAC. This may be due to that these circRNAs may
promote the malignant behaviors of PDAC cells by interacting with
other tumor-related factors or signaling pathways, which in turn
can promote tumor progression, eventually leading to a more
advanced tumor stage in patients with PDAC. For instance, as
reported by a previous study about other circRNAs in PDAC, these
circRNAs might sponge miRNAs to enhance PDAC cell proliferation,
invasion and migration (32).
However, regarding the five candidate circRNAs identified in the
present study, no mechanistic studies have been reported to date;
therefore, in vivo and in vitro studies are urgently
required to verify their effects on PDAC.
In the present study, two candidate circRNAs,
circ_0000515 and circ_0011385, were not only positively associated
with TNM stage, but also negatively associated with OS time in
patients with PDAC. Thus, it was hypothesized that these two
candidate circRNAs may serve as prognostic factors in PDAC
management. To the best of our knowledge, the present study was the
first to report the potential role of these two circRNAs as
biomarkers in PDAC. Similarly, other circRNAs, including
circ_0030235 and circ_0007534, have been identified as prognostic
biomarkers of PDAC (33–36). A previous study suggested that
circ-ASH2L may serve as a prognostic biomarker in patients with
PDAC as its high expression in PDAC tissues was associated with
poor survival outcomes (31).
Another study reported that circ_0001649 expression is
downregulated in PDAC tissues compared with normal tissues, and
multivariate regression analysis revealed that its expression may
be an independent prognostic factor for survival (37). A previous study demonstrated that
circ_0030235 expression is upregulated in PDAC tissues, and its
expression is associated with poor prognosis (35). In terms of the circRNAs identified
in the present study, circ_0000515 and circ_0011385 have not yet
been identified in PDAC. However, they have been reported in
different types of cancer. For example, an in vivo and in
vitro study revealed that circ_0000515 enhances progression of
cervical cancer by sponging miR-326 expression via increasing
E-twenty six transcription factor ELK1 expression (38). Another study reported that
circ_0011385 promotes thyroid cancer development by sponging
miR-361-3p expression in vitro and in vivo (39). The results of the present study may
be explained as follows: Several target miRNAs of these two
candidate circRNAs function as antitumor factors in different types
of cancer. For example, the target miRNA of circ_0000515, miR-939,
and the target miRNA of circ_0011385, miR-615, are both considered
tumor suppressors that inhibit cancer cell invasion and migration
in various types of cancer (40–43).
Thus, it was hypothesized that circ_0000515 and circ_0011385 may
potentially function as regulators in enhancing the malignant
behavior of PDAC by sponging their target miRNAs, which promotes
the tumor progression of PDAC. In addition, circ_0000515 and
circ_0011385 may serve as biomarkers for PDAC management in
clinical practice.
The present study is not without limitations. First,
the statistical power can be diminished due to the relatively small
sample size of 60 patients with PDAC. Secondly, the molecular
functions of the candidate circRNAs in PDAC were not investigated
in the present study, which should be validated by in vitro
and in vivo experiments. Thirdly, microarray analysis may
present some bias (44); however,
the present study performed RT-qPCR analysis to validate the
expression trends of the candidate circRNAs in patients with PDAC.
Furthermore, a control group of healthy individuals was not
included in the present study. However, it was difficult to acquire
pancreatic tissue samples from healthy individuals. Thus,
prospective studies will aim to include this control cohort.
Fourthly, the expression levels of the circRNAs were only assessed
in tissue samples but not peripheral samples, which is inconvenient
for clinical application. This was the case as circRNAs are
abundantly expressed in tissue samples compared with peripheral
blood (19,45).
In conclusion, the results of the present study
provide evidence that circ_0000515 and circ_0011385 may serve as
biomarkers for disease control and determine the prognosis of
patients with PDAC, which implies their potential for guiding the
management of patients with PDAC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YX conceived, designed and supervised the present
study, and revised the manuscript for important intellectual
content. HW performed the experiments and provided technical
support. BW and LW analyzed the data and drafted the initial
manuscript. YX and HW confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tongji Medical College, Huazhong University of Science
and Technology [approval number (2014), ethics approval no.
(S108)]. Written informed consent was provided by all patients or
their legal representatives prior to the study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
circRNA
|
circular RNA
|
|
DEcircRNAs
|
differentially expressed circRNAs
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
LNM
|
lymph node metastasis
|
|
OS
|
overall survival
|
|
ROC
|
receiver operating characteristic
|
|
BFAR
|
bifunctional apoptosis regulator
|
References
|
1
|
Storz P and Crawford HC: Carcinogenesis of
pancreatic ductal adenocarcinoma. Gastroenterology. 158:2072–2081.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Latenstein AEJ, van der Geest LGM, Bonsing
BA, Groot Koerkamp B, Haj Mohammad N, de Hingh IHJT, de Meijer VE,
Molenaar IQ, van Santvoort HC, van Tienhoven G, et al: Nationwide
trends in incidence, treatment and survival of pancreatic ductal
adenocarcinoma. Eur J Cancer. 125:83–93. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter K, Talar-Wojnarowska R, Dąbrowski
A, Degowska M, Durlik M, Gąsiorowska A, Głuszek S, Jurkowska G,
Kaczka A, Lampe P, et al: Diagnostic and therapeutic
recommendations in pancreatic ductal adenocarcinoma.
Recommendations of the working group of the Polish pancreatic club.
Prz Gastroenterol. 14:1–18. 2019.PubMed/NCBI
|
|
6
|
Sarantis P, Koustas E, Papadimitropoulou
A, Papavassiliou AG and Karamouzis MV: Pancreatic ductal
adenocarcinoma: Treatment hurdles, tumor microenvironment and
immunotherapy. World J Gastrointest Oncol. 12:173–181. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rishi A, Goggins M, Wood LD and Hruban RH:
Pathological and molecular evaluation of pancreatic neoplasms.
Semin Oncol. 42:28–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hackeng WM, Hruban RH, Offerhaus GJ and
Brosens LA: Surgical and molecular pathology of pancreatic
neoplasms. Diagn Pathol. 11:472016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vivekanandhan S, Madamsetty VS, Angom RS,
Dutta SK, Wang E, Caulfield T, Pletnev AA, Upstill-Goddard R,
Asmann YW, Chang D, et al: Role of PLEXIND1/TGFβ signaling axis in
pancreatic ductal adenocarcinoma progression correlates with the
mutational status of KRAS. Cancers (Basel). 13:40482021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinn M, Sinn BV, Treue D, Keilholz U, Damm
F, Schmuck R, Lohneis P, Klauschen F, Striefler JK, Bahra M, et al:
TP53 mutations predict sensitivity to adjuvant gemcitabine in
patients with pancreatic ductal adenocarcinoma: Next-generation
sequencing results from the CONKO-001 trial. Clin Cancer Res.
26:3732–3739. 2020.PubMed/NCBI
|
|
11
|
Gao J, Chen X, Li X, Miao F, Fang W, Li B,
Qian X and Lin X: Differentiating TP53 mutation status in
pancreatic ductal adenocarcinoma using multiparametric MRI-derived
radiomics. Front Oncol. 11:6321302021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eger N, Schoppe L, Schuster S, Laufs U and
Boeckel JN: Circular RNA splicing. Adv Exp Med Biol. 1087:41–52.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Tan S, Liu WR, Lei Q, Qiao W, Wu
Y, Liu X, Cheng W, Wei YQ, Peng Y and Li W: RNA-Seq profiling of
circular RNA in human lung adenocarcinoma and squamous cell
carcinoma. Mol Cancer. 18:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dube U, Del-Aguila JL, Li Z, Budde JP,
Jiang S, Hsu S, Ibanez L, Fernandez MV, Farias F, Norton J, et al:
An atlas of cortical circular RNA expression in Alzheimer disease
brains demonstrates clinical and pathological associations. Nat
Neurosci. 22:1903–1912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel Role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ameli-Mojarad M, Ameli-Mojarad M,
Hadizadeh M, Young C, Babini H, Nazemalhosseini-Mojarad E and Bonab
MA: The effective function of circular RNA in colorectal cancer.
Cancer Cell Int. 21:4962021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian T, Zhao Y, Zheng J, Jin S, Liu Z and
Wang T: Circular RNA: A potential diagnostic, prognostic, and
therapeutic biomarker for human triple-negative breast cancer. Mol
Ther Nucleic Acids. 26:63–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu S, Song W, Yang X, Wang J, Zhang R,
Zhang Z, Zhang H and Li H: Microarray expression profile of
circular RNAs in human pancreatic ductal adenocarcinoma. Genom
Data. 5:385–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo S, Xu X, Ouyang Y, Wang Y, Yang J, Yin
L, Ge J and Wang H: Microarray expression profile analysis of
circular RNAs in pancreatic cancer. Mol Med Rep. 17:7661–7671.
2018.PubMed/NCBI
|
|
24
|
Li H, Hao X, Wang H, Liu Z, He Y, Pu M,
Zhang H, Yu H, Duan J and Qu S: Circular RNA expression profile of
pancreatic ductal adenocarcinoma revealed by microarray. Cell
Physiol Biochem. 40:1334–1344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Roessel S, Kasumova GG, Verheij J,
Najarian RM, Maggino L, de Pastena M, Malleo G, Marchegiani G,
Salvia R, Ng SC, et al: International validation of the eighth
edition of the American joint committee on cancer (AJCC) TNM
staging system in patients with resected pancreatic cancer. JAMA
Surg. 153:e1836172018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crea F, Quagliata L, Michael A, Liu HH,
Frumento P, Azad AA, Xue H, Pikor L, Watahiki A, Morant R, et al:
Integrated analysis of the prostate cancer small-nucleolar
transcriptome reveals SNORA55 as a driver of prostate cancer
progression. Mol Oncol. 10:693–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Sang J, Zhang Y, Gao L, Zhao D and
Cao H: Circular RNA ITCH attenuates the progression of
nasopharyngeal carcinoma by inducing PTEN upregulation via miR-214.
J Gene Med. e33912021.(Online ahead of print). PubMed/NCBI
|
|
30
|
Zhu Z, Huang R and Huang B: Predicting
functional circular RNA-based competitive endogenous RNA network in
gastric carcinoma using novel bioinformatics analysis. Exp Biol Med
(Maywood). 153537022110487572021.(Epub ahead of print). PubMed/NCBI
|
|
31
|
Chen Y, Li Z, Zhang M, Wang B, Ye J, Zhang
Y, Tang D, Ma D, Jin W, Li X and Wang S: Circ-ASH2L promotes tumor
progression by sponging miR-34a to regulate Notch1 in pancreatic
ductal adenocarcinoma. J Exp Clin Cancer Res. 38:4662019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo X, Zhou Q, Su D, Luo Y, Fu Z, Huang L,
Li Z, Jiang D, Kong Y, Li Z, et al: Circular RNA circBFAR promotes
the progression of pancreatic ductal adenocarcinoma via the
miR-34b-5p/MET/Akt axis. Mol Cancer. 19:832020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao Y: Construction of a
circRNA-miRNA-mRNA network to explore the pathogenesis and
treatment of pancreatic ductal adenocarcinoma. J Cell Biochem.
121:394–406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Q, Geng S, Yuan H, Li Y, Zhang S, Pu L,
Ge J, Niu X, Li Y and Jiang H: Circular RNA expression profiles in
extracellular vesicles from the plasma of patients with pancreatic
ductal adenocarcinoma. FEBS Open Bio. 9:2052–2062. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Yao Y, Gao P and Cui Y: Upregulated
circular RNA circ_0030235 predicts unfavorable prognosis in
pancreatic ductal adenocarcinoma and facilitates cell progression
by sponging miR-1253 and miR-1294. Biochem Biophys Res Commun.
509:138–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao L, Rong W, Bai L, Cui H, Zhang S, Li
Y, Chen D and Meng X: Upregulated circular RNA circ_0007534
indicates an unfavorable prognosis in pancreatic ductal
adenocarcinoma and regulates cell proliferation, apoptosis, and
invasion by sponging miR-625 and miR-892b. J Cell Biochem.
120:3780–3789. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang Y, Wang T, Yan L and Qu L: A novel
prognostic biomarker for pancreatic ductal adenocarcinoma:
hsa_circ_0001649. Gene. 675:88–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang Q, Chen Z, Zhao L and Xu H: circular
RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical
cancer progression through up-regulation of ELK1. Aging (Albany
NY). 11:9982–9999. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia F, Chen Y, Jiang B, Bai N and Li X:
Hsa_circ_0011385 accelerates the progression of thyroid cancer by
targeting miR-361-3p. Cancer Cell Int. 20:492020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ji Y, Sun Q, Zhang J and Hu H: MiR-615
inhibits cell proliferation, migration and invasion by targeting
EGFR in human glioblastoma. Biochem Biophys Res Commun.
499:719–726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guan X, Zong ZH, Liu Y, Chen S, Wang LL
and Zhao Y: circPUM1 promotes tumorigenesis and progression of
ovarian cancer by sponging miR-615-5p and miR-6753-5p. Mol Ther
Nucleic Acids. 18:882–892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Guo Y and Yan W: lncRNA
RP5-916L7.2 correlates with advanced tumor stage, and promotes
cells proliferation while inhibits cells apoptosis through
targeting miR-328 and miR-939 in tongue squamous cell carcinoma.
Clin Biochem. 67:24–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen A, Liu S, Lu X, Wei L and Chen Y:
Inhibition of microRNA-939 suppresses the development of human
non-small cell lung cancer via the upregulation of tissue inhibitor
of metalloproteinases 2. Mol Med Rep. 18:4831–4838. 2018.PubMed/NCBI
|
|
44
|
Rachinger N, Fischer S, Bohme I, Böhme I,
Linck-Paulus L, Kuphal S, Kappelmann-Fenzl M and Bosserhoff AK:
Loss of gene information: Discrepancies between RNA sequencing,
cDNA microarray, and qRT-PCR. Int J Mol Sci. 22:93482021.
View Article : Google Scholar
|
|
45
|
Xu T, Wu J, Han P, Zhao Z and Song X:
Circular RNA expression profiles and features in human tissues: A
study using RNA-seq data. BMC Genomics. 18 (Suppl 6):S6802017.
View Article : Google Scholar
|