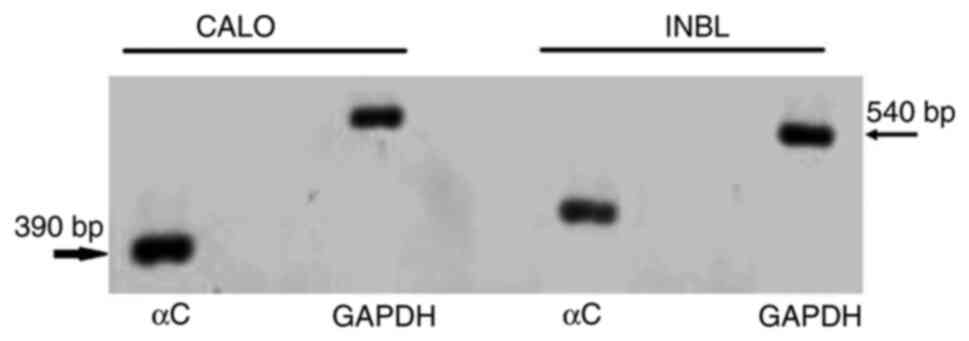
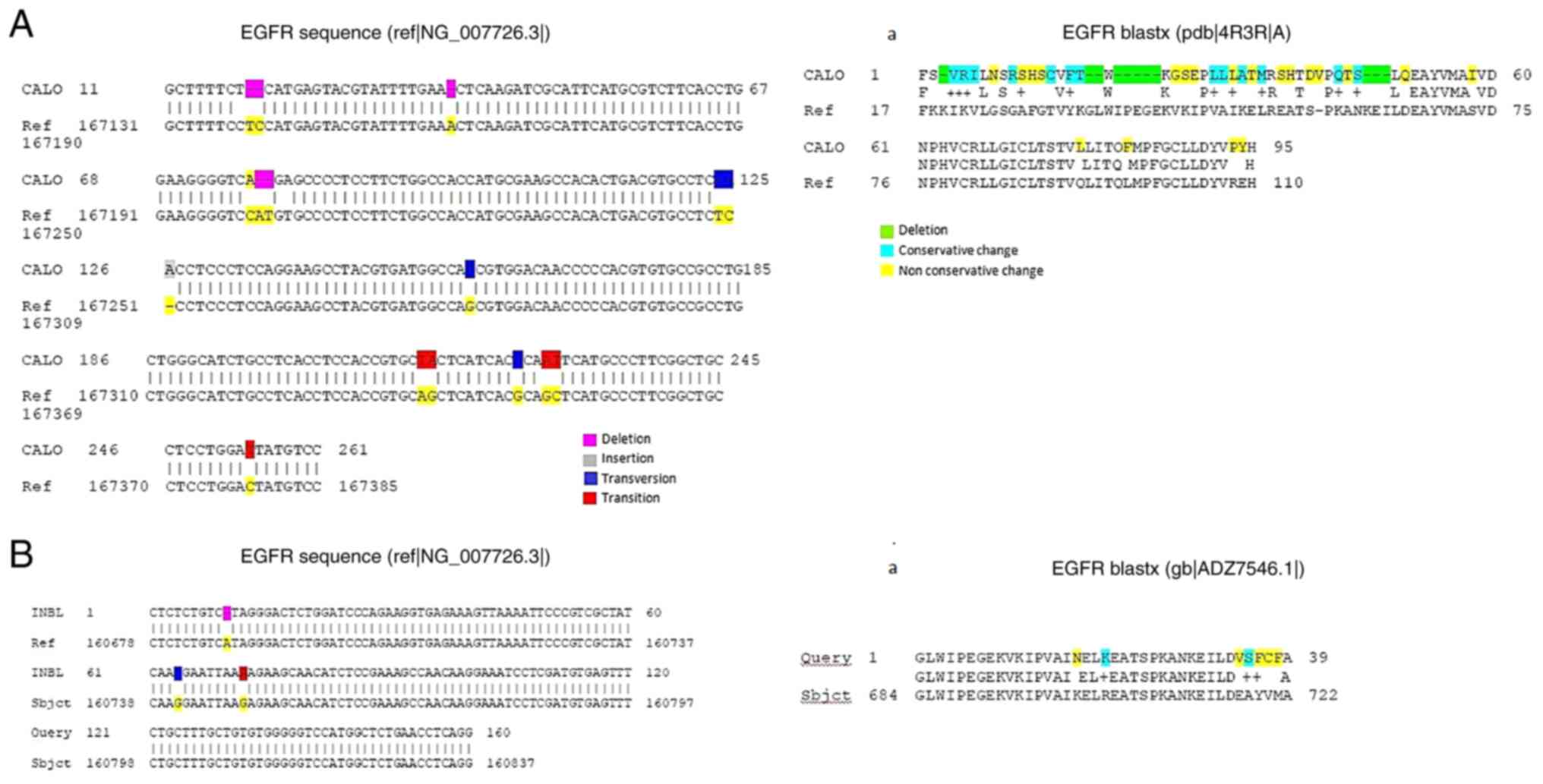
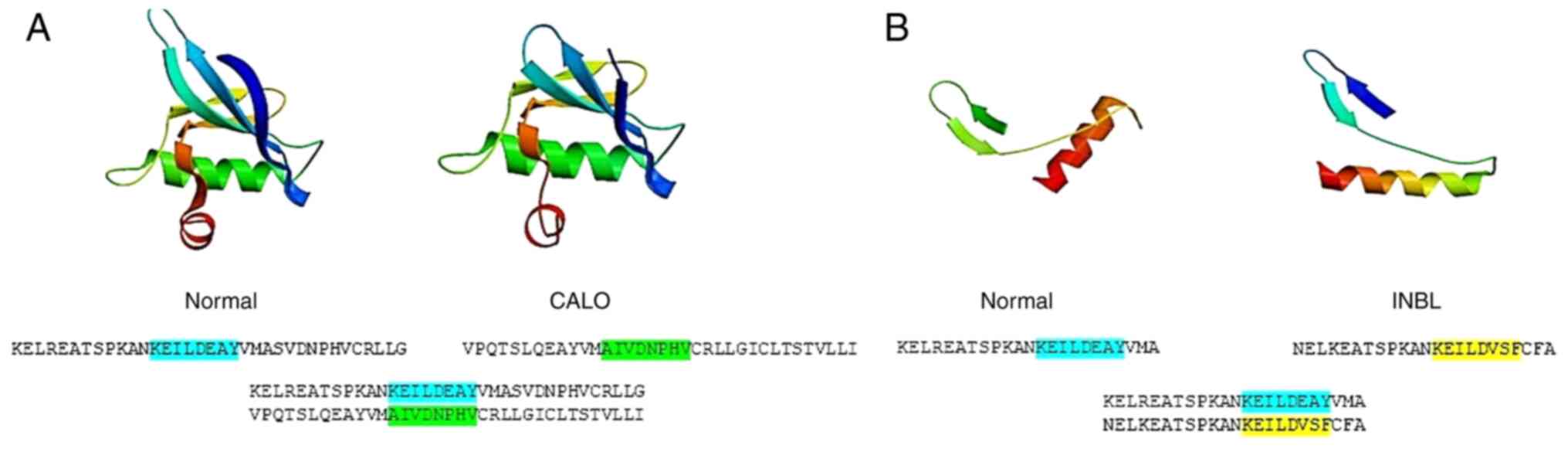
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouvard V, Baan R, Straif K, Grosse Y,
Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part B: Biological
agents. Lancet Oncol. 10:321–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munoz N, Bosch FX, de Sanjose S, Herrero
R, Castellsaque X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group, : Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soonthornthum T, Arias-Pulido H, Joste N,
Lomo L, Muller C, Rutledge T and Verschraegen C: Epidermal growth
factor receptor as a biomarker for cervical cancer. Ann Oncol.
22:2166–2178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stöppler MC, Straight SW, Tsao G, Schlegel
R and Mccance DJ: The E5 gene of HPV16 enhances keratinocyte
immortalization by full-length DNA. Virology. 223:251–254. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomakidi P, Cheng H, Kohl A, Komposch G
and Alonso A: Modulation of the epidermal growth factor receptor by
the human papillomavirus type 16 E5 protein in raft cultures of
human keratinocytes. Eur J Cell Biol. 79:407–412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jura N, Shan Y, Cao X, Shaw DE and Kuriyan
J: Structural analysis of the catalytically inactive kinase domain
of the human EGF receptor 3. Proc Natl Acad Sci USA.
106:21608–21613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sierke SL, Cheng K, Kim HH and Koland JG:
Biochemical characterization of the protein tyrosine kinase
homology domain of the ErbB3 (HER3) receptor protein. Biochem J.
322:757–763. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wallasch C, Weiss FU, Niederfellner G,
Jallal BA, Issing W and Ullrich A: Heregulin-dependent regulation
of HER2/neu oncogenic signaling by heterodimerization with HER3.
EMBO J. 14:4267–4275. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi F, Telesco SE, Liu Y, Radhakrishnan R
and Lemmon MA: ErbB3/HER3 intracellular domain is competent to bind
ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA.
107:7692–7697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor underlying responsiveness of non-small-cell
lung cancer to gefitinib. N Engl J Med. 350:2129–2139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iida K, Nakayama K, Rahman MT, Rahman M,
Ishikawa M, Katagiri A, Yeasmin S, Otsuki Y, Kobayashi H, Nakayama
S and Miyazaki K: EGFR gene amplification is related to adverse
clinical outcomes in cervical squamous cell carcinoma, making the
EGFR pathway a novel therapeutic target. Br J Cancer. 105:420–427.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolber L, Hess S, Bohlken H, Tennstedt P,
Eulenburg C, Simon R, Gieseking F, Jaenicke F, Mahner S and
Choschzinck M: EGFR gene copy number increase in vulvar carcinoma
is linked with poor clinical outcome. J Clin Pathol. 65:133–139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Tang Y, Cheng X, Ji J, Zhang J and
Zhou X: EGFR protein expression and gene amplification in squamous
intraepithelial lesions and squamous cell carcinomas of the cervix.
Int J Clin Exp Pathol. 7:733–741. 2014.PubMed/NCBI
|
|
16
|
Conesa-Zamora P, Torres-Moreno D, Isaac MA
and Pérez-Guillermo M: Gene amplification and immunohistochemical
expression of ERBB2 and EGFR in cervical carcinogenesis.
Correlation with cell-cycle markers and HPV presence. Exp Mol
Pathol. 95:151–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Cheng X, Ji J, Zhang J and Zhou X:
Gene amplification of EGFR and its clinical significance in various
cervical (lesions) lesions using cytology and FISH. Int J Clin Exp
Pathol. 7:2477–2483. 2014.PubMed/NCBI
|
|
18
|
Arias-Pulido H, Joste N, Chavez A, Muller
CY, Dai D, Smith HO and Verschraegen CF: Absence of epidermal
growth factor receptor mutations in cervical cancer. Int J Gynecol
Cancer. 18:749–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soto-Cruz I, Rangel-Corona R,
Valle-Mendiola A, Moreno-Morales X, Santiago-Pérez R, Weiss-Steider
B and Cáceres-Cortés JR: The tyrphostin B42 inhibits cell
proliferation and HER-2 autophosphorylation in cervical carcinoma
cell lines. Cancer Invest. 26:136–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caceres-Cortes JR, Alvarado-Moreno JA,
Waga K, Rangel-Corona R, Monroy-Garcia A, Rocha-Zavaleta L,
Urdiales-Ramos J, Weiss-Steider B, Haman A, Hugo P, et al:
Implication of tyrosine kinase receptor and steel factor in cell
density-dependent growth in cervical cancers and leukemias. Cancer
Res. 61:6281–6290. 2001.PubMed/NCBI
|
|
21
|
Biasini M, Bienert S, Waterhouse A, Arnold
K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli
L and Schwede T: SWISS-MODEL: Modeling protein tertiary and
quaternary structures using evolutionary information. Nucleic Acids
Res. 42:W252–W258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arnold K, Bordoli L, Kopp J and Schwede T:
The SWISS-MODEL workspace: A web-based environment for protein
structure homology modeling. Bioinformatics. 22:195–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benkert P, Biasini M and Schwede T: Toward
the estimation of the absolute quality of individual protein
structure models. Bioinformatics. 27:343–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valle-Mendiola A, Weiss-Steider B,
Rocha-Zavaleta L and Soto-Cruz I: IL-2 enhances cervical cancer
cells proliferation and JAK3/STAT5 phosphorylation at low doses,
while at high doses IL-2 has opposite effects. Cancer Invest.
32:115–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yarden Y and Pines G: The ERBB network: At
last, cancer therapy meets systems biology. Nat Rev Cancer.
12:553–563. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
London M and Gallo E: Epidermal growth
factor receptor (EGFR) involvement in epitelial-derived cancers and
its current antibody-based immunotherapies. Cell Biol Int.
44:1267–1282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, López-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–977. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayati A, Moghimi S, Salarinejad S, Safavi
M, Pouramiri B and Foroumadi A: A review on progression of
epidermal growth factor receptor (EGFR) inhibitors as an efficient
approach in cancer targeted therapy. Bioorg Chem. 99:1038112020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anastasi S, Baietti MF, Frosi Y, Alema S
and Segatto O: The evolutionarily conserved EBR module of RALT/MIG6
mediates suppression of the EGFR catalytic activity. Oncogene.
26:7833–7846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh D, Attri BK, Gill RK and Bariwal J:
Review on EGFR inhibitors: Critical update. Mini Rev Med Chem.
16:1134–1166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Bondt HL, Rosenblatt J, Jancarik J,
Jones HD, Morgan DO and Kim SH: Crystal structure of
cyclin-dependent kinase 2. Nature. 363:595–602. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu W, Doshi A, Lei M, Eck MJ and Harrison
SC: Crystal structure of c-Src reveal features of its
autoinhibitory mechanism. Mol Cell. 3:629–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deindl S, Kadlecek TA, Brdicka T, Cao X,
Weiss A and Kuriyan J: Structural basis for the inhibition of
tyrosine kinase activity of ZAP-70. Cell. 129:735–746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wood ER, Truesdale AT, McDonald OB, Yuan
D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K,
et al: A unique structure for epidermal growth factor receptor
bound to GW572016 (Lapatinib): relationship among protein
conformation, inhibitor off-rate, and receptor activity in tumor
cells. Cancer Res. 64:6652–6659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guy PM, Platko JV, Cantley LC, Cerione RA
and Carraway KL III: Insect cell-expressed p180erbB3 possesses an
impaired tyrosine kinase activity. Proc Natl Acad Sci USA.
91:8132–8136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Gureasko J, Shen K, Cole PA and
Kuriyan J: An allosteric mechanism for activation of the kinase
domain of epidermal growth factor receptor. Cell. 125:1137–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeffrey PD, Russo AA, Polyak K, Gibbs E,
Hurwitz J, Massague J and Pavletich NP: Mechanism of CDK activation
revealed by structure of a cyclinA-CDK2 complex. Nature.
376:313–320. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baselga J and Swain SM: Novel anticancer
targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sergina NV, Rausch M, Wang D, Blair J,
Hann B, Shokat KM and Moasser MM: Escape from HER-family tyrosine
kinase inhibitor therapy by the kinase-inactive HER3. Nature.
445:437–441. 2007. View Article : Google Scholar : PubMed/NCBI
|