Introduction
Ovarian cancer is the second most common
gynaecological malignancy following uterine corpus cancer and it is
considered the fifth leading cause of cancer death in women with
more than 4,000 deaths annually in UK (1). In addition, it has been called a
‘silent killer’ because no specific symptoms have been associated
with the early stages of the disease. The 5-year survival rate for
patients diagnosed with stage II or greater ovarian cancer is ~40%.
Although there have been recent improvements in treatment, notably
the introduction of PARP inhibitors, it still represents a
significant unmet medical problem and new treatments are
required.
Statins are a class of low molecular weight drugs
that are used effectively to control hypercholesterolemia. They
inhibit hydroxymethylglutaryl Coenzyme-A reductase (HMGCR). Statins
reduce production of mevalonate, a precursor in the synthesis of
cholesterol and isoprenoids such as farnesol and geranylgeraniol
(2). A significant body of
evidence suggests that statins may be repurposed to treat cancer
(3). Numerous studies have
reported that statins can induce cancer cell death through
apoptosis [reviewed in (3)] as a
result of reducing the production of geranylgeraniol. This
isoprenoid is necessary for the proper function of small GTPase
oncogenes which are consequently inactivated by statins (4). Retrospective analyses have shown
reduced cancer mortality in patients using statins to control
elevated cholesterol (3). However,
prospective clinical trials of statins in cancer patients have so
far failed to show any survival benefit. We have argued that this
is due in part to failures in trial designs which have not
adequately considered pharmacodynamic and pharmacokinetic factors
(5).
In addition to these factors, diet may affect the
efficacy of statins in treating cancer (6). We showed that pitavastatin can cause
the regression of ovarian cancer xenografts in mice. However, this
required the use of a diet that lacked geranylgeraniol.
Supplementation of the diet with geranylgeraniol restored the
growth of the xenografts in mice receiving pitavastatin (6). Although others have also reported
that statins can affect the growth of cancer xenografts, to our
knowledge no other researchers have observed tumour regression, nor
have they controlled dietary geranylgeraniol. Considering that
several human foods, particularly oils (7), have been shown to contain
geranylgeraniol, this raises the concern that dietary
geranylgeraniol could also interfere with the anti-cancer activity
of statins in patients and that clinical trials of statins may not
be successful unless diet is adequately controlled. Prospective
clinical trials of statins to treat cancer to date have uniformly
failed to consider the effect of dietary geranylgeraniol in their
design (5). Although there are
geranylgeraniol-free complete food products (e.g., Ensure) that
could be used by patients on a statin clinical trial, these liquid
foods may not be suitable for prolonged use due to poor patient
compliance with the liquid diet. Thus, it is desirable to identify
additional foods for trial participants that are less likely to
interfere with the activity of pitavastatin.
We have tested several foods to identify those which
interfere with the cytotoxic activity of statins. We have made use
of the observation that the addition of geranylgeraniol, or the
organic extract of some foods, can suppress the cytotoxic activity
of statins (6,8) against ovarian cancer cells. Here we
use that bioassay to identify several foods which do not interfere
with the activity of pitavastatin and may potentially be eaten by
patients during a clinical trial. Importantly we also identify
foods which suppress the activity of pitavastatin and so should be
avoided during clinical trials of statins.
Materials and methods
Materials
Thirty foodstuffs were obtained for the purposes of
extracting fats and were obtained from different local markets in
the United Kingdom. Pitavastatin (Sequoia Research Products),
geranylgeranyl (Sigma-Aldrich), mevalonate (Enzo Life Sciences),
isopentenol (Sigma-Aldrich), dolichol (Avanti) and Coenzyme Q10
were dissolved in DMSO (20 mM). Cell culture media were obtained
from Lonza.
Preparation of extracts
Extracts of the different foods were prepared
according to the method reported by Muraguchi (9). Each solid foodstuff (50 g) was
homogenized in an electric blender and then transfer to a mortar
and homogenised manually with a pestle in 60 ml methanol. Then, 30
ml of chloroform/methanol (50/50%) were added and the extract
homogenized briefly again. The samples were filtered through fluted
filter paper. Oils (50 g) were directly mixed with methanol and
chloroform/methanol without homogenization. Both types of extracts
were transferred to a separating funnel and the upper layer
collected and evaporated. The dried residues were dissolved in 25
ml 99% ethanol and 25 ml 5 M potassium hydroxide were added and the
solution incubated at 56°C for 1 h in a water bath. After cooling
and neutralisation with 25 ml 5 M hydrochloric acid and addition of
30 ml water, the resulting solution was partitioned with 120 ml
n-hexane. The upper phase was collected and the solvent removed in
a rotary evaporator and then in a freeze dryer overnight. The
residue was dissolved in 1 ml DMSO and stored at −20°C for later
analysis.
Cell growth assays
Ovcar-4 and Fuov-1 cells were grown in RPMI 1640
medium supplemented with 10% FCS, 2 mM glutamine and 50 µg/ml
penicillin/streptomycin. Cells were incubated at 37°C and in a
humidified 5% CO2 atmosphere. Cells were regularly
tested to confirm absence of mycoplasma infection.
Cells (5,000/well) were seeded in 96-well plates in
80 µl of growth medium. After incubation for 24 h, pitavastatin,
geranylgeraniol, or food extracts at the indicated final
concentration, either alone or in combination, were added to cells.
The cells were incubated for a further 72 h, the growth medium
removed and the cells in each well were fixed in 100 µl cold 10%
trichloroacetic acid (TCA) for 30 min on ice. The TCA was removed
and the cells were left to air dry, before staining in 0.4%
sulforhodamine B in 1% acetic acid for 30 min. Excess SRB was
removed by washing the wells three times in 1% acetic acid and the
plates were left to dry. Lastly, the dye was solubilised in 100 µl
10 mM Tris (pH 10) and the absorbance at 570 nm (A570) was
determined using a BioTek Synergy 2 multi-mode microplate
reader.
A parameter ‘rescue’ was defined to quantify the
activity of the extracts in suppressing the activity of
pitavastatin. Rescue
(%)=[(Npe-Np)/(N0-Np)]
× (N0/Ne) ×100 in which the relative biomass
(used as a surrogate for cell number) measured by SRB staining were
measured in samples exposed to either pitavastatin and the extract
(Npe), or pitavastatin alone
(Np), or DMSO (solvent control,
N0) or the extract alone
(Ne). The second term in the equation was
included to control for any effect of the extract itself on cell
growth. However, if the extract on its own was apparently cytotoxic
(here defined as inhibition of growth by more than 15%, i.e.,
Ne/N0 <0.85)
the data were considered unreliable and rejected for further
analysis.
Cell viability assays
To estimate the cell viability by trypan blue
staining, 2 ml of Ovcar-4 cells (1×105 per ml) were
seeded per well of a 6-well plate. After 24 h, 20 µl of medium
containing pitavastatin, geranylgeraniol or extract were added.
After 72 h, the medium (containing any detached cells) was
collected and combined with the adherent cells which had been
detached by trypsinization. The combined samples were centrifuged
(150 g, 3 min), the pellet resuspended in 0.5 ml of medium and
mixed with an equal volume of 0.4% (v/v) trypan blue
(Sigma-Aldrich). The cells were counted using a Neubauer
haemocytometer.
GC-MS analysis
1–2 mg of extracts were dissolved in 200 µl
ethylacetate and sonicated for 5 min at 40°C. Subsequently, 1–2 µl
of the solution was injected into the gas chromatography mass
spectrometer (GC-MS), an Agilent 7890 coupled with Agilent MS type
5975 C MSD (Agilent Technologies). The gas chromatography was
started for two minutes with an initial oven temperature of 60°C
and increased to 300°C at a rate of 10°C/min, followed by 4 min at
300°C to produce a total run of 30 min at a steady helium pressure
(10 psi). Mass spectral data were acquired in scanning mode within
the 40-1,000 m/z range.
Statistical analysis
The data obtained from cell growth assays was
analysed by using the GraphPad Prism software (GraphPad Software,
Inc.). Non-linear regression was used to fit a four-parameter
(Hill-equation) sigmoidal dose-response curve to determine
IC50 values. Statistical significance was assessed using
paired or one sample t-tests where indicated.
Results
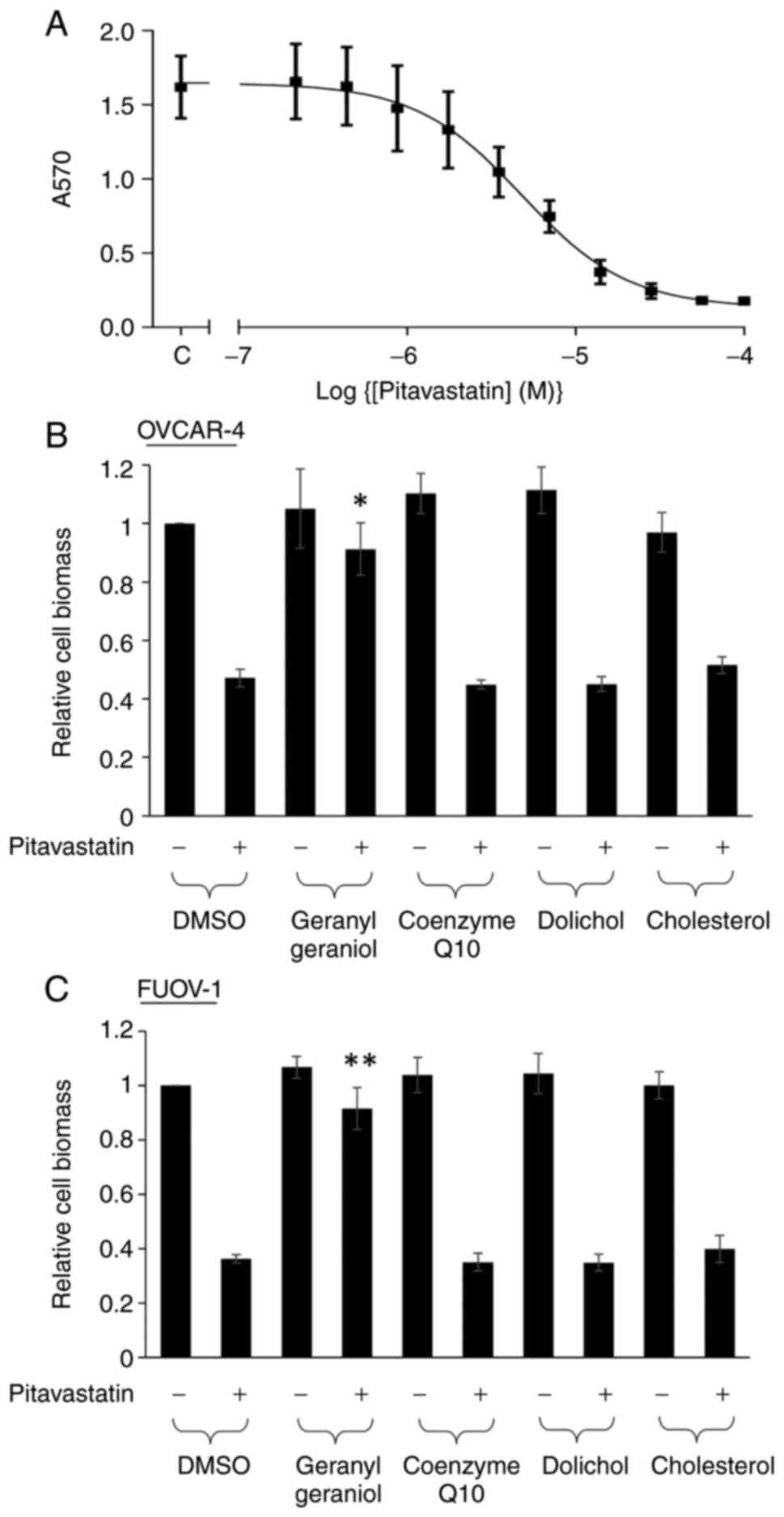
The activity of pitavastatin in Ovcar-4 cell growth
assays was first confirmed. Pitavastatin inhibited the growth of
Ovcar-4 cell with a potency comparable to that measured previously
(IC50=5.2±1.20 µM; Fig.
1A). We have previously shown that geranylgeraniol and
mevalonate, but not farnesol, could suppress the cytotoxic activity
of pitavastatin (6). We tested
other products of the mevalonate pathway and found that neither
dolichol, coenzyme Q10 nor cholesterol appreciably altered the
effect of pitavastatin. As expected, geranylgeraniol was able to
rescue the inhibitory effect of pitavastatin (Fig. 1B). To ensure that these results
were not unique to Ovcar-4 cells, we used a different ovarian
cancer cell line (Fuov-1) and a similar pattern of activity was
observed with the mevalonate pathway metabolites (Fig. 1C).
Next organic solvent extracts (Table I) were prepared from a range of
foodstuffs using a method previously established to extract
geranylgeraniol. A range of concentrations of these extracts were
tested alone and in combination with pitavastatin to evaluate
whether they suppressed the cytotoxic activity of pitavastatin. To
quantify this, we defined a parameter ‘rescue’ (see methods) where
a value of 100% reflects an extract that completely restores the
growth of the cells in the presence of pitavastatin to control
levels and 0% represents an extract that has no effect on growth of
the cells in the presence of pitavastatin. By monitoring the effect
of the extracts in the absence of pitavastatin, we were also able
to determine if any extracts were cytotoxic or promoted cell
growth.
 | Table I.Mass of extract recovered from 50 g of
foodstuff. |
Table I.
Mass of extract recovered from 50 g of
foodstuff.
| Food | Extract mass, mg |
|---|
| Grape seed oil | 580 |
| Corn oil | 370 |
| Ground nut oil | 350 |
| Rape seed oil | 400 |
| Coconut oil | 110 |
| Sesame oil | 160 |
| Sunflower oil | 510 |
| Butter | 180 |
| Oats | 60 |
| Bread | 140 |
| Pasta | 30 |
| Boiled potato | 10 |
| Kiwi | 10 |
| Lettuce | 550 |
| Passion fruit | 40 |
| Pomegranate | 10 |
| Cherry | 30 |
| Fig | 40 |
| Squash | 20 |
| Gooseberry | 10 |
| Pears | 30 |
| Tomato | 130 |
| Pecan nuts | 110 |
| Pasta sauce | 30 |
| Cheese | 60 |
| Milk | 50 |
| Strawberry jam | 80 |
| Boiled egg | 120 |
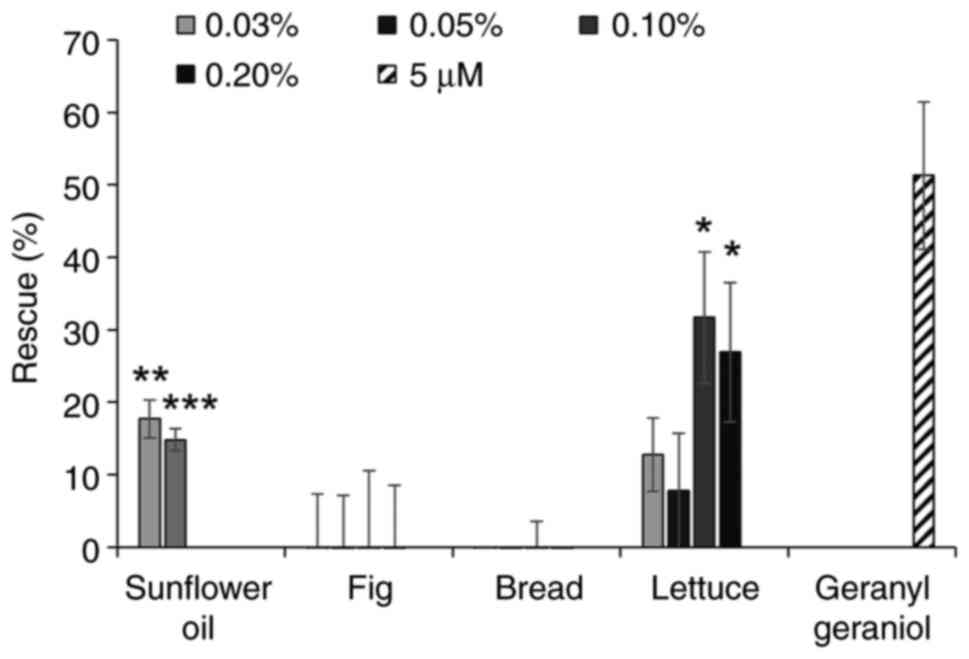
The extracts from several oils were able to inhibit
the activity of pitavastatin, in particular corn oil, sunflower oil
(as previously reported) (6) and
grape seed oil (Fig. 2A). However,
rape seed oil had minimal activity. We next tested extracts from
several fruit and vegetables (Fig.
2B). A number of these did not have any effect on the activity
of pitavastatin, however the extracts from beans and cherries were
partially able to restore the growth of cells in the presence of
pitavastatin. The extract from lettuce substantially suppressed the
activity of pitavastatin, however the extract on its own stimulated
cell growth by up to 40%. Most of the extracts from foods rich in
carbohydrate (Fig. 2C) had no
activity but an extract from oats was also able to rescue the
effects of pitavastatin. Finally, we tested various other foods
stuffs (Fig. 2D). Among these,
extracts from pecan nuts and boiled eggs suppressed the effects of
pitavastatin, although these extracts were toxic on their own when
tested at high concentrations and in the absence of pitavastatin,
precluding full exploration of their activity. We also noted that a
commercially available pasta sauce suppressed the activity of
pitavastatin, possibly reflecting its high sunflower oil content.
To confirm these results were not unique to Ovcar-4 cells, we again
used Fuov-1 cells and tested two extracts that were able to
suppress the activity of pitavastatin in Ovcar-4 cells (sunflower
oil, lettuce) and two extracts which did not (fig, bread). A
similar pattern of activity was seen in the Fuov-1 cells to that
observed in the Ovcar-4 cells (Fig.
3).
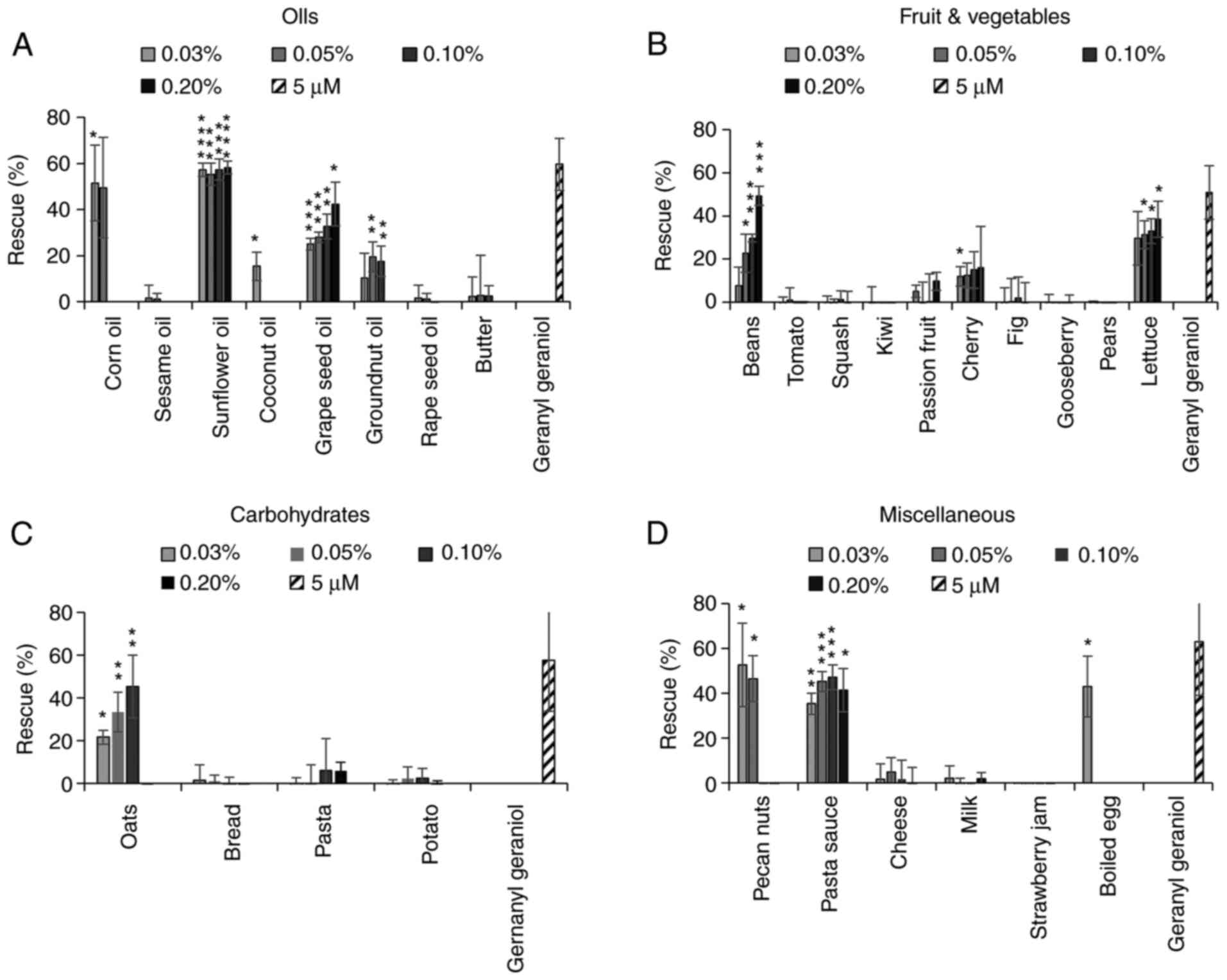 | Figure 2.Effect of food extracts on the
activity of pitavastatin in Ovcar-4 cells. Effects of extracts from
(A) oils, (B) fruit and vegetables, (C) carbohydrates and (D)
miscellaneous other foods on the cytotoxic activity of pitavastatin
were assessed in Ovcar-4 cells. The cells were exposed to
pitavastatin (10 µM) and/or the indicated concentration of food
extract. After 72 h, the cell biomass was assessed by staining with
sulforhodamine B. The results (mean ± SD; n=3) are expressed as the
rescue of the cytotoxic effects of pitavastatin, as defined in the
methods section. Geranylgeraniol (5 µM) was included as a positive
control. Data from corn oil (0.1 and 0.2%), coconut oil (0.1 and
0.2%), groundnut oil (0.2%), rape seed oil (0.2%), sesame oil (0.1
and 0.2%), pecan nuts (0.1 and 0.2%), oats (0.2%) and eggs (0.05,
0.1 and 0.2%) have been omitted because these extracts were toxic
(>15% inhibition of cell growth). *P<0.05, **P<0.01,
***P<0.005 and ****P<0.001 vs. no rescue; one sample
t-test. |
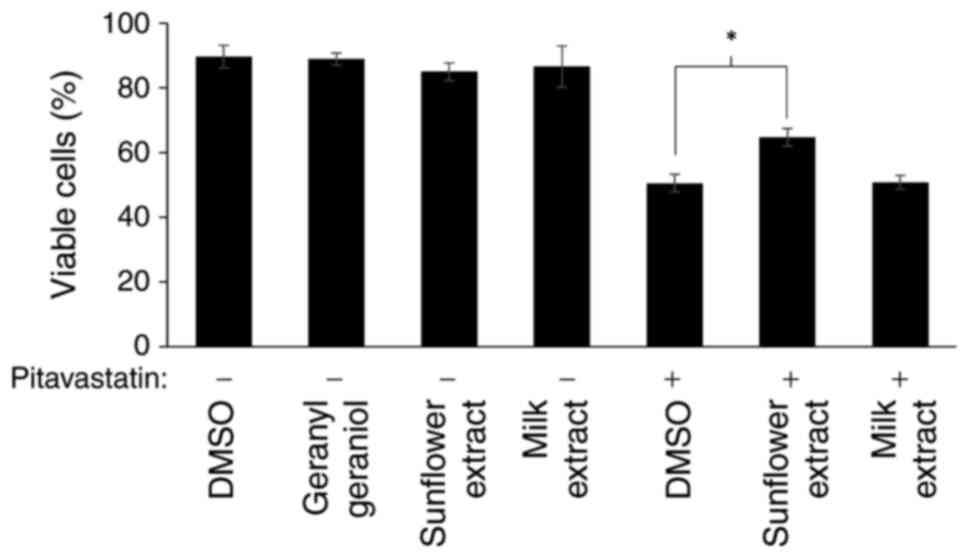
We have previously shown that pitavastatin induces
cell death through apoptosis (8).
To confirm here that the effects in the cell growth assay reflected
cell death, we measured cell viability after exposure to
pitavastatin using two extracts, one which significantly rescued
the activity of pitavastatin in the cell growth assay (sunflower
oil) and one which did not (milk). Consistent with this, exposure
to pitavastatin resulted in cell death when assessed by trypan blue
staining and this was suppressed by addition of sunflower oil
extract, but not by an extract from milk (Fig. 4).
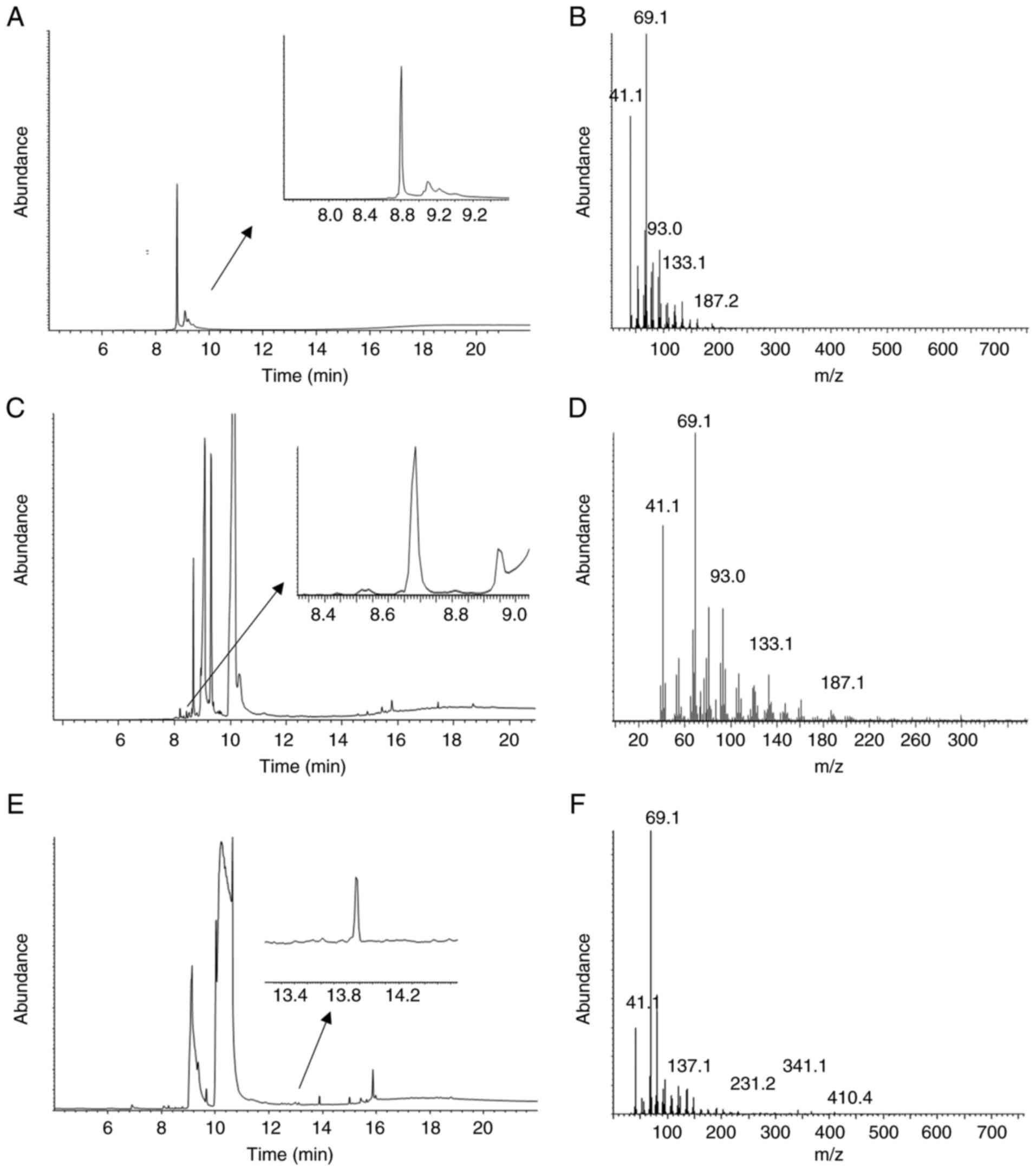
We next used GC-MS to confirm the presence of
geranylgeraniol in two of the extracts that rescued the effect of
pitavastatin (Fig. 5). Analysis of
the lettuce extract identified free geranylgeraniol (Fig. 5C and D). Free geranylgeraniol was
not detected in the bean extract by GCMS (Fig. 5E and F), however a derivative which
contained geranylgeraniol (Fig.
5A) was identified.
Discussion
We have previously shown that the potential activity
of statins as a cancer treatment depends on the reduced production
of geranylgeraniol. However, several human foods have been reported
to contain geranylgeraniol, potentially bypassing the effect of the
statins on the cancer cells. Here we have attempted to enable
clinical trials of statins by beginning to delineate which foods do
not interfere with the cytotoxic activity of statins and so may be
eaten by patients while using statins to treat cancer.
We have previously shown that geranylgeraniol, but
not farnesol, reverses the activity of pitavastatin or simvastatin
(6,8). Here we extended this and tested other
products of the mevalonate pathway whose biosynthesis would also be
anticipated to be blocked by statins. However, addition of coenzyme
Q, dolichol or cholesterol had no effect on the activity of
pitavastatin whereas geranylgeraniol did. We therefore used a
method to prepare extracts from a range of foods (oils, meals,
fruits and vegetables) previously used to extract geranylgeraniol.
However, we used a bioassay to evaluate the activity of these
extracts, rather than quantifying geranylgeraniol by analytical
methods. This approach has the advantage that it makes no
assumption about the nature and quantities of the compounds present
which can interfere with the activity of the statin. There may be
other molecules other than geranylgeraniol which have yet to be
identified which suppress the activity of statins. Consistent with
our previous results, the sunflower extract reversed the activity
of pitavastatin to an extent comparable to that achieved with
geranylgeraniol itself, as did corn oil. On the other hand, some
extracts such as groundnut oil had more modest effects while others
such as rape seed oil showed no significant effect on the
anti-cancer activity of pitavastatin. We also found that foods
other than oils, particularly beans, cherries, eggs and oats,
suppressed the activity of pitavastatin but extracts from several
other carbohydrates and fruits had no effect. The extract from
lettuce also rescued the effect of pitavastatin and GC-MS analysis
confirmed the presence of geranylgeraniol. However, the extract
also stimulated cell growth on its own, confusing interpretation.
It is possible that the extract contains an additional growth
stimulatory molecule that remains to be identified. Taken together,
our data suggest some foods are more likely than others to
interfere with the anti-cancer activity of statins.
To confirm that the observations were not unique to
Ovcar-4 cells, we also tested the ability of mevalonate pathway
metabolites and selected food extracts to suppress the activity of
pitavastatin in Fuov-1 cells. Comparable results were obtained,
although the lettuce extract suppressed the activity of
pitavastatin while having no growth promoting properties in Fuov-1
cells. We have also previously shown that extracts from sunflower
and olive oil and rice suppress the cytotoxic activity of
pitavastatin in Ovcar-3 and Ovcar-8 cells (6). In all of these cell lines,
geranylgeraniol suppressed the activity of pitavastatin. This
suggests that the ability of various extracts to suppress the
cytotoxic activity of pitavastatin are not unique to Ovcar-4 cells
but are likely to apply to ovarian cancer cells in general and
possibility cancer cells from other tissues.
In this study, we measured the effect of the
extracts on the cytotoxicity of pitavastatin primarily by staining
the cells with sulforhodamine B. This assay measures total cellular
protein, which is often used as a surrogate for cell number and
consequently reflects the growth of a cell culture rather than
directly measuring cell death. We confirmed that the pitavastatin
caused cell death and that this could be rescued by certain
extracts by using the trypan blue assay. This method has the
advantage that it measures cell death independently of the mode of
cell death. However, we have previously shown that pitavastatin
induces apoptosis in Ovcar-3, Ovcar-8, Ovsaho, Cov362, SkOv-3,
Igrov-1 and A2780 ovarian cancer cells. This was assessed by
several methods including activation of both executioner
caspases-3/7 and initiator caspases (caspase-8, caspase-9), PARP
cleavage and annexin/PI staining (4,6,10).
In the case of Ovcar-3 and Ovcar-8, we also showed that
geranylgeraniol and extracts from sunflower oil, olive oil and rice
suppress caspase-3/7 activation by pitavastatin (6). These data suggest food extracts can
suppress statin-induced cell death. However, the potential clinical
use of statins in oncology may extend beyond inducing tumour cell
apoptosis. Others have also shown that statins can inhibit other
cancer hallmarks, including migration, invasion and cell
progression (2,3) and further studies to evaluate the
activity of the food extracts on these hallmarks are desirable.
Previous research has used analytical methods such
as GC-MS to identify either free geranylgeraniol or its derivatives
in several human foodstuffs. Geranylgeraniol has been discovered as
a wax ester in several oils, in particular oils prepared from
sunflower (7,11) vegetable (12), linseed (13), soybean (7), sesame (7), hemp (14) and olives (15). The potent effect of the sunflower
oil extract in suppressing the cytotoxic effect of pitavastatin we
observed is particularly significant because apart from its use to
fry food, sunflower oil is also an ingredient found in
approximately 1,000 manufactured food products (16). Oil extracted from hazelnuts, pecans
and almonds also have been reported to contain geranylgeraniol
(17,18). In contrast to these oils, rapeseed
oil lacks at least the common 22 and 24 carbon fatty acid esters of
geranylgeraniol (7) and extracts
from rapeseed oil also failed to inhibit the activity of
pitavastatin in our experiments. This suggests that this oil may be
preferable for patients to use for culinary purposes while they are
receiving statins to treat cancer. Geranylgeraniol is also found in
certain types of rice (9) and we
have previously shown that a rice extract suppresses the cytotoxic
effect of pitavastatin (6). Leaves
from E. persicus, a traditional food in central Asia and the
Middle East (19) and fruit from
Pterodon tree, which his used in ethnomedicine in Brazil
(20) have also been reported to
contain geranylgeraniol. Our own GC-MS analysis identified free
geranylgeraniol in the extract from lettuce. However, it is
possible that the geranylgeraniol is present in another form, such
as an ester, in lettuce itself and that it is liberated by the
alkaline hydrolysis step in the extraction method. The extract
obtained from beans contained a derivative of geranylgeraniol,
rather than free geranylgeraniol. Further investigation is
necessary to confirm its precise chemical identity; it may be a wax
ester that is not susceptible to alkaline hydrolysis but
geranylgeraniol is liberated enzymatically from it in the cancer
cells. Lastly, we note that phytol, derived from the reduction of
geranylgeraniol, is a component of chlorophyll (21). This suggests that at least trace
quantities of geranylgeraniol are likely to occur throughout the
plant kingdom.
Based on these observations, we suggest that
patients may inadvertently consume sufficient geranylgeraniol to
counter the anti-cancer activity of statins. Consequently, a diet
certified to lack geranylgeraniol may be beneficial for patients
using statins to treat cancer. This would preferably be achieved by
establishing a database of foods in which geranylgeraniol and its
derivatives have been systematically quantified by appropriate
analytical methods. Until this is available, the data presented
here may be of help in identifying such a diet. In particular, we
suggest patients using statins to control cancer should consider
avoiding sunflower oil or corn oil, nuts, eggs, oats, beans,
lettuce and cherries. The foods which failed to suppress the
activity of pitavastatin on cells are likely to be improved choices
for patients using statins to treat cancer and which may be
consumed alongside food replacements such as Ensure which we have
shown also do not interfere with the activity of statins (6). It may also be preferable to avoid
pre-prepared food products that are rich in oils or ingredients
that have not been evaluated. For example, a commercial pasta sauce
we tested suppressed the cytotoxic activity of pitavastatin.
Several issues remain to be addressed. We anticipate
that the food extracts are able to restore membrane localization of
key signalling proteins such as small GTPases by restoring their
geranylgeranylation. We have previously shown that pitavastatin
decreases the proportion of rho, ras, cdc42 and rab6A in cell
membranes (4). Furthermore,
statins reduce the amount of Rab7, presumably as a result of
turnover following reduced membrane localization, and that this is
reversed by addition of geranylgeraniol (8). However, we have not yet formally
shown that the food extracts are able to restore
geranylgeranylation and membrane localization of small GTPases. In
addition, in most cases we have neither fully identified nor
quantified the compounds in the foods which suppress the activity
of pitavastatin. Although they are likely to be geranylgeraniol
derivatives, it is possible other compounds can inhibit the
activity of pitavastatin and that remain to be identified. We do
not know the bioavailability of the various geranylgeraniol
derivatives, nor do we know the amount of geranylgeraniol that must
be absorbed to suppress the cytotoxic activity of statins in
patients. Thus, we acknowledge that it remains a formal possibility
that the ingestion of foods containing geranylgeraniol has minimal
effect on the activity of statins as anti-cancer agents because
insufficient geranylgeraniol reaches the systemic circulation from
dietary sources. Further research is essential to address this.
Until then, we consider that when clinical trials of statins in
cancer are conducted, it is prudent to minimize dietary
geranylgeraniol to maximize the chances of the trials being
successful. An indication of anti-cancer activity of a statin in a
prospective clinical trial would provide significant motivation to
carry out a more thorough analysis of geranylgeraniol in human
food. We have only so far tested a limited number of foods and a
wider range would facilitate compliance with a
‘geranylgeraniol-free’ diet. This work also raises the question
whether other targeted cancer therapeutics could be affected by
diet. This overlooked area warrants additional research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Iraqi Ministry of Higher
Education and Scientific research (MOHESR; grant nos. S1884 and
S939).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The project was conceived by AR. The extracts were
prepared and tested by MJJ, SI, MK and CB. GCMS was performed by
MJJ and WL. AR, SI, MK and CB analysed the biological data. WW and
MJJ analysed the GCMS data. AR, MJJ and WW confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rooth C: Ovarian cancer: Risk factors,
treatment and management. Br J Nurs. 22:S23–S30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mullen PJ, Yu R, Longo J, Archer MC and
Penn LZ: The interplay between cell signalling and the mevalonate
pathway in cancer. Nat Rev Cancer. 16:718–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Altwairgi AK: Statins are potential
anticancerous agents (Review). Oncol Rep. 33:1019–1039. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdullah MI, Abed MN and Richardson A:
Inhibition of the mevalonate pathway augments the activity of
pitavastatin against ovarian cancer cells. Sci Rep. 7:80902017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdullah MI, de Wolf E, Jawad MJ and
Richardson A: The poor design of clinical trials of statins in
oncology may explain their failure-lessons for drug repurposing.
Cancer Treat Rev. 69:84–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Wolf E, Abdullah MI, Jones SM, Menezes
K, Moss DM, Drijfhout FP, Hart SR, Hoskins C, Stronach EA and
Richardson A: Dietary geranylgeraniol can limit the activity of
pitavastatin as a potential treatment for drug-resistant ovarian
cancer. Sci Rep. 7:54102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biedermann M, Haase-Aschoff P and Grob K:
Wax ester fraction of edible oils: Analysis by on-line LC-GC-MS and
GCxGC-FID. Eur J Lipid Sci Technol. 110:1084–1094. 2008. View Article : Google Scholar
|
|
8
|
Robinson E, Nandi M, Wilkinson LL,
Arrowsmith DM, Curtis AD and Richardson A: Preclinical evaluation
of statins as a treatment for ovarian cancer. Gynecol Oncol.
129:417–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muraguchi T, Okamoto K, Mitake M, Ogawa H
and Shidoji Y: Polished rice as natural sources of
cancer-preventing geranylgeranoic acid. J Clin Biochem Nutr.
49:8–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdullah MI, Abed MN, Khanim F and
Richardson A: Screening a library of approved drugs reveals that
prednisolone synergizes with pitavastatin to induce ovarian cancer
cell death. Sci Rep. 9:96322019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reiter B and Lorbeer E: Analysis of the
wax ester fraction of olive oil and sunflower oil by gas
chromatography and gas chromatography-mass spectrometry. J Am Oil
Chem Soc. 78:881–888. 2001. View Article : Google Scholar
|
|
12
|
Fedeli E and Jacini G: Lipid composition
of vegetable oils. Adv Lipid Res. 9:335–382. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fedeli E, Capella P, Cirimele M and Jacini
G: Isolation of geranyl geraniol from the unsaponifiable fraction
of linseed oil. J Lipid Res. 7:437–441. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montserrat-de la Paz S, Marin-Aguilar F,
Garcia-Gimenez MD and Fernandez-Arche MA: Hemp (Cannabis
sativa L.) seed oil: Analytical and phytochemical
characterization of the unsaponifiable fraction. J Agric Food Chem.
62:1105–1110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ranalli A, Modesti G, Patumi M and
Fontanazza G: The compositional quality and sensory properties of
virgin olive oil from a new olive cultivar-I-77. Food Chem.
69:37–46. 2000. View Article : Google Scholar
|
|
16
|
Gigandet S: Open food facts, 2021.
https://world.openfoodfacts.org/cgi/search.pl?search_terms=sunflower+oil&search_simple=1&action=processSeptember
16–2021.
|
|
17
|
Fernandes GD, Gómez-Coca RB, Pérez-Camino
MC, Moreda W and Barrera-Arellano D: Chemical characterization of
major and minor compounds of nut oils: Almond, hazelnut, and pecan
nut. J Chem. 2017:26095492017. View Article : Google Scholar
|
|
18
|
Purcaro G, Barp L and Conte L: Comparison
of different injection modes in edible oil minor components
analysis. J Sep Sci. 38:2278–2285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salehi B, Ayatollahi SA, Segura-Carretero
A, Kobarfard F, Contreras MDM, Faizi M, Sharifi-Rad M, Tabatabai SA
and Sharifi-Rad J: Bioactive chemical compounds in Eremurus
persicus (joub. & spach) Boiss. essential oil and their
health implications. Cell Mol Biol (Noisy-Le-Grand). 63:1–7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menna-Barreto R, Laranja GAT, Silva MCC,
Coelho MGP, Paes MC, Oliveira MM and de Castro SL: Anti-trypanosoma
cruzi activity of pterodon pubescens seed oil: Geranylgeraniol as
the major bioactive component. Parasitol Res. 103:111–117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gutbrod K, Romer J and Dormann P: Phytol
metabolism in plants. Prog Lipid Res. 74:1–17. 2019. View Article : Google Scholar : PubMed/NCBI
|