Introduction
Neck and distant metastases are crucial prognostic
factors in the treatment of oral cancer. Prediction of metastases
by identifying prognostic markers could contribute towards the
management and treatment of oral cancer; however, to date, no such
prognostic markers have been identified (1).
Programmed cell death 1 (PD-1), which is expressed
on the surface of activated T cells in healthy conditions,
regulates unnecessary or heightened immune responses, including
self-protective responses. Upon binding to a ligand, it negatively
regulates the signal transduction through an antigen receptor. The
PD-1 pathway is the main immune regulatory switch in cancer cells
to escape the T-cell immune surveillance system (2). Despite its expression in a few normal
tissues, programmed cell death 1 ligand 1 (PD-L1) is overexpressed
in numerous cancer cells since it suppresses T-cell activity
(2). Reportedly, high PD-L1
expression in cancer cells leads to a poor prognosis in various
types of cancer, such as renal cell, hepatocellular, ovarian and
non-small cell lung cancer (3–6).
Zinc finger E-box binding homeobox 1 (ZEB-1), also
known as dEF1, ZFHX1A, Nil-2-a, TCF8, AREB6 or BZP, is a
transcription factor belonging to the human ZEB family. Previous
studies demonstrated that ZEB-1 serves a significant role in
epithelial-to-mesenchymal transition (EMT) during tumor invasion
and metastasis in various types of human cancer (7–9).
It has been reported that PD-L1 expression may be
associated with ZEB-1 expression and poor prognosis in esophageal
cancer since the gene promoter region of PD-L1 contains a binding
site for ZEB-1, a transcription factor associated with EMT
(10). Tsutsumi et al
(10) also reported that small
interfering RNA ZEB-1 suppressed PD-L1 expression through the
ZEB-1/PD-L1 pathway and TGF-β1-induced EMT in esophageal squamous
cell carcinoma (ESCC) cell lines.
Several studies have reported the individual
association of PD-L1 and ZEB-1 with oral squamous cell carcinoma
(OSCC) (11,12). These reports indicated that both
PD-L1 and ZEB-1 have the potential to be useful prognostic
biomarkers in OSCC, individually, as they are in ESCC. However, to
the best of our knowledge, the present study was the first to
explore the association between the expression patterns of those
two markers in patients with OSCC and clinicopathological
characteristics or prognosis.
Materials and methods
Clinical characteristics of the
patients
A total of 169 patients with OSCC initially visited
Tokushima University Hospital (Tokushima, Japan) between April 2008
and March 2014 [including 92 men and 77 women (median age, 69.0
years; range, 24–97 years)] and were enrolled in the present study.
All of the patients underwent radical surgery and were followed up
after treatment (mean follow up period, 61 months; range, 3–132
months). The present study was a retrospective study and was
approved by the Tokushima University Human Investigations Committee
(approval no. 2516; Tokushima, Japan) and adhered to the principles
in the Declaration of Helsinki. OSCC specimens were obtained by
biopsy or surgery after the patients had provided informed consent.
Clinical characteristics of the patients are presented in Table I. The primary tumor was located at
sites including the tongue (71 sites), lower gingiva (46 sites),
upper gingiva (26 sites), oral floor (13 sites), buccal mucosa (12
sites) and hard palate (one site). Oral cancer specimens were
obtained during biopsy or surgery, and all patients were treated
surgically. Tumor size and clinicopathological stage of OSCC were
classified according to the 2002 TNM classification general rules
(13) (all samples used in the
present study were collected before the revision of TNM
classification in 2017), and the numbers of T1, T2, T3 and T4 cases
were 39, 88, 16 and 26, respectively. The histological types of the
obtained specimens were well-differentiated, moderately
differentiated and poorly differentiated in 72, 90 and seven cases,
respectively. Furthermore, in the present study, the
Yamamoto-Kohama (YK) classification, a modified version of the
classification proposed by Jakobsson et al (14) and Willén et al (15), was used to determine the
pathological grade of tumor invasion (16). The number of cases classified under
grades YK 1, YK 2, YK 3, YK 4C and YK 4D were 13, 41, 66, 30 and
14, respectively. In addition, unknown grade was assigned to five
cases, as specimens of these cases were not collected deeply enough
to clearly observe the tumor invasion front. In the present study,
the tumor invasion front was defined as in a previous report, as
the most progressed, with three to six tumor cell layers or
detached tumor cell groups at the advancing edge of the
histological specimen (17).
Finally, the number of patients diagnosed with lymph node
metastasis was counted, and 53 patients were diagnosed between the
initial diagnosis and the end of the 5-year follow-up period.
 | Table I.Clinicopathological characteristics of
the patients. |
Table I.
Clinicopathological characteristics of
the patients.
| Characteristic | Group | Number of
patients |
|---|
| Sex | Male | 92 |
|
| Female | 77 |
| Primary site | Tongue | 71 |
|
| Lower gingiva | 46 |
|
| Upper gingiva | 26 |
|
| Oral floor | 13 |
|
| Buccal mucosa | 12 |
|
| Hard palate | 1 |
| T
classification | T1 | 39 |
|
| T2 | 88 |
|
| T3 | 16 |
|
| T4 | 26 |
| Histological
differentiation | Well
differentiated | 72 |
|
| Moderately
differentiated | 90 |
|
| Poorly
differentiated | 7 |
| YK
classification | YK 1 | 13 |
|
| YK 2 | 41 |
|
| YK 3 | 66 |
|
| YK 4C | 30 |
|
| YK 4D | 14 |
|
| Unknown | 5 |
| N status | Negative | 116 |
|
| Positive | 53 |
Immunohistochemistry
The obtained specimens were fixed in neutral 10%
formalin for 24 h at room temperature and embedded in paraffin
after resection; 5-µm sections were obtained and transferred onto
slides. The sections were deparaffinized in xylene and dehydrated
in graded ethanol. Standard hematoxylin and eosin (H&E)
staining was performed by the Department of Pathology, Tokushima
University Hospital, at room temperature. Endogenous peroxidase
activity was blocked using 3% hydrogen peroxide for 5 min at room
temperature, and antigen retrieval using 10 mM citrate buffer
solution was performed in a microwave oven. Immunostaining was
performed using an avidin-biotin-peroxidase enzyme complex (ABC
kit; cat. no. PK4001; Vector Laboratories, Inc.). Briefly, the
sections were incubated with monoclonal rabbit anti-human PD-L1
(dilution 1:1; clone SP142; cat. no. 518113193; Roche Diagnostics)
and monoclonal rabbit anti-human ZEB-1 (dilution 1:100; clone
EPR17375; cat. no. ab203829; Abcam) antibodies overnight at 4°C,
and subsequently incubated with secondary anti-rabbit antibody (ABC
kit; cat. no. PK4001; Vector Laboratories, Inc.) for 30 min at room
temperature followed by the avidin-biotin complex reagent. The
sections were incubated in substrate 3,3-diaminobenzidine (0.05%)
for 20 min at room temperature and 0.1% hydrogen peroxide for 8
min. The immunohistochemically stained images were observed using a
light microscope (BX43; Olympus) in at least five fields of view,
each at a different magnification (magnification, ×40-400).
Thereafter, the percentage of tumor cells in the images was
evaluated, and the specimens were graded as negative or positive
for PD-L1 and ZEB-1. Briefly, expression of PD-L1 was considered as
positive when >1% of all tumor cells were stained (18), and ZEB-1 was considered as positive
when >10% of all tumor cells were stained (19). In the following sentences, positive
results are indicated as (+), and negative results are indicated as
(−). For example, PD-L1 (+)/ZEB-1 (−) indicates PD-L1-positive and
ZEB-1-negative staining. Additionally, the expression patterns of
PD-L1 and ZEB-1 were divided into four groups: Type A, PD-L1
(+)/ZEB-1 (+); Type B, PD-L1 (+)/ZEB-1 (−); Type C, PD-L1 (−)/ZEB-1
(+); Type D, PD-L1 (−)/ZEB-1 (−).
Statistical analyses
The χ2 test, Fisher's exact test,
Kruskal-Wallis test, and Mann-Whitney U test were used to
statistically analyze the relationship between the expression
levels of PD-L1 and ZEB-1 and clinicopathological factors. Survival
analysis was performed using the Kaplan-Meier method and compared
using the log-rank test. A Cox hazard regression model was used for
multivariate analysis. Analyses were performed using BellCurve for
Excel (Social Survey Research Information Co., Ltd.). The results
were quantified using hazard ratios (HRs) and 95% confidence
intervals (CIs). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PD-L1 and ZEB-1 in
OSCC
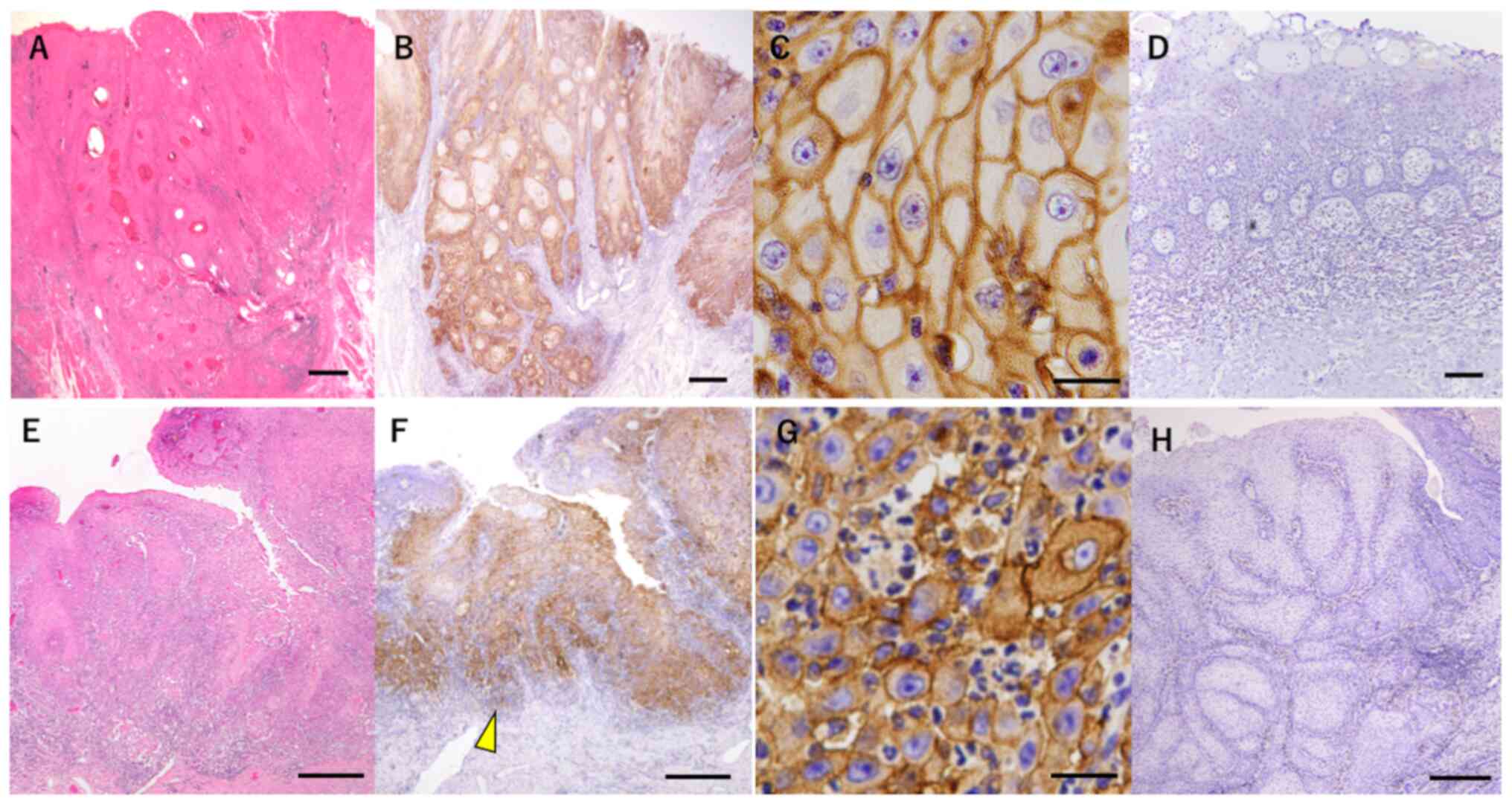
As shown in H&E staining images (Fig. 1A and E), cancer cell nests with
various size were observed. Representative staining images of PD-L1
and ZEB-1 proteins are shown in Fig.
1. The analysis revealed that PD-L1 protein was expressed in
the cell membrane of cancer cells (Fig. 1B and C), whereas ZEB-1 protein was
mainly observed in the intercellular substance and cell membrane of
cancer cells localized at the invasion front of the tumor (Fig. 1F and G).
The expression rates of PD-L1 and ZEB-1 in oral
cancer tissues from 169 patients with OSCC are summarized in
Table II. The positive rates of
PD-L1 and ZEB-1 expression in patients with OSCC were 60.9%
(103/169 patients) and 28.4% (48/169 patients), respectively.
 | Table II.Expression rates of PD-L1 and
ZEB-1. |
Table II.
Expression rates of PD-L1 and
ZEB-1.
| Marker | Patients with
positive expression/total patients | Percentage (%) |
|---|
| PD-L1 | 103/169 | 60.9 |
| ZEB-1 | 48/169 | 28.4 |
Association of PD-L1 and ZEB-1
expression with clinicopathological characteristics
The statistical association between PD-L1 expression
and clinicopathological features is demonstrated in Table III. PD-L1 expression was
significantly associated with YK classification (P=0.02) and lymph
node metastasis (P=0.02), although there was no statistical
difference between PD-L1 expression and tumor size (P=0.97) or
histological differentiation (P=0.64).
 | Table III.Statistical association between PD-L1
expression and clinicopathological features. |
Table III.
Statistical association between PD-L1
expression and clinicopathological features.
| Clinicopathological
feature | Group | PD-L1 (+)
(n=103) | PD-L1 (−)
(n=66) | P-value |
|---|
| T
classification | T1 | 25 | 14 | 0.97a |
|
| T2 | 52 | 36 |
|
|
| T3 | 7 | 9 |
|
|
| T4 | 19 | 7 |
|
| Histological
differentiation | Well
differentiated | 46 | 26 | 0.64a |
|
| Moderately
differentiated | 53 | 37 |
|
|
| Poorly
differentiated | 4 | 3 |
|
| YK
classification | YK 1 | 8 | 5 | 0.02a |
|
| YK 2 | 17 | 24 |
|
|
| YK 3 | 45 | 21 |
|
|
| YK 4C | 20 | 10 |
|
|
| YK 4D | 10 | 4 |
|
|
| YK unknown | 3 | 2 |
|
| N status | Negative | 64 | 52 | 0.02b |
|
| Positive | 39 | 14 |
|
The association between ZEB-1 expression and
clinicopathological features is presented in Table IV. Similar to PD-L1, ZEB-1
expression was significantly associated with YK classification
(P=0.03) and lymph node metastasis (P<0.01). However, there was
no association between ZEB-1 expression and tumor size (P=0.25) or
histological differentiation (P=0.47).
 | Table IV.Statistical association between ZEB-1
expression and clinicopathological features. |
Table IV.
Statistical association between ZEB-1
expression and clinicopathological features.
| Clinicopathological
feature | Group | ZEB-1 (+)
(n=48) | ZEB-1 (−)
(n=121) | P-value |
|---|
| T
classification | T1 | 8 | 31 | 0.25a |
|
| T2 | 27 | 61 |
|
|
| T3 | 3 | 13 |
|
|
| T4 | 10 | 16 |
|
| Histological
differentiation | Well
differentiated | 19 | 53 | 0.47a |
|
| Moderately
differentiated | 24 | 66 |
|
|
| Poorly
differentiated | 5 | 2 |
|
| YK
classification | YK 1 | 1 | 12 | 0.03a |
|
| YK 2 | 9 | 32 |
|
|
| YK 3 | 20 | 46 |
|
|
| YK 4C | 12 | 18 |
|
|
| YK 4D | 5 | 9 |
|
|
| YK unknown | 1 | 4 |
|
| N status | Negative | 21 | 95 |
<0.01b |
|
| Positive | 27 | 26 |
|
The association of PD-L1 and ZEB-1 expression
patterns with clinicopathological features is summarized in
Table V. It should be noted that
PD-L1 and ZEB-1 expression pattern was significantly associated
with cervical lymph node metastasis. Cervical lymph node metastases
were observed in 57.5% (23/40 patients) of Type A [PD-L1 (+)/ZEB-1
(+)] patients (P<0.01), whereas it was observed in 17.2% (10/58
patients) Type D [PD-L1 (−)/ZEB-1 (−)] patients (P<0.01). These
findings suggested that co-expression of PD-L1 and ZEB-1 was
predominantly associated with the development of cervical
metastases. With respect to the invasion mode (YK classification),
the combination of Type A and D showed a P-value near statistical
significance (P=0.053) across all types, even though there was no
statistical significance among all combinations. Briefly, Type A
tended to exhibit a poor invasion mode (YK 4D), whereas Type D
tended to show a clear border between tumor and normal tissue (YK
1). By contrast, tumor size (T classification) and histological
differentiation were not affected by PD-L1 and ZEB-1
expression.
 | Table V.Statistical association between PD-L1
and ZEB-1 expression and clinicopathological features. |
Table V.
Statistical association between PD-L1
and ZEB-1 expression and clinicopathological features.
| Clinicopathological
feature | Group | Type A (n=40) | Type B (n=63) | Type C (n=8) | Type D (n=58) | P-value |
|---|
| T
classification | T1 | 7 | 18 | 1 | 13 | NS |
|
| T2 | 22 | 30 | 5 | 31 |
|
|
| T3 | 2 | 5 | 1 | 8 |
|
|
| T4 | 9 | 10 | 1 | 6 |
|
| Histological
differentiation | Well
differentiated | 15 | 31 | 4 | 22 | NS |
|
| Moderately
differentiated | 21 | 32 | 3 | 34 |
|
|
| Poorly
differentiated | 4 | 0 | 1 | 2 |
|
| YK
classification | YK 1 | 1 | 7 | 0 | 5 | 0.053a |
|
| YK 2 | 6 | 11 | 3 | 21 |
|
|
| YK 3 | 17 | 28 | 3 | 18 |
|
|
| YK 4C | 11 | 9 | 1 | 9 |
|
|
| YK 4D | 4 | 6 | 1 | 3 |
|
|
| YK unknown | 1 | 2 | 0 | 2 |
|
| N status | Negative | 17 (42.5%) | 47 (74.6%) | 4 (50.0%) | 48 (82.8%) |
<0.01b |
|
| Positive | 23 (57.5%) | 16 (25.4%) | 4 (50.0%) | 10 (17.2%) |
|
Survival analysis
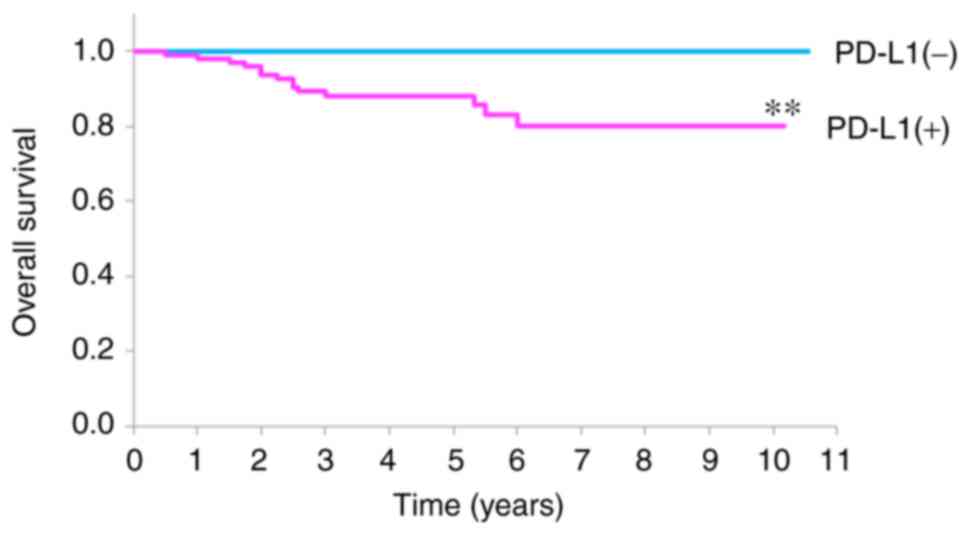
The survival rates of patients with OSCC with and
without PD-L1 expression are demonstrated in Fig. 2. PD-L1 (+) patients had a 5-year
survival rate of 88.2%, whereas PD-L1 (−) patients were alive at
the 5-year point. Briefly, PD-L1 expression was associated with a
significantly worse prognosis for patients when compared with those
without or with lower PD-L1 expression (P=0.0016).
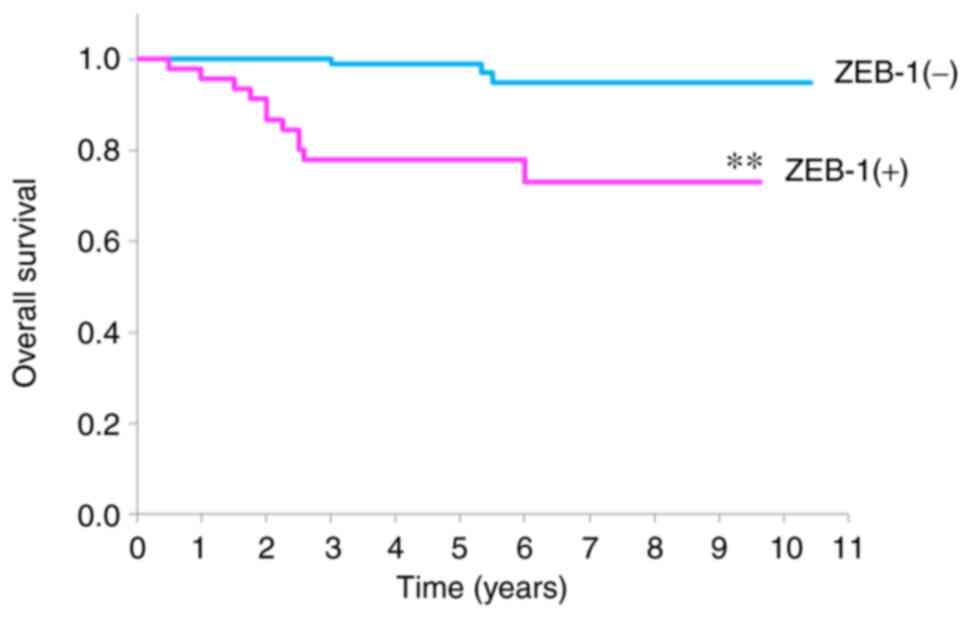
The survival rates of patients with OSCC with and
without ZEB-1 expression are revealed in Fig. 3. ZEB-1 (+) patients demonstrated a
significantly lower survival rate when compared with ZEB-1 (−)
patients; the 5-year survival rate was 77.6% in the ZEB-1 (+)
group, whereas it was 99.0% in the ZEB-1 (−) group
(P<0.001).
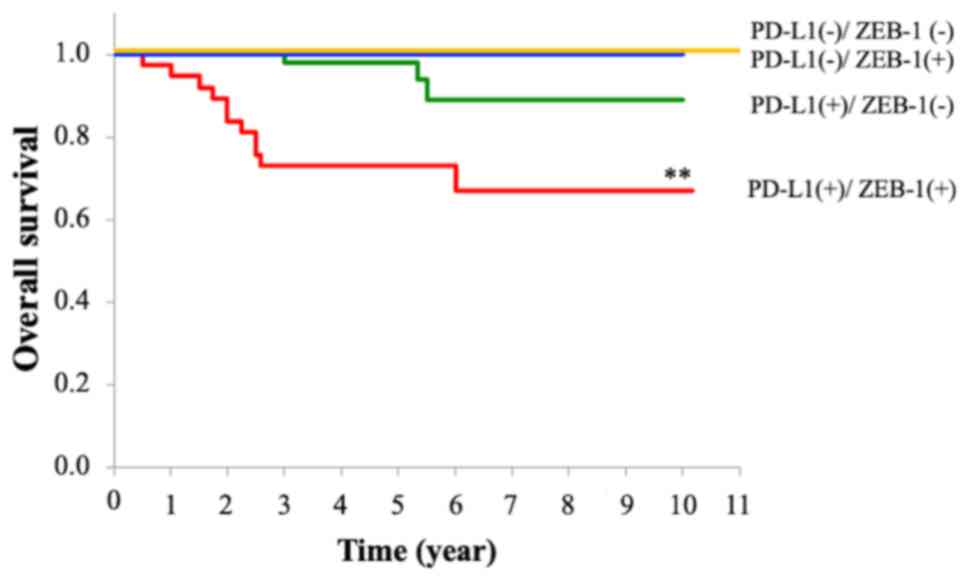
The combined effects of PD-L1 and ZEB-1 expression
on the survival rate of patients with OSCC are summarized in
Fig. 4. As shown, Type D and Type
C patients demonstrated a 100% 5-year survival rate. The 5-year
survival rate of Type B patients was 98.2%. By contrast, Type A
patients showed a significantly worse prognosis than the other
groups, with a 5-year survival rate of 73.1% (P<0.001) across
all groups.
The results of a multivariate analysis of the
factors associated with survival rate are shown in Table VI. The results indicated that N
status (HR, 7.06; 95% CI, 1.24-40.07; P=0.03) and ZEB-1 expression
(HR, 8.32; 95% CI, 1.70-40.07; P=0.004) were significantly
associated with survival rate. Cox analysis for PD-L1 could not be
performed since no deaths were reported in the PD-L1 (−) patient
group in the present study.
 | Table VI.Multivariate analysis of factors
affecting overall survival in oral squamous cell carcinoma. |
Table VI.
Multivariate analysis of factors
affecting overall survival in oral squamous cell carcinoma.
| Factor | HR | 95% CI | P-value |
|---|
| Age | 1.00 | 0.95-1.05 | 0.98 |
| T
classification | 0.74 | 0.37-1.51 | 0.41 |
| Histological
type | 2.04 | 0.53-7.82 | 0.29 |
| YK
classification | 2.26 | 0.84-6.07 | 0.11 |
| N status | 7.06 | 1.24-40.07 | 0.03 |
| ZEB-1 | 8.32 | 1.70-40.07 | 0.004 |
Discussion
The results obtained in the present study
demonstrated that both PD-L1 and ZEB-1 were associated with the
invasion mode and lymph node metastasis of OSCC. Notably,
co-expression of PD-L1 and ZEB-1 was strongly associated with
higher cervical lymph node metastasis and a lower survival rate,
even though it has been hypothesized that the survival rate may be
biased since several cases were at the early stages of OSCC [T1: 39
cases and T2: 88 cases; Total: 127/169 cases (75%)] and inoperable
cases were not included in the present study.
PD-L1 expression has already been identified in
several types of cancer, including head and neck cancer (20–28).
In cancer, PD-L1 is expressed on the surface of tumor cells and
non-transformed cells in the tumor microenvironment (2). PD-L1 is expressed on the plasma
membrane of tumor cells and binds to PD-1 on the surface of
activated T cells, inhibiting T-cell proliferation and activation.
Inactivated T cells then remain in the tumor microenvironment
without migrating, leading to tumor cells being resistant to host
immunity (2). Certain studies have
revealed that PD-L1 expression leads to worse outcomes (25,29–31).
For instance, Lin et al (11) reported that higher PD-L1 expression
levels in OSCC were associated with several clinicopathological
factors, such as female sex and distant metastases. Although there
was no relationship between sex and PD-L1 expression in the present
study, PD-L1 expression was associated with not only invasion mode
and high cervical metastases but also poor prognosis. As for the
relationship between PD-L1 and tumor invasion, it has been
suggested that PD-L1 expression may be involved in EMT via RAS/ERK
signaling in a various types of carcinoma (32).
ZEB-1 is known as one of the transcription factors
that are capable of downregulating E-cadherin expression. Yao et
al (12) suggested that
suppressed E-cadherin expression resulted in a worse prognosis due
to increased OSCC migration and invasion, leading to metastasis.
Tsutsumi et al (10)
experimentally demonstrated that the expression of E-cadherin was
upregulated when ZEB-1 was knocked down. Previous studies have
reported that ZEB-1 overexpression is significantly associated with
aggressive disease and poor clinical prognosis, including increased
metastasis and post-treatment recurrence in other human
malignancies, such as uterine cervical, breast and pancreatic
cancer, and hepatocellular carcinoma (11,12,25–36).
Consistent with these findings, the ZEB-1 (+) group, which
accounted for 28.4% of all patients with OSCC in the present study,
showed a significantly worse type of YK classification and
exhibited a significantly worse clinical outcome i.e., higher lymph
node metastasis and lower overall survival rate.
In terms of the relationship between PD-L1 and
ZEB-1, it has been hypothesized that ZEB-1 has a binding site in
the promoter region of PD-L1. Tsutsumi et al (10) demonstrated using ESCC cell lines
that PD-L1 mRNA and protein expression levels were suppressed upon
ZEB-1 silencing mediated by small interfering RNA. These findings
suggested that the ZEB-1 transcription factor may exist upstream of
the PD-L1 signaling pathway, and ZEB-1 could be one of the
regulation factors of PD-L1 expression, while simultaneously
inducing EMT and evading the immune system, even though it has been
hypothesized that other mechanisms may also affect the regulation
of gene expression.
The present findings revealed that the co-expression
pattern of PD-L1 and ZEB-1 led to poor prognosis in patients with
OSCC, which could have been caused by EMT and the evasion of immune
surveillance mechanisms. Briefly, this situation may have occurred
since ZEB-1, an EMT-related factor, binds to the promoter region of
the PD-L1 gene and regulates the expression of PD-L1, which is
involved in EMT through RAS/ERK signaling. To confirm this
hypothesis, it is necessary to collect more data on patients with
OSCC, and to conduct further in vitro and in vivo
experiments in future studies. Although it is a matter for
speculation, PD-L1 and ZEB-1 could be useful prognostic markers for
OSCC since the expression patterns can be examined by
immunohistochemical staining without additional burden for the
patients, such as a collection of tissue and blood samples.
In conclusion, PD-L1 and ZEB-1 expression was
revealed to be associated with higher cervical lymph node
metastasis and poor prognosis in OSCC. In particular, co-expression
of PD-L1 and ZEB-1 was highly associated with poor prognosis in
OSCC. Therefore, PD-L1 and ZEB-1 could serve as useful markers for
predicting the prognosis of patients with OSCC and their clinical
relevance should be explored further.
Acknowledgements
Not applicable.
Funding
The present study was supported by JSPS KAKENHI (grant no.
18K09745).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NT, NF and YM conceived and designed the study. NT,
KA and KK analyzed and interpreted the data. NT and NF wrote,
reviewed and revised the manuscript. NF and YM supervised the
study. NT, NF, KA and KK confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Tokushima
University Human Investigations Committee (approval no. 2516;
Tokushima, Japan) and adhered to the principles in the Declaration
of Helsinki. OSCC specimens were obtained by biopsy or surgery
after the patients had provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed cell death 1 ligand 1
|
|
ZEB-1
|
zinc finger E-box binding homeobox
1
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
OSCC
|
oral squamous cell carcinoma
|
|
YK classification
|
Yamamoto-Kohama classification
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Ebrahimi A, Zhang WJ, Gao K and Clark JR:
Nodal yield and survival in oral squamous cancer: Defining the
standard of care. Cancer. 117:2917–2925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 22:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlsson J, Sundqvist P, Kosuta V, Fält A,
Giunchi F, Fiorentino M and Davidsson S: PD-L1 expression is
associated with poor prognosis in renal cell carcinoma. Appl
Immunohistochem Mol Morphol. 28:213–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei R, Zhang W, Wang S, Huang X and Zou Y:
Prognostic value of PD-L1 in patients with hepatocellular
carcinoma. Clin Lab. May 1–2019.(Epub ahead of print). doi:
10.7754/Clin.Lab.2018.180839. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Webb JR, Milne K, Kroeger DR and Nelson
BH: PD-L1 expression is associated with tumor-infiltrating T cells
and favorable prognosis in high-grade serous ovarian cancer.
Gynecol Oncol. 141:293–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takada K, Toyokawa G, Shoji F, Okamoto T
and Maehara Y: The significance of the PD-L1 expression in
non-small-cell lung cancer: Trenchant double swords as predictive
and prognostic markers. Clin Lung Cancer. 19:120–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gheldof A, Hulpiau P, van Roy F, De Craene
B and Berx G: Evolutionary functional analysis and molecular
regulation of the ZEB transcription factors. Cell Mol Life Sci.
69:2527–2541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vandewalle C, Van Roy F and Berx G: The
role of the ZEB family of transcription factors in development and
disease. Cell Mol Life Sci. 66:773–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang PJ, Sun YT and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsutsumi S, Saeki H, Nakashima Y, Ito S,
Oki E, Morita M, Oda Y, Okano S and Maehara Y: Programmed
death-ligand 1 expression at tumor invasive front is associated
with epithelial-mesenchymal transition and poor prognosis in
esophageal squamous cell carcinoma. Cancer Sci. 108:1119–1127.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai
HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT and Chen CJ: High
PD-L1 expression correlates with metastasis and poor prognosis in
oral squamous cell carcinoma. PLoS One. 10:e01426562015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao X, Sun S, Zhou X, Zhang Q, Guo W and
Zhang L: Clinicopathological significance of ZEB-1 and E-cadherin
proteins in patients with oral cavity squamous cell carcinoma. Onco
Targets Ther. 10:781–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours. 6th edition. John Wiley &
Sons Inc.; New York, USA: pp. 22–26. 2002
|
|
14
|
Jakobsson PA, Eneroth CM, Killander D,
Moberger G and Mårtensson B: Histologic classification and grading
of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys
Biol. 12:1–8. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willén R, Nathanson A, Moberger G and
Anneroth G: Squamous cell carcinoma of the gingiva. Histological
classification and grading of malignancy. Acta Otolaryngol.
79:146–154. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto E, Miyakawa A and Kohama G: Mode
of invasion and lymph node metastasis in squamous cell carcinoma of
the oral cavity. Head Neck Surg. 6:938–947. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piffko J, Bánkfalvi A, Ofner D, Rasch D,
Joos U and Schmid KW: Standardized demonstration of silver-stained
nucleolar organizer regions-associated proteins in archival oral
squamous cell carcinomas and adjacent non-neoplastic mucosa. Mod
Pathol. 10:98–104. 1997.PubMed/NCBI
|
|
18
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chin D, Boyle GM, Williams RM, Ferguson K,
Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG and Coman
WB: Novel markers for poor prognosis in head and neck cancer. Int J
Cancer. 113:789–797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greaves P and Gribben JG: The role of B7
family molecules in hematologic malignancy. Blood. 121:734–744.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keir ME, Liang SC, Guleria I, Latchman YE,
Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH and Sharpe
AH: Tissue expression of PD-L1 mediates peripheral T cell
tolerance. J Exp Med. 203:883–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ
and Chen L: B7-H1 is a ubiquitous antiapoptotic receptor on cancer
cells. Blood. 111:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strome SE, Dong H, Tamura H, Voss SG,
Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al:
B7-H1 blockade augments adoptive T-cell immunotherapy for squamous
cell carcinoma. Cancer Res. 63:6501–6505. 2003.PubMed/NCBI
|
|
27
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wintterle S, Schreiner B, Mitsdoerffer M,
Schneider D, Chen L, Meyermann R, Weller M and Wiendl H: Expression
of the B7-related molecule B7-H1 by glioma cells: A potential
mechanism of immune paralysis. Cancer Res. 63:7462–7467.
2003.PubMed/NCBI
|
|
29
|
Ghebeh H, Mohammed S, Al-Omair A, Qattan
A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah
A, et al: The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is
expressed in breast cancer patients with infiltrating ductal
carcinoma: Correlation with important high-risk prognostic factors.
Neoplasia. 8:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-H1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M and
Nakajima Y: Clinical significance and therapeutic potential of the
programmed death-1 ligand/programmed death-1 pathway in human
pancreatic cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Xu LJ, Zhu J, Li J, Xue BX, Gao
J, Sun CY, Zang YC, Zhou YB, Yang DR and Shan YX: ATM-JAK-PD-L1
signaling pathway inhibition decreases EMT and metastasis of
androgen-independent prostate cancer. Mol Med Rep. 17:7045–7054.
2018.PubMed/NCBI
|
|
33
|
Ran J, Lin DL, Wu RF, Chen QH, Huang HP,
Qiu NX and Quan S: ZEB1 promotes epithelial-mesenchymal transition
in cervical cancer metastasis. Fertil Steril. 103:1606–1614.e2.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mock K, Preca BT, Brummer T, Brabletz S,
Stemmler MP and Brabletz T: The EMT-activator ZEB1 induces bone
metastasis associated genes including BMP-inhibitors. Oncotarget.
6:14399–14412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|