Introduction
Stereotactic body radiotherapy (SBRT) has been used
for early clinical stage non-small cell lung cancers (NSCLCs).
Generally, SBRT is performed in patients with lung cancer who are
medically inoperable; recently it has also been performed in
operable patients due to clinical outcomes comparable to surgery
(1–3). In addition, various factors have been
reported to influence the prognosis for patients with lung cancer
who underwent SBRT (4–11). For instance, tumor diameter,
standardized uptake value (SUV) on
18F-fluoro-2-deoxyglucose positron emission tomography
(18F-FDG-PET) and low dose distribution have been
reported as prognostic factors for local control (LC) after SBRT
(12,13). Takeda et al (10) reported that maximum SUV
(SUVmax) on 18F-FDG-PET/CT was the strongest
predictor of local failure of localized NSCLC treated with SBRT. In
another study regarding pathologically confirmed NSCLC, high
SUVmax on 18FDG-PET/CT was significantly
correlated with LC after SBRT (11). Tumor size, pretreatment C-reactive
protein (CRP) value, histology types, and pretreatment physical
state were shown to be significantly associated with overall
survival (OS) in multivariate analysis (4). Yamamoto et al (6) indicated that tumor diameter was
identified as a factor for OS as well as LC; large tumors caused
poor LC and increased the tendency for metastasis. Furthermore, our
previous study indicated that decreasing iodine density values
(IDV) correlated with the local recurrence after SBRT (14), and suggested that the low iodine
density tumor area ratio was a useful prognostic factor for lung
cancer after SBRT (15).
Additionally, it has been reported that the surfactant protein-D
(SP-D) screening, a marker for interstitial pneumonia, could
prevent the risk of severe radiation pneumonitis (RP) (16). However, the effective prognostic
factors after SBRT have not fully understood.
Therefore, we retrospectively evaluated lung cancer
patients treated with SBRT to identify the prognostic factors
associated with both OS and LC, with an aim to improve prognosis
prediction after SBRT.
Materials and methods
Patient and tumor characteristics
This study was approved by the institutional review
board of Hirosaki University Hospital, Japan, and written informed
consent was obtained from all patients. From March, 2003 to March,
2020, 497 patients (340 males and 157 females; median age, 77
years; range, 41–91) with 408 primary lung cancers and 89 lung
oligo-metastasis fulfilling the study eligibility criteria, and
treated with SBRT were retrospectively reviewed. Patient and tumor
characteristics were summarized in Table SI. In this study, primary lung
cancer and lung oligo-metastasis were categorized under
‘Diagnosis’. The primary sites for oligo-metastasis in patients
were shown in Table I. The tumors
were classified according to tumor-nude-metastasis (TNM)
Classification of Malignant Tumors (7th Edition). The cases before
2010 were reclassified because they were originally classified
according to the previous criteria.
 | Table I.Primary sites for
oligo-metastasis. |
Table I.
Primary sites for
oligo-metastasis.
| Primary sites | n |
|---|
| Head and Neck | 28 |
| Colorectal | 24 |
| Lung | 9 |
| Esophageal | 5 |
| Uterine | 5 |
| Ovarian | 4 |
| Liver | 3 |
| Skin | 2 |
| Prostate | 1 |
| Renal | 1 |
| Breast | 1 |
| Gastric | 1 |
| Malignant fibrous
histiocytoma | 1 |
| Unknown | 4 |
Treatment and scanning procedures
SBRT treatment was performed using procedures
reported previously with 10-MV X-ray beams from a linear
accelerator (EXL-20TP, Mitsubishi Electric Co. Ltd.) until 2011
(14), and thereafter, 6-MV X-ray
beams from a linear accelerator (Clinac iX, Varian Medical Systems)
in three non-coplanar and three coplanar static ports (17). The median isocentric dose was 50 Gy
(range, 45–60), administered in a median of 5 (range, 5–10)
fractions. Patient fixation was performed using a custom-made head
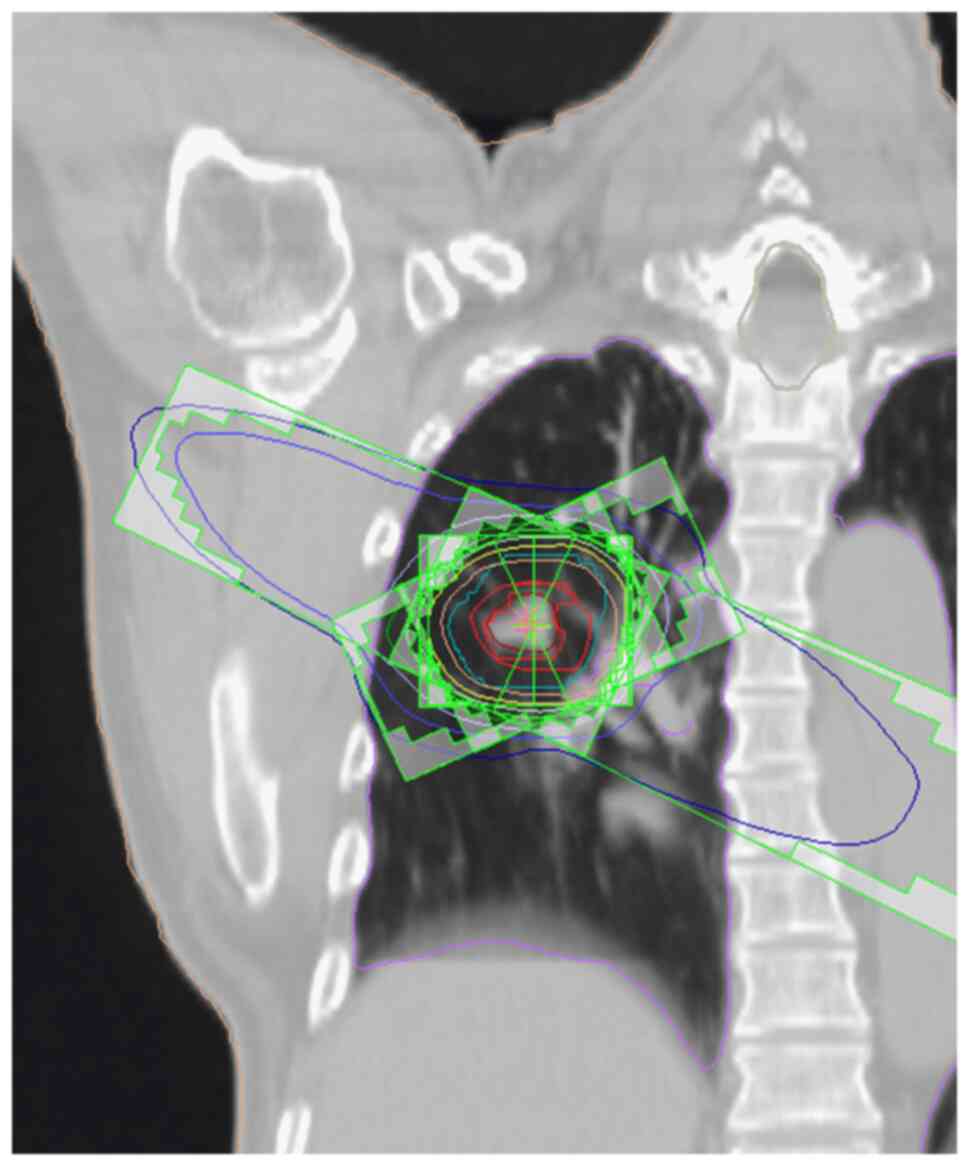
rest and an immobilized system (18). Treatment-planning computed
tomography (CT) was performed using Aquilion (Toshiba Medical
Systems Co. Ltd.) until 2008 and thereafter Optima (GE Healthcare)
with a 1.25-mm thickness. According to our previous study (14), treatment-planning CT was performed
as follows: If respiratory tumor movement was >1 cm, planning CT
was performed through a breath-holding technique using the Abches
system (APEX Medical Inc.), and if it was <1 cm, it was
performed through the 4D-CT technique using a real-time position
management system (Varian Medical Systems). A 3D treatment-planning
system (XiO, version 4.8, ELEKTA) was used for dose calculation
with the following target margins: The clinical target volume (CTV)
was equal to the gross tumor volume (GTV) or internal target volume
(ITV) delineated on CT images displayed at the window level (WL) of
−300 Hounsfield units (HU) and window width (WW) of 1700 HU. The
planning target volume (PTV) was the CTV plus 5–10-mm margin in all
directions, and a 5-mm leaf margin was included around the PTV
(14) (Fig. 1). Dual energy CT (DECT) was
performed using Discovery CT 750 HD (GE Healthcare) with a fast
kilovoltage (kV) switching method for pretreatment evaluation. The
non-ionic low osmolar contrast medium was administrated at 600 mg I
per kg body weight, and iodine content of 300 or 350 mg I/ml. The
total amount of contrast medium was intravenously injected within
30 sec, and the scan was started 25 sec after initiating the
injection. The scanning CT images were transferred to a workstation
(GSI Viewer, GE Healthcare, USA) and were subjected to data
analyses. The slices thickness used for data analysis were 0.63-mm.
The region of interest was set at the maximum cross-sectional
diameter of the tumor in the GSI Viewer, and IDV and water density
values (WDV) were calculated.
Follow-up
Follow-up CT scanning images after SBRT were
obtained at 3–6-month intervals and were used to assess tumor
control and toxicity. Patients were periodically monitored via
medical examinations performed during and after treatment. Local
recurrence was diagnosed based on local tumor enlargement on CT,
which continued for at least 6 months (14). If local recurrence was suspected,
18F-FDG-PET and/or histological confirmation was
recommended, but this was not mandatory.
Statistical analysis
Kaplan-Meier curves were calculated and groups were
statistically compared using a log-rank test; the Holm method was
used to correct for multiple comparisons. If normality was met,
correlations between two continuous variables were performed using
a Pearson correlation, and if not, Spearman non-parametric
statistics were calculated, and confounding factors were
determined. In the multivariate analysis, the factors with a
P-value <0.05 identified via univariate analyses were put in a
stratified Cox proportional hazard regression analysis with the
Akaike information criterion as a stepwise selection. In the
log-rank test and the Cox proportional hazard analysis, continuous
variables with normal values were evaluated using normal values,
while other variables were compared using cut-off values obtained
from the receiver operating characteristic (area under the curve
>0.5). All statistical analyses were performed using EZR version
1.52 (Saitama Medical Center, Jichi Medical University), a
graphical user interface for R (The R Foundation for Statistical
Computing) (19). Statistical
significance was defined as P-value <0.05.
Results
Treatment results
The median follow-up period for all 497 patients was
26.17 months (range, 0.36-194.37). The confounding factors were
assessed by using Spearman's rank correlation coefficient, and a
strong correlation (r>0.7) was observed for each of the
following: Sex-smoking history, sex-brinkman index, WDV-tumor CT
value (TCTV), and the total dose-fraction (Table II).
 | Table II.Correlation coefficient. |
Table II.
Correlation coefficient.
| Correlation
factor | Correlation
coefficient, r | P-value |
|---|
| Sex vs. Smoking
history | 0.746 | <0.01 |
| Sex vs. Brinkman
index | 0.719 | <0.01 |
| Smoking history vs.
Brinkman index | 0.799 | <0.01 |
| WDV
(mg/cm3) vs. TCTV (HU) | 0.915 | <0.01 |
| Total dose (Gy) vs.
Fraction | 0.900 | <0.01 |
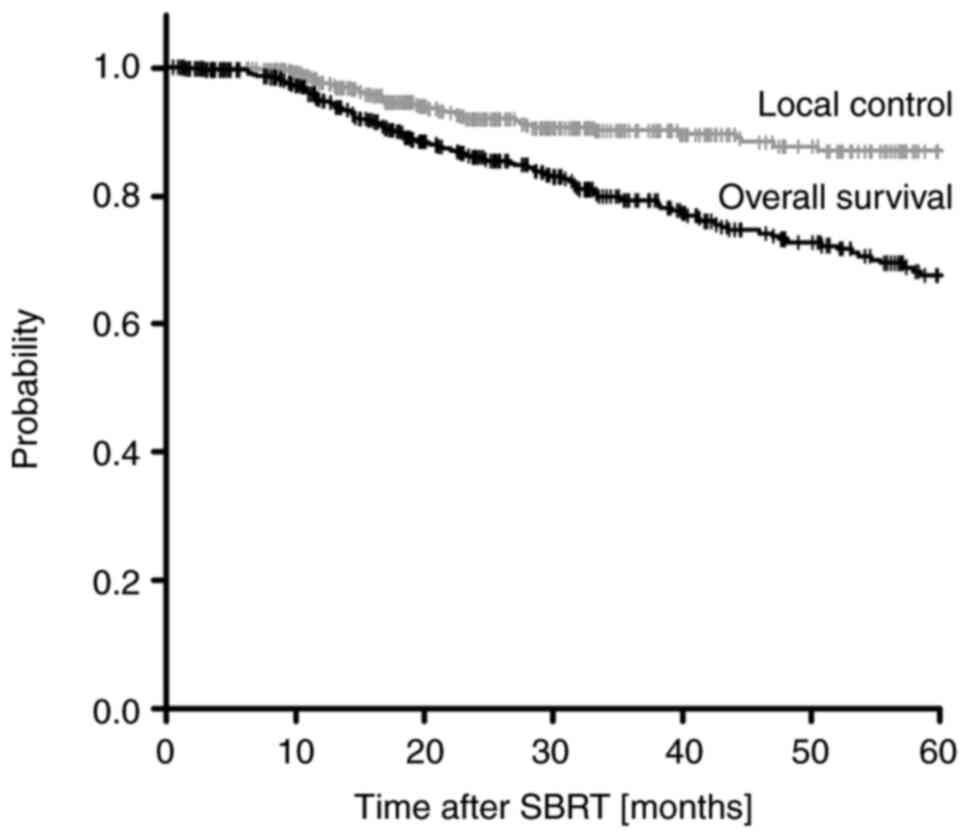
Among the 497 patients followed up, the 1-, 2-, 3-,
4-, and 5-year OS rates were 95.2, 85.3, 77.8, 72.0, and 66.3%,
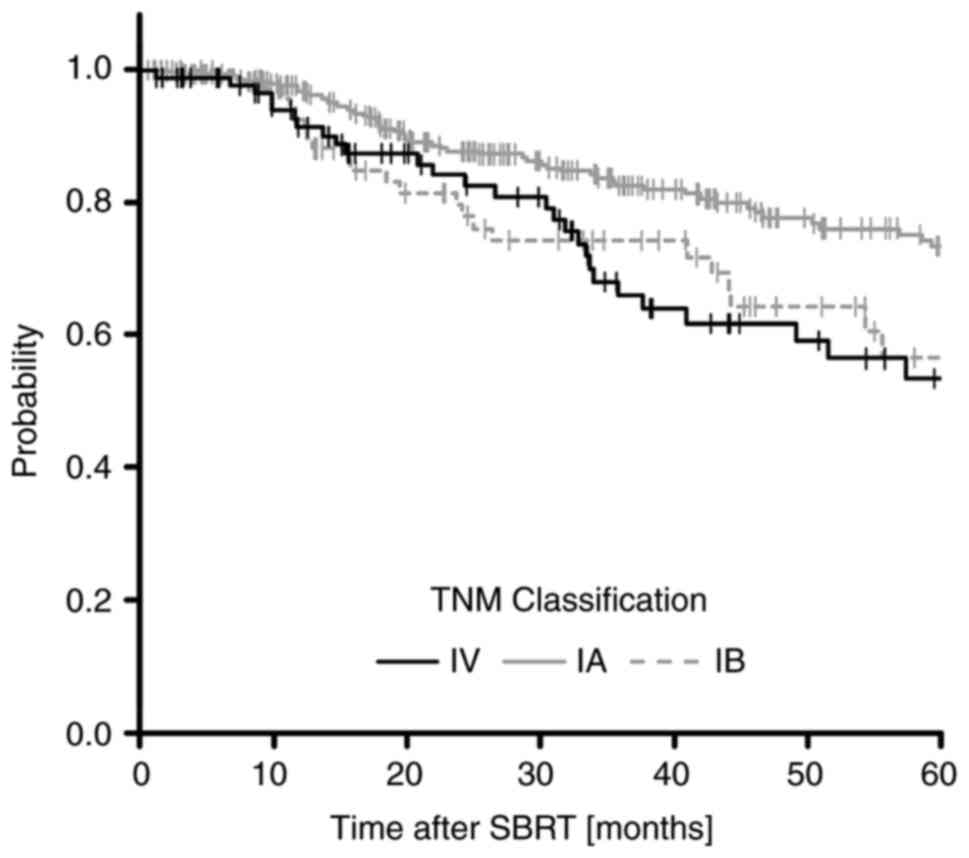
respectively (Fig. 2). The 5-year
OS rates classified by TNM stage were 72.7% (95% confidence
interval (CI): 65.3-78.8%) for stage IA, 55.6% (95% CI: 38.9-69.4%)
for stage IB, and 52.3% (95% CI: 37.3-65.3%) for stage IV. There
were statistically significant differences between stage IA and IB
(P=0.042), and IA and IV (P=0.031) (Fig. 3). In this study, primary lung
cancers were classified as those of stages IA and IB, and
metastatic lung cancers were classified as those of stage IV; no
cases involved cancers of stage II and III. The 1-, 2-, 3-, 4-, and
5-year LC rates were 98.3, 92.1, 89.4, 87.4, and 86.0%,
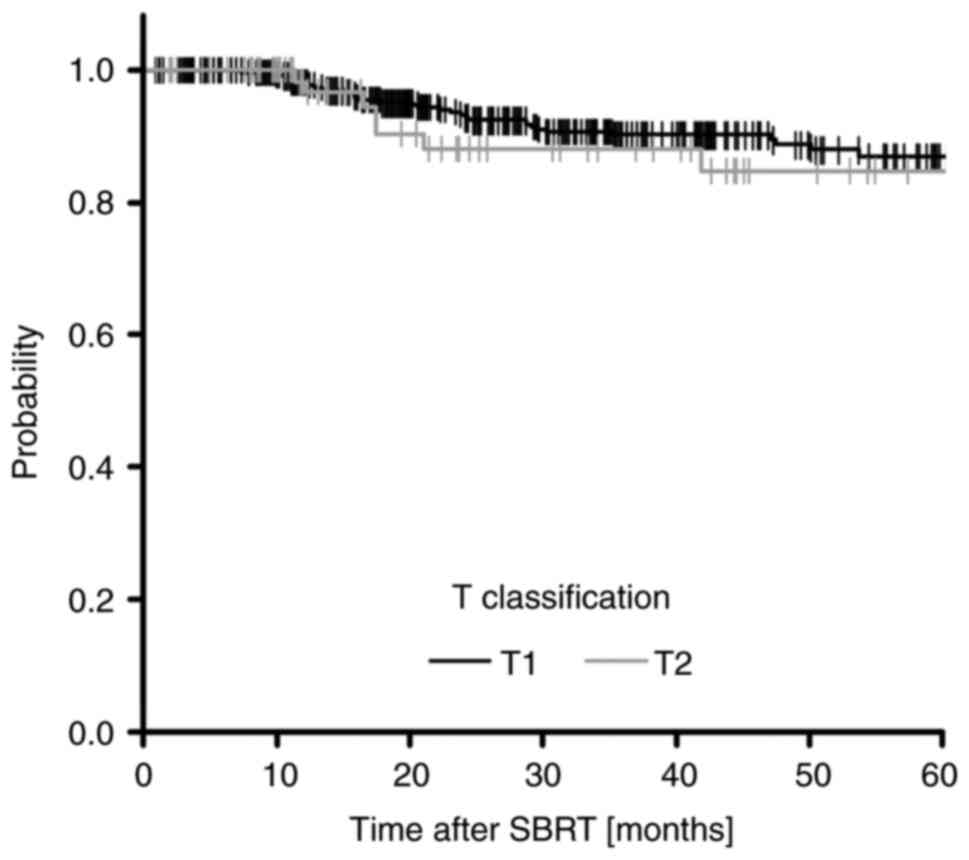
respectively (Fig. 2). The 5-year
LC rates, according to the tumor states, were 86.4% (95% CI:
80.9-90.4%) for T1 and 83.8% (95% CI: 60.8-92.2%) for T2 (Fig. 4).
Evaluation of prognostic factors
We performed the univariate analysis to determine
the association between factors shown in Table SI and OS and LC. There were
statistically significant differences in maximum SUV
(SUV)max, TCTV, IDV, WDV, histology
(adenocarcinoma/squamous cell carcinoma) and respiratory functions
in both OS and LC (Tables SII and
SIII).
In addition to these factors, univariate analysis
identified further factors which showed statistically significant
differences in OS (Table SIV) and
LC (Table III),
respectively.
 | Table III.Five-year LC univariate analysis. |
Table III.
Five-year LC univariate analysis.
| Patient
characteristic | n | 5-yer LC, % | 95% CI | P-value |
|---|
| Histology |
|
|
| 0.015 |
| AD | 202 | 89.8 | 82.4-94.1 |
|
|
SCC | 86 | 73.1 | 55.5-84.6 |
|
| Respiratory
function |
|
|
| 0.03 |
|
Normal | 232 | 91.7 | 86.3-95.0 |
|
|
Abnormal | 242 | 80.4 | 70.8-87.2 |
|
| TCTV, HU |
|
|
| 0.014 |
|
<22.59 | 148 | 92.3 | 84.1-96.3 |
|
|
≥22.59 | 110 | 75.7 | 60.6-85.6 |
|
| IDV,
mg/cm3 |
|
|
| <0.001 |
|
>17.84 | 134 | 92.7 | 81.6-97.2 |
|
|
≤17.84 | 124 | 74.2 | 59.6-84.1 |
|
| WDV,
mg/cm3 |
|
|
| 0.002 |
|
<984.66 | 159 | 93.1 | 85.7-967 |
|
|
≥984.66 | 99 | 72.4 | 56.2-83.4 |
|
| Tumor size, mm |
|
|
| 0.026 |
|
<27 | 364 | 87.8 | 81.8-92.0 |
|
|
≥27 | 133 | 80.6 | 69.3-88.1 |
|
|
SUVmax |
|
|
| <0.001 |
|
<5.2 | 180 | 89.8 | 80.0-95.0 |
|
|
≥5.2 | 105 | 71.6 | 48.2-85.8 |
|
| FEV1.0,
% |
|
|
| 0.023 |
|
>70 | 277 | 90.4 | 84.6-94.1 |
|
|
≤70 | 197 | 80.6 | 70.3-87.7 |
|
Since univariate analysis cannot make it clear
whether the factors shown significantly differences are independent
factors, we further performed the multivariate analysis with these
factors to determine the impact against prognosis. Among the
various factors showed statistically significant differences in the
univariate analysis, SP-D, TCTV, and IDV were selected as factors
for OS (Table IV), and histology
(adenocarcinoma/squamous cell carcinoma), TCTV, and IDV for LC
(Table V).
 | Table IV.Five-year OS multivariate
analysis. |
Table IV.
Five-year OS multivariate
analysis.
| Patient
characteristic | n | 5-year OS, % | HR | 95% CI | P-value |
|---|
| TCTV (HU) |
|
|
|
|
|
|
<34.0 | 173 | 68.7 | 3.381 | 1.7550-6.511 | <0.001 |
|
≥34.0 | 85 | 57.4 |
|
|
|
| IDV
(mg/cm3) |
|
|
|
|
|
|
>14.94 | 169 | 72.7 | 2.58 | 1.334-4.987 | 0.004835 |
|
≤14.94 | 89 | 50.1 |
|
|
|
| SP-D (ng/ml) |
|
|
|
|
|
|
≤109 | 341 | 69.9 | 3.603 | 1.7090-7.594 | <0.001 |
|
>109 | 87 | 48.0 |
|
|
|
 | Table V.Five-year LC multivariate
analysis. |
Table V.
Five-year LC multivariate
analysis.
| Patient
characteristic | n | 5-year LC (%) | HR | 95% CI | P-value |
|---|
| IDV
(mg/cm3) |
|
|
|
|
|
|
>17.84 | 134 | 92.7 | 8.317 | 2.406-28.75 | <0.001 |
|
≤17.84 | 124 | 85.3 |
|
|
|
| TCTV (HU) |
|
|
|
|
|
|
<22.59 | 148 | 92.3 | 2.861 | 1.067-7.669 | 0.03672 |
|
≥22.59 | 110 | 75.7 |
|
|
|
| Histology |
|
|
|
|
|
| AD | 202 | 89.8 | 1.998 | 1.148-3.478 | 0.01433 |
|
SCC | 86 | 73.1 |
|
|
|
|
Unknown | 209 | 86.9 |
|
|
|
Individual treatments took approximately 30 min,
regardless of the fractionation schedule. RP was identified in more
than half of the patients in this study. Grade 1, 2, and 3 RP had
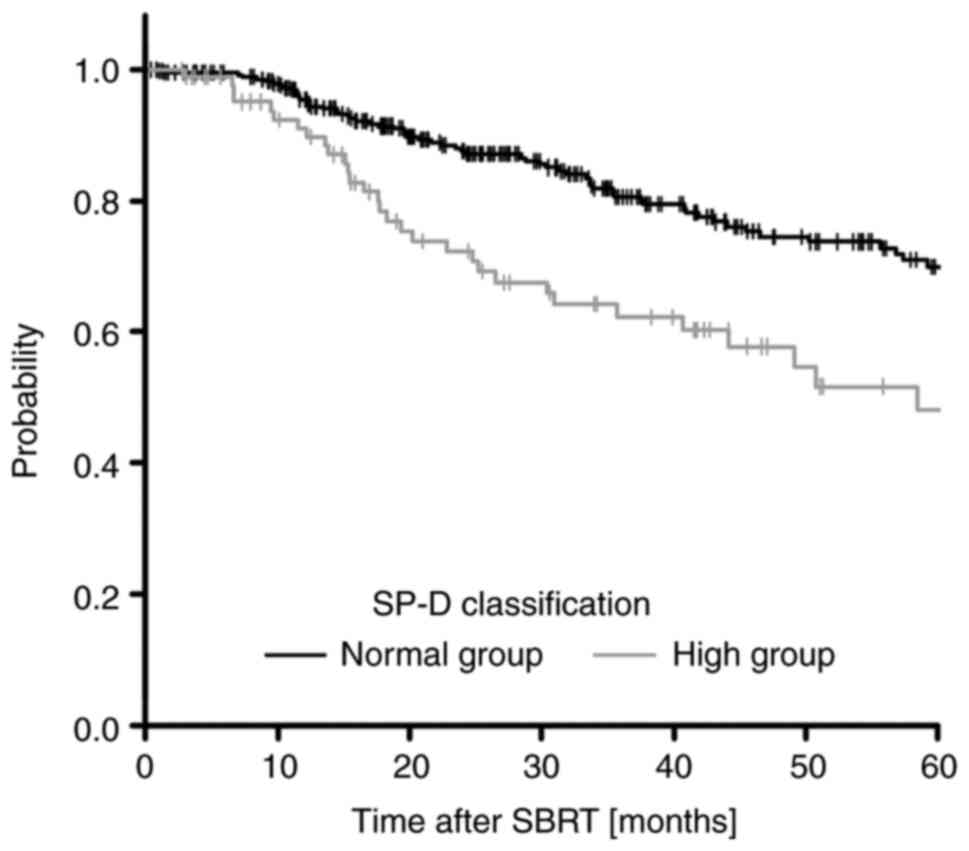
373, 14, and 1 patients, respectively (Table SI). SP-D, which has been reported
as a pneumonia marker (20), was
selected for OS in the multivariate analysis, and the 5-year OS
rates in the SP-D normal group and high group were 69.9 and 48.0%,
respectively (Fig. 5). Thus, we
assessed the relation with the SP-D value and the RP grade. In the
SP-D normal group, the percentage of RP G2 or a higher RP grade was
2.93%, while in the SP-D high group, it was 4.65% (Table VI). There were no statistically
significant differences between these groups. These results
suggested that there are other factors associated with SP-D rather
than RP.
 | Table VI.Radiation pneumonia grade at
SP-D. |
Table VI.
Radiation pneumonia grade at
SP-D.
|
| SP-D normal group
(<109 ng/ml, n=341) | SP-D high group
(>109 ng/ml, n=86) |
|---|
|
|
|
|
|---|
| Grade | n | % | n | % |
|---|
| G0 | 53 | 15.54 | 18 | 20.93 |
| G1 | 258 | 75.66 | 59 | 68.60 |
| G2 | 10 | 2.93 | 3 | 3.49 |
| G3 | 0 | 0.00 | 1 | 1.16 |
| Unknown | 20 | 5.87 | 5 | 5.81 |
Discussion
The development of stereotactic irradiation
techniques has made it possible to focus high-doses radiation on
tumors without increasing the side effects. Moreover, this approach
can significantly reduce the treatment schedule compared to the
conventional methods. In Japan, SBRT is performed for the treatment
of early-stage lung cancer, and recently, it has also been
performed for inoperable and operable patients (2). In this study, the 5-year OS rates was
66.3% (Fig. 2), and the 5-year OS
rates according to stage IA, IB, and IV were 72.7, 55.6, and 52.1%,
respectively (Fig. 3). In
addition, it was suggested that the 5-year LC rates was 86.0%
(Fig. 2), and the 5-year LC rates
according to T1 and T2 were 86.4 and 83.8%, respectively (Fig. 4). It was reported that the
representative 5-year OS rates for surgery against clinical stage
IA and IB NSCLC were approximately 60–75% (IA) and 40–60% (IB),
respectively, and the clinical outcomes of patients with
early-stage NSCLC treated with SBRT were as good as the outcomes of
surgery (3,21). The results of this study supported
these reports. Furthermore, we categorized the cause of death for
patients who died within 5 years after SBRT as a result of lung
cancer or other diseases. The percentages of patients who died from
lung cancer vs. other diseases were 6.6 and 93.4%, respectively,
indicating that the patients who died from lung cancer was small.
Therefore, our results demonstrated that the 5-year OS (66.3%) and
LC (86.0%) rates after SBRT treatment were superior and the
prognosis was favorable.
In the multivariate analysis, SP-D and IDV showed
the highest hazard ratios (HR) for OS and LC, respectively
(Tables IV and V), suggesting that they influence the
prognosis after SBRT. Our results suggested that the 5-year OS
rates in the SP-D normal group and high group were 69.9 and 48.0%,
respectively, and the high group showed a poor prognosis compared
to the 5-year OS rates for all patients (66.3%) (Table IV; Fig. 5). Chong et al (22) reported that high expression of SP-D
in NSCLC correlates with poor prognosis, which was consistent with
our result. SP-D has been known as an effective diagnostic
biomarker for RP (16,20,23–25).
Yamazaki et al (26)
indicated the relationship between SP-D levels in serum and RP.
However, our results demonstrated that 96% of the patients with RP
after SBRT showed G1 and below. Furthermore, 4.65% of the patients
in the SP-D high group, and 2.93% in the normal group showed G2 or
higher. There was no statistically significant difference in these
groups. Therefore, it was suggested that other factors besides RP
may contribute to poor prognosis. Regarding histology, the patients
with squamous cell carcinoma showed high SP-D rates compared to the
patients with adenocarcinoma (35.3% vs. 16.8%, data not shown). The
proportion of deaths in the high SP-D group were 6.4 and 20.6% for
adenocarcinoma and squamous cell carcinoma, respectively. These
results indicated that the cancer histology type, especially
squamous cell carcinoma, was related to the poor prognosis in the
SP-D high group. Thus, combining histology with SP-D may improve
the accuracy of prognostic prediction although histology was not
selected as an OS-related factor in multivariate analysis. However,
since recent reports indicate that the SP-D low group was
correlated with the poor prognosis of patients with lung cancer
(27), further studies are
necessary to use them as prognostic factors.
Our previous study demonstrated that IDV is related
to LC after SBRT (7). This study
identified that TCTV is a new factor related to both OS and LC
after SBRT. Aoki et al (14) reported that the reduction of IDV as
an index of blood flow may reflect the hypoxic cell population
cause of radioresistant in the tumor. In contrast, WDV in the tumor
is presumed to reflect cell density and cell necrosis. Our previous
study suggested that the reduction of the WDV has a positive effect
on the OS after radiotherapy (7).
Although the correlation between WDV and IDV was not confirmed, WDV
tended to decrease with increasing IDV. Thus, the combination of
decreasing IDV and increasing WDV may indicate a poor prognostic
index. However, there is a limitation to its use as a prognostic
factor because WDV details are not fully understood. Our results
indicated a positive correlation between WDV and TCTV, suggesting
that using it as an alternative index to WDV and combining it with
IDV may improve the accuracy of prognostic prediction. Further
studies on TCTV and WDV will improve the validity of these
factors.
In conclusion, the prognosis of patients treated
with SBRT was favorable, and we identified SP-D, TCTV, and IDV as
prognostic factors for OS. Although further studies on these
candidate prognostic factors are necessary, our results indicated
that they might contribute toward improving the accuracy of
prognostic prediction for patients with lung cancer after SBRT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Grant-in-Aid for Scientific
Research (KAKENHI) (grant no. 17K10466). The funders had no role in
the study design, data collection and analysis, decision to
publish, or in the preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HS, FI, YH, MT and MA conceived and designed the
study. HS, FI, KH and RS participated in statistical analysis and
data interpretation, drafted the article, and produced figures and
tables. RS, YH, MT and MA critically reviewed the article. MT and
MA performed the patient treatment, provided patient data and
provided valuable insights from their fields. YH, MT and MA confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Hirosaki University Hospital, Hirosaki, Japan (approval no.
2018-1162). All participants provided oral and written consent for
the collection of data and participation in the study, and signed
an informed consent form.
Patient consent for publication
Patients provided oral and written consent for the
publication of data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagata Y and Kimura T: Stereotactic body
radiotherapy (SBRT) for stage I lung cancer. Jpn J Clin Oncol.
48:405–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Onishi H, Shirato H, Nagata Y, Hiraoka M,
Fujimoto M, Gomi K, Harsawa K, Hayakawa K, Niibe Y, Takai Y, et al:
Stereotactic body radiotherapy (SBRT) for operable stage I
non-small-cell lung cancer: Can SBRT be comparable to surgery? Int
J Radiat Oncol Biol Phys. 81:1352–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palma D, Visser O, Lagerwaard FJ,
Belderbos J, Slotman B and Senan S: Treatment of stage I NSCLC in
elderly patients: A population-based matched-pair comparison of
stereotactic radiotherapy versus surgery. Radiother Oncol.
101:240–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bei Y, Murakami N, Nakayama Y, Okuma K,
Kashihara T, Raturi VP, Okamoto H, Takahashi K, Inaba K, Igaki H
and Itami J: Stereotactic body radiation therapy for early-stage
non-small-cell lung cancer in octogenarians and older: An
alternative treatment. J Radiat Res. 61:586–593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dupic G, Biau J, Molnar I, Chassin V,
Dediue V, Lapeyre M and Bellière-Calandry A: Significant
correlation between overall survival and mean lung dose in lung
stereotactic body radiation therapy (SBRT). Front Oncol.
10:15772020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto T, Jingu K, Shirata Y, Koto M,
Matsushita H, Sugawara T, Kubozono M, Umezawa R, Abe K, Kadoya N,
et al: Outcomes after stereotactic body radiotherapy for lung
tumors, with emphasis on comparison of primary lung cancer and
metastatic lung tumors. BMC Cancer. 14:4642014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoki M, Hatayama Y, Kawaguchi H, Ichise K,
Hirose K and Takai Y: Measurements of substance densities of
non-small cell lung cancer using dual energy computed tomography
are useful for prediction of local control and overall survival
after stereotactic body radiation therapy. Astro. 102:e6712018.
|
|
8
|
Grills IS, Hope AJ, Guckenberger M, Kestin
LL, Werner-Wasik M, Yan D, Sonke JJ, Bissonnette JP, Wilbert J,
Xiao Y and Belderbos J: A collaborative analysis of stereotactic
lung radiotherapy outcomes for early-stage non-small-cell lung
cancer using daily online cone-beam computed tomography
image-guided radiotherapy. J Thorac Oncol. 7:1382–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang JY, Liu H, Balter P, Komaki R, Liao
Z, Welsh J, Mehran RJ, Roth JA and Swisher SG: Clinical outcome and
predictors of survival and pneumonitis after stereotactic ablative
radiotherapy for stage I non-small cell lung cancer. Radiat Oncol.
7:1522012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeda A, Yokosuka N, Ohashi T, Kunieda E,
Fujii H, Aoki Y, Sanuki N, Koike N and Ozawa Y: The maximum
standardized uptake value (SUVmax) on FDG-PET is a strong predictor
of local recurrence for localized non-small-cell lung cancer after
stereotactic body radiotherapy (SBRT). Radiother Oncol.
101:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamamoto Y, Kataoka M, Yamashita M, Nogami
N, Sugawara Y, Kozuki T, Sawada S, Suehisa H, Shinohara S, Nakajim
N and Shinkai T: Factors affecting the local control of
stereotactic body radiotherapy for lung tumors including primary
lung cancer and metastatic lung tumors. Jpn J Radiol. 30:430–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunlap NE, Larner JM, Read PW, Kozower BD,
Lau CL, Sheng K and Jones DR: Size matters: A comparison of T1 and
T2 peripheral non-small-cell lung cancers treated with stereotactic
body radiation therapy (SBRT). J Thorac Cardiovasc Surg.
140:583–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bral S, Gevaert T, Linthout N, Versmessen
H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De
Ridder M and Storme G: Prospective, risk-adapted strategy of
stereotactic body radiotherapy for early-stage non-small-cell lung
cancer: Results of a Phase II trial. Int J Radiat Oncol Biol Phys.
80:1343–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoki M, Hirose K, Sato M, Akimoto H,
Kawaguchi H, Hatayama Y, Fujioka I, Tanaka M, Ono S and Takai Y:
Prognostic impact of average iodine density assessed by dual-energy
spectral imaging for predicting lung tumor recurrence after
stereotactic body radiotherapy. J Radiat Res. 57:381–386. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka M, Ichise K, Fujioka I, Sato M,
Hirose K, Kawaguchi H, Hatayama Y, Takai Y, Tsushima E and Aoki M:
Impact of low iodine density tumor area ratio on the local control
of non-small cell lung cancer through stereotactic body
radiotherapy. J Radiat Res. 62:448–456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamashita H, Kobayashi-Shibata S, Terahara
A, Okuma K, Haga A, Wakui R, Ohtomo K and Nakagawa K: Prescreening
based on the presence of CT-scan abnormalities and biomarkers (KL-6
and SP-D) may reduce severe radiation pneumonitis after
stereotactic radiotherapy. Radiat Oncol. 5:322010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aoki M, Hatayama Y, Kawaguchi H, Hirose K,
Sato M, Akimoto H, Miura H, Ono S and Takai Y: Stereotactic body
radiotherapy for lung metastases as oligo-recurrence: A single
institutional study. J Radiat Res. 57:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aoki M, Abe Y, Kondo H, Hatayama Y,
Kawaguchi H, Fujimori A, Suzaki K, Seino M, Morita T, Souma M, et
al: Clinical outcome of stereotactic body radiotherapy of 54 Gy in
nine fractions for patients with localized lung tumor using a
custom-made immobilization system. J Radiat Res. 25:289–294.
2007.PubMed/NCBI
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasaki R, Soejima T, Matsumoto A, Maruta
T, Yamada K, Ota Y, Kawabe T, Nishimura H, Sakai E, Ejima Y and
Sugimura K: Clinical significance of serum pulmonary surfactant
proteins A and D for the early detection of RP. Int J Radiat Oncol
Biol Phys. 50:301–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyazaki T, Yamazaki T, Nakamura D, Sato
S, Yamasaki N, Tsuchiya T, Matsumoto K, Kamohara R, Hatachi G and
Nagayasu T: Surgery or stereotactic body radiotherapy for elderly
stage I lung cancer? A propensity score matching analysis. Surg
Today. 47:1476–1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong IW, Chang MY, Chang HC, Yu YP, Shue
CC, Tsai JR, Hung JY, Chou SH, Tsai MS, Hwang JJ and Lin SR: Great
potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1
markers for diagnosis of patients with non-small cell lung cancer.
Oncol Rep. 16:981–988. 2006.PubMed/NCBI
|
|
23
|
Yamashita H, Takahashi W, Haga A and
Nakagawa K: Radiation pneumonitis after stereotactic radiation
therapy for lung cancer. World J Radiol. 6:708–715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuno Y, Satoh H, Ishikawa H, Kodama T,
Ohtsuka M and Sekizawa K: Simultaneous measurements of KL-6 and
SP-D in patients undergoing thoracic radiotherapy. Med Oncol.
23:75–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi H, Imai Y, Fujishima T,
Shiratori M, Murakami S, Chiba H, Kon H, Kuroki Y and Abe S:
Diagnostic significance of surfactant proteins A and D in sera from
patients with radiation pneumonitis. Eur Respir J. 17:481–487.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamazaki H, Aibe N, Nakamura S, Sasaki N,
Suzuki G, Yoshida K, Yamada K, Koizumi M, Arimoto T, Iwasaki Y, et
al: Measurement of exhaled nitric oxide and serum surfactant
protein D levels for monitoring radiation pneumonitis following
thoracic radiotherapy. Oncol Lett. 14:4190–4196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Umeda Y, Hasegawa Y, Otsuka M, Ariki S,
Takamiya R, Saito A, Uehara Y, Saijo H, Kuronuma K, Chiba H, et al:
Surfactant protein D inhibits activation of non-small cell lung
cancer-associated mutant EGFR and affects clinical outcomes of
patients. Oncogene. 36:6432–6445. 2017. View Article : Google Scholar : PubMed/NCBI
|