Introduction
Epithelial ovarian cancer (EOC) is a highly lethal
gynecologic malignancy (1). Cancer
cells often produce more endogenous reactive oxygen species (ROS)
due to increased cell growth and metabolic demands for oxygen and
nutrients (2). They are also
persistently exposed to exogenous oxidative stress conditions
(2). To survive oxidative stress
and adapt to ROS exposure, cancer cells have evolved various
defense mechanisms, including antioxidant enzymes, DNA-repair
enzymes, and endoplasmic reticulum stress response (3). A balanced antioxidant system
neutralizes excess endogenous and exogenous ROS through a defense
system that consists of enzymatic and non-enzymatic antioxidants
(2,4). However, increased defense against ROS
is a leading cause of treatment resistance and poor prognosis
(5). Furthermore, cancer stem
cells contain lower intracellular ROS levels due to enhanced ROS
defense than non-cancer stem cells (5). Cancer stem cells represent a small
subtype of tumor cells with unlimited self-renewal,
differentiation, and tumorigenesis capacity. They are a determining
factor contributing to tumor metastasis, recurrence, therapeutic
resistance, and poor prognosis (6). Additionally, the potential reason for
treatment failure is primarily attributed to cancer stem cells
(6). CD44v9 (a variant isoform of
CD44 and a cell surface marker of cancer stem cells) (7) and nuclear factor erythroid 2-related
factor 2 (Nrf2) genes (8,9) are major regulators of ROS defense in
ovarian cancer. Cancer cells can escape oxidative injuries by
producing high levels of intracellular antioxidants via a unique
protection system, such as CD44v9 and Nrf2 genes.
This review focuses on the molecular mechanisms of
redox homeostasis in human cancers and discusses targeted therapies
for ovarian cancer based on redox modifications.
Search strategy and selection criteria
A computerized literature search was performed to
identify relevant studies reported in English. PubMed electronic
databases published between January 1998 and October 2021 were
searched, combining the following keywords: Nrf2, CD44v9,
antioxidant, cancer, ovarian cancer, treatment, inhibitor, and
redox. References of each article were searched to identify
potentially relevant studies. In addition, publications of original
studies and review papers were included. Given the heterogeneity in
the research theme, data from the studies were synthesized using a
descriptive review design with narrative methods. Fig. 1 shows that the first identification
phase includes records identified through a database search. Terms
in the titles and abstracts were focused on in the first
screening stage. However, duplicates were removed during the
second screening phase, and titles, abstracts, and full-text
articles were read to remove inappropriate papers. The final
eligibility phase included the full-text articles for analysis
after excluding those for which detailed data cannot be
extracted.
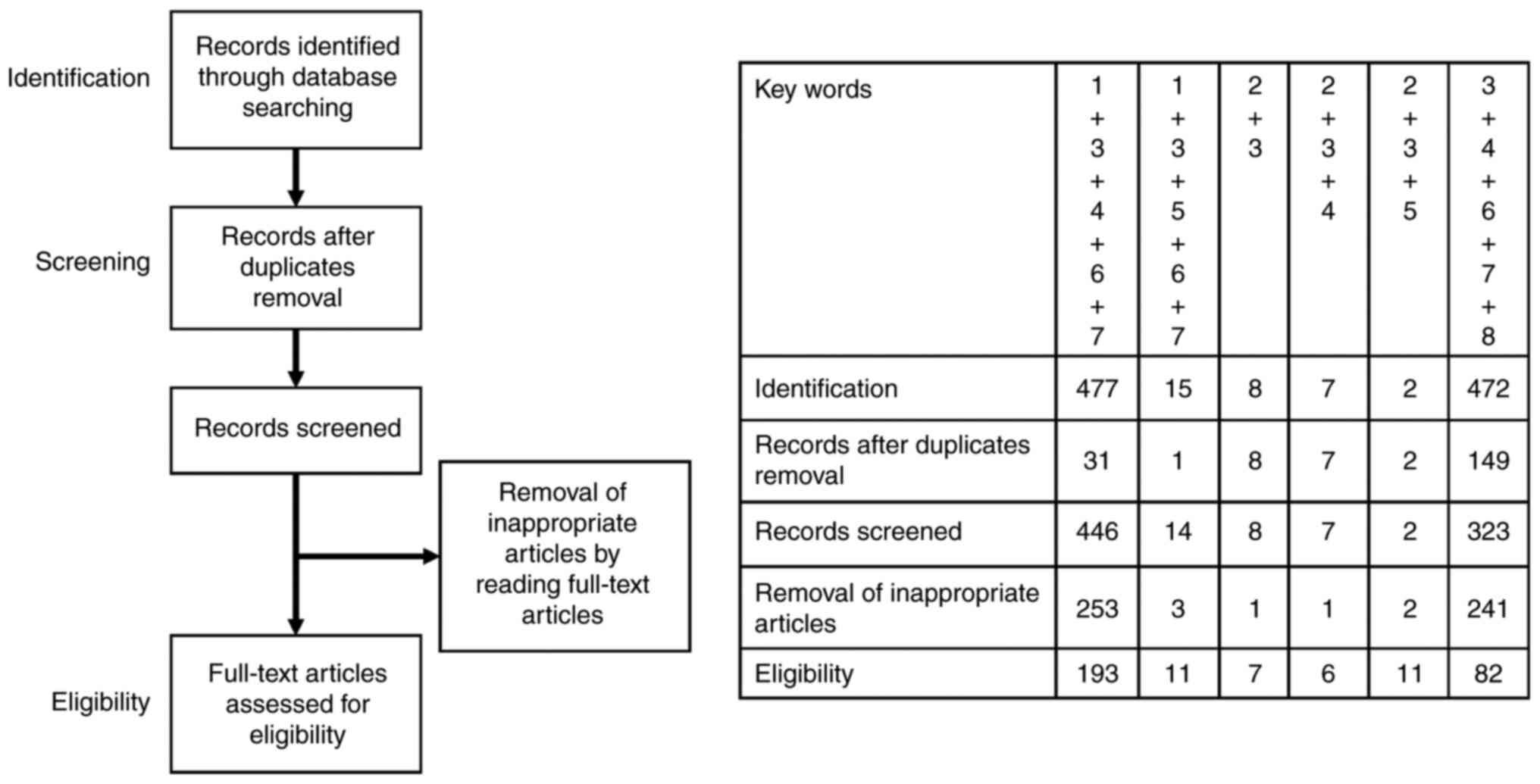 | Figure 1.Number of articles identified by
searching for keyword combinations. This figure shows the number of
articles identified by key word combinations and the number of
records identified through database search, records after duplicate
removal, records screened, removal of inappropriate articles by
reading full-text articles and full-text articles assessed for
eligibility. Key words: 1, nuclear factor erythroid 2-related
factor 2; 2, CD44 variant isoform 9; 3, antioxidant; 4, cancer; 5,
ovarian cancer; 6, treatment; 7, inhibitor; and 8, redox. |
Unique redox homeostasis in cancer
Cancer cells utilize oxygen for adenosine
triphosphate (ATP) production through metabolic reprogramming,
supplying them with energy and fueling their proliferation. ROS,
such as superoxide anion and hydroxyl radicals, are generated
during ATP production through oxidative phosphorylation (OXPHOS) in
mitochondria (10). ROS are
produced by mitochondria, endoplasmic reticulum, and peroxisome;
thus, cancer cells specifically accumulate high ROS levels.
Furthermore, ROS cause oxidative damage to protein, lipid, and DNA
and induces genomic instability, promoting tumor initiation and
malignant progression (10). Also,
ROS concentration with extremely high levels can induce cancer cell
death, making it a promising cancer treatment (2). Therefore, ROS have a positive and
negative impact on cancer evolution, leading to cancer progression
(ROS levels below the threshold) or cell death (ROS levels beyond
the threshold) (11). Therefore,
modulating the unique redox homeostasis may be a promising strategy
to eliminate cancer cells.
Cells encode pivotal defense systems to protect
themselves against oxidative stress (3,10).
Molecular targets as antioxidant defense systems are the
mitochondrial electron transport chain and the OXPHOS system, the
endoplasmic reticulum system, peroxisomal proteins, and the
redox-sensitive signaling pathways (e.g., Nrf2, glutathione, and
thioredoxin). Since the availability of the antioxidant system
determines ROS concentration, this system contributes to
conflicting biological activities, such as cell fate, i.e.,
survival or death. A review targeting redox imbalance for cancer
treatment was published by Narayanan et al (12). Furthermore, researchers have
developed novel therapies targeting oxidative vulnerabilities in
various cancers. For example, gene silencing or pharmacological
inhibition of ROS-scavenging, upregulation of ROS-generating
enzymes, or pro-oxidant therapy can induce excessive oxidative
stress, leading to cell death (12). A redox shift from an antioxidant
condition toward a pro-oxidant state can inhibit tumor development
and progression, improving treatment resistance. The redox balance
is a critical molecular switch that controls cancer stimulation and
suppression (2). Additionally,
targeted therapy turns off this antioxidant switch to convert a
mediator of tumor progression into an accelerator of cell
death.
Therefore, this section discusses recent advances in
cancer treatment strategies that control redox balance. We mainly
summarize the following antioxidant defense systems: 1) redox
cofactors [e.g., nicotinamide adenine dinucleotide phosphate
(NADPH)], 2) antioxidant transcription factors (e.g., Nrf2 and
CD44v9), and 3) detoxifying enzymes and molecular scavengers [e.g.,
glutathione-S-transferase (GST), superoxide dismutase (SOD), and
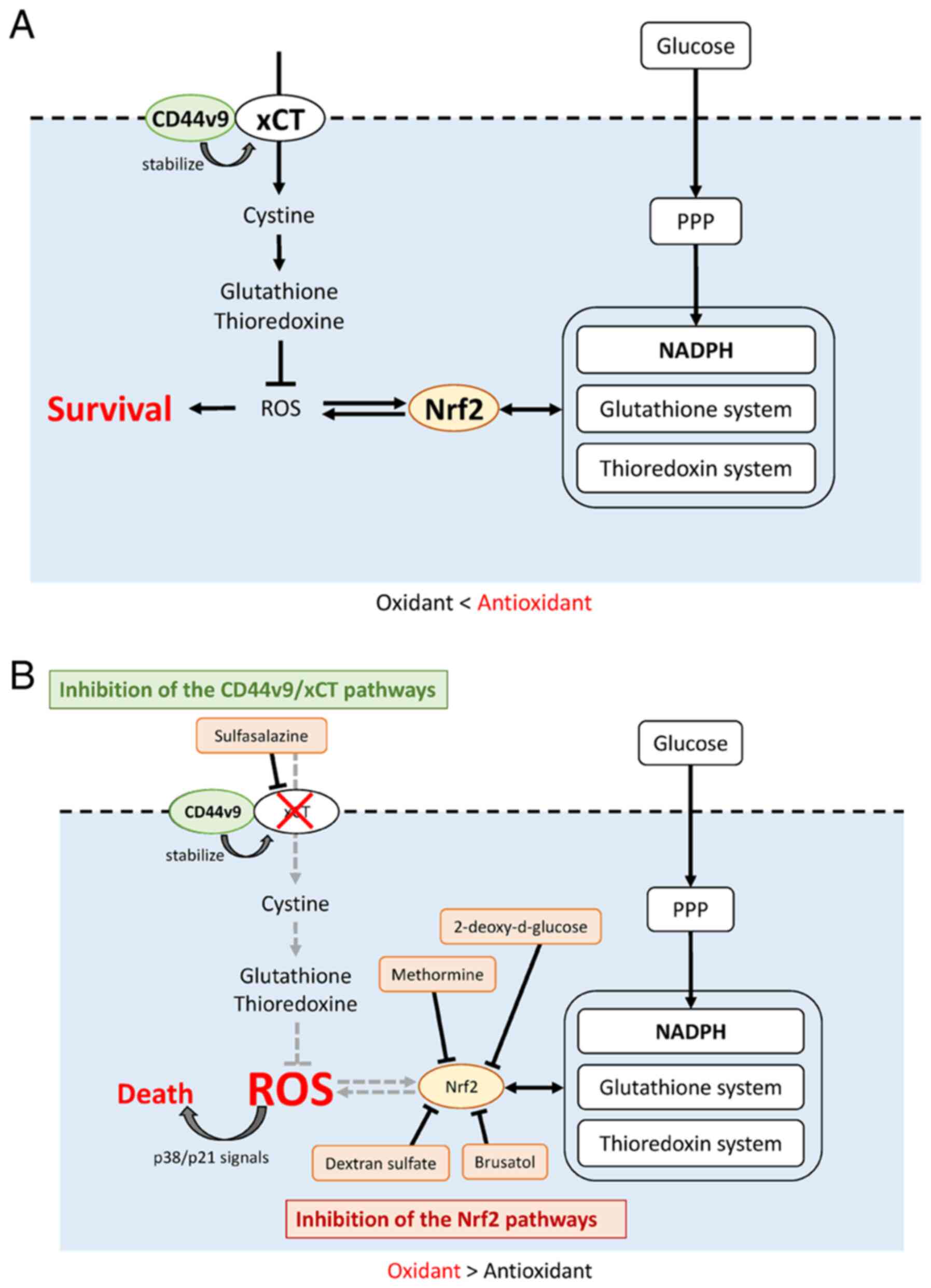
glutathione] (Fig. 2A).
NADPH
Cancer cells mainly rely on aerobic glycolysis
rather than OXPHOS, generating NADPH by activating the pentose
phosphate pathway (PPP), adenosine monophosphate-activated protein
kinase, and reductive glutamine and folate metabolism to prevent a
rapid ROS generation (11,13,14).
NADPH is a high-energy essential electron donor for antioxidants,
such as glutathione and thioredoxin, playing a role in protecting
against redox stress (14)
(Fig. 2A). Furthermore, NADP is
recycled to NADPH by three main enzymes: malic enzyme 1 (ME1),
isocitrate dehydrogenase 1 (IDH1), and oxidative PPP
[glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate
dehydrogenase (PGD)] (14,15). These enzymes are ubiquitous in all
mammals. The first two enzymes are tricarboxylic acid
cycle-associated. ME1 is a multifunctional enzyme that
decarboxylates malate to form pyruvate (16). This enzyme is also essential for
NADPH production, glutamine metabolism, and lactate fermentation
(16). IDH1 is a key metabolic
enzyme involved in the oxidative decarboxylation of isocitrate to
α-ketoglutarate (α-KG), ultimately producing NADPH under cellular
stress (17). The PPP is a major
source of NADPH and plays a critical role in protecting cells from
ROS (14). However, cancer cells
actively produce NADPH for antioxidant defense, promoting tumor
progression and survival in many cancer types (18). Defective NADPH production can cause
cancer cell death (18).
Intracellular NADPH levels are affected by nicotinamide
phosphoribosyltransferase (NAMPT), an enzyme in the NAD salvage
synthesis pathway. Also, NAMPT is overexpressed in various cancers,
such as ovarian, colorectal, breast, prostate, gastric cancer,
osteosarcoma, melanoma, and myeloma (19). Additionally, this enzyme is
involved in cancer cell metabolism, survival, angiogenesis, and
chemoresistance (19). A
preclinical study demonstrated that suppressing NADPH production by
targeting NAMPT enhanced cisplatin-treated cell death in ovarian
cancer (19). NAMPT inhibitors,
such as FK866, have become promising targets for platinum-resistant
ovarian cancer (19). Therefore,
interfering with NADPH production and modulating unique NADPH
homeostasis may be an effective strategy for treating cancer.
Preclinical studies targeting NADHP in cancer have been reviewed in
reference 19.
Transcription factor Nrf2
Nrf2 plays a central role in cellular defense
against oxidative insults, tightly regulating the activation of
specific downstream targets, including glutathione and thioredoxin
systems, detoxification system, NADPH regeneration, and heme and
iron metabolism (20). The Nrf2
pathway is often activated in various types of cancer. Under
unstressed conditions, trapping Nrf2 by Kelch-like ECH-associated
protein 1 (Keap1), degrading Nrf2 by ubiquitination (21). Oxidative stress disrupts Keap1 and
Nrf2 binding, leading to constitutive activation of the Nrf2
transcription factor (22).
Various antioxidant genes, e.g., glutathione and thioredoxin
systems and NADPH production are activated by the Nrf2 gene
(2,23,24)
(Fig. 2A). In addition, cancer
cells promote metabolic reprogramming from mitochondrial OXPHOS to
aerobic glycolysis, thus, fueling the PPP (7,21).
Nrf2 triggers G6PD activation, contributing to the activation of
the PPP, NADPH synthesis, and metabolic reprogramming of cancer
cells (25). The primary function
of Nrf2 is stabilizing intracellular redox potential against
physical and chemical insults involving oxidative stress (8,9).
Nrf2 is a master regulator of cellular antioxidant response.
Furthermore, the Nrf2 pathway induces many genes, regulating redox
homeostasis, detoxification, autophagy, and DNA repair (26). Furthermore, Nrf2 is a pivotal
regulator of stem cell self-renewal and unlimited proliferation
(27).
Table I summarizes
the role of Nrf2 in cancer (2,21,28–34).
Nrf2 has been considered a tumor suppressor because the defense
mechanism against endogenous and exogenous oxidative damage
protects normal cells from neoplastic transformation (2,28).
Nrf2-deficient mice are associated with increased susceptibility to
redox-mediated spontaneous, chemical, and radiation carcinogenesis
(29). However, Nrf2-knockout mice
cannot eliminate cancer cells, suggesting that host-derived Nrf2 is
essential to suppress cancer initiation (29). Therefore, Nrf2 activation in cancer
cells upregulates target antioxidant genes; these alterations
confer many advantages to cancer cells, including malignant
phenotypes leading to tumor growth advantage, progression,
aggressiveness, and poor prognosis (2,29).
Ovarian cancer stem-like cells have increased Nrf2-induced
antioxidant scavengers, protecting cancer cells from oxidative
damage (35). Additionally, Nrf2
activation in cancer cells protects cells against harmful
substances, such as chemotherapeutic agents and radiotherapy,
conferring therapeutic resistance (21,30).
The Nrf2-Keap1 system is responsible for platinum chemotherapy
resistance in ovarian cancer (36).
 | Table I.Summary of the preclinical evidence
of Nrf2 in cancer. |
Table I.
Summary of the preclinical evidence
of Nrf2 in cancer.
| A,
Tumor-suppressing effects |
|---|
|
|---|
| First author/s,
year | In
vitro/in vivo | Human samples | Methods | Summary | (Refs.) |
|---|
| Moon and Giaccia,
2015 |
|
| Review article | Discussing the dual
role of Nrf2 in cancer prevention and progression depending on the
cellular context and environment | (29) |
| Menegon et
al, 2016 |
|
| Review article | A tumor suppressor
due to its cytoprotective functions against exogenous and
endogenous insults, including oxidative stress | (28) |
| Cho et al,
2017 |
| Human epithelial
ovarian cancer samples |
Immunohistochemistry | Patients with high
Nrf2 expression displayed better overall survival and disease-free
survival, but the association was not statistically
significant | (31) |
| Czogalla et
al, 2018 |
| Human ovarian
cancer samples | In vitro and
gene expression analysis | Cytoplasmic Nrf2
expression in the serous ovarian cancer subtype was associated with
longer overall survival (median 50.6 vs. 29.3 months; P=0.04) | (32) |
| Jaganjac et
al, 2020 |
|
| Review article | A tumor suppressor
due to its role in reducing ROS and environmental carcinogens. The
thioredoxin and glutathione systems play a protective role against
carcinogenesis | (2) |
|
| B,
Tumor-promoting effects |
|
| First author/s,
year | In
vitro/in vivo | Human
samples | Methods | Summary | (Refs.) |
|
| Moon and Giaccia,
2015 |
|
| Review article | Discussing the dual
role of Nrf2 in cancer prevention and progression depending on the
cellular context and environment | (29) |
| Harris et
al, 2015 | Isolated primary
mammary epithelial cells |
| In vitro, in
vivo murine tumor and xenograft models, and human tissue
samples | While glutathione
is required for cancer initiation, thioredoxin is a key driver in
cancer progression in already established neoplasm. Inhibition of
both GSH and thioredoxin pathways causes synergistic cancer cell
death | (33) |
| Liew et al,
2015 |
| Human ovarian
cancer samples |
Immunohistochemistry | Nrf2 expression was
associated with poorer overall survival and disease-free survival
in human ovarian cancer | (34) |
| Menegon et
al, 2016 |
|
| Review article | Hyperactivation of
the Nrf2 pathway creates an environment that favors the survival of
malignant cells, protecting them against oxidative stress,
chemotherapeutic agents and radiotherapy | (28) |
| Kitamura and
Motohashi, 2018 |
|
| Review article | Persistently high
levels of Nrf2 activity enhance therapeutic resistance of cancer
cells and show malignant phenotypes leading to poor prognoses in
patients with cancer. Nrf2 also drives metabolic reprogramming to
establish cellular metabolic processes that are advantageous for
cell proliferation | (21) |
| Jaganjac et
al, 2020 |
|
| Review article | Constitutive
activation of Nrf2 contributes not only to the progression in the
already-established tumor cells but also to the tumor development,
revealing its novel role as an oncogene. Thioredoxin and
glutathione systems support carcinogenesis | (2) |
| Li et al,
2021 |
|
| Review article | Nrf2 protects cells
by fighting oxidative stress and defending against harmful
substances, such as chemotherapeutics | (30) |
Nrf2 pathway inhibition represents an attractive
target for developing anticancer drugs (37–46)
(Table II). The core results in
Table II are summarized in
illustrative Fig. 2B. Nrf2
inhibitors include brusatol (37,43),
all-trans retinoic acid (ATRA) (38), ARE expression modulator 1 (AEM1)
(40), ML385 (41), clobetasol propionate (CP) (42), dextran sulfate (45), 1-(2-cyclohexylethoxy)aniline
(IM3829) (2), and malabaricone-A
(MAL-A) (2). Dextran sulfate
suppresses angiogenesis by inhibiting the Nrf2 signaling pathway
and reducing the expression of hypoxia-inducible factor-1alpha
(HIF-1α) in gastric cancer (45).
Brusatol extracted from a family of natural products known as
quassinoids inhibited Nrf2-related cell cycle transition from G2 to
M phase, which depends on cyclin B-cyclin-dependent kinase 1 (CDK1)
complex (43). Furthermore,
brusatol downregulates c-MYC expression, leading to cell death
(37). Thus, other natural
products (low molecular weight organic compounds produced by
plants) and natural product-derived synthetic compounds may be
potential drug candidates for cancer therapy.
 | Table II.Role of Nrf2 inhibitors during cancer
development, progression and determining the therapeutic response
in preclinical cancer models. |
Table II.
Role of Nrf2 inhibitors during cancer
development, progression and determining the therapeutic response
in preclinical cancer models.
| First author/s,
year | Name | Means to suppress
Nrf2 function | Cancer type | In
vitro/in vivo | Summary | (Refs.) |
|---|
| Mata-Greenwood
et al, 2002 | Quassinoid
brusatol | Natural
product | Acute or chronic
myeloid leukemia cell lines | In
vitro | Brusatol
downregulates c-MYC expression. | (37) |
| Wang et al,
2007 | ATRA and RARalpha
agonists |
| A human mammary
MCF7-derived AREc32 reporter cell line | In
vitro/in vivo mouse model | ATRA and RARalpha
agonists reduce the ability of NRF2 | (38) |
| van der Wijst et
al, 2015 | Small interfering
RNA against NRF2 | siRNA-induced
downregulation of Nrf2 signaling | Ovarian cancer | In
vitro | Downregulation of
NRF2 enhances sensitivity to oxidative stress by increasing
intracellular ROS levels, which promotes ovarian cancer cell
death | (39) |
| Bollong et
al, 2015 | AEM1 | A small molecule
inhibitor | Lung adenocarcinoma
cells | A high throughput
screen identified small molecules which decrease NRF2
transcriptional activity at antioxidant response element sites | AEM1 sensitizes
lung adenocarcinoma cells to various chemotherapeutic agents via
downregulation of NRF2 controlled genes, inhibiting the growth of
cancer cells in vitro and in vivo | (40) |
| Singh et al,
2016 | ML385 | A small molecule
inhibitor | Non-small cell lung
cancer cells | A quantitative
high-throughput screen | Combination of
ML385 and carboplatin is an efficient therapeutic approach in
non-small cell lung cancer cells in vitro | (41) |
| Choi et al,
2017 | CP | Clinical compound
screening | Lung cancer | In
vitro/in vivo mouse model | CP prevents nuclear
accumulation and promotes degradation of NRF2 in a glucocorticoid
receptor- and a glycogen synthase kinase 3-dependent manner. CP
could be a repurposed therapeutic agent for cancers with high NRF2
activity | (42) |
| Lin et al,
2018 | Brusatol | A natural product
isolated from the seeds of Brucea | Early mouse
embryo | In
vitro/in vivo | Brusatol inhibits
NRF2-related cell cycle transition from G2 to M phase
that is dependent on the cyclin B-CDK1 complex | (43) |
| Lee et al,
2020 | Small interfering
RNA against NRF2 | siRNA-induced
downregulation of Nrf2 signaling | Colorectal
cancer | In vitro
experiments and human colorectal cancer tissues | Small interfering
RNA against NRF2 successfully inhibits tumor growth and markedly
increases apoptosis | (44) |
| Xu et al,
2021 | DS | Dextran
Sulfate | Gastric cancer | In vitro and
in vivo nude mouse intraperitoneal implantation metastasis
model | DS reduces the
angiogenic potential through suppressing Nrf2 expression in gastric
cancer | (45) |
| Bovilla et
al, 2021 | siNrf2 and
pharmacological inhibition | Pharmacological
inhibition and siRNA-induced downregulation of Nrf2 signaling | Breast cancer | In
vitro/in vivo mouse model/human breast cancer
tissues | Pharmacological
inhibition and siRNA-induced downregulation of NRF2 signaling
results in reduced breast cancer proliferation and migration, cell
cycle arrest, activation of apoptosis, and sensitization of cancer
cells to cisplatin in vitro | (46) |
Additionally, a key role for Nrf2 in treating
ovarian cancer has been validated by siRNA studies (39,47).
Nrf2 downregulation enhanced sensitivity to oxidative stress by
increasing intracellular ROS levels, which maintains an antitumor
effect, and decreases cell viability in ovarian cancer cells
(39). Nrf2 inhibitors
specifically blocked the progression of Nrf2-positive cancers
(39). Pharmacological inhibition
and siRNA-induced downregulation of Nrf2 signaling in breast cancer
cells resulted in sensitization of cancer cells to cisplatin
(46). Nrf2 small-interfering RNA
induces apoptosis and inhibits proliferation in various cancer
cells (39,44–46).
Nrf2 inhibitors also increased the sensitivity of cancer cells to
ionizing radiation and chemotherapeutic drugs. Further, concurrent
inhibition of the thioredoxin and glutathione systems, downstream
targets of Nrf2, promoted cancer cell death in mouse models
(48).
Interestingly, depending on the cellular context and
environment, Nrf2 plays a dynamic tumor-suppressive or -promoting
role. Although in vivo experiments in knockout mice
demonstrate that Nrf2 protected mice from cancer development, the
xenograft animal model showed that Nrf2 promoted cancer growth
(29). Host- and cancer
cell-derived Nrf2 may act as tumor suppressors and tumor promoters,
respectively. Therefore, the Nrf2 pathway is often activated in
various cancer but is thought to play a dual role in cancer
initiation and progression. Preclinical studies demonstrated that
antioxidant pathways might be a promising target for cancer
therapy. However, some clinical studies on the impact of Nrf2
expression on the prognosis of cancer patients have yielded
inconsistent findings (2,29,31,32,34).
Further studies are required to determine whether Nrf2 is
associated with tumor aggressivity and poor prognosis in various
cancers, including HGSC.
CD44v9
CD44 is a transmembrane glycoprotein and surface
receptor for hyaluronan involved in the mutual response between
cells and their microenvironment (49) (Fig.
2A). The variant isoform of CD44 containing v8-v10 (CD44v9) is
an ovarian cancer stem cell surface marker (50). CD44v9 interacts with xCT [also
known as solute carrier family 7 member 11 (SLC7A11)], a
cystine/glutamate transporter, for cystine uptake (50–52).
A continual cystine supply is crucial for de novo synthesis
of glutathione and thioredoxin antioxidant peptides (51). CD44v9 specifically stabilizes redox
potential through antioxidant factors, such as glutathione and
glutathione peroxidases (GPxs) (53). In addition, CD44v9 contributes to
antioxidative response by reducing ROS levels. Various tumor cells,
including ovarian cancer and normal cells, acquire protection and
resistance against oxidative stress by activating CD44v9 (50). This section summarizes the
relationship between the CD44v9 expression level and tumor
progression in various patients with cancer (52,54–65)
(Table III). Studies conducted
on different cancers have shown the dual role of the CD44v9/xCT
pathway in tumor progression and suppression.
 | Table III.Summary of the preclinical evidence
of CD44v9 in cancer. |
Table III.
Summary of the preclinical evidence
of CD44v9 in cancer.
| A,
Tumor-suppressing effects: An increase in CD44v9 expression
suppresses cancer progression |
|---|
|
|---|
| First author/s,
year | In
vitro/in vivo or human samples | Methods | Summary | (Refs.) |
|---|
| Sato et al,
2004 | Oral squamous cell
carcinoma HSC-4 cells | Cell culture
invasion assay and a three-dimensional culture invasion assay | Overexpression of
CD44v9 resulted in downregulation of the invasive potential | (54) |
| Miwa et al,
2017 | Gallbladder cancer
NOZ cells | In vitro
cell migration and invasion assays | CD44v9-positive
cells exhibited decreased invasiveness compared with
CD44v9-negative cells | (55) |
| Sato et al,
2000 | Primary squamous
cell carcinoma of the tongue | Immunohistochemical
study. Biopsy specimens from primary squamous cell carcinoma of the
tongue. | Downregulation of
CD44v9 in squamous cell carcinoma of the tongue may relate to the
detachment of tumor cells from primary lesions, establishment of
lymph node metastasis and consequently the death of patients | (56) |
|
| B,
Tumor-suppressing effects: A decrease in CD44v9 expression promotes
cancer progression |
|
| First author/s,
year | In
vitro/in vivo or human samples | Methods | Summary | (Refs.) |
|
| Sato et al,
2004 | Oral squamous cell
carcinoma HSC-4 cells | Cell culture
invasion assay and a three-dimensional culture invasion assay | Treatment with an
anti-CD44v9 antibody enhanced the invasive potential of oral
squamous cell carcinoma cell lines | (54) |
| Umeda et al,
2016 | Invasive
micropapillary breast carcinoma and ICNST |
Immunohistochemistry. Twenty-one
consecutive cases of mixed invasive micropapillary carcinoma of the
breast. | Immunohistochemical
scores of CD44v9 in the ICNST component of lymph node metastasis
cases of breast cancer were lower compared with cases without lymph
node metastasis | (57) |
|
| C,
Tumor-promoting effects: An increase in CD44v9 expression promotes
cancer progression |
|
| First author/s,
year | In
vitro/in vivo or human samples | Methods | Summary | (Refs.) |
|
| Yasui et al,
1998 | Non-neoplastic
mucosa, adenoma and adenocarcinoma of the stomach |
Immunohistochemistry | Incidence of CD44v9
expression was higher in the cases of stages 3 and 4 in comparison
with that in the stages 1 and 2 cases. The expression of CD44v9 may
be associated with the development as well as progression of
gastric cancer | (58) |
| Okano et al,
1999 | Early colorectal
cancer |
Immunohistochemistry | Immunohistochemical
expression of p53 and CD44v9 provides useful information for
identifying those patients with early colorectal cancer who have a
high risk of developing liver metastases | (59) |
| Koyama et
al, 1999 | Primary gastric and
esophageal carcinomas |
Immunohistochemistry | Upregulation of the
CD44v9 molecule in gastric cancer, especially metastatic
adenocarcinoma, is associated with tumor growth and
progression | (60) |
| Goi et al,
2002 | Colorectal
cancers |
Immunohistochemistry | CD44v9 was
expressed in the primary colorectal cancers in 42% of patients
without pulmonary metastases and 88% of patients with pulmonary
metastases | (61) |
| Bánkfalvi et
al, 2002 | Oral squamous cell
carcinoma |
Immunohistochemistry | In oral squamous
cell carcinoma, an accumulation of CD44v9 was observed at the
invasive tumor front. In metastases and recurrences, an increase of
v9 was recorded. Changes of CD44v9 phenotype within the primary
tumors were associated with poor prognosis | (62) |
| Kakehashi et
al, 2016 | Hepatocellular
carcinoma |
Immunohistochemistry | Patients with
hepatocellular carcinoma with positive CD44v9 expression had poor
overall and recurrence-free survival compared with those with
negative expression | (63) |
| Miwa et al,
2017 | Gallbladder cancer
NOZ cells | In vivo
animal model | CD44v9 cells
exhibited increased tumorigenicity | (55) |
| Ogihara et
al, 2019 | In
vitro/in vivo mouse metastasis model and bladder
cancer |
Immunohistochemistry | CD44v9 expression
was associated with disease recurrence and death in muscle invasive
bladder cancer | (52) |
| Go et al,
2019 | Early gastric
cancer |
Immunohistochemistry | Both positive
CD44v9 and high Ki67 expression are associated with poor prognosis
in early gastric cancer | (64) |
|
| D,
Tumor-promoting effects: A decrease in CD44v9 expression suppresses
cancer progression |
|
| First author/s,
year | In
vitro/in vivo or human samples | Methods | Summary | (Refs.) |
|
| Suwannakul et
al, 2020 | Cholangiocarcinoma
cells | CD44v9 silencing
using siRNA transfection. In vitro/in vivo mouse
xenografts. | CD44v9 silencing
regulates redox system by reducing the expression levels of
cysteine transporter xCT. CD44v9 silencing suppresses cell
proliferation, migration and invasion by induction of apoptosis and
cell cycle arrest. CD44v9 downregulation inhibited tumor growth in
mouse xenografts | (65) |
An increase in CD44v9 expression
suppresses cancer progression
i) Oral squamous cell carcinoma. CD44v9
overexpression downregulated the invasive potential of oral
squamous cell carcinoma HSC-4 cells due to enhanced cell-cell
adhesion (54).
ii) Gallbladder cancer. In gallbladder cancer,
CD44v9-positive cells exhibited decreased invasiveness than
CD44v9-negative cells in in vitro cell invasion assays
(55).
iii) Tongue squamous cell carcinoma. CD44v9
expression was negatively correlated with lymphatic metastasis and
unfavorable outcome in patients with tongue squamous cell carcinoma
(56). Also, increased CD44v9
expression is associated with suppressing some cancer cell invasion
and metastasis, suggesting that targeted CD44v9 activation is a
novel approach to preventing cancer initiation.
A decrease in CD44v9 expression
promotes cancer progression
i) Breast cancer. Immunohistochemical analysis
showed that downregulating CD44v9 expression in the invasive breast
carcinoma of no special type was a risk factor for lymph node
metastasis (57).
ii) Oral squamous cell carcinoma. Reduced CD44v9
expression was correlated with increased invasive potential in oral
squamous cell carcinoma cells (54). However, decreased CD44v9 expression
may be associated with the progression of certain cancer types.
An increase in CD44v9 expression
promotes cancer progression
i) Gastric cancer. CD44v9 expression is identified
in normal gastric epithelium, H. pylori-infected pyloric
gland cells, and primary gastric carcinoma cells (58,60).
CD44v9 is involved in the wound-healing process of the gastric
epithelium after injury (66).
Positive CD44v9 expression is associated with gastric cancer
progression (58,60), correlating with a poor prognosis in
early gastric cancer (64).
ii) Colorectal cancer. CD44v9 significantly impacts
the survival of early colorectal cancer (59) and may be a biomarker for cancer
progression, particularly for predicting pulmonary metastasis, in
patients with colorectal cancer (61).
iii) Oral squamous cell carcinoma.
Immunohistochemistry showed that CD44v9-positive cancer cells were
located at the tip of the invasive front of oral squamous cell
carcinoma (62). Also, a
significant increase in CD44v9 expression was identified in
metastatic and recurrent lesions. CD44v9 is a predictive indicator
of poor prognosis in oral squamous cell carcinoma and may play a
role in patient risk assessment (62).
iv) Hepatocellular carcinoma. Positive CD44v9
expression was significantly associated with poor overall and
recurrence-free survival in patients with hepatocellular carcinoma
than those with CD44v9-negative expression (63). CD44v9 expression was negatively
associated with Ki67 expression, proposing the importance of CD44v9
in maintaining the stemness of cancer stem-like cells.
v) Bladder cancer. CD44v9 may be a clinical
biomarker for predicting poor outcomes in patients with
muscle-invasive bladder cancer (52). Furthermore, CD44v9-positive cells
enhanced tumorigenicity in xenotransplantation models (55).
A decrease in CD44v9 expression
suppresses cancer progression
i) Cholangiocarcinoma. CD44v9 silencing inhibits
proliferation and invasion, thus, promoting apoptosis and cell
cycle arrest by downregulating xCT expression levels (65).
We reviewed several preclinical studies that suggest
a causal effect between CD44v9 expression and increased or
decreased risk of tumor formation. CD44v9 had divergent effects on
cancer initiation and progression in different contexts. Different
antioxidant-signaling pathways may be activated in different
cancers. Furthermore, several documents reported that CD44v9
expression in cancer tissue is predictive of a poor prognosis
(52,55,58–64).
Therefore, drugs targeting CD44v9 and xCT may be effective in
patients with cancer. The CD44v9/xCT pathway inhibitor may provide
a therapeutic option for treating certain cancers (Table IV). Sulfasalazine is an oral
pharmacological inhibitor of xCT (52,67–69).
Sulfasalazine suppressed cell proliferation in lymphoma cells
(67) and induced cell death in
cholangiocarcinoma cells in vitro, possibly by enhancing ROS
levels (68). Sulfasalazine also
enhances chemosensitivity, promotes apoptosis, and suppresses the
growth of various cancer cells (52,67–69).
Combining CDDP treatment with sulfasalazine represents a novel
therapeutic strategy for overcoming CDDP resistance in various
cancers (52,68,69).
Sulfasalazine showed favorable therapeutic effects in various
malignant tumors. Therefore, inhibiting the CD44v9/xCT pathway can
be of therapeutic value.
 | Table IV.Role of inhibiting the CD44v9/xCT
pathway during cancer development, progression and determining the
therapeutic response in preclinical cancer models. |
Table IV.
Role of inhibiting the CD44v9/xCT
pathway during cancer development, progression and determining the
therapeutic response in preclinical cancer models.
| First author/s,
year | Name | Therapeutic
uses | Cancer type | In
vitro/in vivo | Summary | (Refs.) |
|---|
| Gout et al,
2001 | Sulfasalazine | A medication used
to treat rheumatoid arthritis, ulcerative colitis, and Crohn's
disease | Lymphoma | In vitro rat
Nb2 lymphoma cultures and in vivo animal model | Sulfasalazine
(i.p.) markedly inhibited growth of rat Nb2 lymphoma transplants
without apparent side-effects | (67) |
| Thanee et
al, 2016 | Sulfasalazine |
|
Cholangiocarcinoma | In vitro and
in vivo hamster model | Sulfasalazine
inhibited cell growth and activated cell death. Sulfasalazine
enhanced chemosensitivity to chemotherapeutic drugs (e.g.,
gemcitabine) | (68) |
| Wada et al,
2018 | Sulfasalazine |
| Hepatocellular
carcinoma | In vitro and
in vivo nude mouse model and human samples/hepatocellular
carcinoma tissues | High levels of xCT
were expressed in poorly differentiated hepatocellular carcinoma
tissues. Sulfasalazine is involved in enhancing CDDP
chemosensitivity in xenograft tumor models | (69) |
| Ogihara et
al, 2019 | Sulfasalazine |
| Bladder cancer | Human
samples/immunohistochemistry, in vitro and in
vivo | CD44v9 expression
was independently associated with disease recurrence and death in
muscle invasive bladder cancer. Sulfasalazine exerted cytotoxic
effects against MBT-2V cells by inhibiting glutathione levels and
inducing ROS production. Sulfasalazine in combination with CDDP
exerts cytotoxic effects against MBT-2V cells by inhibiting CD44v9
expression and upregulating phospho-p38MAPK expression | (52) |
GST
There are two major antioxidant systems: highly
complex antioxidant enzymatic systems, including SOD, catalase,
GST, GPxs, glutathione S-reductase, and G6PD; and non-enzymatic
antioxidant systems, such as vitamins, such as E, C, and A,
tocopherol, glutathione, and bilirubin (70,71).
GST is a key enzyme that maintains intracellular redox homeostasis
and is often overexpressed in cancer cells (72). Glutathione and GST enzymes, such as
GST P1-1 and GST A1-1, were overexpressed in various cancers,
including ovarian cancer (73).
Preclinical studies showed that GST overexpression had been
commonly associated with high malignant phenotype, chemoresistance,
and poor prognosis (72,74). Several clinical studies have also
showed that GST expression was significantly correlated with drug
resistance and poor prognosis in patients with ovarian cancer
(75,76). For example, high GST-π mRNA
expression correlated with lower three-year survival (76). GST is considered an effective
marker for assessing the efficacy of chemotherapy and predicting
prognosis in patients with ovarian cancer (76). However, we sometimes encounter
conflicting information that GST activity in ovarian cancer tissue
was positively associated with a better prognosis (77).
Researchers have developed several drugs that target
unique redox-related enzymes in tumor tissue. One therapeutic
strategy is to develop specific inhibitors of antioxidant enzymes,
such as GST. The GST inhibitor, 6-(7-nitro-2, 1,
3-benzoxadiazol-4-ylthio) hexanol (NBDHEX), induced caspase
activation, apoptosis, and cell death in human mesothelioma cell
lines by activating c-Jun NH2-terminal kinase and p38
mitogen-activated protein kinase (MAPK) pathways (78). TLK199 (Telintra;
Ezatiostat®), a GST P1-1 inhibitor, has been proposed as
a promising approach to treat patients with myelodysplastic
syndrome (72). GST inhibitors are
novel therapeutic candidates for human cancers in preclinical and
clinical settings. Furthermore, auranofin, a thioredoxin reductase
inhibitor, elicited cytotoxicity by perturbing the cellular redox
balance, and increasing ROS production (79). Phase II study has been conducted to
evaluate the efficacy and tolerability of auranofin in patients
with recurrent EOC (79).
The second promising concept is creating
redox-directed anticancer prodrugs that convert to active parent
drugs in vivo by antioxidant enzymes that are specifically
overexpressed in cancer. Prodrugs activated by GST (e.g.,
doxorubicin and etoposide) have been designed and synthesized as
more effective and less toxic treatment regimens to overcome drug
resistance (74,80). For example, Canfosfamide HCl for
injection (TLK286, TELCYTA) is a prodrug cleaved and activated by
GST to form a glutathione derivative and active metabolite
phosphorodiamidate moiety as an anticancer agent (81). The phase II clinical trials showed
that TLK286 had demonstrated a manageable safety profile as a
single agent in patients with ovarian, non-small cell lung, breast,
and colorectal cancers (82).
TLK286 with a single agent or a combined chemotherapeutic regimen
has shown safe antitumor activity and clinical benefit in phases II
(81) and III (83) clinical trials for treating patients
with ovarian and breast cancers (84). However, the phase III trial of
TLK286 in non-small cell lung cancer could not reach a favorable
efficacy, and further clinical trials are currently underway
(82).
Targeted therapy for ovarian cancer based on
redox modifications
Current status of ovarian cancer
treatment
Surgical debulking and combining paclitaxel and
carboplatin-based chemotherapy are the standard treatments for
ovarian cancer (85). Clinical
studies have shown the safety and efficacy of angiogenesis
inhibitors (e.g., bevacizumab) and the poly(ADP-ribose) polymerase
(PARP) inhibitors (e.g., olaparib and niraparib) (85). Additionally, several studies are
evaluating PARP inhibitors in monotherapy and combined in a
real-world cohort. Patients with ovarian cancer BRCA1/2 mutations
or homologous recombination deficiency can benefit from PARP
inhibitors. Unfortunately, even molecular targeted therapies cause
treatment resistance and tumor recurrence. Therefore, additional
alternative therapies are required. Mechanisms of drug resistance
include epithelial-mesenchymal transition, DNA repair activation,
reduced drug uptake, enhanced drug efflux, changes in pro-apoptotic
and anti-apoptotic genes, changes in tumor cell microenvironment,
and redox imbalance. However, oxidative stress and redox imbalance
play an important role in the initiation, progression, drug
resistance, and recurrence of ovarian cancer. This section
discusses molecular mechanisms and therapeutic strategies for
ovarian cancer, focusing on redox modification.
Mechanism of ovarian
carcinogenesis
Steady progress has been made in the molecular
understanding of the etiology of ovarian cancer, particularly HGSC
(1). Accumulating evidence has
shown that HGSC originates from the fimbriated end of the fallopian
tube secretory epithelial cells (21,86,87).
Also, human endometrial cells and fallopian tube epithelial cells
are constantly exposed to menstrual blood or follicular fluid.
Erythrocytes and follicular fluid induce intracellular ROS
generation (88) and may induce
oxidative injury to fallopian tube fimbria epithelial cells
(89). Fallopian tube cells have
evolved strategies to promote their survival by modulating genetic
and epigenetic profiles, including antioxidative and proliferative
changes (90). Fallopian tube
fimbria epithelial cells acquire protective molecules and pathways,
such as antioxidants, when exposed to oxidative stress
environments. HGSC has been postulated to arise through a stepwise
accumulation of (epi)genetic alterations from normal epithelium to
secretory cell outgrowth, p53 signature, and serous tubal
intraepithelial carcinoma to invasive HGSC (90). Some clones of fallopian tube
fimbria epithelial cells can turn into precursor cells, allowing
them to survive oxidative stress conditions, and eventually grow as
ovarian cancer. Ovarian cancer is exposed to an oxidative stress
environment from an early stage of carcinogenesis to the advanced
stage.
Antioxidant defense system in ovarian
cancer
We have earlier summarized how ovarian cancer cells
regulate their antioxidant defense system to cope with oxidative
stress. Many antioxidants (e.g., NADPH, Nrf2, GST, GPxs,
glutathione, peroxiredoxin, and CD44v9) exist in ovarian cancer
cells to protect against oxidative stress (76,90–93).
The association between these antioxidant-related gene expressions
and clinical outcomes of ovarian cancer has been investigated.
Glutathione, GPxs, and peroxiredoxin overexpressed in ovarian
cancer are associated with an aggressive phenotype and poor
prognosis (76,91–93).
GPx3 upregulated in clear-cell ovarian cancer may promote
chemotherapeutic resistance (94).
Furthermore, a high GPx3 expression was significantly associated
with poor overall survival in patients with HGSC (13). Peroxiredoxins have been implicated
in tumorigenesis, therapeutic resistance, recurrence, and
metastasis of ovarian cancer (95).
Various antioxidant genes, including glutathione,
thioredoxin, and other antioxidants, are downstream targets of the
Nrf2 gene. Aberrant expression and activation of Nrf2 are
frequently observed in ovarian cancer because KEAP1 expression, as
a negative regulator of Nrf2, is downregulated by DNA copy-number
loss (47). Immunohistochemistry
showed that positive staining for Nrf2 was observed in HGSC
(36), showing that constitutive
Nrf2 pathway activation often occurs in HGSC (39,47,96).
Furthermore, altered expression of CD44v9 occurs in the early stage
of HGSC carcinogenesis. Immunohistochemistry revealed that CD44v9
was expressed in normal fallopian tube fimbria epithelial cells
(90). Additionally, a recent
study demonstrated that CD44v9 loss followed by p53 mutation is the
earliest and universal phase of ovarian neoplastic changes, which
may confer positive clonal selection with growth and survival
advantages (90). CD44v9 loss in
fallopian tube fimbria epithelial cells leads to ROS increase.
Furthermore, CD44v9 also reappeared in cancer stem-like cells of
HGSC (90). Constant change by
redox homeostasis may contribute to the high tumor heterogeneity of
malignant cells, including plasticity of cancer stem-like traits
and phenotypic diversity (2). A
high ROS level and upregulation of antioxidant genes are
characteristic features of ovarian cancer (47,97).
HGSC has evolved antioxidant defense strategies to combat
endogenous and exogenous oxidative stress. Therefore, CD44v9 and
Nrf2 pathways may be key regulators of redox balance in ovarian
cancer (92). Ovarian cancer cells
promoted antioxidant defense by upregulating CD44v9 (7) and Nrf2 (39). The antioxidant system supports
cancer progression by suppressing oxidative stress and increasing
ROS-scavenging capacity.
Finally, BRCA1 is a key factor in DNA damage repair
and upregulates, activates, and stabilizes the expression of
multiple genes, including Nrf2, GST oxidoreductases, and other
antioxidant genes involved in the cytoprotective antioxidant
response (98). Genetic deletion
of the BRCA gene significantly disrupted redox balance by
downregulating Nrf2 expression, leading to apoptotic cell death
triggered by excessive ROS production. In addition, BRCA1
deficiency enhanced auranofin (a thioredoxin reductase inhibitor)
sensitivity of ovarian cancer cells (79). The BRCA1 gene plays a critical role
in protecting ovarian cancer cells from oxidative stress by
upregulating and activating the antioxidant defense system.
Furthermore, silencing PARP reduces the expression of
cystine/glutamate exchanger, xCT (SLC7A11), enhancing cell death by
decreasing glutathione biosynthesis (99). PARP inhibitors offer a significant
clinical benefit even in patients without BRCA1/2 mutations. This
effect may be due to a decreased antioxidant capacity induced by
PARP inhibitors. Thus, targeting the antioxidant defense system is
considered a promising therapeutic strategy for ovarian cancer
(39,47).
Potential therapeutic strategies
targeting the antioxidant defense system
Treatments targeting antioxidant defense systems
have conflicting results.
Suppressing tumor progression by
inhibiting ROS generation
It has been proposed that oxidative stress
inhibition may benefit cancer treatment. Polyphenols and
flavonoids, such as resveratrol, genistein, curcumin, and
quercetin, maintain the balance of antioxidant systems by
scavenging ROS (100–103). Furthermore, preclinical studies
showed that these antioxidant phytochemicals inhibit human cancers,
including ovarian cancer, by anticancer activities, including
anti-angiogenic, anti-proliferative, anti-inflammatory, and
pro-apoptotic properties (100–103). The positive effects of
antioxidant therapy have been reported in in vitro and in
vivo animal studies. Additionally, preclinical studies have
shown that inhibiting ROS production by pharmacological inhibitors,
antagonists, or scavengers can inhibit the proliferation of ovarian
cancer cells. Diphenylene iodinenium, a ROS inhibitor, promotes
apoptosis in ovarian cancer cells by inhibiting NADPH oxidase (NOX)
(91). Lysophosphatidic acid
(LPA), a bioactive lipid mediator, induced cell proliferation by
stimulating NOX-dependent ROS generation in ovarian cancer
(104). A specific LPA receptor
antagonist promoted cancer cell apoptosis by inhibiting
LPA/NOX-dependent ROS production (104). Several antioxidants have
exhibited therapeutic potential in preclinical studies.
Furthermore, clinical trials have shown that the vitamin C-treated
group improves survival efficacy in patients with ovarian cancer
better than the non-treated group (105). In addition, vitamin C may improve
tumor drug resistance via its antioxidant and anti-inflammatory
mechanisms (105). These data
provide a rationale to increase the efficacy of conventional
chemotherapies combined with antioxidant therapies for ovarian
cancer. However, few clinical studies have been conducted on cancer
patients. Therefore, it is unclear whether antioxidants provide
clinical benefits in patients with ovarian cancer.
Suppressing tumor progression by
inhibiting the antioxidant defense system
Antioxidant inhibitions have beneficial effects on
cancer therapy. Increased pro-oxidant or decreased antioxidant
defense is considered a promising therapeutic option as excessive
ROS production beyond a threshold value leads to cancer cell death.
Such treatment strategies may benefit patients who already have
ovarian cancer rather than cancer prevention. Here we focus on
CD44v9 and Nrf2, antioxidants highly expressed in HGSC. Nrf2
(Tables I and II) and CD44v9/xCT inhibition (Tables III and IV) can promote cancer initiation or
inhibit cancer progression in a context-dependent manner. Fig. 2B illustrates the therapeutic
strategies based on Nrf2- and CD44v9/xCT-associated signaling
pathways. Results showed that Nrf2 or CD44v9 inhibition delays
tumor progression, but the treatment was insufficient to eradicate
cancer cells. The endogenous antioxidant defense system, such as
Nrf2 and CD44v9, may act coordinately to neutralize ROS, and
protect cells against the biological damage of ROS-induced
oxidative stress. Intracellular ROS elevated by concurrent
inhibition of the Nrf2 and CD44v9 pathways promotes p38 MAPK
activation and modulates the expression or activity of cell cycle
regulators, including cyclin D1, the cyclin-dependent kinase
inhibitors (CDKI; p16 and p21, checkpoint kinase 1 (CHK1), cell
division cycle 25C (CDC25C) and p53 (106,107). p38 shows strong anticancer
effects by inducing G2/M cell cycle arrest and apoptosis (108). However, this is still a
hypothesis, not a clinically proven fact. Oxidative stress may be
involved in the pathophysiology of HGSC, but it remains largely
unknown how CD44v9 and Nrf2 play a regulatory role in HGSC
prevention or development. Thus, further studies on the relevant
mechanisms of Nrf2 and CD44v9 may help improve cancer therapy
outcomes.
In addition to Nrf2 and CD44v9, therapeutic
strategies targeting antioxidant defense systems have been
reported. SOD2 (Mn-SOD) localized in the mitochondrial matrix
detoxifies superoxide radicals. Also, SOD2 is involved in
tumorigenesis, proliferation, invasion, and metastasis in CCC
(109). Reduced SOD2 expression
effectively inhibited the malignant progression of CCC by
upregulating ROS levels (109).
Inhibition of thioredoxin reductase overcame cisplatin resistance
and potentiated antitumor response via glutathione depletion and
increased ROS generation (110).
Also, diselenium nanoparticle encapsulating the cisplatin prodrug
enhanced cisplatin-induced cytotoxicity by depleting glutathione
and increasing ROS (111).
Additionally, antioxidant defense systems also participate in
regulating genes related to energy metabolism in drug-resistant
cancer cells. Cisplatin-resistant ovarian cancer cells can avoid
large amounts of ROS accumulation by stimulating the PPP and
maintaining redox homeostasis (112). A recent study has reported that
concurrent inhibition of endogenous antioxidant enzymes (e.g.,
glutathione biosynthesis) with enzymes involved in ROS production
(e.g., NOX) may improve treatment outcomes (113). Altogether, pro-oxidant and
antioxidant therapies may play contradictory roles in cancer
treatment, depending on the concentration of intracellular ROS.
Lessons from animal studies
Redox homeostasis that controls the dynamic
interaction between oxidative stress and antioxidants can differ
between cancer prevention in cancer-free cases and treatment of
cancer patients (114).
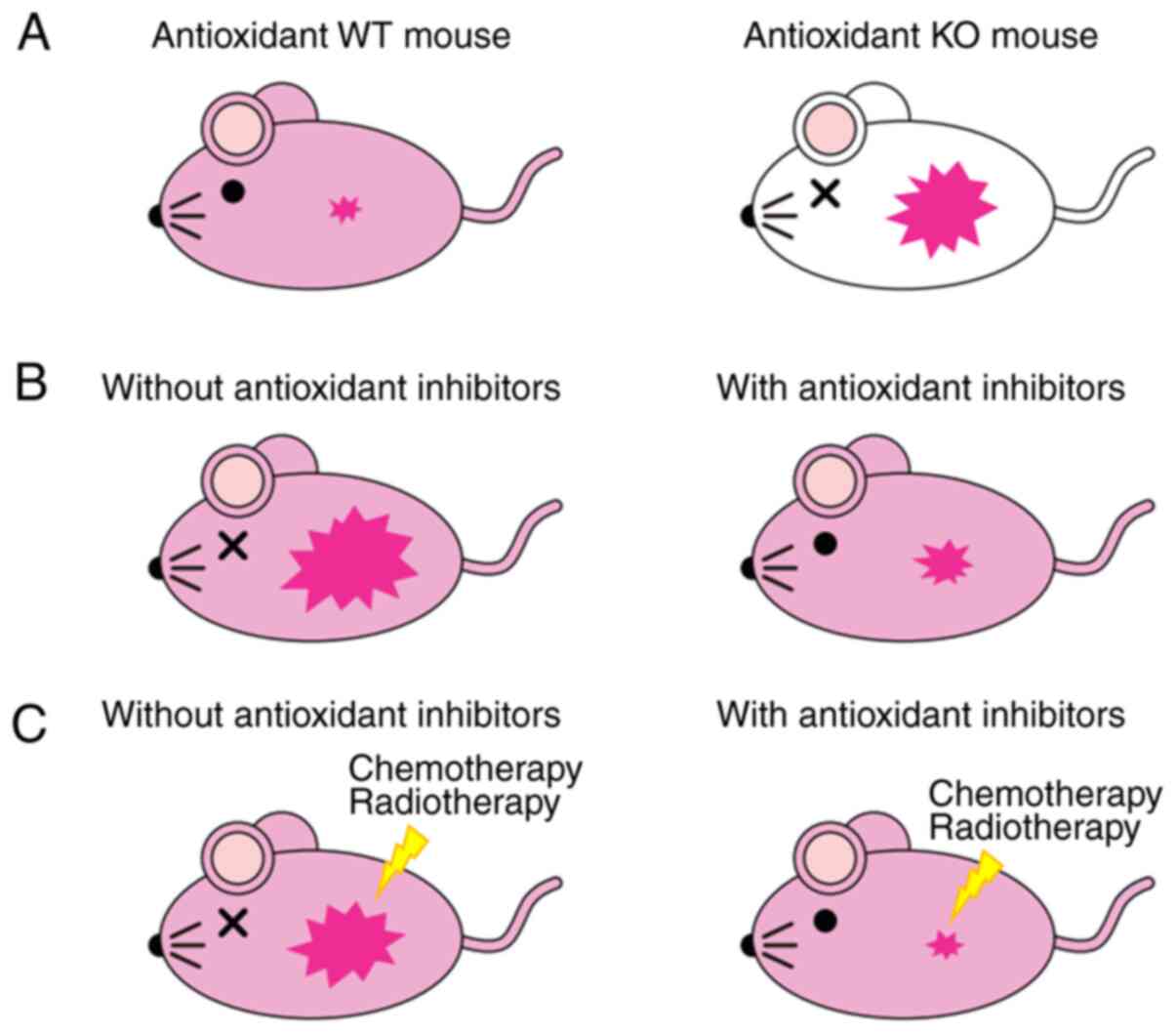
Investigators created various antioxidant knockout mouse models
(Fig. 3A). For example, Nrf2
knockout mice cannot eliminate cancer cells efficiently (29). Also, GPX-1 and −2 double-knockout
spontaneously develop intestinal cancer (115). Loss of GST zeta 1 (GSTZ1), a
downstream target of Nrf2, promotes chemically induced
hepatocellular carcinogenesis (116). Furthermore, antioxidant knockout
mice are more prone to spontaneous or carcinogen-induced
tumorigenesis due to increased endogenous ROS production.
Host-derived antioxidants are essential to suppress carcinogenesis
or cancer initiation. Decreased intracellular ROS-scavenging
ability in the host significantly promotes cancer development
(117). Therefore, antioxidants,
such as CD44v9 and Nrf2, and their downstream targets protect the
host from cancer development.
However, the antioxidant molecules, CD44v9 and
Nrf2, are elevated in ovarian cancer cells, especially cancer stem
cells. In vitro studies and cancer xenograft models
demonstrated that antioxidants play an important oncogenic role in
supporting cancer proliferation and growth (114). After cancer initiation, the
CD44v9 and Nrf2 antioxidant pathways are required for cancer
progression. In contrast, the elevation of ROS level above the
cellular tolerability threshold leads to cell death, suggesting
that high ROS levels can block cancer progression (118). Antioxidant inhibitors suppressed
cancer proliferation in mouse xenograft models (Table II and Fig. 3B). Also, these inhibitors increase
the sensitivity of cancer cells to chemotherapeutic drugs and
ionizing radiation (21) (Fig. 3C). This suggests that antioxidant
inhibitors prevent cancer progression. Nevertheless, the CD44v9/xCT
pathway or Nrf2 inhibitors have limited therapeutic effects.
Concurrent inhibition of both pathways can induce ROS-dependent
lethality in cancer cells. Therefore, the depletion of antioxidant
defense systems and increased ROS concentration can promote ovarian
cancer cell death. Complete loss of antioxidant protein expression
can cause cancer cell death by excessive elevation of ROS, whereas
incomplete suppression may promote cancer progression. The role of
antioxidant systems in inhibiting cancer progression remains
controversial. However, insights into the opposite effects of
antioxidants in initiating tumorigenesis and supporting cancer
proliferation are essential to allow optimal exploitation of CD44v9
and Nrf2 as therapeutic targets.
Conclusion
This review focuses on the redox balance between
ROS production and antioxidant defense systems, discussing proposed
therapeutic strategies for the development and progression of human
cancer, especially ovarian cancer. Cancer cells reprogram their
oxidative metabolism to meet high-energy demand, generating ROS as
undesirable side products. Excessive ROS generation has been
reported in various cancers, including ovarian cancer. Cancer cells
increase their antioxidant defense system to block the harmful
effects of ROS. Antioxidant genes, such as Nrf2, GST, and CD44v9,
and their downstream targets are overexpressed in many types of
cancer, including ovarian cancer. They are involved in cancer
initiation and progression, resulting in chemoresistance and
unfavorable prognosis. Redox balance that controls the dynamic
interaction between oxidative stress and antioxidants plays a key
role in determining the fate of cancer cells. Furthermore,
treatment strategies targeting redox balance can differ between
taking preventive measures in cancer-free women, and treating
patients receiving medical care who already have cancer.
Preclinical studies assessing redox modulators have given
conflicting results. In animal studies, inhibition of excessive ROS
production by host antioxidants plays a vital role in suppressing
carcinogenesis. In contrast, pharmacological inhibition of
antioxidant defense systems may suppress cancer progression in
cancer-bearing animals. Inhibition of antioxidant defense systems
may benefit patients who already have cancer. Concurrent Nrf2 and
CD44v9 inhibition might help develop targeted, personalized
medicine in ovarian cancer. Furthermore, understanding the complex
interplay between ROS and antioxidants in cancer initiation and
progression may spur the development of natural or small-molecule
modulators regulating redox balance. Thus, further research is
needed to validate the therapeutic potential of regulating redox
balance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HK and HS made substantial contributions to
conception and design. SI and HS performed acquisition of data. HS
performed analysis and interpretation of data. SI and HS confirm
the authenticity of all the raw data. The first draft of the
manuscript was written by HK. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 24
(Suppl 10):x16–x21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaganjac M, Milkovic L, Sunjic SB and
Zarkovic N: The NRF2, Thioredoxin, and glutathione system in
tumorigenesis and anticancer therapies. Antioxidants (Basel).
9:11512020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gram M and Åkerström B: Editorial:
Biomarkers of oxidative stress. Front Physiol. 11:3382020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang T, Shigdar S, Gantier MP, Hou Y, Wang
L, Li Y, Shamaileh HA, Yin W, Zhou SF, Zhao X and Duan W: Cancer
stem cell targeted therapy: Progress amid controversies.
Oncotarget. 6:44191–44206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng Y, Fan XY, Yang LJ, Xu BQ, He D, Xu
Z, Wu D, Wang B, Cui HY, Wang SJ, et al: Detachment activated
CyPA/CD147 induces cancer stem cell potential in non-stem breast
cancer cells. Front Cell Dev Biol. 8:5438562020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mvunta DH, Miyamoto T, Asaka R, Yamada Y,
Ando H, Higuchi S, Ida K, Kashima H and Shiozawa T: SIRT1 regulates
the chemoresistance and invasiveness of ovarian carcinoma cells.
Transl Oncol. 10:621–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimizu T, Inoue K, Hachiya H, Shibuya N,
Shimoda M and Kubota K: Frequent alteration of the protein
synthesis of enzymes for glucose metabolism in hepatocellular
carcinomas. J Gastroenterol. 49:1324–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rutkowski DT, Arnold SM, Miller CN, Wu J,
Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D and Kaufman RJ:
Adaptation to ER stress is mediated by differential stabilities of
pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol.
4:e3742006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narayanan D, Ma S and Özcelik D: Targeting
the redox landscape in cancer therapy. Cancers (Basel).
12:17062020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiliro C and Firestein BL: Mechanisms of
metabolic reprogramming in cancer cells supporting enhanced growth
and proliferation. Cells. 10:10562021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Zhang Z, Hoshino A, Zheng HD,
Morley M, Arany Z and Rabinowitz JD: NADPH production by the
oxidative pentose-phosphate pathway supports folate metabolism. Nat
Metab. 1:404–415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang P, Du W, Wang X, Mancuso A, Gao X,
Wu M and Yang X: p53 regulates biosynthesis through direct
inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol.
13:310–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakashima C, Yamamoto K, Fujiwara-Tani R,
Luo Y, Matsushima S, Fujii K, Ohmori H, Sasahira T, Sasaki T,
Kitadai Y, et al: Expression of cytosolic malic enzyme (ME1) is
associated with disease progression in human oral squamous cell
carcinoma. Cancer Sci. 109:2036–2045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X and Gong Y: Isocitrate dehydrogenase
inhibitors in acute myeloid leukemia. Biomark Res. 7:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang P, Du W and Wu M: Regulation of the
pentose phosphate pathway in cancer. Protein Cell. 5:592–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pramono AA, Rather GM, Herman H, Lestari K
and Bertino JR: NAD- and NADPH-contributing enzymes as therapeutic
targets in cancer: An overview. Biomolecules. 10:3582020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta RK, Patel AK, Shah N, Chaudhary AK,
Jha UK, Yadav UC, Gupta PK and Pakuwal U: Oxidative stress and
antioxidants in disease and cancer: A review. Asian Pac J Cancer
Prev. 15:4405–4409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitamura H and Motohashi H: NRF2 addiction
in cancer cells. Cancer Sci. 109:900–911. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malhotra D, Portales-Casamar E, Singh A,
Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler
TW, Wasserman WW and Biswal S: Global mapping of binding sites for
Nrf2 identifies novel targets in cell survival response through
ChIP-Seq profiling and network analysis. Nucleic Acids Res.
38:5718–5734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsuishi Y, Taguchi K, Kawatani Y,
Shibata T, Nukiwa T, Aburatani H, Yamamoto M and Motohashi H: Nrf2
redirects glucose and glutamine into anabolic pathways in metabolic
reprogramming. Cancer Cell. 22:66–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka G, Inoue K, Shimizu T, Akimoto K
and Kubota K: Dual pharmacological inhibition of glutathione and
thioredoxin systems synergizes to kill colorectal carcinoma stem
cells. Cancer Med. 5:2544–2557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YY, Chen J, Liu XM, Zhao R and Zhe H:
Nrf2-Mediated metabolic reprogramming in cancer. Oxid Med Cell
Longev. 2018:93040912018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dodson M, de la Vega MR, Cholanians AB,
Schmidlin CJ, Chapman E and Zhang DD: Modulating NRF2 in disease:
Timing is everything. Annu Rev Pharmacol Toxicol. 59:555–575. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menegon S, Columbano A and Giordano S: The
dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moon EJ and Giaccia A: Dual roles of NRF2
in tumor prevention and progression: Possible implications in
cancer treatment. Free Radic Biol Med. 79:292–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Hong X, Zhao F, Ci X and Zhang S:
Targeting Nrf2 may reverse the drug resistance in ovarian cancer.
Cancer Cell Int. 21:1162021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho HY, Kim K, Kim YB, Kim H and No JH:
Expression patterns of Nrf2 and keap1 in ovarian cancer cells and
their prognostic role in disease recurrence and patient survival.
Int J Gynecol Cancer. 27:412–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Czogalla B, Kahaly M, Mayr D, Schmoeckel
E, Niesler B, Kolben T, Burges A, Mahner S, Jeschke U and Trillsch
F: Interaction of ERα and NRF2 impacts survival in ovarian cancer
patients. Int J Mol Sci. 20:1122018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harris IS, Treloar AE, Inoue S, Sasaki M,
Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA,
et al: Glutathione and thioredoxin antioxidant pathways synergize
to drive cancer initiation and progression. Cancer Cell.
27:211–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liew PL, Hsu CS, Liu WM, Lee YC, Lee YC
and Chen CL: Prognostic and predictive values of Nrf2, Keap1, p16
and E-cadherin expression in ovarian epithelial carcinoma. Int J
Clin Exp Pathol. 8:5642–5649. 2015.PubMed/NCBI
|
|
35
|
Mizuno T, Suzuki N, Makino H, Furui T,
Morii E, Aoki H, Kunisada T, Yano M, Kuji S, Hirashima Y, et al:
Cancer stem-like cells of ovarian clear cell carcinoma are enriched
in the ALDH-high population associated with an accelerated
scavenging system in reactive oxygen species. Gynecol Oncol.
137:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pylväs-Eerola M, Liakka A, Puistola U,
Koivunen J and Karihtala P: Cancer stem cell properties as factors
predictive of chemoresistance in neoadjuvantly-treated patients
with ovarian cancer. Anticancer Res. 36:3425–3431. 2016.PubMed/NCBI
|
|
37
|
Mata-Greenwood E, Cuendet M, Sher D,
Gustin D, Stock W and Pezzuto JM: Brusatol-mediated induction of
leukemic cell differentiation and G(1) arrest is associated with
down-regulation of c-myc. Leukemia. 16:2275–2284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XJ, Hayes JD, Henderson CJ and Wolf
CR: Identification of retinoic acid as an inhibitor of
transcription factor Nrf2 through activation of retinoic acid
receptor alpha. Proc Natl Acad Sci USA. 104:19589–19594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van der Wijst MG, Huisman C, Mposhi A,
Roelfes G and Rots MG: Targeting Nrf2 in healthy and malignant
ovarian epithelial cells: Protection versus promotion. Mol Oncol.
9:1259–1273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bollong MJ, Yun H, Sherwood L, Woods AK,
Lairson LL and Schultz PG: A small molecule inhibits deregulated
NRF2 transcriptional activity in cancer. ACS Chem Biol.
10:2193–2198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh A, Venkannagari S, Oh KH, Zhang YQ,
Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S,
et al: Small molecule inhibitor of NRF2 selectively intervenes
therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem
Biol. 11:3214–3225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi EJ, Jung BJ, Lee SH, Yoo HS, Shin EA,
Ko HJ, Chang S, Kim SY and Jeon SM: A clinical drug library screen
identifies clobetasol propionate as an NRF2 inhibitor with
potential therapeutic efficacy in KEAP1 mutant lung cancer.
Oncogene. 36:5285–5295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin Y, Sui LC, Wu RH, Ma RJ, Fu HY, Xu JJ,
Qiu XH and Chen L: Nrf2 inhibition affects cell cycle progression
during early mouse embryo development. J Reprod Dev. 64:49–55.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee YJ, Kim WI, Bae JH, Cho MK, Lee SH,
Nam HS, Choi IH and Cho SW: Overexpression of Nrf2 promotes colon
cancer progression via ERK and AKT signaling pathways. Ann Surg
Treat Res. 98:159–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Y, Yang Y, Huang Y, Ma Q, Shang J, Guo
J, Cao X, Wang X and Li M: Inhibition of Nrf2/HO-1 signaling
pathway by dextran sulfate suppresses angiogenesis of gastric
cancer. J Cancer. 12:1042–1060. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bovilla VR, Kuruburu MG, Bettada VG,
Krishnamurthy J, Sukocheva OA, Thimmulappa RK, Shivananju NS,
Balakrishna JP and Madhunapantula SV: Targeted inhibition of
anti-inflammatory regulator Nrf2 results in breast cancer
retardation in vitro and in vivo. Biomedicines. 9:11192021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Van der Wijst MG, Brown R and Rots MG:
Nrf2, the Master Redox Switch: The Achilles' heel of ovarian
cancer? Biochim Biophys Acta. 1846:494–509. 2014.PubMed/NCBI
|
|
48
|
Benhar M, Shytaj IL, Stamler JS and
Savarino A: Dual targeting of the thioredoxin and glutathione
systems in cancer and HIV. J Clin Invest. 126:1630–1639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jang BI, Li Y, Graham DY and Cen P: The
Role of CD44 in the pathogenesis, diagnosis, and therapy of gastric
cancer. Gut Liver. 5:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(−) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nagano O, Okazaki S and Saya H: Redox
regulation in stem-like cancer cells by CD44 variant isoforms.
Oncogene. 32:5191–5198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogihara K, Kikuchi E, Okazaki S, Hagiwara
M, Takeda T, Matsumoto K, Kosaka T, Mikami S, Saya H and Oya M:
Sulfasalazine could modulate the CD44v9-xCT system and enhance
cisplatin-induced cytotoxic effects in metastatic bladder cancer.
Cancer Sci. 110:1431–1441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jogo T, Oki E, Nakanishi R, Ando K,
Nakashima Y, Kimura Y, Saeki H, Oda Y, Maehara Y and Mori M:
Expression of CD44 variant 9 induces chemoresistance of gastric
cancer by controlling intracellular reactive oxygen spices
accumulation. Gastric Cancer. 24:1089–1099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sato S, Miyauchi M, Kato M, Kitajima S,
Kitagawa S, Hiraoka M, Kudo Y, Ogawa I and Takata T: Upregulated
CD44v9 expression inhibits the invasion of oral squamous cell
carcinoma cells. Pathobiology. 71:171–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Miwa T, Nagata T, Kojima H, Sekine S and
Okumura T: Isoform switch of CD44 induces different chemotactic and
tumorigenic ability in gallbladder cancer. Int J Oncol. 51:771–780.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sato S, Miyauchi M, Takekoshi T, Zhao M,
Kudo Y, Ogawa I, Kitagawa S, Fujita M and Takata T: Reduced
expression of CD44 variant 9 is related to lymph node metastasis
and poor survival in squamous cell carcinoma of tongue. Oral Oncol.
36:545–549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Umeda T, Ishida M, Murata S, Mori T, Kawai
Y, Itoi N, Tomida K, Tanaka A, Sakai S, Kitamura M, et al:
Immunohistochemical analyses of CD44 variant isoforms in invasive
micropapillary carcinoma of the breast: Comparison with a
concurrent conventional invasive carcinoma of no special type
component. Breast Cancer. 23:869–875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yasui W, Kudo Y, Naka K, Fujimoto J, Ue T,
Yokozaki H and Tahara E: Expression of CD44 containing variant exon
9 (CD44v9) in gastric adenomas and adenocarcinomas: Relation to the
proliferation and progression. Int J Oncol. 12:1253–1258.
1998.PubMed/NCBI
|
|
59
|
Okano K, Shimoda T and Matsumura Y:
Clinicopathologic and immunohistochemical study of early colorectal
cancer with liver metastases. J Gastroenterol. 34:334–340. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Koyama S, Maruyama T and Adachi S:
Expression of epidermal growth factor receptor and CD44 splicing
variants sharing exons 6 and 9 on gastric and esophageal
carcinomas: A two-color flow-cytometric analysis. J Cancer Res Clin
Oncol. 125:47–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Goi T, Koneri K, Katayama K, Hirose K and
Yamaguchi A: Evaluation of clinicopathological factors and the
correlation between the adhesion molecule CD44 variant 9 expression
and pulmonary metastases from colorectal cancers. Int Surg.
87:130–136. 2002.PubMed/NCBI
|
|
62
|
Bánkfalvi A, Krassort M, Buchwalow IB,
Végh A, Felszeghy E and Piffkó J: Gains and losses of adhesion
molecules (CD44, E-cadherin, and beta-catenin) during oral
carcinogenesis and tumour progression. J Pathol. 198:343–351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kakehashi A, Ishii N, Sugihara E, Gi M,
Saya H and Wanibuchi H: CD44 variant 9 is a potential biomarker of
tumor initiating cells predicting survival outcome in hepatitis C
virus-positive patients with resected hepatocellular carcinoma.
Cancer Sci. 107:609–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Go SI, Ko GH, Lee WS, Lee JH, Jeong SH,
Lee YJ, Hong SC and Ha WS: The use of CD44 Variant 9 and Ki-67
combination can predicts prognosis better than their single use in
early gastric cancer. Cancer Res Treat. 51:1411–1419. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Suwannakul N, Ma N, Midorikawa K, Oikawa
S, Kobayashi H, He F, Kawanishi S and Murata M: CD44v9 induces stem
cell-like phenotypes in human cholangiocarcinoma. Front Cell Dev
Biol. 8:4172020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Teal E, Dua-Awereh M, Hirshorn ST and
Zavros Y: Role of metaplasia during gastric regeneration. Am J
Physiol Cell Physiol. 319:C947–C954. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the x(c)-cystine transporter: A new action for an
old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Thanee M, Loilome W, Techasen A, Sugihara
E, Okazaki S, Abe S, Ueda S, Masuko T, Namwat N, Khuntikeo N, et
al: CD44 variant-dependent redox status regulation in liver
fluke-associated cholangiocarcinoma: A target for
cholangiocarcinoma treatment. Cancer Sci. 107:991–1000. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wada F, Koga H, Akiba J, Niizeki T,
Iwamoto H, Ikezono Y, Nakamura T, Abe M, Masuda A, Sakaue T, et al:
High expression of CD44v9 and xCT in chemoresistant hepatocellular
carcinoma: Potential targets by sulfasalazine. Cancer Sci.
109:2801–2810. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lu Z, Wen T, Wang Y, Kan W and Xun G:
Peripheral non-enzymatic antioxidants in patients with
schizophrenia: A case-control study. BMC Psychiatry. 20:2412020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang J, Ye ZW, Janssen-Heininger Y,
Townsend DM and Tew KD: Development of Telintra as an inhibitor of
glutathione S-Transferase P. Handb Exp Pharmacol. 264:71–91. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sprem M, Babić D, Abramić M, Vrhovec I,
Skrk J, Milicić D, Ambriović Ristov A, Kalafatić D and Osmak M:
Glutathione and glutathione S-transferases as early markers for
ovarian carcinomas: Case series. Croat Med J. 42:624–629.
2001.PubMed/NCBI
|
|
74
|
van Gisbergen MW, Cebula M, Zhang J,
Ottosson-Wadlund A, Dubois L, Lambin P, Tew KD, Townsend DM, Haenen
GR, Drittij-Reijnders MJ, et al: Chemical reactivity window
determines prodrug efficiency toward glutathione transferase
overexpressing cancer cells. Mol Pharm. 13:2010–2025. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang J, Yang L, Xiang X, Li Z, Qu K and
Li K: A panel of three oxidative stress-related genes predicts
overall survival in ovarian cancer patients received platinum-based
chemotherapy. Aging (Albany NY). 10:1366–1379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tong X, Zhao J, Zhang Y, Mu P and Wang X:
Expression levels of MRP1, GST-pi, and GSK3beta in ovarian cancer
and the relationship with drug resistance and prognosis of
patients. Oncol Lett. 18:22–28. 2019.PubMed/NCBI
|
|
77
|
Ferrandina G, Scambia G, Damia G,
Tagliabue G, Fagotti A, Benedetti Panici P, Mangioni C, Mancuso S
and D'Incalci M: Glutathione S-transferase activity in epithelial
ovarian cancer: Association with response to chemotherapy and
disease outcome. Ann Oncol. 8:343–350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
De Luca A, Pellizzari Tregno F, Sau A,
Pastore A, Palumbo C, Alama A, Cicconi R, Federici G and Caccuri
AM: Glutathione S-transferase P1-1 as a target for mesothelioma
Treatment. Cancer Sci. 104:223–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Oommen D, Yiannakis D and Jha AN: BRCA1
deficiency increases the sensitivity of ovarian cancer cells to
auranofin. Mutat Res. 784–785. 8–15. 2016.PubMed/NCBI
|
|
80
|
Lyttle MH, Satyam A and Hocker MD:
Glutathione-S-transferase activates novel alkylating agents. J Med
Chem. 37:1501–1507. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kavanagh JJ, Levenback CF, Ramirez PT,
Wolf JL, Moore CL, Jones MR, Meng L, Brown GL and Bast RC Jr: Phase
2 study of canfosfamide in combination with pegylated liposomal
doxorubicin in platinum and paclitaxel refractory or resistant
epithelial ovarian cancer. J Hematol Oncol. 3:92010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tew KD, Manevich Y, Grek C, Xiong Y, Uys J
and Townsend DM: The role of glutathione S-transferase P in
signaling pathways and S-glutathionylation in cancer. Free Radic
Biol Med. 51:299–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Vergote I, Finkler NJ, Hall JB, Melnyk O,
Edwards RP, Jones M, Keck JG, Meng L, Brown GL, Rankin EM, et al:
Randomized phase III study of canfosfamide in combination with
pegylated liposomal doxorubicin compared with pegylated liposomal
doxorubicin alone in platinum-resistant ovarian cancer. Int J
Gynecol Cancer. 20:772–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dourado DF, Fernandes PA, Ramos MJ and
Mannervik B: Mechanism of glutathione transferase P1-1-catalyzed
activation of the prodrug canfosfamide (TLK286, TELCYTA).
Biochemistry. 52:8069–8078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chandrasekaran A and Elias KM: Synthetic
lethality in ovarian cancer. Mol Cancer Ther. 20:2117–2128. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wethington SL, Park KJ, Soslow RA, Kauff
ND, Brown CL, Dao F, Otegbeye E, Sonoda Y, Abu-Rustum NR, Barakat
RR, et al: Clinical outcome of isolated serous tubal
intraepithelial carcinomas (STIC). Int J Gynecol Cancer.
23:1603–1611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kyo S, Ishikawa N, Nakamura K and Nakayama
K: The fallopian tube as origin of ovarian cancer: Change of
diagnostic and preventive strategies. Cancer Med. 9:421–431. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu Y, Qiang W, Xu X, Dong R, Karst AM,
Liu Z, Kong B, Drapkin RI and Wei JJ: Role of miR-182 in response
to oxidative stress in the cell fate of human fallopian tube
epithelial cells. Oncotarget. 6:38983–38998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Scutiero G, Iannone P, Bernardi G,
Bonaccorsi G, Spadaro S, Volta CA, Greco P and Nappi L: Oxidative
stress and endometriosis: A systematic review of the literature.
Oxid Med Cell Longev. 2017:72652382017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sugimoto S, Uchiyama T, Kawahara N,
Ohbayashi C and Kobayashi H: Immunohistochemical expression status
of p53, CD44v9, and Ki-67 in a series of fallopian tube lesions of
High-grade Serous Carcinoma. Int J Gynecol Pathol. 40:419–426.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jiang Z, Fletcher NM, Ali-Fehmi R, Diamond
MP, Abu-Soud HM, Munkarah AR and Saed GM: Modulation of redox
signaling promotes apoptosis in epithelial ovarian cancer cells.
Gynecol Oncol. 122:418–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Khalil HS, Goltsov A, Langdon SP, Harrison
DJ, Bown J and Deeni Y: Quantitative analysis of NRF2 pathway
reveals key elements of the regulatory circuits underlying
antioxidant response and proliferation of ovarian cancer cells. J
Biotechnol. 202:12–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Worley BL, Kim YS, Mardini J, Zaman R,
Leon KE, Vallur PG, Nduwumwami A, Warrick JI, Timmins PF, Kesterson
JP, et al: GPx3 supports ovarian cancer progression by manipulating
the extracellular redox environment. Redox Biol. 25:1010512019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Agnani D, Camacho-Vanegas O, Camacho C,
Lele S, Odunsi K, Cohen S, Dottino P and Martignetti JA: Decreased
levels of serum glutathione peroxidase 3 are associated with
papillary serous ovarian cancer and disease progression. J Ovarian
Res. 4:182011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jia W, Chen P and Cheng Y: PRDX4 and its
roles in various cancers. Technol Cancer Res Treat.
18:15330338198643132019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Martinez VD, Vucic EA, Thu KL, Pikor LA,
Hubaux R and Lam WL: Unique pattern of component gene disruption in
the NRF2 inhibitor KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex in
serous ovarian cancer. Biomed Res Int. 2014:1594592014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hu Y, Rosen DG, Zhou Y, Feng L, Yang G and
Liu J: Mitochondrial manganese-superoxide dismutase expression in
ovarian cancer: Role in cell proliferation and response to
oxidative stress. J Biol Chem. 280:39485–39492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang
HJ, Xu J, Goldberg ID, Jaiswal AK and Rosen EM: BRCA1 induces
antioxidant gene expression and resistance to oxidative stress.
Cancer Res. 64:7893–7909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hong T, Lei G, Chen X, Li H, Zhang X, Wu
N, Zhao Y, Zhang Y and Wang J: PARP inhibition promotes ferroptosis
via repressing SLC7A11 and synergizes with ferroptosis inducers in
BRCA-proficient ovarian cancer. Redox Biol. 42:1019282021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Landis-Piwowar KR, Milacic V, Chen D, Yang
H, Zhao Y, Chan TH, Yan B and Dou QP: The proteasome as a potential
target for novel anticancer drugs and chemosensitizers. Drug Resist
Updat. 9:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shafabakhsh R and Asemi Z: Quercetin: A
natural compound for ovarian cancer treatment. J Ovarian Res.
12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Anticancer efficacy of polyphenols and their combinations.
Nutrients. 8:5522016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tendulkar S and Dodamani S:
Chemoresistance in ovarian cancer: Prospects for new drugs.
Anticancer Agents Med Chem. 21:668–678. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rogers LC, Davis RR, Said N, Hollis T and
Daniel LW: Blocking LPA-dependent signaling increases ovarian
cancer cell death in response to chemotherapy. Redox Biol.
15:380–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nauman G, Gray JC, Parkinson R, Levine M
and Paller CJ: Systematic review of intravenous ascorbate in cancer
clinical trials. Antioxidants (Basel). 7:892018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bulavin DV and Fornace AJ Jr: p38 MAP
kinase's emerging role as a tumor suppressor. Adv Cancer Res.
92:95–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang P, Hong J, Yoon IN, Kang JK, Hwang
JS and Kim H: Clostridium difficile Toxin A induces reactive
oxygen species production and p38 MAPK activation to exert cellular
toxicity in neuronal cells. J Microbiol Biotechnol. 27:1163–1170.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Z, Huang C, Li J, Leonard SS,
Lanciotti R, Butterworth L and Shi X: Vanadate-induced cell growth
regulation and the role of reactive oxygen species. Arch Biochem
Biophys. 392:311–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hemachandra LP, Shin DH, Dier U, Iuliano
JN, Engelberth SA, Uusitalo LM, Murphy SK and Hempel N:
Mitochondrial superoxide dismutase has a protumorigenic role in
ovarian clear cell carcinoma. Cancer Res. 75:4973–4984. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bykov VJ, Zhang Q, Zhang M, Ceder S,
Abrahmsen L and Wiman KG: Targeting of Mutant p53 and the cellular
redox balance by APR-246 as a strategy for efficient cancer
therapy. Front Oncol. 6:212016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wei D, Yu Y, Zhang X, Wang Y, Chen H, Zhao
Y, Wang F, Rong G, Wang W, Kang X, et al: Breaking the
intracellular redox balance with diselenium nanoparticles for
maximizing chemotherapy efficacy on patient-derived xenograft
models. ACS Nano. Dec 7–2020.(Epub ahead of print). View Article : Google Scholar
|
|
112
|
Xu Y, Gao W, Zhang Y, Wu S, Liu Y, Deng X,
Xie L, Yang J, Yu H, Su J and Sun L: ABT737 reverses cisplatin
resistance by targeting glucose metabolism of human ovarian cancer
cells. Int J Oncol. 53:1055–1068. 2018.PubMed/NCBI
|
|
113
|
Liu M, Wang D, Luo Y, Hu L, Bi Y, Ji J,
Huang H, Wang G, Zhu L, Ma J, et al: Selective killing of cancer
cells harboring mutant RAS by concomitant inhibition of NADPH
oxidase and glutathione biosynthesis. Cell Death Dis. 12:1892021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Müller MF, Florian S, Pommer S, Osterhoff
M, Esworthy RS, Chu FF, Brigelius-Flohé R and Kipp AP: Deletion of
glutathione peroxidase-2 inhibits azoxymethane-induced colon cancer
development. PLoS One. 8:e720552013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Krehl S, Loewinger M, Florian S, Kipp AP,
Banning A, Wessjohann LA, Brauer MN, Iori R, Esworthy RS, Chu FF
and Brigelius-Flohé R: Glutathione peroxidase-2 and selenium
decreased inflammation and tumors in a mouse model of
inflammation-associated carcinogenesis whereas sulforaphane effects
differed with selenium supply. Carcinogenesis. 33:620–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li J, Wang Q, Yang Y, Lei C, Yang F, Liang
L, Chen C, Xia J, Wang K and Tang N: GSTZ1 deficiency promotes
hepatocellular carcinoma proliferation via activation of the
KEAP1/NRF2 pathway. J Exp Clin Cancer Res. 38:4382019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Taguchi K and Yamamoto M: The KEAP1-NRF2
system as a molecular target of cancer treatment. Cancers (Basel).
13:462020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Glasauer A and Chandel NS: Targeting
antioxidants for cancer therapy. Biochem Pharmacol. 92:90–101.
2014. View Article : Google Scholar : PubMed/NCBI
|