Introduction
Gastric cancer (GC) is currently one of the most
common malignant tumors diagnosed and is the third leading cause of
cancer-associated mortality worldwide (1,2).
Alpha-fetoprotein-producing GC (AFP-GC) is more aggressive with a
low incidence of 1.2-15% (3,4)
compared with non-AFP GC. At a comparable stage, patients with
AFP-GC displayed significantly lower survival rates and higher
incidence of lymphatic metastasis, liver metastasis and vascular
invasion than those with non-AFP GC (5,6).
Although the aggressive behaviors of AFP-GC have
drawn much attention, whether and how AFP could regulate GC
progression remain unknown. AFP is a glycoprotein produced to a
lesser extent in the fetal gastrointestinal tract but mainly
produced by the yolk sac and liver during fetal development
(7). It has been reported that in
patients with common GC, a high serum AFP level is considered as an
independent predictor of high metastasis and poor prognosis
(8,9).
Amemiya et al (10) reported that patients with AFP-GC
have higher expression of c-Met compared with those with
non-AFP-GC. c-MET protein is encoded by the MET proto-oncogene and
its high-affinity ligand is the hepatocyte growth factor (HGF).
Previous studies indicated that the HGF-Met signaling pathway plays
a vital role in the growth, metastasis and drug resistance in
gastrointestinal cancers (11,12).
Metastasis-associated colon cancer-1 (MACC1) gene, which is ac-Met
transcriptional regulator, has been identified as a colon cancer
oncogene that could promote metastasis (13). Higher expression of MACC1 was found
in GC tissues compared with adjacent non-tumor tissues (14,15)
and is associated with distant metastasis and low survival rate
(15–21). Furthermore, the expression of
MACC1, HGF and c-Metis positively correlated with each other in GC
tissues (14,15).
Considering that high c-Met expression is correlated
with high AFP and MACC1 expression levels, both predict poor
prognosis in GC, we hypothesized that an interaction may exist
between AFP and MACC1 activity, which might subsequently enhance GC
progression. The present study aimed to investigate this
interaction in vitro models mimicking AFP-GC.
Materials and methods
Cell lines
The human GC cell lines, MKN-45 (cat. no. JCRB0254)
and GCIY (Cat. No. TKG0405) were purchased from Biofeng. MKN-45 was
cultured in RPMI-1640 medium (HyClone; Cytiva; cat. no. SH30809.01)
and GCIY was cultured in Minimum Essential Media (MEM) medium
(HyClone; Cytiva; cat. no. SH30024.01), respectively, and both were
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.;
cat. no. 10270-106) and 1% of 10,000 U/ml penicillin-streptomycin
(Thermo Fisher Scientific, Inc.; cat. no. 15140122). All cells were
placed at 37°C in a humidified incubator containing 5%
CO2. Kataoka et al (22) reported the abundant expression of
AFP in GCIY cells and the low expression of AFP in MKN-45 cells.
Therefore these two cell lines were used in the study.
Cell transfection
To overexpress AFP, AFP human untagged clone
(OriGene; cat. no. SC122582) was used and combined with pCMV6-XL5
vector (OriGene Technologies, Inc.). For downregulation of MACC1,
short hairpin (sh)RNA targeting MACC1 (shMACC1; GeneCopoeia, Inc.;
cat. no. HSH009476) was integrated into the pSUPER-retro-puromycin
plasmid (GeneCopoeia, Inc.). The sequence of shMACC1 was
5′-AAGAUUGGACUUGUACACUGC-3′ and the sequence of the negative
control (NC) shRNA was 5′-UUCUCCGAACGUGUCACGUTT-3′. Transfections
were performed using Lipofectamine™ 3000 Transfection Reagent
(Thermo Fisher Scientific, Inc.; cat. no. L3000008) in 37°C for 48
h unless otherwise specified. Subsequent experiments were performed
48 h after transfection.
Reverse transcription quantitative
(RT-q) PCR
Total RNA was extracted from cells using
TRIzol® Reagent (Thermo Fisher Scientific, Inc.; cat.
no. 15596-026) according to the manufacturer's instructions.
Briefly, the sample was homogenized with TRIzol® and
then chloroform (Sinopharm Chemical Reagent Co., Ltd.; cat. no.
10006818) was added. The homogenate was left to stand for at least
5 min at room temperature to allow its separation into an
RNA-containing aqueous phase and a lower organic layer. RNA was
then precipitated from the aqueous layer by adding isopropanol
(Sinopharm Chemical Reagent Co., Ltd.; cat. no. 80109218).
Extracted RNA was dissolved in RNase-free-dH2O. The
quantity and quality of extracted RNA were measured and confirmed
using NanoDrop 2000 (Thermo Fisher Scientific, Inc.), and 1 µg of
total RNA was reverse transcribed into cDNA using commercial
PrimeScript™ RT Master Mix (Perfect Real Time; Takara Bio, Inc.;
cat. no. RR036A) according to the manufacturer's instructions.
Briefly, RNA was diluted to adequate concentrations and added to 2
µl 5X PrimeScript RT Master Mix (Perfect Real Time) and
RNase-free-dH2O was added up to 10 µl. The reaction was
achieved using Veriti™ 96-Well Thermal Cycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.; cat. no. 4375786) according to the
following conditions: Incubation at 37°C for 1 h, then termination
at 85°C for 5 min to inactivate the enzymes. After the termination
reaction, the product was kept at 4°C, after which qPCR was
performed using TB Green® Premix Ex Taq™II (TliRNaseH
Plus; Takara Bio, Inc.; cat. no. RR820A). The reaction was
completed using Applied Biosystems 7300 Real-Time PCR System
(Thermo Fisher Scientific, Inc.) and the conditions of the reaction
were as follows: 95°C for 30 sec, 95°C for 5 sec, and 60°C for 34
sec (1 cycle), for a total of 40 cycles. β-actin was used as the
endogenous control. The relative expression levels were normalized
to endogenous control and were expressed as 2−ΔΔCq
(23). The sequences of the
primers are listed in Table
SI.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology; cat. no. P0013B)
containing 1% phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology; cat. no. ST506) and 1X Protease inhibitor cocktail
(Beyotime Institute of Biotechnology; cat. no. P1005) on ice.
Protein concentration was quantified by Pierce BCA protein assay.
Proteins (20 µg) were separated by 10–15% SDS-PAGE and were
transferred onto PVDF membranes (Sangon Biotech, Co., Ltd.). Then
the membranes were washed with ddH2O for 2 min with
shaking, rinsed with ddH2O twice, and incubated in
SuperSignal™ Western Blot Enhancer (Thermo Fisher Scientific, Inc.;
cat. no. 46640) for 10 min with shaking at room temperature to
enhance detection according to the manufacturer's instructions. The
membranes were blocked in 1% milk in 0.05% Tween-20 in TBST buffer
at room temperature for 1 h after washed with ddH2O for
5 times. Then membranes were incubated with primary antibody
diluted in the Primary Antibody Diluent from the enhancer kit
(1:1,000) against c-Met monoclonal antibody (Invitrogen; cat. no.
37-0100), MACC1 monoclonal antibody (Abcam; cat. no. ab242199),
alpha-fetoprotein monoclonal antibody (Abcam; cat. no. ab3980) and
β-actin monoclonal antibody (Thermo Fisher Scientific, Inc.; cat.
no. AM4302) for 1 h at room temperature. After washing three times
for 5 min with TBST buffer, membranes were incubated with
HRP-labeled Goat-Anti-Mouse IgG secondary antibody (H+L; Beyotime
Institute of Biotechnology; cat. no. A0216) at room temperature for
30 min. After washing membranes four times for 5 min, enhanced
chemiluminescence reagents (Thermo Fisher Scientific, Inc.; cat.
no. 32106) was applied to detect the signal on the membranes. The
data were analyzed via densitometry using Gel-Pro Analyzer Gelpro
32 software (Analytik Jena AG) and normalized to expression of the
internal control β-actin.
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
Cell proliferation was determined using the CCK-8
assay purchased from Dojindo Molecular Technologies, Inc. (cat. no.
CK04). Cells were seeded at a density of 5×103 cells per
well in a 96-well plate for 24 h and were transfected with
overexpression vector and/or shRNA, and further incubated for 24,
48 and 72 h. Subsequently, at 1 h before the endpoint of
incubation, 10 µl CCK-8 reagent was added to each well for 1 h.
Absorbance was read at 450 nm using a microplate reader.
Cell invasion and migration assay
The cell invasion and migration were assessed using
6.5 mm Transwell® migration assay, with 8.0 µm Pore
Polycarbonate Membrane Insert, Sterile (Corning, Inc.; cat. no.
3422). For cell invasion, serum-free medium was mixed with the BD
Matrigel™ hESC-qualified Matrix (BD Biosciences; cat. no. 354277)
in a 1:10 ratio. This mixture (50 µl) was added to the bottom of
the insert. The Matrigel was then incubated at 37°C for 4 h to
solidify. Then, 5×104 cells were transfected and at 24 h
following transfection, cells were harvested by trypsinization,
washed with serum-free medium and placed in the upper chamber of
the Transwell. The lower chamber contained 500 µl medium
supplemented with 10% FBS that was used as chemo-attractant. After
incubation at 37°C with 5% CO2 for 48 h, the cells in
the inner side of the chamber were removed using cotton swabs.
Invaded cells on the lower membrane surface were fixed with
methanol for 15 min at room temperature and stained with 0.1%
crystal violet for 10 min at room temperature. Images of the
invaded cells were taken using a light microscope (Olympus IX71,
×200 magnification) and cell numbers were counted. Cell migration
assay was performed in a similar way except that 1×105
cells were added into the insert without Matrigel pre-coating. Each
experiment was conducted in triplicate and repeated three
times.
Statistical analysis
Data analysis was performed using SAS version 9.4
software (SAS Institute, Inc.). Comparison among multiple groups
was performed using one-way ANOVA followed by Tukey's post-hoc
test. Data in Fig. 5 were analyzed
by two-way ANOVA followed by Tukey's post-hoc test, with group and
post-transfection time considered as independent variables. All
data were presented as the means ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
MACC-1 is upregulated following AFP
overexpression
We used 10% FBS in the medium as indicated in the
culture protocol of the cell line. The additional effect of 10% FBS
on growth can be ruled out by comparing experimental groups with
the respective controls.
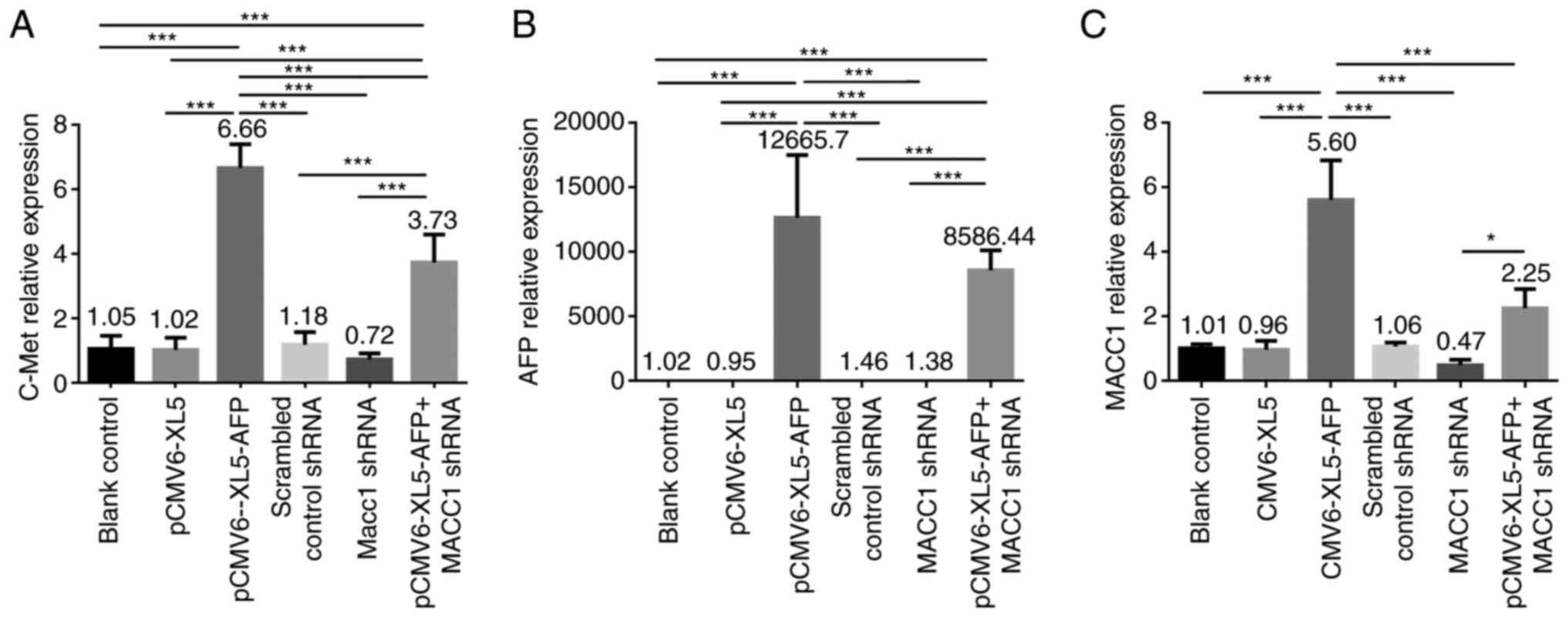
To investigate whether the mRNA level of c-Met, AFP
and MACC1 are regulated following AFP overexpression or MACC1
downregulation, RT-qPCR was performed on MKN-45 cell untreated or
transfected with empty vector (pCMV6-XL5), pCMV6-XL5-AFP, scrambled
control shRNA, MACC1 shRNA and pCMV6-XL5-AFP+MACC1 shRNA. The mRNA
expression level of c-Met was significantly elevated in
AFP-overexpressed and AFP-overexpressed + MACC1-downregulated
groups compared with the control group (Fig. 1A; both P<0.001), and was
significantly higher in AFP-overexpressed group compared with
AFP-overexpressed + MACC1-downregulated group (Fig. 1A; P<0.001).No significant
difference in mRNA expression level of c-Met was observed in
MACC1-downregulated group (Fig.
1A,). The higher expression level of AFP was observed in the
AFP-overexpressed and AFP-overexpressed + MACC1-downregulatedgroups
as seen in Fig. 1B (both
P<0.001). An elevated mRNA level of MACC1 was detected in both
AFP-overexpressed and AFP-overexpressed + MACC1-downregulated
groups (Fig. 1C). Furthermore, the
mRNA level of MACC1 was significantly increased in
AFP-overexpressed + MACC1-downregulated group compared with
MACC1-downregulated group (Fig.
1C; P<0.05).
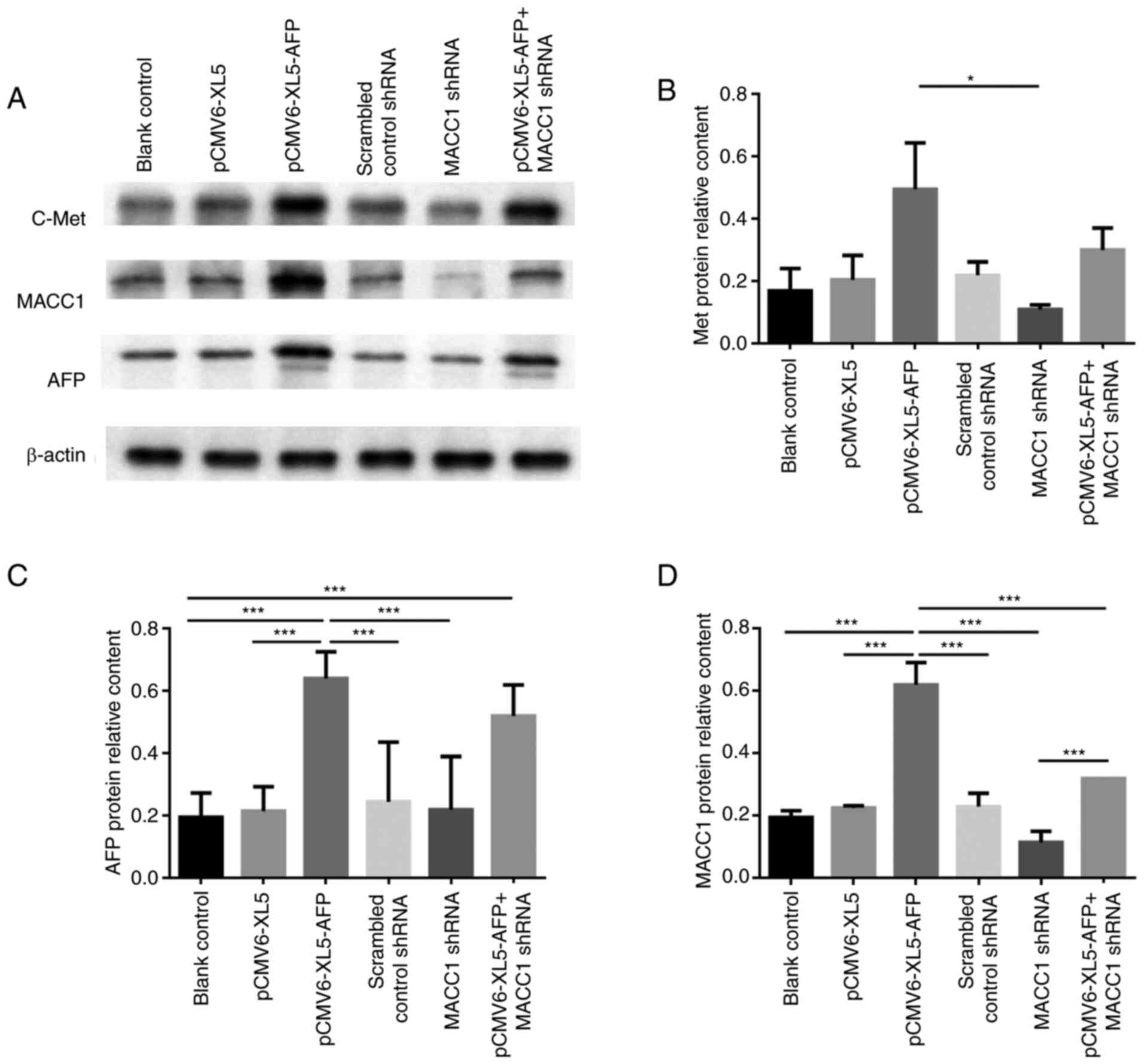
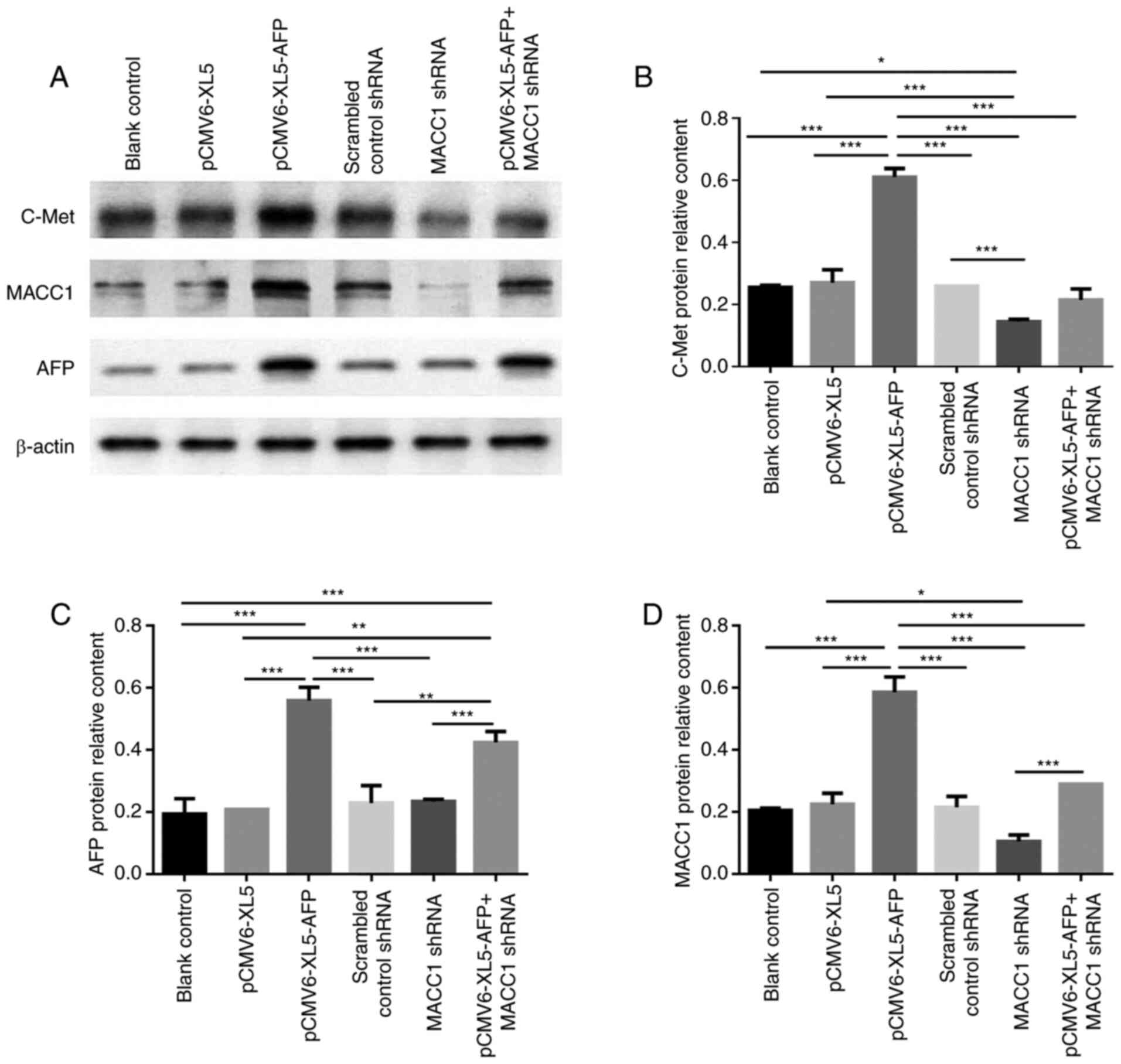
To further investigate the expression of the above
proteins after AFP overexpression or MACC1 downregulation, western
blotting was performed with the same approaches as in the RT-qPCR
experiments (Fig. 2A). In MKN-45
cells, the protein expression of c-Met was significantly higher in
AFP-overexpressed group than MACC1-downregulated group (Fig. 2B; P<0.05). The relative protein
expression of AFP was higher in AFP-overexpressed and
AFP-overexpressed + MACC1-downregulated groups (Fig. 2A and C; P<0.001), while that of
MACC1 was significantly elevated in the AFP-overexpressed group
(Fig. 2A and D; P<0.001).
Furthermore, protein expression of MACC1 was significantly higher
in AFP-overexpressed + MACC1-downregulated group compared with
MACC1-downregulated group (Fig. 2A and
D; P<0.001), suggesting MACC1 was upregulated by AFP.
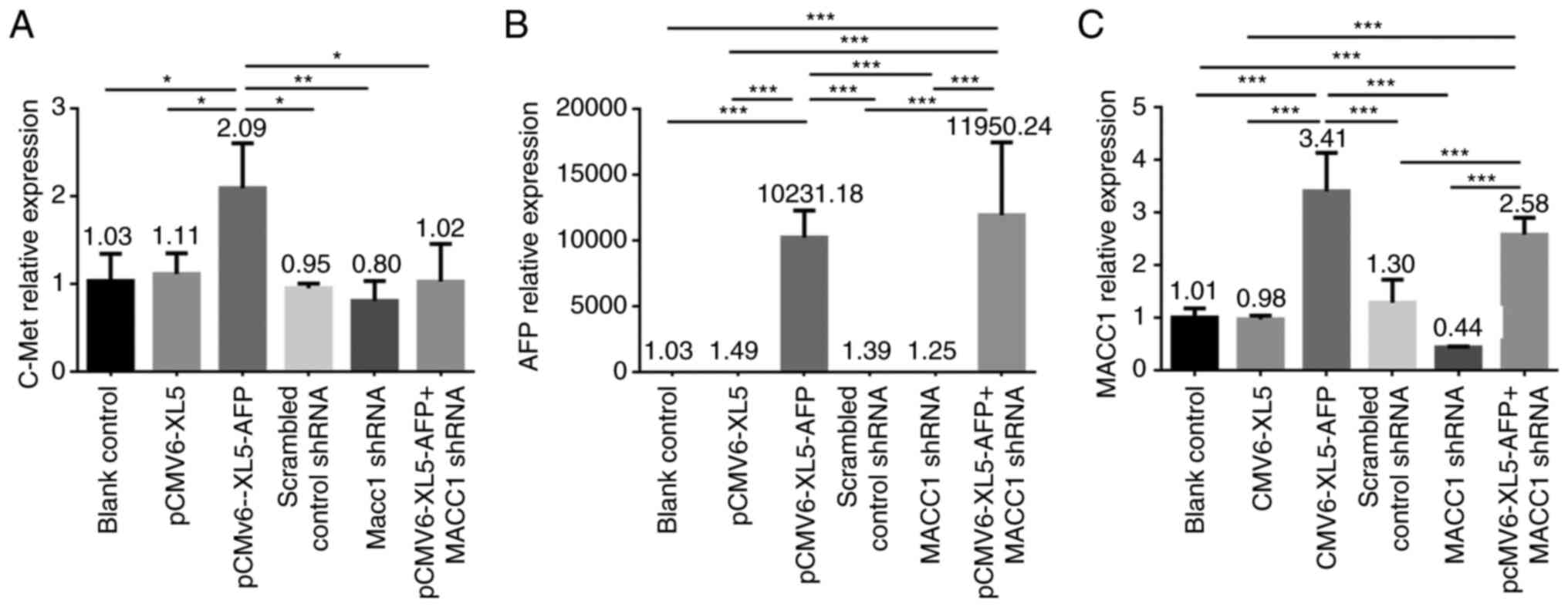
We also investigated the expression levels of c-Met,
AFP, and MACC1 in the GCIY cell line using the same approaches. The
mRNA level of c-Met compared with controls was significantly
elevated in AFP-overexpressed group (Fig. 3A; P<0.05), and no significant
difference was displayed in MACC1-downregulated group (Fig. 3A). The higher expression level of
AFP was observed in AFP-overexpressed and AFP-overexpressed +
MACC1-downregulated groups (Fig.
3B; both P<0.001), and a significantly elevated mRNA level
of MACC1 was detected in both AFP-overexpressed and
AFP-overexpressed + MACC1-downregulated groups (Fig. 3C; P<0.001). In the GCIY cell
line, the expression level of c-Met compared with control group was
significantly increased in the AFP-overexpressed group (Fig. 4A and B; P<0.001). A
significantly higher protein expression level of AFP was detected
in both AFP-overexpressed and AFP-overexpressed +
MACC1-downregulated groups (Fig. 4A
and C; P<0.001), and that of MACC1 was significantly
elevated in the AFP-overexpressed group (Fig. 4A and D; P<0.001). In addition,
the protein expression of MACC1 was significantly higher in
AFP-overexpressed + MACC1-downregulated group compared with
MACC1-downregulated group (Fig. 4A and
D; P<0.001), suggesting that AFP could upregulate MACC1.
Regulatory effect of AFP
overexpression or MACC1 downregulation on GC cell progression
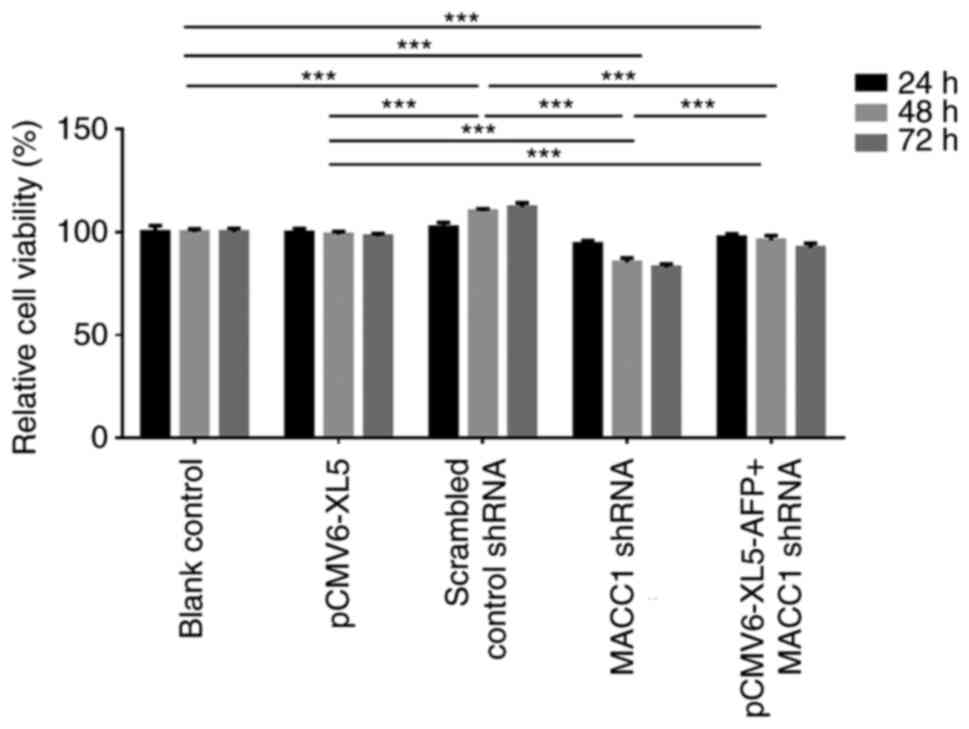
CCK-8 assay was performed in MKN-45 cells to analyze
the effects of AFP overexpression or MACC1 downregulation on cell
proliferation. Cells were treated and grouped similarly as in the
above experiments, except that the scrambled control shRNA group
was omitted. Fig. 5 presents the
relative cell viability normalized to control group at 24, 48 and
72 h following transfection. The results demonstrated that both
transfection time and group were significant sources of variation
(both P<0.001), and post-hoc Tukey's test comparison indicated
that there were differences between groups (P<0.001). Compared
with blank control group at 48 and 72 h, cell viability was
significantly increased in the AFP-overexpressed group
(P<0.001). A significantly decreased cell viability was observed
in the MACC1-downregulated group at all three time points
(P<0.001). Compared with AFP-overexpressed + MACC1-downregulated
group, cell viability was significantly lower in
MACC1-downregulated group (P<0.001) but higher in
AFP-overexpressed group (P<0.001) at the three time points.
These results suggested that AFP and MACC1 may enhance cell
viability.
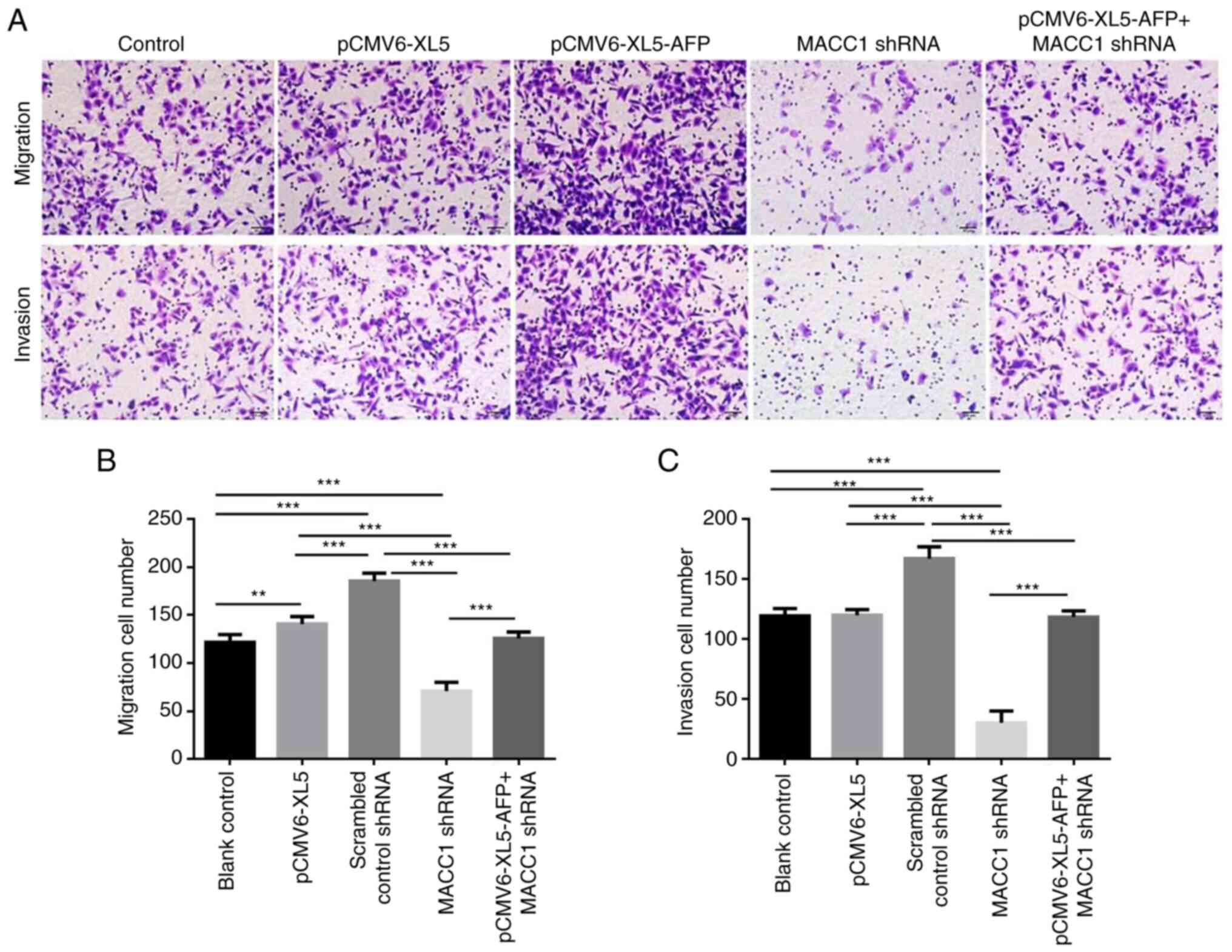
The effect of AFP overexpression or MACC1
downregulation on cell migration and invasion was also evaluated as
presented in Fig. 6A. Compared
with the control group, the number of migrated and invaded cells
was significantly increased in the AFP-overexpressed group and
AFP-overexpressed + MACC1-downregulated group (Fig. 6B and C; P<0.001).
Discussion
To the best of our knowledge, the present study was
the first to reveal that AFP could upregulate MACC1 activity and
thus promote GC proliferation, migration, and invasion. Numerous
previous studies reported that high expression levels of AFP and
MACC1 are significantly associated with higher metastasis and poor
prognosis in GC, respectively (8,9,14,15,24,25).
The findings from the present study demonstrated that AFP could
enhance tumor progression rather than acting as a tumor marker.
This may help illustrating the aggressive behaviors of AFP-GC and
common GC in patients with high AFP serum levels.
Serum AFP level is a prognostic factor for overall
survival and treatment response in patients with AFP-GC (24,25).
In common GC, a higher serum AFP level is also an independent
factor of poor prognosis (8,9).
However, the underlying molecular mechanism of AFP in GC
progression remains unclear. A previous study reported that
activation of the Wnt signaling pathway is responsible for
stimulation of cell proliferation and aggressiveness enhanced by
AFP in AFP-overexpressed GC cells (26). The findings from the present study
demonstrated the enhancement of GC progression by AFP via
upregulation of MACC1. To further confirmation this results, the
correlation between AFP and MACC1 expression levels should be
evaluated in human GC tissues. Additional research is therefore
needed to determine the molecular mechanism underlying AFP-MACC1
regulation on GC progression.
High expression levels of MACC1 in GC is
significantly associated with poor prognosis (15–21).
However, it remains unknown why the malignant progression is
accompanied by upregulation of MACC1. It has been reported that GC
cells escape from glucose deprivation by lowering the expression of
RhoA-specific GTPase-activating protein DLC3 to upregulate its
downstream target MACC1 (27),
suggesting that MACC1 upregulation may respond to metabolic stress.
Considering that the number of proliferated cells as well as MACC1
expression level were significantly higher in the AFP-overexpressed
group compared with the control in the present study, we
hypothesized that MACC1 upregulation may result from metabolic
stress because of an energy deficit due to uncontrolled cell
proliferation enhanced by AFP.
The results from the present study performed on two
GC cell lines showed that AFP upregulated MACC1 activity,
regardless of cellular c-Met protein expression levels. The
inconsistency between the mRNA and protein expression of c-Met and
AFP may be associated with a lack of certain post-transcriptional
factors in in vitro cell line systems. However, further
investigation is required to clarify the underlying mechanisms.
According to the results from the present study, AFP overexpression
enhanced GC cell viability, migration and invasion, thus
contributing to GC progression. Furthermore, knockout of MACC1 by
shRNA attenuated GC cell viability, migration and invasion. GC
progression may therefore be enhanced following activation of
MACC1.
HGF-Met signaling pathway plays an important role in
the proliferation and metastasis of gastrointestinal cancers
(11,12). High c-Met expression has been
reported in AFP-GC tissues (10)
but not in non-AFP GC tissues. The positive rate of c-Met in common
GCs ranges from 18 to 71.1% (26–30).
The gene amplification of c-Met is positively correlated with the
cancer stage, and c-Met was found to be overexpressed in GCs with
deeper invasion and distant metastasis (30,31).
A higher c-Met expression level has been observed in GC with liver
metastasis compared with the other primary tumor types (30). In addition, a positive correlation
between MACC1 and c-Met expression was reported in GC tissues
(15,16), and MACC1 was demonstrated to
mediate c-Met phosphorylation in the HGF/c-Met signaling pathway in
GC (32). However, further
investigation is required to clarify whether and how c-Met might be
involved in AFP-MACC1 regulation on GC progression, especially to
determine the following features: i) Phosphorylated c-Met level in
AFP-overexpressed and control GC cells; and ii) expression level of
AFP and MACC1 when nerve growth factor is upregulated in GC
cells.
This study presented some limitations. Firstly,
co-expression of AFP and MACC1 should be evaluated in human GC
samples to identify the existence of an AFP-MACC1 pathway.
Secondly, the level of phosphorylated c-Met in AFP-overexpressed
and control GC cells should be evaluated to determine whether c-Met
might be involved in AFP-MACC1 pathway. Thirdly, a colony formation
assay should be performed to validate the data from CCK8 assay.
In summary, the present study demonstrated that both
AFP and MACC1 enhance cell viability, migration and invasion of GC
cells. In addition, AFP may enhance GC progression via upregulating
MACC1 activity. Considering that AFP and MACC1 are predictors of
poor prognosis in patients with GC, further investigating this
AFP-MACC1 regulation may provide theoretical information for the
development of therapeutic approaches for GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL designed the study, reviewed the manuscript and
guaranteed the integrity of the entire study. XM performed
literature research, experimental studies and data acquisition and
prepared the manuscript. JW analyzed data, performed statistical
analysis and edited the manuscript. FL and XM confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
alpha-fetoprotein
|
|
AFP-GC
|
AFP-producing gastric cancer
|
|
MACC1
|
metastasis-associated colon cancer
1
|
|
GC
|
gastric cancer
|
|
HGF
|
hepatocyte growth factor
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinjo T, Taniguchi H, Kushima R, Sekine S,
Oda I, Saka M, Gotoda T, Kinjo F, Fujita J and Shimoda T:
Histologic and immunohistochemical analyses of
alpha-fetoprotein-producing cancer of the stomach. Am J Surg
Pathol. 36:56–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inoue M, Sano T, Kuchiba A, Taniguchi H,
Fukagawa T and Katai H: Long-term results of gastrectomy for
alpha-fetoprotein-producing gastric cancer. Br J Surg.
97:1056–1061. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Sheng W and Wang Y: An analysis of
clinicopathological features and prognosis by comparing hepatoid
adenocarcinoma of the stomach with AFP-producing gastric cancer. J
Surg Oncol. 106:299–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun W, Liu B, Chen J, Gong P, Wu X, Zhou
Liu C, Hong B and Gong W: Novel characteristics of
alpha-fetoprotein (AFP)-producing gastric cancer. Oncotarget.
8:101944–101951. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babali A, Cakal E, Purnak T, Biyikoglu I,
Cakal B, Yüksel O and Köklü S: Serum α-fetoprotein levels in liver
steatosis. Hepatol Int. 3:551–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Qu H, Jian M, Sun G and He Q: High
level of serum AFP is an independent negative prognostic factor in
gastric cancer. Int J Biol Markers. 30:e387–e393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng F, Sun L, Liu Z, Liu S, Zheng G, Xu
G, Guo M, Lian X, Fan D and Zhang H: Prognostic values of normal
preoperative serum cancer markers for gastric cancer. Oncotarget.
7:58459–58469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amemiya H, Kono K, Mori Y, Takahashi A,
Ichihara F, Iizuka H, Sekikawa T and Matsumoto Y: High frequency of
c-Met expression in gastric cancers producing alpha-fetoprotein.
Oncology. 59:145–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guinney J, Dienstmann R, Wang X, de
Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradley CA, Salto-Tellez M, Laurent-Puig
P, Bardelli A, Rolfo C, Tabernero J, Khawaja HA, Lawler M, Johnston
PG and Van Schaeybroeck S; MErCuRIC consortium, : Targeting c-MET
in gastrointestinal tumours: Rationale, opportunities and
challenges. Nat Rev Clin Oncol. 14:562–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin L, Huang H and Liao W, Ma H, Liu J,
Wang L, Huang N, Liao Y and Liao W: MACC1 supports human gastric
cancer growth under metabolic stress by enhancing the Warburg
effect. Oncogene. 34:2700–2710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong G, Wang M, Gu G, Li S, Sun X, Li Z,
Cai H and Zhu Z: MACC1 and HGF are associated with survival in
patients with gastric cancer. Oncol Lett. 15:3207–3213.
2018.PubMed/NCBI
|
|
16
|
Guo T, Yang J, Yao J, Zhang Y, Da M and
Duan Y: Expression of MACC1 and c-Met in human gastric cancer and
its clinical significance. Cancer Cell Int. 13:1212013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Wu Y, Lin L, Liu P, Huang H, Liao
W, Zheng D, Zuo Q, Sun L, Huang N, et al: Metastasis-associated in
colon cancer-1 upregulation predicts a poor prognosis of gastric
cancer, and promotes tumor cell proliferation and invasion. Int J
Cancer. 133:1419–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weidle UH, Birzele F and Krüger A:
Molecular targets and pathways involved in liver metastasis of
colorectal cancer. Clin Exp Metastasis. 32:623–635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie C, Wu J, Yun J, Lai J, Yuan Y, Gao Z,
Li M, Li J and Song L: MACC1 as a prognostic biomarker for
early-stage and AFP-normal hepatocellular carcinoma. PLoS One.
8:e642352013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie QP, Xiang C, Wang G, Lei KF and Wang
Y: MACC1 upregulation promotes gastric cancer tumor cell metastasis
and predicts a poor prognosis. J Zhejiang Univ Sci B. 17:361–366.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Y, Zhou K, Zhao W, Han R, Huo X, Yang
F and Chen J: Clinicopathological and prognostic significance of
metastasis-associated in colon cancer-1 in gastric cancer: A
meta-analysis. Int J Biol Markers. 34:27–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kataoka H, Miura Y, Joh T, Seno K, Tada T,
Tamaoki T, Nakabayashi H, Kawaguchi M, Asai K, Kato T and Itoh M:
Alpha-fetoprotein producing gastric cancer lacks transcription
factor ATBF1. Oncogene. 20:869–873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Li B, Yan B, Liu L, Jia Y, Wang Y,
Ma X and Yang F: The clinicopathological features and prognosis of
serum AFP positive gastric cancer: A report of 16 cases. Int J Clin
Exp Pathol. 13:2439–2446. 2020.PubMed/NCBI
|
|
25
|
Wang R, Li J, Xu D, Li R and Gong P:
Dynamic change in serum alpha-fetoprotein level predicts treatment
response and prognosis of alpha-fetoprotein-producing gastric
cancer. Medicine (Baltimore). 99:e233262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen D, Lin X, Zhang C, An G, Li Z, Dong
B, Shen L, Gao J and Zhang X: Activated Wnt signaling promotes
growth and progression of AFP-producing gastric cancer in
preclinical models. Cancer Manag Res. 11:1349–1362. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin L, Liu Y, Pan C, Zhang J, Zhao Y, Shao
R, Huang Z, Su Y, Shi M, Bin J, et al: Gastric cancer cells escape
metabolic stress via the DLC3/MACC1 axis. Theranostics.
9:2100–2114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang TJ, Wang JY, Lin SR, Lian ST and
Hsieh JS: Overexpression of the c-met protooncogene in human
gastric carcinoma-correlation to clinical features. Acta Oncol.
40:638–643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janjigian YY, Tang LH, Coit DG, Kelsen DP,
Francone TD, Weiser MR, Jhanwar SC and Shah MA: MET expression and
amplification in patients with localized gastric cancer. Cancer
Epidemiol Biomarkers Prev. 20:1021–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK,
Lee BL, Bang YJ and Kim WH: MET in gastric carcinomas: Comparison
between protein expression and gene copy number and impact on
clinical outcome. Br J Cancer. 107:325–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakajima M, Sawada H, Yamada Y, Watanabe
A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T and
Nakano H: The prognostic significance of amplification and
overexpression of c-met and c-erb B-2 in human gastric carcinomas.
Cancer. 85:1894–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang Y, Liang B, Jiang Y, Sun F, Zhao Y,
Wu Q, Hu X, Liu Y, Huang Q, Liao W, et al: MET transcriptional
regulator/serine peptidase inhibitor kunitz type 1 panel operating
through HGF/c-MET axis as a prognostic signature in pan-cancer.
Cancer Med. 10:2442–2460. 2021. View Article : Google Scholar : PubMed/NCBI
|