The glycosylation of proteins is a sophisticated
protein modification. Depending on the sugar-amino acid bond,
glycosylation may be divided into two categories: N-glycosylation
and O-glycosylation. Sugars are connected either to the amino
group's lateral chain of asparagine residues (N-linked
glycosylation) or, more commonly, to the hydroxyl group's lateral
chains of serine (Ser) or threonine (Thr) residues (O-linked
glycosylation) (1,2). N-glycosylation has been well studied,
but less is known about O-glycosylation. O-glycosylation is also
frequently referred to as mucin (MUC)-based glycosylation, as
O-glycans make up >80% of the sugar chain (3).
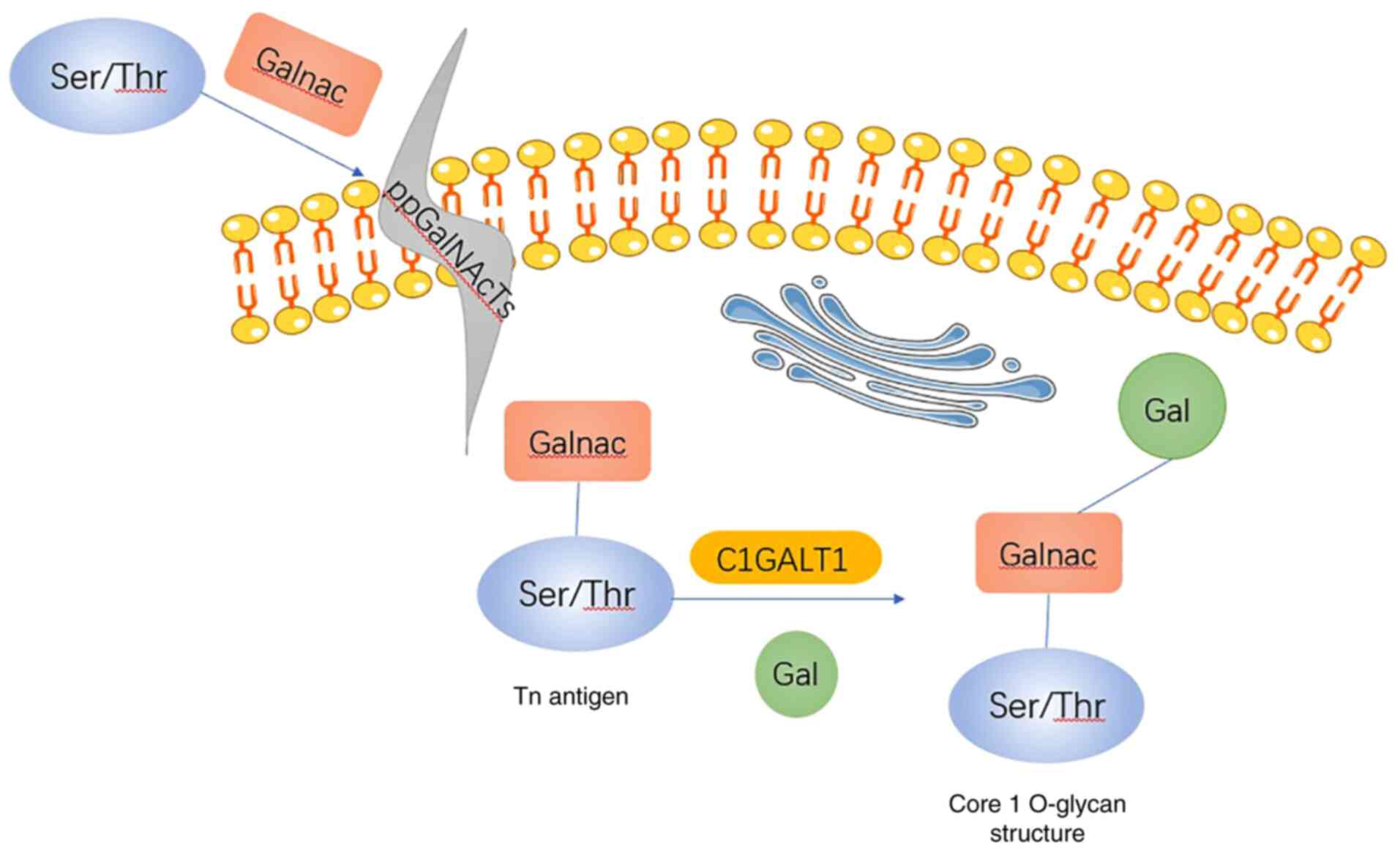
O-glycosylation is initiated in the presence of
polypeptide N-acetylgalactosamine-transferase-(GalNAc-T), which
catalyzes the formation of the GalNAcα1-O-Ser/Thr linkage in
O-glycoproteins. The synthesis process occurs in the Golgi
apparatus (4). The addition of
N-acetylgalactosamine (GalNAc) to Ser/Thr residues forms the GalNAc
α1-Ser/Thr structure [also known as the Thomsen-nouvelle (Tn)
antigen] (5–7). Subsequently, the Tn antigen further
forms core I, II and III structures under the action of
glycosyltransferases, such as core 1β1,3-galactosyltransferase
(C1GALT1), core 2β-1,6-N-acetylglucosaminyltransferase and core
3β1,3 N-acetylglucosaminyltransferase (7,8). The
formation of the core 1 structure is the most frequent modification
of the Tn antigen (9,10).
Glycosylation is important for megakaryocyte
development and platelet production in vivo (11). The pathogenesis of human tumors
suggests that cells acquire a series of characteristic functions,
such as maintenance of proliferation, evasion of growth inhibitors
and perpetual replication, during the transition from the normal to
the tumor state (12). Abnormal
glycosylation affects cell adhesion, migration and proliferation
(13). Glycosylation has recently
been proposed to be associated with the acquisition of labeling
capacity (14) and may be a
biomarker of cancer (13). Based
on the significance of glycosylation in the tumor pathway, the
present review focuses on the key enzyme of glycosylation, C1GALT1,
in tumorigenesis and therapy.
C1GALT1 (also known as core 1 synthase or
T-synthase), a glycosyltransferase with a molecular weight of 42-43
kDa, is encoded by chromosome 7p14-7p13. Analysis of the cDNA
sequence of human C1GALT1 has revealed that it contains three exons
(10). C1GALT1 is a mammalian
cell-specific T-synthase that catalyzes the transfer of galactose
(Gal) from UDP-Gal to the extant GalNAc (Galβ1, 3GalNAcα-O-Ser/Thr)
that forms the core 1 O-glycan (15) (Fig.
1). ClGALT1 has an important role in numerous biological
processes and alterations in its expression may cause developmental
defects and affect the malignant behavior of a tumor (16). Numerous glycoproteins have
regulatory roles in the development of the lymphatic system.
Knockdown of the C1GALT1 gene in endothelial and hematopoietic
cells in mice causes deletion of O-glycan chains in endothelial
cells of blood vessels and lymphatic vessels, resulting in
disruption of vascular-lymphatic connections and fatty liver
(17). C1GALT1 is abnormally
expressed in a variety of malignant tumor types, including
pancreatic cancer (18), gastric
cancer (19), head and neck
squamous cancer (20), laryngeal
cancer (21), ovarian cancer
(22) and liver cancer (23). In summary, C1GALT1 has an important
role in maintaining normal physiological functions and also
participates in the development of tumors.
Synthesis of the Tn antigen is the first step in the
initiation of O-glycosylation; under normal conditions, GalNAc is
first added to serine/threonine via an α-bond to form Tn antigen,
and then galactose is transferred to Tn antigen via a β-glycosidic
bond with the aid of C1GALT1 to form core 1-O glycans. However, in
the absence of C1GALT1, Tn antigen is not properly converted to T
antigen, resulting in overexpression of Tn antigen, followed by
increased expression of sialyl-Tn antigen (sTn antigen) in the
presence of increased Tn (24). Tn
antigen and sTn antigen are expressed in numerous types of tumor,
including colon, lung, cervical, ovarian, prostate and gastric
cancers. The expression of Tn antigen and sTn antigen is positively
associated with metastasis and poor prognosis of patients (25–27).
In addition, Tn or sTn antigen expression is also present in the
hinge region of IgA1 molecules in patients with IgA nephropathy as
a result of abnormal O-glycosylation of IgA1 (28).
Cosmc (or C1GALT1C1) is a unique molecular chaperone
essential for mammalian C1GALT1 (6,29).
Similar to T-synthase, Cosmc was first purified from rat liver and
its molecular weight is similar to that of T-synthase at 36-38 KDa
according to SDS-PAGE analysis (7). Cosmc is located on chromosome Xq24
and includes one encoding exon of ~1 kb and it has 26 homologous
sequences to C1GALT1 (30), which
maintains the stability and folding of C1GALT1 in the endoplasmic
reticulum (29).
Human T-leukemia Jurkat cells produce truncated
O-glycan (Tn antigen) due to deficiency of T-synthase activity
(31). The gene and transcript
level of T-synthase are normal in Jurkat cells, but a T deletion is
present at nucleotide position 478 in the Cosmc cDNA sequence,
causing early appearance of the stop codon (6). However, the addition of wild-type
Cosmc restores T-synthase activity and the normal extension of
O-glycan (6,32). Cosmc is involved in the
cotranslation of C1GALT1 and hinders the unfavorable clustering of
C1GALT1 (29). In the absence of
Cosmc, inactivated T-synthase accumulates and translocates from the
endoplasmic reticulum back into the cytoplasm where it is degraded
in a ubiquitin-/proteasome-dependent manner (33). However, Cosmc may reactivate the
activity of denatured T-synthase (34). Molecular chaperones bind to
non-natural proteins but not to natural proteins, forming stable
complexes that result in efficient folding (35). Regarding the combination of Cosmc
with unnatural C1GALT1, Ju et al (36) indicated that Cosmc does not affect
natural T-synthase but forms stable complexes with unnatural
T-synthase. Cosmc therefore provides a novel mechanism for
regulating protein O-glycosylation. Several studies have indicated
that Cosmc mutations cause C1GALT1 defects, resulting in Tn antigen
exposure across multiple blood cell lineages to form Tn syndromes
(37).
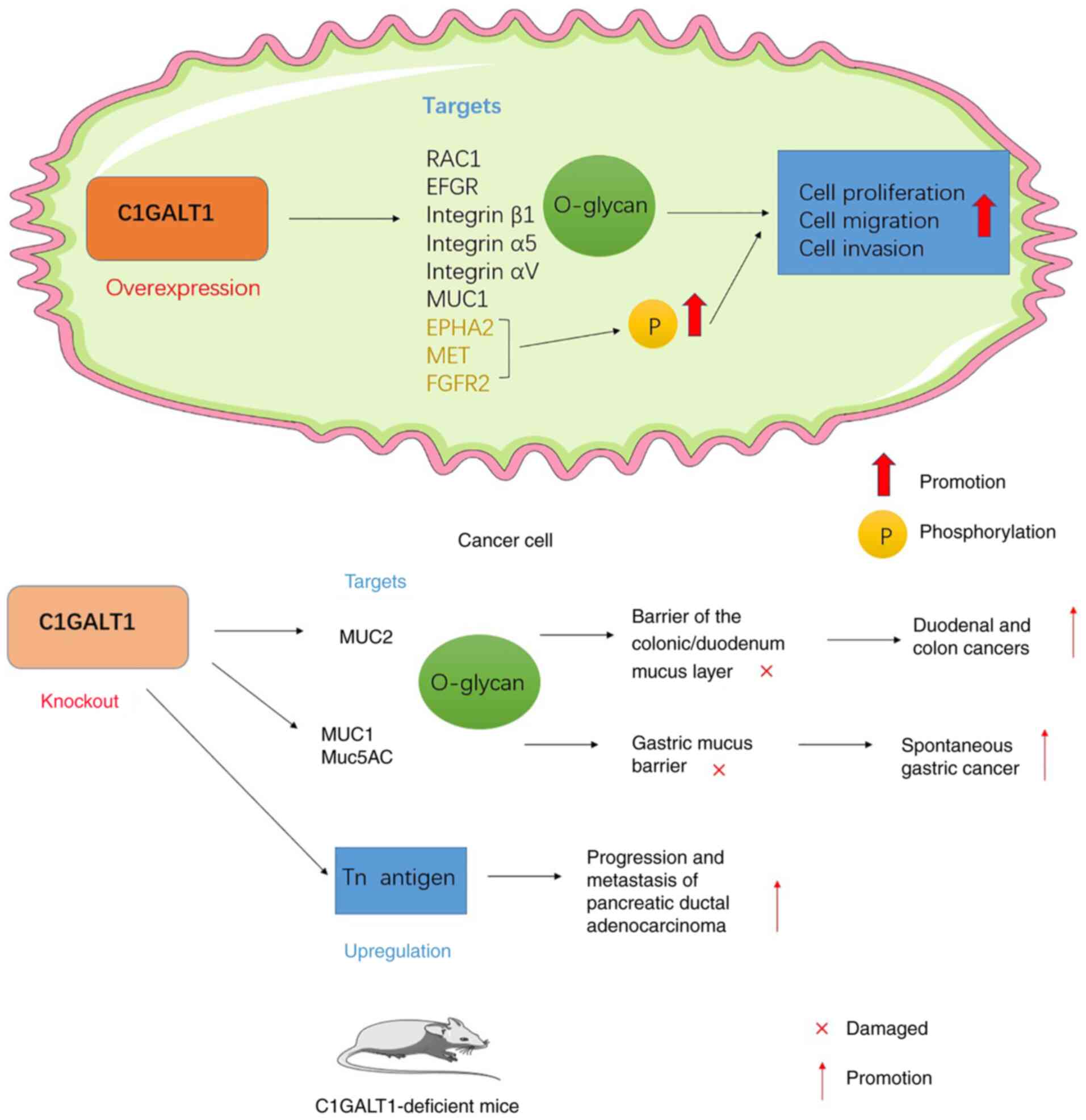
C1GALT1 expression is upregulated in most tumors.
Numerous studies have indicated that C1GALT1 overexpression is
closely associated with the malignant behavior of tumors, which
involves multiple steps, including proliferation, invasion, tumor
spread and immune evasion (38).
In addition, C1GALT1 expression is associated with poor patient
prognosis (39–41). To better analyze the role of
C1GALT1 in tumors, the molecular mechanisms by which C1GALT1 exerts
a regulatory role in cancer are discussed (Table I; Fig.
2).
miRNAs are a class of small noncoding RNAs that
regulate gene expression at the posttranscriptional level (42). miRNAs are involved in numerous
cellular processes, including cell growth, development,
differentiation and apoptosis (43,44).
miR-181d-5p has been reported to affect tumorigenesis and malignant
transformation in different signaling pathways (45,46).
Of note, miR-181d-5p has been indicated to have an oncogenic role
in non-small-cell lung cancer (47). Similarly, in lung adenocarcinoma
(LUAD), the overall survival rate of patients with low miR-181d-5p
expression was lower than that of patients with high miR-181d-5p
expression. On this basis, Dong et al (48) indicated that miR-181d-5p was able
to bind to the C1GALT1 3′UTR and act as an inhibitor of
proliferation, migration and invasion of LUAD cells.
miR-152 has also been reported to exhibit aberrant
expression in a variety of malignancies (49–51).
Dong et al (52) indicated
that in gastric cancer, miR-152 is an upstream regulator of C1GALT1
and able to negatively regulate C1GALT1 expression by binding to
the C1GALT1 3′-UTR. Overexpression of miR-152 decreased C1GALT1
expression, while downregulation of miR-152 increased C1GALT1
expression. They also demonstrated that the promoting effect of
C1GALT1 on the growth and metastasis of gastric cancer was
associated with miR-152.
The Rho family of small GTPases has been identified
as an important signaling effector in the regulation of cellular
morphology and motility. RAC1 is a member of the Rho family of
small GTPases (53) and is
involved in cellular activities, such as phagocytosis, adhesion,
migration, motility and proliferation (54). In recent years, RAC1 has been
reported to be involved in numerous physiological and pathological
processes, including cancer (55).
Aberrant expression of RAC1 is considered a hallmark of cancer and
increases the tumorigenic and metastatic properties of cancer cells
(56). There is increasing
evidence that different factors may affect RAC1 expression. For
instance, lncRNA NR2F2AS1 overexpression promoted Rac1 expression
in clear cell renal cell carcinoma (57), knockdown of RhoGDI2 decreased the
mRNA expression of Rac1 in gastric cancer (58) and knockdown of DDX3 decreased the
expression of RAC1 protein in medulloblastoma (59). De et al (55) indicated that the Rho GTPase
signaling pathway is closely related to C1GALT1 expression, while
RAC1 is a driver of tumor growth and metastasis. They further
reported that RAC1 is positively regulated by C1GALT1 in LUAD, as
high expression of C1GALT1 increased RAC1 expression, while low
expression of C1GALT1 decreases RAC1 expression. Furthermore,
knockdown of RAC1 reversed the oncogenic effect induced by C1GALT1
(48).
RTKs, such as epidermal growth factor receptors
(EGFRs), fibroblast growth factor receptor 2 (FGFR2) and MET, have
been reported to carry O-glycans (23,60–63).
O-glycosylation is involved in the phosphorylation of various RTKs
such as EGFR, FGFR2 and MET (20,23,60).
Evidence suggests that RTKs actively contribute to gastric
carcinogenesis and disease progression and are considered targets
for cancer therapy (64,65). Ephrin receptors are the largest
family of RTKs and affect a variety of developmental processes
(66). The human ephrin receptor
is composed of EPHAs and five EPHBs that preferentially bind their
respective ephrin A and B ligands. Ephrin receptors are highly
expressed in cancer and promote tumor development (67–70).
Therefore, EPH receptors are considered to be attractive targets
for tumor therapy (67,71,72).
EPHA2 and its ligand ephrin A1 are highly expressed in gastric
adenocarcinoma and overexpression of EPHA2 is associated with poor
prognosis (73,74). Yuan et al (75) reported that C1GALT1 is highly
expressed in gastric adenocarcinoma and that overexpression of
C1GALT1 promotes malignant behavior in gastric cancer cells.
C1GALT1 modifies the O-glycan on EPHA2 and regulates soluble ephrin
A1-induced tyrosine phosphorylation of EPHA2, and silencing EPHA2
results in inhibition of gastric cancer cell growth and invasion.
Knockdown of EPHA2 had no significant effect on cell viability but
EPHA2 knockdown diminished cell migration and invasion. By
contrast, high expression of C1GALT1 increased the tyrosine
phosphorylation of EPHA2, promoted the binding of ephrin A1 to the
cell surface and further enhanced soluble ephrin A1-induced
migration of gastric cancer cells (19).
RTKs are reported to have an important role in the
proliferation of hepatocellular carcinoma (76,77).
Previous studies focused on the effect of N-glycans on RTK, but in
recent years, it has been indicated that O-glycosylation is also
able to modulate their activity (62,78).
In hepatocellular carcinoma, MET signaling is aberrantly activated
when cell proliferation is abnormally enhanced (79–81).
Hepatocyte growth factor (HGF)/MET signaling has been indicated to
promote the invasion and metastasis of hepatocellular carcinoma
cells (82,83). Wu et al (23) reported that C1GALT1 was highly
expressed in hepatocellular carcinoma cells and that overexpression
of C1GALT1 enhanced the proliferation of hepatocellular carcinoma
cells, whereas knockdown of C1GALT1 resulted in inhibition of the
proliferation of hepatocellular carcinoma cells in vitro and
in vivo. In addition, C1GALT1 modified the O-glycan chain of
MET in RTK in hepatocellular carcinoma; low expression of C1GALT1
inhibited HGF-mediated phosphorylation of MET kinase, whereas
overexpression of C1GALT1 enhanced the phosphorylation of MET
(23). Receptor dimerization is a
key regulatory step of RTK signaling and C1GALT1 is likely to
regulate MET activity by enhancing its dimerization (84). The proliferative effect of C1GALT1
on hepatocellular carcinoma cells may be achieved by regulating MET
glycosylation and dimerization (23).
FGFR2 and its isoforms are overexpressed in
colorectal cancer and are involved in tumor growth, metastasis and
angiogenesis (85). C1GALT1 is
highly expressed in colorectal cancer; it enhances the
proliferation, migration, invasion, sphere formation and tumor
growth, as well as the metastatic potential of colorectal cancer
cells, and affects patient prognosis. It has been reported that,
when FGFR2 undergoes N-glycosylation, its N-glycan chain affects
FGFR2 activation and intracellular transport (86). Hung et al (60) reported that C1GALT1 was able to
regulate the O-glycan chain structure on FGFR2 in colon cancer
cells, which suggests that FGFR2 carries short O-glycan chains,
such as Tn and T antigen, in colon cancer cells. Furthermore, high
expression of C1GALT1 promoted the phosphorylation of FGFR2 and the
downstream signaling molecules ERK1/2. By contrast, low expression
of C1GALT1 reduced the phosphorylation of FGFR2 and ERK1/2.
Promotion of malignant behavior of colon cancer cells by high
C1GALT1 expression may be achieved by altering the O-glycosylation
and activity of FGFR2, while low C1GALT1 gene expression suppresses
these malignant properties both in vitro and in vivo
(60).
The extracellular structural domain of EGFR is
composed of four subregions, namely structural domains I, II, III
and IV, where structural domains I and III are responsible for
ligand binding (87). EGFR is
overexpressed in head and neck squamous cell carcinoma (HNSCC) and
its signaling pathway has an important role in cell proliferation
and invasion (88). C1GALT is
highly expressed in HNSCC and contributes to malignant behaviors
such as increased cell proliferation, migration and invasion. Of
note, Lin et al (20)
reported that EGFR structural domain III carries an
O-polysaccharide and that C1GALT1 regulates the O-glycan chain on
EGFR. Low expression of the C1GALT1 gene blocks the extension of
the O-glycan chain on EGFR, reduces the EGF-EGFR binding affinity,
inhibits EGFR signaling and acts as a suppressor of malignant
behavior (20). In prostate cancer
cells, C1GALT1 regulates EGFR O-glycosylation to enhance
galectin-4-mediated EGFR phosphorylation. When the C1GALT1 gene is
lowly expressed, it decreases galectin-4-mediated EGFR
phosphorylation but not ligand-mediated EGFR phosphorylation and
downregulates EGFR protein levels (89). By contrast, in HNSCC cells, low
expression of the C1GALT1 gene reduces EGF-mediated phosphorylation
of EGFR without affecting EGFR protein levels. The differential
effect of C1GALT1 on EGFR in prostate and HNSCC cells may be due to
cell-specific O-glycan groups on EGFR (20).
Changes in the interaction between cancer cells and
extracellular matrix (ECM) in the tumor microenvironment contribute
to the metastasis of cancer cells (90–93).
ECM receptors, such as integrins, are involved in cellular
interactions with the ECM, are strongly associated with malignant
tumorigenesis and have emerged as targets for cancer therapy
(91,93). In addition, integrins have been
suggested to be key factors in the invasion of hepatocellular
carcinoma cells (94–96). It has been reported that integrin
β1 is an O-glycosylated protein (97–99).
Various studies have highlighted the critical nature of
glycosyltransferases on ECM interactions through modification of
integrins (100–103). Liu et al (5) indicated that C1GALT1 is overexpressed
in hepatocellular carcinoma cells. C1GALT1 modifies the O-glycan on
integrin β1, and the promotion of cell adhesion, migration and
invasion by C1GALT1 was significantly attenuated by the use of
integrin β1 blockers in high C1GALT1-expressing cells. By contrast,
in cells with low C1GALT1 expression, these C1GALT1-induced
malignant phenotypes were not further inhibited by integrin β1
blockers. In addition, C1GALT1 also affects FAK, a downstream
signal of integrin β1. This indicates that C1GALT1 may regulate the
malignant behavior of hepatocellular carcinoma cells by regulating
the integrin β1 signaling pathway (5).
Integrins consist of a large family of αβ
heterodimeric transmembrane adhesion receptors that regulate
adhesion, survival and motility by activating multiple
intracellular signaling molecules and reorganizing the actin
cytoskeleton (104,105). It also has N- and O-linked
glycosylation sites (106).
Integrin α5 is involved in cancer development and progression by
promoting tumor cell adhesion and migration through the activation
of FAK (107–109). Integrin α5 is also an upstream
regulator of the PI3K/AKT pathway and Wang et al (110) determined that integrin α5 is a
downstream target of C1GALT1 in gastric cancer; low expression of
C1GALT1 inhibited, while high expression of C1GALT1 promoted the
activation of the PI3K/AKT pathway. Of note, the effects of high
C1GALT1 expression on the malignant behavior of tumor cells in
gastric cancer, such as proliferation, migration and invasion, were
suppressed by low expression of integrin α5 (52).
Pancreatic ductal adenocarcinoma (PDAC) is the most
common type of pancreatic cancer with abundant interstitial matrix,
mainly ECM proteins, regulating tumor growth and metastasis
(111). Kuo et al
(18) indicated that C1GALT1 was
overexpressed in PDAC and that overexpression of C1GALT1 promoted
tumor migration and invasion. By contrast, low expression of the
C1GALT1 gene diminished cell proliferation, migration and invasion
and inhibited tumor growth and metastasis (18). In addition, low expression of the
C1GALT1 gene resulted in upregulation of Tn antigen expression on
integrins αv and α5 in PDAC cells, accompanied by reduced cell-ECM
adhesion and phosphorylation of FAK, the most important downstream
signaling molecule of integrins (112,113). FAK is a key downstream signaling
molecule of integrins and is involved in the invasion of numerous
cancer types (113–115). Of note, antibody-mediated
knockdown of integrin αv significantly inhibited C1GALT1-mediated
invasiveness of PDAC. This suggests that integrin αv carries
O-glycan chains and that C1GALT1-mediated O-glycosylation regulates
integrin αv function (18).
MUC1 is a large transmembrane glycoprotein
consisting of a highly glycosylated extracellular portion and a
small cytoplasmic tail; it is a marker of poor prognosis and a
potential therapeutic target (116–118). MUC1 is a type I transmembrane
mucin that contains two subunits, MUC1-N and MUC1-C (119). MUC1 is expressed in almost all
epithelial tissues of the respiratory, gastrointestinal,
genitourinary and hepatobiliary tracts. High expression of MUC1 has
been frequently associated with tumor progression and poor
prognosis in colon, breast, ovarian, lung, prostate and pancreatic
cancers, which has led to the emergence of MUC1 as a major
direction for oncology treatment. Aberrant glycosylation of MUC1 is
observed in cancer. This is due to the N-terminus of MUC1
containing a Variable Number Tandem Repeat segment, which contains
Ser and Thr residues that may be involved in O-glycosylation
binding (120–122). Core 1 glycans on MUC1 increase
the degradation and accumulation of MUC1 (123). It has been reported that C1GALT1
is overexpressed in breast cancers and contributes to the enhanced
invasive and migratory potential of tumors by modifying the
O-glycan chain structure on MUC1, prompting increased detachment of
the MUC1-N subunit from the membrane and activating the ERK
phosphorylation level downstream of MUC1-C in breast cancer cells
(16). MUC1 is able to regulate
the Wnt signaling pathway by forming an intracellular complex with
β-catenin, which in may turn synergistically activate cyclin D1
expression in the nucleus, ultimately promoting tumorigenesis by
allowing cancer cells to avoid apoptosis (124). In addition, in pancreatic cancer,
it has also been indicated that MUC1 glycosylation affects the
invasiveness of pancreatic cancer cells (125). Wang et al (126) demonstrated that both MUC1 and
C1GALT1 were highly expressed in esophageal squamous carcinoma and
were positively correlated. Furthermore, C1GALT1 was determined to
affect the survival and prognosis of patients with esophageal
cancer by regulating the O-glycosylation of MUC1 (126). Abnormal MUC1 glycosylation may
cause shortening of glycans such as the Tn antigen, leading to
hidden antigen exposure; hidden antigens usually have peptide and
carbohydrate properties that make MUC1 antigen epitopes
tumor-specific (127). Transgenic
T cells with the MUC1-Tn chimeric antigen receptor have been
reported to be therapeutically effective in xenograft models of
T-cell leukemia and pancreatic cancer (128). Based on this, Kato et al
(129) screened an antibody
specifically against the MUC1-Tn antigen epitope carrying the Tn
antigen for study in LUAD; they indicated that the antibody
identified by the MUC1-Tn epitope was highly specific for LUAD
cells and that high expression of MUC1-Tn was present in LUAD, but
not in normal lung tissue. Antibodies specific for MUC1-Tn epitopes
are expected to be novel targets for LUAD therapy (129).
In the colorectum, C1GALT1 is essential for the
formation of an important mucus barrier in the gastrointestinal
tract. MUC2 is an important mucin involved in the formation of
major gels of the intestine (130–132). MUC2 has a barrier-stabilizing
role for the microbiota by forming an internal tissue adhesion
layer with the assistance of O-glycans (133). In a mouse model of core 1- and
core 3-derived O-glycan deficiency (C1galt1−/−;
C3GnT−/−), Bergstrom et al (134,135) observed that core 1- and 3-derived
O-glycan deletion causes microbial-dependent colitis as well as
severe colitis-associated cancers by reducing their stability to
bacterial-derived proteases, disrupting the barrier capacity of the
colonic mucus layer and activating epithelial-mesenchymal
transition. It is also accompanied by rapid degradation of MUC2 and
loss in the lumen, loss of mucus in the duodenal lumen and
disruption of homeostasis within the duodenal mucosa, which
triggers duodenal cancer (136).
In addition, O-glycans are also major components of
gastric mucins, including the membrane-bound MUC1 and the mucin
Muc5AC, which is involved in gastric gel formation (137). In mice with deletion of gastric
epithelial O-glycan (GEC C1galt1−/−), Liu et al
(15) determined that those GEC
C1galt1−/− mice develop severe spontaneous chronic
gastritis in the gastric sinus first and then progress to
spontaneous gastric cancer with abnormal expression of Muc5AC and
Muc1. They suggest that this is caused by casp1/11, a mucosal
inflammatory vesicle similar to that of the colon (15).
Dysregulation of C1GALT1 activity causes increased
expression of truncated O-glycans, and such high expression of
truncated O-glycan structures (e.g., Tn and sTn) are observed in
PDACs. To investigate the effect of truncated O-glycans on PDACs,
Chugh et al (138) further
established a C1GALT1 knockout mouse model based on the constructed
pancreatic tumor microenvironment (Kras and p53 mutations) and
observed that C1GALT1 knockout together with Kras and p53 mutations
accelerated the progression of pancreatic cancer and shortened
overall survival. In addition, glycosylation affects the molecular
weight of the protein and the molecular weight of the highly
glycosylated mucin MUC16, which is bound to the membrane in PDAC,
was indicated to decrease with the loss of C1GALT1. This was
accompanied by activation of the MUC16/EFGR//FAK signaling pathway,
which led to increased expression of the mesenchymal markers Slug,
Snail and Vimentin and decreased expression of epithelial markers
such as E-cadherin and Claudin-1, increasing the metastasis of PDAC
(Fig. 2) (138).
Radiotherapy is an effective route for tumor
treatment but radioresistance remains a major obstacle to tumor
outcomes. Several studies have indicated that altered glycosylation
is associated with the acquisition of a multidrug-resistant
phenotype (139–141). Of note, reports suggested that
numerous patients present with abnormal glycosylation when they are
resistant to radiotherapy (21,142–145). Therefore, discovering the cause
of the abnormalities that cause glycosylation is essential to
increase radiosensitivity. After constructing intrinsically
radiation-resistant (Hep-2max) and radiation-sensitive (Hep-2min)
cell lines of the parental laryngeal carcinoma Hep-2 cell line,
Dong et al (21) determined
that the intrinsically radiation-resistant cell line Hep-2max had a
higher content of core-type O-glycan chains than the
radiation-sensitive cell line Hep-2max. By contrast, C1GALT1
modified O-glycan chains on Hep-2max and Hep-2min cells and high
expression of C1GALT1 promoted the malignant behavior of laryngeal
cancer cells. Furthermore, high expression of C1GALT1 was
associated with increased tumor radioresistance, while knockdown of
C1GALT1 increased tumor radiosensitivity. In addition, blocking
integrin β1 attenuated the radioresistance induced by high C1GALT1
expression, suggesting that C1GALT1 radioresistance to laryngeal
cancer cells may be associated with integrin β1 (21). Zhang et al (146) indicated that C1GALT1, a key
enzyme in the glycosylation process, has an important role in the
radioresistance of esophageal cancer. Esophageal cancer cells
exhibiting high expression of C1GALT1 evaded cell death and had
increased resistance to radiotherapy. Low expression of C1GALT1
resulted in reduced resistance to radiotherapy of esophageal cancer
cells (146). Radiation promotes
the invasive potential of certain cancer cells (147). For instance, an increase in
radiation-induced invasiveness has been observed in breast cancer
(148), as well as increased
invasiveness of cancer cells after radiotherapy in pancreatic
cancer (149). In esophageal
cancer, irradiation by X-rays elevated C1GALT1 and core O-glycan
expression in esophageal cancer cells and enhanced invasion, while
knockdown of C1GALT1 diminished the invasive effect of irradiation
on esophageal cancer cells. Normal transduction of FAK signaling
downstream of β1-integrin facilitated cell proliferation and
survival and correlated with the radioresistance of cancer cells
(150). In esophageal squamous
carcinoma cells with C1GALT1 knockdown, the phosphorylation level
of FAK was reduced. Pretreatment with a FAK inhibitor promoted
radiation-induced apoptosis in esophageal squamous carcinoma cells.
It was demonstrated that the anti-radiation regulation of C1GALT1
in esophageal cancer cells was achieved by affecting O-glycosylated
C1GALT1 in β1-integrin, which in turn affected the β1-integrin/FAK
signaling pathway (146).
Itraconazole is a common antifungal drug with anticancer and
antiangiogenic effects (151,152). In HNSCC, Lin et al
(20) indicated that itraconazole
was able to directly interact with C1GALT1 and promote its
proteasomal degradation, resulting in reduced C1GALT1 expression in
HNSCC cells but not C1GALT1 mRNA expression, suggesting that the
effect of itraconazole on C1GALT1 protein levels may be achieved
through posttranslational modifications. In SAS cells, itraconazole
acted as a C1GALT1 inhibitor and partially reversed
C1GALT1-mediated effects on malignant behavior and EFGR activity in
HNSCC cells. In addition, erlotinib and lapatinib also inhibited
C1GALT1-mediated tumor cell viability and malignant behavior
(19,20).
C1GALT1, a key enzyme for O-glycosylation, is
receiving much attention. Recent oncological research has focused
on the role of C1GALT1 in tumor development. C1GALT1 is considered
a biomarker and potential therapeutic target for cancer diagnosis
and prediction of prognosis. The present article reviewed the
currently known mechanisms of action of C1GALT1 on the malignant
behavior of cancer cells and provided a theoretical basis for its
potential clinical role in cancer diagnosis and prognosis
determination. The dual regulatory roles of C1GALT1 in tumors may
be divided into roles of oncogenesis and tumor suppression. Its
role in oncogenic effects is mainly reflected in three pathways.
First, miR-181d-5p and miR-152 negatively regulate C1GALT1 to exert
oncogenic effects. Furthermore, deletion of C1GALT1 triggers
spontaneous gastric and duodenal cancers by disrupting the major
mucus barrier of the gastrointestinal tract. In addition, loss of
C1GALT1 elevates truncated Tn antigen expression, contributing to
higher tumorigenic and metastatic potential. By contrast, C1GALT1
achieves procancer effects by modifying the O-glycans of downstream
targets. Lapatinib, erlotinib and itraconazole, as C1GALT1
inhibitors, are able to exert anticancer effects by blocking the
malignant effects of C1GALT1 in cancer cells.
However, most studies currently focus only on the
predictive and poor prognostic value of high C1GALT1 expression
with cancer, but continued research is required to determine
whether there is consistency in its mechanism of action in
different tumor types. Furthermore, based on the cancer-promoting
effect of low C1GALT1 expression in the constructed mouse model, is
it tempting to speculate that complete deletion of the C1GALT1 gene
may be harmful to tumor patients. More in-depth studies are
required on the clinical significance of low C1GALT1 expression in
cancer patients. With novel methods and the efforts of research
scientists, the understanding of the impact of C1GALT1 expression
on tumor patients will be enhanced.
Not applicable.
This project was supported by the Natural Science Foundation of
Hunan Province (grant no. 2019JJ80022), the Clinical Medical
Technology Innovation Guidance Project of Hunan Province Technology
Innovation Guidance Program (grant no. 2018SK51503).
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
TXia developed the concept for the study and drafted
the manuscript. HX and TXiang reviewed and edited the manuscript.
TXiang performed a literature search and selection. All authors
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lis H and Sharon N: Protein glycosylation.
Structural and functional aspects. Eur J Biochem. 218:1–27. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spiro RG: Protein glycosylation: Nature,
distribution, enzymatic formation, and disease implications of
glycopeptide bonds. Glycobiology. 12:43R–56R. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arike L and Hansson GC: The Densely
O-Glycosylated MUC2 mucin protects the intestine and provides food
for the commensal bacteria. J Mol Biol. 428:3221–3229. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bennett EP, Mandel U, Clausen H, Gerken
TA, Fritz TA and Tabak LA: Control of mucin-type O-glycosylation: A
classification of the polypeptide GalNAc-transferase gene family.
Glycobiology. 22:736–756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CH, Hu RH, Huang MJ, Lai IR, Chen CH,
Lai HS, Wu YM and Huang MC: C1GALT1 promotes invasive phenotypes of
hepatocellular carcinoma cells by modulating integrin β1
glycosylation and activity. PLoS One. 9:e949952014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ju T and Cummings RD: A unique molecular
chaperone Cosmc required for activity of the mammalian core 1 beta
3-galactosyltransferase. Proc Natl Acad Sci USA. 99:16613–16618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ju T, Otto VI and Cummings RD: The Tn
antigen-structural simplicity and biological complexity. Angew Chem
Int Ed Engl. 50:1770–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galvan M, Tsuboi S, Fukuda M and Baum LG:
Expression of a specific glycosyltransferase enzyme regulates T
cell death mediated by galectin-1. J Biol Chem. 275:16730–16737.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ju T, Cummings RD and Canfield WM:
Purification, characterization, and subunit structure of rat core 1
Beta1,3-galactosyltransferase. J Biol Chem. 277:169–177. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ju T, Brewer K, D'Souza A, Cummings RD and
Canfield WM: Cloning and expression of human core 1
beta1,3-galactosyltransferase. J Biol Chem. 277:178–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo T, Sato T, Hagiwara K, Kozuma Y,
Yamaguchi T, Ikehara Y, Hamada M, Matsumoto K, Ema M, Murata S, et
al: C1galt1-deficient mice exhibit thrombocytopenia due to abnormal
terminal differentiation of megakaryocytes. Blood. 122:1649–1657.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vajaria BN and Patel PS: Glycosylation: A
hallmark of cancer? Glycoconj J. 34:147–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–35489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu F, Fu J, Bergstrom K, Shan X, McDaniel
JM, McGee S, Bai X, Chen W and Xia L: Core 1-derived mucin-type
O-glycosylation protects against spontaneous gastritis and gastric
cancer. J Exp Med. 217:e201823252020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou CH, Huang MJ, Chen CH, Shyu MK, Huang
J, Hung JS, Huang CS and Huang MC: Up-regulation of C1GALT1
promotes breast cancer cell growth through MUC1-C signaling
pathway. Oncotarget. 6:6123–6135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu J, Gerhardt H, McDaniel JM, Xia B, Liu
X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, et al:
Endothelial cell O-glycan deficiency causes blood/lymphatic
misconnections and consequent fatty liver disease in mice. J Clin
Invest. 118:3725–3737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo TC, Wu MH, Yang SH, Chen ST, Hsu TW,
Jhuang JY, Liao YY, Tien YW and Huang MC: C1GALT1 high expression
is associated with poor survival of patients with pancreatic ductal
adenocarcinoma and promotes cell invasiveness through integrin αv.
Oncogene. 40:1242–1254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee PC, Chen ST, Kuo TC, Lin TC, Lin MC,
Huang J, Hung JS, Hsu CL, Juan HF, Lee PH and Huang MC: C1GALT1 is
associated with poor survival and promotes soluble Ephrin
A1-mediated cell migration through activation of EPHA2 in gastric
cancer. Oncogene. 39:2724–2740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin MC, Chien PH, Wu HY, Chen ST, Juan HF,
Lou PJ and Huang MC: C1GALT1 predicts poor prognosis and is a
potential therapeutic target in head and neck cancer. Oncogene.
37:5780–5793. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong X, Luo Z, Wang Y, Meng L, Duan Q, Qiu
L, Peng F and Shen L: Altered O-glycosylation is associated with
inherent radioresistance and malignancy of human laryngeal
carcinoma. Exp Cell Res. 362:302–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chou CH, Huang MJ, Liao YY, Chen CH and
Huang MC: C1GALT1 seems to promote in vitro disease progression in
ovarian cancer. Int J Gynecol Cancer. 27:863–871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu YM, Liu CH, Huang MJ, Lai HS, Lee PH,
Hu RH and Huang MC: C1GALT1 enhances proliferation of
hepatocellular carcinoma cells via modulating MET glycosylation and
dimerization. Cancer Res. 73:5580–5590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karsten U and Goletz S: What controls the
expression of the core-1 (Thomsen-Friedenreich) glycotope on tumor
cells? Biochemistry (Mosc). 80:801–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Numa F, Tsunaga N, Michioka T, Nawata S,
Ogata H and Kato H: Tissue expression of Sialyl Tn antigen in
gynecologic tumors. J Obstet Gynaecol (Tokyo 1995). 21:385–389.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laack E, Nikbakht H, Peters A, Kugler C,
Jasiewicz Y, Edler L, Hossfeld DK and Schumacher U: Lectin
histochemistry of resected adenocarcinoma of the lung: Helix
pomatia agglutinin binding is an independent prognostic factor. Am
J Pathol. 160:1001–1008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konno A, Hoshino Y, Terashima S, Motoki R
and Kawaguchi T: Carbohydrate expression profile of colorectal
cancer cells is relevant to metastatic pattern and prognosis. Clin
Exp Metastasis. 19:61–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki H, Moldoveanu Z, Hall S, Brown R,
Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, et al:
IgA1-secreting cell lines from patients with IgA nephropathy
produce aberrantly glycosylated IgA1. J Clin Invest. 118:629–639.
2008.PubMed/NCBI
|
|
29
|
Narimatsu Y, Kubota T, Furukawa S,
Shimojima M, Iwasaki H, Tozawa Y, Tachibana K and Narimatsu H:
Co-translational function of Cosmc, core 1 synthase specific
molecular chaperone, revealed by a cell-free translation system.
FEBS Lett. 585:1276–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alexander WS, Viney EM, Zhang JG, Metcalf
D, Kauppi M, Hyland CD, Carpinelli MR, Stevenson W, Croker BA,
Hilton AA, et al: Thrombocytopenia and kidney disease in mice with
a mutation in the C1galt1 gene. Proc Natl Acad Sci USA.
103:16442–16447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piller V, Piller F and Fukuda M:
Biosynthesis of truncated O-glycans in the T cell line Jurkat.
Localization of O-glycan initiation. J Biol Chem. 265:9264–9271.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ju T, Lanneau GS, Gautam T, Wang Y, Xia B,
Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, et al: Human tumor
antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer
Res. 68:1636–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aryal RP, Ju T and Cummings RD: Tight
complex formation between Cosmc chaperone and its specific client
non-native T-synthase leads to enzyme activity and client-driven
dissociation. J Biol Chem. 287:15317–15329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aryal RP, Ju T and Cummings RD: The
endoplasmic reticulum chaperone Cosmc directly promotes in vitro
folding of T-synthase. J Biol Chem. 285:2456–2462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ellis RJ: The molecular chaperone concept.
Semin Cell Biol. 1:1–9. 1990.PubMed/NCBI
|
|
36
|
Ju T, Aryal RP, Stowell CJ and Cummings
RD: Regulation of protein O-glycosylation by the endoplasmic
reticulum-localized molecular chaperone Cosmc. J Cell Biol.
182:531–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ju T and Cummings RD: Protein
glycosylation: Chaperone mutation in Tn syndrome. Nature.
437:12522005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
An G, Wei B, Xia B, McDaniel JM, Ju T,
Cummings RD, Braun J and Xia L: Increased susceptibility to colitis
and colorectal tumors in mice lacking core 3-derived O-glycans. J
Exp Med. 204:1417–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guda K, Moinova H, He J, Jamison O, Ravi
L, Natale L, Lutterbaugh J, Lawrence E, Lewis S, Willson JK, et al:
Inactivating germ-line and somatic mutations in polypeptide
N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc
Natl Acad Sci USA. 106:12921–12925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brockhausen I: Pathways of O-glycan
biosynthesis in cancer cells. Biochim Biophys Acta. 1473:67–95.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shin S, Jung Y, Uhm H, Song M, Son S, Goo
J, Jeong C, Song JJ, Kim VN and Hohng S: Quantification of purified
endogenous miRNAs with high sensitivity and specificity. Nat
Commun. 11:60332020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Condrat CE, Thompson DC, Barbu MG, Bugnar
OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC: miRNAs
as biomarkers in disease: Latest findings regarding their role in
diagnosis and prognosis. Cells. 9:2762020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen H, Xiao Z, Yu R, Wang Y, Xu R and Zhu
X: miR-181d-5p-FOXP1 feedback loop modulates the progression of
osteosarcoma. Biochem Biophys Res Commun. 503:1434–1441. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang H, Wei H, Wang J, Li L, Chen A and Li
Z: MicroRNA-181d-5p-containing exosomes derived from CAFs promote
EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic
Acids. 19:654–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao LM, Zheng Y, Wang P, Zheng L, Zhang
WL, Di Y, Chen LL, Yin XB, Tian Q, Shi SS and Xu SF:
Tumor-suppressive effects of microRNA-181d-5p on non-small-cell
lung cancer through the CDKN3-mediated Akt signaling pathway in
vivo and in vitro. Am J Physiol Lung Cell Mol Physiol.
316:L918–L933. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong X, Liu Y, Deng X, Shao J, Tian S,
Chen S, Huang R, Lin Z, Chen C and Shen L: C1GALT1, negatively
regulated by miR-181d-5p, promotes tumor progression via
upregulating RAC1 in lung adenocarcinoma. Front Cell Dev Biol.
9:7079702021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng F, Liu H, Chen A, Xia Q, Zhao Y, Jin
X and Huang J: miR-148-3p and miR-152-3p synergistically regulate
prostate cancer progression via repressing KLF4. J Cell Biochem.
120:17228–17239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun J, Tian X, Zhang J, Huang Y, Lin X,
Chen L and Zhang S: Regulation of human glioma cell apoptosis and
invasion by miR-152-3p through targeting DNMT1 and regulating NF2:
MiR-152-3p regulate glioma cell apoptosis and invasion. J Exp Clin
Cancer Res. 36:1002017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma P, Li L, Liu F and Zhao Q:
HNF1A-induced lncRNA HCG18 facilitates gastric cancer progression
by upregulating DNAJB12 via miR-152-3p. Onco Targets Ther.
13:7641–7652. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong X, Chen C, Deng X, Liu Y, Duan Q,
Peng Z, Luo Z and Shen L: A novel mechanism for C1GALT1 in the
regulation of gastric cancer progression. Cell Biosci. 11:1662021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nguyen LK, Kholodenko BN and von
Kriegsheim A: Rac1 and RhoA: Networks, loops and bistability. Small
GTPases. 9:316–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zou T, Mao X, Yin J, Li X, Chen J, Zhu T,
Li Q, Zhou H and Liu Z: Emerging roles of RAC1 in treating lung
cancer patients. Clin Genet. 91:520–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
De P, Aske JC and Dey N: RAC1 Takes the
lead in solid tumors. Cells. 8:3822019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kazanietz MG and Caloca MJ: The Rac GTPase
in Cancer: From old concepts to new paradigms. Cancer Res.
77:5445–5451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen L, Zhang D, Ding T, Liu F, Xu X, Tian
Y, Xiao J and Shen H: LncRNA NR2F2-AS1 upregulates Rac1 to increase
cancer stemness in clear cell renal cell carcinoma. Cancer Biother
Radiopharm. 35:301–306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zeng Y, Ren M, Li Y, Chen C, Su J, Su B,
Xia H, Liu F, Jiang H, Ling H, et al: Knockdown of RhoGDI2
represses human gastric cancer cell proliferation, invasion and
drug resistance via the Rac1/Pak1/LIMK1 pathway. Cancer Lett.
492:136–146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen HH, Yu HI, Cho WC and Tarn WY: DDX3
modulates cell adhesion and motility and cancer cell metastasis via
Rac1-mediated signaling pathway. Oncogene. 34:2790–2800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hung JS, Huang J, Lin YC, Huang MJ, Lee
PH, Lai HS, Liang JT and Huang MC: C1GALT1 overexpression promotes
the invasive behavior of colon cancer cells through modifying
O-glycosylation of FGFR2. Oncotarget. 5:2096–2106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu SY, Shun CT, Hung KY, Juan HF, Hsu CL,
Huang MC and Lai IR: Mucin glycosylating enzyme GALNT2 suppresses
malignancy in gastric adenocarcinoma by reducing MET
phosphorylation. Oncotarget. 7:11251–11262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ,
Chen CH, Huang J, Lai HS, Lee PH, Hsu WM, et al: Mucin
glycosylating enzyme GALNT2 regulates the malignant character of
hepatocellular carcinoma by modifying the EGF receptor. Cancer Res.
71:7270–7279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huang MJ, Hu RH, Chou CH, Hsu CL, Liu YW,
Huang J, Hung JS, Lai IR, Juan HF, Yu SL, et al: Knockdown of
GALNT1 suppresses malignant phenotype of hepatocellular carcinoma
by suppressing EGFR signaling. Oncotarget. 6:5650–5665. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bradley CA, Salto-Tellez M, Laurent-Puig
P, Bardelli A, Rolfo C, Tabernero J, Khawaja HA, Lawler M, Johnston
PG and Van Schaeybroeck S; MErCuRIC consortium, : Targeting c-MET
in gastrointestinal tumours: Rationale, opportunities and
challenges. Nat Rev Clin Oncol. 14:562–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sierra JC, Asim M, Verriere TG, Piazuelo
MB, Suarez G, Romero-Gallo J, Delgado AG, Wroblewski LE, Barry DP,
Peek RM Jr, et al: Epidermal growth factor receptor inhibition
downregulates-induced epithelial inflammatory responses, DNA damage
and gastric carcinogenesis. Gut. 67:1247–1260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kania A and Klein R: Mechanisms of
ephrin-Eph signalling in development, physiology and disease. Nat
Rev Mol Cell Biol. 17:240–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xi HQ, Wu XS, Wei B and Chen L: Eph
receptors and ephrins as targets for cancer therapy. J Cell Mol
Med. 16:2894–2909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vaught D, Brantley-Sieders DM and Chen J:
Eph receptors in breast cancer: Roles in tumor promotion and tumor
suppression. Breast Cancer Res. 10:2172008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Herath NI and Boyd AW: The role of Eph
receptors and ephrin ligands in colorectal cancer. Int J Cancer.
126:2003–2011. 2010.PubMed/NCBI
|
|
70
|
Lisle JE, Mertens-Walker I, Rutkowski R,
Herington AC and Stephenson SA: Eph receptors and their ligands:
Promising molecular biomarkers and therapeutic targets in prostate
cancer. Biochim Biophys Acta. 1835:243–257. 2013.PubMed/NCBI
|
|
71
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Boyd AW, Bartlett PF and Lackmann M:
Therapeutic targeting of EPH receptors and their ligands. Nat Rev
Drug Discov. 13:39–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yuan WJ, Ge J, Chen ZK, Wu SB, Shen H,
Yang P, Hu B, Zhang GW and Chen ZH: Over-expression of EphA2 and
EphrinA-1 in human gastric adenocarcinoma and its prognostic value
for postoperative patients. Dig Dis Sci. 54:2410–2417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nakamura R, Kataoka H, Sato N, Kanamori M,
Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, et
al: EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.
96:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yuan W, Chen Z, Chen Z, Wu S, Guo J, Ge J,
Yang P and Huang J: Silencing of EphA2 inhibits invasion of human
gastric cancer SGC-7901 cells in vitro and in vivo. Neoplasma.
59:105–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zender L, Villanueva A, Tovar V, Sia D,
Chiang DY and Llovet JM: Cancer gene discovery in hepatocellular
carcinoma. J Hepatol. 52:921–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Marquardt JU, Galle PR and Teufel A:
Molecular diagnosis and therapy of hepatocellular carcinoma (HCC):
An emerging field for advanced technologies. J Hepatol. 56:267–275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Herr P, Korniychuk G, Yamamoto Y, Grubisic
K and Oelgeschläger M: Regulation of TGF-(beta) signalling by
N-acetylgalactosaminyltransferase-like 1. Development.
135:1813–1822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kaposi-Novak P, Lee JS, Gòmez-Quiroz L,
Coulouarn C, Factor VM and Thorgeirsson SS: Met-regulated
expression signature defines a subset of human hepatocellular
carcinomas with poor prognosis and aggressive phenotype. J Clin
Invest. 116:1582–1595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu
MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, et al: Role of overexpression of
CD151 and/or c-Met in predicting prognosis of hepatocellular
carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
D'Errico A, Fiorentino M, Ponzetto A,
Daikuhara Y, Tsubouchi H, Brechot C, Scoazec JY and Grigioni WF:
Liver hepatocyte growth factor does not always correlate with
hepatocellular proliferation in human liver lesions: Its specific
receptor c-met does. Hepatology. 24:60–64. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ma PC, Maulik G, Christensen J and Salgia
R: c-Met: Structure, functions and potential for therapeutic
inhibition. Cancer Metastasis Rev. 22:309–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Casaletto JB and McClatchey AI: Spatial
regulation of receptor tyrosine kinases in development and cancer.
Nat Rev Cancer. 12:387–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Matsuda Y, Ueda J and Ishiwata T:
Fibroblast growth factor receptor 2: Expression, roles, and
potential as a novel molecular target for colorectal cancer.
Patholog Res Int. 2012:5747682012.PubMed/NCBI
|
|
86
|
Hatch NE, Hudson M, Seto ML, Cunningham ML
and Bothwell M: Intracellular retention, degradation, and signaling
of glycosylation-deficient FGFR2 and craniosynostosis
syndrome-associated FGFR2C278F. J Biol Chem. 281:27292–27305. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Leemans CR, Snijders PJF and Brakenhoff
RH: The molecular landscape of head and neck cancer. Nat Rev
Cancer. 18:269–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tsai CH, Tzeng SF, Chao TK, Tsai CY, Yang
YC, Lee MT, Hwang JJ, Chou YC, Tsai MH, Cha TL and Hsiao PW:
Metastatic progression of prostate cancer is mediated by autonomous
binding of Galectin-4-O-Glycan to cancer cells. Cancer Res.
76:5756–5767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Marcucci F, Bellone M, Caserta CA and
Corti A: Pushing tumor cells towards a malignant phenotype: Stimuli
from the microenvironment, intercellular communications and
alternative roads. Int J Cancer. 135:1265–1276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fransvea E, Mazzocca A, Antonaci S and
Giannelli G: Targeting transforming growth factor (TGF)-betaRI
inhibits activation of beta1 integrin and blocks vascular invasion
in hepatocellular carcinoma. Hepatology. 49:839–850. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang C, Zeisberg M, Lively JC, Nyberg P,
Afdhal N and Kalluri R: Integrin alpha1beta1 and alpha2beta1 are
the key regulators of hepatocarcinoma cell invasion across the
fibrotic matrix microenvironment. Cancer Res. 63:8312–8317.
2003.PubMed/NCBI
|
|
96
|
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH,
Ding ZB, Wang XY, Devbhandari RP and Fan J: CD151 amplifies
signaling by integrin α6β1 to PI3K and induces the
epithelial-mesenchymal transition in HCC cells. Gastroenterology.
140:1629–1641.e15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee SH, Hatakeyama S, Yu SY, Bao X, Ohyama
C, Khoo KH, Fukuda MN and Fukuda M: Core3 O-glycan synthase
suppresses tumor formation and metastasis of prostate carcinoma PC3
and LNCaP cells through down-regulation of alpha2beta1 integrin
complex. J Biol Chem. 284:17157–17169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Clément M, Rocher J, Loirand G and Le
Pendu J: Expression of sialyl-Tn epitopes on beta1 integrin alters
epithelial cell phenotype, proliferation and haptotaxis. J Cell
Sci. 117:5059–5069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liao WC, Chen CH, Liu CH, Huang MJ, Chen
CW, Hung JS, Chou CH, Chen CH, Che MI, Chang HM, et al: Expression
of GALNT2 in human extravillous trophoblasts and its suppressive
role in trophoblast invasion. Placenta. 33:1005–1011. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Huang MC, Chen HY, Huang HC, Huang J,
Liang JT, Shen TL, Lin NY, Ho CC, Cho IM and Hsu SM: C2GnT-M is
downregulated in colorectal cancer and its re-expression causes
growth inhibition of colon cancer cells. Oncogene. 25:3267–3276.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Park JH, Katagiri T, Chung S, Kijima K and
Nakamura Y: Polypeptide N-acetylgalactosaminyltransferase 6
disrupts mammary acinar morphogenesis through O-glycosylation of
fibronectin. Neoplasia. 13:320–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang H, Meng F, Wu S, Kreike B, Sethi S,
Chen W, Miller FR and Wu G: Engagement of I-branching {beta}-1,
6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and
TGF-{beta} signaling. Cancer Res. 71:4846–4856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chang HH, Chen CH, Chou CH, Liao YF, Huang
MJ, Chen YH, Wang WJ, Huang J, Hung JS, Ho WL, et al:
β-1,4-Galactosyltransferase III enhances invasive phenotypes via
β1-integrin and predicts poor prognosis in neuroblastoma. Clin
Cancer Res. 19:1705–1716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Seguin L, Desgrosellier JS, Weis SM and
Cheresh DA: Integrins and cancer: Regulators of cancer stemness,
metastasis, and drug resistance. Trends Cell Biol. 25:234–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Marsico G, Russo L, Quondamatteo F and
Pandit A: Glycosylation and integrin regulation in cancer. Trends
Cancer. 4:537–552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ju JA, Godet I, Ye IC, Byun J, Jayatilaka
H, Lee SJ, Xiang L, Samanta D, Lee MH, Wu PH, et al: Hypoxia
selectively enhances integrin αβ receptor expression in breast
cancer to promote metastasis. Mol Cancer Res. 15:723–734. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li XQ, Lu JT, Tan CC, Wang QS and Feng YM:
RUNX2 promotes breast cancer bone metastasis by increasing integrin
α5-mediated colonization. Cancer Lett. 380:78–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pantano F, Croset M, Driouch K,
Bednarz-Knoll N, Iuliani M, Ribelli G, Bonnelye E, Wikman H, Geraci
S, Bonin F, et al: Integrin alpha5 in human breast cancer is a
mediator of bone metastasis and a therapeutic target for the
treatment of osteolytic lesions. Oncogene. 40:1284–1299. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang JF, Wang Y, Zhang SW, Chen YY, Qiu Y,
Duan SY, Li BP and Chen JQ: Expression and prognostic analysis of
integrins in gastric cancer. J Oncol. 2020:88622282020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hakamada K: Cancer stroma-targeting
therapy: A new tool for fighting pancreatic cancer? Ann
Gastroenterol Surg. 3:120–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Guan JL: Role of focal adhesion kinase in
integrin signaling. Int J Biochem Cell Biol. 29:1085–1096. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mitra SK, Mikolon D, Molina JE, Hsia DA,
Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG,
et al: Intrinsic FAK activity and Y925 phosphorylation facilitate
an angiogenic switch in tumors. Oncogene. 25:5969–5984. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen CH, Shyu MK, Wang SW, Chou CH, Huang
MJ, Lin TC, Chen ST, Lin HH and Huang MC: MUC20 promotes aggressive
phenotypes of epithelial ovarian cancer cells via activation of the
integrin β1 pathway. Gynecol Oncol. 140:131–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Guan JL: Integrin signaling through FAK in
the regulation of mammary stem cells and breast cancer. IUBMB Life.
62:268–276. 2010.PubMed/NCBI
|
|
116
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Taylor-Papadimitriou J, Burchell JM,
Plunkett T, Graham R, Correa I, Miles D and Smith M: MUC1 and the
immunobiology of cancer. J Mammary Gland Biol Neoplasia. 7:209–221.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Apostolopoulos V, Stojanovska L and
Gargosky SE: MUC1 (CD227): A multi-tasked molecule. Cell Mol Life
Sci. 72:4475–4500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen CH, Wang SW, Chen CW, Huang MR, Hung
JS, Huang HC, Lin HH, Chen RJ, Shyu MK and Huang MC: MUC20
overexpression predicts poor prognosis and enhances EGF-induced
malignant phenotypes via activation of the EGFR-STAT3 pathway in
endometrial cancer. Gynecol Oncol. 128:560–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen CH, Hsiao SM, Chang TC, Wu WY and Lin
HH: Clinical and urodynamic effects of baclofen in women with
functional bladder outlet obstruction: Preliminary report. J Obstet
Gynaecol Res. 42:560–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Razawi H, Kinlough CL, Staubach S, Poland
PA, Rbaibi Y, Weisz OA, Hughey RP and Hanisch FG: Evidence for core
2 to core 1 O-glycan remodeling during the recycling of MUC1.
Glycobiology. 23:935–945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kufe DW: MUC1-C oncoprotein as a target in
breast cancer: Activation of signaling pathways and therapeutic
approaches. Oncogene. 32:1073–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xu HL, Zhao X, Zhang KM, Tang W and Kokudo
N: Inhibition of KL-6/MUC1 glycosylation limits aggressive
progression of pancreatic cancer. World J Gastroenterol.
20:12171–12181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang Y, Liao X, Ye Q and Huang L: Clinic
implication of MUC1 O-glycosylation and C1GALT1 in esophagus
squamous cell carcinoma. Sci China Life Sci. 61:1389–1395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Gendler SJ, Spicer AP, Lalani EN, Duhig T,
Peat N, Burchell J, Pemberton L, Boshell M and Taylor-Papadimitriou
J: Structure and biology of a carcinoma-associated mucin, MUC1. Am
Rev Respir Dis. 144 (Suppl 1):S42–S47. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Posey AD, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR T cells targeting the
cancer-associated Tn-glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kato T, Ujiie H, Hatanaka KC, Nange A,
Okumura A, Tsubame K, Naruchi K, Sato M, Kaga K, Matsuno Y, et al:
A novel Tn antigen epitope-recognizing antibody for MUC1 predicts
clinical outcome in patients with primary lung adenocarcinoma.
Oncol Lett. 21:2022021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Allen A, Hutton DA and Pearson JP: The
MUC2 gene product: A human intestinal mucin. Int J Biochem Cell
Biol. 30:797–801. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tytgat KM, Büller HA, Opdam FJ, Kim YS,
Einerhand AW and Dekker J: Biosynthesis of human colonic mucin:
Muc2 is the prominent secretory mucin. Gastroenterology.
107:1352–1363. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
van Klinken BJ, Einerhand AW, Duits LA,
Makkink MK, Tytgat KM, Renes IB, Verburg M, Büller HA and Dekker J:
Gastrointestinal expression and partial cDNA cloning of murine
Muc2. Am J Physiol. 276:G115–G124. 1999.PubMed/NCBI
|
|
133
|
Johansson MEV, Phillipson M, Petersson J,
Velcich A, Holm L and Hansson GC: The inner of the two Muc2
mucin-dependent mucus layers in colon is devoid of bacteria. Proc
Natl Acad Sci USA. 105:15064–15069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bergstrom K, Fu J, Johansson ME, Liu X,
Gao N, Wu Q, Song J, McDaniel JM, McGee S, Chen W, et al: Core 1-
and 3-derived O-glycans collectively maintain the colonic mucus
barrier and protect against spontaneous colitis in mice. Mucosal
Immunol. 10:91–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q,
Song K, Cui Y, Li Y, McDaniel JM, McGee S, et al: Defective
intestinal Mucin-type O-Glycosylation causes spontaneous
colitis-associated cancer in mice. Gastroenterology.
151:152–164.e11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gao N, Bergstrom K, Fu J, Xie B, Chen W
and Xia L: Loss of intestinal O-glycans promotes spontaneous
duodenal tumors. Am J Physiol Gastrointest Liver Physiol.
311:G74–G83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Johansson MEV, Sjövall H and Hansson GC:
The gastrointestinal mucus system in health and disease. Nat Rev
Gastroenterol Hepatol. 10:352–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chugh S, Barkeer S, Rachagani S,
Nimmakayala RK, Perumal N, Pothuraju R, Atri P, Mahapatra S, Thapa
I, Talmon GA, et al: Disruption of C1galt1 Gene promotes
development and metastasis of pancreatic adenocarcinomas in mice.
Gastroenterology. 155:1608–1624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
da Fonseca LM, da Silva VA, Freire-de-Lima
L, Previato JO, Mendonça-Previato L and Capella MAM: Glycosylation
in Cancer: Interplay between Multidrug resistance and
Epithelial-to-Mesenchymal Transition? Front Oncol. 6:1582016.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ma H, Miao X, Ma Q, Zheng W, Zhou H and
Jia L: Functional roles of glycogene and N-glycan in multidrug
resistance of human breast cancer cells. IUBMB Life. 65:409–422.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wu J, Qin H, Li T, Cheng K, Dong J, Tian
M, Chai N, Guo H, Li J, You X, et al: Characterization of
site-specific glycosylation of secreted proteins associated with
multi-drug resistance of gastric cancer. Oncotarget. 7:25315–25327.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Huang C, Huang M, Chen W, Zhu W, Meng H,
Guo L, Wei T and Zhang J: N-acetylglucosaminyltransferase V
modulates radiosensitivity and migration of small cell lung cancer
through epithelial-mesenchymal transition. FEBS J. 282:4295–4306.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhuo E, He J, Wei T, Zhu W, Meng H, Li Y,
Guo L and Zhang J: Down-regulation of GnT-V enhances nasopharyngeal
carcinoma cell CNE-2 radiosensitivity in vitro and in vivo. Biochem
Biophys Res Commun. 424:554–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Park JJ and Lee M: Increasing the α 2, 6
sialylation of glycoproteins may contribute to metastatic spread
and therapeutic resistance in colorectal cancer. Gut Liver.
7:629–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shen L, Dong XX, Wu JB, Qiu L, Duan QW and
Luo ZG: Radiosensitisation of human glioma cells by inhibition of
β1,6-GlcNAc branched N-glycans. Tumour Biol. 37:4909–4918. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang C, Deng X, Qiu L, Peng F, Geng S,
Shen L and Luo Z: Knockdown of C1GalT1 inhibits radioresistance of
human esophageal cancer cells through modifying β1-integrin
glycosylation. J Cancer. 9:2666–2677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Moncharmont C, Levy A, Guy JB, Falk AT,
Guilbert M, Trone JC, Alphonse G, Gilormini M, Ardail D, Toillon
RA, et al: Radiation-enhanced cell migration/invasion process: A
review. Crit Rev Oncol Hematol. 92:133–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Paquette B, Therriault H, Desmarais G,
Wagner R, Royer R and Bujold R: Radiation-enhancement of MDA-MB-231
breast cancer cell invasion prevented by a cyclooxygenase-2
inhibitor. Br J Cancer. 105:534–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Qian LW, Mizumoto K, Urashima T, Nagai E,
Maehara N, Sato N, Nakajima M and Tanaka M: Radiation-induced
increase in invasive potential of human pancreatic cancer cells and
its blockade by a matrix metalloproteinase inhibitor, CGS27023.
Clin Cancer Res. 8:1223–1227. 2002.PubMed/NCBI
|
|
150
|
Wu J, Li Y, Dang YZ, Gao HX, Jiang JL and
Chen ZN: HAb18G/CD147 promotes radioresistance in hepatocellular
carcinoma cells: A potential role for integrin β1 signaling. Mol
Cancer Ther. 14:553–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Aftab BT, Dobromilskaya I, Liu JO and
Rudin CM: Itraconazole inhibits angiogenesis and tumor growth in
non-small cell lung cancer. Cancer Res. 71:6764–6772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kim J, Tang JY, Gong R, Kim J, Lee JJ,
Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al:
Itraconazole, a commonly used antifungal that inhibits Hedgehog
pathway activity and cancer growth. Cancer Cell. 17:388–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|