Introduction
Glioblastoma multiforme (GBM) is particularly
refractory among tumors and is highly resistant to radiation, with
a 5-year survival rate of 10% or less (1). The average life expectancy of GBM
patients is 1–2 years. GBM diffuses and invades the normal brain
tissue; therefore, it is difficult to surgically remove the tumor
completely. Some cell fractions have been reported to be highly
resistant to cancer therapy. Some of these cells are categorized as
cancer stem cells (CSCs). Even if the tumor shrunk or seemingly
disappeared after treatment with radiation or powerful anti-cancer
drugs, surviving CSCs are thought to cause recurrence or
metastasis. Additionally, CSCs have been reported to cause
difficulty in GBM therapy (2–6).
Our previous report (7) showed that cells immunofluorescently
positive for CD133, a marker of glioblastoma stem cells, are
induced in the hypoxic microenvironment of spheroids, which are
three-dimensional culture models (8). Furthermore, we revealed that spheroid
CD133-positive cells (SCPCs) exhibit some CSC-like characteristics,
including increased migration and inversion abilities and
resistance to radiation and anti-cancer agents (9). Based on these results, we propose
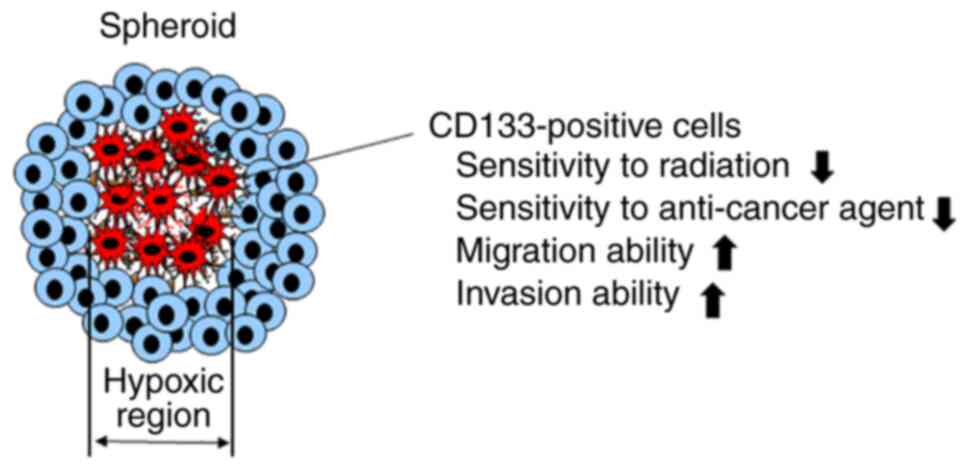
that SCPCs are glioblastoma CSC-like cells, as shown in Fig. 1. Using SCPCs, the present study
examined whether the sensitivity of CSC-like cells to radiation was
enhanced by N-vinylpyrrolidone (NVP)-AUY922.
Heat shock protein 90 (Hsp90) is a well-known
chaperone protein that protects against the degradation of numerous
client proteins that contribute to the maintenance of cancer cells
(10). Inhibition of Hsp90
function may lead to the loss of cancer cell malignancy. Thus,
targeting Hsp90 using Hsp90 inhibitors provides a promising
strategy to develop anti-cancer agents (11) or radiosensitizers (12,13).
Geldanamycin and its derivative
17-allylamino-17-demethoxygeldanamcyin (17-AAG) are commonly used
Hsp90 inhibitors (14). However,
it has been reported that Hsp90 inhibitors cause severe side
effects concerning the kidney and liver and chemoresistance in some
tumors, including GBM (15–17).
NVP-AUY922 is a purine-scaffold derivative and non-geldanamycin
analog of 17-AAG (18). NVP-AUY922
mimics the ATP/adenosine diphosphate (ADP)-binding interface of the
Hsp90 N-terminus, thereby inhibiting client proteins. NVP-AUY922
effectively overcomes 17-AAG resistance in glioblastoma cells with
less pronounced side effects, even at low doses (19). In the present study, we examined
the radiosensitizing effects of NVP-AUY922 on SCPCs and spheroid
CD133-negative cells (SCNCs) derived from spheroids cultured from
T98G cells. Radiosensitization by Hsp90 inhibitors has not yet been
examined in preclinical studies targeting cancer stem cells. The
novelty of the present study is to compare effects of NVP-AUY922 on
radiosensitivity between cancer cells and cancer stem cell-like
cells.
Materials and methods
Cell culture
We used the human glioblastoma cell line, T98G
(provided by JCRB, Setagaya, Tokyo), in the present study. T98G
cells were cultured in α-minimum essential medium (α-MEM)
supplemented with 20 mM 4-(2-hydroxyethyl) piperazine ethane
sulfonic acid, 8 mM NaHCO3, 50 µg/ml streptomycin, 50
U/ml penicillin, and 10% fetal calf serum and maintained in a
humidified incubator at 37°C containing a mixture of 98% air and 2%
CO2.
Spheroid culture
T98G cells were seeded onto non-adherent U-shaped
bottom 96-well plates (PrimeSurface 96U, MS-9096U, Sumitomo
Bakelite Co., Ltd.). The seeded cells were cultured in α-MEM at a
density of 5,000 or 10,000 cells/well for 3 days at 37°C under
conditions of 98% air and 2% CO2. The seeded cells
aggregated and formed a cell mass at the bottom of the nonadherent
U-shaped wells on the plates. The spheroids formed were transferred
to non-adherent 100-mm dishes (PrimeSurface 100Φ, MS-9090×,
Sumitomo Bakelite Co., Ltd.) at a density of 96 spheroids/dish
after 3 days of culture. The transferred spheroids were then
successively cultured for 7–10 days until they reached a diameter
of 300–500 µm.
Preparation of frozen cryostat
sections
After the spheroids were fixed with a solution
containing 10% formalin and 10% sucrose for 1 h, the spheroids were
rinsed with phosphate-buffered saline (PBS) (-). The spheroids were
then embedded in Tissue-Tek O.C.T. Compound (Sakura Finetechnical
Co., Ltd.) and sliced into frozen sections 10–20 µm in thickness
using a cryostat.
Immunofluorescence staining
Cryostat sections on glass slides were incubated for
double immunofluorescent staining using anti-CD133AC133
monoclonal antibody (Miltenyi Biotechnology; 1:10 dilution),
anti-HIF-1α polyclonal antibody (Novus Biologicals; 1:100
dilution), or anti-nestin polyclonal antibody (Santa Cruz
Biotechnology; 1:50 dilution) for 1 h at room temperature.
Subsequently, the sections were washed thrice using PBS (-) and
then incubated with Alexa Fluor 488 anti-rabbit and Alexa Fluor 546
anti-mouse secondary antibodies (Nacalai Tesque; 1:1,000 dilution)
for 1 h at 37°C. After washing with PBS (-), the sections were
embedded with SlowFade Gold antifade reagent containing
4,6-diamidino-2-phenylindole (Invitrogen; Thermo Fisher Scientific,
Inc.), and covered with a glass coverslip. For immunofluorescent
staining of monolayer cultured cells, cells were seeded onto a
glass slide and cultured for 1 day at 37°C with a mixture of 98%
air and 2% CO2. After 1 day, the cells were washed with
PBS (-), fixed with 10% formalin for 1 h, and then incubated for
double immunofluorescent staining using the same method used for
cryostat section staining.
Fluorescence-activated cell
sorting
After culturing the spheroids for 7–10 days, the
spheroids were rinsed once with PBS (-) and then treated with
0.025% trypsin for 5 min at 37°C. Trypsin-treated spheroids were
dispersed into single cells via pipetting. The single cells were
centrifuged at 1,200 × g for 5 min, rinsed thrice using PBS (-),
and treated for 10 min with a PE-labeled anti-CD133 monoclonal
antibody (Miltenyi Biotechnology 1:10 dilution) and
7-aminoactinomycin D (7-AAD; 1:10 dilution) for dead cell-labeling.
PBS containing 1% bovine serum albumin (BSA) was used for dilution.
Subsequently, the single cells were centrifuged, rinsed thrice with
PBS (-), and suspended in PBS containing 1% BSA. BD FACSJazz cell
sorter (BD Biosciences) was used for flow cytometry analysis.
Finally, CD133-positive and 7-AAD-negative cells or CD133-negative
and 7-AAD-negative cells were sorted as SCPCs and SCNCs,
respectively, according to the manufacturer's protocol (BD
Biosciences). The clonogenic cell survival assay was performed
within 1 h of sorting the cells.
Clonogenic cell survival assay
The proportion of surviving cells was measured using
a colony formation assay. Exponentially growing cells were plated
at a density of 1.0×103 or 3.0×103 cells in
three replicate 6-well plates. These cells were then incubated at
37°C for 3 h in a CO2 incubator. Subsequently, the cells
were irradiated in α-MEM with 150 kV X-rays emitted by an
irradiator (Softex, M-150ME) at a dose rate of 1.1 Gy/min under
normoxia at room temperature. Non-irradiated cells were treated
with 0, 10, and 50 nM of NVP-AUY922 (Novartis) or dimethyl
sulfoxide (DMSO) for 24 h, following which the medium containing
NVP-AUY922 or DMSO was replaced with a NVP-AUY922-free or DMSO-free
medium. We used doses of 10 and 50 nM of NVP-AUY922 on the basis of
pre-experiments. Under combined treatment with X-rays and
NVP-AUY922, the cells were irradiated in α-MEM containing
NVP-AUY922 or DMSO. α-MEM containing NVP-AUY922 or DMSO was
replaced with a NVP-AUY922- or DMSO-free medium 24 h after
irradiation. The cells were subsequently incubated at 37°C for
10–14 days in a CO2 incubator. The colonies formed were
stained with crystal violet and dissolved in 20% methanol. Colonies
containing more than 50 cells were counted as survivors. Three
independent experiments were performed.
Statistical analysis
To analyze a significant interaction effect, two-way
ANOVA followed by a post hoc Tukey's HSD test was conducted. Paired
Student's t-test was also used for confirmation of the difference
between CD133-positive and CD133-negative cells. P<0.01 was
considered to indicate a statistically significant difference.
Results
Immunofluorescent double-staining of
CD133AC133 and HIF-1α
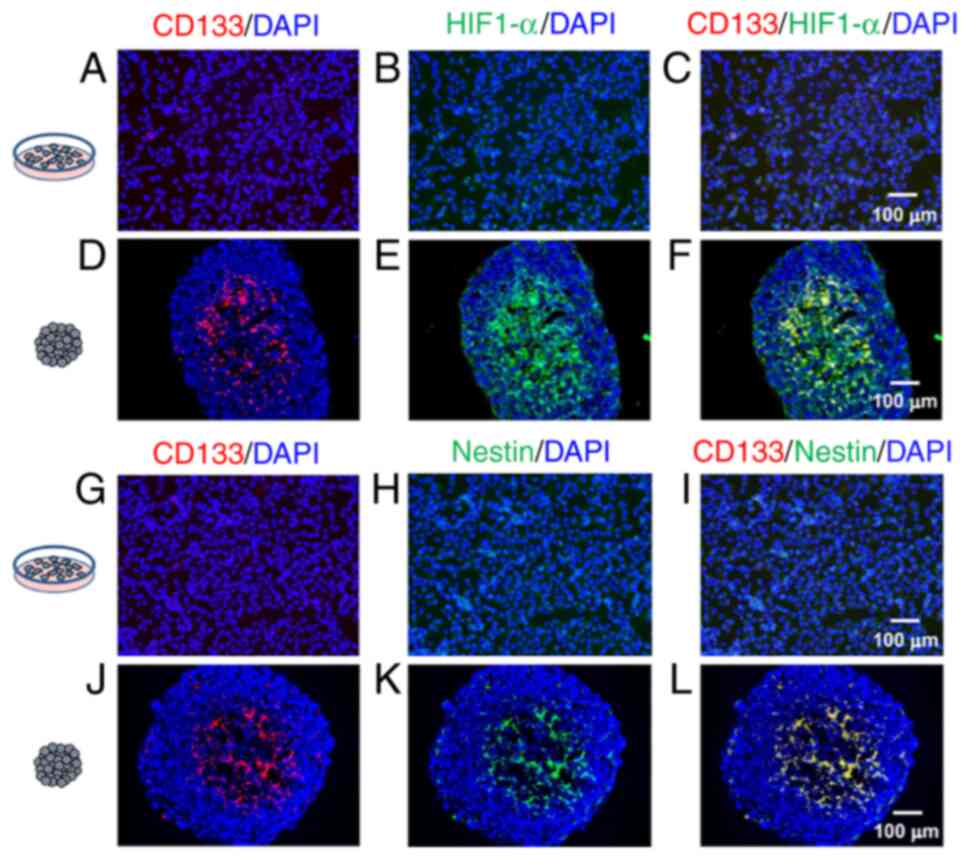
Fig. 2A-F shows
anti-CD133AC133 and anti-HIF-1α antibody
immunofluorescence double-stained images. In monolayer cultured
cells (Fig. 2A-C), only a few
CD133-or HIF-1α-positive cells were observed occasionally. In
contrast, in the cryostat sections from spheroids (Fig. 2D-F), CD133 positive or HIF-1α
positive cells were observed. CD133 positive cells were also
positive for HIF-1α in the hypoxic region, as suggested by HIF-1α
positivity (Fig. 2F).
Immunofluorescent double-staining of
CD133AC133 and nestin
Fig. 2G-L shows
CD133AC133 and nestin (a marker for undifferentiated
neural cell) antibodies double-stained immunofluorescence images.
In monolayer cultured cells (Fig.
2G-I), only a few CD133- and nestin-positive cells were
observed occasionally. In contrast, CD133 positive or
nestin-positive cells were observed in the cryostat sections from
spheroids (Fig. 2J-L). CD133
positive cells were also positive for nestin and were observed in
the central region of spheroids (Fig.
2L).
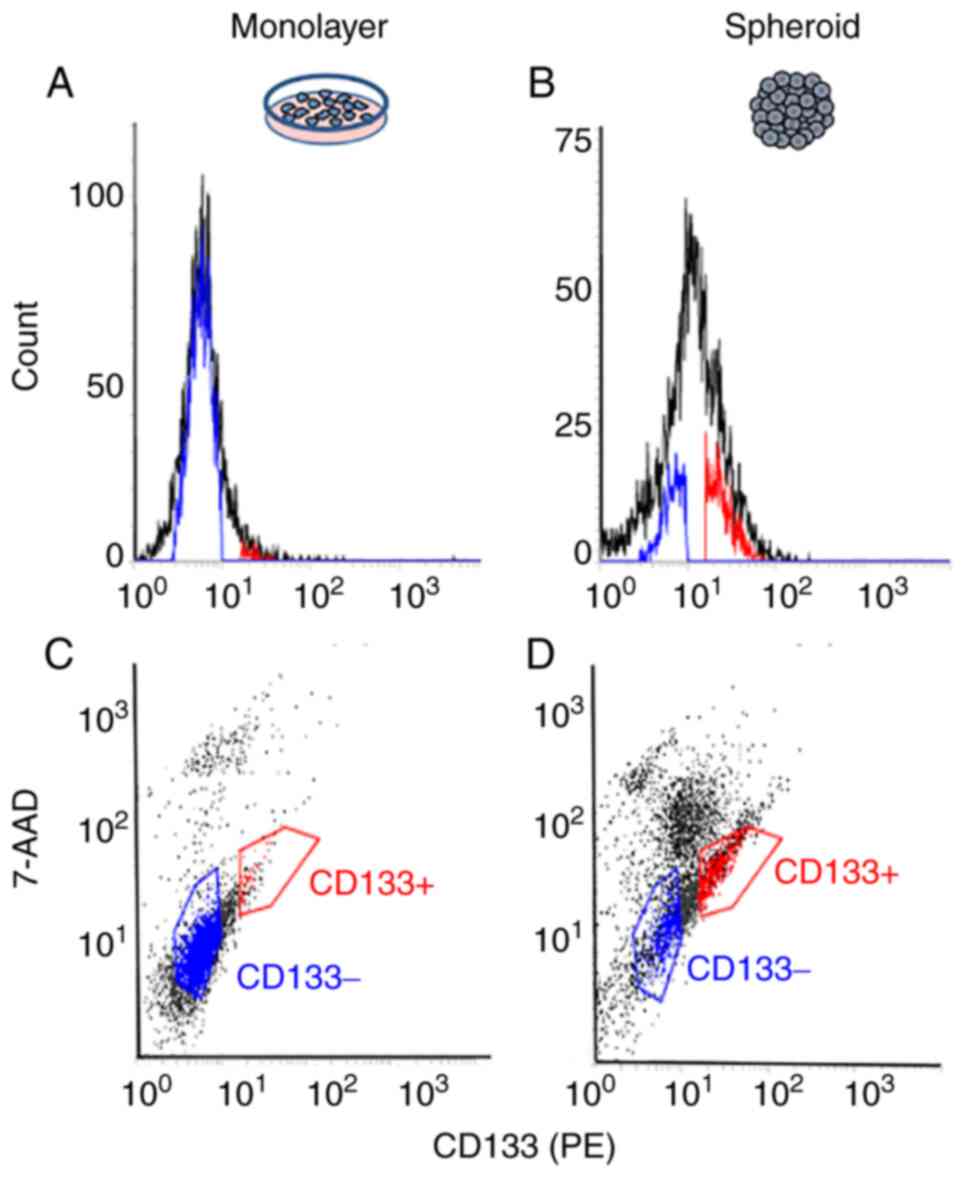
Cell sorting of the CD133-positive and
CD133-negative cells of spheroids
Single cells that were dispersed from spheroids and
stained immunofluorescently with anti-CD133AC133 antibodies were
analyzed. CD133-positive and 7-AAD-negative cells (red-colored
gating) represented ~0.8% of all measured single cells in monolayer
cultured cells (negative control; Fig.
3A and C) and ~15% in the cells from spheroids (Fig. 3B and D). Sorted CD133-positive and
7-AAD-negative cells or CD133-negative and 7-AAD-negative cells
(blue-colored gating) from spheroids were used for the cell
viability assay.
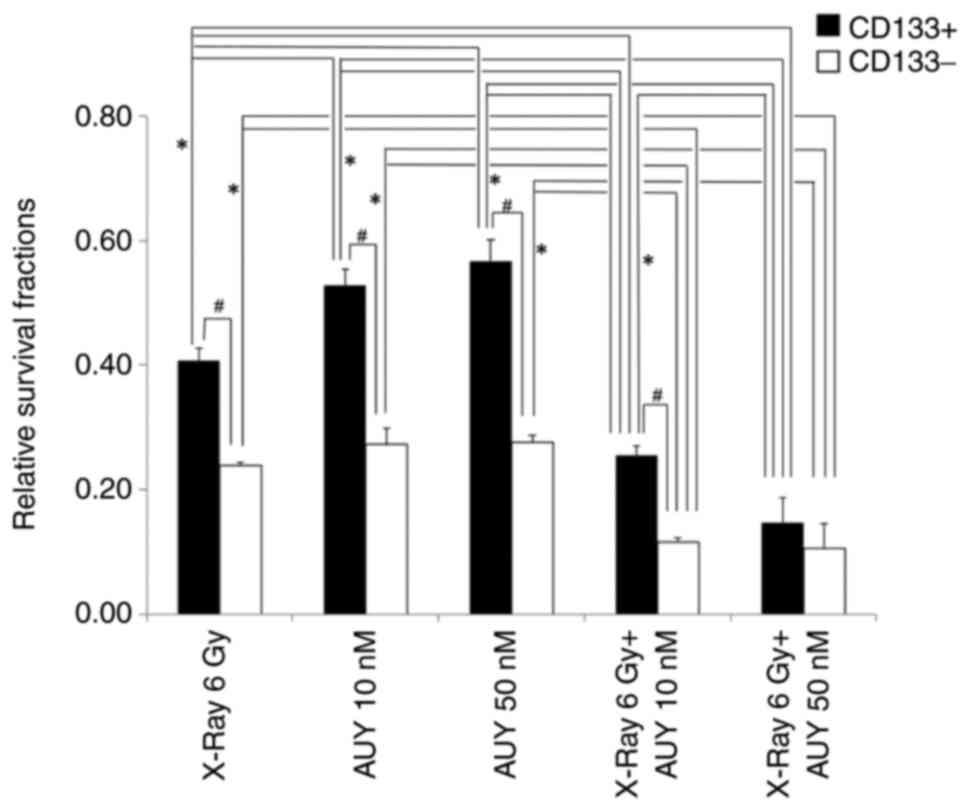
Viability of SCPCs and SCNCs as
determined using colony-formation assay
SCPCs and SCNCs sensitivity to X-rays (6 Gy),
NVP-AUY922 (10 and 50 nM), or X-rays combined with NVP-AUY922 was
examined using a colony-formation assay. As shown in Fig. 4, the survival fractions among SCPCs
groups (X-ray 6 Gy (0.41±0.021) vs. AUY 10 nM (0.53±0.025) or 50 nM
(0.57±0.035), X-ray 6 Gy vs. X-ray 6 Gy + AUY 10 nM (0.26±0.015) or
50 nM (0.15±0.040), AUY 10 or 50 nM vs. X-ray 6 Gy + AUY 10 or 50
nM) were significantly different (P<0.01, Tukey's HSD) except
for the survival fraction between groups of AUY 10 nM and AUY 50 nM
with no significant difference (P>0.05). In the case of SCNCs,
the survival fractions among groups (X-ray 6 Gy (0.24±0.006) vs.
X-ray 6 Gy + AUY 10 nM (0.12±0.006) or 50 nM (0.11±0.040), AUY 10
nM (0.27±0.025) or 50 nM (0.28±0.010) vs. X-ray 6 Gy + AUY 10 or 50
nM) were significantly different (P<0.01) but those among groups
(X-ray 6 Gy vs. AUY 10 or 50 nM, AUY 10 nM vs. AUY 50 nM, X-ray 6
Gy + AUY10 nM vs. X-ray 6 Gy + AUY 50 nM) were not significantly
different (P>0.05). The difference in the proportion of
surviving SCPCs between X-ray 6 Gy and X-ray 6 Gy + AUY 50 nM
(Cohen's d=6.22, effect size) was larger compared with that of
surviving SCNCs between X-ray 6 Gy and X-ray 6 Gy + AUY 50 nM
(d=4.50). In addition, the difference in the proportion of
surviving SCPCs between AUY 10 nM (d=8.79) or 50 nM (d=8.54) and
X-ray 6 Gy + AUY 50 nM was larger compared with that of surviving
SCNCs between AUY 10 nM (d=4.85) or 50 nM (d=5.66) and X-ray 6 Gy +
AUY 50 nM. The larger Cohen's d values in SCPCs indicated that the
sensitivity of SCPCs to X-rays or NVP-AUY was more strongly
enhanced by combined treatment of X-rays and NVP-AUY than that of
SCNCs.
Since the interaction was significant, we also
confirmed the difference between CD133-positive and CD133-negative
cells. The survival fractions of SCPCs exposed to X-ray 6 Gy, AUY
10 nM, and AUY 50 nM were significantly larger (P<0.01,
Student's t-test) than those of SCNCs exposed to X-ray 6 Gy, AUY 10
nM, and AUY 50 nM, respectively, under X-ray irradiation or
NVP-AUY922 treatment conditions, indicating a lower sensitivity of
SCPCs to X-rays and NVP-AUY922. Under treatment with X-ray 6 Gy
irradiation combined with a low concentration of AUY (10 nM), a
significant difference in the proportion of surviving SCPCs and
SCNCs was observed (P<0.01) but that of surviving SCPCs and
SCNCs was not observed (P>0.05) under treatment with X-ray 6 Gy
irradiation combined with a high concentration of AUY (50 nM). We
did not use other HSP90 inhibitors because it was reported that
other inhibitors have high cell toxicity (15–17).
Discussion
The present study confirmed the findings of our
previous study, indicating that glioblastoma SCPCs are induced in
the hypoxic microenvironment of T98G cell line-derived spheroids,
and they are positive for nestin, which is a marker for
undifferentiated neural cells (7).
The sensitivity of SCPCs to X-rays is lower than that of SCNCs and
migration and inversion abilities of SCPCs are higher than those of
SCNCs (9). Therefore, we used T98G
cell line-derived SCPCs as the CSC models in the present study. We
have not yet analyzed the CSC-like properties of SCPCs in other
glioblastoma cell lines. The significance of SCPCs is shown in
Fig. 1. In addition, we reported
that CD133-positive cells are not induced under hypoxia in
monolayer cell culture condition (9). It seems that a combined condition of
two dimensional culture and hypoxia is not enough for the induction
of stem cell-like cells.
Hsp90 protects against the degradation of numerous
client proteins that contribute to the maintenance of cancer cells.
Both SCPCs and SCNCs were sensitive to the Hsp90 inhibitor
NVP-AUY922, while the sensitivity of SCPCs to NVP-AUY922 was lower
than that of SCNCs, as shown in Fig.
4. In general, cancer stem cells are resistant to anti-cancer
agents; thus, SCPCs may show lower sensitivity to NVP-AUY922. The
resistance of cancer stem cells to NVP-AUY922 has not yet been
reported elsewhere.
Inhibition of Hsp90 by NVP-AUY922 has been reported
to induce radiosensitization of cancer cells (13,20–22).
In the present study, radiosensitization by NVP-AUY922 was observed
in glioblastoma SCPCs and SCNCs. Interestingly, the sensitivity of
SCPCs to radiation was more strongly enhanced dose-dependently by
NVP-AUY922 than that of SCNCs, as shown in Fig. 4. This result suggests that
NVP-AUY922 may cause preferential radiosensitization of
glioblastoma cancer stem cells. The preferential radiosensitization
has not yet been reported in other Hsp90 inhibitors. There are
possible mechanisms for the preferential radiosensitization of
SCPCs by NVP-AUY922. NVP-AUY922 may inhibit some cancer stem cell
properties, such as enhanced DNA repair (3,5),
removal of reactive oxygen species (23), and activation of Akt-mediated cell
survival signal transduction (24). In addition, NVP-AUY922 may inhibit
client proteins that maintain cancer cell stemness.
Hypoxia-inducible factor-1 α (HIF-1α) is an Hsp90 client protein
that plays important roles in maintaining cancer cell stemness.
Previous studies using tumor cells in patients and established
cancer cell lines showed that HIF-1α maintains glioma stem cells
(25,26), promotes glioma stem cell expansion
(27), and upregulates CD133
protein expression (28,29). Furthermore, HIF-1α may mediate the
expression of chemoresistance markers in CSCs (30). From these reports, it is assumed
that HIF-1α inhibition by NVP-AUY922 might induce higher
sensitization of cancer stem cells to radiation. In the present
study, we did not examine effects of NVP-AUY922 on cancer stem cell
markers such as HIF-1α, CD133, nestin and others. It is important
to explore the mechanism of preferential radiosensitization by
NVP-AUY922 in glioblastoma cancer stem cells through analyses of
the location or expression of the markers in spheroids.
In summary, NVP-AUY922 enhanced the sensitivity to
radiation more strongly in SCPCs than in SCNCs sorted from T98G
cell line-derived spheroids. The present study suggests for the
first time that an Hsp90 inhibitor, NVP-AUY922, is a candidate for
preferential radiosensitization in glioblastoma cancer stem
cells.
Acknowledgements
The authors would like to thank Professor Kohichi
Iwai (Ibaraki Prefectural University of Health Sciences, Ibaraki,
Japan) for their helpful comments regarding the statistical
analyses.
Funding
This work was supported by Grants-in-Aid from the Ministry of
Education, Science, Sports, Culture and Technology of Japan (grant
no. 16K10399) and a subsidy for Science and Technology Promotion of
Prefectures Locating Electric Power Plants from the Ministry of
Education, Culture, Sports, Science and Technology of Japan to
Ibaraki Prefectural University of Health Sciences.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT performed histological and cytological
examination using cell lines and spheroids and was a major
contributor in writing the manuscript. NT participated in cell
culture and data analysis. KO contributed to the conceptualization,
methodology, review and editing of the manuscript. TT, NT, and KO
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Binger DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beier D, Schulz JB and Beier CP:
Chemoresistance of glioblastoma cancer stem cells-much more complex
than expected. Mol Cancer. 10:1282011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mannino M and Chalmers AJ: Radioresistance
of glioma stem cells: Intrinsic characteristic or property of the
‘microenvironment-stem cell unit’? Mol Oncol. 5:374–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lathia JD, Mack SC, Mulkearns-Hubert EE,
Valentim CL and Rich JN: Cancer stem cells in glioblastoma. Genes
Dev. 29:1203–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohnishi K, Tani T, Bando S, Kubota N,
Fujii Y, Hatano O and Harada H: Plastic induction of
CD133AC133-positive cells in the microenvironment of glioblastoma
spheroid. Int J Oncol. 45:581–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sutherland RM: Cell and environment
interactions in tumor microregions: The multicell spheroid model.
Science. 240:177–184. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohnishi K, Tani T, Tojo N and Sagara JI:
Glioblastoma cell line shows phenotypes of cancer stem cells in
hypoxic microenvironment of spheroids. Biochem Biophys Res Commun.
546:150–154. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuno A, Lee MJ, Lee S, Tomita Y, Rekhtman
D, Moore B and Trepel JB: Clinical evaluation and biomarker
profiling of Hsp90 inhibitors. Methods Mol Biol. 1709:423–441.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dote H, Burgan WE, Camphausen K and
Tofilon PJ: Inhibition of hsp90 compromises the DNA damage response
to radiation. Cancer Res. 66:9211–9220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camphausen K and Tofilon PJ: Inhibition of
Hsp90: A multitarget approach to radiosensitization. Clin Cancer
Res. 13:4326–4330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jhaveri K, Taldone T, Modi S and Chiosis
G: Advances in the clinical development of heat shock protein 90
(Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823:742–755.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaspar N, Sharp SY, Pacey S, Jones C,
Walton M, Vassal G, Eccles S, Pearson A and Workman P: Acquired
resistance to 17-allylamino-17-demethoxygeldanamycin (17-AAG,
tanespimycin) in glioblastoma cells. Cancer Res. 69:1966–1975.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piper PW and Millson SH: Mechanisms of
resistance to Hsp90 inhibitor drugs: A complex mosaic emerges.
Pharmaceuticals (Basel). 4:1400–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jarosz D: HSP90: A global regulator of the
genotype-to-phenotype map in cancers. Adv Cancer Res. 129:225–247.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KH, Lee JH, Han SW, Im SA, Kim TY, Oh
DY and Bang YJ: Antitumor activity of NVP-AUY922, a novel heat
shock protein 90 inhibitor, in human gastric cancer cells is
mediated through proteasomal degradation of client proteins. Cancer
Sci. 102:1388–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaspar N, Sharp SY, Eccles SA, Gowan S,
Popov S, Jones C, Pearson A, Vassal G and Workman P: Mechanistic
evalution of the novel HSP90 inhibitor NVP-AUY92 in adult and
pediatric glioblastoma. Mol Cancer Ther. 9:1219–1233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaidi S, McLaughlin M, Bhide SA, Eccles
SA, Workman P, Nutting CM, Huddart RA and Harrington KJ: The HSP90
inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous
recombination resulting in mitotic entry with unresolved DNA
damage. PLoS One. 7:e354362012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gandhi N, Wild AT, Chettiar ST, Aziz K,
Kato Y, Gajula RP, Williams RD, Cades JA, Annadanam A, Song D, et
al: Novel Hsp90 inhibitor NVP-AUY922 radiosensitizes prostate
cancer cells. Cancer Biol Ther. 14:347–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hashida S, Yamamoto H, Shien K, Ohtsuka T,
Suzawa K, Maki Y, Furukawa M, Soh J, Asano H, Tsukuda K, et al:
Hsp90 inhibitor NVP-AUY922 enhances the radiation sensitivity of
lung cancer cell lines with acquired resistance to EGFR-tyrosine
kinase inhibitors. Oncol Rep. 33:1499–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Man J, Shoemake JD, Ma T, Rizzo AE, Godley
AR, Wu Q, Mohammadi AM, Bao S, Rich JN and Yu JS: Hyperthermia
sensitizes glioma stem-like cells to radiation by inhibiting AKT
signaling. Cancer Res. 75:1760–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heddleston JM, Li Z, McLendon RE,
Hjelmeland AB and Rich JN: The hypoxic microenvironment maintains
glioblastoma stem cells and promotes reprogramming towards a cancer
stem cell phenotype. Cell Cycle. 8:3274–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao XG, Yan M, Xue XY, Zhang X, Ren HG,
Guo G, Wang P, Zhang W and Huo JL: Overexpression of ZNF217 in
glioblastoma contributes to the maintenance of glioma stem cells
regulated by hypoxia-inducible factors. Lab Invest. 91:1068–1078.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soeda A, Park M, Lee D, Mintz A,
Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T,
Kassam AB, et al: Hypoxia promotes expansion of the CD133-positive
glioma stem cells through activation of HIF-1alpha. Oncogene.
28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bar EE, Lin A, Mahairaki V, Matsui W and
Eberhart CG: Hypoxia increases the expression of stem-cell markers
and promotes clonogenicity in glioblastoma neurospheres. Am J
Pathol. 177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79.
2012.PubMed/NCBI
|
|
30
|
Kolenda J, Jensen SS, Aaberg-Jessen C,
Christensen K, Andersen C, Brünner N and Kristensen BW: Effects of
hypoxia on expression of a panel of stem cell and chemoresistance
markers in glioblastoma-derived spheroids. J Neurooncol. 103:43–58.
2011. View Article : Google Scholar : PubMed/NCBI
|