Introduction
Gastric cancer (GC) is the second most common cancer
in China and ranks as the fourth most common type of malignant
cancer worldwide (1,2). Although the survival rate is improved
when patients are treated with radiotherapy, chemotherapy and
targeted therapy after surgery, the overall survival rate of
patients with advanced GC remains low (3). Thus, an understanding of the
molecular mechanisms and identification of potential therapeutic
targets is very important for GC therapy.
Ferroptosis is a newly identified form of programmed
cell death that is mediated by excess iron-dependent lipid
peroxidation (4,5). Glutathione peroxidase 4 (GPX4), a
glutathione (GSH)-dependent selenoenzyme, prevents ferroptosis by
changing toxic lipid hydroperoxides into nontoxic lipid alcohols
(6,7). Once GPX4 activity is suppressed, the
products of lipid peroxidation increase significantly, subsequently
resulting in ferroptosis (8,9).
Solute carrier family 7 member 11 (SLC7A11, xCT) is a
sodium-independent cystine-glutamate antiporter that transfers
extracellular cystine into cells, which is then processed into
cysteine, a rate-limiting substrate for glutathione (GSH) synthesis
(10,11). Nuclear receptor coactivator 4
(NCOA4) is a component of autophagosomes and is reported to mediate
ferritinophagy by interacting with surface arginine residues in
ferritin heavy chain 1 (FTH1) (12,13).
NCOA4 overexpression induces ferroptosis by increasing
intracellular-free iron contents, glutathione production and
reactive oxygen species (ROS) levels (14). Ferroptosis plays a key role in
various diseases, including GC (15–17).
Targeting ferroptosis may be a potential therapeutic strategy for
patients with GC.
Ophiopogonin B (OP-B) is extracted from Radix
Ophiopogon japonicus, which has been extensively applied as
a treatment for pulmonary disease in the past in Southeast Asia.
OP-B has been reported to exert anticancer effects on different
cancer types (18,19). For instance, in colon cancer, OP-B
suppresses cancer cell proliferation and migration by activating
JNK/c-Jun signaling (18). In lung
cancer cells, OP-B exerts anticancer effects by inducing apoptosis,
mitotic catastrophe and autophagy (19). Previous findings showed that OP-B
suppresses the proliferation of SGC-7901 human GC cells (20). However, SGC-7901 cells are reported
to be contaminated with HeLa cells. Thus, we explored whether OP-B
suppresses GC cells and the potential underlying mechanism.
Materials and methods
Patient samples
A total of 60 GC and adjacent normal control tissues
(>5 cm away from the edge of cancer tissues and pathologically
confirmed as normal gastric mucosa) were selected. Once the
specimens were removed, they were immediately stored in RNAsaver
(Beijing Solarbio Science and Technology Co., Ltd.) at −80°C to
minimize RNA degradation. The 60 patients with GC were aged 42–85
years with an average age of 58.9±23.7 years, and included 32 males
and 28 females. The inclusion criteria were: i) Age ≥18 years; ii)
absence of radiotherapy, chemotherapy or any adjuvant therapy
before hospitalization; iii) absence of other malignant tumors; and
iv) absence of a family history of genetic diseases. Exclusion
criteria were: i) Antibiotics <3 months before blood collection;
ii) liver insufficiency and iii) autoimmune system deficiency. In
terms of the degree of tissue differentiation, 32 cases of high and
moderate differentiation and 28 cases of low and no differentiation
were identified.
In terms of the tumor-node-metastasis (TNM) stage,
25 cases were in stage I-II and 35 cases were in stage III-IV. None
of the patients underwent chemotherapy, radiotherapy or
immunotherapy prior to surgery, and all of the patients were
diagnosed via routine histopathology after the operation. The
clinicopathological types of GC patients were summarized as
follows: 2 cases of papillary adenocarcinoma, 25 cases of
well-differentiated adenocarcinoma, 5 cases of moderately
differentiated adenocarcinoma, 24 cases of poorly differentiated
adenocarcinoma, 2 cases of signet-ring cell carcinoma, 2 cases of
mucinous carcinoma.
All 60 patients signed the informed consent form,
and the study was approved by the Ethics Committee of Jiaozhou
Central Hospital (approval no. JZCH-2019JHU4).
Cell culture
The human gastric cancer cell lines AGS and NCI-N87
were purchased from the American Type Culture Collection (ATCC).
The normal human gastric epithelium cell line (GES-1) was purchased
from Procell Life Science & Technology Co., Ltd. (CL-0563).
Cell authenticity was identified using STR files. Cells were
cultured at 37°C with Dulbecco's modified Eagle's medium (DMEM)
(HyClone; GE Healthcare Life Sciences) supplemented with 10% fetal
bovine serum (HyClone; GE Healthcare Life Sciences), 100 U/ml
penicillin (HyClone; GE Healthcare Life Sciences) and 100 g/ml
streptomycin (HyClone; GE Healthcare Life Sciences) in a humidified
atmosphere containing 5% CO2.
Cell Counting Kit-8 (CCK-8) assay
AGS and NCI-N87 cells were seeded in a 96-well plate
at a density of 3,000 cells/well. After incubation for 24 h at
37°C, the cells were treated with OP-B (MW: 722.9, HPLC ≥98%,
abs47001825, Absin Biotech. Co.) at the indicated concentrations
(0.00, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00, 32.00 and 64.00
µM for AGS; 0, 1, 2, 4, 8, 16, 32 and 64 µM NCI-N87).
In addition, AGS and NCI-N87 cells were preincubated
with 10 µM zVAD, 10 µM 3-MA, 10 µM for Nec-1or 1 µM Fer-1 for 1 h.
AGS and NCI-N87 cells were treated with 10 or 20 µM OP-B for 24 h,
respectively. Subsequently, 10 µl of CCK-8 solution (C0038,
Beyotime Biotech) was incubated with the cells for 2 h before
harvest. A Bio-Tek microplate reader (Winooski) was used to read
the optical density (OD) value at 490 nm. Half maximal inhibitory
concentration (IC50) was calculated as: Cell inhibition
rate (%)=1-(experimental group OD value/normal group OD
value)x100%.
Cell apoptosis analysis
After treatment with OP-B for 24 h, AGS and NCI-N87
cells were washed with 1× PBS three times and stained with an
Annexin V-PE/7-AAD apoptosis kit (E-CK-A216, Elabsience) according
to the manufacturer's protocol. The following controls were used to
set up compensation and quadrants: i) Unstained cells; ii) Cells
stained with PE Annexin V (no 7-AAD); iii) Cells stained with 7-AAD
(no PE Annexin V). Cells were analyzed using an FC500 flow
cytometer equipped with CXP software (Beckman Coulter). According
to the instructions, Q1 represented 7-AAD+/PE-cells; Q2 represented
PE+/7-ADD+ cells (necrosis and late apoptosis cells); Q3
represented PE+/7-ADD-cells (early apoptosis cells); Q4 represented
PE-/7-AAD-cells (living cells). Death cells were calculated as: the
necrosis and late apoptosis cells (Q2) + early apoptosis cells
(Q3).
Western blot analysis
Total protein were collected from AGS and NCI-N87
cells in a radioimmunoprecipitation assay buffer (Beijing Solarbio
Science and Technology Co., Ltd.). The protein concentration was
determined using a BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). A total of 30 µg protein was separated on 10%
SDS-PAGE gels, followed by transfer of electrophoresed proteins
onto nitrocellulose membranes. The membranes were blocked with 5%
non-fat milk (Beyotime Biotechnology, Beijing, China) and incubated
with primary antibodies, including GPX4 (no. 52455, 1:1,000, Cell
Signaling Technology, Inc.), xCT (no. 12691, 1:1,000, Cell
Signaling Technology, Inc.), NCOA4 (no. 66849; 1:1,000; Abcam),
FTH-1 (no. 4393; 1:1,000; Abcam), and GAPDH (cat. no. 5174,
1:4,000, Cell Signaling Technology, Inc.), at 4°C overnight.
Subsequently, the cells were incubated with the corresponding
peroxidase-conjugated secondary antibodies (both 1:5,000; cat. no.
ZB-2301 and ZB-2305; Beijing Zhongshan Golden Bridge Biotechnology
Co.). The protein bands were detected using enhanced
chemiluminescence reagent (EMD Millipore). Relative protein
expression was normalized to GAPDH. All the experiments were
repeated three times. ImageJ 1.43b software (National Institutes of
Health) was used for the densitometry analysis.
Quantitative PCR
A total of 5×106 cells were collected in
EP tubes without RNase (Qiagen GmbH) and lysed with RNAVzol
(Vigorous Biotechnology Beijing Co., Ltd.). Chloroform (Vigorous
Biotechnology Beijing Co., Ltd.) was added for 15 sec, incubated at
room temperature for 10 min, and centrifuged at 13,000 × g for 10
min at 4°C. Then, the supernatant was absorbed, and an equal volume
of isopropanol was added. The mixture was gently agitated and
incubated at room temperature for 10 min. The cells were
centrifuged at 13,000 × g for 10 min at 4°C. The supernatant was
then discarded carefully, and the sample precipitated by washing
with 1 ml of 75% ethanol. Subsequently, the supernatant was
discarded after centrifugation at 13,000 × g for 3 min at 4°C, and
the pellet was dissolved in 20 µl of DEPC H2O. The RNA
(1 µg) was reverse transcribed into cDNAs. The target fragment was
amplified using PCR. The reaction system had a total volume of 20
µl, including 10 µl of 2× QuantiTect SYBR Green PCR Master Mix
(Bio-Rad Laboratories, Inc.), 0.25 µl of 10 µM forward primer, 0.25
µl of reverse primer, 5 µl of cDNAs, and 4.75 µl of DEPC
H2O. The reaction conditions were: predenaturation at
95°C for 15 min and 40 cycles of denaturation at 94°C for 30 sec,
annealing at 60°C for 30 sec, and extension at 68°C for 30 sec.
Each experiment was repeated three times. The primers used in the
study were as follows: GAPDH-F: 5′-ACCACAGTCCATGCCATCAC-3′;-R:
5′-CTAGACGGCAGGTCAGGTC-3′; Ptgs2-F: 5′-GAGGGATCTGTGGATGCTTCG-3′;-R:
5′-AAACCCACAGTGCTTGACAC-3′; Chac1-F: 5′-CCCCATCCTGGAACTTGACC-3′;-R:
5′-CTATGGATGGCTGGGCTGAG-3′.
Quantification of MDA, ROS, and
Fe2+ levels
Briefly, AGS and NCI-N87 cells were seeded in 6-well
culture dishes at a density of 2×106 cells/well and
incubated overnight at 37°C. The cells were preincubated with or
without 1 µM ferrostatin-1 (Fer-1, HY-100579, MedChemExpress) for 1
h. Subsequently, 10 or 20 µM OP-B was added to AGS and NCI-N87
cells and incubated for another 24 h at 37°C. Subsequently, the MDA
[Lipid Peroxidation (MDA) Assay Kit, ab118970, Abcam], ROS
[DCFDA/H2DCFDA Cellular ROS Assay kit, ab113851, Abcam,
Cambridge, UK] and Fe2+ [Iron Assay Kit, ab83366, Abcam]
contents were measured using kits according to the protocol.
ROS staining
Briefly, AGS and NCI-N87 cells were seeded in 6-well
culture dishes at a density of 2×106 cells/well and
incubated overnight at 37°C. The cells were preincubated with or
without 1 µM Fer-1 (HY-100579, MedChemExpress) for 1 h.
Subsequently, 10 or 20 µM OP-B was added to AGS and NCI-N87 cells
and incubated for another 24 h at 37°C. Subsequently, the cells
were stained with 1 ml of 1 µM 2′,7′-dichlorofluorescein diacetate
(DCFDA) probe for 30 min at room temperature. The cells were washed
with PBS three times (5 min/wash) and observed under a fluorescence
microscope (×20, Olympus, Japan).
In vivo assay
Four- to six-week-old female nude mice were
purchased from the Peking University Health Science Center
(Beijing, China). AGS cells (1×106 cells, total volume:
100 µl in PBS) were injected into the right posterior flanks of the
nude mice [5 mice/group (n=70 total animals), weight: 14–16 g].
Animal handling and research protocols were approved by the Ethics
Committee of Jiaozhou Central Hospital (JCH-209ZH35). All mice were
housed in a temperature- (20–24°C) and humidity-controlled (45–55%)
environment with free access to food and water. A 12/12h light/dark
cycle was maintained in the animal housing rooms. Seven days later,
tumor-bearing mice were randomly divided into two groups, including
those treated with OP-B (100 µl, 50 mg/kg p.o. daily; n=5) or
CMC-Na (control, 100 µl, p.o. daily; n=5) for 14 days. To evaluate
tumor growth, 5 mice/group were sacrificed every two days prior to
sacrifice on day 14. Finally, after 14 days, the mice were
euthanized by administering an intraperitoneal injection of
pentobarbital sodium (110 mg/kg) (21), and the tumors were collected for
further analysis. Tumor volumes were calculated using the formula:
volume=(length × width2)/2.
Statistical analysis
SPSS 13.0; SPSS, Inc. was used to analyze the data.
The data are presented as the means ± standard deviations from
three independent experiments. A Wilcoxon signed-rank test was
performed to compare ptgs2 and chac1 mRNA levels between GC tissues
and adjacent normal control tissues. Unpaired Student's t-tests
were used for comparisons between two groups. Analysis of variance
followed by Tukey's post hoc test were used for comparisons of more
than 2 groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
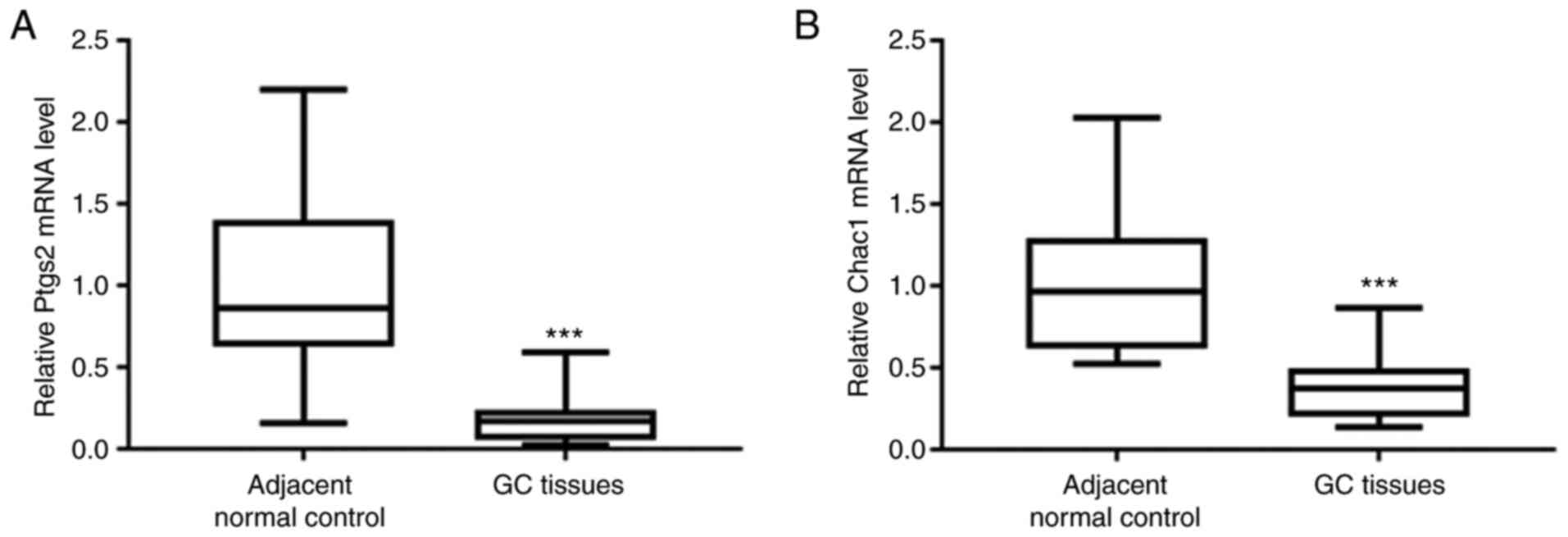
Decreased levels of the Ptgs2 and
Chac1 mRNAs in GC tissues
First, the mRNA levels of ferroptosis markers,
including prostaglandin-endoperoxide synthase 2 (Ptgs2) and ChaC
glutathione-specific gamma-glutamylcyclotransferase 1 (Chac1) were
determined. qPCR analysis revealed significantly reduced levels of
the Ptgs2 and Chac1 mRNAs in GC tissues compared with those in
adjacent normal control tissues (Fig.
1A and B), indicating that ferroptosis was suppressed in GC
tissues.
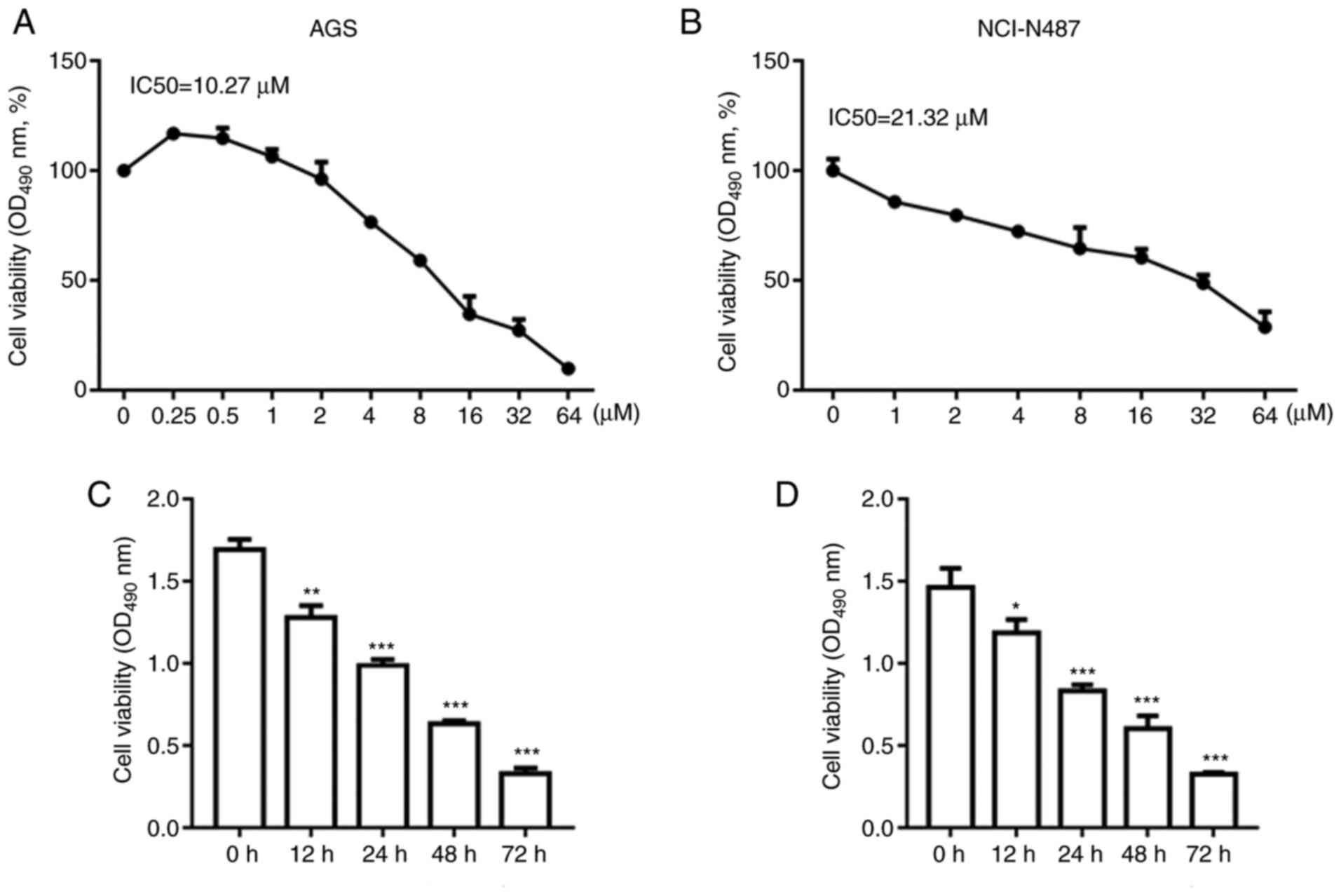
OP-B reduced AGS and NCI-N87 cell
viability in a dose- and time-dependent manner
GC cells were treated with different concentrations
of OP-B as aforementioned. As shown in Fig. 2A and B, treatment with OP-B
decreased the viability of AGS and NCI-N87 cells in a
dose-dependent manner. The IC50 for AGS cells was 10.27
µM and for NCI-N87 cells it was 21.32 µM. Subsequently, AGS and
NCI-N87 cells with 10 or 20 µM OP-B, respectively. OP-B decreased
the viability of AGS and NCI-N87 cells at 12, 24, 48 and 72 h
(Fig. 2C and D).
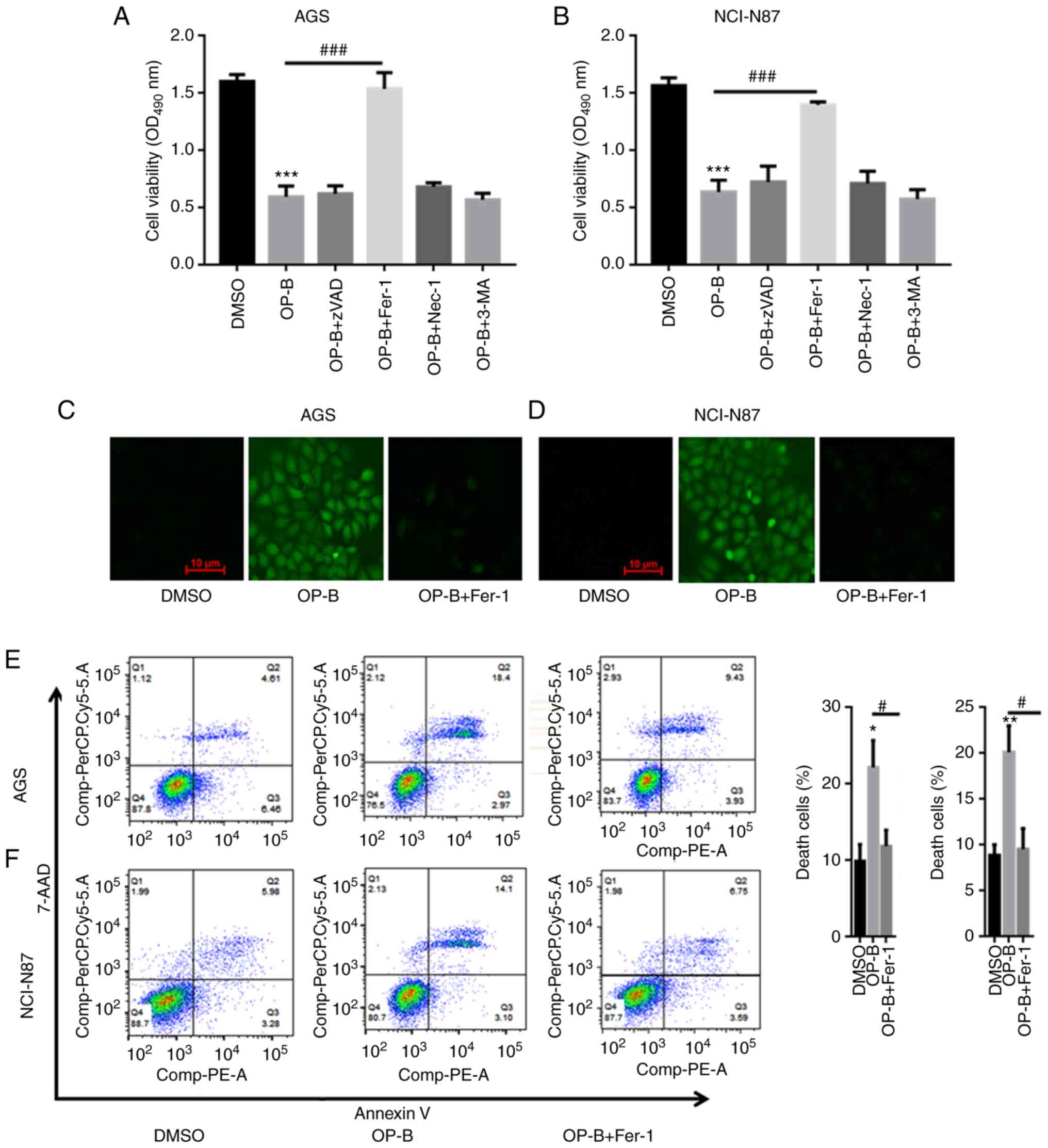
OP-B induced ferroptosis in AGS and
NCI-N87 cells
AGS and NCI-N87 cells were treated with 10 or 20 µM
OP-B to explore whether OP-B induces ferroptosis in GC cells. As
shown in Fig. 3A and B,
preincubation with a pancaspase inhibitor
(benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone, zVAD-fmk),
necrosis inhibitor (necrostatin-1, Nec-1), or autophagy inhibitor
(3-methyladenine, 3-MA) did not abolish OP-B-induced GC cell death.
However, pretreatment with the ferropotosis inhibitor Fer-1
significantly reversed AGS and NCI-N87 cell death induced by OP-B
(Fig. 3A and B). DCFDA staining
showed that OP-B increased the ROS contents in AGS and NCI-NC87
cells, but a preincubation with Fer-1 reversed the increased
production of ROS induced by OP-B (Fig. 3C and D). The flow cytometric
analysis indicated that OP-B significantly induced AGS and NCI-N87
cell death, while a preincubation with Fer-1 partially reversed
OP-B-induced GC cell death (Fig. 3E
and F).
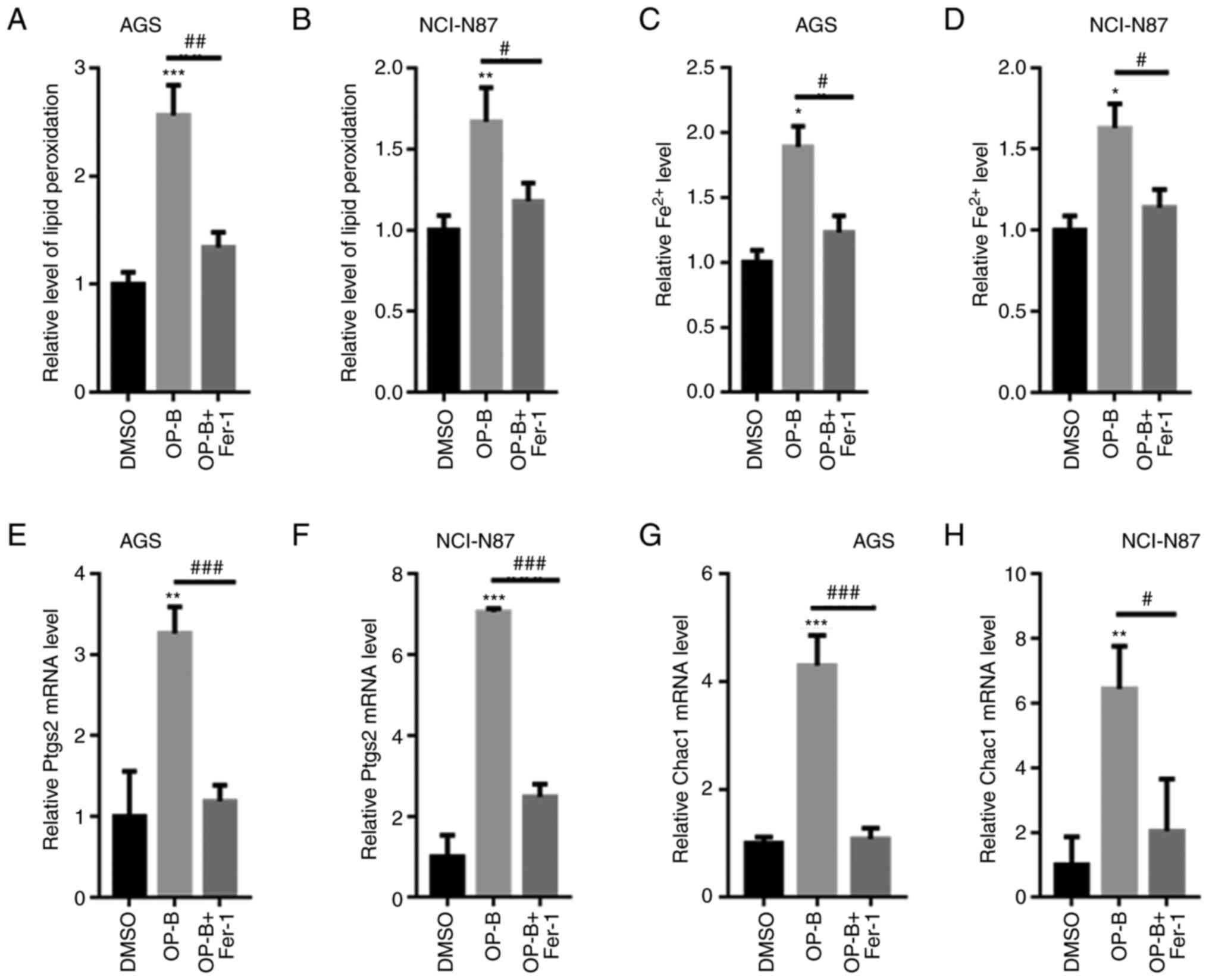
OP-B increased the production of MDA
and Fe2+
MDA and ROS contents were quantified in AGS and
NCI-N87 cells. As shown in Fig. 4A and
B, OP-B significantly increased the MDA content, but
preincubation with Fer-1 decreased the higher MDA content induced
by OP-B in both AGS and NCI-N87 cells. OP-B also increased
Fe2+ levels in AGS and NCI-N87 cells (Fig. 4C and D). In addition, the levels of
ferroptosis-related markers, including Ptgs2 and Chac1, were
significantly increased by OP-B treatment, but preincubation with
Fer-1 reversed these effects (Fig.
4E-H). These data further validated that OP-B induced GC cell
ferroptosis.
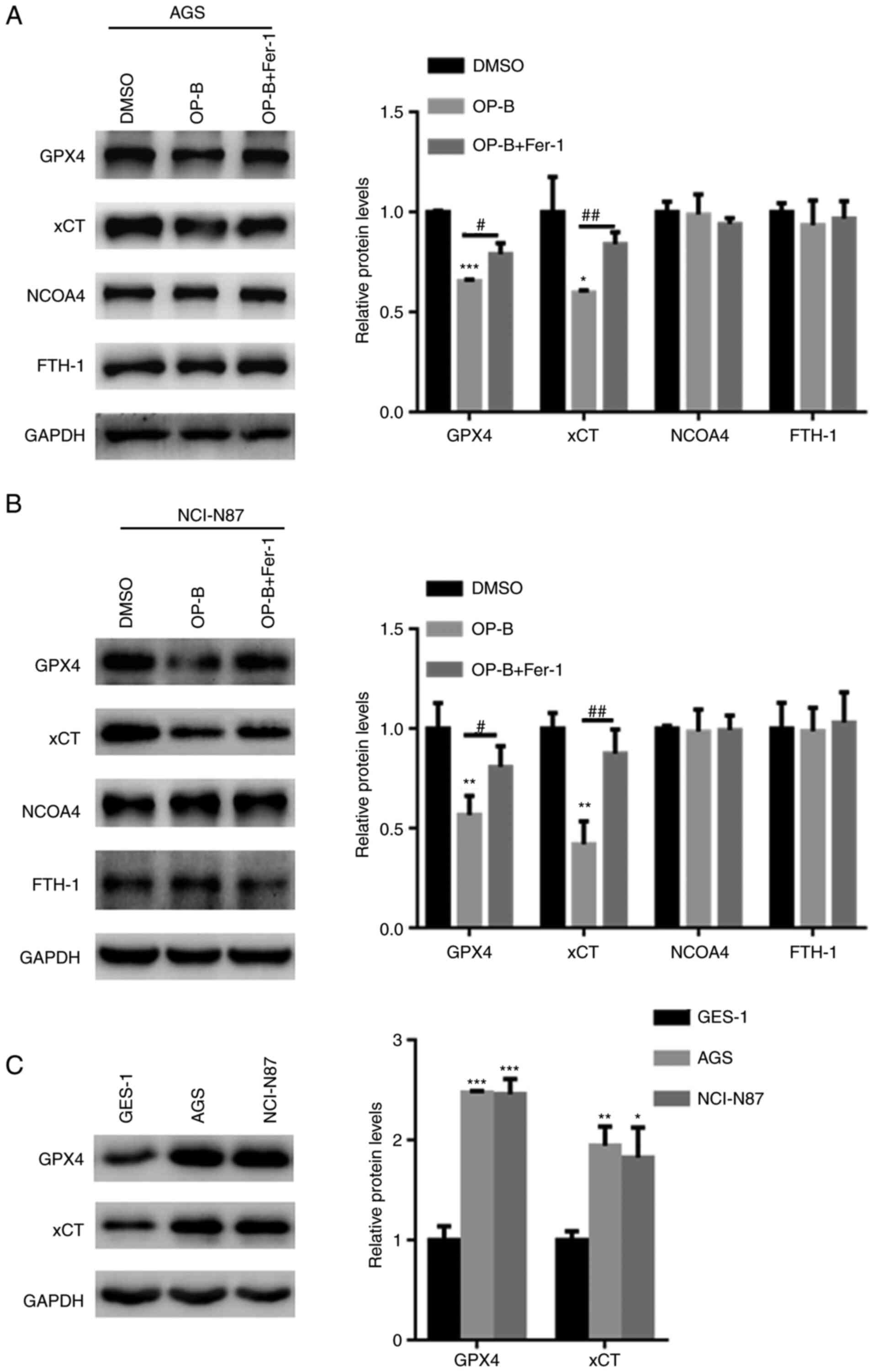
OP-B reduced GPX4 and xCT expression
in GC cells
The underlying mechanism by which OP-B induced
ferroptosis in GC cells was examined. Western blot analysis
revealed that OP-B reduced the expression of GPX4 and xCT in both
AGS and NCI-N87 cells (Fig. 5A and
B). By contrast, pretreatment with Fer-1 partially reversed the
OP-B-induced reductions in GPX4 and xCT levels in AGS and NCI-N87
cells (Fig. 5A and B). However,
OP-B did not alter the expression of NCOA4 and FTH-1, two important
ferritinophagy markers (Fig. 5A and
B). Furthermore, the expression of GPX4 and xCT gene was
explored in gastric cancer and normal cells. Compared with that in
GES-1 cells, the expression of GPX4 and xCT was upregulated in
those of AGS and NCI-N87 cells (Fig.
5C).
OP-B suppressed tumor growth in
vivo
In vivo assays showed that OP-B treatment
significantly decreased xenograft tumor growth after six days
compared with that of the control (Fig. 6A). After 14 days, the final tumor
volumes in the OP-B and control groups were 85.7±8.5 mm3
and 158.35±12.3 mm3, respectively. Compared with the
control group, the tumor weight was also significantly decreased in
the OP-B group (Fig. 6B). In
addition, the levels of GC biomarkers, including CEA and CA19-9,
were also decreased in the serum of the OP-B group compared with
the control group (Fig. 6C and D).
qPCR analysis indicated that OP-B significantly reduced the mRNA
levels of Ptgs2 and Chac1 compared with those of the control group
(Fig. 6E). Western blot analysis
revealed a significantly decreased expression of GPX4 and xCT in
the tumor tissues from the OP-B group compared to those from the
control group (Fig. 6F).
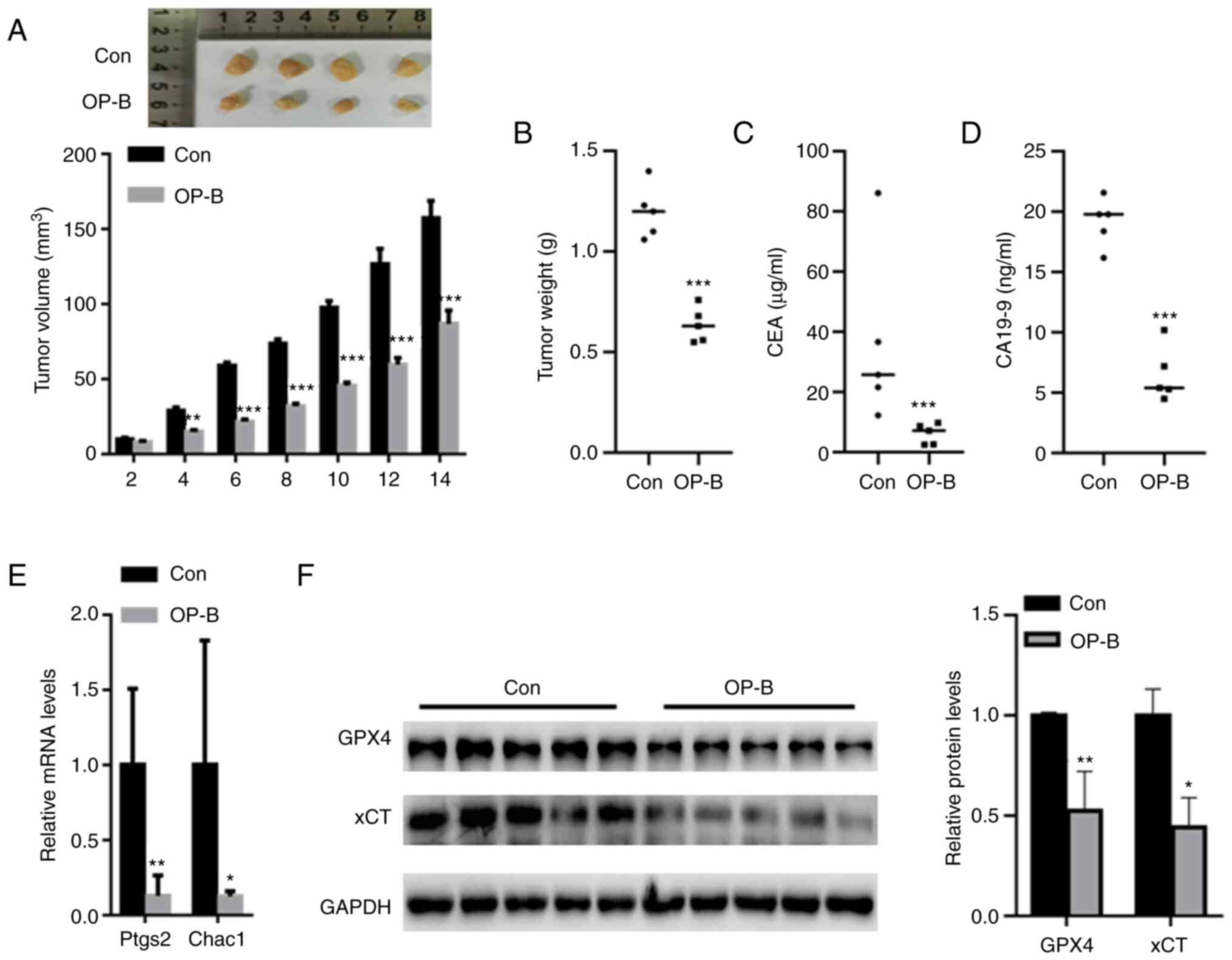 | Figure 6.OP-B suppressed tumor growth in
vivo. (A) The mice (5 mice/group) in the control and OP-B
groups were sacrificed at day 2, 4, 6, 8, 10, 12 and 14,
respectively. OP-B treatment significantly decreased xenograft
tumor growth compared with that of the control. When the mice (5
mice/group) grew until 14 days, the tumor weight, CEA level, CA19-9
level, Ptgs2 and Chac1 mRNA level, as well as the corresponding
protein expression were determined. (B) Compared with the control
group, the tumor weight was also significantly decreased in the
OP-B group. Serum levels of GC biomarkers, including (C) CEA and
(D) CA19-9, were also decreased in the OP-B group compared with the
control group. (E) qPCR analysis indicated that OP-B significantly
reduced the mRNA levels of Ptgs2 and Chac1 compared with those of
the control group. (F) Western blot analysis revealed significantly
decreased GPX4 and xCT levels in the tumor tissues from the OP-B
group compared to those from the control group. *P<0.05,
**P<0.01, and ***P<0.001 compared with the indicated
groups. |
Discussion
Ferroptosis is a novel characterized form of
regulated cell death that is induced by excess intracellular ROS
and iron levels (9). Nanotargeting
of withaferin efficiently leads to ferroptosis and exerts
satisfactory effects on neuroblastoma (22). In addition, anticancer drugs,
including lapatinib and siramesine, have been shown to induce
ferroptosis (23). Based on these
findings, ferroptosis is involved in the anticancer effects of
drugs. However, researchers have not clearly determined whether
OP-B induces ferroptosis in GC cells.
Recent studies have confirmed that ferroptosis plays
a key role in the evolution of GC (15–17).
Consistent with these findings, data of the present study showed
significantly increased levels of ferroptosis biomarkers, including
Ptgs2 and Chac1, in GC tissues compared with adjacent normal
control tissues. The CCK-8 assay indicated that OP-B reduced GC
cell viability in a time- and dose-dependent manner. These findings
indicated a tumor suppressor function of OP-B in GC cells.
OP-B was administered in combination with different
cell death inhibitors, including zVAD, Nec-1, 3-MA and Fer-1. The
results of the present study showed that only the ferroptosis
inhibitor Fer-1 abolished the OP-B-induced death of both AGS and
NCI-N87 cells, but not the other inhibitors. Furthermore, OP-B
significantly increased the production of ROS, MDA and
Fe2+, but preincubation with Fer-1 abolished these
effects. Flow cytometry also confirmed that OP-B increased AGS and
NCI-NC87 cell death. These findings suggest that, OP-B may induce
GC cell ferroptosis, thereby inhibiting GC.
One of the roles of GPX4 is to block lipid ROS
production, and suppression of GPX4 results in the accumulation of
lipid ROS, thereby inducing ferroptosis in various cells (24,25).
xCT is composed of cystine/glutamate transporters, and it mainly
provides a substrate for glutathione synthesis (10). The inhibition of xCT would decrease
the capacity of GPX4 to clear lipid ROS via inadequate glutathione
synthesis and finally induce cell death (10,26).
According to results of the present study, OP-B suppressed the
expression of GPX4 and xCT, suggesting that OP-B-induced
ferroptotic cell death may be achieved by inhibiting the GPX4/xCT
system.
Considering the effect dose and toxicity dose of
OP-B in in vivo studies, it has been reported that 75 mg/kg
OP-B significantly decreases the number of A-549-related metastatic
nodules in contrast to the control treatment group (27). In addition, the toxicity effects of
OP-B were evaluated in ovarian cancer cells in nude mice in
vivo (28). The results show
that both 15 and 75 mg/kg OP-B do not lead to cell degeneration,
necrosis, or infiltration of inflammatory factors in heart, liver,
lung, and kidney in vivo (28). Thus, 50 mg/kg OP-B was selected in
the present study while 50 mg/kg OP-B did not demonstrate toxicity.
The in vivo results were consistent with the in vitro
experiments. In vivo administration of OP-B reduced the
volume and weight of AGS tumors. In addition, expression of GPX4
and xCT was suppressed in nude mice treated with OP-B compared with
control mice.
However, there are limitations to the present study.
First, we did not explore the expression of each gene in the 60 GC
and adjacent normal control specimens. In future studies, analysis
via western blot and RT-qPCR. Secondly, results of the present
study showed that OP-B had an IC50 of 10–20 µM.
Investigation as to whether these results are comparable to
clinical plasma concentration in patients should be conducted.
Thirdly, novel data that OP-B induced GC cell death via GPX4 and
xCT were identified; however, whether GPX4 or xCT contributes more
to the OP-B-induced ferroptosis deserves further study. Fourthly, a
future aim is the translation of the current results into a
clinical trial, which may ameliorate the employment of OP-B in
clinic.
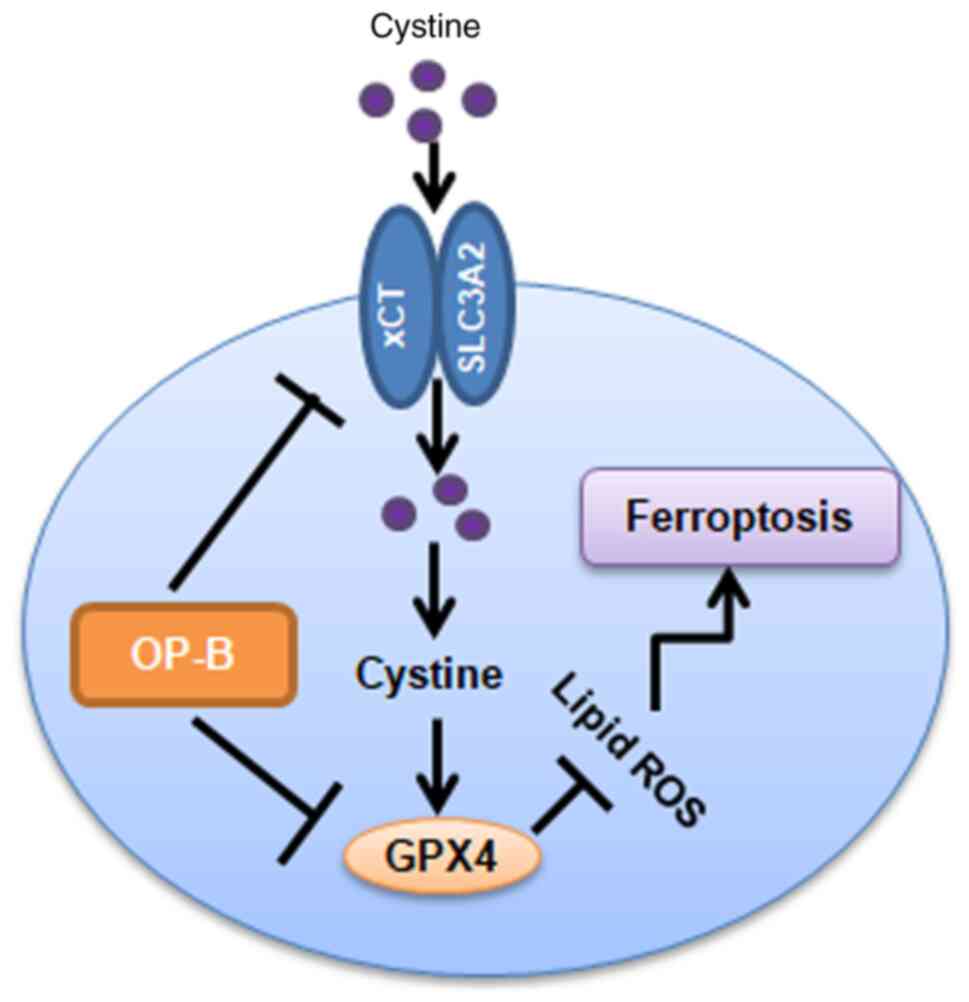
In summary, OP-B induces ferroptosis in gastric
cancer cells by inhibiting the transplasma membrane cysteine redox
shuttle mediated by blocking the GPX4/xCT system (Fig. 7).
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Hunan
Province Supporting Fund of Jiaozhou Central Hospital
(HNJ-20B76CD).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LZ performed the experiments, analyzed the data and
wrote the manuscript. CL, YZ, and JZ performed the RT-qPCR
experiments. XY designed the experiments, analyzed the data and
provided final approval of the version to be published. LZ and XY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jiaozhou Central Hospital. Animal handling and
research protocols were approved by the Ethics Committee of
Jiaozhou Central Hospital (JCH-209ZH35). The approval human
protocol number was JCH-209ZH36, and all the participants provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests to
declare.
References
|
1
|
Ma H, Lian C and Song Y: Fibulin-2
inhibits development of gastric cancer by downregulating β-catenin.
Oncol Lett. 18:2799–2804. 2019.PubMed/NCBI
|
|
2
|
Niu J, Song X and Zhang X: Regulation of
lncRNA PVT1 on miR-125 in metastasis of gastric cancer cells. Oncol
Lett. 19:1261–1266. 2020.PubMed/NCBI
|
|
3
|
Sun Y, Zhao C, Ye Y, Wang Z, He Y, Li Y
and Mao H: High expression of fibronectin 1 indicates poor
prognosis in gastric cancer. Oncol Lett. 19:93–102. 2020.PubMed/NCBI
|
|
4
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong Y, Wang N, Liu N and Dong H: Lipid
peroxidation and GPX4 inhibition are common causes for
myofibroblast differentiation and ferroptosis. DNA Cell Biol.
38:725–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Freitas FP, Seibt T, et al:
Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422 e421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ni J, Chen K, Zhang J and Zhang X:
Inhibition of GPX4 or mTOR overcomes resistance to lapatinib via
promoting ferroptosis in NSCLC cells. Biochem Biophys Res Commun.
567:154–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song X, Wang X, Liu Z and Yu Z: Role of
GPX4-mediated ferroptosis in the sensitivity of triple negative
breast cancer cells to gefitinib. Front Oncol. 10:5974342020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee N, Carlisle AE, Peppers A, Park SJ,
Doshi MB, Spears ME and Kim D: xCT-Driven expression of GPX4
determines sensitivity of breast cancer cells to ferroptosis
inducers. Antioxidants (Basel). 10:3172021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Kong Y, Ma Y, Ni S, Wikerholmen
T, Xi K, Zhao F, Zhao Z, Wang J, Huang B, et al: Loss of COPZ1
induces NCOA4 mediated autophagy and ferroptosis in glioblastoma
cell lines. Oncogene. 40:1425–1439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang Y, Chen X, Tan Q, Zhou H, Xu J and Gu
Q: Inhibiting ferroptosis through disrupting the NCOA4-FTH1
interaction: A new mechanism of action. ACS Cent Sci. 7:980–989.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai Y, Xia C and Sun Z: The inhibitory
effect of 6-gingerol on ubiquitin-specific peptidase 14 enhances
autophagy-dependent ferroptosis and anti-tumor in vivo and in
vitro. Front Pharmacol. 11:5985552020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma R, Shimura T, Yin C, Okugawa Y,
Kitajima T, Koike Y, Okita Y, Ohi M, Uchida K, Goel A, et al:
Antitumor effects of andrographis via ferroptosis-associated genes
in gastric cancer. Oncol Lett. 22:5232021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao L, Peng Y, He S, Li R, Wang Z, Huang
J, Lei X, Li G and Ma Q: Apatinib induced ferroptosis by lipid
peroxidation in gastric cancer. Gastric Cancer. 24:642–654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan Z, Chen J, Li X and Dong N:
Tanshinone IIA induces ferroptosis in gastric cancer cells through
p53-mediated SLC7A11 down-regulation. Biosci Rep.
40:BSR202018072020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao GY, Ma J, Lu P, Jiang X and Chang C:
Ophiopogonin B induces the autophagy and apoptosis of colon cancer
cells by activating JNK/c-Jun signaling pathway. Biomed
Pharmacother. 108:1208–1215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Guo Y, Zhao R, Wang X, Jiang M, Fu
H and Zhang X: Ophiopogonin B induces apoptosis, mitotic
catastrophe and autophagy in A549 cells. Int J Oncol. 49:316–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Zhang Q, Jiang Y, Li F and Xin H:
Effects of ophiopogonin B on the proliferation and apoptosis of
SGC7901 human gastric cancer cells. Mol Med Rep. 13:4981–4986.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aartsen WM, Schuijt MP, Danser AH, Daemen
MJAP and Smits JFM: The role of locally expressed angiotensin
converting enzyme in cardiac remodeling after myocardial infarction
in mice. Cardiovasc Res. 56:205–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hassannia B, Wiernicki B, Ingold I, Qu F,
Van Herck S, Tyurina YY, Bayır H, Abhari BA, Angeli JPF, Choi SM,
et al: Nano-targeted induction of dual ferroptotic mechanisms
eradicates high-risk neuroblastoma. J Clin Invest. 128:3341–3355.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vuckovic AM, Bosello Travain V, Bordin L,
Cozza G, Miotto G, Rossetto M, Toppo S, Venerando R, Zaccarin M,
Maiorino M, et al: Inactivation of the glutathione peroxidase GPx4
by the ferroptosis-inducing molecule RSL3 requires the adaptor
protein 14-3-3ε. FEBS Lett. 594:611–624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Liu W, Wang J and Bai X:
Curculigoside inhibits ferroptosis in ulcerative colitis through
the induction of GPX4. Life Sci. 259:1183562020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Peng S, Cai J and Bao S: Silencing
of PTPN18 induced ferroptosis in endometrial cancer cells through
p-P38-mediated GPX4/xCT down-regulation. Cancer Manag Res.
13:1757–1765. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen M, Hu C, Guo Y, Jiang R, Jiang H,
Zhou Y, Fu H, Wu M and Zhang X: Ophiopogonin B suppresses the
metastasis and angiogenesis of A549 cells in vitro and in
vivo by inhibiting the EphA2/Akt signaling pathway. Oncol Rep.
40:1339–1347. 2018.PubMed/NCBI
|
|
28
|
Yuan S, Xu Y, Yi T and Wang H: The
anti-tumor effect of OP-B on ovarian cancer in vitro and in vivo,
and its mechanism: An investigation using network
pharmacology-based analysis. J Ethnopharmacol. 283:1147062021.
View Article : Google Scholar : PubMed/NCBI
|