Introduction
Colorectal cancer (CRC) is the third most common
type of cancer and ranks as the second most frequent cause of
cancer-related deaths. The incidence in young individuals continues
to increase (1). Tumor metastasis
is the leading cause of death in patients with cancer. At least
half of all patients with CRC experience systemic metastases. The
most frequent metastatic sites are the lungs and liver (2,3).
Once metastasis occurs, the clinical outcomes of conventional
therapies, including surgery, radiotherapy, chemotherapy, and
targeted drug therapy, remain unsatisfactory (4,5).
Defining the molecular mechanisms underlying the progression and
metastasis of CRC will help to identify novel biomarkers and
provide efficient therapeutic strategies to improve CRC
treatment.
Yes-associated protein (YAP) is the main effector of
Hippo signaling and is the key mechanism in the regulation of
cellular proliferation, differentiation, fate determination, and
regeneration (6,7). In mammalian systems, YAP translocates
from the cytoplasm to the nucleus, where it induces the
transcriptional activity of genes associated with cell
proliferation, apoptosis, migration, and invasion by interacting
with DNA-binding transcription factors (8,9).
Accumulating evidence suggests that YAP contributes to the
progression in human cancers, including breast cancer, melanoma,
and lung cancer (10–12). Aberrant YAP expression or
activation is also associated with poor prognosis (13–15).
In addition, YAP is frequently overexpressed in CRC tissues and has
been correlated with pathological grading, lymph node metastasis,
and survival in CRC (16,17). However, it is unclear whether YAP
serves as a useful therapeutic target to inhibit CRC
metastasis.
In the present study, we tested whether YAP could be
the key mechanism involved in CRC migration and invasion. We
investigated whether YAP protein levels are correlated with the
metastatic phenotype of CRC cells and serve as a useful therapeutic
target. Importantly, we found that YAP plays a critical role in the
migration and invasion of DLD-1 cells. Furthermore, we also tested
the potential of verteporfin, a small molecule that inhibits YAP
activation, as a therapeutic agent to inhibit the migration and
invasion of DLD-1 cells. The collective findings indicate potential
therapeutic targets that can help to suppress the migration and
invasion of CRC cells.
Materials and methods
Cell culture
Caco-2, LoVo and Colo-205 cell lines were purchased
from the Riken Cell Bank (Ibaraki, Japan). The DLD-1 cell line was
obtained from the Health Science Research Resources Bank (Osaka,
Japan). These cell lines were grown in RPMI-1640 (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.).
Chemicals and reagents
Verteporfin was purchased from ChemScene. Trametinib
was obtained from LC Laboratories. LY294002 was purchased from
Promega. The reagents were dissolved in dimethyl sulfoxide (DMSO).
Antibodies against lamin A/C were purchased from Santa Cruz
Biotechnology. Antibodies against β-actin were obtained from
Sigma-Aldrich; Merck KGaA. Antibodies against YAP, phosphorylated
(p)-Akt, Akt, p-extracellular signal-regulated kinase (ERK), and
ERK were obtained from Cell Signaling Technology. Small interfering
RNA (siRNA) targeting YAP (HSS115942);
(5′-GCAACTCCAACCAGCAGCAACAGAT-3′) was purchased from Thermo Fisher
Scientific, Inc.
Silencing of YAP
DLD-1 cells were transfected with YAP siRNA (10, 20
and 50 nM) or Stealth™ RNAi Negative Control (NC) (Invitrogen;
Thermo Fisher Scientific, Inc.) using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). After transfection,
the cells were treated according to the experimental
requirements.
Total RNA extraction and reverse
transcription-quantitative PCR
Total RNA was extracted using RNAiso Plus reagent
(Takara Bio) according to the manufacturer's instructions. The RNA
was reverse-transcribed to cDNA using the PrimeScript™ RT reagent
kit (Takara Bio), according to the manufacturer's protocol.
Quantitative PCR was performed with the Thermal Cycler Dice Real
Time system (Takara Bio) using SYBR Premix Ex Taq (Takara Bio). The
PCR conditions were an initial denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation at 94°C for 30 sec, annealing
at 50°C for 30 sec, and extension at 72°C for 30 sec. The following
primers were used: YAP forward, 5′-CCTCGTTTTGCCATGAACCAG-3′ and
reverse, 5′-GTTCTTGCTGTTTCAGCCGCAG-3′; glyceraldehyde 2-phosphate
dehydrogenase (GAPDH) forward, 5′-AAGGTCGGAGTCAACGGATT-3′ and
reverse, 5′-CTCCTGGAAGATGGTGATGG-3′. The expression levels were
normalized to the GAPDH internal control and fold changes in
expression levels were calculated using the 2−ΔΔCq
method.
Western blot analysis
Western blot analysis was performed as previously
described (18). The cells were
lysed using lysis buffer, and the protein concentration was
determined using the BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Proteins were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred to polyvinylidene fluoride membranes (EMD Millipore).
The membranes were treated with primary antibodies at 4°C
overnight, followed by incubation with the appropriate secondary
antibody. The proteins were detected using the Luminata Forte
Western HRP Substrate (EMD Millipore).
Transwell migration and invasion
assay
Transwell migration and invasion assays were
performed as described previously (19). DLD-1 cells were treated for 24 h
with YAP siRNA (10 and 20 nM), verteporfin (10, 50 and 100 nM), and
LY294002 (1 and 5 µM). Cells (2×104) were collected and
seeded in the upper chamber without Matrigel coating for the
migration assay and in the upper chamber precoated with Matrigel
for the invasion assay. After 24 h, cells that had traversed the
membrane were counted using a light microscope (Olympus).
Trypan blue exclusion assay
DLD-1 cells (2×104) were treated with YAP
siRNA (10, 20 and 50 nM), verteporfin (10, 50 and 100 nM), LY294002
(1, 5 and 10 µM), and trametinib (1 and 10 µM). After 72 h of
incubation, the number of stained cells was counted. There are no
images of trypan blue exclusion assay in this study.
Statistical analysis
All experiments were repeated three times. The
results are expressed as the mean ± standard deviation (SD).
Statistical analysis involved analysis of variance (ANOVA) with
Dunnett's test. Statistical significance was set at P<0.05.
Results
Levels of YAP expression are
correlated with migration and invasion of CRC cells
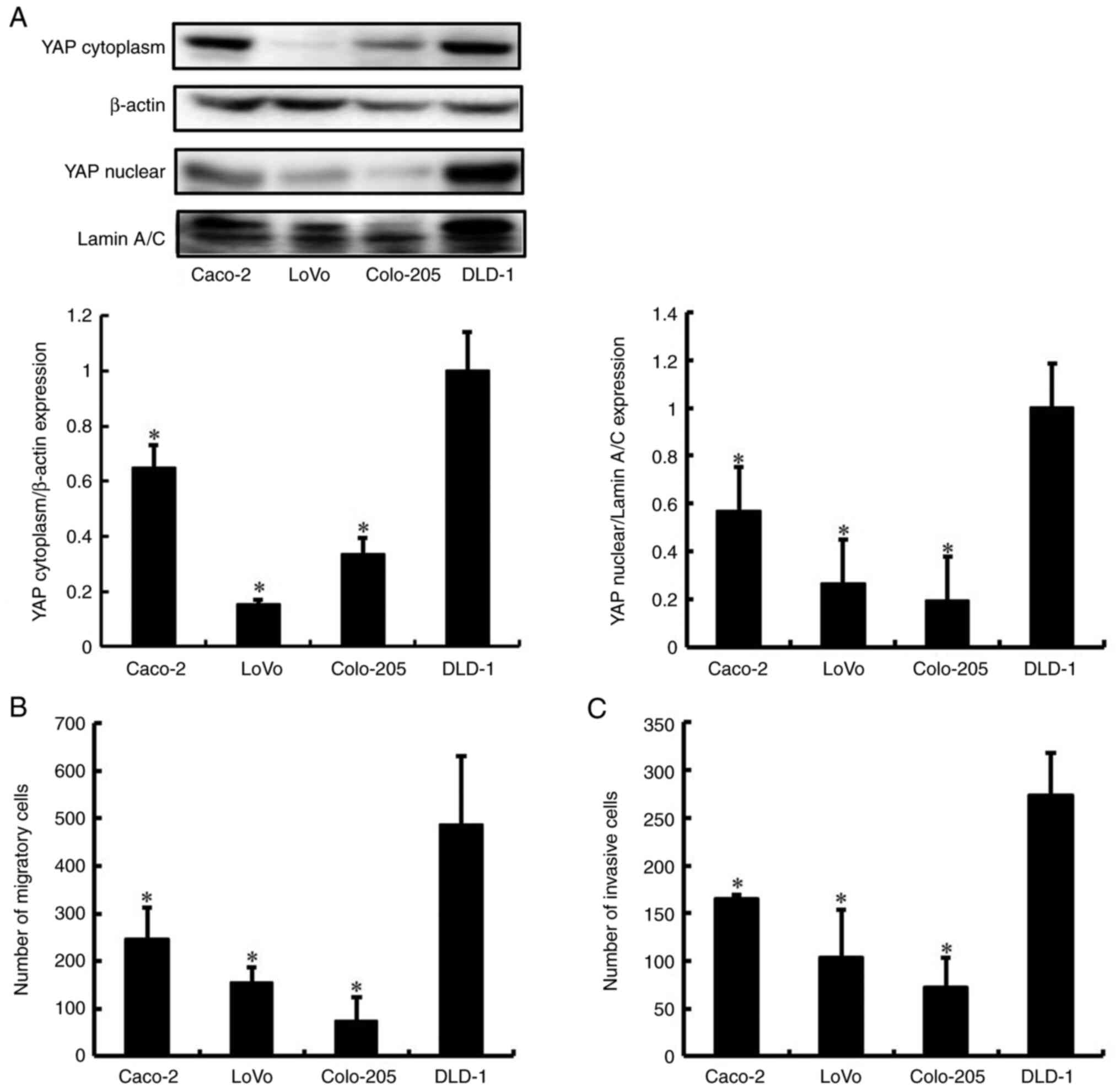
We examined whether high levels of YAP correlated
with the metastatic phenotype of cells from four human CRC cell
lines (Caco-2, LoVo, Colo-205, and DLD-1). We first examined the
expression of YAP in the CRC cells using western blot analysis.
Only DLD-1 cells produced a high level of YAP protein (Fig. 1A). We next investigated the
migration and invasion of the four CRC cell lines using the
Transwell migration and invasion assay. Migration and invasion were
more pronounced for DLD-1 cells compared to those of Caco-2, LoVo,
and Colo-205 cells (Figs. 1B and
C, and S1). These results
support the view that the levels of YAP protein are correlated with
high migration and invasion in CRC cell lines. The DLD-1 cells that
abundantly expressed YAP and displayed a highly metastatic
phenotype were used for subsequent experiments.
YAP promotes migration and invasion of
DLD-1 cells
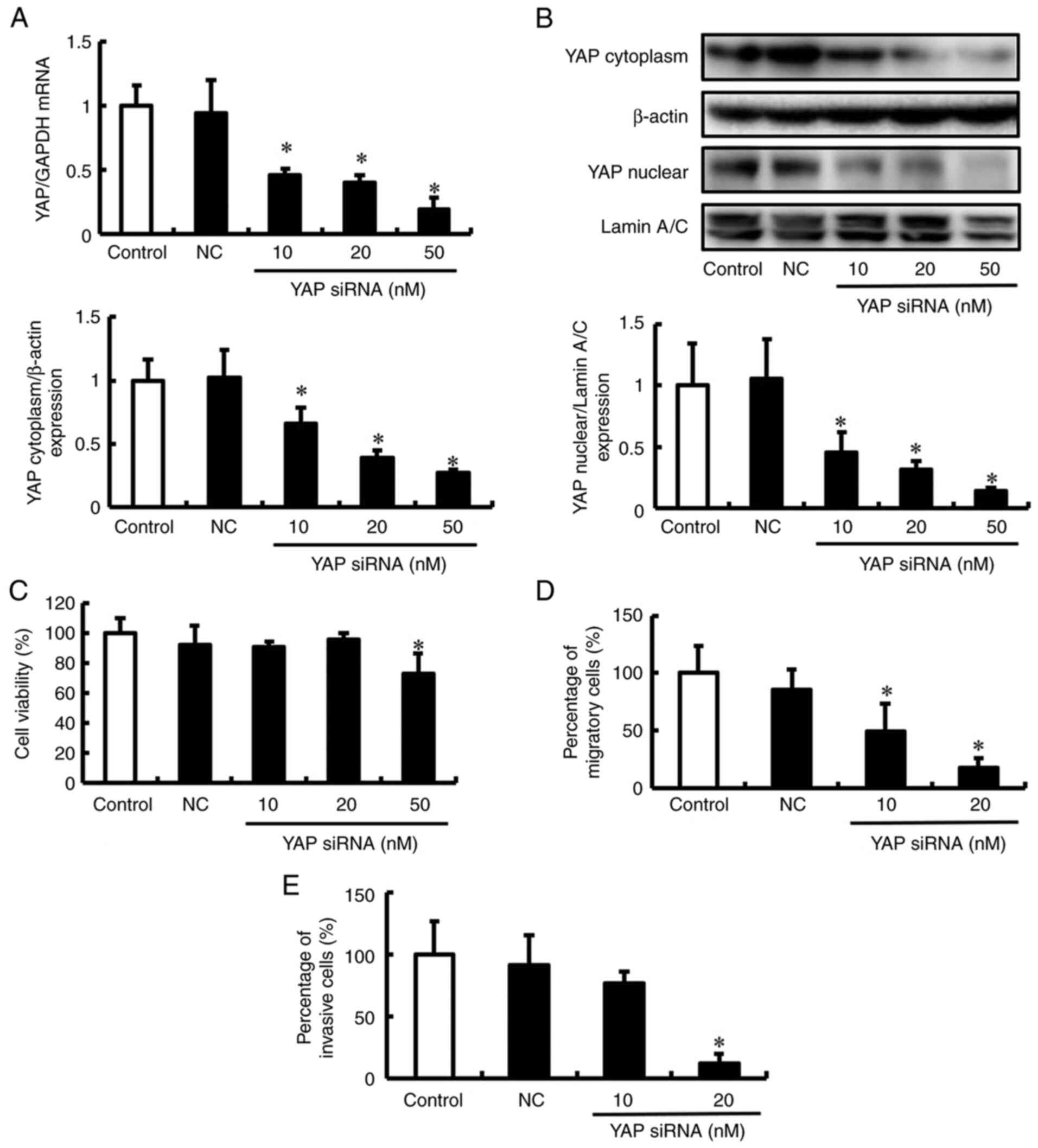
To determine the contribution of YAP to migration
and invasion, these attributes of DLD-1 cells were examined when
YAP was silenced using siRNA. DLD-1 cells were transfected with
siRNA specific for YAP in DLD-1, and the mRNA and protein
expression levels of YAP were determined after 2 days. The levels
of YAP mRNA and protein were suppressed in DLD-1 cells treated with
YAP siRNA in a concentration-dependent manner (Fig. 2A and B). Next, the effect of YAP
siRNA on the viability of DLD-1 cells was assessed using the Trypan
blue exclusion assay. DLD-1 cells treated with 10 and 20 nM YAP
siRNA showed no inhibition of cell viability (Fig. 2C). However, 50 nM YAP siRNA reduced
the viability of DLD-1 cells compared to that of untreated cells.
These conditions were used for subsequent experiments to assess the
effects of YAP siRNA on the migration and invasion of DLD-1 cells.
YAP siRNA inhibited the migration and invasion of DLD-1 cells but
did not affect viability (Fig. 2D and
E, and S2). Although the
phenotypes observed after depletion of YAP mRNA and proteins with
YAP siRNAs are usually attributed to the impaired function of these
proteins, it is possible that they are due to off-target effects of
the siRNAs. These results suggest that YAP is important for the
migration and invasion of DLD-1 cells.
PI3K/Akt pathway regulates YAP
activation and promotes migration and invasion of DLD-1 cells
YAP is regulated by signaling pathways, including
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) and phosphoinositide 3-kinase
(PI3K)/Akt pathways (20,21). In addition, we previously reported
that the YAP-high expressing DLD-1 cell line harbors a K-Ras
mutation and phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit alpha (PIK3CA) mutations (22). To determine whether the PI3K/Akt
and/or MEK/ERK pathways regulate YAP activation and therefore
promote the migration and invasion of DLD-1 cells, we examined the
expression of YAP in cells treated with the PI3K/Akt signaling
pathway inhibitor LY294002 and the MEK/ERK signaling pathway
inhibitor trametinib. LY294002 suppressed YAP activation by
inhibiting Akt phosphorylation (Fig.
3A). However, no change was observed in YAP activation upon
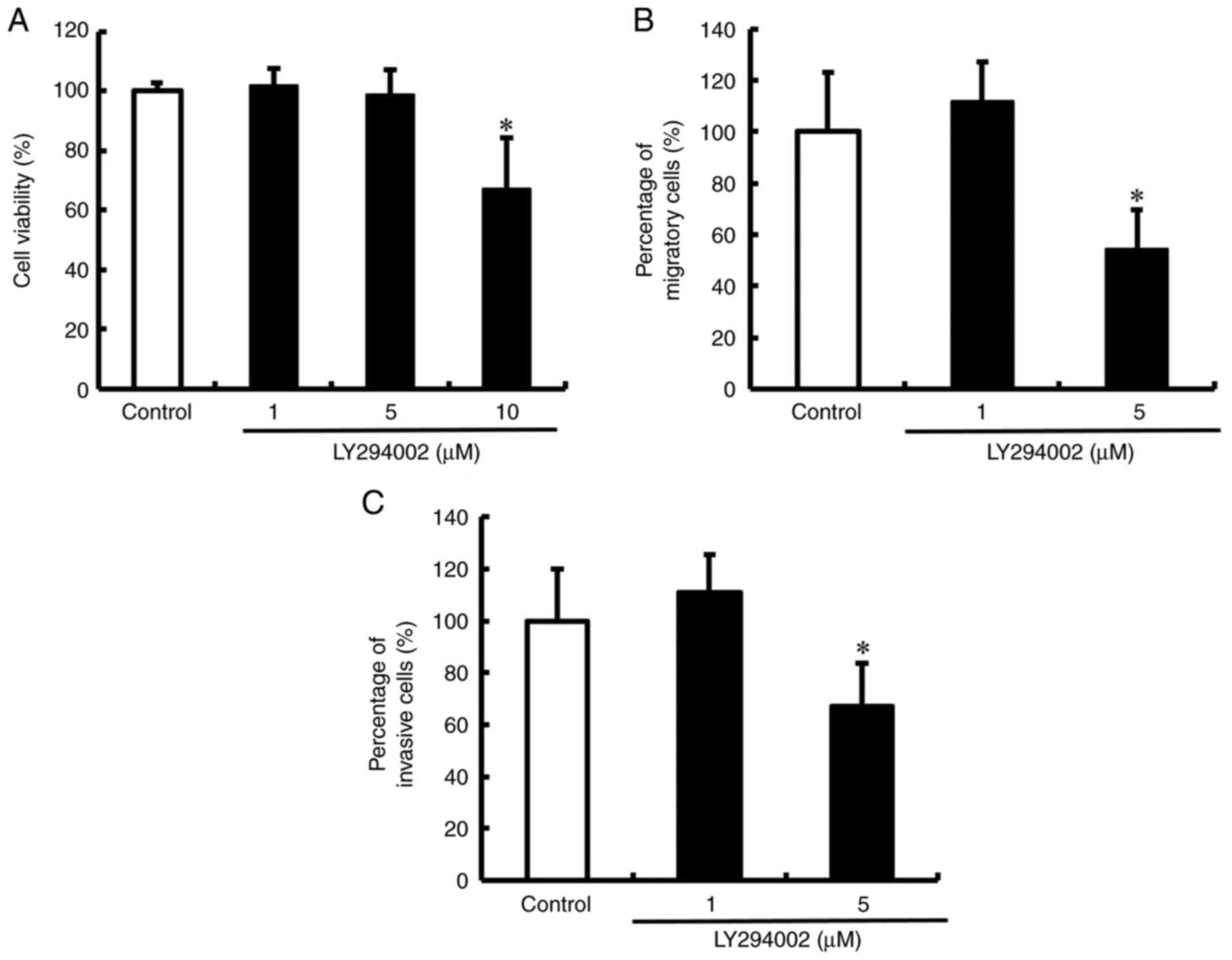
inhibition of ERK phosphorylation by trametinib (Fig. 3B). Next, we tested the effect of
LY294002 on the viability of DLD-1 cells. Viability was not reduced
in DLD-1 cells treated with 1 and 5 µM LY294002 (Fig. 4A). However, 10 µM LY294002 reduced
the viability of DLD-1 cells compared to that of untreated cells.
These conditions were used in subsequent experiments to assess the
effects of LY294002 on the migration and invasion of DLD-1 cells.
LY294002 inhibited the migration and invasion of DLD-1 cells but
did not affect viability (Figs. 4B and
C, and S3). These results
showed that the PI3K/Akt pathway regulates YAP activation and
promotes the migration and invasion of DLD-1 cells.
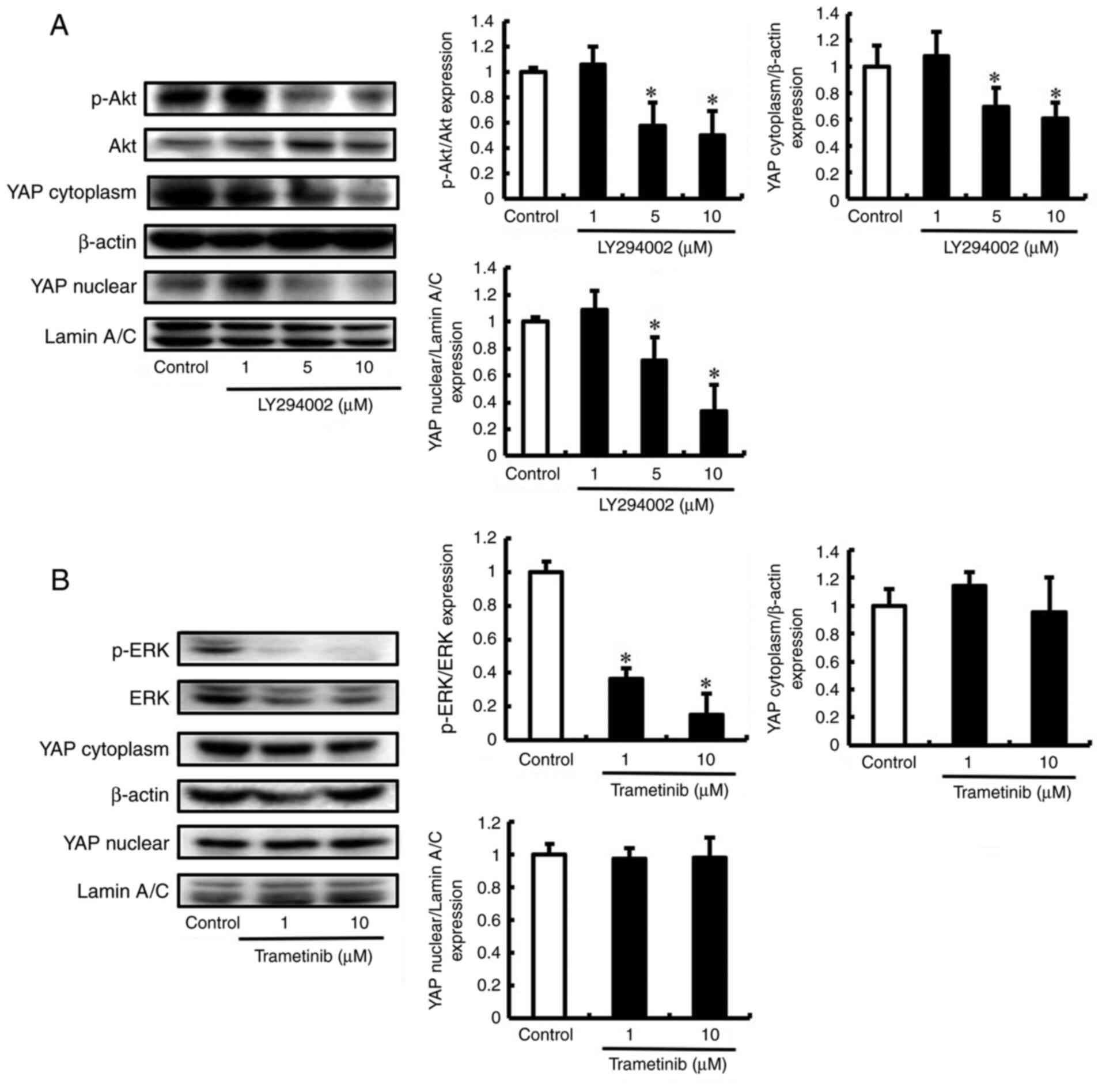 | Figure 3.LY294002 suppresses YAP activation by
inhibiting Akt phosphorylation. (A) DLD-1 cells were untreated
(control) or treated with LY294002 (1, 5 and 10 µM). The expression
of YAP, p-Akt, and Akt was detected by western blot. β-actin and
Lamin A/C were analyzed as an internal control. Bands were
normalized to that of Akt, β-actin and Lamin A/C. (B) DLD-1 cells
were untreated (control) or treated with trametinib (1 and 10 µM).
The expression of YAP, p-ERK, and ERK was detected by western blot.
β-actin and Lamin A/C were analyzed as an internal control. Bands
were normalized to that of ERK, β-actin and Lamin A/C. Data are
mean ± SD and have been repeated three times with similar results.
*P<0.05 compared with control. YAP, yes-associated protein; ERK,
extracellular signal-regulated kinase; SD, standard deviation. |
Verteporfin suppresses migration and
invasion of DLD-1 cells by decreasing YAP production
Verteporfin is primarily used as a photosensitizer
for the treatment of choroidal neovascularization in age-related
macular degeneration in ophthalmology (23). Previous studies have reported that
verteporfin inhibits YAP activation by preventing its binding to
the transcriptional enhancer associate domain (TEAD) (24). Thus, we investigated the potential
of repurposing verteporfin as a new treatment for metastatic CRC.
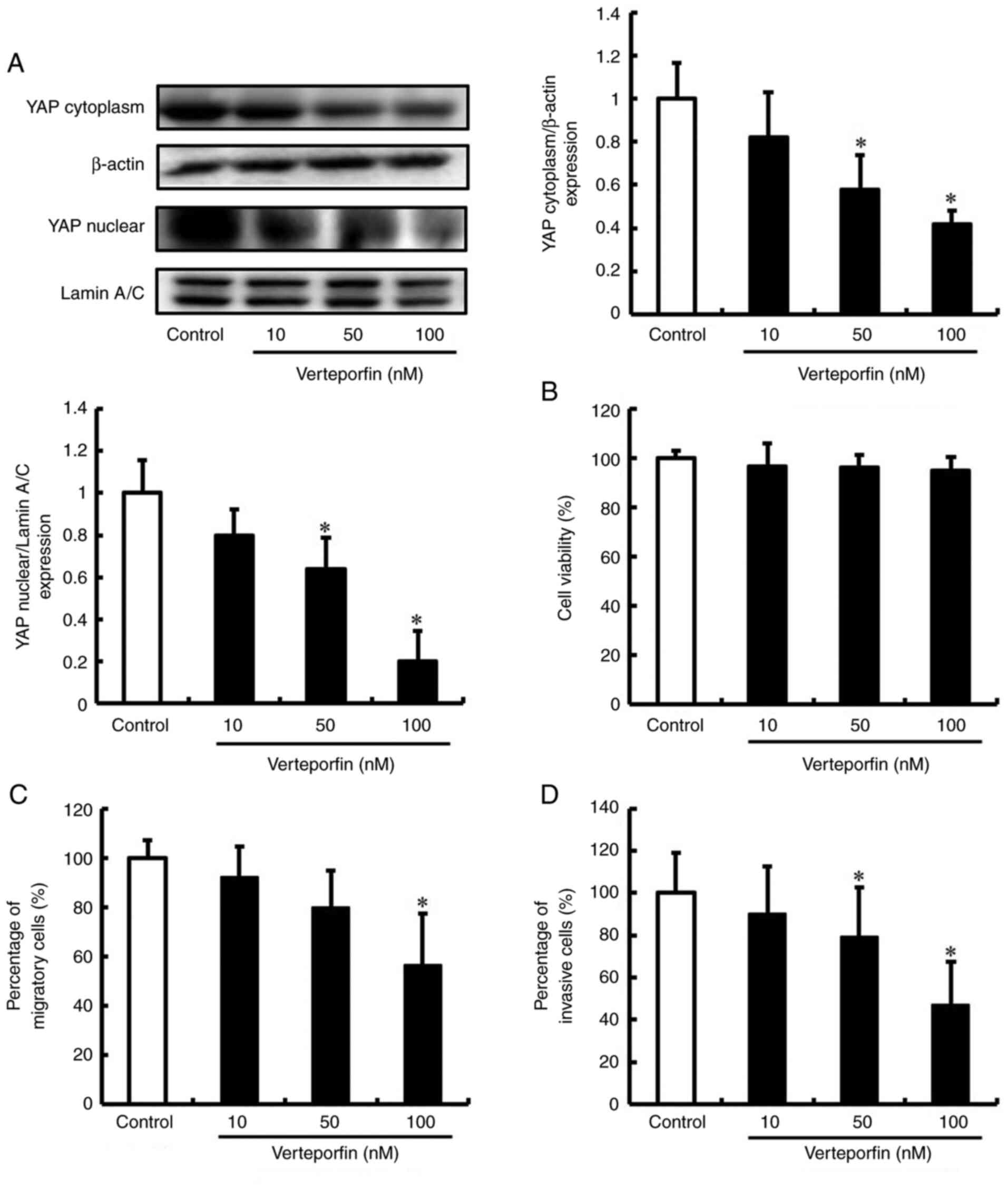
The expression of YAP in cells treated with verteporfin was
assessed by western blot analysis. Verteporfin decreased the
production of YAP in DLD-1 cells in a concentration-dependent
manner (Fig. 5A). Next, the effect
of verteporfin on the viability of DLD-1 cells was assessed using
the Trypan blue exclusion assay. Viability was not significantly
decreased in DLD-1 cells treated with 10, 50 and 100 nM verteporfin
(Fig. 5B). The effects of
verteporfin on the migration and invasion of DLD-1 cells were also
examined. Consistent with YAP knockdown, verteporfin treated DLD-1
cells exhibited significantly decreased migration and invasion
(Figs. 5C and D, and S4). These results indicate that
verteporfin inhibits the expression of YAP and suppresses the
migration and invasion of DLD-1 cells.
Discussion
CRC is one of the most prevalent malignant tumors.
Its poor prognosis is mainly ascribed to the pronounced malignant
invasion and metastasis of the cancer cells. Clarifying the
mechanism of metastasis could facilitate the design of novel and
more effective therapeutic strategies for metastasis in CRC
patients. Numerous studies have confirmed that dysregulation of
signaling pathways is involved in the migration, invasion, and
metastasis of human cancers (25–28).
YAP is involved in the regulation of tissue growth, homeostasis,
and tumor development (29).
Altered expression of YAP has been reported in various cancers that
include breast, ovarian, and liver cancers. The expression levels
of YAP are associated with disease-free survival (DFS) and overall
survival (OS) of patients with tumors (30,31).
In this study, we investigated whether YAP protein levels are
correlated with the metastatic phenotype of CRC cells and thus have
potential as a useful therapeutic target. The level of YAP protein
was correlated with pronounced migration and invasion of CRC cells.
Inhibition of YAP expression decreased the migration and invasion
of DLD-1 cells. Consistent with these findings, inhibition of YAP
reportedly suppressed the migration and invasion of pancreatic
cancer cells (32). These results
suggest that YAP plays an important role in the migration and
invasion of DLD-1 cells.
Although an increasing number of negative regulators
of YAP have been identified, there are few known positive
regulators of YAP (21,33). Recent studies have reported that
the MEK/ERK and PI3K/Akt pathways positively regulate YAP
activation (34,35). In this study, we used the YAP high
expression cell line DLD-1, which harbors KRAS and PIK3CA
mutations. KRAS mutations are frequent in CRC and have the
potential to activate proliferation, survival, migration, and
invasion through MEK/ERK signaling pathways (36). PIK3CA mutation also leads to the
activation of the PI3K/Akt signaling pathway, promoting cancer
growth in CRC (37). Presently,
the PI3K/Akt signaling pathway inhibitor LY294002 suppressed YAP
activation by inhibiting Akt phosphorylation. Furthermore, LY294002
inhibited the migration and invasion of DLD-1 cells. These results
indicate that the PI3K/Akt pathway regulates YAP activation and
promotes the migration and invasion of DLD-1 cells.
Drug repositioning refers to the discovery of new
indications for drugs that are clinically approved for other
indications. For approved drugs that have been clinically used for
a long time, the dosage, safety, dosage, safety, toxicity,
tolerability, and pharmacokinetic features are clear. In addition,
the success rate of drug repurposing approaches accounts for
approximately 30% of new Food and Drug Administration-approved
(FDA) drugs and vaccines in recent years (38). Therefore, repurposed candidate
drugs reduce the time and costs associated with drug development.
Verteporfin is a second-generation photosensitizer approved by the
FDA for photodynamic therapy in macular degeneration as a
photosensitizer (39). The present
evidence demonstrates that verteporfin suppresses the migration and
invasion of DLD-1 cells by decreasing YAP expression. Verteporfin
was recently identified as a disruptor of YAP-TEAD-mediated
transcription, which inhibits the YAP-TEAD complex (40). Moreover, verteporfin was reported
to decrease the expression of YAP protein by increasing the levels
of 14-3-3σ, a YAP chaperone protein (24). These findings support the use of
verteporfin as an effective therapy to suppress CRC migration and
invasion.
This study has a few limitations. Migration and
invasion of cells are important factors in cancer cell metastasis.
In this study, we showed that inhibition of YAP suppresses the
migration and invasion of DLD-1 cells. However, we have only
circumstantial evidence to support the relevance of our findings
in vivo. Further studies are warranted to scientifically
establish the efficacy of YAP inhibition in DLD-1 cells in
vivo. Moreover, the present experiments involved the DLD-1
human CRC cell line. These results were not confirmed in other
human CRC cell lines. The contribution of YAP to migration,
invasion, and metastasis should be more widely studied in other
human CRC cell lines.
In summary, the levels of YAP protein were
correlated with high migration and invasion of CRC cells. YAP siRNA
inhibited the migration and invasion of DLD-1 cells, but did not
influence viability. Furthermore, the Akt inhibitor LY294002
suppressed YAP activation by inhibiting Akt phosphorylation and
decreasing the migration and invasion of DLD-1 cells. Importantly,
verteporfin suppressed the migration and invasion of DLD-1 cells by
decreasing the expression of YAP. The collective findings indicate
that targeting YAP might be valuable for developing therapeutics
against CRC. Verteporfin may be an effective therapy to suppress
the migration and invasion of CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant-in-Aid for Young
Scientists from the Japan Society for the Promotion of Science
(JSPS) (grant no. 20K16343).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SN, TT and, YY designed the study. TT wrote the
manuscript, and performed western blot analysis and silencing of
YAP. YY edited the manuscript, performed western blot analysis,
trypan blue exclusion assay and silencing of YAP. MT performed the
trypan blue exclusion assay and statistical analysis. TM, AK and NS
performed the Transwell migration and invasion assays, western blot
analysis and statistical analysis. SN, TT and YY confirm the
authenticity of all raw data. All authors read, revised, and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
YAP
|
yes-associated protein
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
siRNA
|
small interfering RNA
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
TEAD
|
transcriptional enhancer associate
domain
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang M, Wang H, Cao Y, Zeng Z, Shan X and
Wang L: Nomogram for predicting occurrence and prognosis of liver
metastasis in colorectal cancer: A population-based study. Int J
Colorectal Dis. 36:271–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Y, Zhao M, Yin J, Lu T, Yang X, Yuan
G, Li M, Liu Y, Zhan C and Wang Q: Pulmonary metastasis in newly
diagnosed colon-rectal cancer: A population-based nomogram study.
Int J Colorectal Dis. 34:867–878. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao LL, Pei XF, Qiao X, Yu J, Ye H, Xi CL,
Wang PY and Gong ZL: SERPINA3 silencing inhibits the migration,
invasion, and liver metastasis of colon cancer cells. Dig Dis Sci.
63:2309–2319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding YL, Wang QS, Zhao WM and Xiang L:
Expression of smoothened protein in colon cancer and its prognostic
value for postoperative liver metastasis. Asian Pac J Cancer Prev.
13:4001–4005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dobrokhotov O, Samsonov M, Sokabe M and
Hirata H: Mechanoregulation and pathology of YAP/TAZ via Hippo and
non-Hippo mechanisms. Clin Transl Med. 7:232018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Totaro A, Panciera T and Piccolo S:
YAP/TAZ upstream signals and downstream responses. Nat Cell Biol.
20:888–899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MK, Jang JW and Bae SC: DNA binding
partners of YAP/TAZ. BMB Rep. 51:126–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Ugalde AP, Scheele CLGJ, Dieter SM,
Nagel R, Ma J, Pataskar A, Korkmaz G, Elkon R, Chien MP, et al: A
comprehensive enhancer screen identifies TRAM2 as a key and novel
mediator of YAP oncogenesis. Genome Biol. 22:542021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsubaki M, Genno S, Takeda T, Matsuda T,
Kimura N, Yamashita Y, Morii Y, Shimomura K and Nishida S: Rhosin
suppressed tumor cell metastasis through inhibition of Rho/YAP
pathway and expression of RHAMM and CXCR4 in melanoma and breast
cancer cells. Biomedicines. 9:352021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng X, Arang N, Rigiracciolo DC, Lee JS,
Yeerna H, Wang Z, Lubrano S, Kishore A, Pachter JA, König GM, et
al: A platform of synthetic lethal gene interaction networks
reveals that the GNAQ uveal melanoma oncogene controls the Hippo
pathway through FAK. Cancer Cell. 35:457–472.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu PC, Tian B, Yang YL, Wang YC, Liu S,
Urisman A, Yang CT, Xu Z, Jablons DM and You L: Cucurbitacin E
inhibits the Yes-associated protein signaling pathway and
suppresses brain metastasis of human non-small cell lung cancer in
a murine model. Oncol Rep. 42:697–707. 2019.PubMed/NCBI
|
|
13
|
Warren JSA, Xiao Y and Lamar JM: YAP/TAZ
activation as a target for treating metastatic cancer. Cancers
(Basel). 10:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Han W, He Y, Zhou J, Miao J and
Zhang G: Livin promotes tumor progression through YAP activation in
ovarian cancer. Am J Cancer Res. 10:3179–3193. 2020.PubMed/NCBI
|
|
15
|
Xu Z, Wang H, Gao L, Zhang H and Wang X:
YAP levels combined with plasma CEA levels are prognostic
biomarkers for early-clinical-stage patients of colorectal cancer.
Biomed Res Int. 2019:21708302019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang
A, Wen W and Zhu Q: Overexpression of YAP and TAZ is an independent
predictor of prognosis in colorectal cancer and related to the
proliferation and metastasis of colon cancer cells. PLoS One.
8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mouillet-Richard S and Laurent-Puig P:
YAP/TAZ signalling in colorectal cancer: Lessons from consensus
molecular subtypes. Cancers (Basel). 12:31602020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsubaki M, Ogawa N, Takeda T, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Satou T and Nishida S:
Dimethyl fumarate induces apoptosis of hematopoietic tumor cells
via inhibition of NF-κB nuclear translocation and down-regulation
of Bcl-xL and XIAP. Biomed Pharmacother. 68:999–1005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Ogawa N, Mashimo K, Fujiwara D, Takeda T,
et al: Inhibition of the tumour necrosis factor-alpha autocrine
loop enhances the sensitivity of multiple myeloma cells to
anticancer drugs. Eur J Cancer. 49:3708–3717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You B, Yang YL, Xu Z, Dai Y, Liu S, Mao
JH, Tetsu O, Li H, Jablons DM and You L: Inhibition of ERK1/2
down-regulates the Hippo/YAP signaling pathway in human NSCLC
cells. Oncotarget. 6:4357–4368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Montminy T, Azad T, Lightbody E,
Hao Y, SenGupta S, Asselin E, Nicol C and Yang X: PI3K positively
regulates YAP and TAZ in mammary tumorigenesis through multiple
signaling pathways. Mol Cancer Res. 16:1046–1058. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsubaki M, Takeda T, Noguchi M, Jinushi M,
Seki S, Morii Y, Shimomura K, Imano M, Satou T and Nishida S:
Overactivation of Akt Contributes to MEK inhibitor primary and
acquired resistance in colorectal cancer cells. Cancers (Basel).
11:18662019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Zhu X, Feng W, Yu Y, Jeong K, Guo
W, Lu Y and Mills GB: Verteporfin inhibits YAP function through
up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J
Cancer Res. 6:27–37, eCollection 2016.2015. PubMed/NCBI
|
|
25
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
26
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: A
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ming H, Li B, Zhou L, Goel A and Huang C:
Long non-coding RNAs and cancer metastasis: Molecular basis and
therapeutic implications. Biochim Biophys Acta Rev Cancer.
1875:1885192021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeda T, Tsubaki M, Asano R, Itoh T,
Imano M, Satou T and Nishida S: Dimethyl fumarate suppresses
metastasis and growth of melanoma cells by inhibiting the nuclear
translocation of NF-κB. J Dermatol Sci. 99:168–176. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coffey K: Targeting the Hippo pathway in
prostate cancer: What's new? Cancers (Basel). 13:6112021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson BJ: YAP/TAZ: Drivers of tumor
growth, metastasis, and resistance to therapy. Bioessays.
42:e19001622020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sorrentino G, Ruggeri N, Zannini A,
Ingallina E, Bertolio R, Marotta C, Neri C, Cappuzzello E, Forcato
M, Rosato A, et al: Glucocorticoid receptor signalling activates
YAP in breast cancer. Nat Commun. 8:140732017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang S, Zhang L, Purohit V, Shukla SK,
Chen X, Yu F, Fu K, Chen Y, Solheim J, Singh PK, et al: Active YAP
promotes pancreatic cancer cell motility, invasion and
tumorigenesis in a mitotic phosphorylation-dependent manner through
LPAR3. Oncotarget. 6:36019–36031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li XJ, Leem SH, Park MH and Kim SM:
Regulation of YAP through an Akt-dependent process by 3,
3′-diindolylmethane in human colon cancer cells. Int J Oncol.
43:1992–1998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin X, Li J, Sun J, Liu L, Chen D and Liu
Y: Low shear stress induces ERK nuclear localization and YAP
activation to control the proliferation of breast cancer cells.
Biochem Biophys Res Commun. 510:219–223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang C and Fakih M: Targeting KRAS in
colorectal cancer. Curr Oncol Rep. 23:282021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu YF, Yu BH, Li DL, Ke HL, Guo XZ and
Xiao XY: PI3K expression and PIK3CA mutations are related to
colorectal cancer metastases. World J Gastroenterol. 18:3745–3751.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pillaiyar T, Meenakshisundaram S, Manickam
M and Sankaranarayanan M: A medicinal chemistry perspective of drug
repositioning: Recent advances and challenges in drug discovery.
Eur J Med Chem. 195:1122752020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miller JW, Schmidt-Erfurth U, Sickenberg
M, Pournaras CJ, Laqua H, Barbazetto I, Zografos L, Piguet B,
Donati G, Lane AM, et al: Photodynamic therapy with verteporfin for
choroidal neovascularization caused by age-related macular
degeneration: Results of a single treatment in a phase 1 and 2
study. Arch Ophthalmol. 117:1161–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brodowska K, Al-Moujahed A, Marmalidou A,
Meyer Zu Horste M, Cichy J, Miller JW, Gragoudas E and Vavvas DG:
The clinically used photosensitizer Verteporfin (VP) inhibits
YAP-TEAD and human retinoblastoma cell growth in vitro without
light activation. Exp Eye Res. 124:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|