Autophagy is an evolutionarily conserved
lysosome-mediated recycling process that is essential for the
maintenance of cellular homeostasis and nutrient recycling
(1,2). Autophagy is characterized by the
formation of autophagosomes, which are vesicles with a lipid
bilayer (1–3). Cytoplasmic constituents are engulfed
into the autophagosome, which is delivered to the lysosome for
degradation or nutrient recycling (1,2).
Autophagosome formation, elongation, maturation and degradation are
regulated in a time-dependent manner by autophagy-related genes
(ATGs), such as Beclin 1, VPS34, ATG14, VPS15 and LC3 (1,3).
EGFR is a member of the ErbB tyrosine kinase
transmembrane receptor family that is activated following binding
to its ligand, EGF (4). EGFR
signaling pathways serve important roles in cell survival, growth,
proliferation and differentiation (5,6).
EGFR can regulate epithelial tissue development and homeostasis
under physiological conditions; however, it can also become a
driver of tumorigenesis (7). In
addition, EGFR also regulates the response of cancer cells to
metabolic stress (6). Various
types of cancer, such as non-small cell lung cancer, and head and
neck carcinomas, are associated with EGFR mutations or upregulation
(8). Targeted therapy for EGFR
sensitive mutations such as 19 deletion, L858R and G719X with
EGFR-tyrosine kinase inhibitors (TKIs) has become an important
therapeutic method (8,9). However, drug resistance often
develops following EGFR-TKI therapy (10). It has been demonstrated that
autophagy serves an important role in drug resistance of EGFR-TKIs
(11,12). A recent study has shown that EGFR
signaling suppresses autophagy (13).
The LAPTM4B gene is located on chromosome 8q22.1 and
was first identified in human hepatocellular carcinoma (HCC).
LAPTM4B is a novel tetratransmembrane protein primarily localized
in the late endosomes and lysosomes (14). Notably, endosomes may provide a
membrane source for autophagosome (15). Lysosomes are essential for
autophagosome maturation and degradation (3). It is also reported that LAPTM4B is
required for autophagy initiation (16). Furthermore, LAPTM4B knockdown
inhibits autophagosome maturation and autophagic flux in the
context of a metabolic stress microenvironment (16–18).
However, the role and mechanism of action of LAPTM4B in autophagy
remains unclear. The present review proposes that LAPTM4B
participates in regulating autophagy through the EGFR pathway.
LAPTM4B is upregulated in several types of cancer
and associated with cancer cell proliferation, survival, drug
resistance and poor prognosis (19). For instance, the LAPTM4B-35 protein
encoded by the AY219176 allele of LAPTM4B is upregulated in HCC,
lung cancer, extrahepatic cholangiocarcinoma, gallbladder cancer
and breast cancer (22–25). The LAPTM4B-35 protein promotes
proliferation and chemotherapy resistance of carcinoma cells
through the AKT signaling pathway and contributes to cell
migration, invasion and metastasis via its proline-rich domain
(PPRP motif), which can interact with SH3 domain-containing
signaling molecules that are involved in several signaling pathways
(25–27). A previous meta-analysis
demonstrated that the AY219177 allele of LAPTM4B, encoding the
40-kDa protein, is a high-risk factor for cancer (24). LAPTM4B promotes chemotherapy
resistance by increasing drug efflux and decreasing drug nuclear
localization and drug-induced DNA damage in vitro and in
vivo (18,27,28).
LAPTM4B upregulation is associated with tumorigenesis, drug
resistance and poor prognosis in hepatocellular, gallbladder,
extrahepatic cholangiocarcinoma, ovarian, gastric and colon
carcinoma (26,29–34)
through autophagy (35). LAPTM4B
silencing using short interfering (si)RNA inhibits autophagic flux,
whereas its overexpression rescues cell autophagy flux in the
context of metabolic stress (16,18).
LAPTM4B can regulate autophagy by activating ATG3 transcription,
which has been predicted using Gene Ontology analysis and animal
experiments (17). However, the
role of LAPTM4B in autophagy and the underlying mechanism remains
to be elucidated.
Autophagy is a conserved lysosome-mediated type-II
programmed cell death, which is specifically regulated by ATGs.
Basal autophagy is essential for homeostasis and can be upregulated
in response to metabolic stresses as a cell survival mechanism.
Excess autophagy results in cell death by catabolizing essential
cellular components (36,37).
Autophagy has a dual function, as it can act both as
a tumor suppressor and promoter. In normal cells, homeostatic
autophagy can keep cells from malignant transformation by degrading
intracellular toxic components. Nevertheless, autophagy can
counteract hypoxia, nutrient starvation and exposure to
chemotherapy, allowing cancer cells to adapt to these stress
conditions and promoting their survival. Autophagy also serves an
important role in chemoresistance in osteosarcoma, as well as
ovarian and lung carcinoma (38–44).
A series of clinical trials have investigated the role of autophagy
inhibitors, such as hydroxychloroquine (HCQ) or chloroquine (CQ),
and autophagy inducers, including rapamycin, in tumor therapy
(45–49). These results showed that HCQ or CQ
inhibits autophagy, resulting in increased tumor shrinkage. HCQ
(the only clinically-approved autophagy inhibitor) with an improved
toxicity profile compared with CQ has been studied in several phase
I/II clinical trials alone or in combination with other
chemotherapeutic regimens such as cisplatin (35). However, rapamycin as an autophagy
inducer also shows cytotoxic effects in tumor targeted therapy
(35). Recent studies show that
ATGs are involved in mediating resistance to chemotherapy and may
represent potential therapeutic targets (50–54).
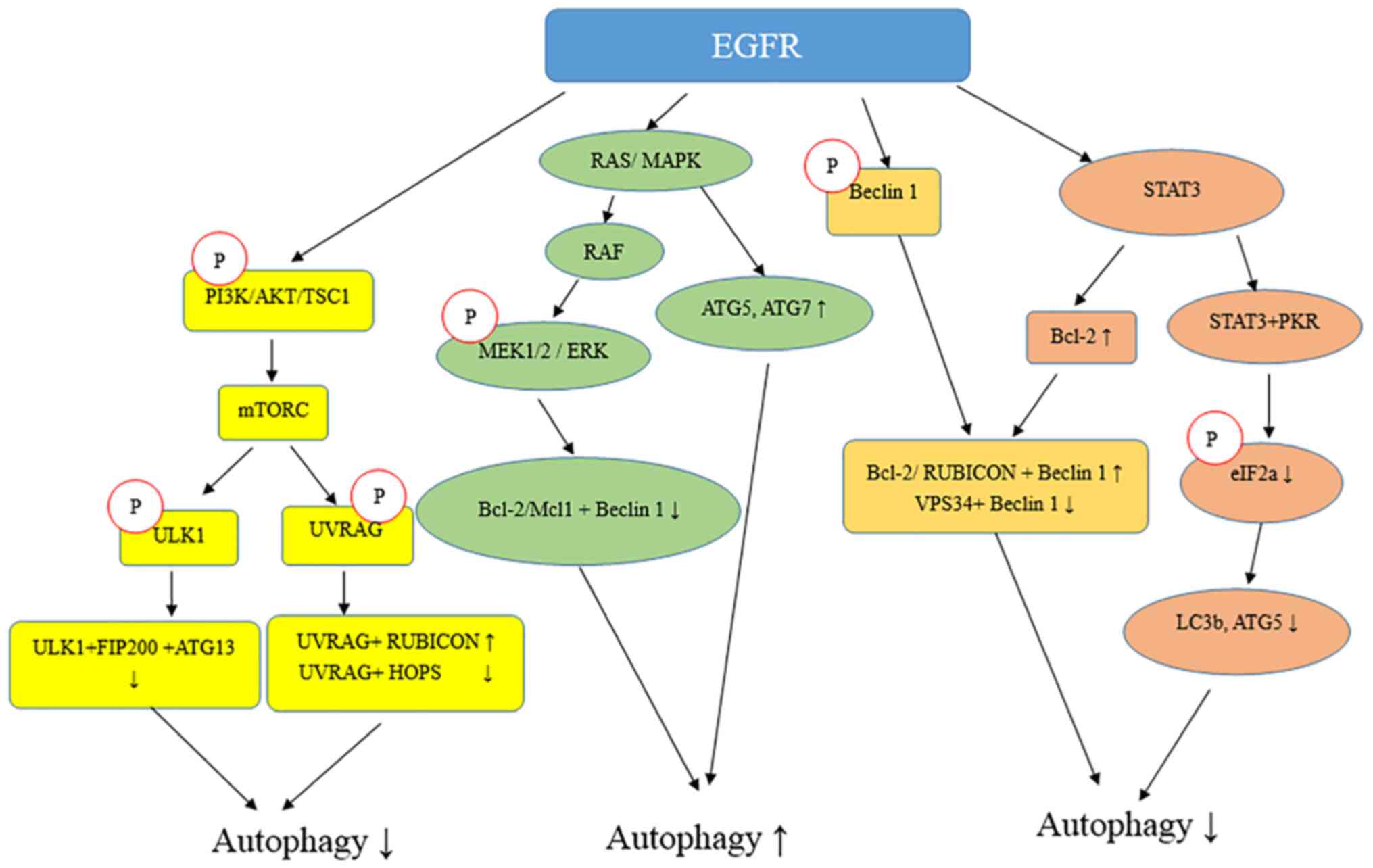
EGFR serves an important role in determining whether autophagy will
serve a tumor-suppressive or an oncogenic role (6).
EGFR is a transmembrane tyrosine kinase receptor
that can regulate DNA synthesis and cell proliferation and serves
critical roles both in physiological conditions and in cancer
(8,55). Various types of cancer, such as
non-small cell lung cancer, and head and neck carcinomas, are
associated with EGFR mutations or upregulation (8). Notably, it has been reported that the
activation of EGFR by its ligand, EGF, inhibits autophagy (13,16).
The aforementioned role of EGFR in autophagy is
kinase-dependent. It has been reported that inactive EGFR
colocalized with LAPTM4B in both late and early endosomes and that
these two molecules could interact and stabilize each other in
cells grown in the absence of serum (16). As aforementioned, LAPTM4B is
required for autophagy initiation. LAPTM4B silencing using siRNA
inhibits autophagosome maturation and autophagic flux in the
context of metabolic stress (16,18).
It may be hypothesized that LAPTM4B is involved in autophagy
through the EGFR pathway.
LAPTM4B and EGFR are upregulated or mutated in the
majority of types of cancer, including lung cancer and breast
cancer, and associated with cancer cell proliferation and survival,
drug resistance and poor prognosis (8,19).
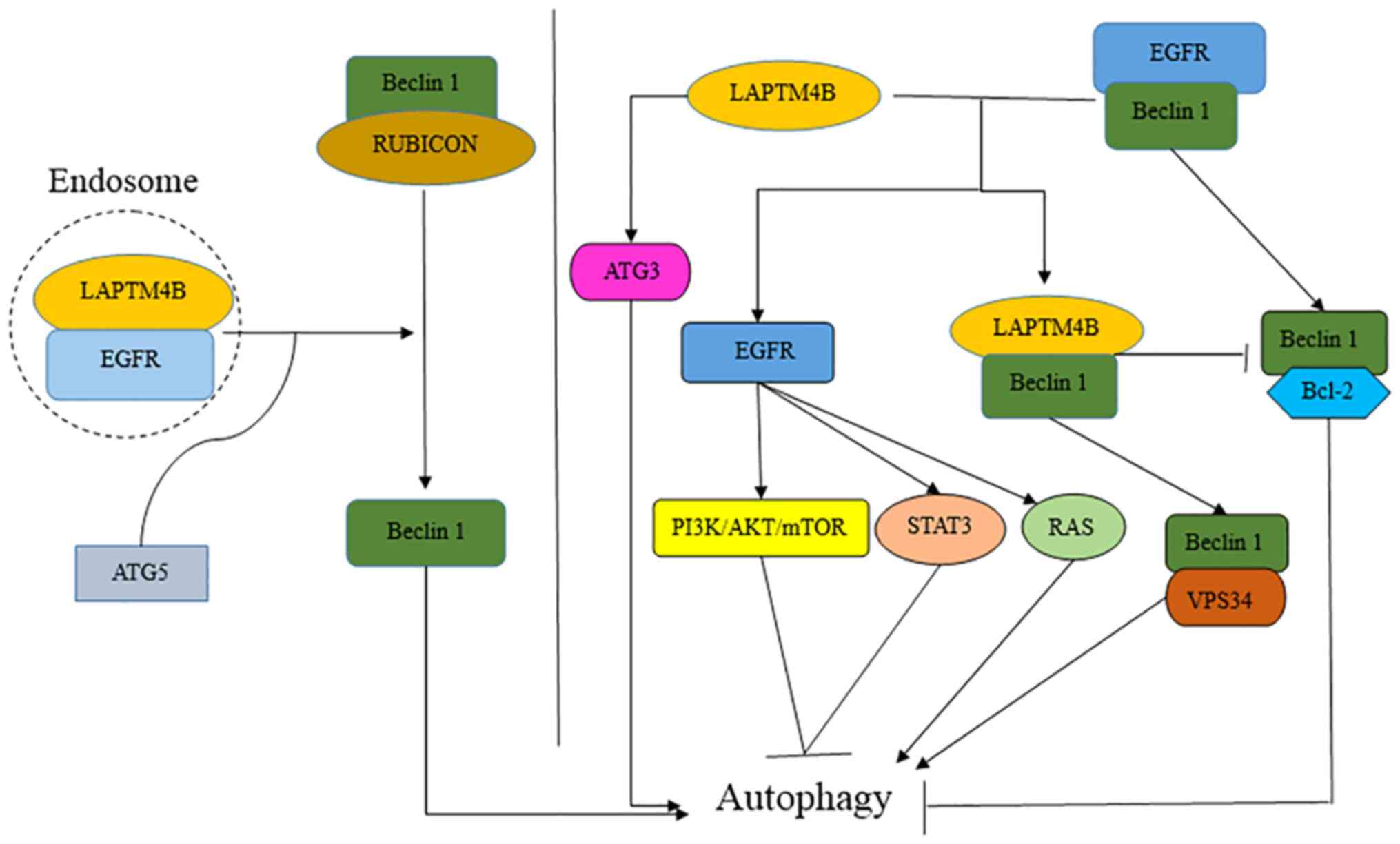
Tan et al (16) reported
that inactive EGFR and LAPTM4B interact and stabilize each other at
endosomes. However, as an endosomal protein, LAPTM4B could modulate
inactive EGFR endosomal accumulation and inhibit EGF-stimulated
EGFR lysosomal sorting (16). As
aforementioned, EGFR phosphorylation is required for autophagy. A
previous study has demonstrated that EGFR-TKIs or neutralizing
antibody such as cetuximab induce autophagy and exert
cytoprotective roles on cancer cells (71). In addition, EGFR silencing using
siRNA strongly inhibits autophagosome formation (16). Notably, it has been demonstrated
that LAPTM4B serves a key role in autophagy and EGFR gene mutations
in clinical tumor samples (16,18,72).
LAPTM4B is required for the endosomal accumulation of inactive EGFR
and autophagy, and LAPTM4B is a cofactor for inactive EGFR-driven
autophagy (16).
LAPTM4B regulates autophagy either by directly
activating ATG3 transcription, or through the EGFR pathway (whether
active or inactive). The present review aimed to describe the role
and mechanism of action of LAPTM4B in EGFR-mediated autophagy.
EGFR-TKI therapy for EGFR sensitive mutations induces autophagy
contributing to cancer cell survival. Considering the role of
LAPTM4B in autophagy and chemotherapy resistance, full
understanding of the association between LAPTM4B, EGFR and
autophagy may provide insight into more effective therapeutic
strategies for tumors such as non-small-cell lung carcinoma.
LAPTM4B may be a potential candidate for future tumor treatment
options, particularly in combination with other cancer driver gene
targeted therapy such as EGFR-TKIs. The present review may provide
useful information for future studies on the development of more
effective targeted therapies compared with broad spectrum
chemotherapy.
Not applicable.
Funding: No funding was received.
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
XJ and HM drafted the manuscript. XJ and YD designed
the study, supervised preparation of the manuscript and gave final
approval of the version to be published. Data authentication is not
applicable. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena AA, Adachi H, Adams CM, Adams PD, Adeli
K, et al: Guidelines for the use and interpretation of assays for
monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng Y, He D, Yao Z and Klionsky DJ: The
machinery of macroautophagy. Cell Res. 24:24–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henson ES and Gibson SB: Surviving cell
death through epidermal growth factor (EGF) signal transduction
pathways: Implications for cancer therapy. Cell Signal.
18:2089–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jutten B and Rouschop KMA: EGFR signaling
and autophagy dependence for growth, survival, and therapy
resistance. Cell Cycle. 13:42–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henson E, Chen Y and Gibson S: EGFR family
members' regulation of autophagy is at a crossroads of cell
survival and death in cancer. Cancers (Basel). 9:272017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendelsohn X and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye KJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Majem M and Remon J: Tumor heterogeneity:
Evolution through space and time in EGFR mutant non small cell lung
cancer patients. Transl Lung Cancer Res. 2:226–237. 2013.PubMed/NCBI
|
|
11
|
Feng Y, Gao S, Gao Y, Wang X and Chen Z:
Anti-EGFR antibody sensitizes colorectal cancer stem-like cells to
Fluorouracil-induced apoptosis by affecting autophagy. Oncotarget.
7:81402–81409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J,
Ge W, Feng L, Lin X, Wang X, et al: EGFR tyrosine kinase inhibitors
activate autophagy as a cytoprotective response in human lung
cancer cells. PLoS One. 6:e186912011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei Y, Zou Z, Becker N, Anderson M,
Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et
al: EGFR-mediated phosphorylation of beclin 1 in autophagy
suppression, tumor progression and tumor chemoresistance. Cell.
154:1269–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XR, Zhou RL, Zhang QY, Zhang Y, Jin
YY, Lin M, Rui JA and Ye DX: Structure analysis and expressions of
a novel tetratransmembrane protein, lysosoma-associated protein
transmembrane 4 beta associated with hepatocellular carcinoma.
World J Gastroenterol. 10:1555–1559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tooze SA and Yoshimori T: The origin of
the autophagosomal membrane. Nat Cell Biol. 12:831–835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan X, Thapa N, Sun Y and Anderson RA: A
kinase independent role for EGF receptor in autophagy initiation.
Cell. 160:145–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Wu H, Zhang S, Lu J, Lu Y, Zhan P,
Fang Q, Wang F, Zhang X, Xie C and Yin Z: LAPTM4B facilitates tumor
growth and induces autophagy in hepatocellular carcinoma. Cancer
Manag Res. 11:2485–2497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Zhang Q, Tian R, Wang Q, Zhao JJ,
Iglehart JD, Wang ZC and Richardson AL: Lysosomal transmembrane
protein LAPTM4B promotes autophagy and tolerance to metabolic
stress in cancer cells. Cancer Res. 71:7481–7489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng Y, Wang L, Chen D, Chang Y, Zhang M,
XU JJ, Zhou R and Zhang QY: LAPTM4B: An oncogene in various solid
tumors and its functions. Oncogene. 35:6359–6365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Zhang QY and Zhou RL: Relationship
between LAPTM4B gene polymorphism and susceptibility of primary
liver cancer. Ann Oncol. 23:1864–1869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Xu JJ, Zhou R and Zhang QY:
Association of LAPTM4B gene polymorphism with nasopharyngeal
carcinoma susceptibility in a Chinese population. Med Oncol.
30:4702013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang H, Tian H, Yue W, Li L, Li S, Gao C,
Si L, Qi L, Lu M and Hu W: LAPTM4B polymorphism is associated with
nonsmall cell lung cancer susceptibility and prognosis. Oncol Rep.
31:2454–2460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaker O, Taha F, Salah M and El-Marzouky
M: LAPTM4B gene expression and polymorphism as diagnostic markers
of breast cancer in Egyptian patients. J Med Biochem. 34:393–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia LZ, Yin ZH, Ren YW, Shen L, Wu W, Li
XL, Guan P and Zhou BS: The relationship between LAPTM4B
polymorphisms and cancer risk in Chinese Han population: A
meta-analysis. Springerplus. 4:1792015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Xiong F, Wei X, Yang Y, McNutt MA
and Zhou RL: Overexpression of LAPTM4B-35 promotes growth and
metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer
Lett. 294:236–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Xiong F, Wei X, Yang H and Zhou R:
LAPTM4B-35, a novel tetratransmembrane protein and its PPRP motif
serve critical roles in proliferation and metastatic potential of
hepatocellular carcinoma cells. Cancer Sci. 100:2335–2340. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Wei XH, Pan YP, Li HC, Yang H, He
QH, Pang Y, Shan Y, Xiong FX, Shao GZ and Zhou RL: LAPTM4B: A novel
cancer-associated gene motivates multidrug resistance through
efflux and activating PI3K/AKT signaling. Oncogene. 29:5785–5795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zou L, Li Q, Haibe-Kains B, Tian R,
Li Y, Desmedt C, Sotiriou C, Szallasi Z, Iglehart JD, et al:
Amplification of LAPTM4B and YWHAZ contributes to chemotherapy
resistance and recurrence of breast cancer. Nat Med. 6:214–218.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, He XD, Yu JC, Zhou RL, Yang H, Qu
Q and Rui JA: Overexpression of LAPTM4B promotes growth of
gallbladder carcinoma cells in vitro. Am J Surg. 199:515–521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, He XD, Cui QC, Zhou WX, Qu Q, Zhou
RL, Rui JA and Yu JC: Expression of LAPTM4B-35: A novel marker of
progression, invasiveness and poor prognosis of extrahepatic
cholangiocarcinoma. Cancer Lett. 264:209–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Liu Y, Zhou R, Meng F, Gao Y, Yang
S, Li X, Yang M and Lou G: LAPTM4B polymorphisms is associated with
ovarian cancer susceptibility and its prognosis. Jpn J Clin Oncol.
42:413–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Yang H, McNutt MA, Xiong F, Xiu N,
Li L and Zhou R: LAPTM4B overexpression is an independent
prognostic marker in ovarian carcinoma. Oncol Rep. 20:1077–1083.
2008.PubMed/NCBI
|
|
33
|
Zhang H, Tian B, Yu H, Yao H and Gao Z:
LAPTM4B-35 protein as a potential therapeutic target in gastric
cancer. Tumour Biol. 35:12737–12742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng XJ, Xu W, Zhang QY and Zhou RL:
Relationship between LAPTM4B gene polymorphism and susceptibility
of colorectal and esophageal cancers. Ann Oncol. 19:527–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Usman RM, Razzaq F, Akbar A, Farooqui AA,
Iftikhar A, Latif A, Hassan H, Zhao J, Carew JS, Nawrocki ST and
Anwer F: Role and mechanism of autophagy-regulating factors in
tumorigenesis and drug resistance. Asia Pac J Clin Oncol.
17:193–208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scarlatti F, Granata R, Meijer AJ and
Codogno P: Does autophagy have a license to kill mammalian cells?
Cell Death Differ. 16:12–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan B, Chen D, Huang J, Wang R, Feng B,
Song H and Chen L: HMGB1-mediated autophagy promotes docetaxel
resistance in human lung adenocarcinoma. Mol Cancer. 13:1652014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao H, Chen F, Liu H, Hu Y, Wang Y and Li
H: Wnt/β-catenin signaling pathway activation reverses gemcitabine
resistance by attenuating beclin1-mediated autophagy in the MG63
human osteosarcoma cell line. Mol Med Rep. 16:1701–1706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ying H, Qu D, Liu C, Ying T, Lv J, Jin S
and Xu H: Chemoresistance is associated with Beclin-1 and PTEN
expression in epithelial ovarian cancers. Oncol Lett. 9:1759–1763.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Zhao L, Ju Y, Li W, Zhang M, Jiao
Y, Zhang J, Wang S, Wang Y, Zhao M, et al: A novel androstenedione
derivative induces ROS-mediated autophagy and attenuates drug
resistance in osteosarcoma by inhibiting macrophage migration
inhibitory factor (MIF). Cell Death Dis. 5:e13612014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eum KH and Lee M: Targeting the autophagy
pathway using ectopic expression of beclin 1 in combination with
rapamycin in drug-resistant v-Ha-ras-transformed NIH 3T3 cells. Mol
Cells. 31:231–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu MY, Fu J, Xu J, O'Malley BW and Wu RC:
Steroid receptor coactivator 3 regulates autophagy in breast cancer
cells through macrophage migration inhibitory factor. Cell Res.
22:1003–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pietrocola F, Pol J, Vacchelli E, Baracco
EE, Levesque S, Castoldi F, Maiuri MC, Madeo F and Kroemer G:
Autophagy induction for the treatment of cancer. Autophagy.
12:1962–1964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chude CI and Amaravadi RK: Targeting
autophagy in cancer: Update on clinical trials and novel
inhibitors. Int J Mol Sci. 18:12792017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pellegrini P, Strambi A, Zipoli C,
Hägg-Olofsson M, Buoncervello M, Linder S and Milito AD: Acidic
extracellular pH neutralizes the autophagy-inhibiting activity of
chloroquine: Implications for cancer therapies. Autophagy.
10:562–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fung C, Chen X, Grandis JR and Duvvuri U:
EGFR tyrosine kinase inhibition induces autophagy in cancer cells.
Cancer Biol Ther. 13:1417–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gorzalczany Y, Gilad Y, Amihai D, Hammel
I, Sagi-Eisenberg R and Merimsky O: Combining an EGFR directed
tyrosine kinase inhibitor with autophagy-inducing drugs: A
beneficial strategy to combat non-small cell lung cancer. Cancer
Lett. 310:207–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pan B, Chen Y, Song H, Xu Y, Wang R and
Chen L: Mir-24-3p downregulation contributes to VP16-DDP resistance
in small-cell lung cancer by targeting ATG4A. Oncotarget.
6:317–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu J, Li Y, Tian Z, Hua X, Gu J, Li J,
Liu C, Jin H, Wang Y, Jiang G, et al: ATG7 overexpression is
crucial for tumorigenic growth of bladder cancer in vitro and in
vivo by targeting the ETS2/miRNA196b/FOXO1/p27 axis. Mol Ther
Nucleic Acids. 7:299–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen J, Zhang L, Zhou H, Wang W, Luo Y,
Yang H and Yi H: Inhibition of autophagy promotes cisplatin-induced
apoptotic cell death through Atg5 and beclin 1 in A549 human lung
cancer cells. Mol Med Rep. 17:6859–6865. 2018.PubMed/NCBI
|
|
53
|
Wu J, Li W, Ning J, Yu W, Rao T and Cheng
F: Long noncoding RNA UCA1 targets miR-582-5p and contributes to
the progression and drug resistance of bladder cancer cells through
ATG7-mediated autophagy inhibition. Onco Targets Ther. 12:495–508.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu Z, Cai L, Lu J, Wang CD, Guan J, Chen
X, Wu J, Zheng W, Wu Z, Li Q and Su Z: MicroRNA-93 mediates
cabergoline-resistance by targeting ATG7 in prolactinoma. J
Endocrinol. Sep 1–2018.(Epub ahead of print).
|
|
55
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Botti J, Djavaheri-Mergny M, Pilatte Y and
Codogno P: Autophagy signaling and the cogwheels of cancer.
Autophagy. 2:67–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim MJ, Woo SJ, Yoon CH, Lee JS, An S,
Choi YH, Hwang SG, Yoon G and Lee SJ: Involvement of autophagy in
oncogenic K-ras-induced malignant cell transformation. J Biol Chem.
286:12924–12932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Elgendy M, Sheridan C, Brumatti G and
Martin SJ: Oncogenic ras-induced expression of noxa and beclin-1
promotes autophagic cell death and limits clonogenic survival. Mol
Cell. 42:23–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG and
Zhu YP: Resveratrol induces apoptosis and autophagy in T-cell acute
lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating
p38-MAPK. Biomed Environ Sci. 26:902–911. 2013.PubMed/NCBI
|
|
60
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Schmukler E, Kloog Y and Pinkas-Kramarski
R: Ras and autophagy in cancer development and therapy. Oncotarget.
5:577–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim YM, Jung CH, Seo M, Kim EK, Park JM,
Bae SS and Kim DH: mTORC1 phosphorylates UVRAG to negatively
regulate autophagosome and endosome maturation. Mol Cell.
57:207–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu SY, Lan SH, Cheng DE, Chen WK, Shen CH,
Lee YR, Zuchini R and Liu HS: Ras-related tumorigenesis is
suppressed by BNIP3-mediated autophagy through inhibition of cell
proliferation. Neoplasia. 13:1171–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Byun JY, Yoon CH, An S, Park IC, Kang CM,
Kim MJ and Lee SJ: The Rac1/MKK7/JNK pathway signals upregulation
of Atg5 and subsequent autophagic cell death in response to
oncogenic ras. Carcinogenesis. 30:1880–1888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu D, Lin J, Su J, Chen X, Jiang P and
Huang K: Glutamine deficiency promotes PCV2 infection through
induction of autophagy via activation of ROS-mediated JAK2/STAT3
signaling pathway. J Agric Food Chem. 66:11757–11766. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Quesnelle KM, Boehm AL and Grandis JR:
STAT-mediated EGFR signaling in cancer. J Cell Biochem.
102:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rouschop KMA, van den Beucken T, Dubois L,
Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W,
Voncken JW, et al: The unfolded protein response protects human
tumor cells during hypoxia through regulation of the autophagy
genes MAP1LC3B and ATG5. J Clin Invest. 120:127–141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shen S, Niso-Santano M, Adjemian S,
Takehara T, Malik SA, Minoux H, Souquere S, Mariño G, Lachkar S,
Senovilla L, et al: Cytoplasmic STAT3 represses autophagy by
inhibiting PKR activity. Mol Cell. 48:667–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eimer S, Belaud-Rotureau MA, Airiau K,
Jeanneteau M, Laharanne E, Véron N, Vital A, Loiseau H, Merlio JP
and Belloc F: Autophagy inhibition cooperates with erlotinib to
induce glioblastoma cell death. Cancer Biol Ther. 11:1017–1027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang L, Meng Y and Zhang QZ: LAPTM4B is a
novel diagnostic and prognostic marker for lung adenocarcinoma and
associated with mutant EGFR. BMC Cancer. 19:2932019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tian M, Chen Y, Tian D, Qiao X, Ma Z and
Li J: Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in
gastric cancer cells. Gene. 626:48–53. 2017. View Article : Google Scholar : PubMed/NCBI
|