Introduction
The survival of patients with multiple myeloma (MM)
has been remarkably extended because of the development of new
proteasome inhibitors (PIs) and immunomodulatory drugs (1,2).
However, most patients treated with these drugs ultimately relapse
owing to the development of chemotherapeutic resistance in MM cells
(3).
Sphingosine-1 phosphate (S1P) was originally
identified as a bioactive lipid and has been reported to be
involved in the regulation of various physiological cell functions,
such as cell proliferation, apoptosis, and angiogenesis (4). A molecule of S1P contains a ceramide
backbone; sphingosine (SP) is catalyzed by two enzymes, namely,
sphingosine kinase 1 (SK1) and sphingosine kinase 2 (SK2). S1P
exerts its activity, both inside and outside the cell membrane, by
interacting with a G protein-coupled S1P receptor (S1PR) on the
cell membrane. Five isotypes of S1PR have been identified
(S1PR1-S1PR5), and their respective functions have been reported
(5,6). Several studies have reported that S1P
influences cancer progression (7–10).
Indeed, high activity of S1P and its synthetases, SKs, combined
with high expression of specific S1PR isotypes has been reported in
numerous cancer types (8,10). Fingolimod is an S1PR1 receptor
antagonist that was recently adopted as a therapeutic drug for
multiple sclerosis, and its efficacy on various tumors in inducing
apoptosis and reducing angiogenesis has been previously reported
(11). Moreover, small-molecule SK
inhibitors with anti-cancer potential against cancer cell survival
and proliferation have been identified (12–17).
SK1-I is synthesized as a sphingosine analog and specifically
inhibits SK1. It reportedly inhibits growth and survival by
inducing apoptosis in leukemia cells (12). ABC294640 is an SK2-specific
inhibitor. This compound reportedly inhibits tumor proliferation
and migration by promoting autophagic cell death (13,14).
However, the role of S1P in regulating myeloma cell
proliferation is unclear. We hypothesized that the bioactivity of
S1P affects myeloma cell proliferation or the acquisition of
chemotherapeutic resistance. Thus, targeting S1PR or the enzymes
involved in S1P biosynthesis may serve as a novel therapeutic
strategy for MM. To test this hypothesis, we evaluated the
potential anti-cancer effects of fingolimod and SK inhibitors in
myeloma cells and investigated the effects of S1P-induced
chemoresistance and neovascularization on MM cell proliferation.
Moreover, we evaluated circulating S1P levels in the serum of
patients with MM and monoclonal gammopathy of undetermined
significance (MGUS) to identify candidate biomarkers capable of
detecting disease progression of MM or its advancement to a later
disease stage.
Materials and methods
Cell lines and primary myeloma cell
culture
The human myeloma cell lines RPMI8226, MM1S, MM1R,
and human umbilical vein endothelial cells (HUVECs) were purchased
from ATCC. Primary myeloma cells were derived from the peripheral
blood of two patients diagnosed with plasma cell leukemia (PCL).
Mononuclear cells were separated using Lymphosepar
(Immuno-biological Laboratories Co.). All cells were cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum and 1% penicillin/streptomycin in a humidified
incubator containing 5% CO2 at 37°C. The study protocol
was approved by the Institutional Review Board of Tokyo Medical
University (no. SH2408). Written informed consent was obtained from
all patients in accordance with the tenets of the Declaration of
Helsinki.
Cell proliferation assay
Cell proliferation was assessed using
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay
with the Cell Euros Kit-8 (Dojindo Molecular Technologies) in
accordance with the manufacturer's protocol.
Immunoblotting
Immunoblot analysis was performed as previously
described (18). After appropriate
treatment, the cells were washed with ice-cold PBS twice and lysed
with a radioimmunoprecipitation assay lysis buffer. Forty
micrograms of total protein extract was separated on 4–20%
polyacrylamide gels and electro-transferred to a polyvinylidene
difluoride membrane. Thereafter, the membrane was probed using the
primary antibodies of interest at 1:1,000 dilutions for 1 h at 25 ±
1°C. We then used the Amersham ECL chemiluminescence kit (GE
Healthcare) in accordance with the manufacturer's instructions. We
used the following primary antibodies: anti-phospho-S6 ribosomal
protein (Ser235/236), anti-cleaved caspase-3, and
anti-poly-ADP-ribose polymerase (PARP), purchased from Cell
Signaling. Antibodies against MAPK ERK1 and β-actin were purchased
from Santa Cruz Biotechnology. The experiments were carried out in
three independent replicates. Protein band intensity was evaluated
using ImageJ software (National Institutes of Health).
Reverse-transcription PCR
Reverse-transcription PCR (RT-PCR) was performed as
previously described (1). Total
RNA was extracted form MM cells, HUVECs, and primary patient
samples using the RNA queous®−4PCR kit (Life
Technologies Japan, Ltd.). The RNA concentration was determined
spectrophotometrically. Next, 82 ng RNA was used to synthesize cDNA
using a first-strand cDNA synthesis kit (OriGene Technologies)
under the following reaction conditions: 1 cycle at 22°C for 5 min,
1 cycle at 42°C for 30 min, and then1 cycle at 85°C for 5 min,
followed by a hold at 4°C. RT-PCR was performed using a PCR Master
Mix (Promega Corporation) and the Roche Light Cyber 2.0 detection
system (Roche Diagnosis Gmbh). Thermocycling conditions were as
follows: 95°C for 5 min, then 40 cycles at 95°C for 30 sec, 55.5°C
for 30 sec, and 72°C for 1 min. The primer sequences were as
follows: GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and GAPDH
reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The GAPDH primer was purchased
from Life Technology Japan, Ltd. The specific PCR primers of S1PR1,
S1PR2, S1PR3, S1PR4, S1PR5, SK1, and SK2 were purchased from Santa
Cruz Biotechnology. The information of the sequences of these
primers could not be provided from the company then the sequences
are not publicly available.
Lentiviral SK1 and S1PR1 shRNA
We purchased lentiviral vector shRNAs of shS1PR1,
SK1, and scramble shRNA from Vector Builder Japan. The method of
lentiviral vector transduction into RPMI8226 cells followed the
Addgene protocol (http://www.addgene.org/tools/protocols/plko#E). We
used Polybrene for enhancing lentiviral transduction with cells,
the concentration of Polybrene 5 µg/ml and 2 µl shRNA was mixed and
cultured overnight. The effect of shRNA knockdown for SK1 and S1PR1
expression was estimated by real time PCR and immunoblotting. Total
RNA extraction and cDNA synthesis did the same method as
previously. Real time PCR was performed using a Fast Strand
Essential DNA Green Master and Roche Light Cyber 2.0 detection
system (Roche Diagnosis Gmbh). Thermocycling conditions were as
follows: 95°C for 10 min, then 45 cycles at 95°C for 10 sec, 60°C
for 30 sec. The primer sequences were as follows: S1PR1 forward,
5′-GGCTATGTTGAGTACGTAGGCTGTG-3′ and S1PR1 reverse,
5′-TCCCGCTTACATGGAAACTTTG-3′, SphK1 forward,
5′-CTGGCAGCTTCCTTGAACCAT-3′ and SphK1 reverse,
5′-TGTGCAGAGACAGCAGGTTCA-3′ (Takara Bio). The immunoblotting
technique was the same method as previous mentioned. Anti-S1PR1 and
SphK1 rabbit polyclonal antibodies were purchased from
Proteintech®. The membrane was probed using the primary
antibodies of interest at 1:300 dilutions. The shRNA knockdown
cells were cultured with carfilzomib and the suppressive ability
for cell proliferation was assessed by caspase-3/7 activity. We
used the kit, Caspase-Glo® 3/7 Assay (Promega
Corporation).
Chemotaxis assay
The chemotaxis assay for HUVECs was performed using
a Boyden chamber with an 8-µm pore size (Corning, Inc.) (19). In the upper chamber, we seeded
HUVECs. In the lower chamber, indicated materials (supernatant of
cell line, S1P and anti-S1P agents) were added with medium (0.2%
FBS DMEM). The cells were incubated at 37°C for 4 h in humidified
air with 5% CO2. Non-migrated cells were removed by a
cotton swab, and migrated cells were stained by May-Giemsa method.
The stained migrated cells were counted by microscopic ×100 field
of vision in three random fields. The cell number was average of
three random fields.
Assessment of serum S1P concentration
among MM patients, MGUS patients, and healthy adults via ELISA
We determined serum S1P levels in 13 patients who
were newly diagnosed with MM, in five patients with MGUS, and
age-matched 16 healthy volunteers. The specimens were harvested in
2013, and patients and healthy volunteers provided informed consent
to participate in the study (approved no. ‘SH2408’). However,
sample size determination, randomization, and blinding were not
performed as some people could not consent when we planned to
design another clinical study for S1P measurement. Furthermore, the
serum S1P levels were assessed using an ELISA Sphingosine
1-phosphate Assay Kit (Echlon, Inc.).
Statistical analysis
The Mann-Whitney U-test was used to estimate
serum S1P levels among MM patients, MGUS patients, and healthy
volunteers, and between MM patients with or without symptoms. The
Student's t-test was used to assess the effects of drug
treatment in comparison with the control group. P<0.05 was
considered significant. All data were analyzed using IBM SPSS
statistic ver.28 and Prism8.
Results
mRNA expression levels of SK1, SK2 and
S1PR1-S1PR5 in HUVECs and MM cells
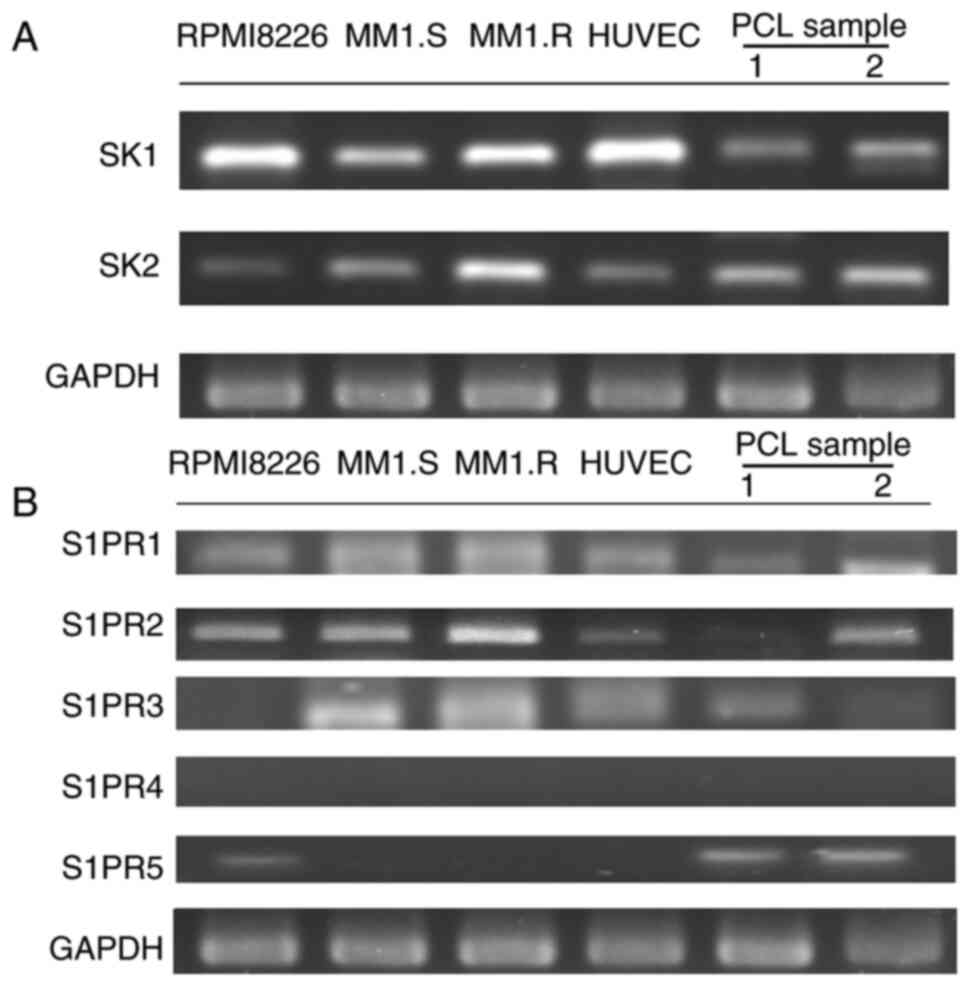
We performed RT-PCR to confirm the mRNA expression
of both SK1 and SK2 in the following samples: HUVECs, primary
myeloma cells from two patients with PCL, and three MM-derived cell
lines, RPMI8226, MM1.S and MM1.R (Fig.
1A). As S1P exerts its effects by binding to S1PRs, we examined
the mRNA expression of the five S1PR isotypes (S1PR1-S1PR5) to
determine their expression patterns in our cell lines. All tested
cell lines expressed S1PR1 mRNA, the target of fingolimod, but not
S1PR4 mRNA. S1PR2 mRNA was expressed in all cells, except for one
of the PCL samples (sample A). Finally, we observed S1PR3 mRNA
expression in MM1.S, MM1.R, PCL sample A, and HUVECs (Fig. 1B). It has been reported that S1PR1,
S1PR2 and S1PR3 are expressed in almost all tissues and organs;
conversely, S1PR4 and S1PR5 are expressed mainly in lymphoid tissue
(20). Our results are consistent
with these previous observations.
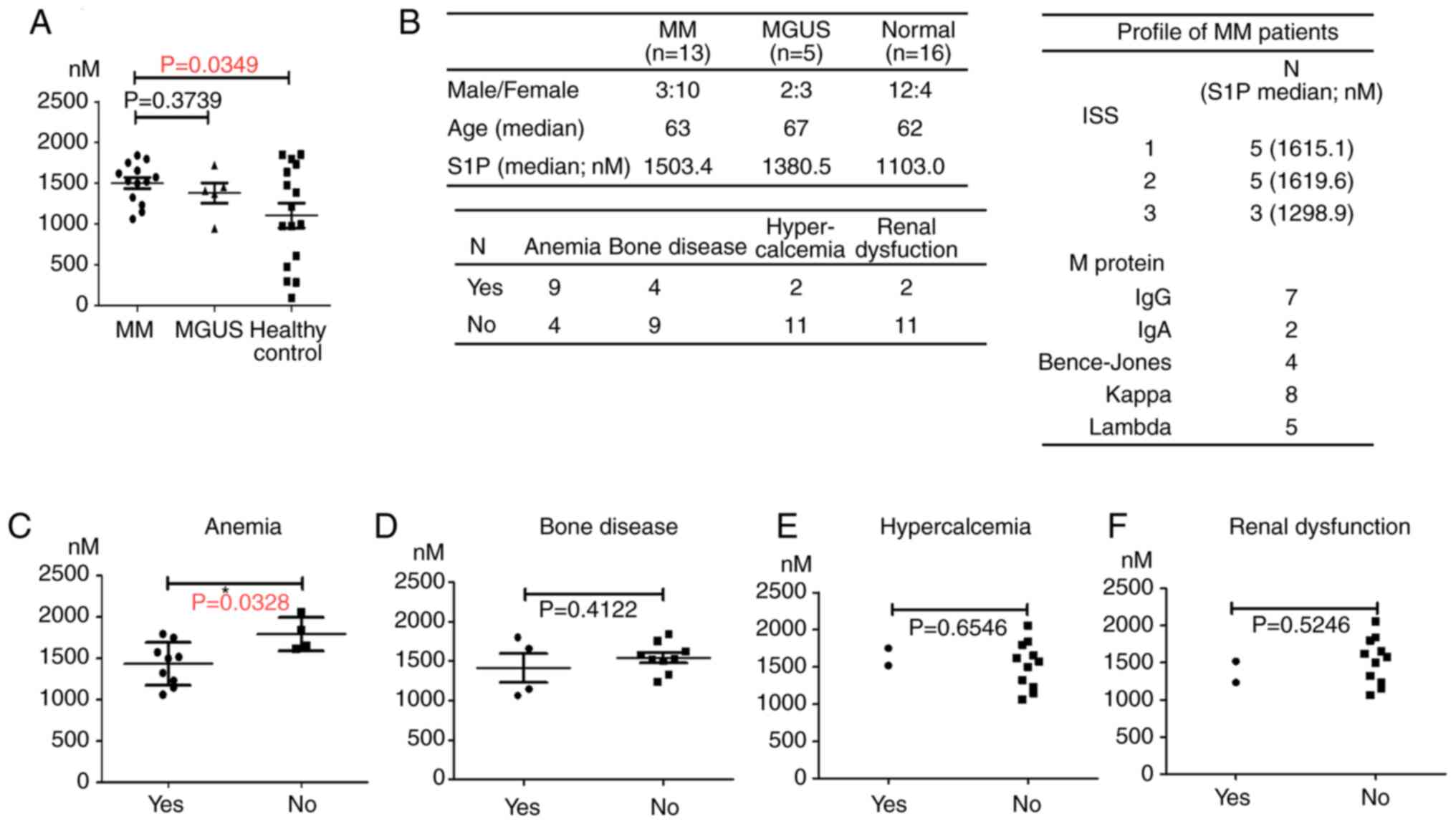
Serum S1P levels increased in patients
with MM
We next determined the serum levels of S1P in MM
patients using ELISA. The results showed that the serum
concentration of S1P was higher in MM patients and MGUS patients
than in healthy age-matched controls. The difference between MM and
healthy age-matched controls was significant (P<0.05; Fig. 2A). Moreover, among MM patients with
anemia as a complication, the median serum S1P level was
significantly lower than that in the group without anemia
(P<0.05; Fig. 2B). Regarding
other complications, including the co-occurrence of bone disease,
hypercalcemia, and renal dysfunction, we did not observe a
significant change in serum S1P levels (P<0.05; Fig. 2C-F). The group profile (MM, MGUS,
and healthy control) and the number of patients with MM symptoms
are shown in Fig. 2F.
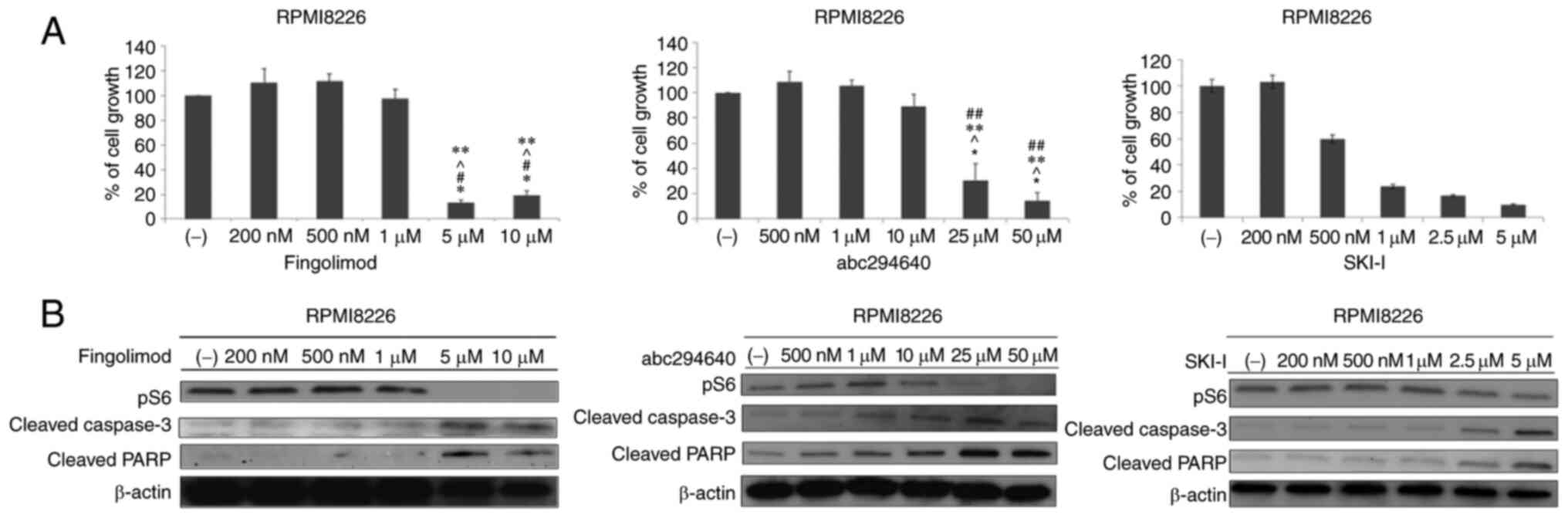
Fingolimod, SKI–I, and ABC294640
inhibit the proliferation of MM cell lines
We investigated the effect of fingolimod, SKI–I, and
ABC294640 on the proliferation of MM cells. All agents inhibited
the growth of RPMI8226, MM1.S, and MM1.R cell lines at
concentrations of 5 and 10 µM (P<0.05; Figs. 3A and S1A and B). Thereafter, we investigated
the effects of the three anti-S1P agents on intracellular signaling
in RPMI8226 cells. RPMI8226 cells were treated with the indicated
anti-S1P agents for 24 h. At high concentrations, the three
molecules inhibited the phosphorylation of the S6 ribosomal
protein. At the same concentration, we observed induction of PARP
and cleaved caspase-3 (Fig. 3B).
To confirm the relation between inhibition of SK or S1PR function
and myeloma cell proliferation, we assessed the effect of SK1 and
S1PR1 mRNA knockdown. Western blotting revealed that caspases were
related to cell apoptosis induced by SKs and S1PRs, and therefore,
we estimated and confirmed caspase activity. We concluded that
suppression of the S1P pathway induced caspase-mediated myeloma
cell death. We next evaluated whether caspase-3/7 activity was
affected by shRNA and culturing with carfilzomib. shRNA knockdown
was performed by lentiviral infection to RPMI8226, and we confirmed
the transfection efficiency by RT-qPCR and immunoblotting. The
result of immunoblotting and RT-qPCR showed the knock down
efficiency for both protein and DNA of S1PR1 and SK1 by shRNA
(Fig. S1C and D). RPMI8226 that
was performed bySK1 or S1PR1 knockdown using shRNA showed a
significantly higher caspase activity than did non-knockdown cells.
When carfilzomib was added to the culture, the caspase-3/7 activity
was higher than that in knockdown cells without carfilzomib. These
results demonstrated that S1PR1 and SK1 were concerned with myeloma
cell survival and S1P interfered the cytotoxic effect of proteasome
inhibitor for myeloma cell. (Fig. S1E
and F).
Use of fingolimod, SKI–I, and
ABC294640 in combination with carfilzomib protease inhibitor
enhanced anti-tumor activity in MM cell lines and primary
cells
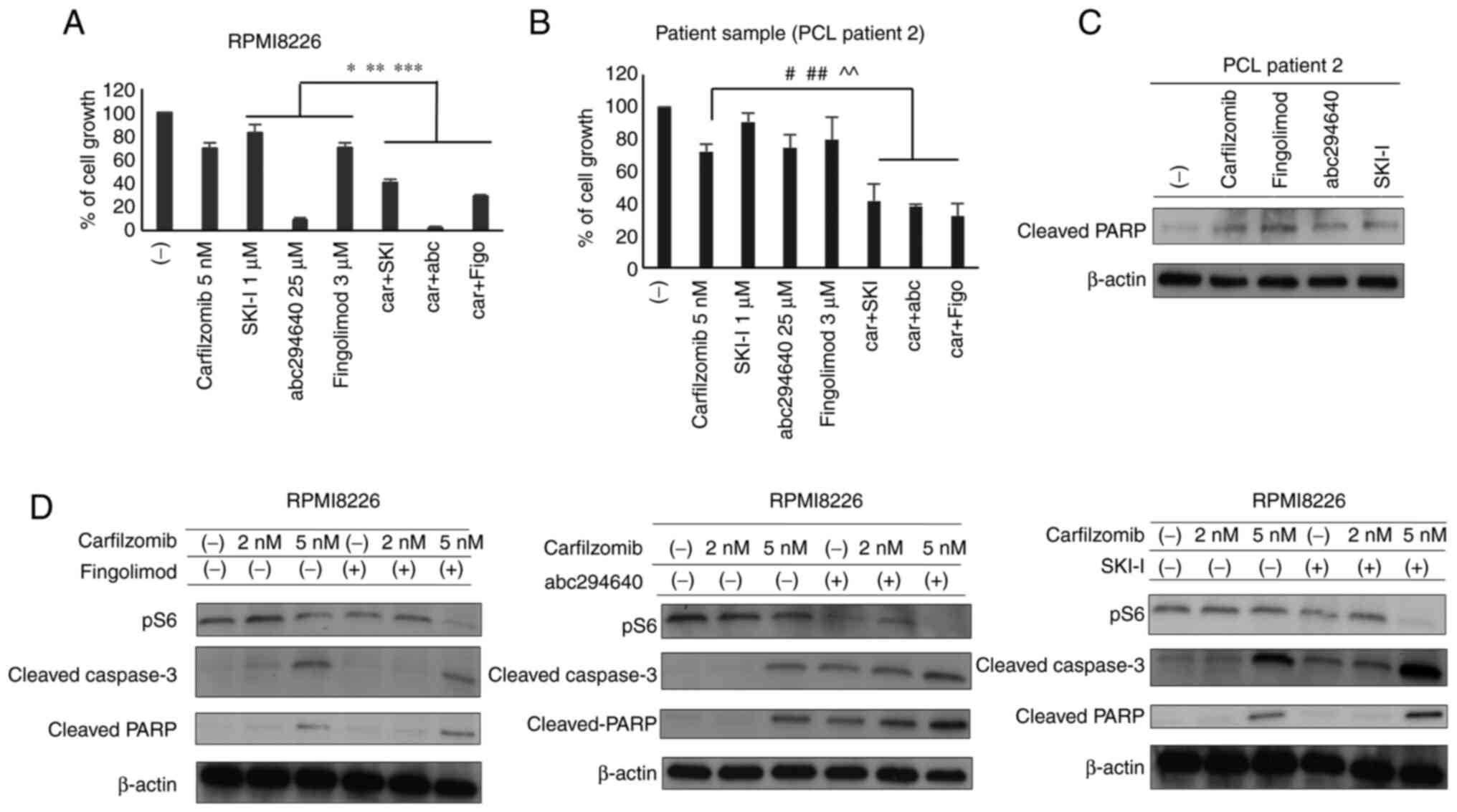
Three agents were further assessed in combination
with the PI carfilzomib in RPMI8226 MM cells. Because we observed
more effective for cell reduction at 5 nM carfilzomib than at 2 nM
carfilzomib previously (Fig.
S1F), we evaluated the impact of the combination of 5 nM
carfilzomib with fingolimod, SKI–I, or ABC294640 on cell growth at
the concentration that suppressed MM cell growth (Figs. 3A and S1A and B). The results showed that
fingolimod (5 µM), SKI–I (2.5 µM), and ABC294640 (25 µM) in
combination with carfilzomib significantly increased cell growth
compared to 5 nM carfilzomib treatment alone (Fig. 4A). We then assessed the effect of
these combinations on the proliferation of PCL sample A.
Consequently, the combination of fingolimod (3 µM), SKI–I (2.5 µM),
or ABC294640 (25 µM) with 5 nM carfilzomib synergistically
inhibited the growth of primary MM cells (Fig. 4B). In each bar plot of Fig. 4A and 4B, the mean and SD of three independent
replicates are shown.
We next investigated the effects of the three
anti-S1P agents in combination with carfilzomib on intracellular
signaling in RPMI8226 cells. RPMI8226 cells were treated with
fingolimod, SKI–I, or ABC294640, with or without carfilzomib, for
24 h. We observed that combinatorial treatment exerted the same
effect as that observed with the three agents alone, resulting in
similar protein expression patterns (Figs. 3B and 4D). Notably, the combinatorial treatment
inhibited the S6 ribosomal protein more strongly than carfilzomib
monotherapy, which was consistent with the observed synergistic
cell growth inhibition. Moreover, we observed increased cleaved
PARP activation upon treating cells with the same amount of
inhibitor used for cells harvested from patients with PCL (Fig. 4C).
S1P attenuates the PI-mediated
anti-tumor effect in MM cells, which is recovered by co-treatment
with anti-S1P agents
After confirming that the serum S1P level was higher
in patients with MM than in healthy controls, we examined the
effect of the addition of exogenous S1P to RPMI8226 cells
co-cultured with carfilzomib alone or through sequential addition
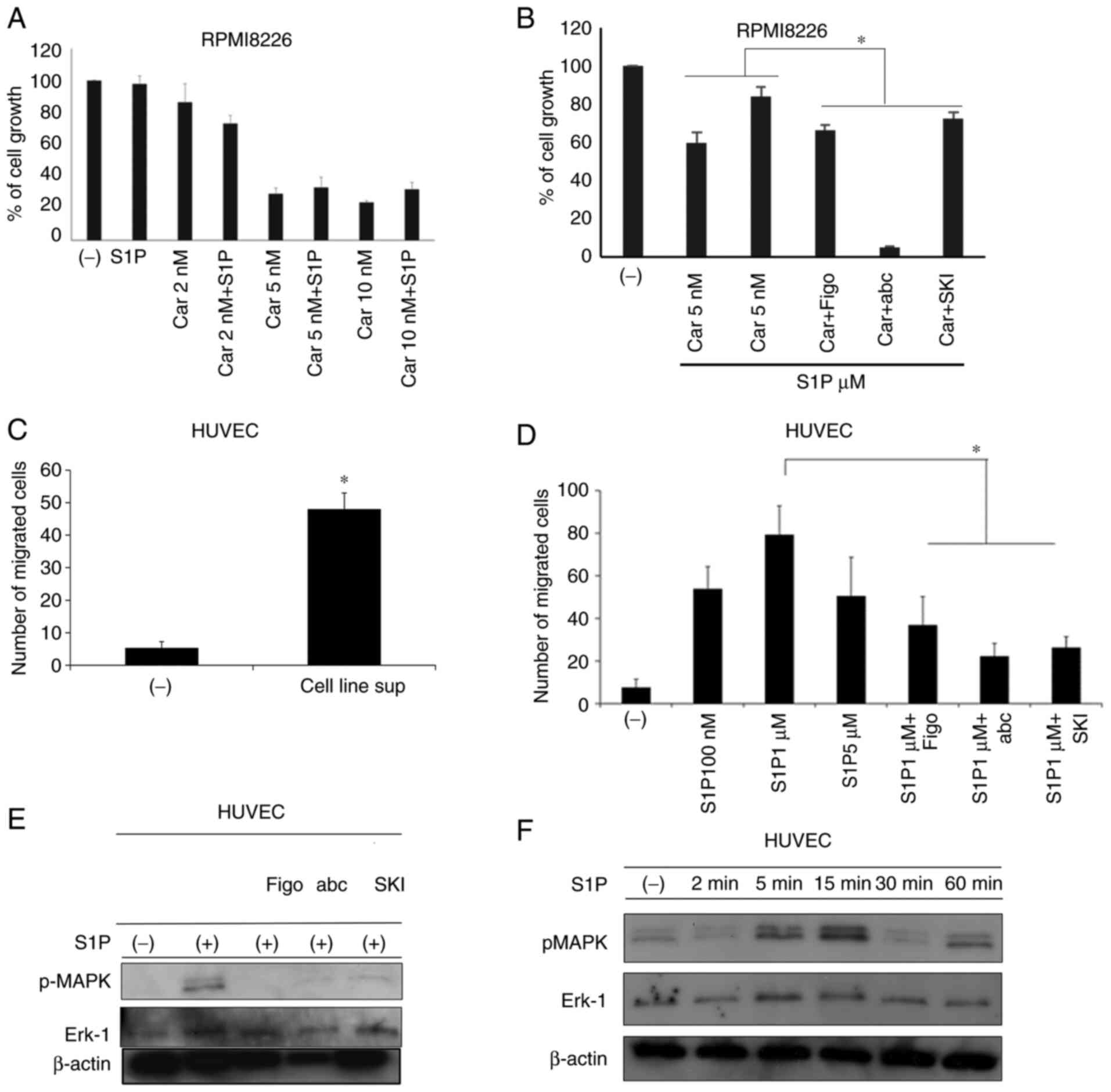
of fingolimod, SKI–I, or ABC294640. As shown in Fig. 5A, the addition of exogenous S1P
attenuated the anti-tumor activity of carfilzomib at 5 and 10 nM.
In particular, 10 nM carfilzomib reduced cell growth by 72%, while
S1P addition resulted in only a 45% reduction. However, the
anti-tumor effect of carfilzomib in the presence of S1P was
restored when used in combination with fingolimod, SKI–I, or
ABC294640 (Fig. 5B). In each bar
plot of Fig. 5A-B, the mean and SD
of three independent replicates are shown (P<0.05).
Anti-S1P agents inhibit S1P-promoted
migration of HUVECs
We next evaluated the chemotactic response of HUVECs
to S1P or its inhibitors. We observed that the addition of the
supernatant of MM cells significantly induced HUVEC migration
(Fig. 5C). This was probably S1P
dependent, as incubation with S1P for 4 h significantly induced the
migration of HUVECs compared with the control medium (with a peak
effect detected at 100 nM S1P). However, simultaneous treatment
with fingolimod, SKI–I, or ABC294640 inhibited S1P-induced cell
migration (Fig. 5D; P<0.05).
Moreover, we observed increases in pMAPK and ERK1 within 5–15 min
after adding S1P, followed by decreased pMAPK thereafter (until 60
min) (Fig. 5E). The addition of
the three anti-S1P agents repressed S1P-induced MAPK
phosphorylation (Fig. 5F).
Discussion
In this study, we assessed the serum S1P levels
among patients with MM or MGUS compared with those in healthy
individuals. Notably, our results show that serum S1P levels were
significantly higher among patients with MM than in healthy
individuals.
S1P and SKs are involved in numerous cancer types,
influencing cell growth, cell survival, mortality, transformation,
and chemotherapy resistance (7,10,16,17).
However, although some recent studies suggested that S1P and SKs
are involved in MM, their association with MM is unclear. For
example, Yasui et al (21)
reported that fingolimod has anti-cancer effects in MM cell lines.
In addition, S1P may play important roles in the adhesion of MM
cells, which is dependent on the α4β1 integrin (22). Venkata et al (23) reported that SK2 is overexpressed in
both MM cell lines and primary cells and demonstrated the efficacy
of SK2 inhibitors in inhibiting cell growth. Thus, further
assessment of the roles of S1P and SKs in MM cell proliferation may
facilitate the development of new treatment strategies for MM.
Xia et al (16) reported that SK1 activation, which
depends on V12 RAS, promotes NIH/3T3 fibroblast transformation to
fibrosarcoma, suggesting that SK1 has oncogenic activity. Thus, S1P
activation via SKs is potentially associated with MM progression.
The median serum S1P level among MM patients was higher than that
of healthy volunteers. Our study is the first to report serum S1P
elevation in MM patients. The proliferation of MM cells might be
associated with S1P-related signaling. Anemia in MM is typically
observed when the tumor is abundant, or the disease is advanced
(24). The S1P level of MM
patients with anemia were lower than that of those without anemia.
The main supplier of serum S1P is red blood cells (RBC), and thus
the low level of S1P in MM patients with anemia might be related to
a reduction in RBC. However, the median S1P level of MM patients
with anemia were higher than that of healthy volunteers, indicating
that S1P level might be constantly high among MM patients.
This study showed that the addition of exogenous S1P
reduced the efficacy of PI in the RPMI8226 myeloma cell line,
suggesting that S1P is involved in increased PI resistance in MM
cells. These results show that the tested anti-S1P agents enhanced
the PI-dependent cytotoxic effect in MM cell lines and primary
myeloma cells, even in the presence of S1P, which can reduce
PI-dependent antimyeloma effects. Taken together, these findings
suggest the involvement of S1P in regulating proteasome activity.
Moreover, some studies have reported associations among SK1, S1P,
and the ubiquitin-proteasome system (UPS). For instance, S1P
promotes NF-κB activation by interacting with the E3 ubiquitin
ligase TNF receptor-associated factor 2 as a cofactor (25). This interaction activates
proteasome and induces inflammation. Other studies have reported
that S1P accumulation leads to UPS activation owing to the
concomitant downregulation of the deubiquitinating enzyme (26,27).
Accordingly, myeloma cell proliferation may be promoted by the
upregulation of S1P and SKs through increased UPS activation.
Therefore, inhibition of S1P signaling may suppress UPS and
increase the efficacy of PI. S1P signaling is also involved in
other signaling pathways. S1P potentially activates the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(AKT)/mammalian target of rapamycin (mTOR) pathway through S1PR1
(28). When MM cells were cultured
with an anti-S1P agent, alone or in combination with carfilzomib,
we observed a stronger reduction in pS6 than with carfilzomib
monotherapy. Furthermore, PI3K signaling is very active in myeloma
cells (29). Therefore, inhibition
of S1P-mediated MM cell proliferation may be PI3K
signaling-dependent. Moreover, all the tested anti-S1P agents
promoted apoptosis in MM cells by increasing the levels of cleaved
caspase-3 and PARP. Thus, our results suggest that the tested
inhibitors promote caspase-induced apoptosis and suppress UPS and
PI3K signaling by inactivating the S6 ribosomal protein, thereby
repressing translation.
The bone marrow microenvironment plays an important
role in the pathophysiology and progression of MM. In particular,
angiogenesis is strongly associated with myeloma cell proliferation
(30). In vascular endothelial
cells, S1P regulates proliferation, migration, and angiogenesis.
This study shows that the inhibition of S1P activity by fingolimod
or SK inhibitors suppressed the migration of HUVECs. Therefore, the
use of these inhibitors potentially reduces S1P-mediated
angiogenesis in the bone marrow microenvironment. Similarly,
LaMontagne et al (11)
reported that fingolimod suppressed tumor angiogenesis and
proliferation in a mouse model. We observed that upon culturing
HUVECs with S1P, the levels of pMAPK and ERK-1 increased. However,
the expression levels reverted to baseline upon treatment with S1P
antagonists. These results suggest that S1P signaling affects
angiogenesis by modulating endothelial cell migration and
proliferation through the MAPK signaling pathway.
Overall, our results indicate that inactivation of
S1P by an S1PR1 antagonist and two SK inhibitors affected MM cell
growth and apoptosis. In addition, these inhibitors displayed
synergistic effects with PI carfilzomib treatment, even in the
presence of S1P-mediated resistance. Moreover, inhibition of S1PR1
and SKs impaired the migration of endothelial cells, which is a
critical mechanism involved in angiogenesis in the bone marrow
microenvironment.
In conclusion, the results of the present study
suggest that combinatorial treatment of PI with fingolimod or SK
inhibitors constitutes a novel approach to treat MM and to overcome
chemotherapeutic resistance. However, it is uncertain whether these
agents can be developed as new therapeutic drugs for MM. Even
though this study is limited by its small sample size and use of
only in vitro data, the identified association between
myeloma cell growth and S1P signaling is a new finding and might be
helpful in decision making when choosing existing myeloma drugs and
biomarkers for disease prognosis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Miss Sayaka Ohmura
and Dr Tomohiro Umezu (Tokyo Medical University, Division of
Hematology) for providing technical support.
Funding
This work was supported by grants-in-aid for Scientific Research
from the Ministry of Education, Culture, Sports Science and
Technology (MEXT; grant no. 24701036) and the Support Centre of
Doctor, Student and Researcher, Tokyo Medical University.
Availability of data and materials
The datasets used and/or analyzed are available from
the corresponding author on reasonable request.
Authors' contributions
YT and SO designed the research, performed the
experiments, confirmed the authenticity of all the raw data and
wrote the manuscript. SO, YT, KO and AG developed the methodology.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
of Tokyo Medical University (approval ID, SH2408l; March 3,
2012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuehl WM and Bergsagel PL: Molecular
pathogenesis of multiple myeloma and its premalignant precursor. J
Clin Invest. 122:3456–3463. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kyle RA and Rajkumar SV: An overview of
the progress in the treatment of multiple myeloma. Expert Rev
Hematol. 7:5–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nooka AK, Kastritis E, Dimopoulos MA and
Lonial S: Treatment options for relapsed and refractory multiple
myeloma. Blood. 125:3085–3099. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pyne S and Pyne NJ: Sphingosine
1-phosphate signaling in mammalian cells. Biochem J. 349:385–402.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiegel S and Milstein S: Sphingosine
1-phosphate: An enigmatic signaling lipid. Nat Rev Mol Cell Biol.
4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannun YA and Obeid LM: Principles of
bioactive lipids signaling: Lessons from sphingolipids. Nat Rev Mol
Biol. 9:139–150. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pyne NJ, El Buri A, Adams DR and Pyne S:
Sphingosine 1-phosphate and cancer. Adv Biol Regul. 68:97–106.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pyne S, Lee SC, Long J and Pyne NJ: Role
of sphingosine kinases and lipid phosphate phosphatases in
regulating spatial sphingosine 1-phosphate signalling in health and
disease. Cell Signal. 21:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pyne NJ and Pyne S: Sphingosine
1-phosphate and cancer. Nat Rev Cancer. 10:489–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
LaMontagne K, Littlewood-Evans A, Schnell
C, O'Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau
G, et al: Antagonist of sphingosine 1-phosphate receptors by FTY720
inhibits anginogenesis and tumor vascularization. Cancer Res.
66:221–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paugh SW, Paugh BS, Rahmani M, Kapitonov
D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S
and Spiegel S: A selective sphingosine kinase1 inhibitor integrates
multiple molecular therapeutic targets in human leukemia. Blood.
112:1382–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
French KJ, Zhuang Y, Maines LW, Gao P,
Wang W, Beljanski V, Upson JJ, Green CL, Keller SN and Smith CD:
Pharmacology and antitumor activity of ABC294640, a selective
inhibitor of sphingosine kinase-2. J Pharm Exp Ther. 333:129–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beljanski V, Knaak C and Smith CD: A novel
sphingosine kinase inhibitor induced autophagy in tumor cells. J
Pharm Exp Ther. 333:454–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neubauer HA and Pitson SM: Roles,
regulation and inhibitors of sphingosine kinase 2. FEBS J.
280:5317–5336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia P, Gamble JR, Wang L, Pitson SM,
Moretti PA, Wattenberg BW, D'Andrea RJ and Vadas MA: An oncogenic
role of sphingosine kinase. Curr Biol. 10:1527–1530. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akao Y, Banno Y, Nakagawa Y, Hasegawa N,
Kim TJ, Murate T, Igarashi Y and Nozawa Y: High expression of
sphingosine kinase 1 and S1P receptors in chemotherapy-resistant
prostate cancer PC-3 cells and their camptothecin-induced
up-regulation. Biochem Biophys Res Commun. 342:1284–1290. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okabe S, Tanaka Y, Tauchi T and Ohyashiki
K: Copanlisib, a novel phosphoinositide 3-kinase inhibitor,
combined with carfilzomib inhibits multiple myeloma cell
proliferation. Ann Hematol. 98:723–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HC: Boyden chamber assay. Methods Mol
Biol. 294:15–22. 2005.PubMed/NCBI
|
|
20
|
Ishii I, Fukushima N, Ye X and Chun J:
Lysophospholipid receptors: Signaling and biology. Annu Rev
Biochem. 73:321–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yasui H, Hideshima T, Raje N, Roccaro AM,
Shiraishi N, Kumar S, Hamasaki M, Ishitsuka K, Tai YT, Podar K, et
al: FTY720 induces apoptosis in multiple myeloma cells and
overcomes drug resistance. Cancer Res. 65:7478–7484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanz-Rodríguez F, Hidalgo A and Teixidó J:
Chemokine stromal cell-derived factor-1α modulates VLA-4
integrin-mediated multiple myeloma cell adhesion to
CS-1/fibronectin and VCAM-1. Blood. 97:346–351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venkata JK, An N, Stuart R, Costa LJ, Cai
H, Coker W, Song JH, Gibbs K, Matson T, Garrett-Mayer E, et al:
Inhibition of sphingosine kinase 2 downregulates the expression of
c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood.
124:1915–1925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alvarez SE, Harikumar KB, Hait NC,
Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T,
et al: Sphingosine-1-phosphate is a missing cofactor for the E3
ubiquitin ligase TRAF2. Nature. 465:1084–1088. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mitroi DN, Deutschmann AU, Raucamp M,
Karunakaran I, Glebov K, Hans M, Walter J, Saba J, Gräler M,
Ehninger D, et al: Sphingosine 1-phosphate lyase ablation disrupts
presynaptic architecture and function via ubiquitin-proteasome
mediated mechanism. Sci Rep. 6:1–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wallington-Beddoe CT, Bennett MK, Vandyke
K, Davies L, Zebol JR, Moretti PA, Pitman MR, Hewett DR, Zannettino
AC and Pitson SM: Sphingosine kinase 2 inhibition synergises with
bortezomib to target myeloma by enhancing endoplasmic reticulum
stress. Oncotarget. 8:43602–42616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banno Y, Takuwa Y, Akao Y, Okamoto H,
Osawa Y, Naganawa T, Nakashima S, Suh PG and Nozawa Y: Involvement
of phospholipase D in sphingosine 1-phosphate-induced activation of
phosphatidylinositol 3-kinase and Akt in Chinese hamster ovary
cells overexpressing EDG3. J Biol Chem. 276:35622–35628. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikeda H, Hideshima T, Fulciniti M, Perrone
G, Miura N, Yasui H, Okawa Y, Kiziltepe T, Santo L, Vallet S, et
al: PI3K/p110Δ is a novel therapeutic target in multiple myeloma.
Blood. 116:1460–1468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar S, Fonseca R, Dispenzieri A, Lacy
MQ, Lust JA, Wellik L, Witzig TE, Gertz MA, Kyle RA, Greipp PR and
Rajkumar SV: Prognostic value of angiogenesis in solitary bone
plasmacytoma. Blood. 101:1715–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|