Introduction
Glioma is the most common and most malignant brain
tumor in adults, composing most of all brain malignancy diagnoses
in this population (1,2). Typically, the clinical outcomes of
this condition are devastating, although aggressive multimodal
treatments, including surgery, radiotherapy, and chemotherapy-which
mainly is composed of drugs in the temozolomide (TMZ) and
nitrosourea classes-can have some effect (3,4). For
cases of recurrent glioblastoma, which is the most common and most
malignant type of glioma, almost all patients eventually experience
a recurrence and die within six months after diagnosis of
recurrence (5,6).
There is no consensus regarding salvageable options
for recurrent and TMZ-resistant adult glioma (7–10).
Among the current treatments, procarbazine, lomustine, and
vincristine (PCV) chemotherapy is one of the representative
salvageable options for recurrent adult glioma (11–14).
However, the various and severe toxicities of this treatment,
including hematologic toxicity from lomustine and peripheral
neurotoxicity from vincristine, often result in its reduction or
discontinuation in glioma patients (15–17).
In addition to its toxicity, vincristine is composed of relatively
heavy molecules (825 Daltons), and concerns exist about its
successful crossing of the blood-brain barrier (18).
In this context, a few studies have suggested
procarbazine and lomustine (PC) chemotherapy without vincristine as
an alternative to PCV chemotherapy with lesser toxicity and no loss
of efficacy (19–22). Vesper et al first suggested
that PC chemotherapy protocols might be as effective as PCV
chemotherapy while avoiding the toxicity of vincristine (20). In addition, Webre et al
suggested that PC chemotherapy can achieve comparable clinical
outcomes to PCV chemotherapy with lesser neurotoxicity in
anaplastic oligodendroglioma (21). We have also tested the modified PC
chemotherapy protocol of reduced dose of lomustine (75
mg/m2, day 1) and procarbazine (60 mg/m2,
days 11–24) every four weeks in recurrent glioblastoma patients
with expectation of lesser toxicity and non-inferior efficacy
(19).
In this study, we retrospectively analyzed the
efficacy and safety of modified PC chemotherapy in recurrent glioma
patients compared with those of conventional PCV chemotherapy. This
study tried to validate that the modified PC chemotherapy is less
toxic than PCV chemotherapy, and that the survival outcomes of PC
chemotherapy are noninferior to those of PCV chemotherapy in
patients with recurrent adult glioma.
Materials and methods
Study population
This retrospective study was approved by the
institutional review board of our institution. The electronic
medical records of adult glioma patients treated at our institution
between 2010 and 2020 were examined. The study inclusion criteria
were i) glioma pathologically confirmed by craniotomy or biopsy,
ii) glioma recurrence confirmed radiologically and/or
pathologically, iii) receipt of PC or PCV chemotherapy following
recurrence diagnosis, and iv) accessible baseline clinical
variables and survival data. The study exclusion criteria were i)
received PC or PCV chemotherapy as adjuvant therapy after initial
diagnosis, ii) a medical history of hematologic or rheumatologic
disease, iii) failure to complete the first cycle of PC or PCV
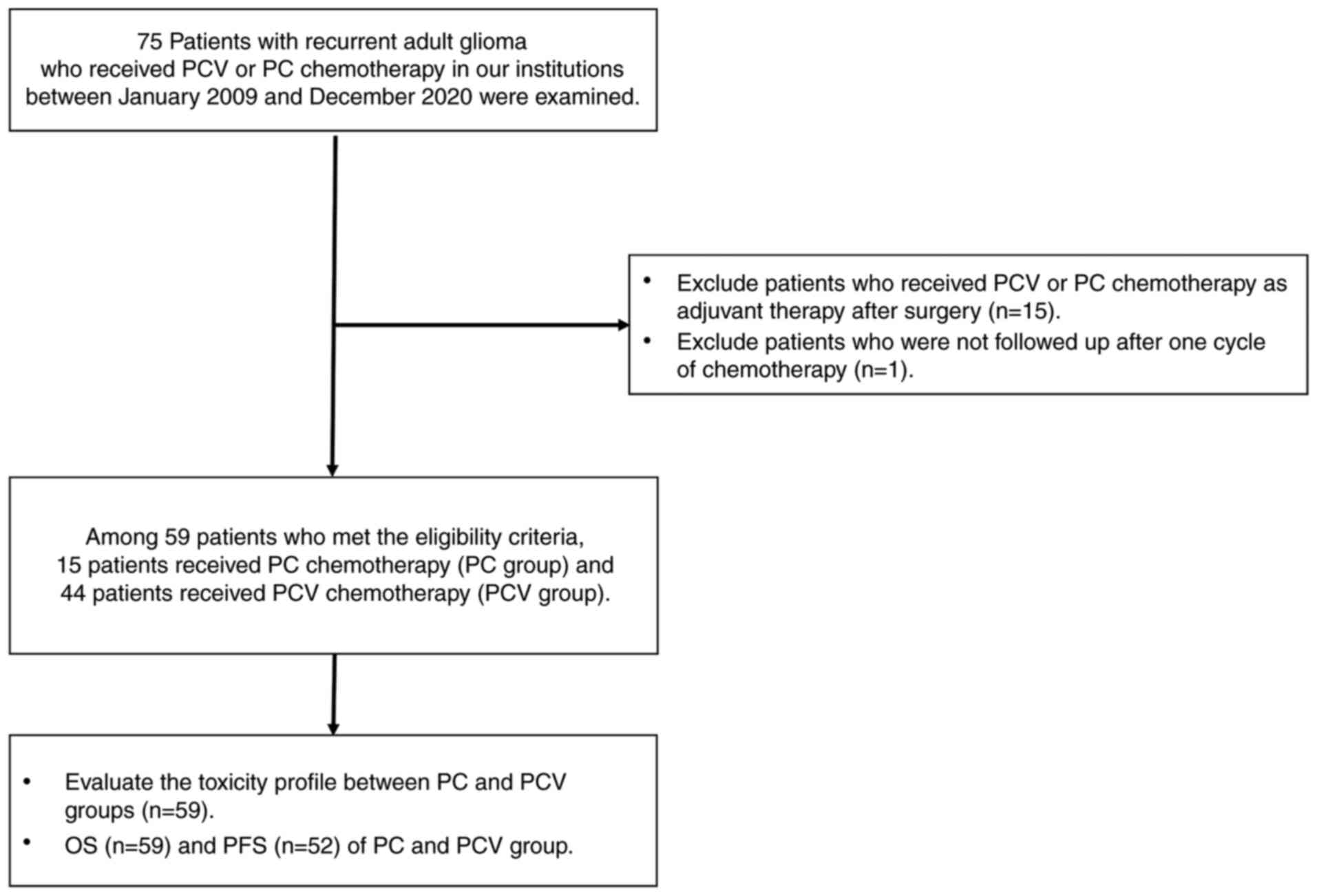
chemotherapy. A flow of the study design is presented in Fig. 1.
Treatment protocols
After maximal safe resection at initial surgery, we
performed adjuvant therapy following the best treatment protocol(s)
by glioma subtype. If the diagnosis was glioblastoma, we performed
concomitant chemoradiation (TMZ dose: 75 mg/m2) and six
cycles of adjuvant TMZ chemotherapy (TMZ dose: 150–200
mg/m2). If the diagnosis was grade II or III glioma, we
conducted adjuvant radiotherapy, in which the dosage was either
5,940 cGy for 33 fractions or 6,000 cGy for 30 fractions. In the
case of grade III glioma, we added adjuvant chemotherapy of PCV or
PC chemotherapy. When recurrence occurred, the first chemotherapy
considered was TMZ; when the use of TMZ chemotherapy was not
possible due to various reasons, such as prior history of TMZ
administration within 6 months, and swallowing difficulty, then
bevacizumab or nitrosourea-based chemotherapy including the
modified PC chemotherapy or the conventional PC chemotherapy, was
considered according to clinician preference.
PC chemotherapy was composed of lomustine (75
mg/m2, day 1) and procarbazine (60 mg/m2,
days 11–24) administered orally every four weeks. This modified
protocol was discussed in a previous study of one of our authors
(19). PCV chemotherapy was
administered to recurrent glioma patients, with lomustine (110
mg/m2, day 1) and procarbazine (60 mg/m2,
days 8–21) administered orally but vincristine administered
intravenously [1.4 mg/m2 (maximum of 2 mg), days 8 and
29] every six weeks.
Clinical variables
The clinical variables of sex; age; pathological
diagnosis, including molecular features; prior history of surgery,
radiation, or chemotherapy; radiological findings; performance
status; and survival status and/or death date were collected.
Diagnosis of recurrent glioma was performed by two
neuropathologists according to the 2016 World Health Organization
classification of the central nervous system. IDH mutation was
evaluated by immunohistochemistry or directing sequencing. If
necessary, IDH 2 mutation was evaluated by directing sequencing.
The presence of a 1p19q co-deletion was examined using fluorescence
in situ hybridization. The
O6-methylguanine-DNA-methyltransferase (MGMT) gene
methylation status was evaluated by polymerase chain reaction.
Performance status was estimated according to the scale of the
Eastern Cooperative Oncology Group (ECOG). All kinds of toxicities
were evaluated according to the Common Terminology Criteria for
Adverse Events (CTCAE) version 5.0. Also, the related impact of the
toxicity of PC or PCV chemotherapy on the course of the treatment
schedule was classified into four categories: delay of a cycle,
dose reduction, discontinuation of drug(s), or total cessation of
chemotherapy. Radiographic responses on magnetic resonance imaging
(MRI) were determined by specialized neuroradiologists according to
the response assessment in neuro-oncology (RANO) criteria. The date
of recurrence was defined as the date of MRI showing recurrence.
Survival status and/or death date were collected from the Korea
Central Cancer Registry database.
Statistical analysis
The overall survival (OS) after recurrence was
defined as days from the starting date of PC or PCV chemotherapy to
death, while progression-free survival (PFS) was defined as days
from the starting date of PC or PCV chemotherapy to disease
progression was confirmed by MRI. Patients who were confirmed to be
alive on March 31, 2021, were censored. The mean duration of
follow-up was 424.6 days (range: 55–2,491 days). All clinical
variables were considered with descriptive statistics. The
differences of clinical variables between the two treatment groups
were compared using Fisher's exact test or the Chi-square test. The
normality test was performed for continuous variables. Kaplan-Meier
survival analysis and the log-rank test were used to calculate the
median OS and PFS values of the groups. Univariate and multivariate
analyses were conducted using a Cox proportional regression model.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were
calculated. Multivariate analysis was performed on the variables
with P-values <0.2, and P-values <0.05 were considered to
indicate statistical significance. All statistical analyses were
conducted using the R version 4.0.5 software program (R Foundation
for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Among a total of 59 patients enrolled in this study,
15 received PC chemotherapy (PC group) and 44 patients received PCV
chemotherapy (PCV group) as salvage treatment for recurrent
gliomas. Clinical characteristics, including sex, age, initial
diagnosis, IDH mutation, 1p19q co-deletion, MGMT promoter
methylation, and prior history of radiotherapy were not
statistically different between the two groups. However, the median
interval from radiation to chemotherapy in the PC group was shorter
than that of the PCV group [4.0 (range: 0–42) months vs. 22.0
(range: 0–167) months, P=0.004]. Also, fewer patients in the PC
group had prior history of any chemotherapy (33.3 vs. 84.7%;
P=0.003), while there was no difference in the median interval from
the last chemotherapy session to initiation of PC or PCV
chemotherapy between the two groups. The summarized baseline
characteristics of these patients are described in Table I.
 | Table I.Baseline characteristics of patients
with recurrent adult glioma who received PC or PCV
chemotherapy. |
Table I.
Baseline characteristics of patients
with recurrent adult glioma who received PC or PCV
chemotherapy.
| Characteristic | PC group (n=15) | PCV group (n=44) | P-value |
|---|
| Male sex, n (%) | 11 (73.3) | 27 (61.4) | 0.600 |
| Age at chemotherapy
(years), n (range) | 52.2 (20–79) | 49.6 (21–73) | 0.528 |
| Initial diagnosis, n
(%) |
|
| >0.999 |
| GBM | 5 (33.3) | 14 (31.8) |
|
|
Non-GBM | 10 (66.7) | 30 (68.2) |
|
| IDH mutation, n
(%) |
|
| 0.103 |
| Yes | 3 (20.0) | 13 (29.5) |
|
| No | 10 (66.7) | 31 (70.5) |
|
|
Unknown | 2 (13.3) | 0 (0.0) |
|
| 1p19q co-deletion, n
(%) |
|
| 0.392 |
| Yes | 2 (13.3) | 7 (15.9) |
|
| No | 11 (73.3) | 24 (54.5) |
|
|
Unknown | 2 (13.3) | 13 (29.5) |
|
| MGMT methylation, n
(%) |
|
| 0.311 |
| Yes | 8 (53.4) | 16 (36.4) |
|
| No | 4 (26.7) | 16 (36.4) |
|
|
Unknown | 3 (20.0) | 12 (27.3) |
|
| Prior radiation
therapy, n (%) |
|
| >0.999 |
|
Yes | 15 (100.0) | 43 (97.7) |
|
| No | 0 (0.0) | 1 (2.3) |
|
| Median interval
from radiation to PC or PCV (months), | 4.0 (0–42) | 22.0 (0–167) | 0.004 |
| n (range) |
|
|
|
| Prior chemotherapy,
n (%) |
|
| <0.003 |
|
Never | 10 (66.7) | 7 (16.3) |
|
|
TMZ | 5 (33.3) | 30 (69.8) |
|
| TMZ,
bevacizumab | 0 (0.0) | 1 (2.3) |
|
| TMZ,
PCV | 0 (0.0) | 5 (11.6) |
|
| Median interval
from last chemotherapy to PC or PCV | 1.0 (0–13) | 2.0 (0–61) | 0.610 |
| (months), n
(range) |
|
|
|
| ECOG score, n
(%) |
|
| 0.311 |
|
0-1 | 7 (46.7) | 29 (65.9) |
|
| ≥2 | 8 (53.3) | 15 (34.1) |
|
Toxicity experiences and the impact of
toxicity on chemotherapy schedule
The PC group presented a significantly lower
hematology toxicity profile (anemia: 6.7 vs. 45.5%; P=0.017 and
thrombocytopenia: 20.0 vs. 70.4%; P<0.001) and liver function
(elevated liver enzymes: 0 vs. 25.0%; P=0.078). CTCAE grade III or
IV toxicities were less frequently observed in the PC group,
although there was no statistically significant difference. Rates
of other toxicities, including neutropenia, kidney injury, allergic
skin reactions, and peripheral neurotoxicity, were not
significantly different between the two groups. Detailed
information about toxicities is presented in Table II.
 | Table II.Toxicity in patients with recurrent
adult glioma who received PC or PCV chemotherapy. |
Table II.
Toxicity in patients with recurrent
adult glioma who received PC or PCV chemotherapy.
|
| All
gradesa | Grades III and
IVa |
|---|
|
|
|
|
|---|
| Toxic
indicator | PC group
(n=15) | PCV group
(n=44) | P-value | PC group
(n=15) | PCV group
(n=44) | P-value |
|---|
| Anemia, n (%) | 1 (6.7) | 20 (45.5) | 0.017 | 1 (6.7) | 6 (13.6) | 0.796 |
| Neutropenia, n
(%) | 3 (20.0) | 17 (38.6) | 0.317 | 3 (20.0) | 8 (18.2) | >0.999 |
| Thrombocytopenia, n
(%) | 3 (20.0) | 31 (70.4) | <0.001 | 3 (20.0) | 14 (31.8) | 0.587 |
| Elevated liver
enzymes, n (%) | 0 (0.0) | 11 (25.0) | 0.078 | 0 (0.0) | 6 (13.6) | 0.310 |
| Elevated
creatinine, n (%) | 0 (0.0) | 2 (4.6) | 0.989 | 0 (0.0) | 1 (2.3) | >0.999 |
| Allergic reaction,
n (%) | 0 (0.0) | 1 (2.3) | >0.999 | 0 (0.0) | 0 (0.0) | >0.999 |
| Peripheral
neurotoxicity, n (%) | 0 (0.0) | 5 (11.4) | 0.408 | 0 (0.0) | 0 (0.0) | >0.999 |
We describe the adverse impacts of toxicity on
chemotherapy schedule in Table
III. The PC group significantly less frequently experienced any
of delay of cycle, dose reduction, discontinuation of one of the
chemotherapeutic drugs, or cessation of the entire chemotherapy
regimen than did the PCV group (26.7 vs. 68.2%; P=0.012). Each type
of toxicity was less frequently observed in the PC group, although
this result failed to show statistical significance.
 | Table III.Adverse impacts of the toxicity of PC
or PCV on the course of chemotherapy for recurrent adult
glioma. |
Table III.
Adverse impacts of the toxicity of PC
or PCV on the course of chemotherapy for recurrent adult
glioma.
| Adverse impact | PC group
(n=15) | PCV group
(n=44) | P-value |
|---|
| Total, n (%) | 4 (26.7) | 30 (68.2) | 0.012 |
| Delay of a cycle, n
(%) | 3 (20.0) | 14 (31.8) | 0.587 |
| Dose reduction, n
(%) | 1 (6.7) | 6 (13.6) | 0.796 |
| Drug
discontinuation, n (%) | 0 (0.0) | 8 (18.2) | 0.180 |
| Chemotherapy
cessation, n (%) | 0 (0.0) | 2 (4.5) | 0.989 |
Comparison of clinical outcomes
between the PC and PCV groups
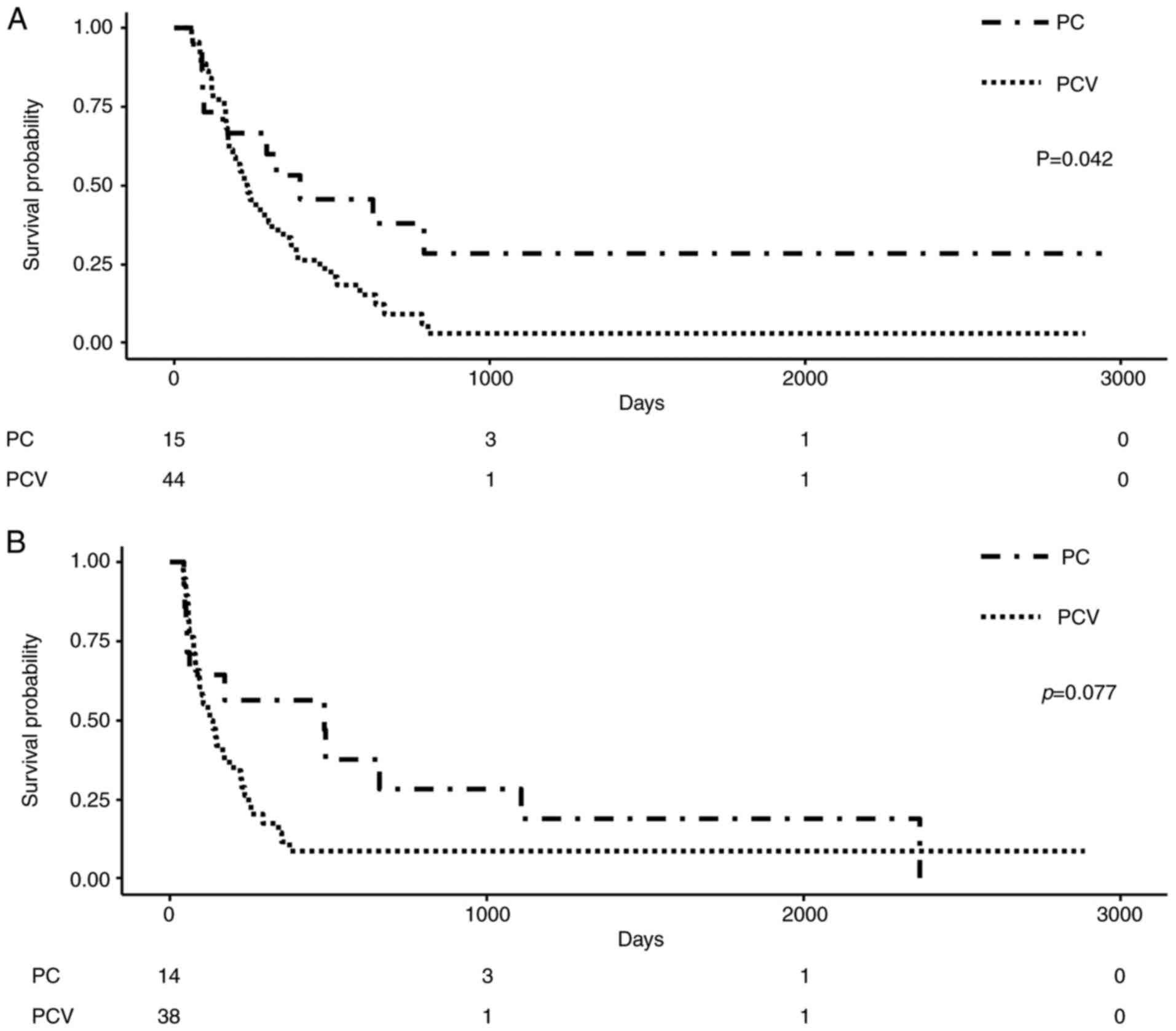
The OS of the PC group was significantly longer than
that of the PCV group (396 vs. 232 days; P=0.042), while there was
no significant difference in PFS between the two groups (284.5 vs.
131 days; P=0.077). The Kaplan-Meier survival curves of OS and PFS
in the two groups are illustrated in Fig. 2. Univariate and multivariate Cox
analyses for OS were performed and are described in Table IV. In multivariate analysis for
OS, the PCV group (HR 2.09 CI, 1.07–4.25, P=0.023) and older age
(≥65) (HR 3.12 CI, 1.12–8.66, P=0.029) were associated with
inferior OS, while presence of 1p19q co-deletion (HR 0.34 CI,
0.13–50.87, P=0.024) was associated with superior OS.
 | Table IV.Univariate and multivariate Cox
regression analysis for overall survival. |
Table IV.
Univariate and multivariate Cox
regression analysis for overall survival.
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Male vs.
female | 1.04 (0.58,
1.86) | 0.903 | – | – |
| Age ≥65 vs. <65
(years) | 3.45 (1.49,
8.00) | 0.004 | 3.12 (1.12,
8.66) | 0.029 |
| Non-glioblastoma
vs. glioblastoma | 0.60 (0.33,
1.07) | 0.081 | 0.66 (0.32,
1.35) | 0.251 |
| IDH mutated vs.
non-mutated | 0.45 (0.22,
0.93) | 0.032 | 0.73
(0.58, 3.26) | 0.472 |
| 1p19q co-deleted
vs. not-deleted | 0.35 (0.13,
0.90) | 0.030 | 0.34
(0.13, 0.87) | 0.024 |
| MGMT methylated vs.
non-methylated | 0.45 (0.21,
0.98) | 0.045 | 0.26 (0.08,
0.86) | 0.028 |
| Prior history of
chemotherapy vs. no | 1.61 (0.84,
3.11) | 0.152 | 0.78 (0.35,
1.76) | 0.557 |
| history of
chemotherapy |
|
|
|
|
| Prior history of
radiotherapy | 2.67 (0.36,
19.58) | 0.330 | – | – |
| vs. no history of
radiotherapy |
|
|
|
|
| PCV group vs. PC
group | 2.06 (1.01,
4.18) | 0.046 | 2.09 (1.07,
4.25) | 0.023 |
Discussion
In our retrospective and comparative study, we tried
to compare the safety and the efficacy of our modified PC
chemotherapy, compared to those of PCV chemotherapy. We also tried
to evaluate how the toxicity of these chemotherapies affected the
course of chemotherapy in patients with recurrent adult glioma. Our
findings showed that anemia and thrombocytopenia were significantly
more frequent in PCV groups than in the PC groups (anemia: 45.5 vs.
6.7%; P=0.017 and thrombocytopenia: 70.4 vs. 20.0%; P<0.001,
respectively). Anemia of higher than CTCAE grade III was also more
frequent in the PCV group than the PC group. Peripheral
neurotoxicity, which is a major concern with vincristine, was not
observed in the PC group, while it was observed in 11.4% of
patients in the PCV group. In addition, frequent and severe adverse
events in the PCV group also resulted in greater disruption to the
course of chemotherapy, such as delay of a cycle, dose reduction,
discontinuation of vincristine, and cessation of salvage
chemotherapy (68.2 vs. 26.7%; P=0.012). In contrast, regarding
concerns about inferior efficacy when omitting vincristine, our
findings suggest that survival outcomes were not different between
the two groups. Interestingly, the OS of the PC group was
significantly superior to that of the PCV group (396 vs. 232 days;
P=0.042), while the PFS of the PC group was not different from that
of the PCV group (284.5 vs. 131 days; P=0.077). This may be
explained by numerous studies showing that the occurrence of less
toxicity after chemotherapy correlates with better prognosis
(23,24). In summary, our modified PC
chemotherapy, which omitted vincristine and reduced the dose of
lomustine, showed lower toxicity and non-inferior efficacy for
adult recurrent glioma patients compared to those of conventional
PCV chemotherapy.
There were significant differences regarding prior
history of chemotherapy and the interval from radiation to
chemotherapy between the two groups, although several baseline
characteristics, including initial diagnosis, molecular features,
and prior history of radiotherapy, were not significantly different
between the two groups. In detail, significantly fewer patients in
the PC group had a prior history of any chemotherapy and in the PC
group, 5 patients were diagnosed with primary glioblastoma at
initial diagnosis, and these patients received concomitant
chemoradiation therapy followed by TMZ. Regarding to the difference
of the median interval from radiation to chemotherapy, we thought
that the PCV group had a longer period of stable state after
radiation compared that of the PC group. Another explanation is
that the PC group received earlier chemotherapy as salvage
treatment than did the PCV group.
When considering chemotherapeutic drugs for
recurrent glioma, there have been options identified to date,
including TMZ rechallenged or continuously administered with
low-dose, bevacizumab, and PCV-based chemotherapy (4,25–28).
As a salvage therapy after TMZ for recurrent glioma, numerous
clinical trials have assessed the efficacy of PCV-based
chemotherapy (11,12,14).
However, in clinics, toxicities including hematologic, neurologic,
liver, kidney, and skin problems were diagnosed frequently and
sometimes very severe, which is a major hindrance when choosing PCV
chemotherapy as salvage therapy for recurrent glioma patients,
especially in those who are elderly or with a lower performance
status (15,29). In addition, there have been
concerns about the efficacy of vincristine because its molecular
weight (825 Daltons) might be too high to penetrate the blood-brain
barrier (18). In this context, a
few studies have put forth the idea of adopting a modified PC-based
chemotherapy regimen without vincristine for glioma patients
(19–22). Vesper et al retrospectively
analyzed clinical outcomes and toxicities of 315 patients with
oligodendroglial brain tumors who received PCV or PC chemotherapy
as adjuvant treatment after surgical resection and radiation. Their
study showed that the PFS of patients who received PC chemotherapy
was not different from that of patients who received PCV
chemotherapy, with significantly fewer hematologic and neurological
toxicities (20). Webre et
al also evaluated 97 patients with primary anaplastic
oligodendroglioma who received PCV or PC chemotherapy as adjuvant
treatment, reporting that the clinical outcomes of PC chemotherapy
for primary anaplastic oligodendroglial tumors were not different
from those of patients who received PCV chemotherapy, with lower
hematologic toxicities (21).
In accordance with two previous studies exploring
the use of PC chemotherapy in primary oligodendroglial patients
(20,21), we added evidence that our modified
PC chemotherapy is as beneficial as PCV chemotherapy but with
significantly less toxicity due to omission of vincristine and
reduction of the dose of lomustine. Taken together, we suggest that
PC chemotherapy can be an alternative option to PCV chemotherapy,
especially for use in patients expected to be intolerable to PCV
chemotherapy, including elderly patients or those with lower
performance.
Our study should be considered within the scope of
several limitations. First, our study included heterogeneous
recurrent gliomas, and the unknown molecular status of 1p19q
co-deletion in about 30% of these patients could cause severe bias.
Second, although several baseline characteristics, including
initial diagnosis, molecular features, and prior history of
radiotherapy, were not significantly different between the two
groups, there were significant differences regarding prior history
of chemotherapy and the interval from radiation to chemotherapy
between the two groups, which can cause several biases in both
toxicity profile and clinical outcomes. Third, this is not a
randomized study, and selection bias about treatment group have
affected the results. Although our institution tried to minimize
clinician biases through multi-disciplinary discussion, treatment
characteristics could not be identical between the two groups.
Fourth, due to the retrospective nature of this study, not all
adverse reactions were considered. Fifth, the number of patients is
insufficient to draw a strong conclusion. Therefore, further
prospective and larger studies are needed to validate whether PC
chemotherapy could be an alternative to PCV chemotherapy as a
secondary salvage option for recurrent glioma patients.
In conclusion, this study showed significantly fewer
toxicities after our modified PC chemotherapy than after PCV
chemotherapy in recurrent glioma patients. The OS and PFS of the
modified PC chemotherapy were noninferior to those of PCV
chemotherapy. Further prospective and larger studies are needed to
validate the modified PC chemotherapy without vincristine as an
alternative option for the conventional PCV chemotherapy for
recurrent glioma patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datsets used and/or analyzed are available from
the corresponding author on reasonable request.
Authors' contributions
SA and YIK wrote the manuscript. SA, YIK, JSP, JYS,
CY, and YSL collected and analyzed the data. JSP, CY, YSL, YKH, SSJ
and SHY supervised the current study. JSP, CY, YSL, YKH, SSJ and
SHY wrote, reviewed and edited the manuscript. YKH, SSJ and SHY
conceptualized the present study. SA, YIK, and JSP confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Seoul St. Mary's
Hospital approved the current study (approval no. XC21RIDI0089).
Due to the retrospective manner of the study, the requirement for
informed consent to patriciate was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: primary brain and other central nervous system tumors
diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molinaro AM, Taylor JW, Wiencke JK and
Wrensch MR: Genetic and molecular epidemiology of adult diffuse
glioma. Nat Rev Neurol. 15:405–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weller M, van den Bent M, Preusser M, Le
Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven
L, et al: EANO guidelines on the diagnosis and treatment of diffuse
gliomas of adulthood. Nat Rev Clin Oncol. 18:1702021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, Van Den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Linde ME, Brahm CG, de Witt Hamer PC,
Reijneveld JC, Bruynzeel AME, Vandertop WP, van de Ven PM,
Wagemakers M, van der Weide HL, Enting RH, et al: Treatment outcome
of patients with recurrent glioblastoma multiforme: A retrospective
multicenter analysis. J Neurooncol. 135:183–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen PY, Weller M, Lee EQ, Alexander BM,
Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM,
Chiocca EA, et al: Glioblastoma in adults: A society for
neuro-oncology (SNO) and European society of neuro-oncology (EANO)
consensus review on current management and future directions. Neuro
Oncol. 22:1073–1113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alexander BM and Cloughesy TF: Adult
glioblastoma. J Clin Oncol. 35:2402–2409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weller M and Le Rhun E: How did lomustine
become standard of care in recurrent glioblastoma? Cancer Treat
Rev. 87:1020292020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDuff SGR, Dietrich J, Atkins KM, Oh KS,
Loeffler JS and Shih HA: Radiation and chemotherapy for high-risk
lower grade gliomas: Choosing between temozolomide and PCV. Cancer
Med. 9:3–11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt F, Fischer J, Herrlinger U, Dietz
K, Dichgans J and Weller M: PCV chemotherapy for recurrent
glioblastoma. Neurology. 66:587–589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Happold C, Roth P, Wick W, Steinbach JP,
Linnebank M, Weller M and Eisele G: ACNU-based chemotherapy for
recurrent glioma in the temozolomide era. J Neurooncol. 92:45–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brada M, Stenning S, Gabe R, Thompson LC,
Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K,
et al: Temozolomide versus procarbazine, lomustine, and vincristine
in recurrent high-grade glioma. J Clin Oncol. 28:4601–4608. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parasramka S, Talari G, Rosenfeld M, Guo J
and Villano JL: Procarbazine, lomustine and vincristine for
recurrent high-grade glioma. Cochrane Database Syst Rev.
7:CD0117732017.PubMed/NCBI
|
|
15
|
Jutras G, Bélanger K, Letarte N, Adam JP,
Roberge D, Lemieux B, Lemieux-Blanchard É, Masucci L, Ménard C,
Bahary JP, et al: Procarbazine, lomustine and vincristine toxicity
in low-grade gliomas. Curr Oncol. 25:e33–e39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keogh RJ, Aslam R, Hennessy MA, Coyne Z,
Hennessy BT, Breathnach OS, Grogan L and Morris PG: One year of
procarbazine lomustine and vincristine is poorly tolerated in low
grade glioma: A real world experience in a national neuro-oncology
centre. BMC Cancer. 21:1402021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SB, Goldstein D, Krishnan AV, Lin CS,
Friedlander ML, Cassidy J, Koltzenburg M and Kiernan MC:
Chemotherapy-induced peripheral neurotoxicity: A critical analysis.
CA Cancer J Clin. 63:419–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Zhou F, Kruh GD and Gallo JM:
Influence of blood-brain barrier efflux pumps on the distribution
of vincristine in brain and brain tumors. Neuro Oncol.
12:1043–1049. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SH, Yoo H, Chang JH, Kim CY, Chung DS,
Kim SH, Park SH, Lee YS and Yang SH: Procarbazine and CCNU
chemotherapy for recurrent glioblastoma with MGMT promoter
methylation. J Korean Med Sci. 33:e1672018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vesper J, Graf E, Wille C, Tilgner J,
Trippel M, Nikkhah G and Ostertag C: Retrospective analysis of
treatment outcome in 315 patients with oligodendroglial brain
tumors. BMC Neurol. 9:332009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Webre C, Shonka N, Smith L, Liu D and De
Groot J: PC or PCV, that is the question: Primary anaplastic
oligodendroglial tumors treated with procarbazine and CCNU with and
without vincristine. Anticancer Res. 35:5467–5472. 2015.PubMed/NCBI
|
|
22
|
Yang SH, Hong YK, Yoon SC, Kim BS, Lee YS,
Lee TK, Lee KS, Jeun SS, Kim MC and Park CK: Radiotherapy plus
concurrent and adjuvant procarbazine, lomustine, and vincristine
chemotherapy for patients with malignant glioma. Oncol Rep.
17:1359–1364. 2007.PubMed/NCBI
|
|
23
|
Cha JY, Park JS, Hong YK, Jeun SS and Ahn
S: Impact of body mass index on survival outcome in patients with
newly diagnosed glioblastoma: A retrospective single-center study.
Integr Cancer Ther. 20:15347354219912332021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trestini I, Carbognin L, Bonaiuto C,
Tortora G and Bria E: The obesity paradox in cancer: Clinical
insights and perspectives. Eat Weight Disord. 23:185–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seystahl K, Hentschel B, Loew S, Gramatzki
D, Felsberg J, Herrlinger U, Westphal M, Schackert G, Thon N,
Tatagiba M, et al: Bevacizumab versus alkylating chemotherapy in
recurrent glioblastoma. J Cancer Res Clin Oncol. 146:659–670. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai Y, Jiang YG, Wang M, Jiang ZH and Tan
ZG: A comparative study of the effectiveness and safety of combined
procarbazine, lomustine, and vincristine as a therapeutic method
for recurrent high-grade glioma: A protocol for systematic review
and meta-analysis. Medicine (Baltimore). 99:e222382020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toft A, Urup T, Christensen IJ, Michaelsen
SR, Lukram B, Grunnet K, Kosteljanetz M, Larsen VA, Lassen U,
Broholm H and Poulsen HS: Biomarkers in recurrent grade III glioma
patients treated with bevacizumab and irinotecan. Cancer Invest.
36:165–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wick W and Winkler F: Regimen of
procarbazine, lomustine, and vincristine versus temozolomide for
gliomas. Cancer. 124:2674–2676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabouret E, Reyes-Botero G, Dehais C,
Daros M, Barrie M, Matta M, Petrirena G, Autran D, Duran A, Bequet
C, et al: Relationships between dose intensity, toxicity, and
outcome in patients with oligodendroglial tumors treated with the
PCV regimen. Anticancer Res. 35:2901–2908. 2015.PubMed/NCBI
|