Introduction
Lung cancer can be classified into small cell lung
cancer (SCLC) and non-SCLC (NSCLC). NSCLC accounts for 85% of all
LC cases and can be further divided into three major pathological
subtypes: Adenocarcinoma, squamous cell carcinoma and large cell
carcinoma (1). Lung cancer is one
of the most common types of malignant tumors in the world and a
serious threat to human health and life. Lung cancer is associated
with high morbidity and mortality and a poor prognosis and has a
5-year survival rate of <15%. A total of 1.8 million individuals
are diagnosed with lung cancer annually, 1.6 million of which
succumb to the disease (2). Early
lung cancer is mainly treated by surgery, chemotherapy and
radiotherapy and advanced lung cancer can be treated using
molecular targeted therapy and immunotherapy. However, the 5-year
survival rate is still very low and the malignant metastasis rate
of lung cancer is as high as 93% (3). Therefore, adopting multitarget
combination therapy, combining chemotherapy with chemobiologic
therapy or exploring new target drugs could be used as an
alternative lung cancer treatment.
In nature, flavonoids are widely distributed in
plants and have extensive pharmacological activities, such as
liver-protective, antioxidant and antitumor activities. For
example, isoliquiritigenin (ISL), a flavonoid extracted from
liquorice, has been confirmed to exhibit antitumor,
anti-inflammatory and antioxidant effects in vitro and in
vivo (4–6). In addition, ISL has been shown to
have a quinone reductase activity, that can promote cancer
chemoprevention (7). ISL inhibits
several tumor activities, such as tumor proliferation, metastasis
and apoptosis (6). In recent
years, various antitumor mechanisms of ISL have been elucidated.
For example, in endometrial cancer cells, ISL could induce cell
cycle arrest in the G1 or G2/M phase via the p53/p21 pathway and
promote apoptosis and autophagy through the activation of the
extracellular signal-regulated kinase pathway. In addition, ISL has
been reported to induce cell apoptosis and inhibit proliferation
via the PI3K/AKT signaling pathway (8).
2,2′,4′-Trihydroxychalcone (7a) is a flavonoid and
an isomer of ISL (Fig. 1). The
only difference between them is that a hydroxyl group is
substituted in a different place on the benzene ring. Therefore, 7a
may also exhibit antitumor effects similar to those of ISL. In the
present study, the effects of 7a on the proliferation, migration,
invasion, vasculogenic mimicry (VM) formation, heterogeneous
adhesion and apoptosis of the A549 human lung cancer cell line were
first examined. At the same time, 7a had almost no effect on the
proliferation of BEAS-2B human lung epithelial cells and human
venous endothelial cells (HUVECs), suggesting that 7a had exhibited
low cytotoxicity in normal cells. Furthermore, 7a downregulated the
expression of N-cadherin, vascular endothelial growth factor (VEGF)
and metalloproteinase (MMP)-2/9, while increasing the expression of
E-cadherin, Bax, caspase-3 and Bcl-2 in A549 cells. Simultaneously,
7a significantly inhibited the PI3K/AKT/NF-κB signaling pathway in
A549 cells. Therefore, 7a may be a potential compound for the
treatment of lung cancer.
Materials and methods
Materials
RPMI-1640 medium, DMEM, fetal bovine serum (FBS) and
L-glutamine were purchased from Gibco (Thermo Fisher Scientific,
Inc.). 7a and ISL were provided by the Natural Medicinal Chemistry
Laboratory of Qingdao University (Shandong, China). Enhanced
chemiluminescence (ECL) reagent was purchased from Beyotime
Institute of Biotechnology. The subcellular structure of the
cytoplasm and cell nucleoprotein extraction kit were obtained from
Boster Biological Technology. Cell Counting Kit-8 (CCK-8), bovine
serum albumin, Vybrant DiO cell labelling solutions, RIPA lysis
buffer and PMSF were purchased from Beijing Solarbio Science &
Technology Co., Ltd. Culture dishes, 6-well plates, 24-well plates
and 24-well Transwell chambers with 8.0-ml polycarbonate filters
were obtained from Corning, Inc. Matrigel® was purchased
from BD Biosciences. An Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit was purchased from Beyotime Institute of
Biotechnology.
Cell lines
A549 human lung cancer cells, BEAS-2B human lung
epithelial cells and HUVECs were obtained from the Shanghai Culture
Collection of the Chinese Academy of Sciences. A549 and BEAS-2B
cells were cultured in RPMI-1640 medium containing 10% FBS. HUVECs
were cultured in DMEM containing 10% FBS. All media were
supplemented with 100 U/ml streptomycin/penicillin. All cells were
cultured in an incubator at 37°C with 5% CO2.
Cell proliferation assay
A cell suspension (100 µl; 1×103
cells/well) was inoculated in a 96-well plate. The plates were
precultured in an incubator for 24 h at 37°C with 5%
CO2. A total of 10 µl of different concentrations (0.0,
2.5, 5.0, 10.0, 20.0 and 40.0 µM) of 7a was added to the culture
plate and incubated in the incubator for 24, 48 and 72 h. CCK-8
solution (10 µl) was added to each well and the plates were
incubated for 1–4 h. Absorbance was measured at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc). Based on the
optical density values, a curve was drawn to analyze cell
proliferation inhibition. The results of three independent
experiments were analyzed.
Wound-healing assay
A wound-healing assay was performed in 6-well
plates, as previously described by Liang et al (9). A549 cells were harvested and seeded
on 6-well plates at a density of 4×105 cells in 2 ml
complete RPMI-1640 medium and incubated at 37°C for 24 h. A
straight line was scrapped on the monolayer cells using a 200-µl
pipette tip. Different concentrations of 7a solution were added to
the cells and incubated in RPMI-1640 medium without FBS. Scratch
images were then captured using a Leica DFC420 camera (Leica
Microsystems, Inc.) under an inverted microscope at 0, 18 and 36 h.
The gap width was analyzed along the scratch with a scale plate
under an inverted microscope (Olympus Corporation; magnification,
×100). At least six points were measured for each scratch. The
results of three independent experiments were analyzed.
Cell invasion assay
An invasion assay was performed using 24-well
Matrigel-coated Transwell chambers. Martigel was diluted 1:15 in
serum-free RPMI-1640 and added into the upper chamber of Transwell
chamber, and then incubated at 37°C for 1 h. A549 cells were
harvested and suspended in serum-free RPMI-1640 medium at a density
of 1×106 cells/well. Next, 50 µl suspended cells were
seeded in the upper chamber of each well and the bottom chamber was
filled with 600 µl RPMI-1640 medium supplemented with 10% FBS.
Following incubation at 37°C for 24 h, cells that did not traverse
the Matrigel were removed using cotton swabs. Following washing
with PBS, cells that traversed the Matrigel were fixed by
paraformaldehyde (4%) at room temperature for 20 min and stained
with crystal violet at room temperature for 30 min. Following
washing with PBS, cells that penetrated the Matrigel were counted
in 10 randomly selected fields using an inverted microscope with a
20 objective (magnification, ×200).
VM assay
First, Matrigel matrix glue stored at −20°C was left
to thaw at 4°C overnight. Next, 50 µl matrix was added to a 96-well
plate and placed in a cell incubator for 1 h incubation at 37°C
prior to solidification. Then, 50 µl A549 cell suspension
(5×l04/well) and 50 µl different concentrations (0.0,
2.5, 5.0 and 10.0 µM) of 7a-containing medium were added. At the
same time, the solvent control group was set up. The cells were
then incubated in the cell incubator for 8 h at 37°C, and the
formation of tubules was observed under a microscope and images
captured. The tubule branch length was counted using ImageJ
software v1.8.0 (National Institutes of Health).
ELISA
A549 cells were collected and inoculated into
24-well plates (5×104/well). After the cells were
attached to the wall, the original medium was discarded and
drug-containing media of different concentrations (0.0, 2.5, 5.0
and 10.0 µM) of 7a were added. Meanwhile, the negative control (NC)
group was set up and incubation was continued in a cell incubator
with 5% CO2 for 24 h at 37°C. The supernatant was then
transferred to a centrifugal tube for centrifugation at 150 × g for
5 min at room temperature. The supernatant was then transferred to
a new centrifugal tube. The VEGF levels in each group were tested
using a VEGF ELISA kit (cat. no. KE00216; Proteintech Group, Inc.),
according to the manufacturer's instructions.
Cancer cell-endothelial adhesion
assay
Cancer cell-endothelial adhesion was performed as
previously described (10). A549
cells were cultured in 6-well plates with different concentrations
(0.0, 2.5, 5.0 and 10.0 µM) of 7a and HUVECs were cultured in
24-well plates. Following incubation at 37°C for 48 h, A549 cells
were washed with PBS and labelled using 5 µM DiO fluorescent cell
labelling solution in serum-free RPMI-1640 medium for 30 min at
37°C. Next, tumor cells were washed with PBS and removed from the
culture plates. A549 cells were collected using centrifugation at
150 × g for 3 min at room temperature. Following washing,
5×104 cells were applied to the HUVEC monolayer cultured
in 24-well plates for 1 h at 37°C. Next, the 24-well plates were
gently washed with PBS and the fluorescently labelled cells were
counted in 10 randomly selected fields using an Olympus B51
fluorescence microscope (Olympus Corporation) with a 10 objective
(magnification, ×100).
Cell apoptosis analysis
A549 cells were either treated with different
concentrations (0, 5, 10 and 20 µM) of 7a or 0.1% DMSO for 48 h at
37°C. The cell suspension (~10×104) was collected and
centrifuged at 150 × g for 5 min at room temperature. The
supernatant was discarded and the cells were gently resuspended in
195 µl Annexin V-FITC binding solution. Annexin V-FITC (5 µl) was
added to the cells and mixed gently. Propidium iodide (PI) staining
solution (5 µl) was added and mixed gently. The cells were
incubated in the dark for 10–20 min at room temperature and then
placed in an ice bath. Early + late apoptotic cells were then
detected via flow cytometer (FACSVantage; BD Biosciences) using the
apoptosis detection kit, following the manufacturer's instructions.
Annexin V-FITC fluorescence was green and PI fluorescence was red.
The flow cytometry results were analyzed using FlowJo software
v7.6.5 (FlowJo LLC). The results were analyzed from three
independent experiments.
Western blot analysis
A549 cells were seeded in 6-well plates
(2×105 cells per well). A total of 24 h later, the cells
were treated with different concentrations (0.0, 2.5, 5.0 and 10.0
µM) of 7a. Following incubation at 37°C for 48 h, cell lysates were
collected in RIPA lysis buffer PMSF (99:1, v/v) and centrifuged at
15,000 × g for 15 min at 4°C. The total protein concentrations were
then determined using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Adequate buffer (6X) was added to the total
protein and mixed completely. The mixture was then heated at 100°C
for 5 min. Next, 15–30 µg total protein was fractionated using 10%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes (MilliporeSigma).
The membranes were blocked with 5% non-fat milk at room temperature
for 4 h, and then cultured overnight at 4°C with the following
primary antibodies: E-cadherin (1:1,000; cat. no. 14472s; mouse
monoclonal), N-cadherin (1:1,000; cat. no. 4061s; rabbit
monoclonal), MMP-9 (dilution, 1:1,000; cat. no. 13667Ts; rabbit
monoclonal), MMP-2 (dilution, 1:1,000; cat. no. 4022s; rabbit
polyclonal), VEGF (dilution, 1:1,000; cat. no. 65373s; rabbit
polyclonal), E-Selectin (1:1,000; cat. no. 20894-1-AP; rabbit
polyclonal), Bcl-2 (1:1,000; cat. no. 15071s; mouse monoclonal),
Bax (1:1,000; cat. no. 5023s; mouse monoclonal), Cleaved-PARP
(dilution, 1:1,000; cat. no. 5625s; rabbit monoclonal), Caspase-3
(dilution, 1:1,000; cat. no. 9662s; rabbit polyclonal), PI3K
(dilution, 1:1,000; cat. no. 4249s; rabbit monoclonal), p-PI3K
(dilution, 1:1,000; cat. no. 17366s; rabbit monoclonal), AKT
(dilution, 1:1,000; cat. no. 9272s; rabbit polyclonal), p-AKT
(dilution, 1:1,000; cat. no. 4060s; rabbit monoclonal), mTOR
(dilution, 1:1,000; cat. no. 2972s; rabbit polyclonal), p-mTOR
(dilution, 1:1,000; cat. no. 5536s; rabbit monoclonal), PCNA
(dilution, 1:1,000; cat. no. 13110T; rabbit monoclonal) and β-actin
(dilution, 1:1,000; cat. no. 4970s; rabbit monoclonal). Primary
E-Selectin antibody was from ProteinTech Group, Inc. Other primary
antibodies were from Cell Signaling Technology, Inc. According to
the sources of the different primary antibodies, the membranes were
incubated with horseradish peroxidase conjugated goat anti-rabbit
or goat anti-mouse IgG secondary antibodies (cat. nos. SA00001-2
and SA00001-1, respectively; dilution, 1:10,000; ProteinTech Group,
Inc.) at room temperature for 1 h and then laid on the developer
board of the gel image processing instrument. ECL (Beyotime
Institute of Biotechnology) was added to allow visualization of the
signals. Image Lab software v3.0 (Bio-Rad Laboratories, Inc.) was
used for band processing and analysis and β-actin was used as an
internal reference for semi-quantitative analysis of protein
expression in each group.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
A549 cells were seeded in 6-well plates
(2×105 cells per well) and incubated at 37°C for 24 h.
The cells were treated with different concentrations (0, 2.5, 5 and
10 µM) of 7a and incubated at 37°C for 48 h. Total RNA was
extracted from the 6-well plates using TRIzol® reagent
(Thermo Fisher Scientific, Inc.). The RNA concentration was
determined by measuring ultraviolet absorption at 260 and 280 nm.
The A260/A280 ratio was calculated to assess RNA quality and
purity. Next, 1 mg RNA was reverse-transcribed using a PrimeScript
RT reagent kit (Takara Bio, Inc.). cDNA was diluted (2 µl) and
selectively amplified using a PCR with SYBR Green I (Takara Bio,
Inc.) and specific primers. Primers were designed by Shanghai
Bioengineering, as follows: NF-κB, forward
5′-GCTGCATCCACAGTTTCCAG-3′, reverse 5′-TCCCCACGCTGCTCTTCTAT-3′;
GAPDH, forward 5′-AATGGGCAGCCGTTAGGAAA-3′, reverse
5′-GCCCAATACGACCAAATCAGAG-3′. The samples were amplified using a
Roche LightCycler 480II (Roche Diagnostics). All procedures were
performed according to the manufacturer's protocols. The relative
quantity of NF-κB mRNA was calculated using a comparative method
(2−ΔΔCq) against a GAPDH endogenous control (n=3)
(11).
Statistical analysis
All experiments were repeated three times. SPSS
software 22.0 (IBM Corp.) was used for the statistical analysis of
all data and the experimental results were expressed as the mean ±
standard deviation. The difference between the groups was analysed
using one-way ANOVA. Tukey's post hoc test was performed after
one-way ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
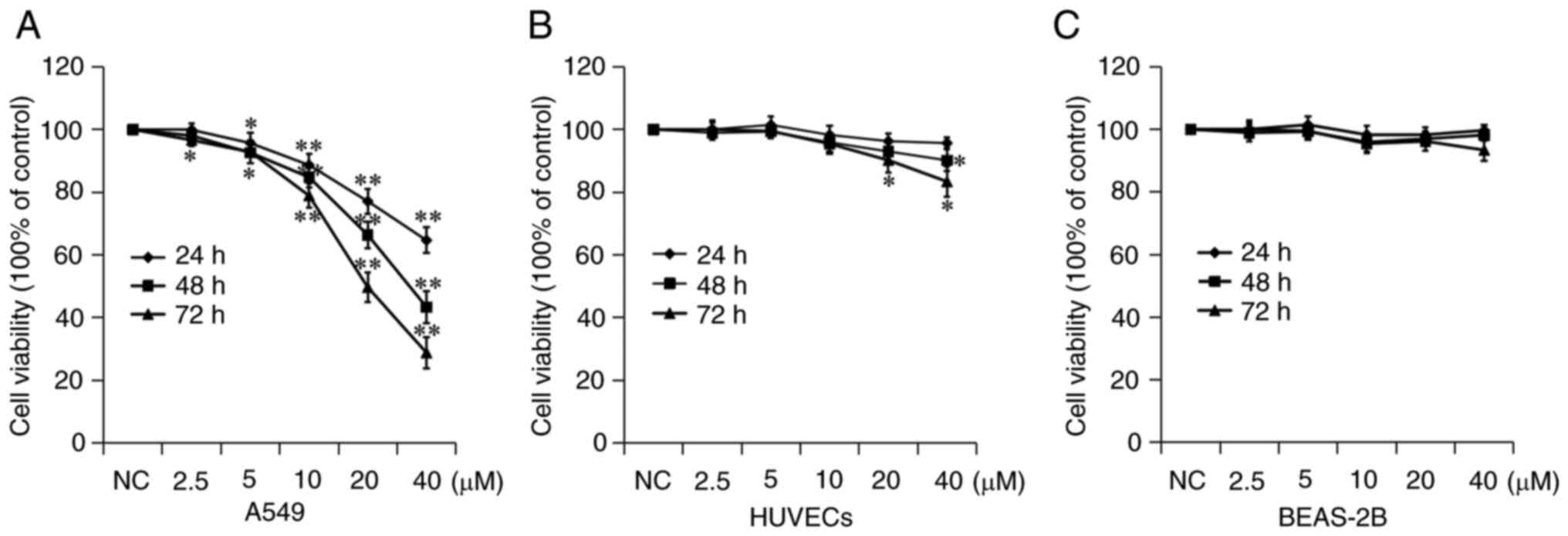
7a inhibits A549 cell
proliferation
The inhibitory effect of 7a on the proliferation of
A549 cells was measured using a CCK-8 assay. A549 cells were
treated with different concentrations of 7a for 24, 48 and 72 h.
The viability of A549 cells was determined and the growth curves of
A549 cells were drawn. As shown in Fig. 2, 7a inhibited the proliferation and
growth of A549 cells in a time- and dose-dependent manner. The
IC50 value of 7a against A549 cells was calculated
(Fig. 2A). The results showed that
the IC50 of 7a on A549 cell growth was 65.72±4.20,
33.46±4.11 and 19.86±2.33 µM at 24, 48 and 72 h, respectively.
These results indicated that 7a could inhibit the proliferation of
A549 cells at higher concentrations and for longer durations.
The toxic effects of 7a were examined on two types
of normal human tissue cells. Normal BEAS-2B human lung epithelial
cells and HUVECs were selected. The CCK-8 test results showed that
7a had no significant inhibitory effect on the proliferation of
these two types of cells. Among them, although HUVECs were
relatively sensitive to 7a, the maximum proliferation inhibition
rate was <20% at 40 µM and 72 h. These results indicated that 7a
had a selective tumor cell-killing effect, but had no obvious
toxicity or side effects on normal cells, suggesting its clinical
application potential.
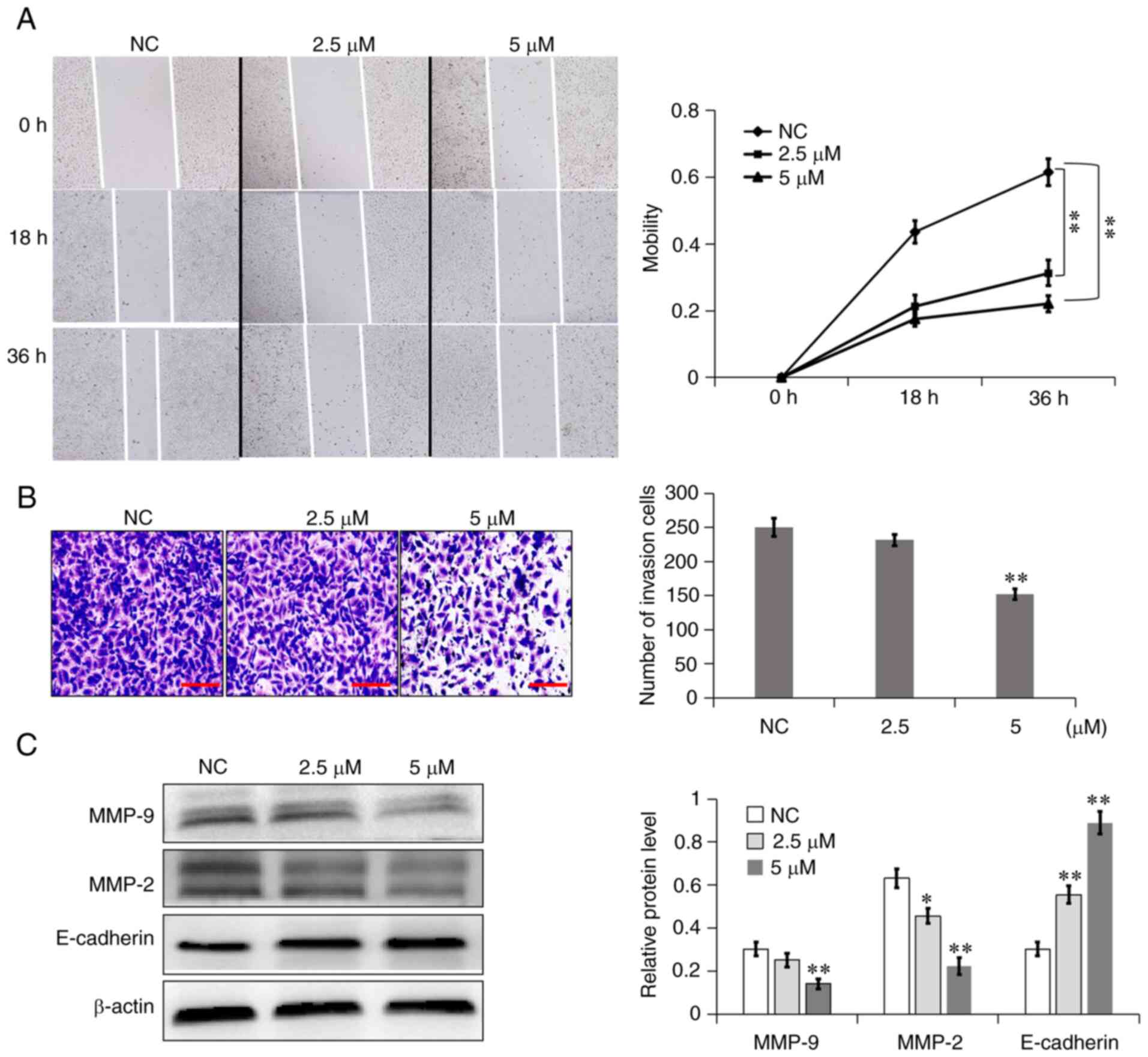
7a inhibits A549 cell migration and
invasion
According to the results of the CCK-8 experiment,
2.5 and 5 µM were selected as the low cytotoxic drug concentrations
for the migration and invasion experiments. First, the effect of 7a
on the migratory ability of A549 cells was tested using a cell
scratch assay and the results showed that 7a could significantly
inhibit the migratory ability of A549 cells. The migration rate of
NC, 2.5 and 5 µM group was respectively ~43.61, 21.30 and 17.52% at
18 h and 61.50, 31.22 and 22.10% at 36 h (Fig. 3A). The effect of 7a on the invasive
ability of A549 cells was also examined, using a Transwell invasion
assay. As compared with the NC group, the number of A549 cells
passing through the compartment was reduced in the 7a group at 48
h, especially in the high-concentration (5 µM) group (Fig. 3B). These results demonstrated that
7a could markedly inhibit the migration and invasion of A549 cells
at non-cytotoxic concentrations.
To understand the mechanism through which 7a
inhibits the migration and invasion of A549 cells, the expression
of cell metastasis-related proteins was further investigated. As
shown in Fig. 3C, 7a could
significantly increase the expression of E-cadherin while at the
same time decreasing the expression of MMP-9 and −2 suggesting that
the 7a-induced inhibition of migration and invasion may be due to
the regulation of the expression of these proteins.
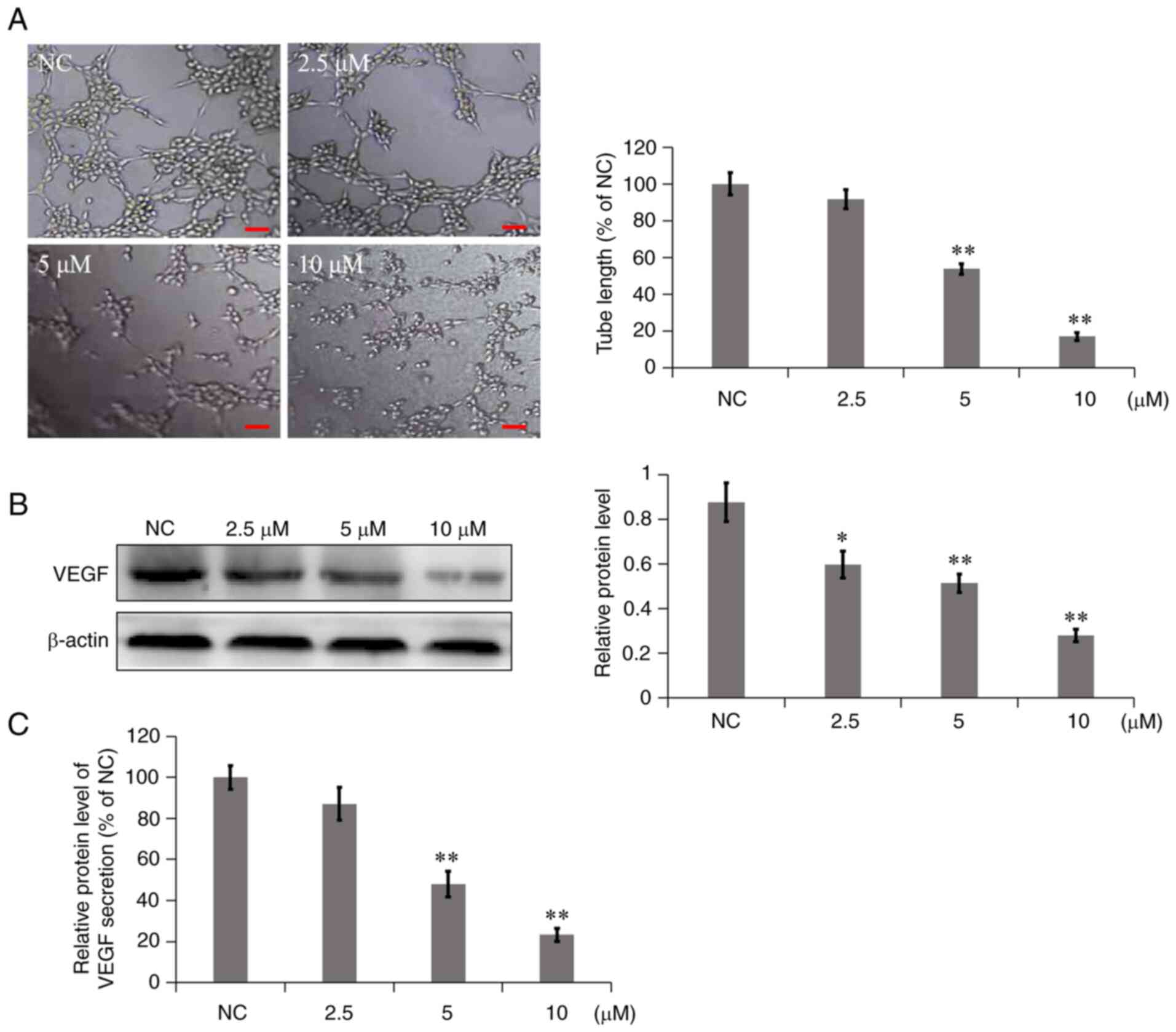
7a inhibits VM
Highly invasive tumor cells, such as NSCLC, can form
mimicry vessels; that is, tumor cells exhibit certain
characteristics that are similar to vascular endothelial cells and
can degrade the basement membrane to connect and form a network
structure (12,13). Therefore, in the in vitro
mimicry angiogenesis experiments, Matrigel was used to simulate the
basement membrane and the simulated angiogenesis of tumor cells in
each group observed. In the NC group, A549 cells were connected to
form multiple grid structures (Fig.
4A). However, in the 7a dosage group, the branch length of
mimetic vessels was significantly reduced, particularly following
treatment with 10 µM 7a for 8 h. The cells were dispersed, almost
no tubules were formed and the inhibition rate of mimetic vessel
formation was as high as 82.89%. These results indicated that 7a
could significantly inhibit the formation of mimicry vessels in
A549 cells in a concentration-dependent manner.
As a proangiogenic factor, VEGF not only serves an
important role in angiogenesis but is also associated with tumor
cells (14). Western blot analysis
and ELISA were used to determine the effect of 7a on the expression
and secretion of VEGF in A549 cells and culture media. As shown in
Fig. 4B, 7a inhibited the
expression of VEGF in A549 cells compared with the NC group. ELISA
results (Fig. 4C) showed that in
A549 cells treated with different concentrations of 7a for 24 h,
VEGF secretion was significantly reduced in a
concentration-dependent manner. The results suggested that 7a could
significantly inhibit the expression and secretion of VEGF in 549
cells.
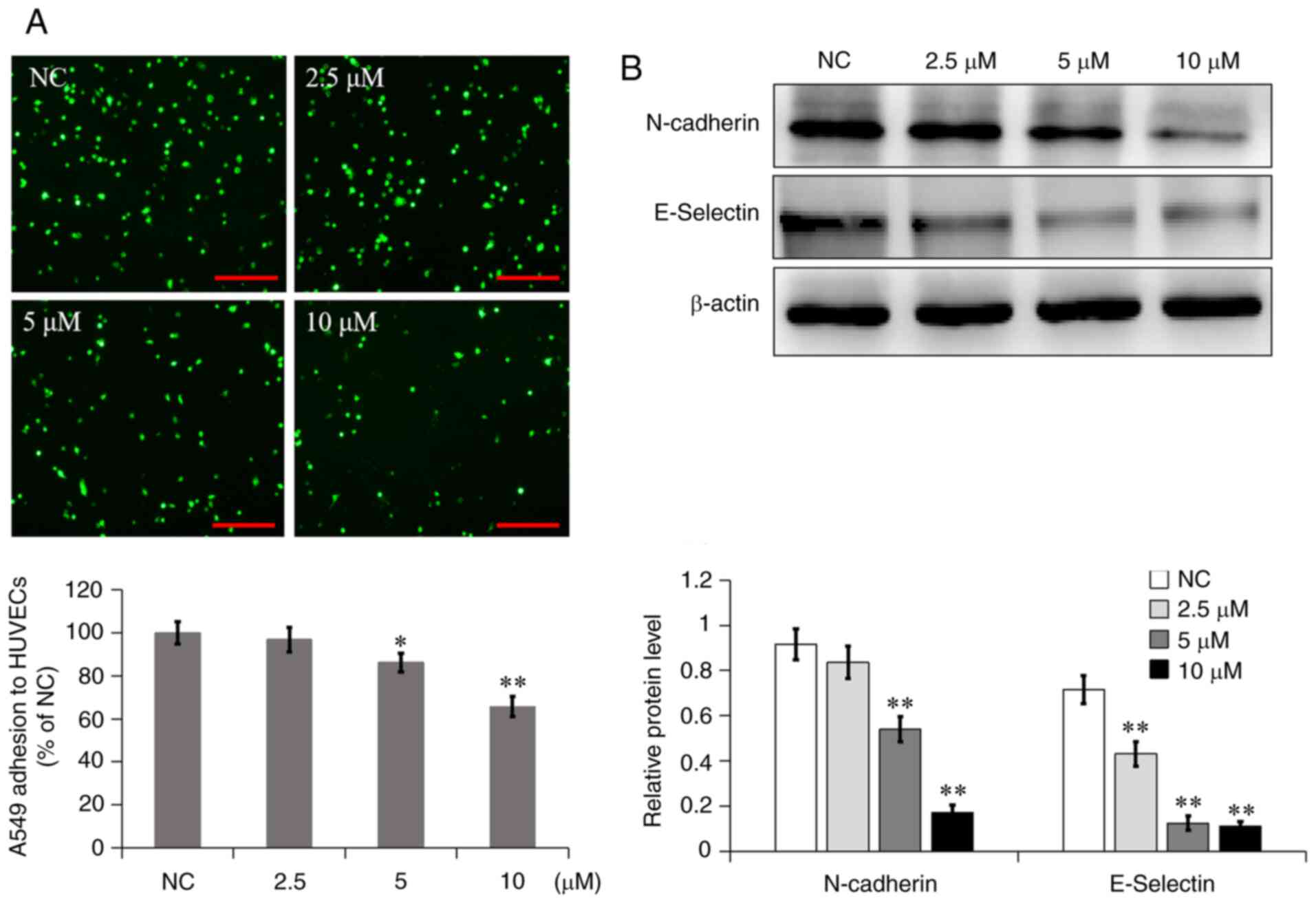
7a inhibits A549 cell adhesion to
HUVECs
The heterogeneous adhesion of tumor cells is a
pivotal step in tumor metastasis. A549 cells treated with different
concentrations of 7a were stained with DiO dye and then added to
24-well plates covered with a single layer of HUVECs, which could
mimic the adhesion of tumor cells to the lining of blood vessels.
The adhesion of A549 cells to HUVECs was examined following
incubation with different concentrations of 7a for 48 h. The
fluorescent cells above the HUVEC monolayer were A549 cells. The
results showed that 2.5, 5 and 10 µM 7a significantly reduced A549
cell adhesion to HUVECs by 3.12, 13.8 and 34.4%, respectively
(Fig. 5A). The results suggested
that 7a could inhibit the adhesion of A549 cells to HUVECs in a
concentration-dependent manner.
Next, the molecular mechanism through which 7a
regulates the adhesion of A549 cells to vascular endothelial cells
was explored. The relevant adhesion protein levels were detected
using western blot analysis. As shown in Fig. 5B, the expression of N-cadherin and
E-selectin was significantly reduced following treatment with
different concentrations of 7a. These two adhesion molecules have
been proven to be involved in adhesion between tumor cells and
HUVECs (10,15). Adhesion could then promote tumor
cell aggregation in the blood vessels, which could avoid anoikis
and increase the tumor metastasis potential. Therefore, 7a may
inhibit A549 cell metastasis by inhibiting the expression of
N-cadherin and E-selectin.
7a promotes A549 cell apoptosis
The effects of different concentrations of 7a on the
apoptosis of A549 cells were quantitatively determined via Annexin
V-FITC/PI double staining. Normal live, early apoptotic, necrotic
and late apoptotic cells could be distinguished by using flow
cytometry. As shown in Fig. 6A,
the apoptosis rate of A549 cells in the NC group was 5.4%. The
total apoptotic rate of A549 cells increased to 9.6, 16 and 28.2%
following treatment with 5, 10 and 20 µM 7a for 48 h, respectively.
To further explore the mechanism through which 7a induces A549 cell
apoptosis, the effect of 7a on the expression of mitochondrial
apoptosis-related proteins was detected via western blot analysis.
As shown in Fig. 6B, the
expression levels of the proapoptotic proteins Bax, cleaved poly
(ADP-ribose) polymerase (PARP) and caspase-3 were significantly
increased, while the expression of the antiapoptotic protein Bcl-2
was significantly decreased. This result indicated that the
promotion of A549 cell apoptosis by 7a may be associated with the
mitochondrial apoptosis pathway.
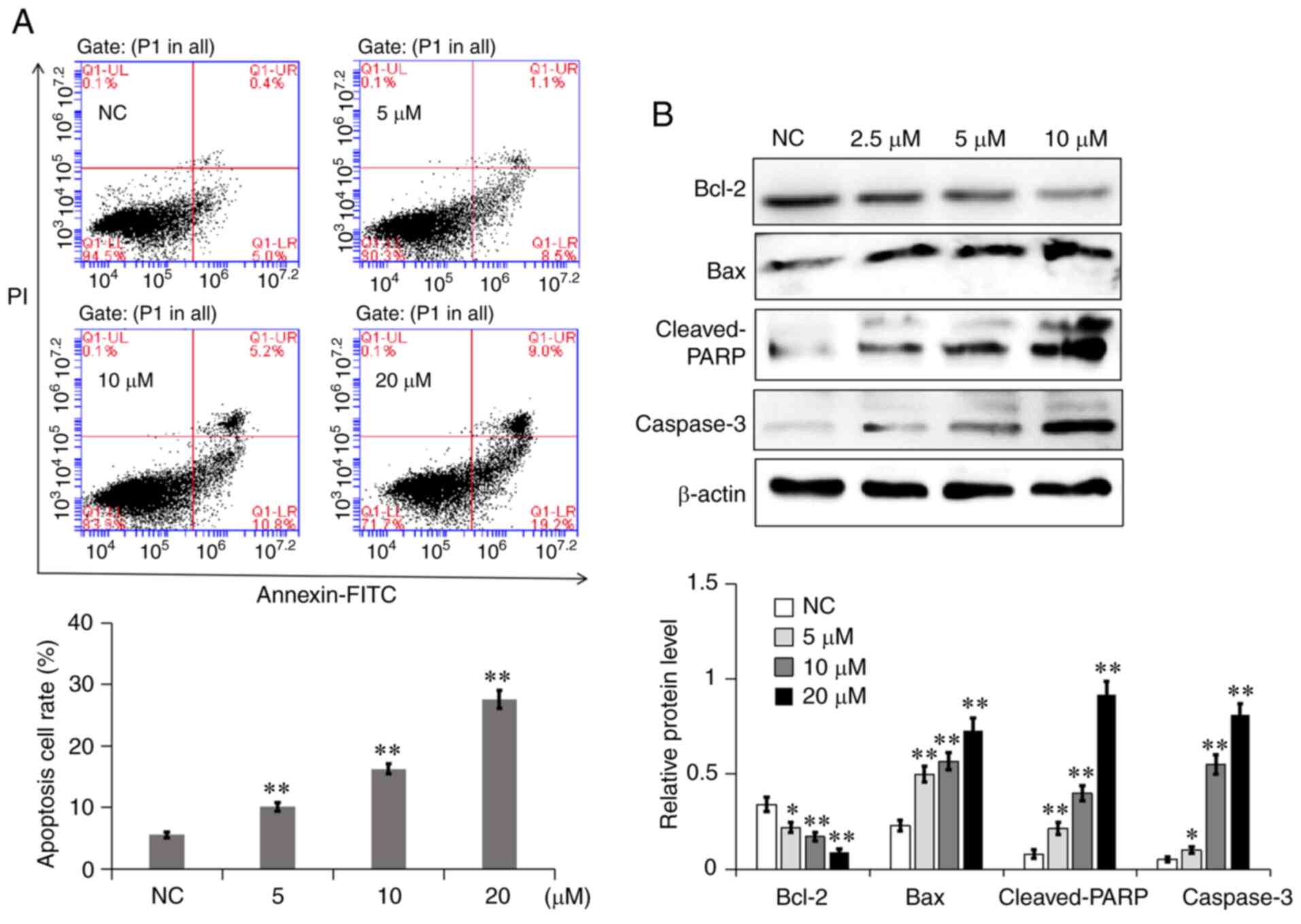 | Figure 6.7a induces A549 cell apoptosis
through the mitochondrial apoptosis pathway. (A) Detection of
apoptotic A549 cells after Annexin V/PI using flow cytometry,
following their treatment with different concentrations of 7a for
48 h. LL, LR, UR and UL represent normal, early apoptotic, late
apoptotic and necrotic cells, respectively. The percentage of
apoptotic cells (both early and late) was scored in three separate
experiments. (B) The expression of four apoptosis-related proteins
in A549 cells following 7a treatment for 48 h was measured using
western blot analysis. The semiquantitative expression levels of
the proteins were measured using exposure grey value. Data are
presented as the mean ± SD from three independent experiments.
*P<0.05 and **P<0.01 vs. NC. 7a, 2,2′,4′-trihydroxychalcone;
NC, negative control; LL, Lower left; LR, Lower right; UR, Upper
right; UL, Upper left; PARP, poly (ADP-ribose) polymerase. |
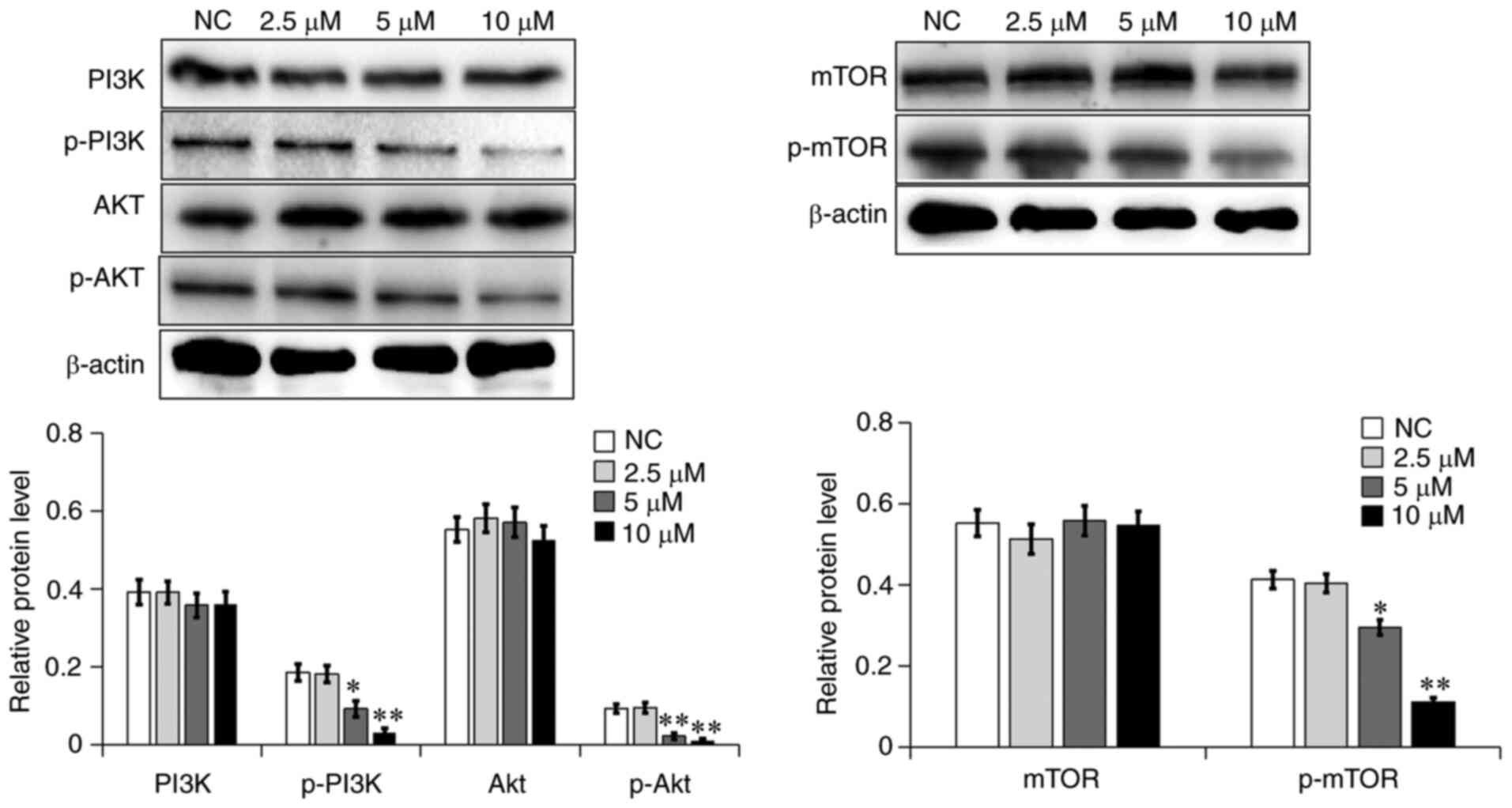
7a inhibits the activation of the
PI3K/AKT signaling pathway
A wide range of biological processes in a variety of
tumor cells is regulated by the PI3K/AKT signaling pathway,
including cell proliferation, apoptosis, survival, growth and
movement. The AKT and mTOR proteins have been reported to serve an
important role in tumor cell viability and metastasis (16). In the present study, the
aforementioned proteins were investigated in the PI3K/AKT signaling
pathway using western blot analysis. As shown in Fig. 7, the phosphorylation levels of
PI3K, AKT and mTOR in the 7a dosage group were significantly
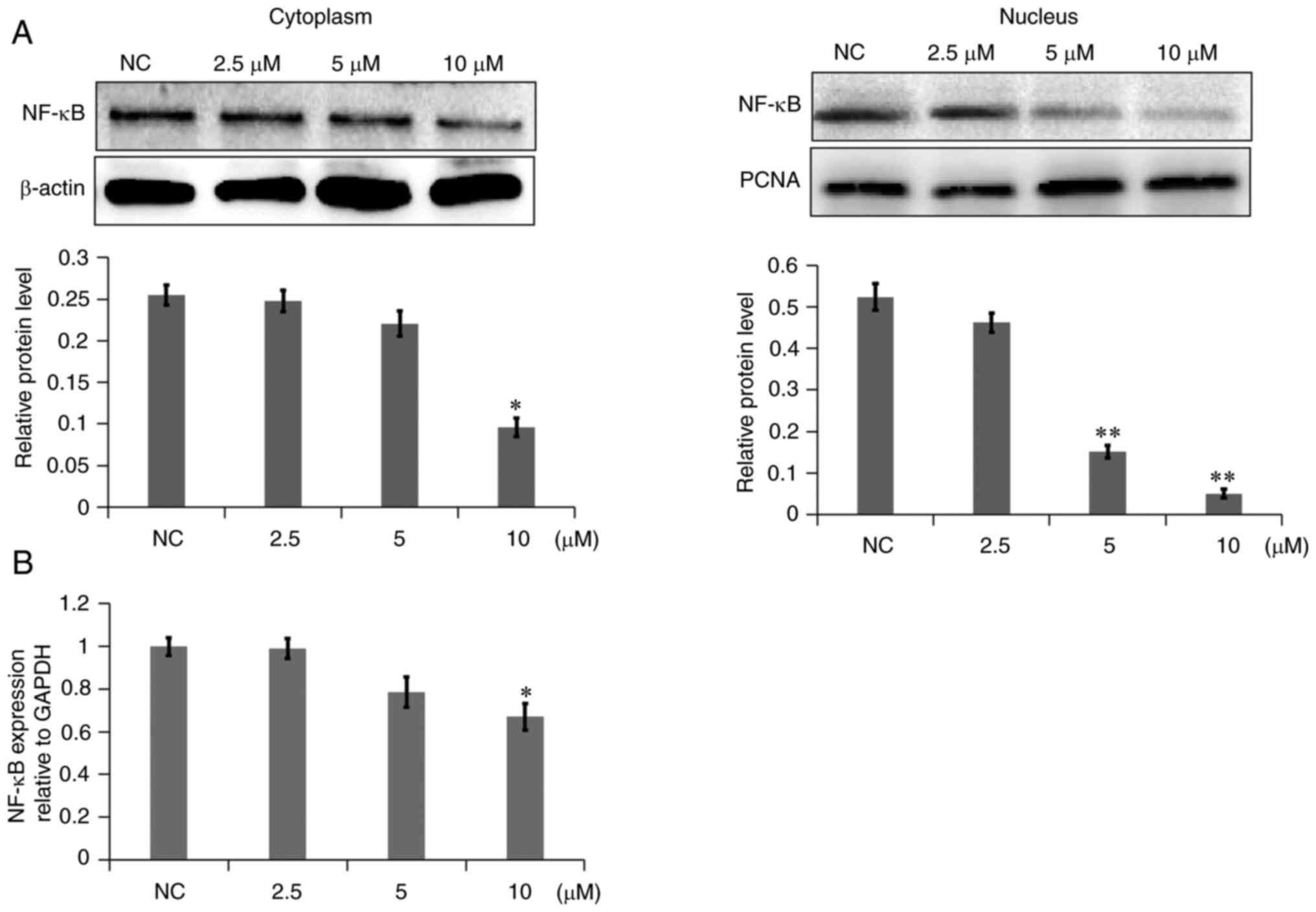
decreased in A549 cells. In addition, the NF-κB level was examined
in the cytoplasm and nucleus. As shown in Fig. 8A, NF-κB levels in the cytoplasm of
the 7a dosage group were slightly decreased, while NF-κB levels in
the nucleus were markedly decreased, as compared with those in the
NC group. The gene expression levels of NF-κB were also decreased
(Fig. 8B). These results
demonstrated that 7a may inhibit the PI3K/AKT/NF-κB signaling
pathway.
Discussion
Distant metastasis and high recurrence are the
leading causes of lung cancer-related mortality (3). Traditional chemotherapy drugs become
tolerated and have numerous serious adverse reactions, which have
become a challenge in the treatment of lung cancer. In recent
years, despite the application of various targeted drugs, the
global 5-year survival rate remains very low (3,17).
Therefore, more new compounds with antitumor effects and low
toxicity need to be explored. Antitumor effects of certain
hydroxychalcone compounds have captured the attention of several
researchers. For example, studies have shown that ISL can inhibit
the proliferation and induce the apoptosis of the lung
adenocarcinoma cell lines A549 and NCI-H1975 (8,18).
However, according to the results of previous studies, the
antitumor activity of ISL is relatively weak. Therefore, more
effective hydroxychalcone compounds need to be explored and
understanding the antitumor mechanisms of these compounds may help
identify new lung cancer treatments. The present study first aimed
to investigate the antitumor effect of 7a on the biological
behaviors of NSCLC cells. 7a was found to significantly inhibit the
proliferation, migration, invasion and heterogeneous adhesion of
A549 cells and induce cell apoptosis, indicating that 7a may have
an anticancer effect on the proliferation and metastasis of A549
cells.
First, the effect of 7a on the proliferation of A549
cells was studied and it was found that 7a strongly inhibited A549
cell growth. 7a significantly inhibited migration and invasion by
upregulating the expression of E-cadherin and downregulating the
expression of N-cadherin in A549 cells. E-cadherin and N-cadherin
are markers of epithelial-mesenchymal transition (EMT), which is a
pivotal mechanism involved in the modulation of cell migration and
invasion. However, in the process of EMT, the regulation of their
expression occurs oppositely; E-cadherin expression is
downregulated, while N-cadherin expression is often upregulated
(19). The decreased expression of
E-cadherin leads to the loss of E-cadherin-mediated isotype
adhesion between epithelial cells, driving the high metastatic
potential of tumor cells (12).
In addition, MMP-2 and MMP-9 expression was also
decreased slightly in the presence of 7a. MMPs have proteolytic
properties and can degrade the extracellular matrix (ECM) and
participate in various biological processes, particularly tumor
invasion and metastasis. During invasion and metastasis, tumor
cells first bind to the basement membrane and then release MMPs,
which degrade the basement membrane and ECM and finally enable
tumor cells to move to the periphery along the damaged site, thus
causing invasion and metastasis (20,21).
Among the MMPs, MMP-2 and MMP-9 serve a crucial role in tumor
invasion and metastasis. MMP-2 and MMP-9 not only degrades the
matrix of cells and promotes the invasion and metastasis of tumor
cells, but also participates in the occurrence and development of
tumors by promoting the formation of capillaries (20,22).
Thus, the present results suggested that 7a may suppress the
migration and invasion of A549 cells by inhibiting the process of
EMT and decreasing MMP-2/9 expression.
Heterogeneous tumor adhesion is also a pivotal step
in hematogenous cancer metastasis. Cellular adhesion molecules are
transmembrane proteins located on the cell membrane and the major
regulators of this process, regulating cell-cell or ECM
interactions. It has been reported that cell surface adhesion
molecules, such as N-cadherin, CD44 and E-selectin, can promote
adhesion between tumor cells and vascular endothelial cells, which
eventually prevented anoikis in the blood circulation (16,23–25).
In the present study, 7a was found to inhibit adhesion between A549
cells and HUVECs in a concentration-dependent manner. At the same
time, N-cadherin and E-selectin expression was decreased. In
addition, tumor cell angiogenesis was found to be closely
associated with tumor cell invasion and metastasis. Tumor cell
growth and proliferation is dysregulated and disorderly, which
disrupts the previous cell balance and requires the supply of
sufficient oxygen and nutrients (26). Therefore, blood vessel formation
serves an essential role in tumor metastasis. In an early study, a
novel tumor angiogenesis modality, VM, was first proposed to
describe the ability of highly aggressive melanomas to
dedifferentiate in order to acquire multiple cellular phenotypes
and endothelial-like characteristics (27). This process leads to the formation
of vascular-like structures of blood vessels and red blood cells,
which in turn leads to angiogenesis and the insertion of a
vascular-like matrix into a network of blood vessels that promotes
circulation. Subsequently, several studies have reported that some
malignant tumors, such as breast, ovarian and prostate cancer and
NSCLC, could also form mimetic blood vessels (28–30).
The present study found that 7a could inhibit the formation of VM
by reducing the expression of VEGF. Specifically, in the 10 µM
group, the tubular structure almost disappeared (Fig. 4). Various studies have shown that
VEGF is an important pro-VM factor (14,31).
VEGF is considered to promote VM by activating the PI3K/AKT
signaling pathway (14).
In addition to the metastasis process, 7a could also
promote A549 cell apoptosis in a concentration-dependent manner.
Apoptosis, a tightly regulated process of cell death, is associated
with organized stability, tumors and autoimmune and
neurodegenerative diseases (32).
7a was found to reduce Bcl-2 expression while increasing Bax,
cleaved PARP and caspase-3 expression. Although Bcl-2 and Bax
belong to the Bcl-2 gene family, they have the opposite effect on
tumor apoptosis (33). Bcl-2
inhibits the release of cytochrome c from mitochondria to the
cytoplasm, thereby inhibiting apoptosis, while the overexpression
of Bax can antagonize the protective effect of Bcl-2 and lead to
cell death (34). Increased
cytochrome c release in tumor cells can trigger the caspase cascade
and PARP is then cleaved; cleaved PARP is a substrate of caspase-3
in the semicarpal protease family, eventually resulting in tumor
cell apoptosis (35).
Several studies have shown that the PI3K/AKT/mTOR
signaling pathway serves a crucial role in the regulation of tumor
growth, apoptosis, metabolism, invasion and metastasis, as well as
angiogenesis (36–38). Therefore, the regulation of this
pathway has become of interest in the treatment of lung cancer. The
activation of the PI3K/AKT signaling pathway can lead to the
activation of several antiapoptotic proteins, such as Bcl-2, and
inhibit a series of proapoptotic proteins, such as Bax, caspase and
p53, thereby preventing apoptotic factors from being released from
mitochondria, which inhibits tumor cell apoptosis (39). The present study found that 7a
could significantly reduce the phosphorylation of key proteins of
the PI3K/AKT signaling pathway, such as p-AKT, p-PI3K and p-mTOR.
As reported by Chien et al (40), the inhibition of the PI3K/AKT
signaling pathway in cells following treatment with specific
inhibitors of PI3K (such as wortmannin) could reduce the protein
expression of MMP-2 and MMP-9. Another study reported that the
inhibition of the PI3K/AKT/p70S6K1 signaling pathway can
significantly reduce the expression of VEGF (41). NF-κB is a downstream signaling
molecule in the PI3K/AKT signaling pathway and its activation is
closely associated with a variety of pathologies, such as
inflammation, adhesion, invasion, metastasis and angiogenesis
(42). NF-κB is considered to
promote the formation of EMT by upregulating E-cadherin and
downregulating N-cadherin (43)
and simultaneously upregulating MMP-2/9 (44). Furthermore, the inhibition of NF-κB
activity by the adenovirus-mediated expression of a
dominant-negative NF-κB or by the proteasome inhibitor MG132
decreases VEGF mRNA in MDA-MB-231 cells (45). NF-κB, as a multifunctional
transcription factor, promotes gene transcription mainly by
entering the nucleus. The present study therefore examined the
NF-κB protein expression in the cytoplasm and nucleus separately.
7a treatment resulted in a marked decrease of NF-κB protein
expression in the nucleus and a slight decrease in the cytoplasm.
In addition, the RT-qPCR results showed that 7a also reduced the
mRNA expression of NF-κB. Therefore, 7a may inhibit A549 cell
activity via the PI3K/AKT/NF-κB signaling pathway.
In the present study, there was a noteworthy
phenomenon; while inhibiting A549 cell migration and invasion,
vasculogenic mimicry and adhesion to HUVECs, 7a might lead to
apoptosis at the same concentration. How to exclude the possibility
that these inhibitions of metastasis were independent of cell
death? First, during the HUVEC adhesion test, most apoptotic cells
were discarded after centrifugation and apoptotic cells could not
be stained with DiO. The same number of viable cells was selected
by cell counting and the cells were then added to a 24-well plate
filled with HUVEC monolayers. So the experimental results were
almost unaffected by apoptosis. During the migration, invasion and
angiogenic mimicry experiments, apoptosis might have some effect,
but the effect was not significant. The cell apoptosis rate was not
high when the concentration of 7a was ≤5 µM for 48 h. The apoptotic
rate of the 2.5 µm group was ~7.1% (the apoptosis result of this
concentration was obtained but not included in Fig. 6), which was only 1.7% higher than
that of the control group. The apoptosis rate of the 5 µM group was
9.6%, which was 4.2% higher than that of control group. This was
much lower than the inhibition rate of 7a on migration, invasion
and angiogenic mimicry. Second, the expression of related proteins
also showed a trend of change, which was consistent with the
experimental findings. These data indicated that 7a could indeed
inhibit the A549 cell metastasis.
However, there were still some shortcomings in the
present study. For example, it did not explore the molecular
mechanism differences between 7a and its isomer ISL. To the best of
the authors' knowledge, this was the first study to examine whether
7a had antitumor activity. So, it is not clear whether the
molecular mechanisms of 7a are different from those of ISL. Thus,
subsequent studies will comprise an in-depth exploration of
antitumor molecular mechanisms of 7a. In addition, there were no
in vivo experiments conducted and no use of a common
antitumor drug as a control to support our observations. Therefore,
further studies are required to verify the present findings.
In conclusion, the present results showed that 7a
may inhibit A549 lung cancer cell proliferation and metastasis and
induce apoptosis through the PI3K/AKT/NF-κB signaling pathway.
Thus, 7a may be a promising flavonoid with antitumor activities
that can inhibit the progression of lung cancer. Further research
is required to investigate the detailed mechanism through which 7a
inhibits lung cancer.
Acknowledgements
The authors acknowledge the Institute of Natural
Medicinal Chemistry, Qingdao University for providing flavonoid
7a.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81903872) and Natural Science
Foundation of Shandong Province (grant no. ZR2020MH418).
Availability of data and materials
All data generated or analyzed in the present study
are included in this published article.
Authors' contributions
PL conceived and designed the study. The experiments
were performed by JLS, ZQC and SWS. ZHS contributed to the writing
of the manuscript and designed the primers for RT-qPCR experiments.
The statistical analysis was performed by SHS and JY. JLS wrote the
manuscript. PL revised the manuscript. All authors have read and
approved the final manuscript. JLS, ZQC and PL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Affiliated Hospital of Qingdao
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quint LE, Tummala S, Brisson LJ, Francis
IR, Krupnick AS, Kazerooni EA, Iannettoni MD, Whyte RI and Orringer
MB: Distribution of distant metastases from newly diagnosed
non-small cell lung cancer. Ann Thorac Surg. 62:246–250. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaya J, Belinky PA and Aviram M:
Antioxidant constituents from licorice roots: Isolation, structure
elucidation and antioxidative capacity toward LDL oxidation. Free
Radic Biol Med. 23:302–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan SC, Chang YS, Wang JP, Chen SC and
Kuo SC: Three new flavonoids and antiallergic, anti-inflammatory
constituents from the heartwood of Dalbergia odorifera. Planta Med.
64:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto S, Aizu E, Jiang H, Nakadate T,
Kiyoto I, Wang JC and Kato R: The potent anti-tumor-promoting agent
isoliquiritigenin. Carcinogenesis. 12:317–323. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cuendet M, Guo J, Luo Y, Chen S, Oteham
CP, Moon RC, van Breemen RB, Marler LE and Pezzuto JM: Cancer
chemopreventive activity and metabolism of isoliquiritigenin, a
compound found in licorice. Cancer Prev Res (Phila). 3:221–232.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian T, Sun J, Wang J, Liu Y and Liu H:
Isoliquiritigenin inhibits cell proliferation and migration through
the PI3K/AKT signaling pathway in A549 lung cancer cells. Oncol
Lett. 16:6133–6139. 2018.PubMed/NCBI
|
|
9
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andrews N Yu LG, Zhao Q, McKean D,
Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J,
Kasai K and Rhodes JM: Galectin-3 interaction with
thomsen-friedenreich disaccharide on cancer-associated MUC1 causes
increased cancer cell endothelial adhesion. J Biol Chem.
282:773–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–11108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herzig M, Savarese F, Novatchkova M, Semb
H and Christofori G: Tumor progression induced by the loss of
E-cadherin independent of beta-catenin/Tcf-mediated wnt signaling.
Oncogene. 26:2290–2298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Passalidou E, Trivella M, Singh N,
Ferguson M, Hu J, Cesario A, Granone P, Nicholson AG, Goldstraw P,
Ratcliffe C, et al: Vascular phenotype in angiogenic and
non-angiogenic lung non-small cell carcinomas. Br J Cancer.
86:244–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Zong Y, Gao Y, Sun X, Zhao H, Luo W
and Jia S: VEGF induce vasculogenic mimicry of choroidal melanoma
through the PI3k signal pathway. Biomed Res Int. 2019:39091022019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Z, Hao Z, Xin M, Yu L, Wang L, Zhang
Y, Zhang X and Guo X: Endogenous and exogenous galectin-3 promote
the adhesion of tumor cells with low expression of MUC1 to HUVECs
through upregulation of N-cadherin and CD44. Lab Invest.
98:1642–1656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu G, Zhang W, Bertram P, Zheng XF and
McLeod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
17
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung SK, Lee MH, Lim DY, Kim JE, Singh P,
Lee SY, Jeong CH, Lim TG, Chen H, Chi YI, et al: Isoliquiritigenin
induces apoptosis and inhibits xenograft tumor growth of human lung
cancer cells by targeting both wild type and L858R/T790M mutant
EGFR. J Biol Chem. 289:35839–35848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Merchant N, Nagaraju GP, Rajitha B,
Lammata S, Jella KK, Buchwald ZS, Lakka SS and Ali AN: Matrix
metalloproteinases: Their functional role in lung cancer.
Carcinogenesis. 38:766–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thi MU, Trocmé C, Montmasson MP, Fanchon
E, Toussaint B and Tracqui P: Investigating metalloproteinases
MMP-2 and MMP-9 mechanosensitivity to feedback loops involved in
the regulation of in vitro angiogenesis by endogenous mechanical
stresses. Acta Biotheor. 60:21–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makrilia N, Kollias A, Manolopoulos L and
Syrigos K: Cell adhesion molecules: Role and clinical significance
in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Q, Barclay M, Hilkens J, Guo X,
Barrow H, Rhodes JM and Yu LG: Interaction between circulating
galectin-3 and cancer-associated MUC1 enhances tumour cell
homotypic aggregation and prevents anoikis. Mol Cancer. 9:1542010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Q, Guo X, Nash GB, Stone PC, Hilkens
J, Rhodes JM and Yu LG: Circulating galectin-3 promotes metastasis
by modifying MUC1 localization on cancer cell surface. Cancer Res.
69:6799–6806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Popper HH: Progression and metastasis of
lung cancer. Cancer Metastasis Rev. 35:75–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shirakawa K, Tsuda H, Heike Y, Kato K,
Asada R, Inomata M, Sasaki H, Kasumi F, Yoshimoto M, Iwanaga T, et
al: Absence of endothelial cells, central necrosis and fibrosis are
associated with aggressive inflammatory breast cancer. Cancer Res.
61:445–451. 2001.PubMed/NCBI
|
|
29
|
Sood AK, Seftor EA, Fletcher MS, Gardner
LM, Heidger PM, Buller RE, Seftor RE and Hendrix MJ: Molecular
determinants of ovarian cancer plasticity. Am J Pathol.
158:1279–1288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma N, Seftor RE, Seftor EA, Gruman LM,
Heidger PM Jr, Cohen MB, Lubaroff DM and Hendrix MJ: Prostatic
tumor cell plasticity involves cooperative interactions of distinct
phenotypic subpopulations: Role in vasculogenic mimicry. Prostate.
50:189–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mei J, Gao Y, Zhang L, Cai X, Qian Z,
Huang H and Huang W: VEGF-siRNA silencing induces apoptosis,
inhibits proliferation and suppresses vasculogenic mimicry in
osteosarcoma in vitro. Exp Oncol. 30:29–34. 2008.PubMed/NCBI
|
|
32
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown R: The bcl-2 family of proteins. Br
Med Bull. 53:466–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knight T, Luedtke D, Edwards H, Taub JW
and Ge Y: A delicate balance-The BCL-2 family and its role in
apoptosis, oncogenesis and cancer therapeutics. Biochem Pharmacol.
162:250–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia J, Dai L, Wang L and Zhu J: Ganoderic
acid DM induces autophagic apoptosis in non-small cell lung cancer
cells by inhibiting the PI3K/Akt/mTOR activity. Chem Biol Interact.
316:1089322020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Z, Zhu J, Huang Y, Li W and Cheng H:
MYO18B promotes hepatocellular carcinoma progression by activating
PI3K/AKT/mTOR signaling pathway. Diagn Pathol. 13:852018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krencz I, Sztankovics D, Danko T,
Sebestyen A and Khoor A: Progression and metastasis of small cell
lung carcinoma: The role of the PI3K/Akt/mTOR pathway and metabolic
alterations. Cancer Metastasis Rev. 27:doi:
10.1007/s10555-021-10012-4. 2021.PubMed/NCBI
|
|
39
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through down-regulation of p53-dependent Bcl-2
expression and inhibition of Mcl-1 protein stability. PLoS One.
9:e955882014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong XS, Zheng JZ, Reed E and Jiang BH:
SU5416 inhibited VEGF and HIF-1alpha expression through the
PI3K/AKT/p70S6K1 signaling pathway. Biochem Biophys Res Commun.
324:471–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou P, Wang C, Hu Z, Chen W, Qi W and Li
A: Genistein induces apoptosis of colon cancer cells by reversal of
epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin
pathway. BMC Cancer. 17:8132017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Lau GKK, Chen L, Dong SS, Lan HY,
Huang XR, Li Y, Luk JM, Yuan YF and Guan XY: Interleukin 17A
promotes hepatocellular carcinoma metastasis via NF-κB induced
matrix metalloproteinases 2 and 9 expression. PLoS One.
6:e218162011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shibata A, Nagaya T, Imai T, Funahashi H,
Nakao A and Seo H: Inhibition of NF-kappaB activity decreases the
VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast
Cancer Res Treat. 73:237–243. 2002. View Article : Google Scholar : PubMed/NCBI
|