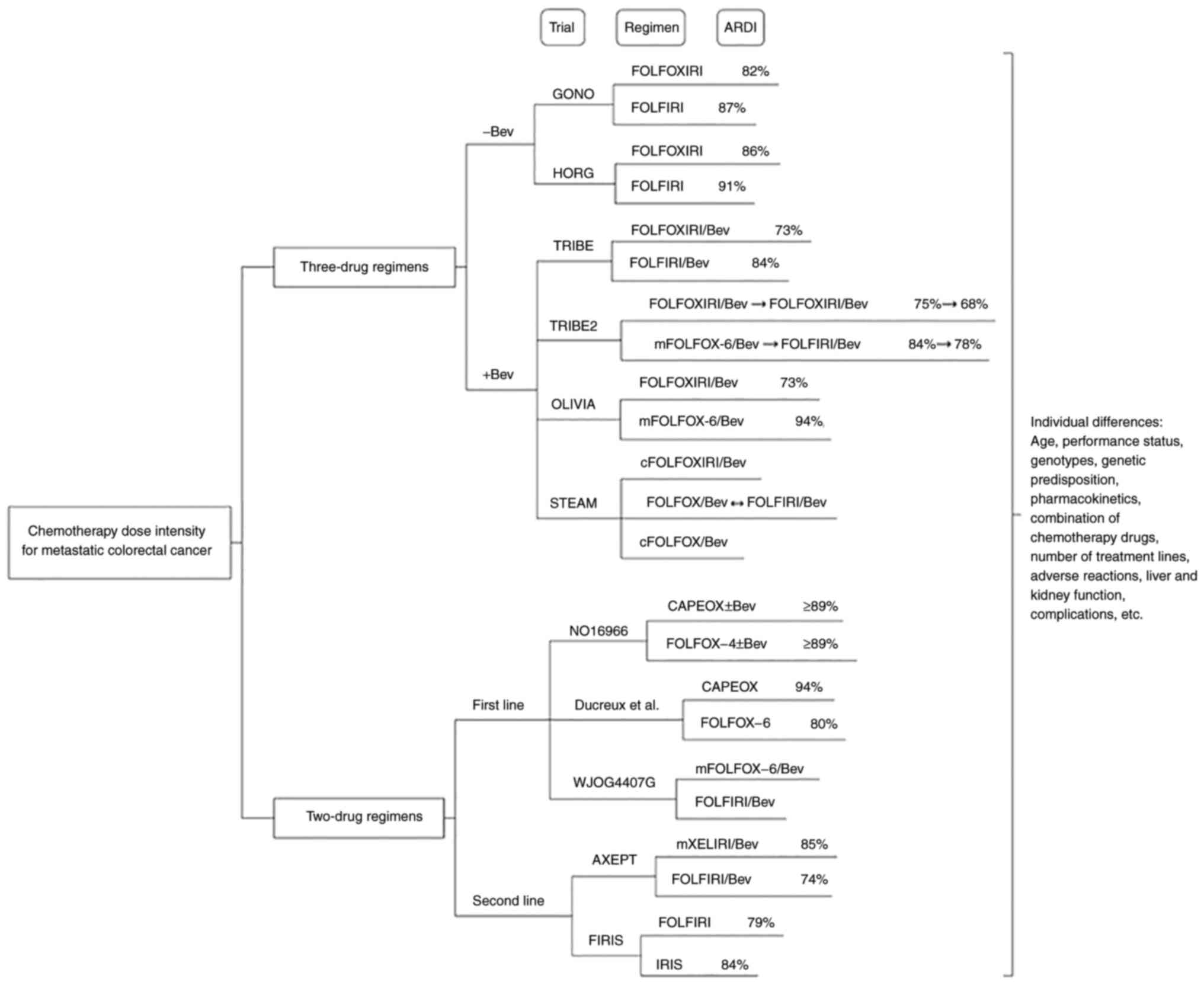
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin J, Bai Z, Zhang J, Zheng Z, Yao H, Ye
P, Li J, Gao X and Zhang Z: Burden of colorectal cancer in China,
1990–2017: Findings from the Global Burden of Disease Study 2017.
Chin J Cancer Res. 31:489–498. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antoniotti C, Borelli B, Rossini D,
Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F,
Tamberi S, Corallo S, et al: AtezoTRIBE: A randomised phase II
study of FOLFOXIRI plus bevacizumab alone or in combination with
atezolizumab as initial therapy for patients with unresectable
metastatic colorectal cancer. BMC Cancer. 20:6832020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das S, Ciombor KK, Haraldsdottir S and
Goldberg RM: Promising new agents for colorectal cancer. Curr Treat
Options Oncol. 19:292018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nightingale G, Schwartz R, Kachur E, Dixon
BN, Cote C, Barlow A, Barlow B and Medina P: Clinical pharmacology
of oncology agents in older adults: A comprehensive review of how
chronologic and functional age can influence treatment-related
effects. J Geriatr Oncol. 10:4–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frei E, Elias A, Wheeler C, Richardson P
and Hryniuk W: The relationship between high-dose treatment and
combination chemotherapy: The concept of summation dose intensity.
Clin Cancer Res. 4:2027–2037. 1998.PubMed/NCBI
|
|
8
|
Lyman GH: Impact of chemotherapy dose
intensity on cancer patient outcomes. J Natl Compr Canc Netw.
7:99–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dodwell DJ, Gurney H and Thatcher N: Dose
intensity in cancer chemotherapy. Br J Cancer. 61:789–794. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ariyoshi Y and Ogawa M: Dose intensity in
cancer chemotherapy (including high dose chemotherapy). Gan To
Kagaku Ryoho. 21:2699–2707. 1994.(In Japanese). PubMed/NCBI
|
|
11
|
Alberto P: Dose intensity in cancer
chemotherapy: Definition, average relative dose intensity and
effective dose intensity. Bull Cancer. 82 (Suppl 1):3s–8s. 1995.(In
French). PubMed/NCBI
|
|
12
|
Martin JH and Dimmitt S: The rationale of
dose-response curves in selecting cancer drug dosing. Br J Clin
Pharmacol. 85:2198–2204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paci A, Veal G, Bardin C, Levêque D,
Widmer N, Beijnen J, Astier A and Chatelut E: Review of therapeutic
drug monitoring of anticancer drugs part 1-cytotoxics. Eur J
Cancer. 50:2010–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erku D, Schneider J and Scuffham P: A
framework for economic evaluation of therapeutic drug
monitoring-guided dosing in oncology. Pharmacol Res Perspect.
9:e008622021. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lakkunarajah S, Breadner DA, Zhang H,
Yamanaka E, Warner A and Welch S: The influence of adjuvant
chemotherapy dose intensity on five-year outcomes in resected colon
cancer: A single Centre retrospective analysis. Curr Oncol.
28:4031–4041. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozer H and Diasio RB: Perspectives in the
treatment of colorectal cancer. Semin Oncol. 31:14–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Phase III trial of infusional fluorouracil,
leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with
infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as
first-line treatment for metastatic colorectal cancer: The Gruppo
Oncologico Nord Ovest. J Clin Oncol. 25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong C, Ding Y, Weng S, Li G, Huang Y, Hu
H, Zhang Z, Zhang S and Yuan Y: Update in version 2021 of CSCO
guidelines for colorectal cancer from version 2020. Chin J Cancer
Res. 33:302–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Messersmith WA: NCCN Guidelines updates:
Management of metastatic colorectal cancer. J Natl Compr Canc Netw.
17:599–601. 2019.PubMed/NCBI
|
|
20
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum.
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Souglakos J, Androulakis N, Syrigos K,
Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S,
Kouroussis Ch, Vamvakas L, et al: FOLFOXIRI (folinic acid,
5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic
acid, 5-fluorouracil and irinotecan) as first-line treatment in
metastatic colorectal cancer (MCC): A multicentre randomised phase
III trial from the Hellenic Oncology Research Group (HORG). Br J
Cancer. 94:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrarotto R and Hoff PM: Antiangiogenic
drugs for colorectal cancer: Exploring new possibilities. Clin
Colorectal Cancer. 12:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cremolini C, Antoniotti C, Rossini D,
Lonardi S, Loupakis F, Pietrantonio F, Bordonaro R, Latiano TP,
Tamburini E, Santini D, et al: Upfront FOLFOXIRI plus bevacizumab
and reintroduction after progression versus mFOLFOX6 plus
bevacizumab followed by FOLFIRI plus bevacizumab in the treatment
of patients with metastatic colorectal cancer (TRIBE2): A
multicentre, open-label, phase 3, randomised, controlled trial.
Lancet Oncol. 21:497–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gruenberger T, Bridgewater J, Chau I,
García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F,
Waterkamp D and Adam R: Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in
patients with initially unresectable liver metastases from
colorectal cancer: The OLIVIA multinational randomised phase II
trial. Ann Oncol. 26:702–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hurwitz HI, Tan BR, Reeves JA, Xiong H,
Somer B, Lenz HJ, Hochster HS, Scappaticci F, Palma JF, Price R, et
al: Phase II randomized trial of sequential or concurrent
FOLFOXIRI-bevacizumab versus FOLFOX-bevacizumab for metastatic
colorectal cancer (STEAM). Oncologist. 24:921–932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brouquet A and Nordlinger B: Neoadjuvant
and adjuvant therapy in relation to surgery for colorectal liver
metastases. Scand J Gastroenterol. 47:286–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F and
Saltz L: XELOX vs FOLFOX-4 as first-line therapy for metastatic
colorectal cancer: NO16966 updated results. Br J Cancer. 105:58–64.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ducreux M, Bennouna J, Hebbar M, Ychou M,
Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L,
et al: Capecitabine plus oxaliplatin (XELOX) versus
5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line
treatment for metastatic colorectal cancer. Int J Cancer.
128:682–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimizu T, Satoh T, Tamura K, Ozaki T,
Okamoto I, Fukuoka M and Nakagawa K:
Oxaliplatin/fluorouracil/leucovorin (FOLFOX4 and modified FOLFOX6)
in patients with refractory or advanced colorectal cancer:
Post-approval Japanese population experience. Int J Clin Oncol.
12:218–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buchler T, Pavlik T, Melichar B, Bortlicek
Z, Usiakova Z, Dusek L, Kiss I, Kohoutek M, Benesova V, Vyzula R,
et al: Bevacizumab with 5-fluorouracil, leucovorin, and oxaliplatin
versus bevacizumab with capecitabine and oxaliplatin for metastatic
colorectal carcinoma: Results of a large registry-based cohort
analysis. BMC Cancer. 14:3232014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petrioli R, Francini E, Cherri S, Torre P,
Fiaschi AI, Miano ST, Marrelli D, Rovello F and Francini G:
Capecitabine plus oxaliplatin and bevacizumab, followed by
maintenance treatment with capecitabine and bevacizumab for
patients aged >75 years with metastatic colorectal cancer. Clin
Colorectal Cancer. 17:e663–e669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neupane R, Boddu SHS, Abou-Dahech MS,
Bachu RD, Terrero D, Babu RJ and Tiwari AK: Transdermal delivery of
chemotherapeutics: Strategies, requirements, and opportunities.
Pharmaceutics. 13:9602021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamazaki K, Nagase M, Tamagawa H, Ueda S,
Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T,
et al: Randomized phase III study of bevacizumab plus FOLFIRI and
bevacizumab plus mFOLFOX6 as first-line treatment for patients with
metastatic colorectal cancer (WJOG4407G). Ann Oncol. 27:1539–1546.
2016. View Article : Google Scholar : View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishi T, Hamamoto Y, Warita E, Miyamoto J,
Akutsu N, Yamanaka Y, Nagase M and Fujii H: Retrospective analysis
of the international standard-dose FOLFIRI (plus bevacizumab)
regimen in Japanese patients with unresectable advanced or
recurrent colorectal carcinoma. Int J Clin Oncol. 16:488–493. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Parikh AR, Lee FC, Yau L, Koh H, Knost J,
Mitchell EP, Bosanac I, Choong N, Scappaticci F, Mancao C, et al:
MAVERICC, a Randomized, Biomarker-stratified, Phase II study of
mFOLFOX6-bevacizumab versus FOLFIRI-bevacizumab as first-line
chemotherapy in metastatic colorectal cancer. Clin Cancer Res.
25:2988–2995. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu RH, Muro K, Morita S, Iwasa S, Han SW,
Wang W, Kotaka M, Nakamura M, Ahn JB, Deng YH, et al: Modified
XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin,
fluorouracil, and irinotecan), both either with or without
bevacizumab, as second-line therapy for metastatic colorectal
cancer (AXEPT): A multicentre, open-label, randomised,
non-inferiority, phase 3 trial. Lancet Oncol. 19:660–671. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: A randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fuchs CS, Marshall J, Mitchell E,
Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D,
Soufi-Mahjoubi R, Wang B and Barrueco J: Randomized, controlled
trial of irinotecan plus infusional, bolus, or oral
fluoropyrimidines in first-line treatment of metastatic colorectal
cancer: Results from the BICC-C study. J Clin Oncol. 25:4779–4786.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Köhne CH, De Greve J, Hartmann JT, Lang I,
Vergauwe P, Becker K, Braumann D, Joosens E, Müller L, Janssens J,
et al: Irinotecan combined with infusional 5-fluorouracil/folinic
acid or capecitabine plus celecoxib or placebo in the first-line
treatment of patients with metastatic colorectal cancer. EORTC
study 40015. Ann Oncol. 19:920–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Q, Zhang P, Wang X, Zhang M, Liao W and
Li Q: Cost-effectiveness of capecitabine + irinotecan versus
leucovorin + Fluorouracil + irinotecan in the second-line treatment
of metastatic colorectal cancer in China. Clin Ther.
42:2148–2158.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hisada H, Takahashi Y, Kubota M, Shimura
H, Itobayashi E, Shimura K and Nakamura A: Clinical and therapeutic
features and prognostic factors of metastatic colorectal cancer
over age 80: A retrospective study. BMC Gastroenterol. 21:1992021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ward BW and Schiller JS: Prevalence of
multiple chronic conditions among US adults: Estimates from the
National Health Interview Survey, 2010. Prev Chronic Dis.
10:E652013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S, Yang T, Qiang W, Shen A, Zhao Z,
Yang H and Liu X: The prevalence of frailty among breast cancer
patients: A systematic review and meta-analysis. Support Care
Cancer. 30:2993–3006. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vargas-Román K, Tovar-Gálvez MI,
Liñán-González A, Cañadas de la Fuente GA, de la Fuente-Solana EI
and Díaz-Rodríguez L: Coping strategies in elderly colorectal
cancer patients. Cancers (Basel). 14:6082022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Marmorino F, Rossini D, Lonardi S, Moretto
R, Zucchelli G, Aprile G, Dell'Aquila E, Ratti M, Bergamo F, Masi
G, et al: Impact of age and gender on the safety and efficacy of
chemotherapy plus bevacizumab in metastatic colorectal cancer: A
pooled analysis of TRIBE and TRIBE2 studies. Ann Oncol.
30:1969–1977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang IG, Kwon M, Kim JW, Kim SH, Lee YG,
Kim JY, Koh SJ, Ko YH, Shin SH, Hong S, et al: Prevalence and
predictive factors for upfront dose reduction of the first cycle of
first-line chemotherapy in older adults with metastatic solid
cancer: Korean Cancer Study Group (KCSG) multicenter study. Cancers
(Basel). 13:3312021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seymour MT, Thompson LC, Wasan HS,
Middleton G, Brewster AE, Shepherd SF, O'Mahony MS, Maughan TS,
Parmar M, Langley RE, et al: Chemotherapy options in elderly and
frail patients with metastatic colorectal cancer (MRC FOCUS2): An
open-label, randomised factorial trial. Lancet. 377:1749–1759.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim JW, Lee KW, Kim KP, Lee JH, Hong YS,
Kim JE, Kim SY, Park SR, Nam BH, Cho SH, et al: Efficacy and safety
of FOLFIRI regimen in elderly versus nonelderly patients with
metastatic colorectal or gastric cancer. Oncologist. 22:293–303.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Folprecht G, Seymour MT, Saltz L,
Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E,
Rougier P, Mitry E, et al: Irinotecan/fluorouracil combination in
first-line therapy of older and younger patients with metastatic
colorectal cancer: Combined analysis of 2,691 patients in
randomized controlled trials. J Clin Oncol. 26:1443–1451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sargent DJ, Köhne CH, Sanoff HK, Bot BM,
Seymour MT, de Gramont A, Porschen R, Saltz LB, Rougier P,
Tournigand C, et al: Pooled safety and efficacy analysis examining
the effect of performance status on outcomes in nine first-line
treatment trials using individual data from patients with
metastatic colorectal cancer. J Clin Oncol. 27:1948–1955. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cremolini C, Antoniotti C, Lonardi S,
Rossini D and Falcone A: Updated results of TRIBE2, a phase III,
randomized strategy study by GONO in the 1st- and 2nd-line
treatment of unresectable mCRC. J Clin Oncol. 37:3508. 2019.
View Article : Google Scholar
|
|
58
|
Haller DG, Cassidy J, Clarke SJ,
Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB,
Schmoll HJ, Allegra C, et al: Potential regional differences for
the tolerability profiles of fluoropyrimidines. J Clin Oncol.
26:2118–2123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marcuello E, Páez D, Paré L, Salazar J,
Sebio A, del Rio E and Baiget M: A genotype-directed phase I–IV
dose-finding study of irinotecan in combination with
fluorouracil/leucovorin as first-line treatment in advanced
colorectal cancer. Br J Cancer. 105:53–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsunedomi R, Hazama S, Fujita Y, Okayama
N, Kanekiyo S, Inoue Y, Yoshino S, Yamasaki T, Suehiro Y, Oba K, et
al: A novel system for predicting the toxicity of irinotecan based
on statistical pattern recognition with UGT1A genotypes. Int J
Oncol. 45:1381–1390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen Y, Wang Y, Shi Y and Dai G: Timing of
chemotherapy-induced neutropenia predicts prognosis in metastatic
colon cancer patients: A retrospective study in mFOLFOX6-treated
patients. BMC Cancer. 17:2422017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abdel-Rahman O and Karachiwala H: Impact
of age on toxicity and efficacy of 5-FU-based combination
chemotherapy among patients with metastatic colorectal cancer; a
pooled analysis of five randomized trials. Int J Colorectal Dis.
34:1741–1747. 2019. View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schraa SJ, Frerichs KA, Agterof MJ,
Hunting JCB, Los M and de Jong PC: Relative dose intensity as a
proxy measure of quality and prognosis in adjuvant chemotherapy for
breast cancer in daily clinical practice. Eur J Cancer. 79:152–157.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kepka L and Socha J: Dose and
fractionation schedules in radiotherapy for non-small cell lung
cancer. Transl Lung Cancer Res. 10:1969–1982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sallustio BC and Boddy AV: Is there scope
for better individualisation of anthracycline cancer chemotherapy?
Br J Clin Pharmacol. 87:295–305. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bridoux F, Cockwell P, Glezerman I,
Gutgarts V, Hogan JJ, Jhaveri KD, Joly F, Nasr SH, Sawinski D and
Leung N: Kidney injury and disease in patients with haematological
malignancies. Nat Rev Nephrol. 17:386–401. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pellacani C and Eleftheriou G:
Neurotoxicity of antineoplastic drugs: Mechanisms, susceptibility,
and neuroprotective strategies. Adv Med Sci. 65:265–285. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kurniali PC, Hrinczenko B and Al-Janadi A:
Management of locally advanced and metastatic colon cancer in
elderly patients. World J Gastroenterol. 20:1910–1922. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nightingale G, Schwartz R, Kachur E, Dixon
BN, Cote C, Barlow A, Barlow B and Medina P: Clinical pharmacology
of oncology agents in older adults: A comprehensive review of how
chronologic and functional age can influence treatment-related
effects. J Geriatr Oncol. 10:4–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lue JK and O'Connor OA: A perspective on
improving the R-CHOP regimen: From Mega-CHOP to ROBUST R-CHOP, the
PHOENIX is yet to rise. Lancet Haematol. 7:e838–e850. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hotchkiss KM and Sampson JH: Temozolomide
treatment outcomes and immunotherapy efficacy in brain tumor. J
Neurooncol. 151:55–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silvestris N, Argentiero A, Natalicchio A,
D'Oronzo S, Beretta GD, Acquati S, Adinolfi V, Di Bartolo P, Danesi
R, Faggiano A, et al: Antineoplastic dosing in overweight and obese
cancer patients: An Associazione Italiana Oncologia Medica
(AIOM)/Associazione Medici Diabetologi (AMD)/Societa Italiana
Endocrinologia (SIE)/Societa Italiana Farmacologia (SIF)
multidisciplinary consensus position paper. ESMO Open.
6:1001532021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kojima T, Mizokami F and Akishita M:
Geriatric management of older patients with multimorbidity. Geriatr
Gerontol Int. 20:1105–1111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Swinson D, Hall P, Seymour M, Lord S,
Marshall H, Ruddock S, Cairns D, Waters J, Wadsley J, Falk S, et
al: Optimising chemotherapy for frail and/or elderly patients with
advanced gastroesophageal cancer (AGOAC): The GO2 phase III trial.
J Geriatr Oncol. 10 (Suppl 1):S82019. View Article : Google Scholar
|
|
75
|
Xu X, Wu Y, Qian X, Wang Y, Wang J, Li J,
Li Y and Zhang Z: Nanomedicine strategies to circumvent intratumor
extracellular matrix barriers for cancer therapy. Adv Healthc
Mater. 11:e21014282022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Handa M, Beg S, Shukla R, Barkat MA,
Choudhry H and Singh K: Recent advances in lipid-engineered
multifunctional nanophytomedicines for cancer targeting. J Control
Release. 340:48–59. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Abadi AJ, Mirzaei S, Mahabady MK, Hashemi
F, Zabolian A, Hashemi F, Raee P, Aghamiri S, Ashrafizadeh M, Aref
AR, et al: Curcumin and its derivatives in cancer therapy:
Potentiating antitumor activity of cisplatin and reducing side
effects. Phytother Res. 36:189–213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen M, May BH, Zhou IW, Xue CC and Zhang
AL: FOLFOX 4 combined with herbal medicine for advanced colorectal
cancer: A systematic review. Phytother Res. 28:976–991. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010. View Article : Google Scholar : PubMed/NCBI
|