Colorectal cancer is a highly prevalent digestive
tract malignancy with the third highest incidence and the second
highest mortality worldwide in cancer according to global cancer
statistics in 2018 (1). The
current major therapeutic modalities include surgery, chemotherapy,
targeted therapy, radiotherapy and immunotherapy (2). The majority of the patients with
metastatic colorectal cancer (mCRC) lose the opportunity of
resection surgery at the initial diagnosis, while several problems
have been encountered in the selection of the treatment population
and the course of disease in radiotherapy, targeted therapy and
immunotherapy (3,4). For example, radiotherapy is used in
some patients with rectal cancer, and targeted therapy is used in
patients with potential targets, such as cetuximab in RAS/BRAF
wild-type patients. PD-1 monoclonal antibody can be used for
patients with MSI-H or dMMR in first-line therapy. Chemotherapy is
the main treatment for patients with mCRC due to its broad
applicability (4). Patients with
advanced malignant tumors experience complications with advanced
age, cardiovascular and cerebrovascular diseases, abnormal liver
and kidney function and tumor progression (5). In clinical practice, the dose of
chemotherapeutic drugs is adjusted according to the actual
situation as described above (for example, age or cardiovascular
diseases), resulting in the occurrence of dose reduction, delay of
the set cycle and adjustment of the chemotherapeutic regimen, which
render the clinical medication incapable to match the standard
scheme and dose of the guidelines (6).
The two most important variables in the regulation
of the efficacy of the antitumor chemotherapeutic drugs are the
adjustments of their combination and dose, which involve multiple
dose-related concepts, as seen below (7). It has been reported that the dose
intensity (DI) of chemotherapy can be further altered by adjusting
the time interval of administration or the dosage (8,9). The
DI of chemotherapy refers to the dose of chemotherapeutic drugs
received by the patients per unit time. It is independent from the
route of administration and is calculated by the dose of
chemotherapeutic drugs received per square meter of body surface
every week (7). The average
relative DI (ARDI) refers to the average value of the actual DI of
each chemotherapeutic drug, which is calculated as a percentage of
the standard DI in the combined chemotherapy regimen (10,11).
Pharmacodynamic evaluation involves the estimation of the
dose-effect curve and suggests that the higher the effective drug
dose, the higher the improvement in the antitumor effect and the
stronger the toxicity (12,13).
It is important to address the influence of the change of the
chemotherapy DI on the curative effect and disease prognosis. It
remains unknown whether the reduction of the DI of chemotherapy can
reduce drug efficacy and disease prognosis (14–16).
In clinical practice, numerous factors affect the DI of
chemotherapy and then affect the overall efficacy, prognosis and
safety. By examining multiple clinical trials of mCRC, the present
review analyzed the variations of chemotherapy DI in clinical
trials and discussed the relationship between chemotherapy DI and
treatment efficacy, disease prognosis and incidence of adverse
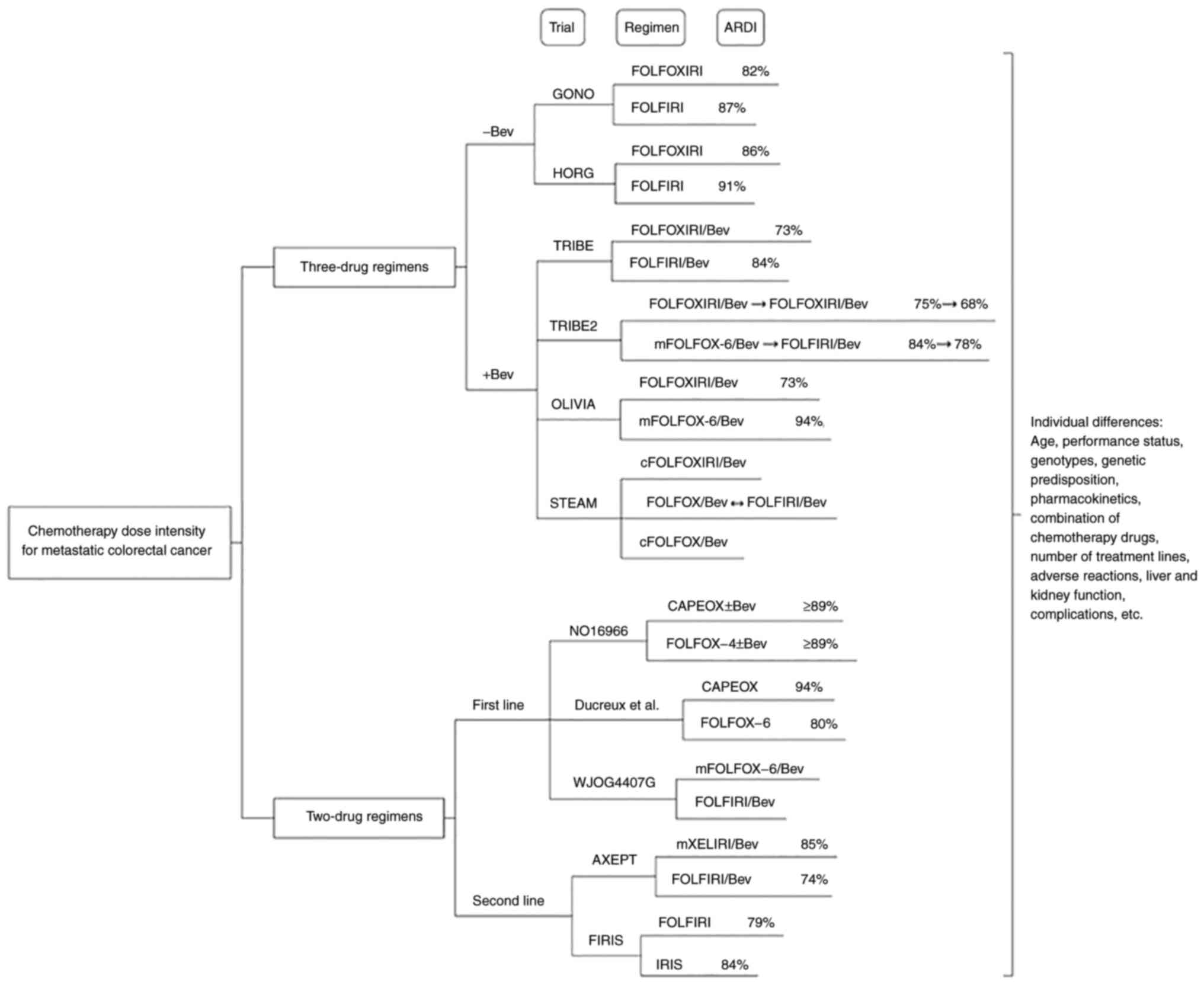
reactions (Fig. 1).
FOLFOXIRI is a combination of three core cytotoxic
drugs: 5-fluorouracil (5-FU)/folinic acid, oxaliplatin (L-OHP) and
irinotecan (CPT-11) (17). The
FOLFOXIRI regimen has a relatively high DI. Based on the present
medical guidelines, the Chinese Society of Clinical Oncology
guidelines incorporated FOLFOXIRI into a first-level recommended
regimen for patients with potentially resectable mCRC who are
suitable for intensive treatment and are positive for RAS/BRAF
mutations (18). The National
Comprehensive Cancer Network (NCCN) guidelines recommend this
regimen for patients with higher performance status scores
(19). The Japanese Society for
Cancer of the Colon and Rectum guidelines and the European Society
for Medical Oncology guidelines recommend this regimen for patients
who are suitable for high-intensity first-line chemotherapy
(20,21).
The results of the Gruppo Oncologico Nord Ovest
(GONO) phase III clinical trial in 2007 and the Hellenic Oncology
Research Group (HORG) phase III clinical trial in 2006 enabled the
development of the FOLFOXIRI protocol, which was included in the
NCCN colon cancer guidelines (17,22).
Both clinical trials explored the efficacy and safety of the
FOLFOXIRI regimen in the first-line treatment of mCRC and employed
the mature 5-FU, folinic acid and CPT-11 (FOLFIRI, a two-drug
combination chemotherapy) regimen as the control. The preset doses
of the three chemotherapeutic drugs in the GONO clinical trial were
higher than those of the HORG clinical trial. Nevertheless, the
former trial indicated a lower dose than the latter one in terms of
final ARDI data, as summarized in Table I. In both trials, it was found that
the incidence of chemotherapy delay and the reduction of the
three-drug regimen was more significant than that of the two-drug
regimen (P<0.05). Regarding efficacy, the GONO trial indicated
significant improvements in the following endpoints: Objective
response rate (ORR), margin-negative resection rate of liver
metastasis, progression-free survival (PFS) and overall survival
(OS; P<0.05). No significant differences were noted in the HORG
trial. It is considered that the latter outcome is interrelated to
the age of the enrolled subjects, their poor performance status and
the low preset chemotherapy dose. In addition, the incidence of
adverse events in the three-drug regimen group was significantly
increased in both trials (P<0.01).
The GONO and HORG data indicated that the three-drug
regimen had a high clinical efficacy. However, the prospect of
combining chemotherapeutic with antiangiogenic drugs requires
further investigation to evaluate the changes in ARDI, treatment
efficacy, prognosis and safety. Angiogenesis is crucial to the
development and progression of cancer and its inhibitory effect has
been shown to be beneficial in patients with several different
malignancies (23). Bevacizumab
(Bev) has been shown to be beneficial to the survival of patients
with mCRC in the following trials (24): TRIBE (25,26),
TRIBE2 (27), OLIVIA (28) and STEAM trials (29). The TRIBE trial was a multicenter
randomized phase III clinical trial conducted by the Italian GONO
cooperation group, which mainly studied the efficacy and safety of
the FOLFOXIRI three-drug regimen combined with Bev. This trial used
FOLFIRI combined with Bev as the control group. The OLIVIA clinical
trial was jointly carried out by 16 centers in Austria, France,
Spain and the UK and mainly assessed the influence of the
three-drug regimen combined with Bev on the resection rate of liver
metastasis. Modified 5-FU/folinic acid and L-OHP (mFOLFOX-6)
combined with Bev was used as the control group. The STEAM trial
examined the efficacy of 5-FU/folinic acid plus L-OHP (FOLFOX)
combined with Bev as the control group and mainly studied the
feasibility of the three-drug regimen combined with the Bev
synchronous regimen (continuous administration of three drugs
combined with Bev for eight cycles) or the sequential regimen
(FOLFOX or FOLFIRI combined with Bev alternating every two cycles).
This was based on the consideration of the sequential selection of
the treatment regimen and on the assessment of whether the American
patient population could benefit from the three-drug regimen. The
TRIBE2 trial was based on the satisfactory results of the TRIBE
trial and focused on the sequential selection of specific treatment
schemes. Moreover, this trial studied the feasibility of
re-applying the FOLFOXIRI/Bev regimen following disease
progression. This application of the FOLFIRI/Bev regimen, which was
performed after the progression of the mFOLFOX-6/Bev regimen, was
considered as the control group.
The aforementioned four clinical trials reported
that the ARDI of the chemotherapy was decreased to a higher extent
following the addition of Bev with that of the standard regimen, as
summarized in Table II. The
incidence of reduction and delay of the three-drug regimen combined
with Bev was more significant than that of the two-drug regimen
combined with Bev (P<0.001). The TRIBE2 trial demonstrated that
the ARDI of the second-line treatment was lower than that of the
first-line treatment. Furthermore, the ARDI of the three-drug
regimen combined with Bev was decreased by 7% and that of the
two-drug regimen combined with Bev was decreased by 6%. Regarding
the assessment of efficacy, the results indicated that the
three-drug or two-drug chemotherapy regimens combined with Bev
displayed improved efficacy and prognosis compared with those noted
in the chemotherapy alone group. Moreover, it was shown that OS,
PFS and ORR were improved. The incidence of adverse reactions, such
as neutropenia, was approximately the same as that noted in the
HORG and GONO trials. It should be noted that the addition of Bev
increased the incidence of adverse reactions related to
antiangiogenic targeted drugs, such as hypertension.
Following analysis of several classic clinical
trials that included three-drug regimens, it was found that the DI
of the three-drug regimen was higher than that of the two-drug
regimen, whereas the actual DI of chemotherapy would descend due to
patient tolerance and other problems encountered in the clinical
environment (25–29). Subsequently, the DI was decreased
following the combination with Bev. The increase in the number of
drugs indicated improved benefits in efficacy and prognosis.
However, considerable emphasis should be paid to the increase of
the number of adverse reactions. The ARDI of the first-line
treatment with the two-drug regimen could reach 83–94%, whereas
that of the three-drug regimen could be maintained at 73–85% and
that of the second-line treatment could be reduced by 5–7%
(25–29). The actual DI of the chemotherapy is
far more likely to decline in the subsequent line of treatment and
that of the three-drug regimen is higher than that of the two-drug
regimen. The tumor load of colorectal cancer and metastases in
patients with advanced primary unresectable tumors is an important
factor affecting survival (30). A
significant correlation has been noted between ORR and the
resection rate (30). The
three-drug regimen can significantly improve ORR and increase the
resection rate of metastatic lesions, which has important clinical
value in reducing metastatic lesions and promoting secondary
resection.
The first-line two-drug regimen of mCRC, which is
commonly used, includes FOLFOX, capecitabine (Cap) plus L-OHP
(CAPEOX) and FOLFIRI. FOLFOX-4, FOLFOX-6 and mFOLFOX-6 are
different in dosage and time, and both belong to FOLFOX. The
two-drug regimen selects the combination of core cytotoxic drugs
for the treatment of mCRC. The NO16966 trial compared CAPEOX and
FOLFOX-4 and Ducreux et al compared CAPEOX and FOLFOX-6
(31–33). The preset dosage of L-OHP in
FOLFOX-4, CAPEOX and FOLFOX-6 was 85 mg/m2 q14d (once
every 14 days), 130 mg/m2 q21d (once every 21 days) and
100 mg/m2 q14d, respectively, whereas the DI of L-OHP
increased in FOLFOX-4, CAPEOX and FOLFOX-6. The DI of 5-FU in
FOLFOX-6 was higher than that in FOLFOX-4. Based on the final ARDI
data of the two trials, the actual DI of the FOLFOX-6 group was the
lowest and was estimated to be only 80%, as shown in Table III. The comparison of the
survival data of the simple chemotherapy group (group without Bev)
of the two trials indicated that OS represented a gradually
increasing trend in the FOLFOX-4, CAPEOX and FOLFOX-6 groups.
However, the difference was not statistically significant. From
this evidence, it can be deduced that the slight change of the
chemotherapy DI exhibited no significant impact on the overall
patient survival. In the NO16966 trial, the survival benefit of the
chemotherapy plus Bev was higher than that of the chemotherapy
alone, which was consistent with the aforementioned conclusion. In
the FOLFOX-6 group, the patients received a longer treatment
duration, higher cumulative dose of L-OHP and demonstrated
significant neurotoxicity, which led to the decrease of ARDI and
the adjustment of the regimen in the subsequent stage of the
treatment. The incidence of neurotoxicity was low when the FOLFOX-4
regimen was used with low DI. Based on the occurrence of adverse
reactions, the FOLFOX-6 regimen was further modified and optimized
to form mFOLFOX-6 regimen with a reduction in the dose of L-OHP
from 100 mg/m2 q14d to 85 mg/m2 q14d. This
facilitated the application of the mFOLFOX-6 regimen (34).
A meta-analysis aiming to compare CAPEOX and FOLFOX
included six randomized controlled trials. Although the dose and
infusion mode of the FOLFOX evolution regimens were different in
the six trials, no significant difference was noted between PFS and
OS (35). The difference noted in
the FOLFOX evolution regimens was mainly interrelated to the
incidence of adverse reactions, suggesting that researchers and
clinicians should seek the lowest point of DI and adverse reactions
on the premise of maintaining survival benefits. CAPEOX and FOLFOX
have shown approximate efficacy in multiple large clinical trials
and improved benefits were demonstrated in combination with Bev
(35,36). The two regimens are a combination
of 5-FU and L-OHP and the difference is mainly reflected in the
incidence of adverse reactions. The CAPEOX regimen exhibits
significant gastrointestinal toxicity and the FOLFOX regimen
exhibits significant myelosuppressive toxicity. The advantage of
the CAPEOX regimen lies in the convenience brought by oral
chemotherapy drugs and the reduction of medical costs (37,38).
Therefore, the selection of the appropriate treatment regimen and
DI according to the individual differences of patients reflects the
concept of precision medicine.
The WJOG4407G trial in Japan compared two
intravenous chemotherapy regimens, as shown in Table III. The first was mFOLFOX-6
combined with Bev and the second FOLFIRI combined with Bev
(39). The preset dose of CPT-11
in the FOLFIRI/Bev protocol was 150 mg/m2 according to
the Japanese guidelines (40). The
trial demonstrated that, with the increase of the number of
treatment lines, the reduction trend of L-OHP was 85, 65 and 50
mg/m2, whereas that of CPT-11 was 150, 120 and 100
mg/m2. The median withdrawal time periods of L-OHP and
CPT-11 were 5.1 and 8.5 months, respectively. L-OHP was withdrawn
significantly earlier. The WJOG4407G trial indicated that the
administration time of L-OHP was limited by the cumulative
toxicity. The curative effects of the two regimens were
approximate, while the prognostic trend of the FOLFIRI/Bev group
was improved. This result was associated with a longer treatment
duration, which was similar to the results of the MAVERICC trial,
in which the dose used of CPT-11 was 180 mg/m2 (41).
The DI changes of the second-line chemotherapy
regimen were analyzed by the AXEPT trial and the Japanese FIRIS
trial (42,43). Most notably, the study subjects of
the two trials were Asians. The AXEPT trial compared the CPT-11 and
Cap (mXELIRI)/Bev and the FOLFIRI/Bev regimens and the FIRIS trial
in Japan compared CPT-11 plus S-1 (IRIS) with the FOLFIRI regimen.
According to the comparative analysis of the two trials, the ARDI
of the second-line treatment (mXELIRI/Bev and IRIS regimens) was
decreased to a higher extent compared with the other types of
treatment. The ARDI of the FOLFIRI regimen for the second-line
chemotherapy was decreased by ~10% and the ARDI of the mXELIRI/Bev
and IRIS regimens remained at ~84.5%, as shown in Table IV. The DI of CPT-11 in the FOLFIRI
regimen was higher than that noted in the mXELIRI/Bev and IRIS
regimens. Nevertheless, in terms of efficacy and prognosis,
mXELIRI/Bev and IRIS exhibited slightly improved performance. In
addition, the incidence of adverse reactions was low. The two
regimens balanced the efficacy and adverse reactions and exhibited
the advantages of oral chemotherapeutic drugs. They were suitable
for the second-line treatment of the mCRC population in Asia. The
predecessor of mXELIRI is XELIRI regimen. The clinical application
of the XELIRI regimen was limited due to severe diarrhea (44,45).
Based on that, XELIRI was improved and modified in subsequent
clinical trials to form mXELIRI, whereas the dose of CPT-11 was
reduced from 250 to 200 mg/m2 and that of Cap from 1,000
to 800 mg/m2, which was approximately equal to the
improvement of the FOLFOX regimen (43,46).
By analyzing the results of the clinical trials of
the first and second-line two-drug regimens, it can be deduced that
an optimal DI balance point can be achieved in the efficacy and
incidence of adverse reactions, such as that noted by the
ameliorated exploration of the mFOLFOX-6 regimen. The FOLFOX
regimen is characterized by a transition process of FOLFOX from −1
to −7. The dose of L-OHP, the infusion mode and the dose of 5-FU
were adjusted to balance the incidence of adverse reactions and the
treatment efficacy. At present, the mFOLFOX-6 regimen is mainly
recommended in the guidelines and has become widely accepted in
clinical practice (18–21). The three first-line two-drug
regimens are almost equivalent regarding their curative effects and
they are frequently used as a back-line treatment or alternative
treatment in the clinic. In addition, it is necessary to address
the differences in the incidence of adverse reactions of the
different regimens, the dose-limiting toxicity of L-OHP and the
dosage form of chemotherapeutic drugs as well as their effect on
the medical costs. The IRIS and mXELIRI regimens have been explored
in the Asian patient population and have provided additional
choices of second-line treatment regimens for patients with mCRC.
Clinicians can select the optimal treatment regimen to balance the
extent of the curative effects and the incidence of the adverse
reactions in accordance with the specific situation of the
patients. In order to ensure that the curative effect is not
significantly reduced, the ARDI of the first-line treatment should
be maintained at >90% and that of the second-line treatment at
>80%.
Chemotherapy reduction and delay often occur in the
elderly (>65 years) and frail patient population in the clinical
environment, which is closely interrelated to poor tolerance and
recovery ability (47,48). It is important to note that limited
data were available for the elderly and frail patients, which
account for >50% of the advanced malignant tumor cases (49,50).
The research on the DI of chemotherapy requires extensive
exploration of the population under examination. The summary
analysis of the TRIBE and TRIBE2 trials demonstrated that ORR and
PFS were not associated with sex and age. However, additional
analysis indicated that the incidence of adverse reactions in the
elderly and female patients was higher (51). The trial proposed that, for
patients with mCRC aged 70–75 years, the initial dose ought to be
reduced and the pretreatment prior to chemotherapy should be
performed when using the FOLFOXIRI/Bev regimen. The Korean Cancer
Study Group conducted a multicenter trial on the reduction of the
first cycle of first-line chemotherapy for elderly advanced
malignant tumors and evaluated the incidence, chemotherapy
compliance and efficacy of the reduction in the first cycle of
chemotherapy (52). Among the 296
patients, the median age was 75 years (70–93 years). A total of
59.8% of the patients underwent treatment decrement. The average
percentage of decrement in the whole patient population was 19.2%
(4–47%) of the standard dose. In addition, the patients who
received standard-dose chemotherapy in the first cycle were more
likely to have it reduced in the second cycle. The trial
demonstrated that the patients with reduced chemotherapy in the
first cycle exhibited improved tolerance and chemotherapy
compliance and lower incidence of adverse reactions compared with
those who received the standard dose. It is important to note that
non-significant differences were noted in OS and PFS between the
two-dose regimens. The FOCUS2 trial conducted a clinical study on
the decrement of the initial chemotherapy in elderly and frail
patients with mCRC who were not suitable for standard-dose
chemotherapy (53). A total of 459
patients with mCRC were included in the trial and received 80% of
the standard chemotherapy dose as the starting dose. When the
patients tolerated chemotherapy for six weeks, the dose was
increased to reach the concentration levels of the standard dose.
The median age of the patients was 74 years old (35–87 years old).
A total of 68% of the patients were old and 71% of the patients
were weak. It was deduced that the decrement of the initial dose of
chemotherapy could result in an improved therapeutic effect,
notably for elderly or frail patients. Furthermore, the trial
recommended that the effect of combined chemotherapy was improved
compared with that of the single drug. A clinical trial explored
the efficacy and safety of FOLFIRI in the treatment of elderly and
non-elderly patients with mCRC (54). It was found that the actual
relative DI of CPT-11 and 5-FU was significantly higher in
non-elderly patients than that noted in elderly patients
(P<0.001). The relative DI of CPT-11 was 81±15% (<70 years
old), 75±15% (70–74 years old) and 75±16% (≥75 years old). The
relative DI of 5-FU was 72±25% (<70 years old), 67±26% (70–74
years old) and 50±25% (≥75 years old). Although the relative DI
received by the elderly patients was comparatively low, the PFS and
OS did not exhibit significant differences between the non-elderly
and the elderly patients. A meta-analysis compared the FOLFIRI
regimen with the 5-FU/folinic acid regimen in the first-line
treatment of elderly and non-elderly patients with mCRC. The age
limit was set to 70 years. The summary analysis of 2,691 patients
indicated that both elderly and non-elderly patients exhibited
higher survival benefits in the combined chemotherapy regimen
(55).
The hierarchical analysis of the HORG trial
indicated that the OS of the patients with performance status (PS)
0–1 in the FOLFOXIRI group was 24 months. Notably, the OS of the
patients with PS=2 was 6.6 months (P=0.0001). The same trend was
observed in the FOLFIRI group. The OS of the patients in the
FOLFIRI group with PS=0-1 was 20 months compared with 6.4 months
(P=0.03) noted in patients with PS=2. This suggested the importance
of the differences in the population functional status to disease
prognosis and indicated that the PS score was an important factor
affecting survival (22). The
summary of nine clinical trials that examined patients with mCRC
who were treated with first-line treatment demonstrated that the
median PFS of the patients with PS=2 was 4.9 months, whereas the OS
was estimated to be 8.5 months and the ORR 32%. The incidence of
adverse reactions in the patients receiving standard-dose
chemotherapy was higher (56). The
updated subgroup analysis of the TRIBE2 trial indicated that the
mCRC population with PS=0 exhibited higher benefit from the
three-drug regimen with high DI (P=0.05) (57). It is suggested that the suitable
population for the different DI of chemotherapy differs. High DI
chemotherapy is suitable for patients with improved performance
status evaluation and the PS score can be used as an excellent
screening parameter.
More specifically, certain differences have been
reported in the response of different populations to the DI of
chemotherapy. It has been found that the American population has
poor tolerance to 5-FU (58).
Therefore, the recommended dose of 5-FU in the three-drug regimen
according to the NCCN guidelines is 2,400 mg/m2, whereas
that corresponding to the European and Chinese populations is 3,200
mg/m2. Moreover, several population differences have
been reported in the tolerated dose of CPT-11. The recommended dose
of CPT-11 according to the NCCN guidelines is 180 mg/m2,
whereas the dose based on the Japanese guidelines is 150
mg/m2. A first-line dose exploration study of patients
with mCRC based on different UDP glucuronosyltransferase family 1
member A (UGT1A) genotypes indicated that patients with different
UGT1A genotypes should be treated with different doses of CPT-11.
The maximum tolerated dose of patients with a genotype of 1/*1 was
450 mg/m2, whereas, for the *1/*28 and *28/*28
genotypes, these doses were 390 mg/m2 and 150
mg/m2, respectively (59,60).
The AXEPT trial applied the UGT1A genotype to guide the dosage of
CPT-11 (42). In addition,
individual differences in the patients' genetic predisposition and
pharmacokinetic profile can lead to differences in the local drug
dose or drug sensitivity of tumor cells (61). Neutropenia caused by chemotherapy
can be used as an indicator of the efficiency of chemotherapy,
which was also an important factor affecting survival (61). Although no significant differences
were noted in the relative DI among patients without neutropenia,
early-onset neutropenia and late-onset neutropenia, the local
chemotherapeutic dose and treatment response were different.
Neutropenia reflects the response rate and survival as an adverse
reaction, which can improve the adjustment of the clinical dose
(61).
Numerous clinical trials have suggested that the
population containing elderly and frail patients was more prone to
the decline of chemotherapy DI (62). In summary, a certain degree of
decline has a limited impact on the survival benefit. In contrast
to these findings, it should be noted that the incidence of adverse
reactions in this part of the population is relatively high. In
specific populations, the combined regimen is also improved
compared with the single-drug regimen, suggesting that it is
feasible to increase the dose of chemotherapeutic drugs (53). In addition, the exploration of an
optimal low-DI regimen in specific populations required further
investigation. The elderly patient population and the patients with
poor performance status present with different responses in drug
efficacy and different incidence of adverse reactions (55,56).
The survival benefit of the population with poor performance status
is significantly low, which should be further distinguished.
Furthermore, the difference in dose tolerance of CPT-11 in patients
with different genotypes suggests that the markers related to
chemotherapy efficacy and prognosis are worthy of further
exploration (59). The differences
in the pharmacokinetic parameters and in the genetic predisposition
suggest that the local drug dose and sensitivity of tumor cells
should be viewed from an accurate perspective. In addition, the
occurrence time of neutropenia can be used as an alternative index
to guide the adjustment of the chemotherapeutic dose (61).
Chemotherapy DI is a highly important factor to be
considered for the balance of drug efficacy and adverse reactions.
It can be used as a proxy measure of chemotherapy quality and
prognosis (63). In the past
decade, additional research has been conducted on the
identification of novel drugs and regimens. The chemotherapy dose
requires adjustment according to each patient and must not always
follow the dosage recommended by the guidelines. The dose and
frequency are adjusted mostly in radiotherapy research or in the
treatment of certain rare malignant diseases (64). The pharmacokinetic study of drugs
should not only be applied under specific experimental conditions
since there are various complex parameters to be taken into
consideration (65). The
chemotherapy DI is affected by multiple factors, such as age,
performance status, genotype, genetic predisposition,
pharmacokinetics, a combination of chemotherapeutic drugs, the
number of treatment lines, adverse reactions, liver and kidney
function, complications and the psychological acceptance of
patients (66–69). The development of precision
medicine has led to the focus on individualized differences. The
exploration related to refining the scheme has been carried out in
different types of malignant tumors and therapeutic drugs (70,71).
Cancer societies have initiated the establishment of consensus and
guidelines on the dosage of drugs for specific patient populations,
such as the elderly, obese and overweight patients (72,73).
This disagrees with our conventional thinking, which involves the
use of the body surface area and the bodyweight to calculate the
dose of chemotherapy while ignoring the discrepancies noted in the
response of different populations to treatment.
The clinical trials of the three-drug and two-drug
regimens reflected the changes in the chemotherapy DI of the
clinical trial environment. In clinical treatment, the baseline
condition of the patient population is more complex and the
implementation of the treatment regimen and clinical benefits vary
greatly among individuals. This suggests that clinicians should
reasonably arrange the chemotherapy regimen according to each
individual, including regimen and dose, time arrangement,
administration mode and combined administration. It was found that
the survival benefit of the treatment mode of the combined
administration (increasing chemotherapeutic drugs or combined
targeted drugs) was outstanding, whereas the incidence of adverse
reactions was increased. The elderly and frail patient population
is more prone to the development of adverse reactions and exhibits
higher therapeutic utility in low-dose intensive chemotherapy
(74). The exploration of the
optimal treatment scheme and DI for different populations requires
substantial research in order to maximize the effectiveness of
clinical treatment. At present, certain studies focus on optimizing
the physical and chemical properties of drugs and increasing the
response of patients to anticancer drugs by increasing the local
concentration following drug absorption (75,76).
In addition, the reduction of adverse reactions and the improvement
of the performance status can increase the DI of chemotherapy and
improve the curative effect (77,78).
As a regulator of CPT-11 in patients with mCRC, Huangqin Decoction
can reduce gastrointestinal toxicity and reduce the events of
chemotherapy reduction caused by toxicity (79). Therefore, it is worth assessing the
effective adjuvant treatment methods or complementary alternative
medicine, such as Traditional Chinese Medicine, to reduce adverse
reactions and improve performance status.
Not applicable.
Funding: No funding was received.
Not applicable.
XC and PX performed data analysis and manuscript
writing. SZ conceived and reviewed the paper for intellectual
content. All authors read and approved the final manuscript. Data
sharing is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin J, Bai Z, Zhang J, Zheng Z, Yao H, Ye
P, Li J, Gao X and Zhang Z: Burden of colorectal cancer in China,
1990–2017: Findings from the Global Burden of Disease Study 2017.
Chin J Cancer Res. 31:489–498. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antoniotti C, Borelli B, Rossini D,
Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F,
Tamberi S, Corallo S, et al: AtezoTRIBE: A randomised phase II
study of FOLFOXIRI plus bevacizumab alone or in combination with
atezolizumab as initial therapy for patients with unresectable
metastatic colorectal cancer. BMC Cancer. 20:6832020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das S, Ciombor KK, Haraldsdottir S and
Goldberg RM: Promising new agents for colorectal cancer. Curr Treat
Options Oncol. 19:292018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nightingale G, Schwartz R, Kachur E, Dixon
BN, Cote C, Barlow A, Barlow B and Medina P: Clinical pharmacology
of oncology agents in older adults: A comprehensive review of how
chronologic and functional age can influence treatment-related
effects. J Geriatr Oncol. 10:4–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frei E, Elias A, Wheeler C, Richardson P
and Hryniuk W: The relationship between high-dose treatment and
combination chemotherapy: The concept of summation dose intensity.
Clin Cancer Res. 4:2027–2037. 1998.PubMed/NCBI
|
|
8
|
Lyman GH: Impact of chemotherapy dose
intensity on cancer patient outcomes. J Natl Compr Canc Netw.
7:99–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dodwell DJ, Gurney H and Thatcher N: Dose
intensity in cancer chemotherapy. Br J Cancer. 61:789–794. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ariyoshi Y and Ogawa M: Dose intensity in
cancer chemotherapy (including high dose chemotherapy). Gan To
Kagaku Ryoho. 21:2699–2707. 1994.(In Japanese). PubMed/NCBI
|
|
11
|
Alberto P: Dose intensity in cancer
chemotherapy: Definition, average relative dose intensity and
effective dose intensity. Bull Cancer. 82 (Suppl 1):3s–8s. 1995.(In
French). PubMed/NCBI
|
|
12
|
Martin JH and Dimmitt S: The rationale of
dose-response curves in selecting cancer drug dosing. Br J Clin
Pharmacol. 85:2198–2204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paci A, Veal G, Bardin C, Levêque D,
Widmer N, Beijnen J, Astier A and Chatelut E: Review of therapeutic
drug monitoring of anticancer drugs part 1-cytotoxics. Eur J
Cancer. 50:2010–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erku D, Schneider J and Scuffham P: A
framework for economic evaluation of therapeutic drug
monitoring-guided dosing in oncology. Pharmacol Res Perspect.
9:e008622021. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lakkunarajah S, Breadner DA, Zhang H,
Yamanaka E, Warner A and Welch S: The influence of adjuvant
chemotherapy dose intensity on five-year outcomes in resected colon
cancer: A single Centre retrospective analysis. Curr Oncol.
28:4031–4041. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozer H and Diasio RB: Perspectives in the
treatment of colorectal cancer. Semin Oncol. 31:14–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Phase III trial of infusional fluorouracil,
leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with
infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as
first-line treatment for metastatic colorectal cancer: The Gruppo
Oncologico Nord Ovest. J Clin Oncol. 25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong C, Ding Y, Weng S, Li G, Huang Y, Hu
H, Zhang Z, Zhang S and Yuan Y: Update in version 2021 of CSCO
guidelines for colorectal cancer from version 2020. Chin J Cancer
Res. 33:302–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Messersmith WA: NCCN Guidelines updates:
Management of metastatic colorectal cancer. J Natl Compr Canc Netw.
17:599–601. 2019.PubMed/NCBI
|
|
20
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum.
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Souglakos J, Androulakis N, Syrigos K,
Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S,
Kouroussis Ch, Vamvakas L, et al: FOLFOXIRI (folinic acid,
5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic
acid, 5-fluorouracil and irinotecan) as first-line treatment in
metastatic colorectal cancer (MCC): A multicentre randomised phase
III trial from the Hellenic Oncology Research Group (HORG). Br J
Cancer. 94:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrarotto R and Hoff PM: Antiangiogenic
drugs for colorectal cancer: Exploring new possibilities. Clin
Colorectal Cancer. 12:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cremolini C, Antoniotti C, Rossini D,
Lonardi S, Loupakis F, Pietrantonio F, Bordonaro R, Latiano TP,
Tamburini E, Santini D, et al: Upfront FOLFOXIRI plus bevacizumab
and reintroduction after progression versus mFOLFOX6 plus
bevacizumab followed by FOLFIRI plus bevacizumab in the treatment
of patients with metastatic colorectal cancer (TRIBE2): A
multicentre, open-label, phase 3, randomised, controlled trial.
Lancet Oncol. 21:497–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gruenberger T, Bridgewater J, Chau I,
García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F,
Waterkamp D and Adam R: Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in
patients with initially unresectable liver metastases from
colorectal cancer: The OLIVIA multinational randomised phase II
trial. Ann Oncol. 26:702–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hurwitz HI, Tan BR, Reeves JA, Xiong H,
Somer B, Lenz HJ, Hochster HS, Scappaticci F, Palma JF, Price R, et
al: Phase II randomized trial of sequential or concurrent
FOLFOXIRI-bevacizumab versus FOLFOX-bevacizumab for metastatic
colorectal cancer (STEAM). Oncologist. 24:921–932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brouquet A and Nordlinger B: Neoadjuvant
and adjuvant therapy in relation to surgery for colorectal liver
metastases. Scand J Gastroenterol. 47:286–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F and
Saltz L: XELOX vs FOLFOX-4 as first-line therapy for metastatic
colorectal cancer: NO16966 updated results. Br J Cancer. 105:58–64.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ducreux M, Bennouna J, Hebbar M, Ychou M,
Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L,
et al: Capecitabine plus oxaliplatin (XELOX) versus
5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line
treatment for metastatic colorectal cancer. Int J Cancer.
128:682–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimizu T, Satoh T, Tamura K, Ozaki T,
Okamoto I, Fukuoka M and Nakagawa K:
Oxaliplatin/fluorouracil/leucovorin (FOLFOX4 and modified FOLFOX6)
in patients with refractory or advanced colorectal cancer:
Post-approval Japanese population experience. Int J Clin Oncol.
12:218–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buchler T, Pavlik T, Melichar B, Bortlicek
Z, Usiakova Z, Dusek L, Kiss I, Kohoutek M, Benesova V, Vyzula R,
et al: Bevacizumab with 5-fluorouracil, leucovorin, and oxaliplatin
versus bevacizumab with capecitabine and oxaliplatin for metastatic
colorectal carcinoma: Results of a large registry-based cohort
analysis. BMC Cancer. 14:3232014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petrioli R, Francini E, Cherri S, Torre P,
Fiaschi AI, Miano ST, Marrelli D, Rovello F and Francini G:
Capecitabine plus oxaliplatin and bevacizumab, followed by
maintenance treatment with capecitabine and bevacizumab for
patients aged >75 years with metastatic colorectal cancer. Clin
Colorectal Cancer. 17:e663–e669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neupane R, Boddu SHS, Abou-Dahech MS,
Bachu RD, Terrero D, Babu RJ and Tiwari AK: Transdermal delivery of
chemotherapeutics: Strategies, requirements, and opportunities.
Pharmaceutics. 13:9602021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamazaki K, Nagase M, Tamagawa H, Ueda S,
Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T,
et al: Randomized phase III study of bevacizumab plus FOLFIRI and
bevacizumab plus mFOLFOX6 as first-line treatment for patients with
metastatic colorectal cancer (WJOG4407G). Ann Oncol. 27:1539–1546.
2016. View Article : Google Scholar : View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishi T, Hamamoto Y, Warita E, Miyamoto J,
Akutsu N, Yamanaka Y, Nagase M and Fujii H: Retrospective analysis
of the international standard-dose FOLFIRI (plus bevacizumab)
regimen in Japanese patients with unresectable advanced or
recurrent colorectal carcinoma. Int J Clin Oncol. 16:488–493. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Parikh AR, Lee FC, Yau L, Koh H, Knost J,
Mitchell EP, Bosanac I, Choong N, Scappaticci F, Mancao C, et al:
MAVERICC, a Randomized, Biomarker-stratified, Phase II study of
mFOLFOX6-bevacizumab versus FOLFIRI-bevacizumab as first-line
chemotherapy in metastatic colorectal cancer. Clin Cancer Res.
25:2988–2995. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu RH, Muro K, Morita S, Iwasa S, Han SW,
Wang W, Kotaka M, Nakamura M, Ahn JB, Deng YH, et al: Modified
XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin,
fluorouracil, and irinotecan), both either with or without
bevacizumab, as second-line therapy for metastatic colorectal
cancer (AXEPT): A multicentre, open-label, randomised,
non-inferiority, phase 3 trial. Lancet Oncol. 19:660–671. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: A randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fuchs CS, Marshall J, Mitchell E,
Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D,
Soufi-Mahjoubi R, Wang B and Barrueco J: Randomized, controlled
trial of irinotecan plus infusional, bolus, or oral
fluoropyrimidines in first-line treatment of metastatic colorectal
cancer: Results from the BICC-C study. J Clin Oncol. 25:4779–4786.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Köhne CH, De Greve J, Hartmann JT, Lang I,
Vergauwe P, Becker K, Braumann D, Joosens E, Müller L, Janssens J,
et al: Irinotecan combined with infusional 5-fluorouracil/folinic
acid or capecitabine plus celecoxib or placebo in the first-line
treatment of patients with metastatic colorectal cancer. EORTC
study 40015. Ann Oncol. 19:920–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Q, Zhang P, Wang X, Zhang M, Liao W and
Li Q: Cost-effectiveness of capecitabine + irinotecan versus
leucovorin + Fluorouracil + irinotecan in the second-line treatment
of metastatic colorectal cancer in China. Clin Ther.
42:2148–2158.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hisada H, Takahashi Y, Kubota M, Shimura
H, Itobayashi E, Shimura K and Nakamura A: Clinical and therapeutic
features and prognostic factors of metastatic colorectal cancer
over age 80: A retrospective study. BMC Gastroenterol. 21:1992021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ward BW and Schiller JS: Prevalence of
multiple chronic conditions among US adults: Estimates from the
National Health Interview Survey, 2010. Prev Chronic Dis.
10:E652013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S, Yang T, Qiang W, Shen A, Zhao Z,
Yang H and Liu X: The prevalence of frailty among breast cancer
patients: A systematic review and meta-analysis. Support Care
Cancer. 30:2993–3006. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vargas-Román K, Tovar-Gálvez MI,
Liñán-González A, Cañadas de la Fuente GA, de la Fuente-Solana EI
and Díaz-Rodríguez L: Coping strategies in elderly colorectal
cancer patients. Cancers (Basel). 14:6082022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Marmorino F, Rossini D, Lonardi S, Moretto
R, Zucchelli G, Aprile G, Dell'Aquila E, Ratti M, Bergamo F, Masi
G, et al: Impact of age and gender on the safety and efficacy of
chemotherapy plus bevacizumab in metastatic colorectal cancer: A
pooled analysis of TRIBE and TRIBE2 studies. Ann Oncol.
30:1969–1977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang IG, Kwon M, Kim JW, Kim SH, Lee YG,
Kim JY, Koh SJ, Ko YH, Shin SH, Hong S, et al: Prevalence and
predictive factors for upfront dose reduction of the first cycle of
first-line chemotherapy in older adults with metastatic solid
cancer: Korean Cancer Study Group (KCSG) multicenter study. Cancers
(Basel). 13:3312021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seymour MT, Thompson LC, Wasan HS,
Middleton G, Brewster AE, Shepherd SF, O'Mahony MS, Maughan TS,
Parmar M, Langley RE, et al: Chemotherapy options in elderly and
frail patients with metastatic colorectal cancer (MRC FOCUS2): An
open-label, randomised factorial trial. Lancet. 377:1749–1759.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim JW, Lee KW, Kim KP, Lee JH, Hong YS,
Kim JE, Kim SY, Park SR, Nam BH, Cho SH, et al: Efficacy and safety
of FOLFIRI regimen in elderly versus nonelderly patients with
metastatic colorectal or gastric cancer. Oncologist. 22:293–303.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Folprecht G, Seymour MT, Saltz L,
Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E,
Rougier P, Mitry E, et al: Irinotecan/fluorouracil combination in
first-line therapy of older and younger patients with metastatic
colorectal cancer: Combined analysis of 2,691 patients in
randomized controlled trials. J Clin Oncol. 26:1443–1451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sargent DJ, Köhne CH, Sanoff HK, Bot BM,
Seymour MT, de Gramont A, Porschen R, Saltz LB, Rougier P,
Tournigand C, et al: Pooled safety and efficacy analysis examining
the effect of performance status on outcomes in nine first-line
treatment trials using individual data from patients with
metastatic colorectal cancer. J Clin Oncol. 27:1948–1955. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cremolini C, Antoniotti C, Lonardi S,
Rossini D and Falcone A: Updated results of TRIBE2, a phase III,
randomized strategy study by GONO in the 1st- and 2nd-line
treatment of unresectable mCRC. J Clin Oncol. 37:3508. 2019.
View Article : Google Scholar
|
|
58
|
Haller DG, Cassidy J, Clarke SJ,
Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB,
Schmoll HJ, Allegra C, et al: Potential regional differences for
the tolerability profiles of fluoropyrimidines. J Clin Oncol.
26:2118–2123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marcuello E, Páez D, Paré L, Salazar J,
Sebio A, del Rio E and Baiget M: A genotype-directed phase I–IV
dose-finding study of irinotecan in combination with
fluorouracil/leucovorin as first-line treatment in advanced
colorectal cancer. Br J Cancer. 105:53–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsunedomi R, Hazama S, Fujita Y, Okayama
N, Kanekiyo S, Inoue Y, Yoshino S, Yamasaki T, Suehiro Y, Oba K, et
al: A novel system for predicting the toxicity of irinotecan based
on statistical pattern recognition with UGT1A genotypes. Int J
Oncol. 45:1381–1390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen Y, Wang Y, Shi Y and Dai G: Timing of
chemotherapy-induced neutropenia predicts prognosis in metastatic
colon cancer patients: A retrospective study in mFOLFOX6-treated
patients. BMC Cancer. 17:2422017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abdel-Rahman O and Karachiwala H: Impact
of age on toxicity and efficacy of 5-FU-based combination
chemotherapy among patients with metastatic colorectal cancer; a
pooled analysis of five randomized trials. Int J Colorectal Dis.
34:1741–1747. 2019. View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schraa SJ, Frerichs KA, Agterof MJ,
Hunting JCB, Los M and de Jong PC: Relative dose intensity as a
proxy measure of quality and prognosis in adjuvant chemotherapy for
breast cancer in daily clinical practice. Eur J Cancer. 79:152–157.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kepka L and Socha J: Dose and
fractionation schedules in radiotherapy for non-small cell lung
cancer. Transl Lung Cancer Res. 10:1969–1982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sallustio BC and Boddy AV: Is there scope
for better individualisation of anthracycline cancer chemotherapy?
Br J Clin Pharmacol. 87:295–305. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bridoux F, Cockwell P, Glezerman I,
Gutgarts V, Hogan JJ, Jhaveri KD, Joly F, Nasr SH, Sawinski D and
Leung N: Kidney injury and disease in patients with haematological
malignancies. Nat Rev Nephrol. 17:386–401. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pellacani C and Eleftheriou G:
Neurotoxicity of antineoplastic drugs: Mechanisms, susceptibility,
and neuroprotective strategies. Adv Med Sci. 65:265–285. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kurniali PC, Hrinczenko B and Al-Janadi A:
Management of locally advanced and metastatic colon cancer in
elderly patients. World J Gastroenterol. 20:1910–1922. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nightingale G, Schwartz R, Kachur E, Dixon
BN, Cote C, Barlow A, Barlow B and Medina P: Clinical pharmacology
of oncology agents in older adults: A comprehensive review of how
chronologic and functional age can influence treatment-related
effects. J Geriatr Oncol. 10:4–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lue JK and O'Connor OA: A perspective on
improving the R-CHOP regimen: From Mega-CHOP to ROBUST R-CHOP, the
PHOENIX is yet to rise. Lancet Haematol. 7:e838–e850. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hotchkiss KM and Sampson JH: Temozolomide
treatment outcomes and immunotherapy efficacy in brain tumor. J
Neurooncol. 151:55–62. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silvestris N, Argentiero A, Natalicchio A,
D'Oronzo S, Beretta GD, Acquati S, Adinolfi V, Di Bartolo P, Danesi
R, Faggiano A, et al: Antineoplastic dosing in overweight and obese
cancer patients: An Associazione Italiana Oncologia Medica
(AIOM)/Associazione Medici Diabetologi (AMD)/Societa Italiana
Endocrinologia (SIE)/Societa Italiana Farmacologia (SIF)
multidisciplinary consensus position paper. ESMO Open.
6:1001532021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kojima T, Mizokami F and Akishita M:
Geriatric management of older patients with multimorbidity. Geriatr
Gerontol Int. 20:1105–1111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Swinson D, Hall P, Seymour M, Lord S,
Marshall H, Ruddock S, Cairns D, Waters J, Wadsley J, Falk S, et
al: Optimising chemotherapy for frail and/or elderly patients with
advanced gastroesophageal cancer (AGOAC): The GO2 phase III trial.
J Geriatr Oncol. 10 (Suppl 1):S82019. View Article : Google Scholar
|
|
75
|
Xu X, Wu Y, Qian X, Wang Y, Wang J, Li J,
Li Y and Zhang Z: Nanomedicine strategies to circumvent intratumor
extracellular matrix barriers for cancer therapy. Adv Healthc
Mater. 11:e21014282022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Handa M, Beg S, Shukla R, Barkat MA,
Choudhry H and Singh K: Recent advances in lipid-engineered
multifunctional nanophytomedicines for cancer targeting. J Control
Release. 340:48–59. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Abadi AJ, Mirzaei S, Mahabady MK, Hashemi
F, Zabolian A, Hashemi F, Raee P, Aghamiri S, Ashrafizadeh M, Aref
AR, et al: Curcumin and its derivatives in cancer therapy:
Potentiating antitumor activity of cisplatin and reducing side
effects. Phytother Res. 36:189–213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen M, May BH, Zhou IW, Xue CC and Zhang
AL: FOLFOX 4 combined with herbal medicine for advanced colorectal
cancer: A systematic review. Phytother Res. 28:976–991. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010. View Article : Google Scholar : PubMed/NCBI
|