Introduction
Landmark discoveries in molecular oncology have
started to shed light both on the underlying causes of cancer onset
and progression, and on unmet clinical needs that have hastened
re-interpretation of the recently emerging landscape of deregulated
signaling pathways. Overexpression of oncogenes, loss of tumor
suppressors, intra- and inter-tumor heterogeneity, and drug
resistance are among the most extensively studied mechanisms
(1–8).
Groundbreaking discoveries from the past decades
have paradigmatically shifted the conceptual understanding of
non-coding (nc)RNAs from being merely ‘junk’ transcriptional
products to being considered as multifunctional regulators that
contextually modulate a number of cellular processes, including
transcription, post-transcriptional processing, chromatin
remodeling and the regulation of cell signaling cascades. Together
with an expansion of knowledge regarding the transcriptome space,
it has become evident that a wide variety of RNA transcripts
contain different microRNA (miRNA/miR)-binding sites (9–11).
Rapidly accumulating scientific evidence has enabled
a transition to be made from a purely phenomenological to a more
detailed mechanistic understanding that all RNA transcripts that
contain miRNA-binding sites are able to regulate and communicate
with each other by specifically competing for shared miRNAs, and
they thereby serve as competing endogenous RNAs (ceRNAs). The
diversity and complexity of known ceRNA interactions have increased
exponentially with the discovery of an ever-increasing number of
oncogenic and tumor suppressor long ncRNAs (lncRNAs) (12–15)
and circular RNAs (16–18).
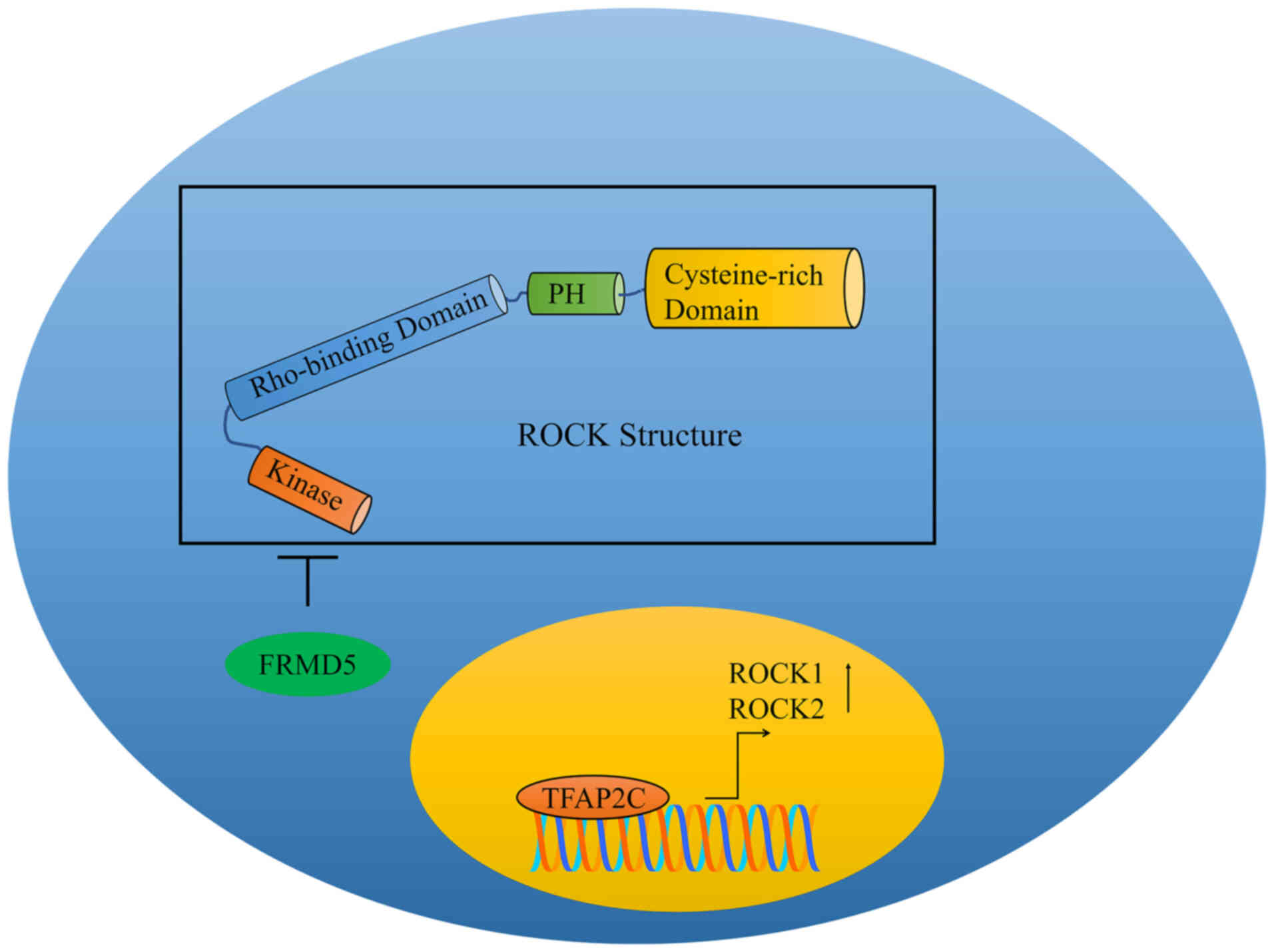
Rho-associated protein kinase (ROCK) is a
serine/threonine protein kinase that was identified as a
RhoGTP-binding protein, having a molecular mass of ~160 kDa
(19,20). To date, two isoforms encoded by two
different genes of ROCK (ROCK1 and ROCK2) have been investigated.
ROCK1 and ROCK2 have been shown to fulfill major roles in
carcinogenesis. These two proteins share an overall sequence
similarity in their kinase domains of 92%, and at the amino-acid
level, a similarity of 65% (21).
Myosin light chain (MLC) is an important downstream substrate of
ROCK1 that is phosphorylated by ROCK1 at Ser-19.
The present review exclusively focuses on
cancer-related roles of lncRNAs, circular RNAs and ROCK1/2. PubMed
(https://pubmed.ncbi.nlm.nih.gov/) was
independently searched using the keywords ‘lncRNA’, ‘ROCK1’ and
‘ROCK2’. All the articles were carefully screened and shortlisted
for inclusion in the manuscript. Only those articles were selected
which provided the findings about ncRNAs and ROCK1/ROCK2
exclusively in cancers.
The present review aims to summarize the interplay
between ncRNAs and ROCK proteins in different types of cancer.
First, a mechanistic overview of the ROCK proteins, and their key
role in carcinogenesis, is provided. Subsequently, the review
features an exclusive focus on how ncRNAs, particularly lncRNAs and
circular RNAs (circRNAs), have been shown to interact with ROCK1
and ROCK2 in a wide variety of different types of cancer.
Overview of the role of ROCKs in different
types of cancer
Transcription factor AP2-γ (TFAP2C) has been shown
to enhance chemoresistance in colorectal cancer cells by
stimulating the expression of ROCK1 and ROCK2 (22). Treatment with 5-fluorouracil
induced regression of tumors in mice inoculated subcutaneously with
TFAP2C-silenced HCT116 cells. TFAP2C also transcriptionally
upregulates ROCK1 and ROCK2 in colorectal cancer cells (Fig. 1). Administration of Y-27632, a
ROCK1/ROCK2 inhibitor, caused a considerable decrease in
chemoresistance and stemness in TFAP2C-overexpressing cells
(22).
FERM domain-containing protein 5 (FRMD5) has been
shown to serve as a tumor suppressor, markedly restricting the
motility of cancer cells (23).
FRMD5 also physically interacts with ROCK1 and inhibits its
activity (Fig. 1). The
FERM-associated domain of FRMD5 was shown to be critical for
interaction with the N-terminal domain of ROCK1. FRMD5 knockdown
induced an increase in the phosphorylated levels of MLC, whereas
FRMD5 overexpression inhibited the phosphorylation of MLC (23). Collectively, these findings
suggested that FRMD5 is able to structurally interact with ROCK1,
interfering with the ROCK1-mediated phosphorylation of MLC.
Therefore, FRMD5 may inhibit the migration of lung cancer cells
through the inhibition of ROCK1 kinase activity.
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase
3 (PFKFB3) has been shown to play a critical role in the metastasis
of osteosarcoma (24). In a
previous study, ROCK2 downregulation led to an obvious decrease in
the migratory and invasive abilities of 143B and U2-OS cells,
whereas PFKFB3 upregulation rescued the ROCK2 knockdown-induced
effects. Furthermore, ROCK2 inhibition caused a marked decrease in
the proliferative capabilities of 143B and U2-OS cells, whereas
PFKFB3 upregulation restored the proliferative abilities of the
osteosarcoma cells. ROCK2 prevented ubiquitin-mediated degradation
of PFKFB3; moreover, ROCK2 inhibition enhanced the process of
PFKFB3 ubiquitination, whereas, conversely, overexpression of ROCK2
led to a decrease in the levels of ubiquitinated PFKFB3. ROCK2
inhibition reduced the levels of PFKFB3 in osteosarcoma cells, and
finally, lung metastasis was not observed in mice inoculated with
ROCK2-silenced osteosarcoma cells (24).
Matrix metalloproteinase 2 (MMP2) is another widely
studied protein that is reportedly involved in the positive
regulation of metastasis (25).
ROCK2 was shown to both prevent degradation of MMP2 and to induce
an increase in MMP2 levels in hepatic cellular carcinoma (HCC)
cells (25).
Forkhead box M1 (FOXM1) has been shown to modulate
ROCK-driven signaling (26). ROCK2
has been reported to be an important FOXM1D-binding protein (FOXM1D
being a novel isoform of FOXM1). In a previous study, ROCK
inhibitors (fasudil and Y-27632) induced actin depolymerization,
markedly decrease the levels of phosphorylated MLC and altered the
shape of FOXM1D-overexpressing colorectal cancer LoVo and SW-480
cells. FOXM1D-induced activation of ROCK also contributed to the
destruction of cell junctions and enhanced cell motility.
Downregulation of E-cadherin could also potentially be a
contributory factor towards the destruction of cell junctions
(26).
The RNA-binding protein Lin28A, which contains a
CCHC-zinc finger RNA-binding domain and cold shock domain (27), has also been shown to physically
interact with ROCK2 and promote metastasis. The growth rates of
tumors in mice injected with ROCK2-silenced ovarian cancer cells
were found to be markedly lowered. There was also a marked decrease
in the number of metastatic nodules present on lung surfaces of the
mice injected with ROCK2-silenced ovarian cancer cells (27).
Introduction of ncRNAs
Evidence from genome-wide analyses and preclinical
studies, supported by recently identified molecular insights, has
improved our understanding of the fundamental role of ncRNAs in
different types of cancer. miRNAs are small (ranging from 18–24
nucleotides), single-stranded ncRNAs. Ever since their discovery in
1993, it has been generally understood that these small molecules
fulfill important roles in gene regulation; their mechanism of
action is based on their binding to the 3′-untranslated region of
mRNA transcripts (28). lncRNAs
also regulate gene transcription, although they consist of >200
nucleotides and are transcribed predominantly by RNA polymerase II.
Similar to mRNAs, lncRNAs are also characterized by the presence of
a 3′polyadenine tail (29).
Regarding their role in cancer, both miRNAs and lncRNAs are
considered to fulfill key roles in tumorigenesis and in tumor
progression. Downregulation of certain tumor suppressor miRNAs is a
common finding in breast, gynecological, prostate and lung cancer
and brain tumors (30). The term
‘oncomiR’, which is used for several miRNAs, is indicative of the
role of those miRNAs that have oncogenic functions. lncRNAs are
also important in cancer, and a greatly expanding list of them has
been noted to be correlated with particular types of cancer. A
number of reviews have been recently published that provide a
comprehensive overview of landmark discoveries in the field of
ncRNAs (31–35).
Gaze through a ‘molecular lens’: ROCK-driven
signaling
ROCK1-driven downstream signaling has been reported
to occupy a central role in enhancing the invasive potential of
cancer cells. The upcoming section focuses exclusively on lncRNAs
and circRNAs reportedly involved in positive and negative
regulation of ROCK in different cancer types.
Positive regulation of ROCK1 by lncRNAs:
Tumorigenic role of lncRNAs
Cyclin-dependent kinase inhibitor 2B
antisense RNA 1 (CDKN2B-AS1)
CDKN2B-AS1 (also known as ANRIL) is an lncRNA that
is frequently overexpressed in laryngeal squamous cell cancer
(36). In a study by Liu et
al (36) CDKN2B-AS1 knockdown
was shown to cause the arrest cells in the G1 phase and
to decrease the number of cells in the S phase. Furthermore, the
levels of proliferating cell nuclear antigen, an indicator of cell
proliferation, were shown to be markedly decreased in cells where
CDKN2B-AS1 had been knocked down. However, the levels of
apoptosis-associated markers, in particular cleaved caspase-3 and
cleaved poly (ADP-ribose) polymerase, were found to be markedly
increased. Further experiments revealed that CDKN2B-AS1 knockdown
induced apoptosis in AMC-HN-8 cells. Mechanistically, CDKN2B-AS1
regulated ROCK1 by blocking the activity of miRNA-324-5p in
AMC-HN-8 cells. Taken together, the molecular analyses clearly
suggested that miRNA-324-5p directly targeted ROCK1, whereas
CDKN2B-AS1 sequestered miRNA-324-5p away from ROCK1, thereby
relieving its inhibitory effects on ROCK1 (36).
Epidermal growth factor
receptor-antisense RNA 1 (EGFR-AS1)
EGFR-AS1 overexpression was shown to enhance the
migratory and invasive capabilities of esophageal squamous cell
carcinoma (ESCC) cells (37).
miR-145 negatively regulated ROCK1 and decreased the invasive
potential of ESCC cells. However, EGFR-AS1 sponged away miR-145 and
promoted ROCK1 expression. Therefore, EGFR-AS1 was demonstrated to
act as an oncogenic lncRNA that effectively potentiated ROCK1
expression (37).
Opa-interacting protein 5 antisense
RNA 1 (OIP5-AS1)
OIP5-AS1 is a cytoplasmic lncRNA (38). OIP5-AS1 inhibition was revealed to
exert repressive effects on cell proliferation, and OIP5-AS1 also
acted as an inducer of apoptotic cell death in cervical cancer
cells. Accordingly, ROCK1 was quantitatively controlled by
miR-143-3p; however, OIP5-AS1 could interfere with the
miR-143-3p-driven targeting of ROCK1 and potentiate its expression
(38).
Differentiation antagonizing
non-protein-coding RNA (DANCR)
DANCR, a novel lncRNA, was found to be overexpressed
in cervical cancer cells. Notably, DANCR stimulated the expression
of ROCK1 mainly by interfering with the miR-335-5p-induced
inhibition of ROCK1 (Fig. 2).
Transfection of cervical cancer cells with miRNA-335-5p mimics or
targeted inhibition of ROCK1 reversed the effects of upregulated
DANCR (39).
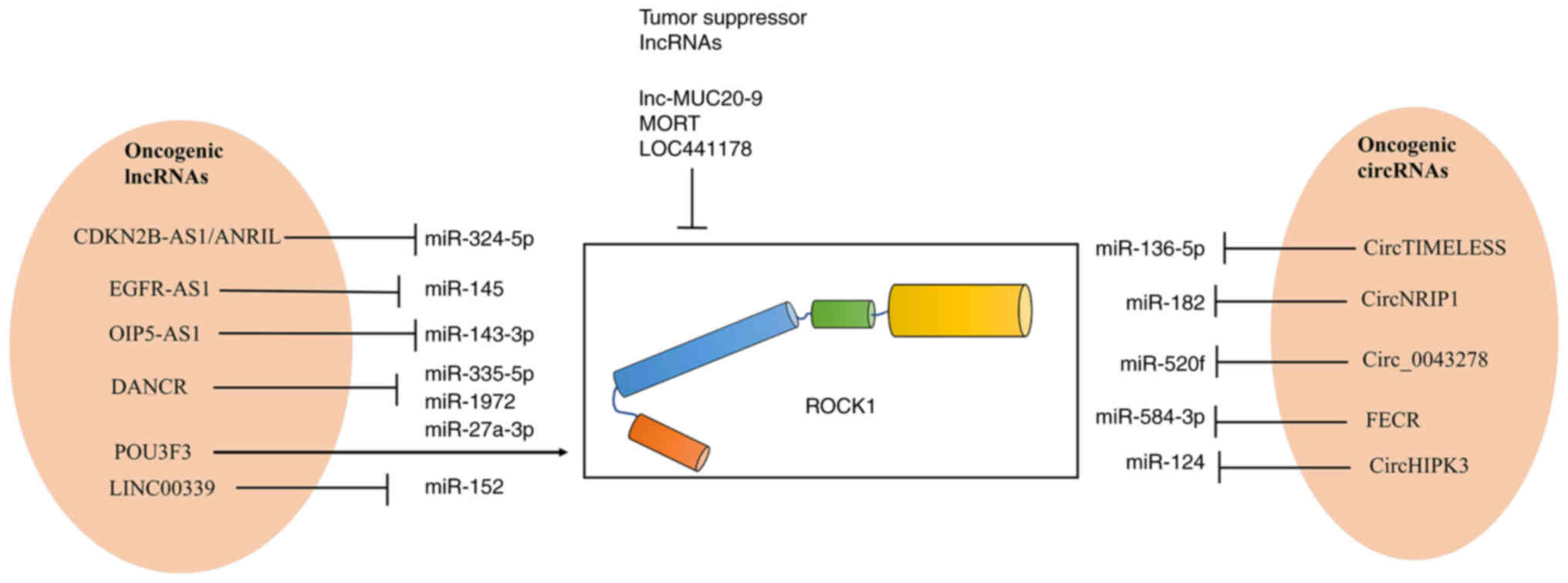 | Figure 2.Oncogenic lncRNA- and
circRNA-mediated inhibition of the ROCK1-targeting activity of
tumor suppressor miRNAs. lncRNA, long non-coding RNA; circRNA,
circular RNA; ROCK, rho-associated protein kinase; miR, microRNA;
CDKN2B AS1, cyclin dependent kinase inhibitor 2B antisense RNA 1;
EGFR AS1, epidermal growth factor receptor antisense RNA 1; OIP5
AS1, opa interacting protein 5 antisense RNA 1; DANCR,
differentiation antagonizing non-protein coding RNA; POU3F3, POU
domain class 3 transcription factor 2; NEAT1, nuclear paraspeckle
assembly transcript 1; MORT, mortal obligate RNA transcript; FECR,
FLI1 exonic circRNA. Arrow indicates activation; ┴
indicates inhibition. |
Higher expression levels of DANCR were previously
reported to be associated with a poor prognosis in clinical
patients with osteosarcoma (40).
miR-335-5p and miR-1972 both directly targeted ROCK1 mRNA
expression. Transfection of cells with mimics of miR-1972 and
miR-335-5p led to abrogation of DANCR-induced ROCK1 upregulation.
DANCR overexpression also served a vital role in the metastasis of
osteosarcoma cells to the lungs in xenografted mice (40).
In addition, DANCR was shown to stimulate both the
proliferation and the metastasizing potential of HCC cells, whereas
knockdown of DANCR exerted the opposite effects (41). Metastatic nodules on the surface of
lungs were found to be considerably decreased in size in a
xenograft mouse model established using DANCR-silenced cancer
cells. Collectively, these experiments revealed that DANCR could
act as a ceRNA, sequestering away miR-27a-3p to potentiate the
expression of LIM domain kinase 1 (LIMK1) in HCC cells. DANCR
activated the ROCK1/LIMK1/COFILIN1 signaling axis via inhibition of
miR-27a-3p (41).
POU domain class 3 transcription factor 2
(POU3F3)
POU3F3 acts as an oncogenic lncRNA, promoting an
increase in the expression level of ROCK1 in prostate cancer cells
(42). POU3F3 overexpression has
also been shown to induce an increase in ROCK1 expression in
prostate cancer cells (42).
Nuclear paraspeckle assembly transcript 1
(NEAT1)
High-grade endometrioid and serous endometrial
cancer are therapeutically resistant (43). NEAT1, an oncogenic lncRNA, was
observed to be overexpressed in this cancer type; it acted as a
molecular sponge and sequestered miR-361 away from STAT3. The
orchestrated interaction of a myriad of oncogenic proteins was
shown to induce drug resistance in endometrial cancer. The addition
of miR-361 mimics significantly decreased paclitaxel resistance,
whereas STAT3 overexpression enhanced paclitaxel resistance in
SPAC-1-L and HI cells. Furthermore, NEAT1 inhibition resulted in
decreases in the levels of ROCK1, STAT3, VEGF-A and WNT7A in
SPAC-1-L cells (43).
LINC00339
LINC00339 has been shown to potentiate ROCK1
expression (44). miR-152 directly
targeted ROCK1, although LINC00339 was shown to protect ROCK1 from
being targeted by miR-152 in HCC cells. The metastatic spread of
LINC00339-overexpressing HCC cells was also observed to be notably
higher. However, LINC00339-silenced HCC cells did not metastasize
to the lungs (44).
Small nucleolar RNA host gene 1
(SNHG1)
SNHG1 was shown to interact with a tumor suppressor
miRNA-101-3p, blocking its activity and potentiating ROCK1
expression (45). Levels of ROCK1,
phosphorylated (p)-phosphoinositide 3-kinase (PI3K) and p-AKT were
also found to be lowered in osteosarcoma cells transfected with
miR-101-3p mimics; however, miR-101-3p knockdown by miR-101-3p
inhibitor led to a robust increase in the levels of ROCK1, p-PI3K
and p-AKT (45).
Taurine-upregulated gene 1 (TUG1)
TUG1 has been shown to effectively sequester
miR-145-5p away from ROCK1, also stimulating ROCK1 expression
(46). TUG1 suppression resulted
in suppression of RhoA, ROCK1, MMP2 and MMP9 in laryngeal carcinoma
cells (46).
E2F-mediated cell proliferation enhancing
lncRNA (EPEL)
EPEL has also been shown to promote ROCK1 expression
(47). EPEL overexpression
promoted both the migratory and invasive capabilities of
osteosarcoma cells, and induced ROCK1 overexpression (47).
Terminal differentiation-induced
non-coding RNA (TINCR)
TINCR was also found to promote the migration and
invasion of HCC cells (48). ROCK1
is a target of miR-214-5p. miR-214-5p targeted ROCK1 and markedly
decreased the invasive potential of HCC cells; however, TINCR
protected ROCK1 from miR-214-5p-mediated targeting (48).
LINC00452
LINC00452 was shown to promote ovarian
carcinogenesis by antagonizing the miR-501-3p-mediated targeting of
ROCK1. Additionally, LINC00452 physically interacted with ROCK1,
thereby protecting it from ubiquitination. LINC00452 overexpression
significantly promoted tumor growth in a xenograft model, although
the simultaneous inhibition of ROCK1 markedly decreased the growth
of the tumors in spite of the overexpression of LINC00452. Tumors
developed from CaOV3 cells with overexpression of LINC00452, but
where ROCK1 had been knocked down, were observed to be smaller in
size (49).
LINC01087
LINC01087 has also been shown to act as an oncogenic
lncRNA, as it effectively enhanced ROCK1 expression by blockade of
miR-335-5p-mediated targeting of ROCK1 (50).
KCNMB2 antisense RNA 1 (KCNMB2-AS1)
Ectopically expressed miR-374a-3p was shown to
effect a significant reduction in the luciferase activity of ROCK1
in SK-MES-1 and H460 cells. However, KCNMB2-AS1 induced an increase
in the expression of ROCK1 by sponging away miR-374a-3p. A marked
decrease in the growth of the tumors developed from
KCNMB2-AS1-silenced H460 cells was also observed. The level of
KCNMB2-AS1 was notably decreased, whereas the expression of
miR-374a-3p was elevated, in the tumor tissues of mice inoculated
with KCNMB2-AS1-silenced H460 cells (51).
LINC00346
LINC00346 is capable of sponging miR-340-5p away
from ROCK1 in glioma cells. In one study, tumor growth rates were
found to be markedly decreased in mice subcutaneously injected with
LINC00346-silenced U251 cells (52).
LINC00941
LINC00941 was found to enhance the metastatic
potential of pancreatic cancer cells. miR-335-5p was shown to
target ROCK1 and inhibit the metastatic spread. However, LINC00941
caused blockade of the miR-335-5p-mediated targeting of ROCK1.
Tumors derived from LINC00941-silenced PANC-1 cells were also
observed to be smaller in size. LINC00941 inhibition resulted in a
marked decrease in the number of metastatic lesions on the surface
of the liver and lungs of tumor-bearing mice (53).
Negative regulation of ROCK1 by lncRNAs
Lnc-MUC20-9 has been demonstrated to act as a tumor
suppressor lncRNA, inhibiting the migratory potential of bladder
cancer cells (54). Lnc-MUC20-9
has been reported to bind to ROCK1, thereby inhibiting its
expression. Tumor growth was shown to be markedly decreased in mice
transplanted with lnc-MUC20-9-overexpressing bladder cancer cells
(54).
Mortal obligate RNA transcript (MORT) is a tumor
suppressor lncRNA (55).
Overexpression of MORT markedly decreased the proliferative ability
of oral squamous cell carcinoma (OSCC) cells, and led to the
downregulation of ROCK1. However, ROCK1 overexpression led to a
significant increase in the proliferative ability of the OSCC
cells. Furthermore, ROCK1 overexpression interfered with the
inhibitory effects of MORT on the proliferation of OSCC cells
(55).
LOC441178 also negatively regulated ROCK1 in OSCC
cells (56). LOC441178 knockdown
was shown to induce an increase in the ROCK1 levels (56).
Regulation of ROCK2 by lncRNAs: Oncogenic
role
miR-4435-2HG, an oncogenic lncRNA, was shown to
promote the expression of ROCK2, and inhibited the apoptotic death
of ovarian cancer cells (57).
miR4435-2HG overexpression induced the upregulation of ROCK2 in
ovarian cancer UWB1.289 cells (57). Similarly, other lncRNAs that
promote carcinogenesis have also been identified in ovarian cancer.
TTN-AS1 blocked the ROCK2-targeting activity of miR-139-5p in SKOV3
cells, and the sizes and masses of subcutaneous tumors were
observed to be significantly decreased in mice subcutaneously
injected with TTN-AS1-silenced SKOV3 cells (58).
ZNFX1 antisense RNA 1 (ZFAS1) was also shown to
promote the expression of ROCK2 by interfering with
miR-3924-mediated targeting of ROCK2 in pancreatic cancer cells
(59). Significant inhibition of
liver metastasis was observed, although the extent of lung
metastasis was not shown to be decreased in mice transplanted with
ZFAS1-depleted SW1990 cells (59).
These findings are of note, and future studies should concentrate
on the identification of the underlying mechanisms.
EGFR-AS1 has also been found to be frequently
overexpressed in bladder cancer cells (60). In bladder cancer HT-1197 cells,
miR-381 directly targeted ROCK2 and decreased the invasive
capability of the cells. miR-381-mediated targeting of ROCK2 was
inhibited by EGFR-AS1 (60).
LINC01638 overexpression induced ROCK2 upregulation in bladder
cancer cells, although overexpression of ROCK2 did not exert a
significant influence on LINC01638 expression (61). Overexpression of LINC01638 and
ROCK2, however, led to an increase in both the migratory and
invasive potentials of the bladder cancer cells. More importantly,
ROCK2 inhibition abrogated the LINC01638-induced increase in the
invasive potential of the cancer cells (61).
Tumor suppressor lncRNAs
HCC is a multifaceted disease, and lncRNAs have been
shown to fulfill fundamental roles in the onset and progression of
cancer. Tumor suppressor lncRNAs have major roles with respect to
inhibiting tumor invasion and spread. Overexpression of MAGI1
antisense RNA 3 (MAGI2-AS3) has been shown to induce the
downregulation of ROCK2 (62).
MAGI2-AS3 overexpression decreased the cell migratory and invasion
rates, whereas ROCK2 abolished the effects of overexpression of
MAGI2-AS3 (62). Taken together,
these findings clearly suggested that the MAGI2-AS3-induced
decrease in the cell migration and invasion rates was reversed by
overexpression of ROCK2. Similarly, HAND2 antisense RNA 1
(HAND2-AS1) was found to induce downregulation of ROCK2 in HCC
cells. HAND2-AS1 overexpression inhibited, whereas overexpression
of ROCK2 potently enhanced, the migratory and invasive abilities of
HCC cells (63).
circRNA-mediated regulation of ROCK1:
Cancer-promoting roles of circRNAs and ROCK1
Recent advancements in circRNA-specific
computational tools and high-throughput RNA sequencing have greatly
helped in the development of state-of-the-art techniques for
identification of circRNAs. circTIMELESS (hsa_circ_0000408) has
been shown to serve as an oncogenic circRNA in lung squamous cell
carcinoma (64). miR-136-5p
negatively regulated ROCK1, although circTIMELESS antagonized the
miR-136-5p-mediated targeting of ROCK1 and stimulated its
expression (Fig. 2). Tumor growth
was also markedly decreased in experimental mice injected with
circTIMELESS-silenced cancer cells (64).
circNRIP1 has also been demonstrated to promote the
expression of ROCK1, thereby enhancing carcinogenesis. The
luciferase activity of ROCK1-expressing MGC-803 and AGS cells was
significantly inhibited by overexpression of miR-182 (65). circNRIP1 sequestered miR-182 away
from ROCK1, and promoted the expression of ROCK1 in gastric cancer
cells. A marked decrease in Bcl-2 levels, with a concomitant
increase in Bax levels, was also identified in circNRIP1-silenced
gastric cancer cells (65).
circ_0043278 is another oncogenic circRNA that has
been reported to be involved in enhancing the proliferative and
migratory capabilities of non-small cell lung cancer (NSCLC) cells
(66). miR-520f acted as a tumor
suppressor and inhibited the growth and migration of NSCLC cells;
however, miR-520f-mediated targeting of ROCK1 was impaired by
circ_0043278 (66).
FLI1 exonic circRNA (FECR) has been shown to have
critical roles in the migration and metastasis of cancer cells
(67). FECR relieved the
inhibitory effects of miR-584-3p on ROCK1 by sponging the miRNA
away from its target. ROCK1 was found to be upregulated in
miR-584-3p inhibitor-transfected SCLC cells. FECR silencing induced
a decrease in the levels of ROCK1 in NCI-H446 and NCI-H1688 SCLC
cells, whereas ectopic expression of FECR caused a marked
upregulation of ROCK1 in NCI-H446 cells. Development of the tumors
was observed to be markedly decreased in mice injected with
FECR-silenced NCI-H446 cells. In addition, metastatic spread to the
lungs and liver was significantly suppressed in mice inoculated
with FECR-silenced NCI-H446 cells (67).
circHIPK3 has been shown to promote the
proliferation of gallbladder cancer cells (68). Ectopic overexpression of circHIPK3
promoted the proliferative ability of gallbladder cancer cells,
whereas an enforced expression of circHIPK3 exerted inhibitory
effects on the levels of miR-124. ROCK1 was directly targeted by
miR-124, although circHIPK3 was able to impair the
tumor-suppressive effects exerted by miR-124 (68).
circ0001591 has also been shown to enhance the
metastasizing potential of melanoma cells. Overexpression of
circ0001591 caused an increase in the levels of PI3K and p-AKT.
ROCK1 was directly targeted by miR-431-5p in melanoma cells.
Furthermore, circ0001591 antagonized the miR-431-5p-mediated
targeting of ROCK1 in melanoma cells (69). circ_101141 has also been shown to
serve as an oncogenic circRNA, blocking miR-1297-mediated
inhibition of ROCK1. The sizes of tumors were markedly decreased in
mice injected with circ_101141-silenced Hep3B cells (70).
circRNAs and ROCK2 as tumor suppressors
Cisplatin-resistant gastric cancer cell lines have
been demonstrated to have significantly lower ROCK2 and circCUL2
levels, and significantly higher miR-142-3p levels. In a previous
study, circCUL2 acted as a tumor suppressor circRNA, sequestering
away miR-142-3p. miR-142-3p was shown to act as an oncogenic miRNA,
directly targeting ROCK2 and promoting carcinogenesis. Extensive
tumor shrinkage was observed in mice injected with
circCUL2-overexpressing SGC-7901 cells (71).
Cancer-promoting roles of circRNAs and
ROCK2
circ_HN1 has been demonstrated to promote cancer
progression by blocking the targeting of ROCK2 by miR-302b-3p.
Tumors derived from circ_HN1-silenced HGC-27 cells were found to be
smaller in size. The levels of circ_HN1 and ROCK2 were also shown
to be decreased in the tumor tissues of xenograft model mice
established with circ_HN1-silenced HGC-27 cells (72).
Concluding remarks
The present review has critically discussed recent
information associated with the regulation of ROCK1/2 by lncRNAs
and circRNAs, providing an updated translational perspective that
may be useful in terms of guiding the selection of optimal targets
and disease-tailored interventions. ROCK-driven signaling has been
shown to serve as a linchpin during various steps of cancer.
Pharmacological and pharmaceutical researchers have started to
focus their attention on the design and development of chemical
inhibitors for ROCK1 and ROCK2. Nevertheless, much further research
needs to be done, since sufficient experimental evidence associated
with epigenetic regulation of ROCK1/2 in different types of cancer
remains lacking. However, a series of cutting-edge studies have
started to shed light on the post-transcriptional regulation of
ROCK1/2 by lncRNAs and circRNAs in different types of cancer. These
aspects are of note and allow researchers to analyze various
lncRNAs that are involved in the positive regulation of ROCK1/2 in
different cancer types. lncRNAs regulate the expression of ROCK1/2
by interfering with the miRNA-mediated targeting of ROCK, and this
knowledge has already been carried forward to tests conducted in
preclinical models. Therefore, there is a need to pursue the
interplay between ncRNAs and ROCK1/2 more comprehensively in order
for scientists to critically evaluate the efficacy of ROCK
inhibitors in different types of cancer.
Acknowledgements
Not applicable.
Funding
This study was financed through a statutory subsidy by the
Minister of Science and Higher Education as a part of the research
grant SUB.C280.21.023 (record number in the Simple System) and
statutory resources of Hirszfeld Institute of Immunology and
Experimental Therapy Polish Academy of Scienses (2021/06).
Availability of data and materials
Not applicable.
Authors' contributions
RZ and HN collected raw data after extensive
browsing through Scopus and PubMed. JS, RM and EP shortlisted the
most relevant and English language based research articles for
inclusion in this review. AAF, RA, MG and RB wrote the manuscript.
RZ and HN constructed the figures. JS, RM and EP carefully edited
the manuscript for technical errors and accurate scientific
presentation. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they do not have any
competing interests.
References
|
1
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber J, Braun CJ, Saur D and Rad R: In
vivo functional screening for systems-level integrative cancer
genomics. Nat Rev Cancer. 20:573–593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin A and Sheltzer JM: Discovering and
validating cancer genetic dependencies: Approaches and pitfalls.
Nat Rev Genet. 21:671–682. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allam M, Cai S and Coskun AF: Multiplex
bioimaging of single-cell spatial profiles for precision cancer
diagnostics and therapeutics. NPJ Precis Oncol. 4:112020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noble ME, Endicott JA and Johnson LN:
Protein kinase inhibitors: Insights into drug design from
structure. Science. 303:1800–1805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reubold TF and Eschenburg S: A molecular
view on signal transduction by the apoptosome. Cell Signal.
24:1420–1425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janse van Rensburg HJ and Yang X: The
roles of the Hippo pathway in cancer metastasis. Cell Signal.
28:1761–1772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′UTR as in the 3′UTR. Proc Natl Acad Sci USA. 104:9667–9672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khraiwesh B, Arif MA, Seumel GI, Ossowski
S, Weigel D, Reski R and Frank W: Transcriptional control of gene
expression by microRNAs. Cell. 140:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cabili MN, Dunagin MC, McClanahan PD,
Biaesch A, Padovan-Merhar O, Regev A, Rinn JL and Raj A:
Localization and abundance analysis of human lncRNAs at single-cell
and single-molecule resolution. Genome Biol. 16:202015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiu HS, Somvanshi S, Patel E, Chen TW,
Singh VP, Zorman B, Patil SL, Pan Y, Chatterjee SS; Cancer Genome
Atlas Research Network, ; et al: Pan-cancer analysis of lncRNA
regulation supports their targeting of cancer genes in each tumor
context. Cell Rep. 23:297–312.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata H, Oishi K, Yamagiwa A, Matsumoto
M, Mukai H and Ono Y: PKNbeta interacts with the SH3 domains of
Graf and a novel Graf related protein, Graf2, which are GTPase
activating proteins for Rho family. J Biochem. 130:23–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishizaki T, Maekawa M, Fujisawa K, Okawa
K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N
and Narumiya S: The small GTP-binding protein Rho binds to and
activates a 160 kDa Ser/Thr protein kinase homologous to myotonic
dystrophy kinase. EMBO J. 15:1885–1893. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leung T, Manser E, Tan L and Lim L: A
novel serine/threonine kinase binding the Ras-related RhoA GTPase
which translocates the kinase to peripheral membranes. J Biol Chem.
270:29051–29054. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Sun D, Tai J, Chen S, Yu M, Ren D
and Wang L: TFAP2C promotes stemness and chemotherapeutic
resistance in colorectal cancer via inactivating hippo signaling
pathway. J Exp Clin Cancer Res. 37:272018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu J, Niu M, Li X, Lu D, Cui J, Xu W, Li
G, Zhan J and Zhang H: FERM domain-containing protein FRMD5
regulates cell motility via binding to integrin β5 subunit and
ROCK1. FEBS Lett. 588:4348–4356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng X, Yi X, Deng J, Zou Y, Wang S, Shan
W, Liu P, Zhang Z, Chen L and Hao L: ROCK2 promotes osteosarcoma
growth and metastasis by modifying PFKFB3 ubiquitination and
degradation. Exp Cell Res. 385:1116892019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang D, Du X, Yuan R, Chen L, Liu T, Wen
C, Huang M, Li M, Hao L and Shao J: Rock2 promotes the invasion and
metastasis of hepatocellular carcinoma by modifying MMP2
ubiquitination and degradation. Biochem Biophys Res Commun.
453:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Zhang L, Du Y, Zheng H, Zhang P,
Sun Y, Wang Y, Chen J, Ding P, Wang N, et al: A novel FOXM1
isoform, FOXM1D, promotes epithelial-mesenchymal transition and
metastasis through ROCKs activation in colorectal cancer. Oncogene.
36:807–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong Y, Yang S, Wang W, Wei P, He S, Ma
H, Yang J, Wang Q, Cao L, Xiong W, et al: The interaction of
Lin28A/Rho associated coiled-coil containing protein kinase2
accelerates the malignancy of ovarian cancer. Oncogene.
38:1381–1397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu PW, Huang HD, Hsu SD, Lin LZ, Tsou AP,
Tseng CP, Stadler PF, Washietl S and Hofacker IL: miRNAMap: Genomic
maps of microRNA genes and their target genes in mammalian genomes.
Nucleic Acids Res. 34:(Database Issue). D135–D139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du Z, Sun T, Hacisuleyman E, Fei T, Wang
X, Brown M, Rinn JL, Lee MG, Chen Y, Kantoff PW and Liu XS:
Integrative analyses reveal a long noncoding RNA-mediated sponge
regulatory network in prostate cancer. Nat Commun. 7:109822016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nair L, Chung H and Basu U: Regulation of
long non-coding RNAs and genome dynamics by the RNA surveillance
machinery. Nat Rev Mol Cell Biol. 21:123–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Santer L, Bär C and Thum T: Circular RNAs:
A novel class of functional RNA molecules with a therapeutic
perspective. Mol Ther. 27:1350–1363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Xiao Y, Ma L and Wang J: Regulating
of cell cycle progression by the lncRNA CDKN2B-AS1/miR-324-5p/ROCK1
axis in laryngeal squamous cell cancer. Int J Biol Markers.
35:47–56. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng Z, Li X, Qiu M, Luo R, Lin J and Liu
B: LncRNA EGFR-AS1 Upregulates ROCK1 by sponging miR-145 to promote
esophageal squamous cell carcinoma cell invasion and migration.
Cancer Biother Radiopharm. 35:66–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song L, Wang L, Pan X and Yang C: lncRNA
OIP5-AS1 targets ROCK1 to promote cell proliferation and inhibit
cell apoptosis through a mechanism involving miR-143-3p in cervical
cancer. Braz J Med Biol Res. 53:e88832020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang H, Zhang C, Guan H, Liu J and Cui Y:
LncRNA DANCR promotes cervical cancer progression by upregulating
ROCK1 via sponging miR-335-5p. J Cell Physiol. 234:7266–7278. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17:892018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo D, Li Y, Chen Y, Zhang D, Wang X, Lu
G, Ren M, Lu X and He S: DANCR promotes HCC progression and
regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1
pathway. Cell Prolif. 52:e126282019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan X, Xiang J, Zhang Q and Bian C: Long
noncoding RNA POU3F3 promotes cancer cell proliferation in prostate
carcinoma by upregulating rho-associated protein kinase 1. J Cell
Biochem. Nov 26–2018.(Epub ahead of print). doi:
10.1002/jcb.28101.
|
|
43
|
Dong P, Xiong Y, Yue J, Xu D, Ihira K,
Konno Y, Kobayashi N, Todo Y and Watari H: Long noncoding RNA NEAT1
drives aggressive endometrial cancer progression via
miR-361-regulated networks involving STAT3 and tumor
microenvironment-related genes. J Exp Clin Cancer Res. 38:2952019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen K and Zhang L: LINC00339 regulates
ROCK1 by miR-152 to promote cell proliferation and migration in
hepatocellular carcinoma. J Cell Biochem. 120:14431–14443. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng R, Zhang J and Chen J: lncRNA SNHG1
negatively regulates miRNA-101-3p to enhance the expression of
ROCK1 and promote cell proliferation, migration and invasion in
osteosarcoma. Int J Mol Med. 43:1157–1166. 2019.PubMed/NCBI
|
|
46
|
Zhuang S, Liu F and Wu P: Upregulation of
long noncoding RNA TUG1 contributes to the development of
laryngocarcinoma by targeting miR-145-5p/ROCK1 axis. J Cell
Biochem. 120:13392–13402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Liu Z, Lu S and Hu B: EPEL
promotes the migration and invasion of osteosarcoma cells by
upregulating ROCK1. Oncol Lett. 17:3133–3140. 2019.PubMed/NCBI
|
|
48
|
Hu M, Han Y, Zhang Y, Zhou Y and Ye L:
lncRNA TINCR sponges miR-214-5p to upregulate ROCK1 in
hepatocellular carcinoma. BMC Med Genet. 21:22020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang J, Wang WG and Zhang KQ: LINC00452
promotes ovarian carcinogenesis through increasing ROCK1 by
sponging miR-501-3p and suppressing ubiquitin-mediated degradation.
Aging (Albany NY). 12:21129–21146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
She JK, Fu DN, Zhen D, Gong GH and Zhang
B: LINC01087 is highly expressed in breast cancer and regulates the
malignant behavior of cancer cells through miR-335-5p/Rock1. Onco
Targets Ther. 13:9771–9783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang H and Wang Z and Wang Z: Long
noncoding RNA KCNMB2-AS1 increases ROCK1 expression by sponging
microRNA-374a-3p to facilitate the progression of non-small-cell
lung cancer. Cancer Manag Res. 12:12679–12695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen X, Li D, Chen L, Hao B, Gao Y, Li L,
Zhou C, He X and Cao Y: Long noncoding RNA LINC00346 promotes
glioma cell migration, invasion and proliferation by up-regulating
ROCK1. J Cell Mol Med. 24:13010–13019. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang J, He Z, Xu J, Chen P and Jiang J:
Long noncoding RNA LINC00941 promotes pancreatic cancer progression
by competitively binding miR-335-5p to regulate ROCK1-mediated
LIMK1/Cofilin-1 signaling. Cell Death Dis. 12:362021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dai R, Zhou Y, Chen Z, Zou Z, Pan Z, Liu P
and Gao X: Lnc-MUC20-9 binds to ROCK1 and functions as a tumor
suppressor in bladder cancer. J Cell Biochem. 121:4214–4225. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jin Z, Jiang S, Jian S and Shang Z: Long
noncoding RNA MORT overexpression inhibits cancer cell
proliferation in oral squamous cell carcinoma by downregulating
ROCK1. J Cell Biochem. Feb 25–2019.(Epub ahead of print). doi:
10.1002/jcb.28449. View Article : Google Scholar
|
|
56
|
Xu K, Tian H, Zhao S, Yuan D, Jiang L, Liu
X, Zou B and Zhang J: Long Noncoding RNA LOC441178 reduces the
invasion and migration of squamous carcinoma cells by targeting
ROCK1. Biomed Res Int. 2018:43576472018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu J, Wang L, Zhao W, Huang Y, Wang Z and
Shen H: mi-R4435-2HG promotes proliferation and inhibits apoptosis
of cancer cells in ovarian carcinoma by upregulating ROCK2. Oncol
Lett. 19:1305–1309. 2020.PubMed/NCBI
|
|
58
|
Liu X, Li Y, Wen J, Qi T and Wang Y: Long
non-coding RNA TTN-AS1 promotes tumorigenesis of ovarian cancer
through modulating the miR-139-5p/ROCK2 axis. Biomed Pharmacother.
125:1098822020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu J, Zhu Y and Ge C: LncRNA ZFAS1
promotes pancreatic adenocarcinoma metastasis via the RHOA/ROCK2
pathway by sponging miR-3924. Cancer Cell Int. 20:2492020.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yuan S, Luan X, Chen H, Shi X and Zhang X:
Long non-coding RNA EGFR-AS1 sponges micorRNA-381 to upregulate
ROCK2 in bladder cancer. Oncol Lett. 19:1899–1905. 2020.PubMed/NCBI
|
|
61
|
Yuan S, Luan X, Han G, Guo K, Wang S and
Zhang X: LINC01638 lncRNA mediates the postoperative distant
recurrence of bladder cancer by upregulating ROCK2. Oncol Lett.
18:5392–5398. 2019.PubMed/NCBI
|
|
62
|
Fang G, Wang J, Sun X, Xu R, Zhao X, Shao
L, Sun C and Wang Y: LncRNA MAGI2-AS3 is downregulated in the
distant recurrence of hepatocellular carcinoma after surgical
resection and affects migration and invasion via ROCK2. Ann
Hepatol. 19:535–540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiang L, He Y, Shen G, Ni J, Xia Z, Liu H,
Cao Y and Li X: lncRNA HAND2-AS1 mediates the downregulation of
ROCK2 in hepatocellular carcinoma and inhibits cancer cell
proliferation, migration and invasion. Mol Med Rep. 21:1304–1309.
2020.PubMed/NCBI
|
|
64
|
Zhang W, Shi J, Cheng C and Wang H:
CircTIMELESS regulates the proliferation and invasion of lung
squamous cell carcinoma cells via the miR-136-5p/ROCK1 axis. J Cell
Physiol. 235:5962–5971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liang L and Li L: Down-regulation of
circNRIP1 promotes the apoptosis and inhibits the migration and
invasion of gastric cancer cells by miR-182/ROCK1 Axis. Onco
Targets Ther. 13:6279–6288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui J, Li W, Liu G, Chen X, Gao X, Lu H
and Lin D: A novel circular RNA, hsa_circ_0043278, acts as a
potential biomarker and promotes non-small cell lung cancer cell
proliferation and migration by regulating miR-520f. Artif Cells
Nanomed Biotechnol. 47:810–821. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li L, Li W, Chen N, Zhao H, Xu G, Zhao Y,
Pan X, Zhang X, Zhou L, Yu D, et al: FLI1 Exonic circular RNAs as a
novel oncogenic driver to promote tumor metastasis in small cell
lung cancer. Clin Cancer Res. 25:1302–1317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kai D, Yannian L, Yitian C, Dinghao G, Xin
Z and Wu J: Circular RNA HIPK3 promotes gallbladder cancer cell
growth by sponging microRNA-124. Biochem Biophys Res Commun.
503:863–869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yin D, Wei G, Yang F and Sun X: Circular
RNA has circ 0001591 promoted cell proliferation and metastasis of
human melanoma via ROCK1/PI3K/AKT by targeting miR-431-5p. Hum Exp
Toxicol. 40:310–324. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang T, Zhang L, Han D, Tursun K and Lu
X: Circular RNA hsa_Circ_101141 as a competing endogenous RNA
facilitates Tumorigenesis of hepatocellular carcinoma by regulating
miR-1297/ROCK1 pathway. Cell Transplant. 29:9636897209480162020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Peng L, Sang H, Wei S, Li Y, Jin D, Zhu X,
Li X, Dang Y and Zhang G: circCUL2 regulates gastric cancer
malignant transformation and cisplatin resistance by modulating
autophagy activation via miR-142-3p/ROCK2. Mol Cancer. 19:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang D, Jiang X, Liu Y, Cao G, Zhang X and
Kuang Y: Circular RNA circ_HN1 facilitates gastric cancer
progression through modulation of the miR-302b-3p/ROCK2 axis. Mol
Cell Biochem. 476:199–212. 2021. View Article : Google Scholar : PubMed/NCBI
|