Introduction
Pancreatic cancer is an exocrine tumor that
originates mainly from glandular duct cells. Since its symptoms are
non-specific, pancreatic cancer is often diagnosed only after the
tumor has spread, which accounts for the 5-year survival rate being
<10% (1). By the time it is
diagnosed, ~90% of patients have locally advanced tumors that have
invaded retroperitoneal structures, spread to local lymph nodes or
have metastasized to the liver and the lungs, making pancreatic
cancer one of the tumor types with the poorest prognosis for
patients today (2,3). Treatment of pancreatic cancer is
supplemented by chemotherapy with gemcitabine or capecitabine, as
well as radiotherapy in addition to pancreaticoduodenectomy
(4). Pancreatic adenocarcinoma
(PAAD) comprises 85% of pancreatic malignancies and is often poorly
treated with or without these adjuvant therapies; by 2020, the
5-year survival rate for PAAD has increased to 10% (4,5).
Pseudogenes are non-functional gene fragments on the
genome that are related to their parental genes but are themselves
unable to be translated into functional proteins; they may be
transcribed as long non-coding (lnc)RNAs or translated into
non-functional polypeptides (6).
The majority of the pseudogenes in the human genome are processed
pseudogenes that originate from the retrotransposition of mature
mRNAs; a number of these processed pseudogenes have lost the
protein-coding capacity of their parental gene, and they function
as lncRNAs that interact with other proteins or regulate the
expression of their parental genes by competitively binding to
microRNAs (miRNAs) (6), such as
the cases of BRAF (7), KRAS
(8) and PTEN (9) pseudogenes. These lncRNA-encoding
pseudogenes have also been recognized as important regulators in
cancer development, making them novel prognostic factors and
potential targets for cancer management (10).
Cancer cells communicate with other tumor cells,
tumor associated fibroblasts and immune cells to shape the tumor
microenvironment to a cancer-supportive status by secreting
exosomes (11–13). lncRNAs loaded in the cancer
cell-secreted exosomes can influence gene expression in the
recipient cells by interacting with protein, mRNA and miRNA
molecules that have significant impact on the biology of these
cells (14). In a preliminary
study, exosomal lncRNAs derived from the blood samples of cancer
patients were investigated in comparison with those derived from
the blood samples of healthy individuals documented in the exorBase
website (15). It was identified
that the level of lncRNA encoded by the adenylate kinase 4
pseudogene 1 (AK4P1) was significantly increased in the exosomes
from the blood samples of patients with PAAD compared with the
levels in circulating exosomes from healthy individuals. According
to the records in the Ensemble database (https://asia.ensembl.org/index.html), the pseudogene
AK4P1 is located on human chromosome 17 and is 2,386 base pairs
long. It is transcribed into a lncRNA of 672 bases in length from
the positive strand; its expression and function in cancer biology
have not yet been examined. Adenylate kinase 4 (AK4), the parent
gene of AK4P1, has been proposed as a novel cancer-promoting gene
in various types of solid tumors, such as lung (16), breast (17), ovarian (18) and bladder cancer (19), but the function of this gene in
pancreatic cancer has not been evaluated. In the present study, The
Cancer Genome Atlas (TCGA)-PAAD data were downloaded from the Xena
platform (20), and the
association between the increased transcription levels of AK4 and
AK4P1 with the development of PAAD and the decrease in overall
survival of patients was identified. The clinical significance of
the expression level of these two genes was further confirmed in
the present study, and AK4P1 was identified as a PAAD-driving
pseudogene, and this function may be through regulating the
expression of AK4.
Materials and methods
Cell culture and transfection
Primary PAAD cells (cat. no. GPC0158) derived from
pathological tissue samples of human pancreatic cancer obtained by
surgery were purchased from China Center for Type Culture
Collection. The present study was approved by the ethical review
committee of Suizhou Hospital affiliated to Hubei University of
Medicine (Suizhou, China). The primary PAAD cells were cultured in
a serum-free complete culture medium for human pancreatic cancer
primary cells (cat. no. CM-H153; Procell Life Science &
Technology Co., Ltd.) in an incubator with humidified atmosphere at
37°C with 5% CO2. The cells were passaged at 1:2 ratio
once every 3 days and were used in experiments within 10 passages
upon arrival.
Overexpression of AK4P1 in primary PAAD cells was
achieved by transfection with AK4P1 overexpression plasmids
(pGPU6-eGFP-Puro) constructed by Shanghai GenePharma Co., Ltd. at a
final concentration of 1 µg/ml. The empty vector was used as
negative control. AK4 gene expression was suppressed in primary
PAAD cells (cell density, 60–70%) by transfection with
AK4-targeting siRNA (A09011; GenePharma) constructed by GenePharma
at a final concentration of 50 nM. The siRNA sequences were as
follows: AK4-siRNA: 5′-CCCAGAACTTTGGTCTCCAGCATCT-3′; and AK4-SiRNA:
5′-GCACCGAAGTTGGTGAGATGGCAAA-3′. The negative control without
homology with the target gene sequence was purchased from
GenePharma (A06001; GenePharma; the sequence is not available by
the company). Lipofectamine® 3000 (cat. no. L3000075;
Invitrogen; Thermo Fisher Scientific, Inc.) was used as the
transfection reagent for transient transfection at room temperature
for 10–15 min following the manufacturer's protocol. After 24 h of
transfection, further experiments with cells followed.
AK4 knockout in primary PAAD cells was achieved
using a customized CRISPR/Cas9 gene knockout kit [cat. no. L00694;
GenScript; single guide (sg)RNA was designed on the CRISPR Design
Tool (http://crispr.mit.edu/); the pU6gRNACas9
plasmid (C11002; GenePharma) was used for transfection; sgRNA
sequence, 5′-GGGATGCGCTCCCCGGCCAT-3′, targets the promoter region
of the AK4 gene] constructed by GenScript following the
manufacturer's protocol and was confirmed by the negative results
of RT-qPCR and western blotting assays evaluating the transcription
and protein expression levels of this gene, respectively (data are
shown in Fig. S1).
Analysis of PAAD tissue specimens and
clinical data of patients
The present study was approved by the ethical review
committee of Suizhou Hospital affiliated to Hubei University of
Medicine; written informed consent was provided by each participant
or the legal representative/guardian of the participant. The
transcriptomic and survival data of the patients in the Genomic
Data Commons (GDC) and the Cancer Genome Atlas (TCGA)-PAAD dataset
were downloaded from Xena platform (20). The data from the exorBase database
(http://www.exorbase.org/exoRBase/download/toIndex)
were downloaded to explore exosomal lncRNAs with significant
differences in their abundances in circulating exosomes derived
from patients with PAAD and from healthy volunteers. PAAD
pathological tissue specimens and the paired adjacent non-cancerous
tissue specimens (~2 cm from the tumoral tissue) were collected
during surgery from 31 patients with PAAD who received radical
surgery before adjuvant chemo/radiotherapy in our facility between
January 2019 and June 2020. The inclusion criteria were as follows:
i) Patients were 19–75 years old; ii) patients with PAAD were
diagnosed by CT and MRI before surgery, and confirmed by
pathological examinations after surgery; iii) Patients with PAAD
had not received radiotherapy and chemotherapy before surgery; and
iv) the basic information of the patients was complete. The
exclusion criteria were as follows: i) Metastatic pancreatic
cancer; ii) pancreatic tissue that had not been confirmed by
pathological examination; and iii) the data were incomplete and
difficult to be included in statistical analysis. The tissue
specimens were stored in liquid nitrogen before analysis.
Circulating exosomes were extracted from frozen plasma samples
derived from each of the 31 PAAD patient or 25 healthy individuals
who received routine medical check-ups at our facility by
ultracentrifugation following a previously described procedure
(21) and were verified by
transmission electronic microscopy before cryopreservation (TEM
image of the exosomes is shown in Fig. S2). In short, peripheral blood was
centrifuged at 2,000 × g for 10 min at 4°C to collect plasma, and
then centrifuged at 4,000 × g for 20 min at 4°C, 12,000 × g for 30
min at 4°C, filtered with a 0.22-µm filter to collect the plasma
and centrifuged at 100,000 × g for 60 min at 4°C twice, and finally
the pellet was resuspended in PBS for use in follow-up analysis.
Exosomes (20 µl) were pipetted onto the copper net dropwise and
left for 5 min at RT. The excess liquid was absorbed at the side
using filter paper, then 30 µl of 2% phosphotungstic acid (cat. no.
C0681090235; Nanjing Reagent) was added dropwise, and negative
staining conducted for 3 min at RT. After the negative staining,
the excess liquid was again absorbed with filter paper, and baking
was performed under an incandescent lamp for 10 min. The shape and
size of the exosomes was examined with a transmission electron
microscope at 80 kV acceleration voltage. Clinicopathological
characteristics of the patients are shown in Table SI. Paraffin-embedded specimens of
83 patients diagnosed with PAAD in Suizhou Hospital between March
2012 and July 2018 were collected. qPCR was performed to detect the
expression levels of AK4 and AK4P1. These patients were followed up
by telephone for survival analysis. The clinicopathological
characteristics of these patients are presented in Table SII. The patients were divided into
high and low groups based on the median of the transcription levels
of AK4P1 and AK4 as detected by RT-qPCR in their tissue specimens
and the overall survival of these patients was compared.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from all samples was extracted by
TRIzol® method (Invitrogen; Thermo Fisher Scientific,
Inc.). Primers were designed for the specific detection of AK4 or
AK4P1 gene transcript and were constructed by GeneCopoeia, Inc. The
primer sequences were as follows: AK4 forward,
5′-CTTGTGCCAGCTTCCCCGGCTC-3′ and reverse,
5′-CCTCGCGAAGGCAATGGCT-3′; AK4P1 forward,
5′-GTTGTCTTAAAAGTCTCTCCTTCCCCCTGT-3′ and reverse,
5′-CCTCGCGAAGGCAATGGCT-3′; and β-actin forward,
5′-CTCTTCCAGCCTTCCTTCCT-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′.
qPCR was performed using BlazeTaq One-Step SYBR Green RT-qPCR Kit
(GeneCopoeia, Inc.) and a thermocycler using 1 µg cDNA as the
template in each assay. The thermocycling conditions were as
follows: Pre-denaturation at 95°C for 10 min, denaturation at 95°C
for 15 sec and extension at 60°C for 30 sec (40 cycles); and
melting curve: 95°C for 15 sec, 60°C for 1 min and 95°C for 15
sec). β-actin was used as housekeeping gene for the comparison of
AK4P1 or AK4 gene transcription levels among tissue specimens or
cells cultured in vitro and AK4P1 transcription levels in
the exosome samples. Data were analyzed by the 2−∆∆Cq
method (22).
Western blotting
Protein levels of AK4 in the tissue specimens or
cells cultured in vitro were assessed by western blotting
using β-actin as internal reference gene. In brief, total protein
from tissue specimens or cells cultured in vitro was
extracted using harsh RIPA lysis buffer (cat. no. 89900; Thermo
Fisher Scientific, Inc.). The protein concentration was determined
using a BCA protein concentration assay kit (cat. no. 23227; Thermo
Fisher Scientific, Inc.). A total of 30 µg protein was used in each
western blot assay. The protein sample was separated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and the protein
was transferred to a PVDF membrane (cat. no. ab133411; Abcam). The
PVDF membrane was incubated for 2 h in a blocking solution (TBS
with 0.1% Tween-20 solution containing 5% BSA (cat. no. A8010;
Beijing Solarbio Science & Technology Co., Ltd.; pH 7.4) at
room temperature. Then, the PVDF membrane was incubated with
antibody at 4°C overnight. The membrane was then incubated with
horseradish peroxidase-labeled goat anti-rabbit IgG (1:3,000; cat.
no. ab6721; Abcam) at room temperature for 3 h. Digital imaging and
signal quantification were performed using the ECL Plus Western
Blotting Substrate (cat. no. 32134; Thermo Fisher Scientific, Inc.)
using Bio-Rad Image Lab Software (version 4.0; Bio-Rad
Laboratories, Inc.). Primary antibodies used in western blot assays
were as follows: Anti-AK4 mouse monoclonal antibody (dilution,
1:1,000; cat. no. sc-271161; Santa Cruz Biotechnology, Inc.) and
anti-β-actin rabbit monoclonal antibody (dilution, 1:1,000; cat.
no. ab115777; Abcam).
Cell viability and apoptosis
assays
PAAD cell viability was evaluated by Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Inc.). Briefly, the cells were
inoculated on a 96-well-plate at an initial density of
5×103 cells/well and were cultured in serum-free
complete culture medium for human pancreatic cancer primary cells
at 37°C for 48 h; after which, the cells were incubated with 10 µl
CCK-8 working solution at 37°C for 1 h. Absorbance was measured at
an optical density of 450 nm using a microplate reader.
Apoptosis of the PAAD cells in response to
gemcitabine challenge was evaluated using TUNEL method. The cells
were inoculated on a 96-well-plate at a density of 2×104
cells/well, before they were treated with gemcitabine (LY-188011;
Abmole Bioscience Inc.) at a final concentration of 10 nM for 24 h.
Apoptosis was assessed by TUNEL staining using a One Step TUNEL
Apoptosis Assay Kit (Beyotime Institute of Biotechnology) according
to the manufacturer's protocol and fluorimetry using a fluorescence
microscope (excitation wavelength, 450–500 nm; emission wavelength,
515–565 nm). Briefly, the cells were washed with PBS, fixed with
immunostaining fixative (P0098; Beyotime Institute of
Biotechnology) for 30–60 min and washed with PBS, and PBS
containing 0.1% Triton X-100 was added. The cells were incubated in
an ice bath for 2 min. After washing with PBS, 50 µl TUNEL
detection solution was added to the cells. The cells were incubated
for 60 min in the dark at 37°C. Subsequently, the section was
counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). After
washing with PBS, the cells were re-suspended with 250–500 µl PBS
and detected by fluorescence microscope (Olympus) at an objective
magnification of ×800, with filter blocks for DAPI and fluorescein.
At least 300 cells in each group were randomly selected for
quantification, and the apoptosis rate was evaluated by the number
of TUNEL staining positive cells.
Statistical analysis
GraphPad Prism software (version 9.02; GraphPad
Software, Inc.) was used for statistical analysis. All data were
normalized to the mean value of the data in the control group under
the same experimental settings and are presented as the mean ± SD,
unless otherwise indicated. An unpaired Student's t-test was used
for comparing two groups of data. One-way analysis of variance was
used for multi-group comparison followed by Tukey's post hoc test.
Association analysis was performed by simple linear regression.
Kaplan-Meier survival curve and log-rank test was used for survival
analysis. A difference was considered statistically significant
when P<0.05.
Results
Analysis of the transcription levels
of AK4 and AK4P1 in publicly available data
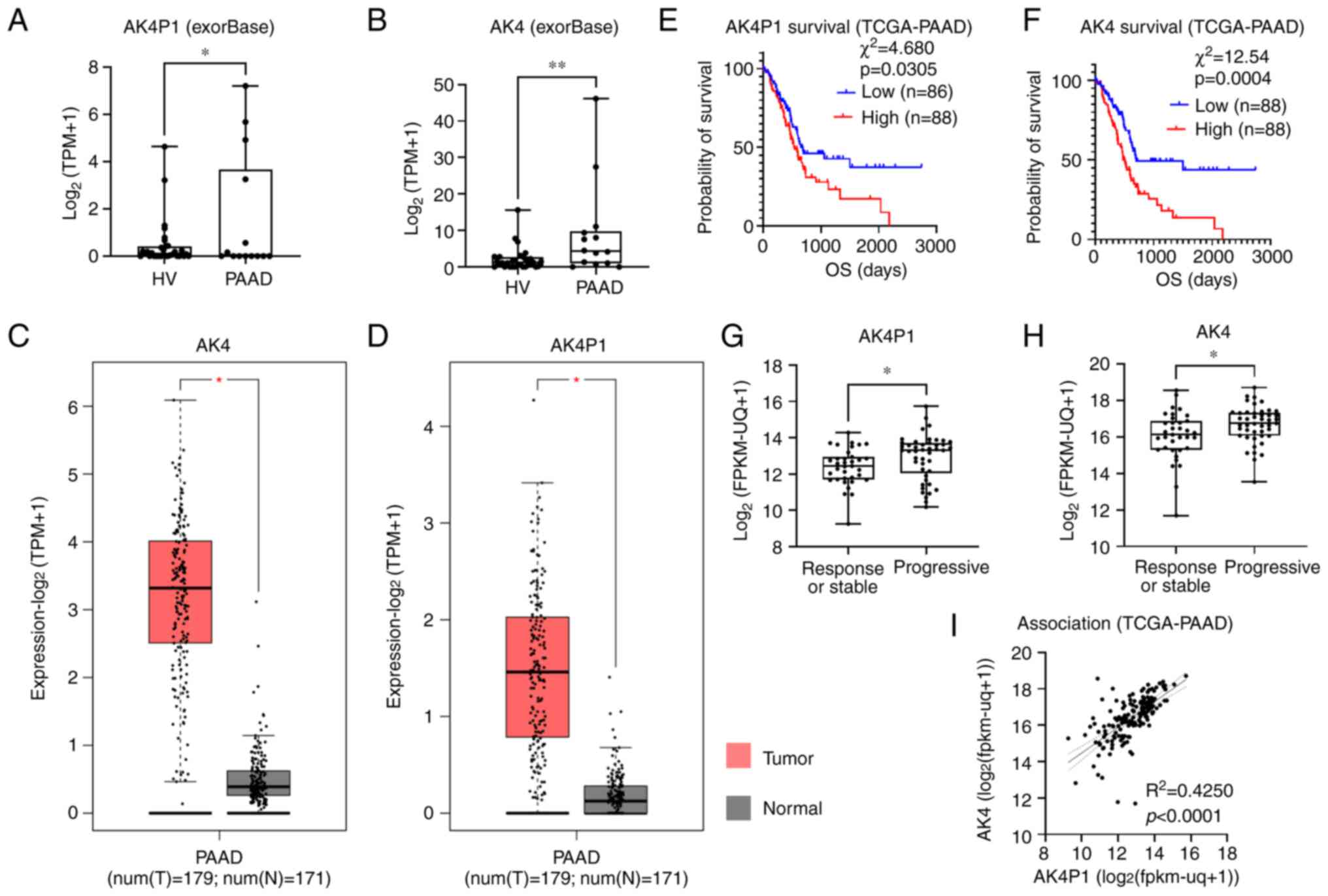
AK4P1 was identified as an exosomal lncRNA whose
abundance in the circulating exosomes derived from patients with
PAAD was significantly higher compared with that in the circulating
exosomes derived from healthy individuals (Fig. 1A). It was also found that the
abundance of AK4 mRNA in the circulating exosomes derived from
patients with PAAD was significantly higher compare with that in
the circulating exosomes derived from healthy individuals (Fig. 1B). To further explore the
expression levels of AK4P1 and AK4 mRNA in publicly available data,
the transcriptomic and survival data of patients in the GDC
TCGA-PAAD dataset were downloaded from the Xena platform (Fig. 1C and D). The high transcription
levels of either AK4P1 or AK4 mRNA were significantly associated
with the decrease in the overall survival and tumor progression of
patients with PAAD (Fig. 1E-H).
The transcription levels of AK4P1 were significantly associated
with those of AK4 mRNA in the tissue specimens of 179 patients with
PAAD (Fig. 1I).
Upregulation in AK4P1 and AK1 gene
expression in PAAD is associated with shortened overall survival of
patients
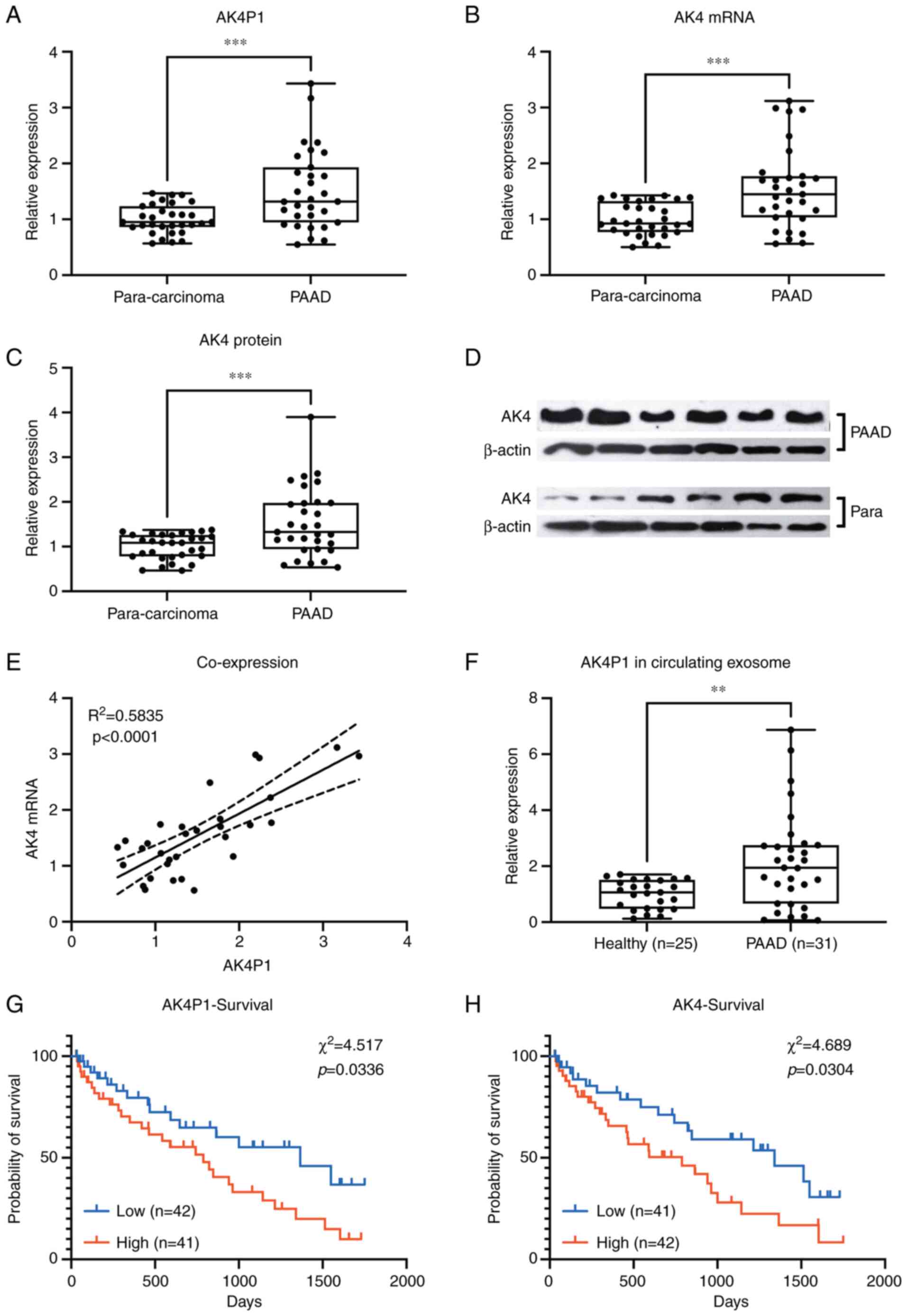
The expression levels of AK4 gene or AK4P1
pseudogene in 31 pairs of PAAD pathologic tissue specimens and
para-carcinoma tissue specimens were compared. RT-qPCR results
demonstrated that the transcription levels of AK4 and AK4P1 were
significantly higher in PAAD tissue specimens compared with those
in the para-carcinoma tissue specimens (Fig. 2A and B, respectively). Western blot
results also showed a significant increase in AK4 protein
expression levels in the PAAD tissue specimens compared with those
in the para-carcinoma tissue specimens (Fig. 2C and D).
The expression levels of AK4 mRNA or AK4P1
pseudogene were further divided into high and low groups based on
the median value, and it was observed that the mRNA expression
levels of AK4 significantly associated with the transcription
levels of lncRNA AK4P1 in the 31 PAAD tissue specimens (Fig. 2E). In addition, it was found that
the exosomes obtained from patients with PAAD contained
significantly higher levels of AK4P1 transcripts compared with
those in the circulating exosomes derived from healthy individuals,
as revealed by RT-qPCR (Fig. 2F).
By integrating the AK4 and AK4P1 transcription levels in
cryopreserved PAAD tissue specimens and the survival of each
patient, it was identified that high expression levels of AK4 and
AK4P1 in PAAD tissue specimens were significantly associated with
decreased overall survival of patients (Fig. 2G and H).
Overexpression of AK4P1 pseudogene
increases AK4 gene expression and affects the cellular biological
functions of PAAD cells in vitro
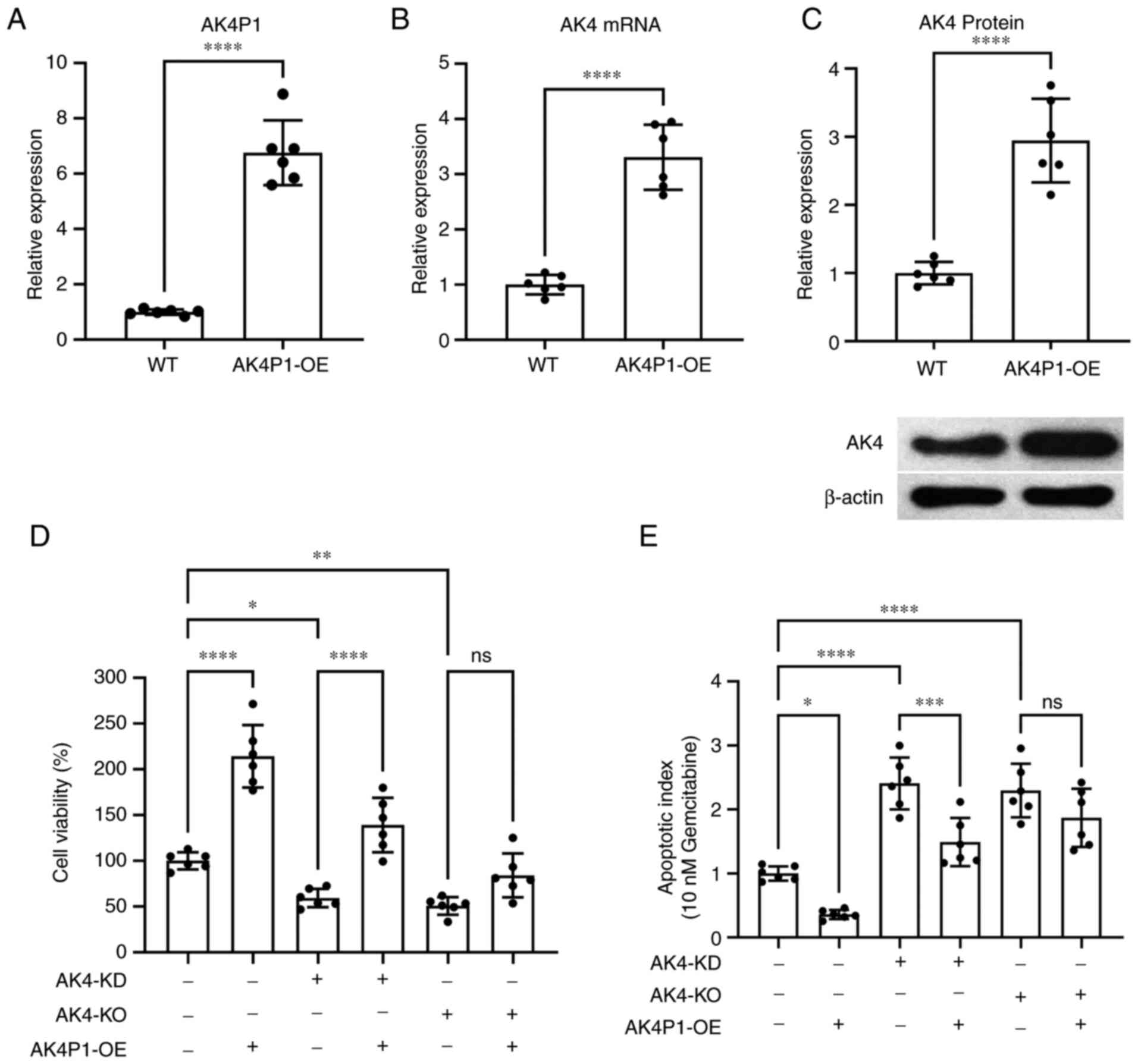
To investigate the function of AK4P1 pseudogene in
PAAD cells, the cells were transfected with AK4P1 overexpression
vectors (Fig. 3A). It was observed
that AK4P1 overexpression significantly upregulated the mRNA and
protein levels of AK4 in PAAD cells in vitro (Fig. 3B and C, respectively). It was also
found that AK4P1 overexpression significantly increased PAAD cell
viability in vitro (Fig.
3D), and this upregulation was weakened by siRNA-mediated AK4
gene knockdown or abrogated by AK4 gene knockout achieved using
CRISPR/Cas9 machinery. The cell apoptosis rate was normalized to
the PAAD cells treated with gemcitabine (which could induce tumor
cell apoptosis), and the cell apoptosis rate of different groups
was compared (Fig. 3E), showing
that AK4P1 overexpression significantly rescued PAAD cells from
gemcitabine-induced cell apoptosis. In addition, when AK4P was
overexpressed, the increase in apoptosis caused by AK4-KD was
rescued, compared with the PAAD cells with siRNA-mediated AK4 gene
knockdown. However, when AK4 gene was completely knocked out in
PAAD cells, the increase in apoptosis level could not be rescued by
AK4P1 overexpression (Fig. 3E).
The representative TUNEL staining images are shown in Fig. S3.
Exosomal AK4P1 regulates cell
proliferation and apoptosis in recipient PAAD cells in vitro
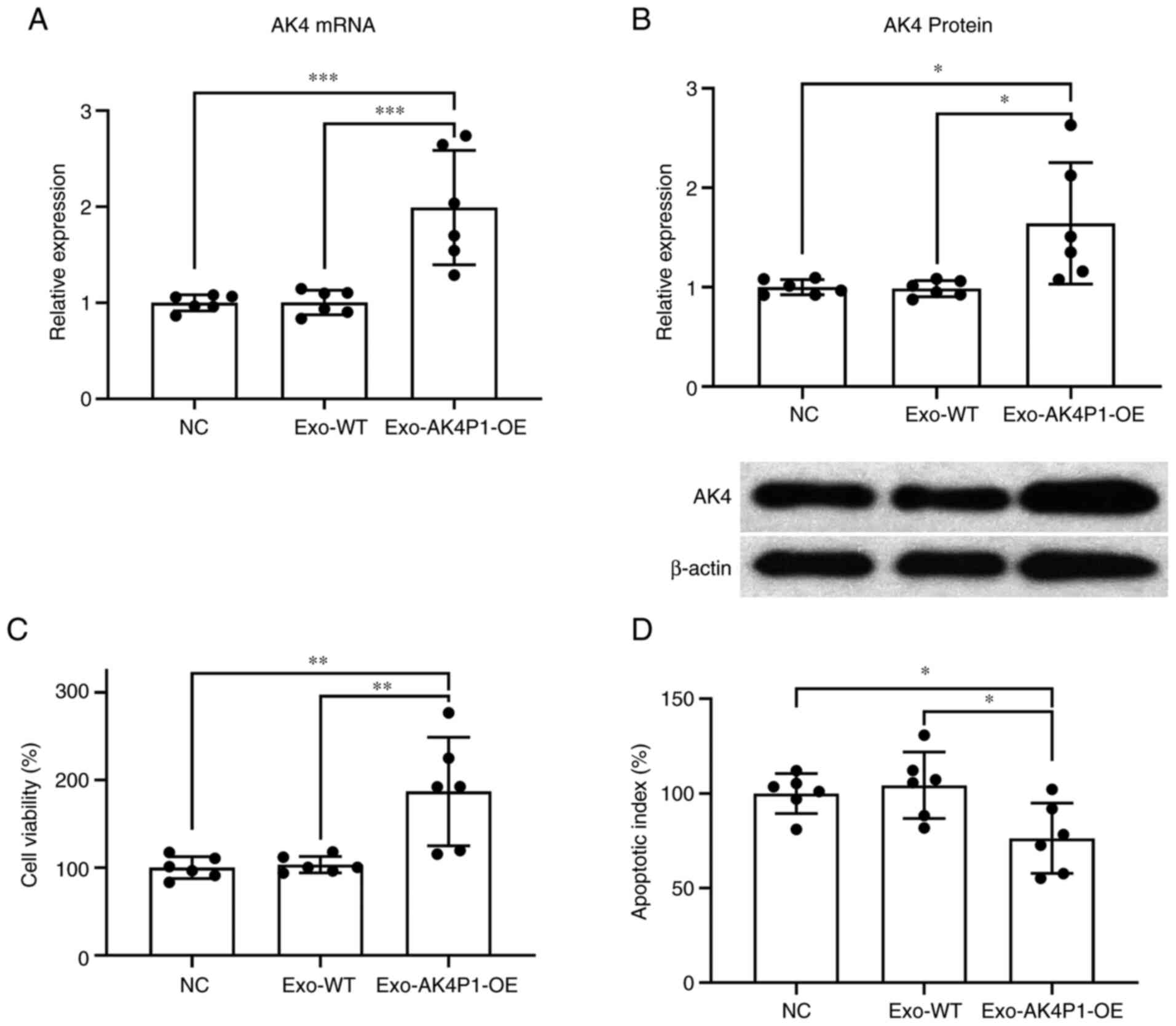
Since the upregulation in AK4P1 levels in the
circulating exosomes of patients with PAAD compared was confirmed,
it was hypothesized that pancreatic cancer cells with high AK4P1
expression may deliver AK4P1 transcripts through exosomes, thereby
affecting recipient cell growth and apoptosis. To investigate this,
exosomes in the cell culture media conditioned by PAAD cells with
or without AK4P1 overexpression were collected and used to incubate
wild-type PAAD cells. RT-qPCR and western blot results showed that
treatment with exosomes derived from AK4P1-overexpressing PAAD
cells significantly increased the mRNA and protein expression
levels of AK4 in the recipient PAAD cells compared with those
treated with exosomes derived from donor cells without AK4P1
overexpression (Fig. 4A and B). It
was further demonstrated that treatment with AK4P1-overexpressing
PAAD cell-derived exosomes resulted in a significant increase in
cell viability and a significant decrease in gemcitabine-induced
cell apoptosis in the recipient cells compared with the recipients
treated with exosomes derived from donor cells without AK4P1
overexpression (Fig. 4C and D).
The representative TUNEL staining images are shown in Fig. S4.
Discussion
AK4 has been proposed as a novel cancer-promoting
gene in several types of solid tumors. In breast cancer cells, for
instance, high AK4 expression levels have been reported to be
associated with the progression of Her-2 positive breast cancer,
and its knockdown significantly reduced the colony formation,
proliferation and migration of breast cancer cells in vitro
as well as the growth of xenografted tumors on nude mice (17). Similar findings were also reported
in lung (16), ovarian (18) and bladder cancer (19). Upregulated AK4 protein expression
was also found to be associated with the development of therapeutic
resistance of cancer cells, such as tamoxifen resistance in breast
cancer cells (23), platinum-based
drug resistance in HeLa cells (24) and radio-resistance in oral squamous
cell carcinoma cells (25),
possibly by modulating mitochondrial metabolism and disrupting the
mitochondria-mediated activation of the intrinsic apoptotic pathway
(24,26). Hypoxia responsive transcription
factor HIF-1 was found to initiate the transcription of AK4 in
colorectal cancer cells (27), and
AK4 protein was shown to interact with and stabilize HIF-1α protein
in lung cancer cells (28). This
positive feedback loop may underlie the hypoxia tolerance conferred
to cancer cells by the increased expression of AK4 (24). The meta-analysis by Atay using
publicly available data proposed AK4 as one possible prognostic
marker in pancreatic ductal adenocarcinoma (29). However, the relationship between
the expression level of this gene and PAAD progression has neither
been evaluated in clinic nor in the laboratory.
Eukaryotic-derived exosomes contain receptors on
their lipid bilayer membranes and carry inside them a variety of
biomolecules obtained from parent cells, such as proteins, nucleic
acids and lipids (30). Exosomes
mediate short- and long-range communication between cells (30). An increasing number of studies have
shown that exosomes secreted from tumor tissues can serve as
potential biomarkers for cancer diagnosis, as they can reflect
intracellular alterations in the parent cells (31,32).
The exosomal membrane protein, Glypican-1, was found to be markedly
more highly expressed in exosomes from cancerous than non-cancerous
tissue, implying its clinical value as an exosomal biomarker for
early diagnosis of pancreatic cancer (33). In the present study, bioinformatics
analysis results and clinical findings showed a significant
increase in the expression levels of both AK4 and AK4P1 in
pathological tissue specimens and in circulating exosomes of
patients with PAAD as well as its association with the decreased
overall survival of patients. These results not only suggested AK4
and AK4P1 as potential biomarkers for the prognosis of PAAD but
also implied their participation in its development. Based on the
high degree of transcript sequence identity, pseudogenes such as
BRAFP1, KRASP1 and PTENP1 are all considered to assist in the
expression of their parental genes in tumor cells by competitively
binding to and sequestering miRNAs (10). Considering the significant
association in the transcription levels between AK4P1 and AK4, as
found in the present study, it was speculated that AK4P1 may
facilitate the expression of AK4 gene in PAAD cells through a
similar competing endogenous RNA mechanism; considering that
gemcitabine is frequently used in clinical practice as adjuvant
chemotherapy for pancreatic cancer; in the present study this drug
was used as an apoptosis inducer. The data showed that AK4P1
overexpression could significantly increase the expression of AK4
gene in PAAD cells in vitro; siRNA-mediated AK4 knockdown
significantly inhibited the growth of PAAD cells and increased
their apoptotic rates induced by gemcitabine treatment, which were
significantly attenuated by AK4P1 overexpression. Interestingly, it
was observed that AK4P1 failed to restore the proliferation and
survival of PAAD cells with AK4 gene knockout, showing that the
pro-malignant role of AK4P1 requires the intact AK4 gene in PAAD
cells, which suggested that the AK4P1 transcript regulated the
expression of AK4 gene in a post-transcriptional fashion. It was
further identified that treatment with exosomes derived from
AK4P1-overexpressing PAAD cells significantly increased the
expression of AK4 gene in the recipient cells, which indicated that
PAAD cell-derived exosomes may shape the malignancy of the
recipient cells by transferring AK4P1, similar to the mechanisms
previously reported (34–36). However, certain limitations exist
in the present study. It was not possible to verify the function of
cytobiology, such as apoptosis, in PAAD cells with AK4P1 knockdown,
which will be the focus of our future research.
Collectively, in the present study, the
cancer-promoting role of AK4P1 transcript was reported for the
first time, to the best of our knowledge. The significant
upregulation of AK4P1 expression levels and its parental gene AK4
in PAAD was identified, which significantly was associated with the
decreased overall survival of patients; elevated levels of AK4P1
transcripts in peripheral blood exosomes of patients with PAAD may
contribute to the early screening and intervention of this disease,
consequently improving prognosis of patients. The effect of AK4P1
on PAAD cells required intact AK4 gene transcription, and AK4P1
transcript from the donor cells may exert cancer-promoting effects
in the recipient cells through exosome delivery.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MX conceptualized and designed the study. LL, TD and
QZ analyzed pancreatic adenocarcinoma tissue specimens and clinical
data of patients. YY and YL performed cell viability and apoptosis
assays. LY and LL conducted cell culture and transfection assays.
LL and MX confirm the authenticity of all the raw data. LL was the
major contributor in writing the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethical Review
Committee of Suizhou Hospital Affiliated to Hubei University of
Medicine (Suizhou, China). All research complied with The
Declaration of Helsinki and the relevant rules of the Ethics
Committee. Written informed consent was provided by each
participant or the legal representative/guardian of the
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo
W, Zhao F, You L, Zheng L, Zhang T and Zhao Y: Targeting hypoxic
tumor microenvironment in pancreatic cancer. J Hematol Oncol.
14:142021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao W, Maitra A and Ying H: Recent
insights into the biology of pancreatic cancer. EBioMedicine.
53:1026552020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheetham SW, Faulkner GJ and Dinger ME:
Overcoming challenges and dogmas to understand the functions of
pseudogenes. Nat Rev Genet. 21:191–201. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karreth FA, Reschke M, Ruocco A, Ng C,
Chapuy B, Léopold V, Sjoberg M, Keane TM, Verma A, Ala U, et al:
The BRAF pseudogene functions as a competitive endogenous RNA and
induces lymphoma in vivo. Cell. 161:319–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haddadi N, Lin Y, Travis G, Simpson AM,
Nassif NT and McGowan EM: PTEN/PTENP1: ‘Regulating the regulator of
RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy.
Mol Cancer. 17:372018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lou W, Ding B and Fu P: Pseudogene-derived
lncRNAs and their miRNA sponging mechanism in human cancer. Front
Cell Dev Biol. 8:852020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mashouri L, Yousefi H, Aref AR, Ahadi AM,
Molaei F and Alahari SK: Exosomes: Composition, biogenesis, and
mechanisms in cancer metastasis and drug resistance. Mol Cancer.
18:752019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng W, Hao Y, He C, Li L and Zhu G:
Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer.
18:572019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Z, Yang S, Zhou Q, Wang G, Song J, Li
Z, Zhang Z, Xu J, Xia K, Chang Y, et al: Emerging role of
exosome-derived long non-coding RNAs in tumor microenvironment. Mol
Cancer. 17:822018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y,
Zheng Q, Li Y, Wang P, He X and Huang S: exoRBase: A database of
circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids
Res. 46(D1): D106–D112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jan YH, Tsai HY, Yang CJ, Huang MS, Yang
YF, Lai TC, Lee CH, Jeng YM, Huang CY, Su JL, et al: Adenylate
kinase-4 is a marker of poor clinical outcomes that promotes
metastasis of lung cancer by downregulating the transcription
factor ATF3. Cancer Res. 72:5119–5129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Yin YT, Wu CH, Qiu RL, Jiang WJ,
Deng XG and Li ZX: AK4 promotes the progression of HER2-positive
breast cancer by facilitating cell proliferation and invasion. Dis
Markers. 2019:81860912019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang M, Qin X, Wang Y and Mao F:
Identification of AK4 as a novel therapeutic target for serous
ovarian cancer. Oncol Lett. 20:3462020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xin F, Yao DW, Fan L, Liu JH and Liu XD:
Adenylate kinase 4 promotes bladder cancer cell proliferation and
invasion. Clin Exp Med. 19:525–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldman MJ, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al:
Visualizing and interpreting cancer genomics data via the Xena
platform. Nat Biotechnol. 38:675–678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
André Mdo R, Pedro A and Lyden D: Cancer
exosomes as mediators of drug resistance. Methods Mol Biol.
1395:229–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Gonzalez G, Dai X, Miao W, Yuan J,
Huang M, Bade D, Li L, Sun Y and Wang Y: Adenylate kinase 4
modulates the resistance of breast cancer cells to tamoxifen
through an m6A-based epitranscriptomic mechanism. Mol
Ther. 28:2593–2604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujisawa K, Terai S, Takami T, Yamamoto N,
Yamasaki T, Matsumoto T, Yamaguchi K, Owada Y, Nishina H, Noma T
and Sakaida I: Modulation of anti-cancer drug sensitivity through
the regulation of mitochondrial activity by adenylate kinase 4. J
Exp Clin Cancer Res. 35:482016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Bao C, Zhang X, Lin X and Fu Y:
Knockdown of LINC00662 represses AK4 and attenuates radioresistance
of oral squamous cell carcinoma. Cancer Cell Int. 20:2442020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanning NJ, Looyenga BD, Kauffman AL,
Niemi NM, Sudderth J, DeBerardinis RJ and MacKeigan JP: A
mitochondrial RNAi screen defines cellular bioenergetic
determinants and identifies an adenylate kinase as a key regulator
of ATP levels. Cell Rep. 7:907–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu L, Huan L, Guo T, Wu Y, Liu Y, Wang Q,
Huang S, Xu Y, Liang L and He X: LncRNA SNHG11 facilitates tumor
metastasis by interacting with and stabilizing HIF-1α. Oncogene.
39:7005–7018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jan YH, Lai TC, Yang CJ, Lin YF, Huang MS
and Hsiao M: Adenylate kinase 4 modulates oxidative stress and
stabilizes HIF-1α to drive lung adenocarcinoma metastasis. J
Hematol Oncol. 12:122019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atay S: Integrated transcriptome
meta-analysis of pancreatic ductal adenocarcinoma and matched
adjacent pancreatic tissues. PeerJ. 8:e101412020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Record M, Subra C, Silvente-Poirot S and
Poirot M: Exosomes as intercellular signalosomes and
pharmacological effectors. Biochem Pharmacol. 81:1171–1182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo
X, Wei Q, Wang J, Xiong H, Chen C, Xu B, et al: Exosome: Emerging
biomarker in breast cancer. Oncotarget. 8:41717–41733. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kok VC and Yu CC: Cancer-derived exosomes:
Their role in cancer biology and biomarker development. Int J
Nanomedicine. 15:8019–8036. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma W, Zhou Y, Liu M, Qin Q and Cui Y: Long
non-coding RNA LINC00470 in serum derived exosome: A critical
regulator for proliferation and autophagy in glioma cells. Cancer
Cell Int. 21:1492021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun J, Jia H, Bao X, Wu Y, Zhu T, Li R and
Zhao H: Tumor exosome promotes Th17 cell differentiation by
transmitting the lncRNA CRNDE-h in colorectal cancer. Cell Death
Dis. 12:1232021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Z, Wang X, Yang Y, Chen W, Zhang K,
Teng B, Huang C, Zhao Q and Qiu Z: Hypoxic tumor-derived exosomal
long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2
in pancreatic cancer. Mol Ther Nucleic Acids. 22:179–195.
2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|