Introduction
Malignant mesothelioma is an extremely aggressive
tumor with a poor prognosis; its occurrence is increasing
worldwide, primarily due to past and/or present occupational and/or
environmental asbestos exposure (1). Malignant mesothelioma is still
predominant in developed countries, including Japan, but a shift in
disease occurrence is anticipated, as asbestos use has recently
increased in developing countries (2). The molecular mechanism of
carcinogenesis and progression of malignant mesothelioma remains
unclear, and effective therapy has not yet been established.
Ferritin is a multifunctional protein that functions
intracellularly or extracellularly and contributes to
proliferation, angiogenesis, immune suppression, and iron delivery
in both non-tumor and tumor cells. Ferritin consists of two
subunits, light chain and heavy chain, and these subunits are
functionally and genetically distinct (3). However, it has been reported that
ferritin light chain (FTL) and ferritin heavy chain (FTH) may be
involved in mesothelioma in relation to iron metabolism (4,5),
particularly FTH (6). High protein
or mRNA expression of FTL is associated with tumor malignancy in
colorectal cancers; an increase in FTL is negatively correlated
with survival and promotes migration, invasion, and metastasis
(7). In gastric cancers, patients
with low FTL expression have longer overall survival and
recurrence-free survival (8). In
glioblastomas, FTL expression is higher in glioblastoma patients
than in low-grade glioma patients, and knockdown of FTL reduces
cell growth (9). Increase FTH
expression is reported to be associated with malignant tumor grade
of renal cell carcinoma (10), FTL
and FTH have no relation to each other in previous reports of
tumorigenesis of malignant tumors. Mohr et al reported that
the expression levels of FTL mRNA were increased among 302
overexpressed genes in malignant mesothelioma (11); however, the molecular role of FTL
in malignant mesothelioma remains unclear. In this study, we
investigated the role of FTL in malignant mesothelioma.
Materials and methods
Investigation of expression levels of
FTL in malignant mesothelioma
The expression levels of FTL in malignant
mesothelioma were examined using the Cancer Cell Line Encyclopedia
(CCLE, Broad Institute, http://www.broadinstitute.org/ccle/) (12), which shows gene expression levels
in various tumors, on October 30, 2020. We also used the DepMap
Portal (Broad Institute, http://depmap.org/portal/) to search for FTL
expression levels in mesothelioma cell lines, distinguishing
between subtypes. We reanalyzed the gene expression data that we
had previously obtained for six epithelioid mesotheliomas and six
lung adenocarcinomas (13).
Mesothelioma cell lines
The ACC-MESO-1 cell line was purchased from RIKEN
BioResearch Center (Tsukuba, Japan), and the CRL-5915 cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). Mesothelioma cells were maintained in Roswell Park Memorial
Institute 1640 medium with GlutaMAX and sodium pyruvate (RPMI-1640)
supplemented with 1% kanamycin/fungizone and 5% fetal bovine serum
(FBS) in a humidified incubator with 5% CO2 at 37°C (all
purchased from Gibco/Thermo Fisher Scientific K.K., Tokyo,
Japan).
Transfection of mRNA inhibitors to
mesothelioma cells
Mesothelioma cell lines were transfected with
Silencer Select siRNAs to reduce FTL mRNA levels (sense:
CCUGGAGACUCACUUCCUATT; antisense: UAGGAAGUGAGUCUCCAGGAA; Thermo
Fisher Scientific, Tokyo, Japan) and negative control (NC; Select
Negative Control No. 1 siRNA, Cat# 4390843, Thermo Fisher
Scientific K.K.) using Lipofectamine RNAiMAX and Opti-MEM (Thermo
Fisher Scientific K.K.) according to the manufacturer's
protocols.
Real-time reverse transcription
polymerase chain reaction
The two mesothelioma cell lines (2×105
cells) were transfected with 25 pmol of FTL siRNA or NC
siRNA in 6-well plates for 72 h. RNA was extracted from the cells
using Maxwell RSC SimplyRNA Cells Kits on a Maxwell RSC Instrument
(Promega Japan) according to the manufacturer's protocols. The
extracted RNA was reverse transcribed with SuperScript IV VILO
Master Mix (Thermo Fisher Scientific K.K.) and amplified using
PowerUp SYBR Green Master Mix (Thermo Fisher Scientific K.K.) on an
AriaMx Real-Time PCR System (Agilent Technologies, Tokyo, Japan).
Relative expression levels were calculated using the comparative Cq
method. Expression levels were normalized to that of glyceraldehyde
3-phosphate dehydrogenase (GAPDH). The primer sequences were
as follows: forward, 5-GGCTTCTATTTCGACCGCGA-3; FTL reverse,
5-TTTCATGGCGTCTGGGGTTT-3; GAPDH forward,
5-ACAACTTTGGTATCGTGGAAGG-3; GAPDH reverse,
5-GCCATCACGCCACAGTTTC-3.
Western blot analysis
A total of 1.5×105 cells of the two
mesothelioma cell lines were transfected with 25 pmol FTL siRNA or
NC siRNA in 6-well plates for 48 h. Cell lysates were obtained
using the RIPA Lysis Buffer System (Santa Cruz Biotechnology), and
the protein concentration in the lysates was measured using a Qubit
Fluorometer (Thermo Fisher Scientific K.K.). Thirty micrograms of
protein were electrophoresed on a sodium dodecyl
sulfate-polyacrylamide gel (SureCast Acrylamide Gel, Thermo Fisher
Scientific K.K.) at 200 V for 40 min and immediately transferred
onto polyvinylidene difluoride membranes using a Mini Blot Module
(Thermo Fisher Scientific K.K.) at 20 V for 60 min. After blocking
with 5% bovine serum albumin in Tris-buffered saline with Tween-20,
the membranes were incubated overnight with primary antibodies
(anti-FTL antibody [D-9, 1:500, mouse monoclonal, sc-74513; Santa
Cruz Biotechnology), anti-GAPDH antibody (1:5,000, rabbit
monoclonal, sc-25778; Santa Cruz Biotechnology), and then incubated
with secondary antibody (1:2,000, anti-mouse IgG, HRP-linked
antibody 7076P2; Cell Signaling Technology, Tokyo, Japan);
(1:5,000, anti-rabbit IgG, HRP-linked antibody 7074S, Cell
Signaling Technology)] for 45 min. Membranes were then stained with
ImmunoStar LD (Wako Pure Chemical Industries), and
chemiluminescence signals were detected using a C-DiGit Blot
Scanner (LI-COR Biosciences, Lincoln, NE, USA). In addition to
FTL and GAPDH, we investigated the expression of p21,
p27, CDK2, and pRb. The antibodies used are listed in Table I. After first blotting GAPDH to
confirm that the recovered proteins were correctly quantified, the
other proteins were tested under the same conditions (except for
the primary and secondary antibody concentrations) on different
membranes.
 | Table I.List of Primary Antibodies. |
Table I.
List of Primary Antibodies.
| Primary
antibody | Concentration | Lot number | Catalogue
number | Type | Source |
|---|
| FTL | 1:500 | D-9 | sc-74513 | Mouse
monoclonal | SCB |
| p21 | 1:2,000 | 12D1 | 2947T | Rabbit
monoclonal | CST |
| p27 | 1:2,000 | D69C12 | 3686S | Rabbit
monoclonal | CST |
| CDK2 | 1:2,000 | 78B2 | 2546T | Rabbit
monoclonal | CST |
| pRb | 1:2,000 | D20B12 | 8516S | Rabbit
monoclonal | CST |
| GAPDH | 1:5,000 | FL-335 | sc-25778 | Rabbit
monoclonal | SCB |
Cell morphology
The morphology of the two mesothelioma cell lines
was observed after NC/FTL siRNA transfection at 0, 24, 48, and 72 h
using a CKX53 microscope equipped with a DP21 digital camera
(Olympus).
Cell proliferation assay
The two mesothelioma cell lines (1×104)
were transfected with 1 pmol FTL siRNA or NC siRNA in 96-well
plates. The proliferation rates were determined at 48 and 72 h by
quantifying ATP using the Cell Titer-Glo 2.0 reagent and GloMax
Explorer microplate reader (Promega,), according to the
manufacturer's protocols.
Cell cycle assay
The two mesothelioma cell lines (5×104)
were transfected with 10 pmol FTL or NC siRNA in 24-well plates for
72 h. Then, the cells were collected and fixed in ice-cold 70%
ethanol in 15 ml centrifuge tubes for approximately 1 h. After
ethanol removal, the Guava Cell Cycle Reagent (Luminex) containing
propidium iodide was used to determine the number of cells at
different stages of the cell cycle by labeling the cellular DNA.
The labeling signal intensity was measured using a Guava EasyCyte
Mini flow cytometer (Guava Technologies) according to the
manufacturer's protocol.
Cell invasion assay
The two mesothelioma cell lines (3×104)
were cultured in BD FluroBlok culture inserts containing 8 µm pores
(BD Biosciences) coated with Geltrex Matrigel (Thermo Fisher
Scientific, K.K.) after transfection with 10 pmol FTL siRNA or NC
siRNA according to the manufacturer's protocols. After 48 h,
invaded cells were stained with Hoechst 33324 (Thermo Fisher
Scientific, K.K.) for 10 min, and the number of cells visualized
using an IX81 fluorescent microscope equipped with a DP80 digital
camera (Olympus) was counted using CellProfiler cell imaging
software (14).
Cell migration assay
The two mesothelioma cell lines (1×104)
were cultured in BD FluroBlok culture inserts containing 8 µm pores
(BD Biosciences) after transfection with 10 pmol FTL siRNA or NC
siRNA according to the manufacturer's protocols. After 48 h, the
migrated cells were stained with Hoechst 33324 (Thermo Fisher
Scientific K.K.) for 10 min, and the number of cells visualized
with a fluorescent microscope was counted using CellProfiler cell
imaging software (14).
Apoptosis and necrosis assays
The two mesothelioma cell lines (1×105)
were incubated with the FTL/NC siRNA in 96-well plates for 24 h,
and the RealTime Glo Annexin V Apoptosis Assay reagent (Promega)
was added to the cells after transfection. Relative levels of
apoptosis and necrosis were measured at 0, 3, 6 and 9 h after
transfection by analyzing luminescence and fluorescence with a
GloMax microplate reader (Promega) according to the manufacturer's
recommended protocol.
Statistical analysis
The data are expressed as mean ± standard deviation
(SD) from three independent experiments, and a paired t-test was
used to compare the data using Microsoft Excel for Mac (version 16;
Microsoft Corporation). P<0.05 was considered to indicate a
statistically significant difference.
Results
FTL mRNA expression was upregulated in
malignant mesothelioma
The CCLE database showed a higher expression of FTL
mRNA in mesothelioma than in other malignant tumors (Fig. 1A). We also searched for the
expression of FTL in mesothelioma cell lines by distinguishing
subtypes using the DepMap Portal database. Eleven epithelioid
mesotheliomas, two sarcomatoid mesotheliomas, and six biphasic
mesotheliomas showed relatively high expression among the diverse
malignancies (Fig. 1B). In
addition, reanalysis of our previously obtained gene expression
data of malignant mesothelioma (epithelioid mesothelioma) showed
higher expression of FTL mRNA in all six cases, as shown in the
line graph (Fig. 1C). In the
reanalysis of gene expression data, the six lung adenocarcinomas
also showed high FTL mRNA expression.
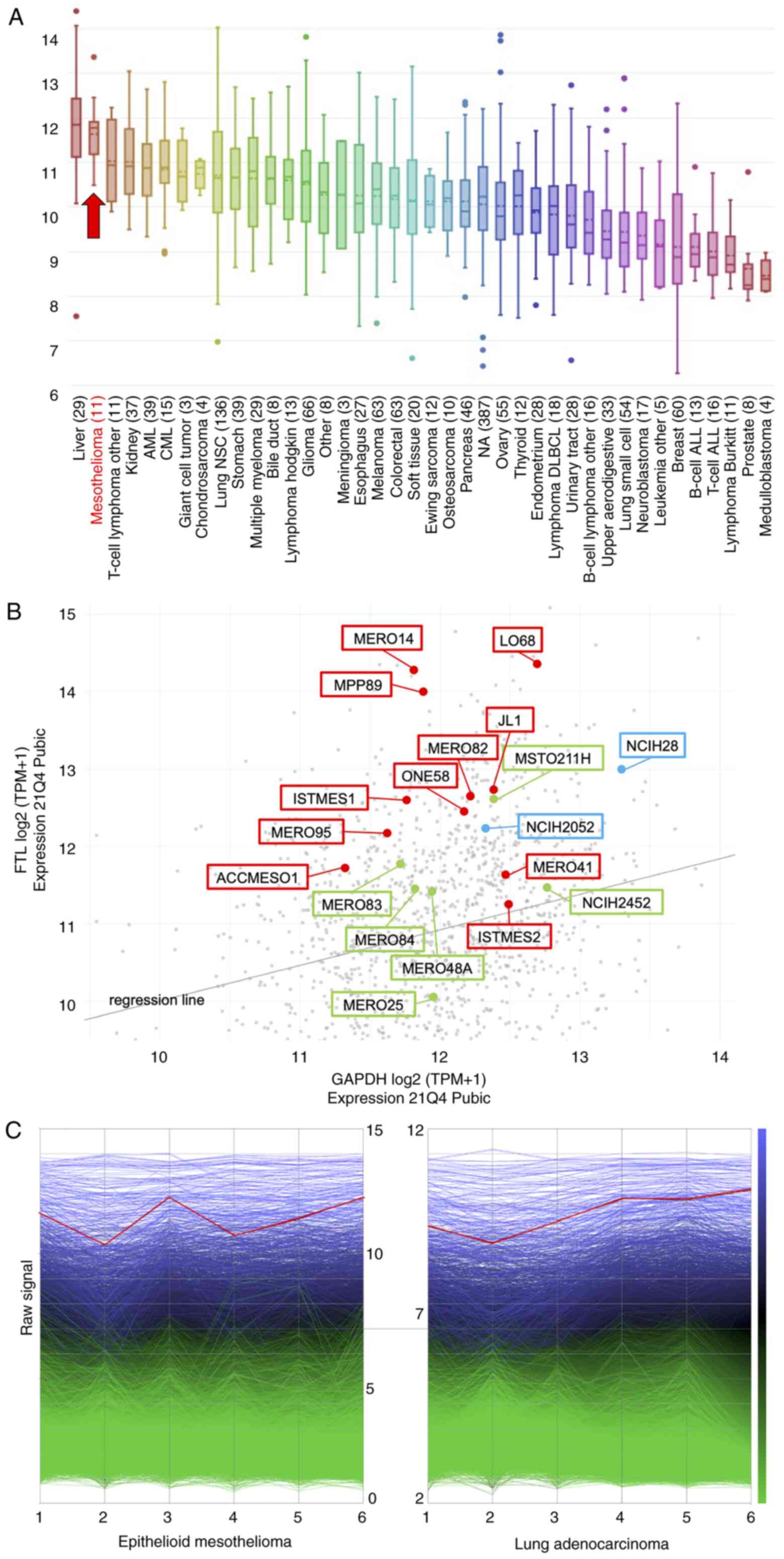 | Figure 1.Gene expression levels of FTL among
malignant tumors, including mesothelioma. (A) In the online
database, Cancer Cell Line Encyclopedia, FTL, indicated by red
arrow, shows higher expression in malignant mesothelioma compared
with other malignant tumors. (B) DepMap Portal database showed
relatively high expression of FTL in three subtypes of malignant
mesothelioma; red dots indicate epithelioid, blue dots indicate
sarcomatoid and green dots indicate biphasic subtypes, amongst the
other malignant tumors, indicated by gray dots. (C) Gene expression
analysis in six epithelioid mesotheliomas and six lung
adenocarcinomas shows high expression of FTL in both epithelioid
mesothelioma and lung adenocarcinoma, indicated by the red line.
Other genes are shown as blue or green lines. FTL, ferritin light
chain. |
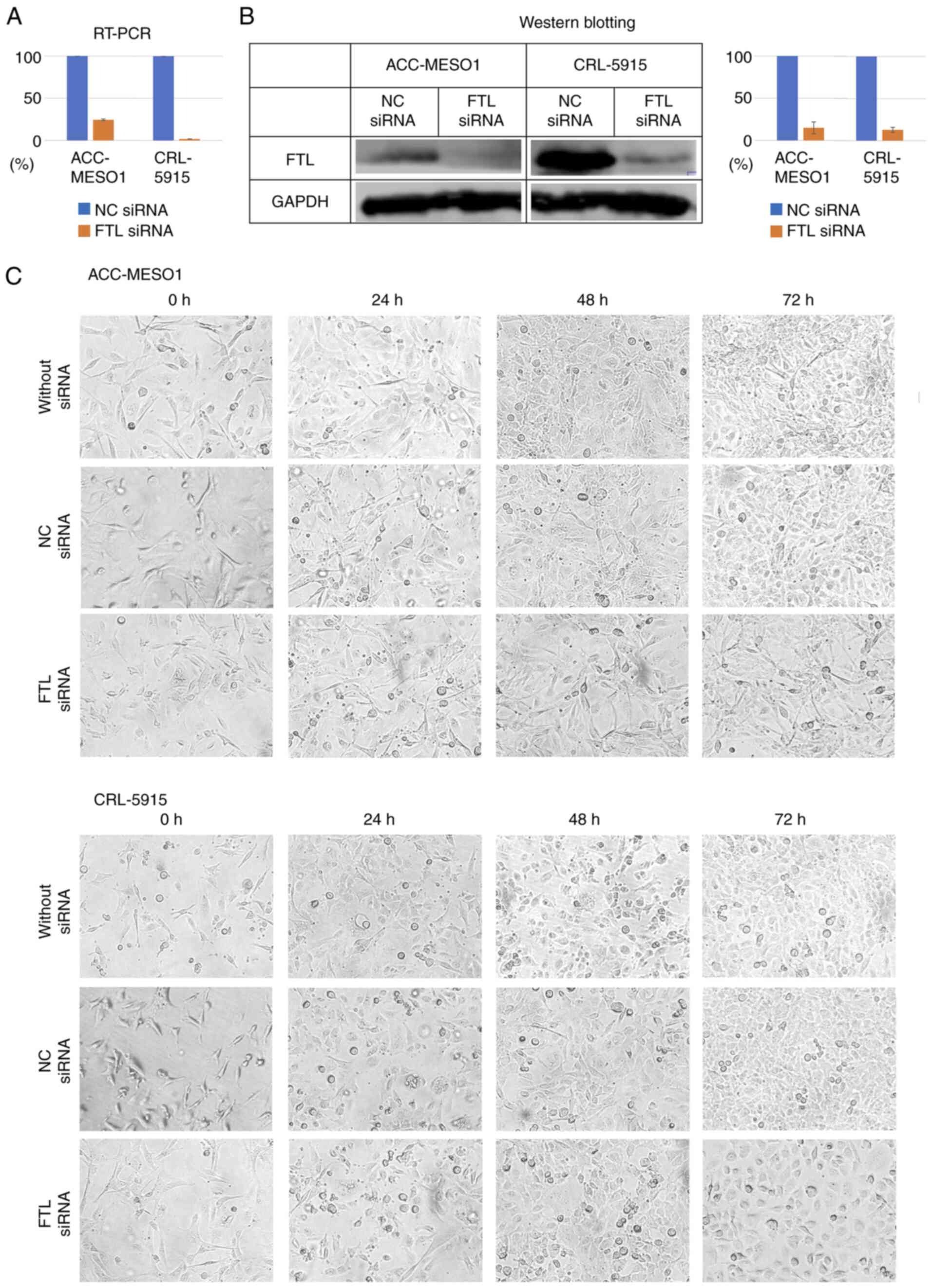
Transfection of FTL siRNA into
mesothelioma cell lines downregulated expression levels of FTL
Compared with cells transfected with NC siRNA, the
expression levels of FTL mRNA, examined by RT-PCR, were suppressed
in cells transfected with FTL siRNA by 75.5% (the Cq of the
NC-siRNA group was 10.92, 10.97, and 10.97, and that of the
FTL-siRNA group was 12.99, 12,92 and 13.03) in ACC-MESO-1 and 98.2%
(the Cq of the NC-siRNA group was 10.0, 10.05, and 10.14, and that
of the FTL-siRNA group was 15.9, 15.81, and 15.87) in CRL-5915
cells (Fig. 2A), and the
expression levels of FTL protein, examined by western blotting,
were suppressed by 85% in ACC-MESO-1 and 87% in CRL-5915 cells
(Fig. 2B).
Transfection of FTL siRNA did not
change morphology of mesothelioma cells
Observation of cell lines at 0, 24, 48, and 72 h
after FTL siRNA transfection did not change the morphology of short
spindle to pleomorphic cells in ACC-MESO-1 and mainly changed
polygonal cells in CRL5915 compared to cells transfected with NC
siRNA. The morphology of the FTL siRNA-transfected group appeared
to be more pronounced, but this was due to suppressed proliferation
and low density (Fig. 2C).
Transfection of FTL siRNA suppressed
proliferation of mesothelioma cell lines
Cell lines transfected with FTL siRNA showed
significantly reduced proliferation compared to that of NC. The
proliferation ability of ACC-MESO-1 cells was reduced by 11.0% at
48 h and 19.5% at 72 h, and that of CRL-5915 cells was reduced by
4.02% at 48 h and 8.96% at 72 h (Fig.
3A).
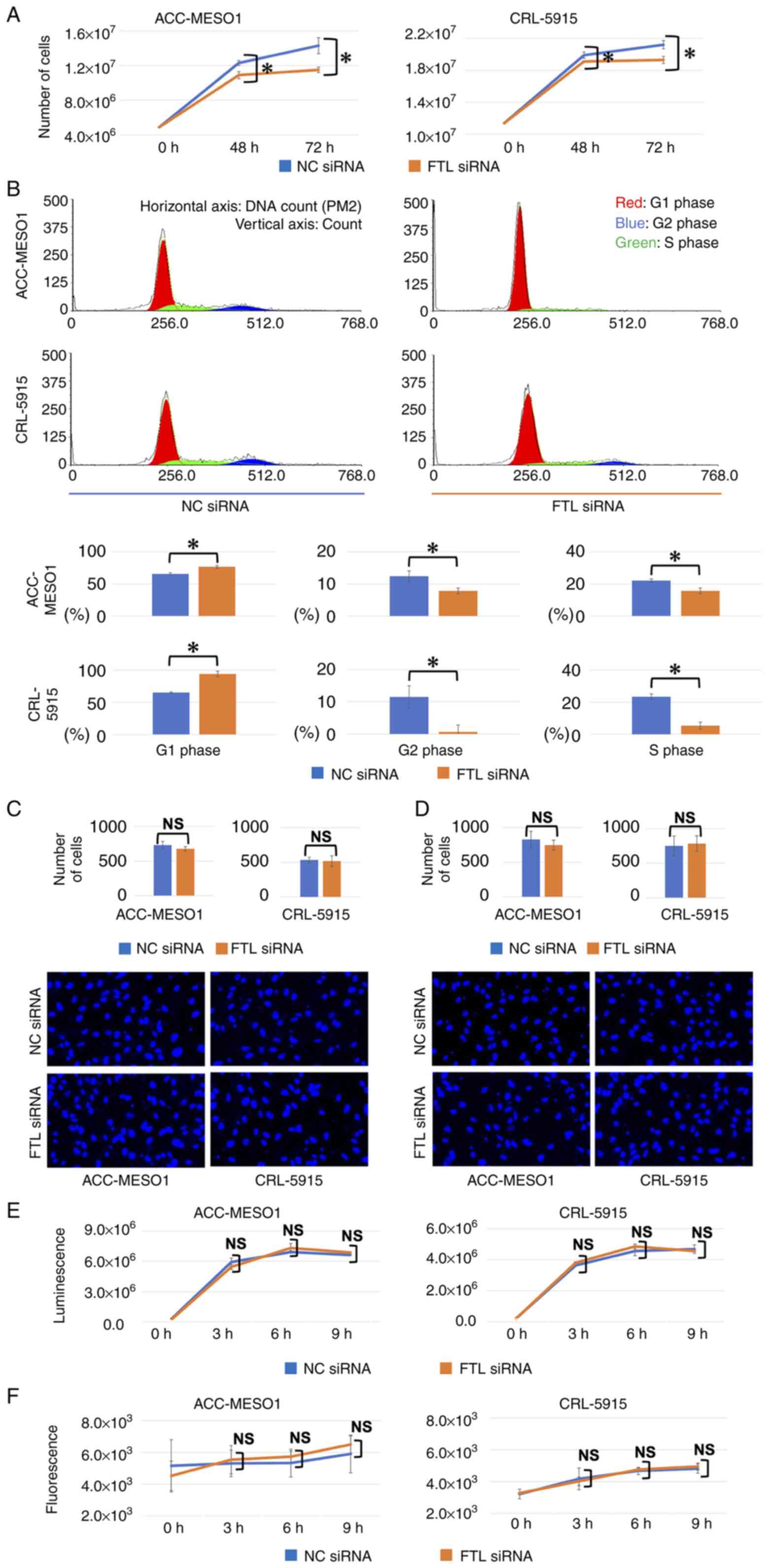 | Figure 3.Results of the proliferation assay,
cell cycle assay, invasion assay and migration assay comparing
cells transfected with FTL siRNA and NC in two mesothelioma cell
lines. (A) FTL downregulation significantly reduced proliferation
of mesothelioma cell lines compared with NC siRNA-transfected
cells. The vertical axis indicates number of cells. (B) By
downregulation of FTL, the number of cells in G1 phase
of the cell cycle increased from 65.6 to 76.4% in ACC-MESO-1 cells
and from 65.3 to 93.9% in CRL-5915 cells compared with cells
transfected with NC siRNA. The vertical axis indicates the count of
cells and the horizontal axis indicates DNA count (PM2) in the
upper four figures and the percentage of cells at each phase in the
lower six figures. (C) Downregulation of FTL does not affect the
invasion ability of mesothelioma cell lines. In the graph, the
vertical axis indicates the number of cells. Representative
fluorescent microscopy images from each group are shown
(magnification, ×200). (D) Downregulation of FTL did not affect
ability of migration of mesothelioma cell lines. In the graph, the
vertical axis indicates the number of cells. Representative
fluorescent microscopy images from each group are shown
(magnification, ×200). (E) Downregulation of FTL does not affect
the apoptosis of mesothelioma cell lines. The vertical axis
indicates the luminescence level. (F) Downregulation of FTL does
not affect the necrosis of mesothelioma cell lines. The vertical
axis indicates the fluorescence level. *P<0.05. FTL, ferritin
light chain; siRNA, short interfering RNA; NC, negative
control. |
FTL siRNA-transfection in mesothelioma
cell lines increased the number of cells at the G1
phase
Transfection with FTL siRNA increased the number of
ACC-MESO-1 and CRL-5915 cells in the G1 phase of the
cell cycle from 65.6 to 76.4% and from 65.3 to 93.9%, respectively,
compared to NC. In contrast, the number of cells in the
G2 and S phases decreased from 14.3 to 8.2% and from
21.7 to 14.0% for ACC-MESO-1 cells, and from 14.1 to 0% and from
20.3 to 10.6% for CRL-5915 cells, respectively (Fig. 3B).
Transfection of FTL siRNA did not
affect the ability of invasion, migration, apoptosis, and necrosis
of mesothelioma cell lines
Transfection of ACC-MESO-1 and CRL-5915 cell lines
with FTL siRNA had no significant effect on their invasion
(Fig. 3C), migration (Fig. 3D), apoptosis (Fig. 3E), or necrosis ability (Fig. 3F), compared to cells transfected
with NC siRNA.
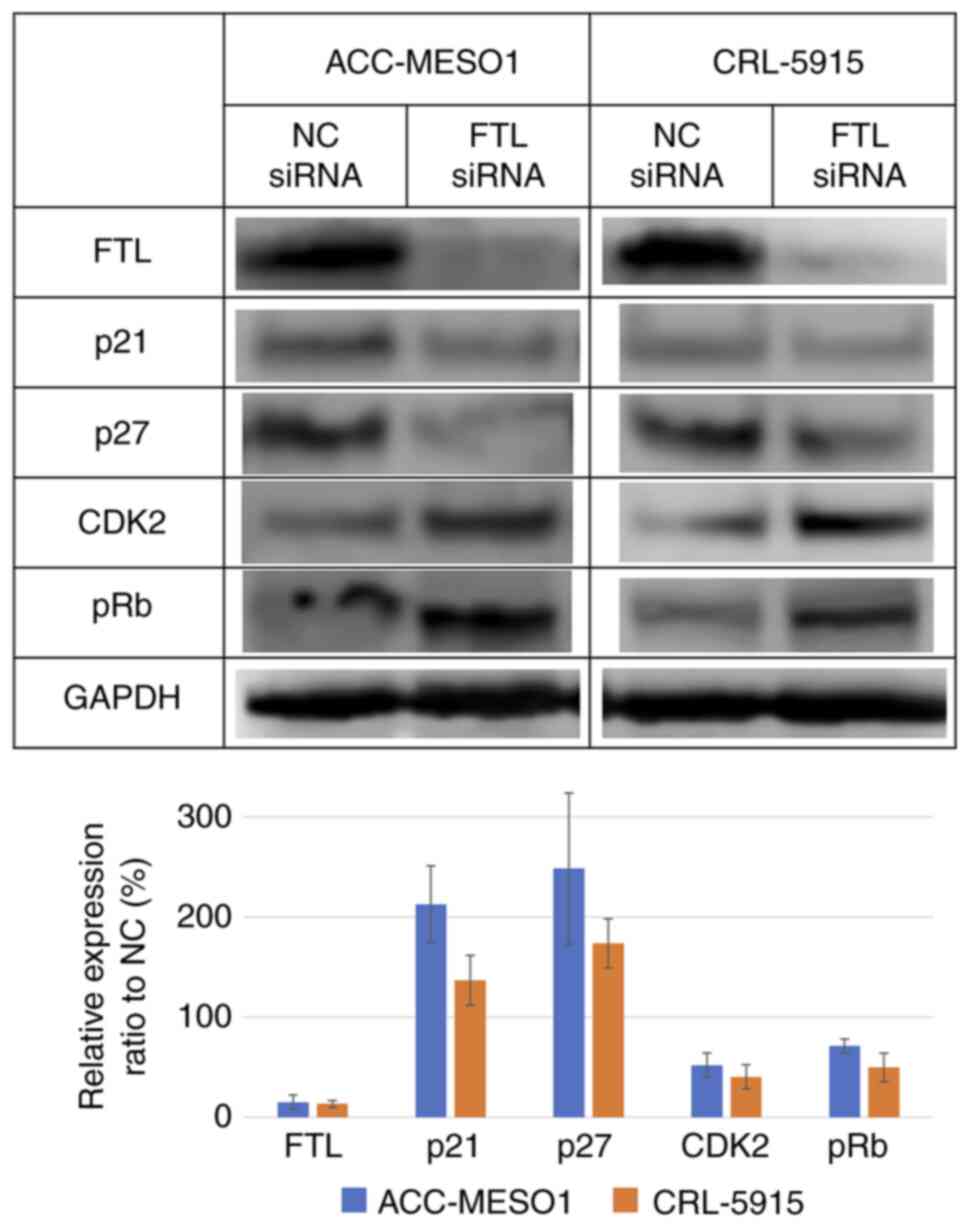
Downregulation of FTL increased
expression of p21 and p27 and decreased expression of CDK2 and
pRb
Western blotting showed an increase in the
expression levels of p21 and p27 and decreased levels of CDK2 and
pRb in FTL siRNA-transfected mesothelioma cell lines compared to
cells transfected with NC siRNA. For ACC-MESO-1/CRL-5915, p21
increased by 113/37%, p27 increased by 148/74%, CDK2 decreased by
48/60%, and pRb decreased by 29/50% (Fig. 4).
Discussion
Malignant mesothelioma is a highly aggressive tumor
with extremely poor prognosis, and its incidence is increasing
worldwide (1), but the molecular
mechanism of carcinogenesis and progression of malignant
mesothelioma remains unclear, and effective therapy has not been
established. Pleurectomy/decortication (P/D) or extra-pleural
pneumonectomy (EPP), selected as surgical therapy for malignant
pleural mesothelial patients, improves patients' overall survival
(15,16). P/D and EPP aim for macroscopic
complete resection of the tumor, but microscopic complete resection
is almost impossible because malignant mesothelioma occurs in the
pleura and anatomically, there is no margin between the tumor and
stromal tissue. Radiation therapy has not been fully investigated
because of the small number of studies (4). A platinum-pemetrexed combination is
the standard for first-line chemotherapy; however, no standard
second-line treatment has been discovered. To determine the optimal
treatment for individual patients, cytotoxic agents, targeted
therapy, and immunotherapy are under investigation (5). Recently, a study showed improvement
in the overall survival of malignant mesothelioma patients treated
with nivolumab plus ipilimumab (17). Despite these developments, an ideal
treatment protocol for malignant mesothelioma is not yet available,
and a more detailed understanding of this disease is required.
Ferritin is a protein involved in iron metabolism
that functions intracellularly or extracellularly and contributes
to proliferation, angiogenesis, immunosuppression, and iron
delivery, not only in non-tumor cells but also in tumor cells.
Ferritin consists of two subunits, light chain (L-ferritin, FTL)
and heavy chain (H-ferritin), which are functionally and
genetically distinct. H-ferritin possesses enzymatic activity and
can oxidize ferrous iron to ferric iron. L-ferritin lacks enzymatic
activity and thus does not contribute to iron oxidization and
uptake; however, L-ferritin has a higher number of carboxyl groups
lining the ferritin cavity, which serve as iron nucleation sites
that mineralize iron faster. Moreover, the L-ferritin monomer
contains a salt bridge within its helical fold, which confers
greater stability to the ferritin complex (3). Recently, high expression of FTL
protein or mRNA has been associated with tumor malignancy. In
colorectal cancer, FTL is upregulated and promotes
resistance to 5-fluoro-uracil, migration, invasion, and metastasis
of tumor cells (7). High
expression of FTL protein in gastric cancer patients has been
proportionally associated with the depth of tumor invasion,
differentiation grade, lymph node metastasis, and TNM stage, and
inversely associated with recurrence-free survival and overall
survival (8). The expression of
FTL is higher in glioblastoma than in low-grade gliomas. FTL
colocalizes with GADD45A in the nucleus of glioblastoma cells and
regulates the GADD45A/JNK pathway, which contributes to tumor cell
growth (9). Previous studies have
reported the association of ferritin, including L-ferritin and
H-ferritin, with tumor malignancy (3), the possibility that FTL and FTH are
involved in mesothelioma in relation to iron metabolism (18,19),
and Mohr et al reported 302 upregulated genes, including
FTL, and 160 downregulated genes in epithelioid mesothelioma
cells of ex vivo resected specimens compared to mesothelial
cells by microarray gene expression analysis (11), but the overall molecular mechanism
is still unclear.
The CCLE database showed relatively high expression
of FTL in mesothelioma compared to other malignant tumors, and
reanalysis of our previous microarray data at the gene level showed
high FTL expression in epithelioid mesotheliomas. We then
investigated the function of FTL in malignant mesothelioma in
vitro. Downregulation of FTL mRNA using siRNA induced a
decrease in proliferation of mesothelioma cell lines compared to
that in cells transfected with NC siRNA. Furthermore, mesothelioma
cells were arrested in the G1 phase: the number of cells
in the G1 phase increased and the number of cells in the
S and G2 phases decreased. In contrast, FTL
downregulation did not affect cell morphology, migration, invasion,
apoptosis, or necrosis. These results indicate that FTL influences
mesothelioma growth purely by promoting the cell cycle; therefore,
we examined the relationship between FTL and cell cycle-related
genes. CDK2 has a considerably broad substrate profile and
phosphorylates a large number of proteins involved in cell cycle
progression (e.g., p27KIP1 and RB), DNA replication, histone
synthesis, and centrosome duplication (20–22).
Much of the control over CDK2 involves the synthesis and
availability of cyclins. RB and E2F regulate the expression of
CDK2, cyclin E1, and cyclin E2 transcripts and proteins (23–27).
Recently, it has become clear that deregulation of CDK2 also occurs
frequently in certain types of cancer (28). The p21CIP1 acts as a DNA damage
checkpoint, which is a critical downstream target gene of p53 that
inhibits DNA synthesis, whereas p27KIP1 is responsive to mitogenic
signaling as a further control of deregulated proliferation
(29,30). Western blotting analysis showed
higher expression of p21 and p27, and lower expression of CDK2 and
pRb in mesothelioma cell lines transfected with FTL siRNA compared
to those transfected with NC siRNA. This suggests that FTL may
downregulate p21 and p27 and upregulate CDK2, which induces
phosphorylation of Rb, promoting the cell cycle at the
G1 phase in malignant mesothelioma cells. There was no
difference in cyclin E1 expression between cell lines transfected
with FTL siRNA and NC siRNA (data not shown). Although these are
the results of experiments using epithelioid mesothelioma cell
lines, the function of FTL in sarcomatoid and biphasic mesothelioma
is worth examining because the expression of the FTL gene
was relatively higher in sarcomatoid and biphasic mesothelioma than
in other tumors in the DepMap Portal. In addition, in vivo
experiments will be conducted to determine whether FTL functions in
malignant mesothelioma in vivo as it does in
vitro.
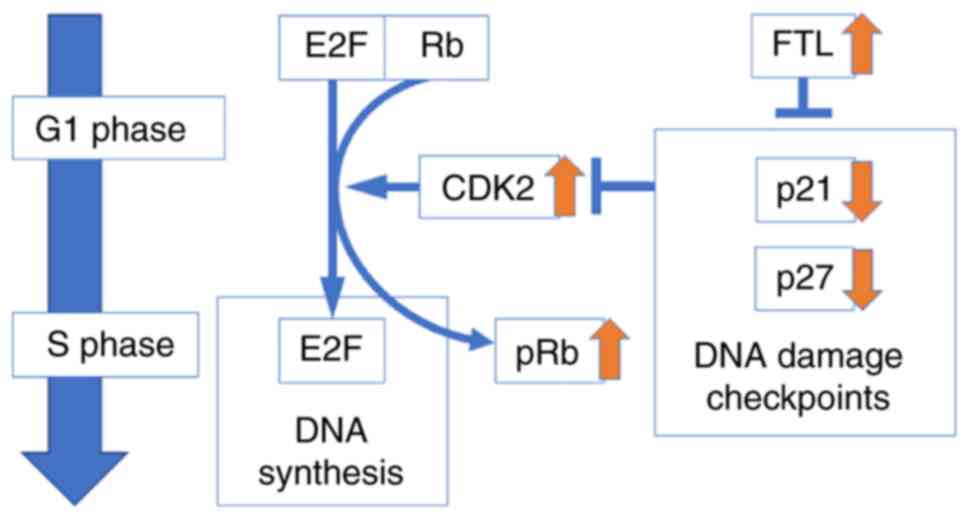
From these results, we concluded that FTL increases
proliferation in malignant mesothelioma by suppressing DNA
checkpoint-related genes, p21 and p27, inducing
activation of CDK2 and inactivation of Rb, which inactivates E2F
and promotes the cell cycle (Fig.
5). Therefore, FTL is expected to be a new therapeutic target
or diagnostic marker for malignant mesothelioma. Further
investigation of the biological mechanism of FTL in malignant
mesothelioma is required.
Acknowledgements
The authors would like to thank Ms Yukari Go and Mr
Tatsuya Nakagawa (Technical Center, Hiroshima University,
Hiroshima, Japan) for their technical assistance and Ms Naomi
Fukuhara (Department of Pathology, Hiroshima University, Hiroshima,
Japan) for administrative support.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, VJA and YT designed the study. VJA and YT
supervised and facilitated the study. TK, KK, YF and IE performed
the experiments. TK and VJA analyzed the data and wrote the
manuscript. TK and VJA interpreted the results. VJA and YT confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Takahiro Kambara (E-mail: kambara0213@hiroshima-u.ac.jp).
References
|
1
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delgermaa V, Takahashi K, Park EK, Le GV,
Hara T and Sorahan T: Global mesothelioma deaths reported to the
World Health Organization between 1994 and 2008. Bull World Health
Organ. 89:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alkhateeb AA and Connor JR: The
significance of ferritin in cancer: Anti-oxidation, inflammation
and tumorigenesis. Biochim Biophys Acta. 1836:245–254.
2013.PubMed/NCBI
|
|
4
|
Gomez DR, Rimner A, Simone CB II, Cho BCJ,
de Perrot M, Adjei AA, Bueno R, Gill RR, Harpole DH Jr, Hesdorffer
M, et al: The use of radiation therapy for the treatment of
malignant pleural mesothelioma: Expert opinion from the National
Cancer Institute thoracic malignancy steering committee,
International Association for the Study of Lung Cancer, and
Mesothelioma Applied Research Foundation. J Thorac Oncol.
14:1172–1183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Gooijer CJ, Baas P and Burgers JA:
Current chemotherapy strategies in malignant pleural mesothelioma.
Transl Lung Cancer Res. 7:574–583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aung W, Hasegawa S, Furukawa T and Saga T:
Potential role of ferritin heavy chain in oxidative stress and
apoptosis in human mesothelial and mesothelioma cells: Implications
for asbestos-induced oncogenesis. Carcinogenesis. 28:2047–2052.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Liu J, Chen H, Zhang Y, Shi H, Huang
L, Tao J, Shen R and Wang T: Ferritin light chain (FTL) competes
with long noncoding RNA Linc00467 for miR-133b binding site to
regulate chemoresistance and metastasis of colorectal cancer.
Carcinogenesis. 41:467–477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Chen Z and Xu A: FTL: A novel
predictor in gastric cancer. Int J Clin Exp Pathol. 10:7865–7872.
2017.PubMed/NCBI
|
|
9
|
Wu T, Li Y, Liu B, Zhang S, Wu L, Zhu X
and Chen Q: Expression of ferritin light chain (FTL) is elevated in
glioblastoma, and FTL silencing inhibits glioblastoma cell
proliferation via the GADD45/JNK pathway. PLoS One.
11:e01493612016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang H, Qiu Y, Huang G, Zhou X, Zhou X
and Luo W: Value of Ferritin Heavy Chain (FTH1) Expression in
diagnosis and prognosis of renal cell carcinoma. Med Sci Monit.
19:3700–3715. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohr S, Bottin MC, Lannes B, Neuville A,
Bellocq JP, Keith G and Rihn BH: Microdissection, mRNA
amplification and microarray: A study of pleural mesothelial and
malignant mesothelioma cells. Biochimie. 86:13–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The cancer cell line encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuraoka M, Amatya VJ, Kushitani K, Mawas
AS, Miyata Y, Okada M, Kishimoto T, Inai K, Nishisaka T, Sueda T
and Takeshima Y: Identification of DAB2 and Intelectin-1 as novel
positive immunohistochemical markers of epithelioid mesothelioma by
transcriptome microarray analysis for its differentiation from
pulmonary adenocarcinoma. Am J Surg Pathol. 41:1045–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamprecht MR, Sabatini DM and Carpenter
AE: CellProfiler: Free, versatile software for automated biological
image analysis. Biotechniques. 42:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao C, Tian D, Park J, Allan J, Pataky KA
and Yan TD: A systematic review and meta-analysis of surgical
treatments for malignant pleural mesothelioma. Lung Cancer.
83:240–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flores RM, Riedel E, Donington JS, Alago
W, Ihekweazu U, Krug L, Rosenzweig K, Adusumilli PS, Carbone M and
Pass HI: Frequency of use and predictors of cancer-directed surgery
in the management of malignant pleural mesothelioma in a
community-based (Surveillance, Epidemiology, and End Results
[SEER]) population. J Thorac Oncol. 5:1649–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Jiang L, Chew SH, Hirayama T, Sekido
Y and Toyokuni S: Carbonic anhydrase 9 confers resistance to
ferroptosis/apoptosis in malignant mesothelioma under hypoxia.
Redox Biol. 26:1012972019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi L, Ito F, Wang Y, Okazaki Y, Tanaka H,
Mizuno M, Hori M, Hirayama T, Nagasawa H, Richardson DR and
Toyokuni S: Non-thermal plasma induces a stress response in
mesothelioma cells resulting in increased endocytosis, lysosome
biogenesis and autophagy. Free Radic Biol Med. 108:904–917. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma T, Van Tine BA, Wei Y, Garrett MD,
Nelson D, Adams PD, Wang J, Qin J, Chow LT and Harper JW: Cell
cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in
Cajal bodies promotes histone gene transcription. Genes Dev.
14:2298–2313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuda M, Horn HF, Tarapore P, Tokuyama Y,
Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE and
Fukasawa K: Nucleophosmin/B23 is a target of CDK2/cyclin E in
centrosome duplication. Cell. 103:127–140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sever-Chroneos Z, Angus SP, Fribourg AF,
Wan H, Todorov I, Knudsen KE and Knudsen ES: Retinoblastoma tumor
suppressor protein signals through inhibition of cyclin-dependent
kinase 2 activity to disrupt PCNA function in S phase. Mol Cell
Biol. 21:4032–4045. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera RE, Sah VP, Williams BO, Mäkelä
TP, Weinberg RA and Jacks T: Altered cell cycle kinetics, gene
expression, and G1 restriction point regulation in Rb-deficient
fibroblasts. Mol Cell Biol. 16:2402–2407. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, Giangrande PH and Nevins JR: E2Fs
link the control of G1/S and G2/M transcription. EMBO J.
23:4615–4626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Markey MP, Angus SP, Strobeck MW, Williams
SL, Gunawardena RW, Aronow BJ and Knudsen ES: Unbiased analysis of
RB-mediated transcriptional repression identifies novel targets and
distinctions from E2F action. Cancer Res. 62:6587–6597.
2002.PubMed/NCBI
|
|
26
|
Möröy T and Geisen C: Cyclin E. Int J
Biochem Cell Biol. 36:1424–1439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren B, Cam H, Takahashi Y, Volkert T,
Terragni J, Young RA and Dynlacht BD: E2F integrates cell cycle
progression with DNA repair, replication, and G(2)/M checkpoints.
Genes Dev. 16:245–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scaltriti M, Eichhorn PJ, Cortés J,
Prudkin L, Aura C, Jiménez J, Chandarlapaty S, Serra V, Prat A,
Ibrahim YH, et al: Cyclin E amplification/overexpression is a
mechanism of trastuzumab resistance in HER2+ breast cancer
patients. Proc Natl Acad Sci USA. 108:3761–3766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polyak K, Lee MH, Erdjument-Bromage H,
Koff A, Roberts JM, Tempst P and Massagué J: Cloning of p27Kip1, a
cyclin-dependent kinase inhibitor and a potential mediator of
extracellular antimitogenic signals. Cell. 78:59–66. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van den Heuvel S and Harlow E: Distinct
roles for cyclin-dependent kinases in cell cycle control. Science.
262:2050–2054. 1993. View Article : Google Scholar : PubMed/NCBI
|