Lung adenocarcinoma (LUAD) is the most common
subtype of non-small cell lung cancer (NSCLC), which accounts for
approximately 40% of all lung cancer (1). The two most common NSCLC histologic
types are LUAD and lung squamous cell carcinoma (LUSC) (2). LUAD cells develop from small airway
epithelial cells and the most distal epithelial cells of the lung
(3). From adenocarcinoma in
situ to minimally invasive adenocarcinoma to overt invasive
adenocarcinoma, LUAD progresses in stages (4). In addition, LUAD cells may easily
invade the walls of blood vessels and lymphatic vessels and thus
metastasize, resulting in poor patient prognosis (5). Although surgical resection, radiation
therapy and immunotherapy have made great progress in recent years,
the 5-year relative overall survival rate of LUAD patients is
approximately 18% (6).
Angiogenesis occurs mainly at the expanding borders
of tumor cells in primary LUAD in a hypoxic environment (7). In hypoxic conditions, tumor cells
produce and secrete pro-angiogenic cytokines, such as vascular
endothelial growth factor (VEGF), which activate endothelial cells
(ECs) (8). Coincidentally,
proliferative ECs have been observed near the alveolar
microvasculature. Furthermore, both ECs and tumor cells secrete
matrix metalloproteinases (MMPs), which degrade the extracellular
matrix (ECM) and basement membranes. Primary sprouts form tubes and
then capillary loops, which are followed by pericyte recruitment,
synthesis of a new basement membrane, and vessel maturation
(9). Low dose of cadmium (Cd) may
promote angiogenesis through upregulation of VEGF expression and
secretion and promote the development of LUAD (10). In addition, reducing VEGF signaling
may effectively inhibit the development of LUAD (11).
Anti-angiogenic drugs that inhibit VEGF signaling
pathways, such as ramucirumab and bevacizumab, have been considered
a promising option for patients with advanced NSCLC (including
LUAD) (12). However, some side
effects such as proteinuria, hypertension, and hand and foot
syndrome often accompany the treatment with angiogenesis inhibitors
that include sorafenib, bevacizumab, and ramucirumab (13,14).
The domains of VWF are ordered symmetrically as
follows: D1-D2-D'D3-A1-A2-A3-D4-C1-C2-C3-C4-C5-C6-CK (19) (Fig.
1). The D regions are divided into smaller lobes or modules:
the D3, D2, and D1 domains are divided into E, TIL, C8, and VWD
modules, respectively (19). D',
on the other hand, only has the subdomains TIL' and E (19).
By tethering platelets to areas of endothelial
injury and acting as a carrier for coagulation factor VIII, VWF
promotes hemostasis. In addition, other functions of VWF have been
identified, including immune response (20), tumor metastasis (21), and leukocyte recruitment (22). Recent evidence suggests the
potential clinical detection value and potential prognostic value
of plasma VWF in patients with acute myocardial infarction
(23,24), type 2 diabetes mellitus with
cardiovascular complications (25)
and coronary artery disease with major adverse cardiovascular
events (26). In addition, the
results of in vivo and in vitro research suggest that
VWF controls angiogenesis and that deficiency of VWF leads to
increased angiogenesis (27).
Plasma VWF levels are higher in patients presenting
with several types of cancer (28,29).
Elevated VWF remains an independent predictor of venous thrombosis
in cancer patients after adjusting for patient-related factors
(30,31). Higher VWF levels in cancer patients
are associated with cancer progression and metastasis (21). Endothelial secretion of VWF
contributes to the adhesion and transendothelial migration of
breast cancer cells (32).
Furthermore, new evidence reveals that VWF regulates tumor cell
proliferation and apoptosis (32).
Plasma VWF and VWF/ADAMTS-13 ratios were found to be
significantly increased in patients with advanced NSCLC (including
LUAD and LUSC), while the levels of ADAMTS-13 were decreased
(28). ADAMTS-13 cleaves VWF in
blood in the A2 domain. Furthermore, a marked increase in the
VWF/ADAMTS-13 ratio is associated with fibrinogen, D-dimers and
coagulation factor VIII (28). ECs
of certain microvessels and small vessels in the lung express
abundant VWF mRNA. The alveolar-capillary ECs do not express
VWF (33). Conversely, other
vessels, including the larger vessels, arterioles and bronchial
capillaries in the lung, consistently express VWF (33). Xu et al (34) discovered that VWF was overexpressed
in tumor vessels of LUAD compared to vessels of adjacent tissues.
Consistently, VWF expression was found to be elevated in ECs of
transplanted mouse LUAD tissues and fresh human LUAD tissues
(34). Similarly, Jin et al
(33) discovered that VWF
expression is elevated in normal alveolar-capillary ECs near areas
of EC germination and tumor invasion. Meanwhile, the cytoplasm of
capillary ECs was enlarged and had increased Weibel-Palade bodies
(WPBs), which contain VWF, Ang-2, and other angiogenesis mediators
(33). However, alveolar-capillary
ECs in LUAD developed new reactivity to VWF (35). The Cancer Genome Atlas (TCGA) and
The Gene Expression Omnibus (GEO) dataset GSE43458 were used to
explore differentially co-expressed genes between LUAD and normal
tissues (36). The VWF
expression was downregulated in LUAD compared to normal tissue
(36).
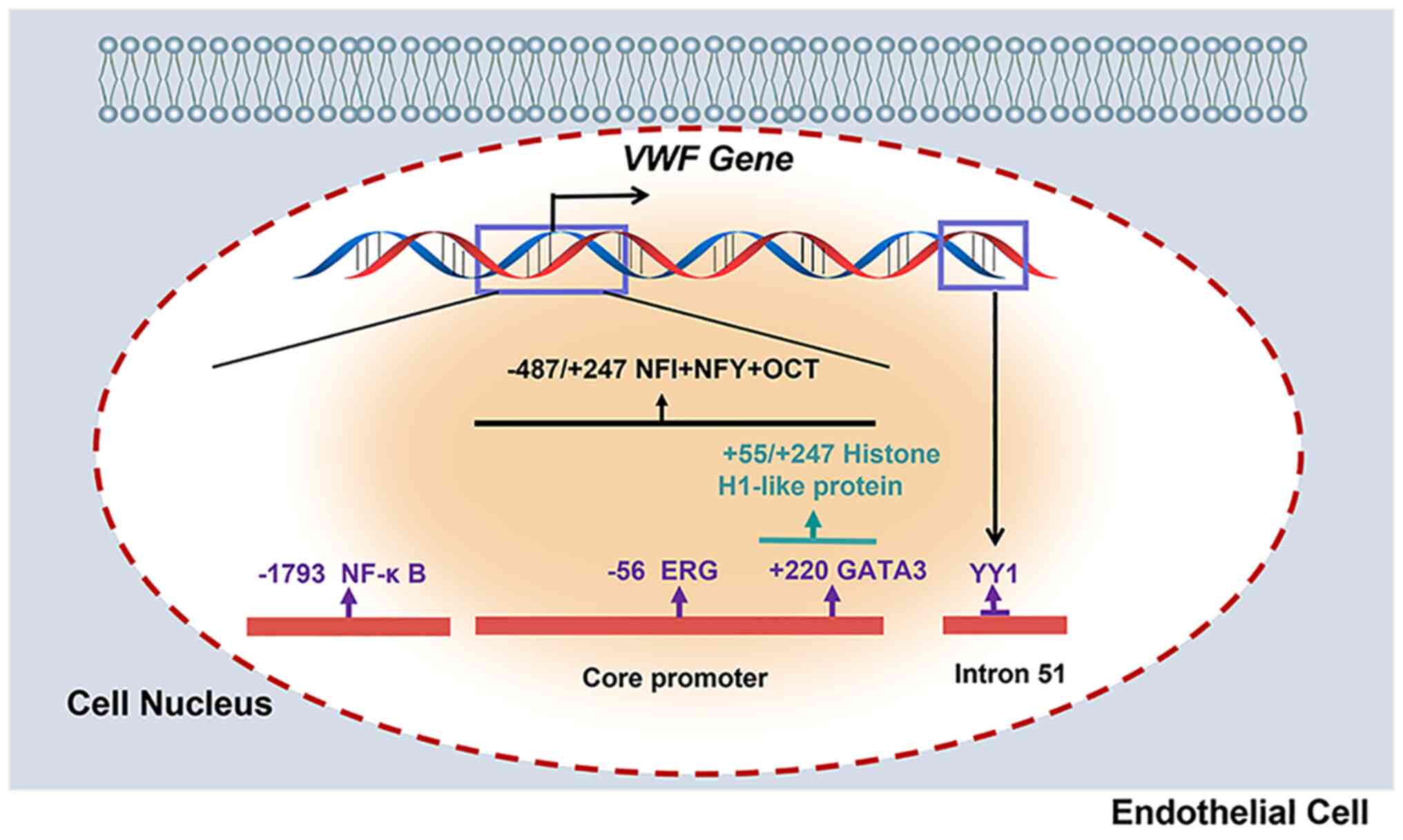
The ETS-related gene (ERG) is an ETS family
transcription factor specifically expressed in ECs (39), and regulates a series of
EC-specific genes (40). By
binding with the −56 ETS motif of the VWF promoter, ERG
maintains basal expression of VWF (37). In addition, ERG mediates cadmium
(Cd)-mediated VWF expression, suggesting that ERG is involved in
the transcriptional control of VWF in pathological
situations (41). However, the
protein and mRNA levels of ERG were unchanged with A549-derived
conditioned medium (CM) (34).
GATA protein 3 (GATA3) is a transcription factor
that belongs to the zinc finger protein family and can recognize
(A/T)GATA(A/G) and related sequences. In vivo research has
shown that loss of GATA-1 expression in megakaryocytes causes
decreased levels of VWF mRNA (42). A GATA-binding motif can be found in
the VWF promoter at position +220 (38). Furthermore, GATA3 expression was
found to be increased in A549-CM co-cultured human umbilical vein
ECs by binding GATA3 to the +220 GATA binding motif in the
VWF promoter (34).
Therefore, GATA3 may upregulate VWF expression in LUAD (34).
Yin Yang 1 (YY1) is a ubiquitous transcription
factor that has both activating and repressive effects. The AATGG
sequence is shown to the core consensus binding site of
transcription factor YY1 (43). A
region in intron 51 of the VWF gene is DNase I
hypersensitive (HSS)-specific in non-endothelial cells and
interacts with a specific complex of endothelial and
non-endothelial cells containing YY1 (43). In addition, the HSS sequence of
intron 51 of the VWF gene contains a cis-acting
element that is required for VWF gene transcription in a
subpopulation of lung ECs (43).
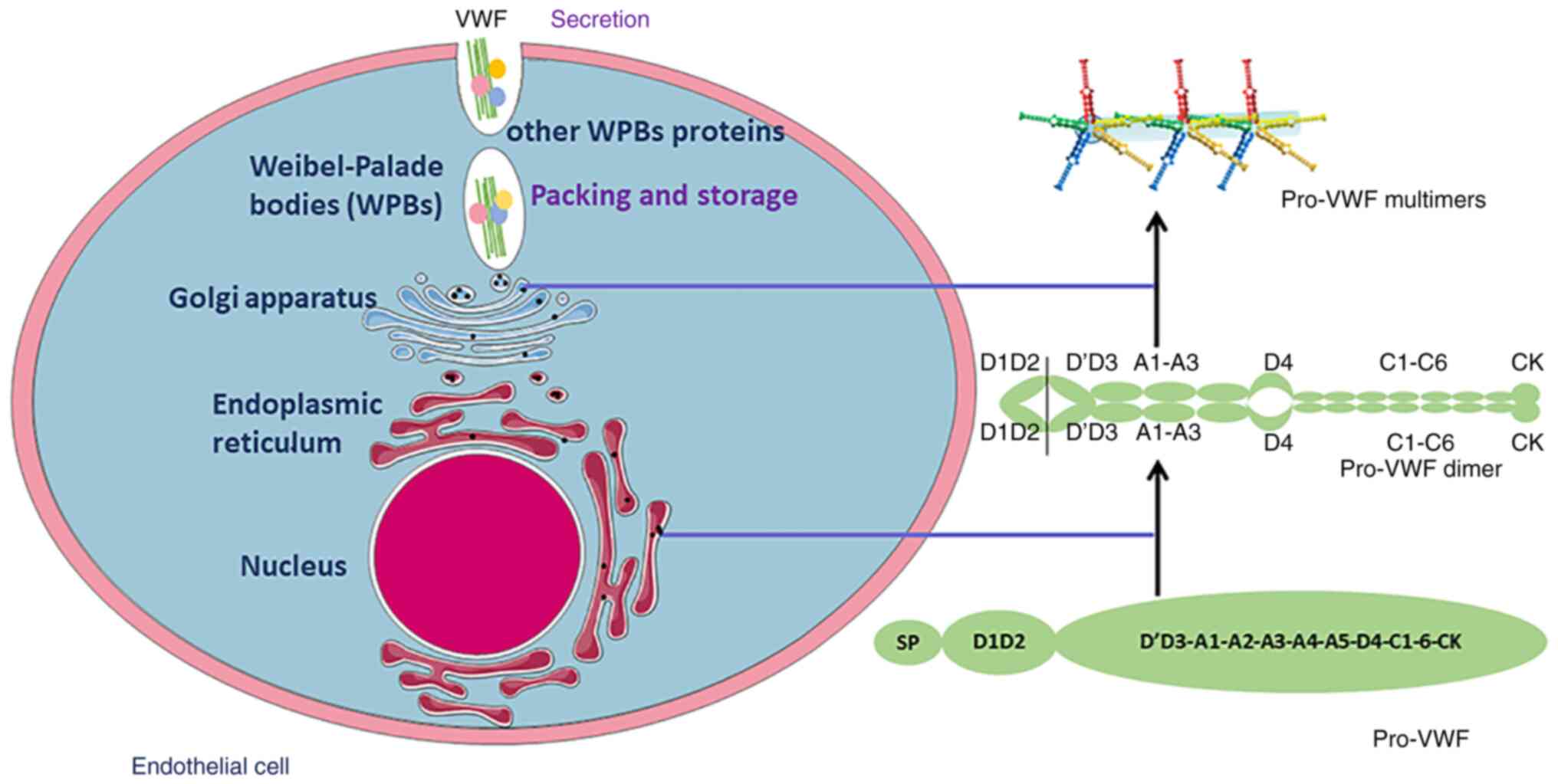
The biosynthesis of VWF includes several
posttranslational modifications in ECs and megakaryocytes (46). The polypeptide of VWF contains 741
characteristic amino acid residues, 22 amino acid long signal
peptides, and 2,050 amino acid residue long mature polypeptides
(46). In the endoplasmic
reticulum, VWF is a dimer (called pro-VWF) through disulfide bonds.
Thereafter, this dimer is transported to the Golgi, and it is
multimerized through a disulfide bond between the D'D3 structural
domains. Subsequently, VWF multimers form tubules and are stored in
Weibel-Palade bodies (WPBs) (47).
VWF is secreted through two main pathways. One is
regulatory and responds to secretion, and the other is continuous
and does not require cellular stimulation (46). Secretion of VWF from specialized
storage granules, called WPBs, is triggered by several substances
(48). When WPBs are stimulated by
a variety of substances such as histamine, thrombin, and phorbol
myristate acetate, they release amounts of ultra-large VWF. Once
released, they are cleaved in the A2 domain by ADAMTS-13. Thus, VWF
circulates in plasma in the form of a series of multimers ranging
in size from 500 to 20,000 kDa (42). Plasma VWF is almost exclusively
derived from endothelial secretion, and VWF secreted into the
subendothelium has a role in EC adhesion and extracellular matrix
binding (49).
The VWF may regulate angiogenesis and vascular
homeostasis by binding integrin αvβ3 (51). Under certain conditions, integrin
αvβ3 can inhibit VEGFR-2 activity and downstream signaling to
suppress angiogenesis (52). The
absence of VWF in ECs leads to integrin αvβ3 expression
decrease, which may cause VEGFR-2 signaling increase (53). Interestingly, VWF also interacts
with integrin αvβ3 on vascular smooth muscle cells via the Notch
signaling pathway (54). However,
pharmacological inhibition of integrin αvβ3 inhibits blood vessel
generation in experimental models (55). Thus, integrin αvβ3 may have a
bimodal effect in regards to angiogenesis, which acts as an
activator or an inhibitor depending on the stage of angiogenesis
and the different extracellular matrix ligands.
VWF may promote the formation of WPBs, which contain
angiopoietin-2 (Ang-2) and VWF (56). Reduced or dysfunctional VWF leads
to a reduction in WPBs, resulting in the component release of WPB
components such as Ang-2 (56).
Barton et al found an increase in Ang-2 in VWF-deficient ECs
in vitro (57). This has
now been confirmed in vivo, with a significant increase in
Ang-2 levels in the brains of Vwf −/− mice
(58). In addition, the binding of
Ang-2 to its receptor Tie-2 can act synergistically with VEGFR-2
signaling to promote angiogenesis (59). Excessive and dysregulated VEGF
signaling can lead to the formation of fragile and leaky blood
vessels (60). For example,
patients with VWD show a high prevalence of gastrointestinal
vascular malformations.
The angiogenic factors VEGF and fibroblast growth
factor-2 (FGF-2), which are abundant in the tumor microenvironment,
have been shown to upregulate VWF expression. Treatment with
bevacizumab, an anti-VEGF, has been demonstrated to lower VWF
levels in the blood (63). In
vitro, VWF binds to VEGF-A through the heparin-binding domain
(HBD) within the VWF A1 domain (64). Incorporation of the A1-HBD domain
of VWF protein into fibrin matrices enables sequestration and slows
release of incorporated VEGF-A (64).
Plasma VWF and the VWF/ADAMTS-13 ratio have been
found to be substantially increased, whereas ADAMTS-13 levels were
found to be decreased in patients with advanced NSCLC (28). Tumor cells directly induce
activation of ECs, leading to WPB extravasation and release of
ultra-large VWF multimers (65).
Ultra-large VWF multimers are discharged into the plasma, where the
plasma VWF-cleaving protease ADAMTS-13 rapidly degrades them into
smaller VWF multimers (66).
Smaller VWF multimers are more rapidly cleared from the circulation
than ultra-large VWF (67).
Increased ultra-large VWF in plasma disrupts the balance between
VWF and ADAMTS-13 levels, resulting in an increased VWF/ADAMTS-13
ratio (68). The VWF/ADAMTS-13
ratio has been used to diagnose hypercoagulability caused by an
imbalance in VWF secretion and ADAMTS-13 in patients with organ
failure (69). In patients with
advanced NSCLC, a marked increase in the VWF/ADAMTS-13 ratio was
found to be positively correlated with D-dimers, fibrinogen and
coagulation factor VIII (28).
Therefore, elevated VWF/ADAMTS-13 levels implicate a highly
thrombotic state, resulting in thrombosis in cancer patients.
VWF/ADAMTS-13 in plasma has the potential to be used as a marker of
prognosis in patients with LUAD.
VWF was found to be preferentially overexpressed in
tumor vessels of LUAD compared to vessels of adjacent tissues
(34). Consistently,
overexpression of VWF was found in ECs of transplanted mouse LUAD
tissues and fresh human LUAD tissues (34). However, VWF was recently found to
be expressed in normal lung tissue, but low or undetectable levels
were found in LUAD tissue (36).
Furthermore, survival analysis showed that LUAD patients with low
VWF expression in tissues had a poorer prognosis (70). Thus, VWF may be differentially
expressed in different stages of LUAD. The association between VWF
levels and LUAD staging may be explored and potentially used for
prognosis.
The mechanism of VWF regulation of tumor
angiogenesis in LUAD has not been elucidated. VWF may act as a
negative regulator of VEGF-dependent angiogenesis through pathways
involving integrin αvβ3 and Ang-2 (50). In addition to integrin αvβ3 and
Ang-2, VWF interacts with galectin-3 and galectin-1, which are
involved in the control of angiogenesis. Supplementation with VWF
analogs may inhibit tumor angiogenesis in LUAD. There are several
medications available to elevate VWF with no significant side
effects. Desmopressin (dDAVP) is a treatment for patients with VWD
and stimulates the release of endogenous VWF into the plasma
(71). MINIRIN® (dDAVP)
is supplied by Ferring/Valeas (71). The recommended dosage is 0.3 µg/kg
by slow i.v. infusion or fixed doses of 150 µg in children and 300
µg in adults by intranasal spray (71). A human recombinant VWF (rVWF),
vonicog alfa, was found to increase VWF levels in VWD patients,
making treatment independent of plasma supply (72). rVWF is a purified glycoprotein
synthesized in a genetically engineered CHO cell line (72). The doses of 50 and 80 U/kg VWF have
been used for evaluation (72).
These drugs may treat LUAD by increasing VWF in the blood to
inhibit tumor angiogenesis. Paradoxically, VWF has the potential to
promote tumor metastasis (21).
Tumor cells of nonendothelial origin may acquire de novo VWF
expression and show enhanced EC adhesion and extravasation
(21). In addition, tumor cells
directly induce EC activation resulting in WPB exocytosis and the
release of ultra-large VWF strings (21). VWF binds to platelets via GPIbα and
GPIIb/IIIa receptors and to tumor cells via GPIIb/IIIa receptors or
their semi-homologous twin integrin αvβ3 (65), and, therefore, may tether platelets
and tumor cells along the endothelium (21). This interaction may increase tumor
cell adhesion to the vascular endothelium and promote extravasation
(21). The balance of the
potential benefits and risks of VWF treatment on LUAD should be
carefully considered. Given that VWF inhibits angiogenesis and thus
LUAD growth, VWF supplementation may achieve therapeutic effects in
LUAD. However, VWF may also promote tumor metastasis. VWF
supplementation is not recommended for patients with early-stage
LUAD to avoid the risk of tumor metastasis. Anti-angiogenesis
therapy is essential for patients with advanced LUAD, thus VWF
supplementation may be attempted together with conventional
chemotherapy.
Not applicable.
This study is supported by Shandong Provincial Administration of
Traditional Chinese Medicine (grant no. 2017-218).
Not applicable.
ZL conceived and designed the review. XL wrote the
first draft. XL and ZL participated in writing of the manuscript.
All authors contributed to the article and read and approved the
final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kuhn E, Morbini P, Cancellieri A, Damiani
S, Cavazza A and Comin CE: Adenocarcinoma classification: Patterns
and prognosis. Pathologica. 110:5–11. 2018.
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hynds RE, Ben Aissa A, Gowers KHC, Watkins
TBK, Bosshard-Carter L, Rowan AJ, Veeriah S, Wilson GA, Quezada SA,
Swanton C, et al: Expansion of airway basal epithelial cells from
primary human non-small cell lung cancer tumors. Int J Cancer.
143:160–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding Y, Zhang L, Guo L, Wu C, Zhou J, Zhou
Y, Ma J, Li X, Ji P, Wang M, et al: Comparative study on the
mutational profile of adenocarcinoma and squamous cell carcinoma
predominant histologic subtypes in Chinese non-small cell lung
cancer patients. Thorac Cancer. 11:103–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X and Adjei AA: Lung cancer and
metastasis: New opportunities and challenges. Cancer Metastasis
Rev. 34:169–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B and Wei C: Hypoxia induces
overexpression of CCL28 to recruit treg cells to enhance
angiogenesis in lung adenocarcinoma. J Environ Pathol Toxicol
Oncol. 40:65–74. 2021. View Article : Google Scholar
|
|
8
|
Zahn LM: Effects of the tumor
microenvironment. Science. 355:1386–1388. 2017. View Article : Google Scholar
|
|
9
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar
|
|
10
|
Liu F, Wang B, Li L, Dong F, Chen X, Li Y,
Dong X, Wada Y, Kapron CM and Liu J: Low-dose cadmium upregulates
VEGF expression in lung adenocarcinoma cells. Int J Environ Res
Public Health. 12:10508–10521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Li Y, Dong F, Li L, Masuda T, Allen
TD and Lobe CG: Trichostatin A suppresses lung adenocarcinoma
development in Grg1 overexpressing transgenic mice. Biochem Biophys
Res Commun. 463:1230–1236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frezzetti D, Gallo M, Maiello MR,
D'Alessio A, Esposito C, Chicchinelli N, Normanno N and De Luca A:
VEGF as a potential target in lung cancer. Expert Opin Ther
Targets. 21:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar
|
|
14
|
Kurzrock R and Stewart DJ: Exploring the
Benefit/Risk associated with antiangiogenic agents for the
treatment of non-small cell lung cancer patients. Clin Cancer Res.
23:1137–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Starke RD, Ferraro F, Paschalaki KE,
Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD,
Cutler DF, et al: Endothelial von Willebrand factor regulates
angiogenesis. Blood. 117:1071–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Löf A, Müller JP and Brehm MA: A
biophysical view on von Willebrand factor activation. J Cell
Physiol. 233:799–810. 2018. View Article : Google Scholar
|
|
17
|
Kremer Hovinga JA, Coppo P, Lämmle B,
Moake JL, Miyata T and Vanhoorelbeke K: Thrombotic thrombocytopenic
purpura. Nat Rev Dis Primers. 3:170202017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sadler JE: Pathophysiology of thrombotic
thrombocytopenic purpura. Blood. 130:1181–1188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou YF, Eng ET, Zhu J, Lu C, Walz T and
Springer TA: Sequence and structure relationships within von
Willebrand factor. Blood. 120:449–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Schroeder JA, Luo X and Shi Q: The
impact of von Willebrand factor on factor VIII memory immune
responses. Blood Adv. 1:1565–1574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Sullivan JM, Preston RJS, Robson T and
O'Donnell JS: Emerging roles for von willebrand factor in cancer
cell biology. Semin Thromb Hemost. 44:159–166. 2018. View Article : Google Scholar
|
|
22
|
Kawecki C, Lenting PJ and Denis CV: von
Willebrand factor and inflammation. J Thromb Haemost. 15:1285–1294.
2017. View Article : Google Scholar
|
|
23
|
Wang X, Zhao J, Zhang Y, Xue X, Yin J,
Liao L, Xu C, Hou Y, Yan S and Liu J: Kinetics of plasma von
Willebrand factor in acute myocardial infarction patients: A
meta-analysis. Oncotarget. 8:90371–90379. 2017. View Article : Google Scholar
|
|
24
|
Li Y, Li L, Dong F, Guo L, Hou Y, Hu H,
Yan S, Zhou X, Liao L, Allen TD and Liu JU: Plasma von Willebrand
factor level is transiently elevated in a rat model of acute
myocardial infarction. Exp Ther Med. 10:1743–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng X, Wang X, Fan M, Zhao J, Lin L and
Liu J: Plasma levels of von Willebrand factor in type 2 diabetes
patients with and without cardiovascular diseases: A meta-analysis.
Diabetes Metab Res Rev. 36:e31932020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan M, Wang X, Peng X, Feng S, Zhao J,
Liao L, Zhang Y, Hou Y and Liu J: Prognostic value of plasma von
Willebrand factor levels in major adverse cardiovascular events: A
systematic review and meta-analysis. BMC Cardiovasc Disord.
20:722020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Randi AM, Smith KE and Castaman G: von
Willebrand factor regulation of blood vessel formation. Blood.
132:132–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo R, Yang J, Liu X, Wu J and Chen Y:
Increased von Willebrand factor over decreased ADAMTS-13 activity
is associated with poor prognosis in patients with advanced
non-small-cell lung cancer. J Clin Lab Anal. 32:e222192018.
View Article : Google Scholar
|
|
29
|
Marfia G, Navone SE, Fanizzi C, Tabano S,
Pesenti C, Abdel Hadi L, Franzini A, Caroli M, Miozzo M, Riboni L,
et al: Prognostic value of preoperative von Willebrand factor
plasma levels in patients with Glioblastoma. Cancer Med.
5:1783–1790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Obermeier HL, Riedl J, Ay C, Koder S,
Quehenberger P, Bartsch R, Kaider A, Zielinski CC and Pabinger I:
The role of ADAMTS-13 and von Willebrand factor in cancer patients:
Results from the vienna cancer and thrombosis Study. Res Pract
Thromb Haemost. 3:503–514. 2019. View Article : Google Scholar
|
|
31
|
Pépin M, Kleinjan A, Hajage D, Büller HR,
Di Nisio M, Kamphuisen PW, Salomon L, Veyradier A, Stepanian A and
Mahé I: ADAMTS-13 and von Willebrand factor predict venous
thromboembolism in patients with cancer. J Thromb Haemost.
14:306–315. 2016. View Article : Google Scholar
|
|
32
|
Qi Y, Chen W, Liang X, Xu K, Gu X, Wu F,
Fan X, Ren S, Liu J, Zhang J, et al: Novel antibodies against GPIbα
inhibit pulmonary metastasis by affecting vWF-GPIbα interaction. J
Hematol Oncol. 11:1172018. View Article : Google Scholar
|
|
33
|
Jin E, Ghazizadeh M, Fujiwara M, Nagashima
M, Shimizu H, Ohaki Y, Arai S, Gomibuchi M, Takemura T and Kawanami
O: Angiogenesis and phenotypic alteration of alveolar capillary
endothelium in areas of neoplastic cell spread in primary lung
adenocarcinoma. Pathol Int. 51:691–700. 2001. View Article : Google Scholar
|
|
34
|
Xu Y, Pan S, Liu J, Dong F, Cheng Z, Zhang
J, Qi R, Zang Q, Zhang C, Wang X, et al: GATA3-induced vWF
upregulation in the lung adenocarcinoma vasculature. Oncotarget.
8:110517–110529. 2017. View Article : Google Scholar
|
|
35
|
Morishita C, Jin E, Kikuchi M, Egawa S,
Fujiwara M, Ohaki Y, Ghazizadeh M, Takemura T and Kawanami O:
Angiogenic switching in the alveolar capillaries in primary lung
adenocarcinoma and squamous cell carcinoma. J Nippon Med Sch.
74:344–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He Y, Liu R, Yang M, Bi W, Zhou L, Zhang
S, Jin J, Liang X and Zhang P: Identification of VWF as a novel
biomarker in lung adenocarcinoma by comprehensive analysis. Front
Oncol. 11:6396002021. View Article : Google Scholar
|
|
37
|
Liu J, Yuan L, Molema G, Regan E, Janes L,
Beeler D, Spokes KC, Okada Y, Minami T, Oettgen P and Aird WC:
Vascular bed-specific regulation of the von Willebrand factor
promoter in the heart and skeletal muscle. Blood. 117:342–351.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Kanki Y, Okada Y, Jin E, Yano K,
Shih SC, Minami T and Aird WC: A +220 GATA motif mediates basal but
not endotoxin-repressible expression of the von Willebrand factor
promoter in Hprt-targeted transgenic mice. J Thromb Haemost.
7:1384–1392. 2010. View Article : Google Scholar
|
|
39
|
Yuan L, Sacharidou A, Stratman AN, Le Bras
A, Zwiers PJ, Spokes K, Bhasin M, Shih SC, Nagy JA, Molema G, et
al: RhoJ is an endothelial cell-restricted Rho GTPase that mediates
vascular morphogenesis and is regulated by the transcription factor
ERG. Blood. 118:1145–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu F, Liu Q, Yuan F, Guo S and Liu J, Sun
Z, Gao P, Wang Y, Yan S and Liu J: Erg mediates downregulation of
claudin-5 in the brain endothelium of a murine experimental model
of cerebral malaria. FEBS Lett. 593:2585–2595. 2019. View Article : Google Scholar
|
|
41
|
Wang X, Dong F, Wang F, Yan S, Chen X,
Tozawa H, Ushijima T, Kapron CM, Wada Y and Liu J: Low dose cadmium
upregulates the expression of von Willebrand factor in endothelial
cells. Toxicol Lett. 290:46–54. 2018. View Article : Google Scholar
|
|
42
|
Stockschlaeder M, Schneppenheim R and
Budde U: Update on von Willebrand factor multimers: Focus on
high-molecular-weight multimers and their role in hemostasis. Blood
Coagul Fibrinolysis. 25:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kleinschmidt AM, Nassiri M, Stitt MS,
Wasserloos K, Watkins SC, Pitt BR and Jahroudi N: Sequences in
intron 51 of the von Willebrand factor gene target promoter
activation to a subset of lung endothelial cells in transgenic
mice. J Biol Chem. 283:2741–2750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nassiri M, Liu J, Kulak S, Uwiera RR, Aird
WC, Ballermann BJ and Jahroudi N: Repressors NFI and NFY
participate in organ-specific regulation of von Willebrand factor
promoter activity in transgenic mice. Arterioscler Thromb Vasc
Biol. 30:1423–1429. 2010. View Article : Google Scholar
|
|
45
|
Harvey PJ, Keightley AM, Lam YM, Cameron C
and Lillicrap D: A single nucleotide polymorphism at
nucleotide-1793 in the von Willebrand factor (VWF) regulatory
region is associated with plasma VWF: Ag levels. Br J Haematol.
109:349–353. 2000. View Article : Google Scholar
|
|
46
|
Lenting PJ, Christophe OD and Denis CV:
von Willebrand factor biosynthesis, secretion, and clearance:
Connecting the far ends. Blood. 125:2019–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zeng J, Shu Z, Liang Q, Zhang J, Wu W,
Wang X and Zhou A: Structural basis of Von Willebrand Factor
multimerization and tubular storage. Blood. 5–Feb;2022.doi:
10.1182/blood.2021014729. View Article : Google Scholar
|
|
48
|
van den Biggelaar M, Bierings R, Storm G,
Voorberg J and Mertens K: Requirements for cellular co-trafficking
of factor VIII and von Willebrand factor to Weibel-Palade bodies. J
Thromb Haemost. 5:2235–2242. 2007. View Article : Google Scholar
|
|
49
|
Lopes da Silva M and Cutler DF: von
Willebrand factor multimerization and the polarity of secretory
pathways in endothelial cells. Blood. 128:277–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Randi AM and Laffan MA: Von Willebrand
factor and angiogenesis: Basic and applied issues. J Thromb
Haemost. 15:13–20. 2017. View Article : Google Scholar
|
|
51
|
Brooks PC, Montgomery AM, Rosenfeld M,
Reisfeld RA, Hu T, Klier G and Cheresh DA: Integrin alpha v beta 3
antagonists promote tumor regression by inducing apoptosis of
angiogenic blood vessels. Cell. 79:1157–1164. 1994. View Article : Google Scholar
|
|
52
|
Sartori A, Portioli E, Battistini L,
Calorini L, Pupi A, Vacondio F, Arosio D, Bianchini F and Zanardi
F: Synthesis of Novel c(AmpRGD)-sunitinib dual conjugates as
molecular tools targeting the αvβ3
Integrin/VEGFR2 couple and impairing tumor-associated angiogenesis.
J Med Chem. 60:248–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Somanath PR, Malinin NL and Byzova TV:
Cooperation between integrin alphavbeta3 and VEGFR2 in
angiogenesis. Angiogenesis. 12:177–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lagrange J, Worou ME, Michel JB, Raoul A,
Didelot M, Muczynski V, Legendre P, Plénat F, Gauchotte G,
Lourenco-Rodrigues MD, et al: The VWF/LRP4/αVβ3-axis represents a
novel pathway regulating proliferation of human vascular smooth
muscle cells. Cardiovasc Res. 118:622–637. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Patsenker E, Popov Y, Stickel F, Schneider
V, Ledermann M, Sägesser H, Niedobitek G, Goodman SL and Schuppan
D: Pharmacological inhibition of integrin alphavbeta3 aggravates
experimental liver fibrosis and suppresses hepatic angiogenesis.
Hepatology. 50:1501–1511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cossutta M, Darche M, Carpentier G, Houppe
C, Ponzo M, Raineri F, Vallée B, Gilles ME, Villain D, Picard E, et
al: Weibel-Palade bodies orchestrate pericytes during angiogenesis.
Arterioscler Thromb Vasc Biol. 39:1843–1858. 2019. View Article : Google Scholar
|
|
57
|
Barton WA, Tzvetkova-Robev D, Miranda EP,
Kolev MV, Rajashankar KR, Himanen JP and Nikolov DB: Crystal
structures of the Tie2 receptor ectodomain and the
angiopoietin-2-Tie2 complex. Nat Struct Mol Biol. 13:524–532. 2006.
View Article : Google Scholar
|
|
58
|
Xu H, Cao Y, Yang X, Cai P, Kang L, Zhu X,
Luo H, Lu L, Wei L, Bai X, et al: ADAMTS13 controls vascular
remodeling by modifying VWF reactivity during stroke recovery.
Blood. 130:11–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Scholz A, Plate KH and Reiss Y:
Angiopoietin-2: A multifaceted cytokine that functions in both
angiogenesis and inflammation. Ann N Y Acad Sci. 1347:45–51. 2015.
View Article : Google Scholar
|
|
60
|
Siveen KS, Prabhu K, Krishnankutty R,
Kuttikrishnan S, Tsakou M, Alali FQ, Dermime S, Mohammad RM and
Uddin S: Vascular endothelial growth factor (VEGF) signaling in
tumour vascularization: Potential and challenges. Curr Vasc
Pharmacol. 15:339–351. 2017. View Article : Google Scholar
|
|
61
|
Saint-Lu N, Oortwijn BD, Pegon JN, Odouard
S, Christophe OD, de Groot PG, Denis CV and Lenting PJ:
Identification of galectin-1 and galectin-3 as novel partners for
von Willebrand factor. Arterioscler Thromb Vasc Biol. 32:894–901.
2012. View Article : Google Scholar
|
|
62
|
Tamura K, Hashimoto K, Suzuki K, Yoshie M,
Kutsukake M and Sakurai T: Insulin-like growth factor binding
protein-7 (IGFBP7) blocks vascular endothelial cell growth factor
(VEGF)-induced angiogenesis in human vascular endothelial cells.
Eur J Pharmacol. 610:61–67. 2009. View Article : Google Scholar
|
|
63
|
Pace A, Mandoj C, Antenucci A, Villani V,
Sperduti I, Casini B, Carosi M, Fabi A, Vidiri A, Koudriavtseva T
and Conti L: A predictive value of von Willebrand factor for early
response to Bevacizumab therapy in recurrent glioma. J Neurooncol.
138:527–535. 2018. View Article : Google Scholar
|
|
64
|
Ishihara J, Ishihara A, Starke RD,
Peghaire CR, Smith KE, McKinnon TAJ, Tabata Y, Sasaki K, White MJV,
Fukunaga K, et al: The heparin binding domain of von Willebrand
factor binds to growth factors and promotes angiogenesis in wound
healing. Blood. 133:2559–2569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bauer AT, Suckau J, Frank K, Desch A,
Goertz L, Wagner AH, Hecker M, Goerge T, Umansky L, Beckhove P, et
al: von Willebrand factor fibers promote cancer-associated platelet
aggregation in malignant melanoma of mice and humans. Blood.
125:3153–3163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lancellotti S, Sacco M, Basso M and De
Cristofaro R: Mechanochemistry of von Willebrand factor. Biomol
Concepts. 10:194–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kappler S, Ronan-Bentle S and Graham A:
Thrombotic microangiopathies (TTP, HUS, HELLP). Hematol Oncol Clin
North Am. 31:1081–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Takaya H, Uemura M, Fujimura Y, Matsumoto
M, Matsuyama T, Kato S, Morioka C, Ishizashi H, Hori Y, Fujimoto M,
et al: ADAMTS13 activity may predict the cumulative survival of
patients with liver cirrhosis in comparison with the
Child-Turcotte-Pugh score and the Model for End-stage liver disease
score. Hepatol Res. 42:459–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Claus RA, Bockmeyer CL, Budde U, Kentouche
K, Sossdorf M, Hilberg T, Schneppenheim R, Reinhart K, Bauer M,
Brunkhorst FM and Lösche W: Variations in the ratio between von
Willebrand factor and its cleaving protease during systemic
inflammation and association with severity and prognosis of organ
failure. Thromb Haemost. 101:239–247. 2009. View Article : Google Scholar
|
|
70
|
Yang R, Zhou Y, Du C and Wu Y:
Bioinformatics analysis of differentially expressed genes in tumor
and paracancerous tissues of patients with lung adenocarcinoma. J
Thorac Dis. 12:7355–7364. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Federici AB: The use of desmopressin in
von Willebrand disease: The experience of the first 30 years
(1977–2007). Haemophilia. 14 (Suppl 1):S5–S14. 2008. View Article : Google Scholar
|
|
72
|
Gill JC, Castaman G, Windyga J, Kouides P,
Ragni M, Leebeek FW, Obermann-Slupetzky O, Chapman M, Fritsch S,
Pavlova BG, et al: Hemostatic efficacy, safety, and
pharmacokinetics of a recombinant von Willebrand factor in severe
von Willebrand disease. Blood. 126:2038–2046. 2015. View Article : Google Scholar : PubMed/NCBI
|