Introduction
Cancer statistics for 2021 estimated there were
83,730 new cases of bladder cancer (64,280 men and 19,450 women) in
USA, leading to 17,200 deaths (12,260 men and 4,940 women)
(1). Despite surgical treatments
(transurethral resection or radical cystectomy) that reduce the
quality of life of the patients, bladder cancer can not only be
recurrent but also spread via metastasis, becoming a highly
life-threatening disease (2).
When a non-invasive tumor grows beyond the limit of
oxygen and nutrient diffusion (>1-mm), a new blood supply is
required to allow subsequent growth of the tumor. The cells are
then placed in a hypoxic environment with the risk of necrosis for
unsupplied cells (3,4). The metabolism of cells may be altered
and growth stopped (5,6). Nevertheless, cells can also react to
this change in their environment (7,8) and
escape from the tumor to reach a more hospitable location (9–11). In
order to produce metastases, cancer cells have to migrate through
the extracellular matrix, enter the blood/lymph vessels and
disperse. This process involves the following: i) The decrease of
cell-cell attachment, ii) the expression of factors promoting or
helping migration, both are part of epithelial-mesenchymal
transition (EMT); iii) the degradation of the extracellular matrix
(ECM) through the secretion and activation of matrix
metalloproteinases (MMP); iv) the ability to survive in the
blood/lymph environment (for example, in oxidative stress and
anoikis); and v) the ability to colonize other organs (12). Cancer is a complex biological
phenomenon, and all aspects are linked; therefore, investigating a
specific effect without considering other biological responses
should be avoided. For example, a change in the metabolism (Warburg
or reverse Warburg) is linked to EMT (13), cell proliferation, angiogenesis
(14) and migration, in addition to
metastases (15) and apoptotic
resistance (16). Thus, non-invasive
bladder cancer must remain localized to be efficiently cured. EMT,
proliferation, migration and resistance to apoptosis remain the key
elements to control in order to prevent cancer cell dispersion from
a localized tumor (17,18).
As in numerous other types of cancer, the presence
of hypoxia markers have been recognized as predictors of poor
prognosis for bladder cancer evolution and treatment (19,20). It
was previously demonstrated that hypoxic bladder cancer cells
enhance their malignant nature (proliferation, migration, invasion
and chemotherapy resistance) by remodeling their tumor
microenvironment (9,10) and by enhancing glycolysis (21). Therefore, it is important to study
the effect of hypoxia on bladder cancer cells to obtain clinically
relevant results. One of the novelties of the present study is the
choice of two grade 1 bladder cancer cell lines compared with two
grade 3 bladder cancer cell lines; whereas, to the best of our
knowledge, the majority of previous studies only used grade 2 and 3
bladder cell lines. Indeed, low-grade bladder cancer can progress
to more aggressive ones and potentially recur after initial
transurethral resection (22). Also,
in order to control the progression of bladder cancer,
understanding the difference between low-grade and high-grade tumor
responses to their environment could be helpful. Specifically, it
has been demonstrated that hypoxia can predispose metastases in
several different cancer types (23,24).
The present study aimed to illustrate that standard
cell culture parameters are inadequate to mimic cancer biology and
that the use of such models could explain the relatively
unsuccessful translation to humans of discoveries in cancer
research (25). Modifying only one
cell culture parameter, such as O2 concentration,
resulted in substantial changes in several aspects of cell biology.
A number of parameters differ between standard cell culture and
actual tumour microenvironment. Recently, research on the effect of
hypoxia in cell biology have been awarded the Nobel prize, paving
the way for more research focusing on this important aspect of the
cancer microenvironment in the future (https://www.nobelprize.org/prizes/medicine/2019/advanced-information/;
accessed 27 January 2022).
Materials and methods
Cell lines
The present study used four bladder cancer cell
lines: Dr Y Fradet's lab generously provided MGHU-3 from the
transitional cell carcinoma of a 76-year-old Caucasian male (grade
1; CVCL_9827) (26), SW-780 (grade
1; CRL-2169; American Type Culture Collection) from the
transitional cell carcinoma of an 80-year-old Caucasian female,
SW-1710 (grade 3; ACC 426; German Collection of Microorganisms and
Cell Cultures GmbH) from bladder tumor of an 84-year-old Caucasian
female, and T24 (grade 3; HTB-4; American Type Culture Collection)
from the transitional cell carcinoma of an 81-year-old Caucasian
female. Cells were cultivated in Dulbecco-Vogt modification of
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% bovine growth serum (HyClone; Cytiva), 100
U/ml penicillin (Sigma-Aldrich; Merck KGaA), 25 µg/ml gentamicin
(Schering AG) in 8% CO2 at 37°C. Media were changed
three times a week.
Hypoxic culture conditions
A desiccator cabinet (Nalgene; Thermo Fisher
Scientific, Inc.) was modified to become a hypoxic chamber
(27). The hypoxic chamber was
placed in a standard cell culture incubator at 37°C. A gas canister
(Linde Canada, Inc.) containing an O2-reduced gas mix of
2% O2, 8% CO2 and 90% N2 (2%) was
connected to the chamber. After all operations in an open chamber,
the atmosphere was renewed with the appropriate
O2-reduced gas mix. During the gas exchange, a pipe
evacuated the old gas from the incubator. O2 pressure
was confirmed using a Pac 5500 O2 (Drägerwerk AG &
Co. KGaA). Time of exposure of bladder cancer cells to hypoxia or
normoxia was set at 72 h to mimic a chronic exposure.
Semi-quantification by western
blotting
Cells cultivated for 72 h in the normoxic atmosphere
(20% O2) or hypoxic conditions (2% O2) were
rinsed two times with PBS and lysed directly in the cell culture
plate in a buffer (31 mM Tris, 10% glycerol, 3% SDS, pH 6.8) and
collected with cell scrapers. Cell lysates were sonicated [3 pulse
of 3 sec at an amplitude of 20% in an ice water bath with a digital
sonifier (Branson Ultrasonics Corporation)]. Protein amount was
determined using MicroBCA protein assay kit according to the
manufacturer's protocol (Themo Fisher Scientific, Inc.). All
experiments were performed with independent triplicates for each of
the four cell lines. An equal amount of proteins, 10 µl (1 µg/µl)
of each sample, were separated by SDS-PAGE (10% gels) and
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.).
Non-specific binding sites were blocked for 1 h at room temperature
with 5% (w/v) non-fat powdered milk (BioBasic, Inc.) before an
overnight incubation at 4°C with the specific antibodies (Table I). Secondary antibodies were
immunopure antibodies (Thermo Fisher Scientific, Inc.) goat
anti-rabbit (cat. no. 31460) or anti-mouse (cat. no. 31430) IgG
(H+L) conjugated to horse radish peroxidase. These antibodies were
incubated at room temperature for 1 h at the concentration
indicated in the Table I. A
SuperSignal™ West Dura Extended Duration substrate
(Thermo Fisher Scientific, Inc.) was used, and the light signal was
detected using Fusion Fx7 (Vilber Lourmat). ImageJ version 1.53e
software (National Institutes of Health) was used to estimate the
relative amount of protein.
 | Table I.Antibodies used for western
blotting. |
Table I.
Antibodies used for western
blotting.
| Target protein | Host species | Company | 1st antibody
dilution | 2nd antibody
dilution | Cat. no. |
|---|
| β-actin | Mouse | Sigma-Aldrich;
Merck KGaA | 1/20,000 | 1/20,000 | A5441 |
| E-Cadherin | Mouse | Abcam | 1/1,000 | 1/1,000 | Ab1416 |
| EpCAM | Rabbit | Abcam | 1/500 | 1/1,000 | Ab32392 |
| N-Cadherin | Mouse | MilliporeSigma | 1/1,000 | 1/1,000 | 05-915 |
| TSP-1 | Rabbit | Cell Signaling
Technology, Inc. | 1/1,000 | 1/1,000 | 37879 |
| Vimentin | Rabbit | Abcam | 1/2,500 | 1/2,000 | Ab45939 |
Doubling time determination
The four cancer cell lines were seeded in 12-well
plates at a density of 20,000 cells per well (12% confluence). They
were cultivated in a normoxic atmosphere (20% O2,
control) or hypoxic conditions (2% O2). The next day
(Day 1), water-soluble tetrazolium (WST)-1 assay (Roche
Diagnostics) was used following the manufacturer's instructions.
Relaxing time of 1 h in normoxic conditions was allowed for all
cultures before being treated with WST-1 to avoid inhibition of the
mitochondrial oxidative chain. Cell density was also measured at
days 2 and 3. Doubling time was determined by the classic formula,
D=Ln(2)/g, where g is the exponential coefficient of the slope.
Overall, three independent experiments in octuplicate were
performed for each of the four cell lines.
Acidity measurement
Cell culture medium (DMEM supplemented with 10%
bovine growth serum, 100 U/ml penicillin, 25 µg/ml gentamicin)
conditioned for 72 h by cells in the normoxic atmosphere (20%
O2, control) or hypoxic conditions (2% O2)
were collected from plate into tubes. The pH was measured using
MQuant pH indicator strips (MilliporeSigma). The pH was used to
determine the level of acidity associated with metabolism. All
experiments were performed in independent triplicates for each of
the four cell lines.
Cell viability measurement
For cell viability measurements, WST-1 (Roche
Diagnostics) was used following the manufacturer's instructions.
All cell cultures were performed in the normoxic atmosphere (20%
O2) or hypoxic conditions (2% O2). Cells were
plated at 50% confluence. Subsequently, 24 h later, DMEM containing
ascorbic acid (Sigma-Aldrich; Merck KGaA) at various concentrations
(0, 1, 2 or 4 mM) was added. The culture was continued for 24 h,
and then WST-1 was added. A relaxing time of 1 h in normoxic
conditions was allowed for all cultures before being treated with
WST-1 to avoid inhibition of the mitochondrial oxidative chain.
Incubation with this reagent took place in normoxia and
measurements were collected after 2 h, when the maximal value of
optical density was between 0.8 and 1.2. A total of three
independent experiments in octuplicate were performed for each of
the four cell lines.
MMP activity measurement
Supernatant from 72 h cell cultures in the normoxic
atmosphere (20% O2) or hypoxic conditions (2%
O2) was collected. Total MMP activity was determined in
cell culture supernatants using the SensoLyte™ 520
Generic MMP assay kit (cat. no. AS711-58; AnaSpec). This kit can
simultaneously detect the activity of MMP-1, 2, 7, 8, 9, 12, 13 and
14. APMA was not used; therefore, inactive forms of MMP were not
detected. The results reflect the overall active MMP and
metalloproteinase inhibitor balance. A total of three independent
experiments in triplicate were performed for each of the four cell
lines.
Cell migration assay
The four bladder cancer cell lines were seeded at
80% confluence and cultured for 48 h in a normoxic atmosphere (20%
O2, control) or hypoxic conditions (2% O2).
After 24 h, the cell culture medium (DMEM supplemented with 10%
bovine growth serum, 100 U/ml penicillin, 25 µg/ml gentamicin) was
exchanged for serum-free cell culture medium (DMEM supplemented
with 100 U/ml penicillin, 25 µg/ml gentamicin) and culture
continued for another 24 h. At this time, a scratch was performed
using a 200 µl-pipette tip. Plates were rinsed three times with PBS
at 37°C to remove detached cells and culture was continued in
serum-free medium for 24 h. At the indicated time points, images
were captured using a CKX41 light microscope (Olympus Corporation)
with an E-620 camera (Olympus Corporation) at a magnification of
40X and analyzed using ImageJ software (National Institutes of
Health). All experiments were performed two times in quadruplicate
for each of the four cell lines. For MGHU-3 and SW-1710 bladder
cancer cell lines, additional experiments were performed using
factors to modulate migratory responses to hypoxia: MG-132 (20 µM;
Enzo Life Sciences, Inc.), cobalt chloride (200 µM; Sigma-Aldrich;
Merck KGaA), hypoxia-inducible factor (HIF)-2 antagonist (10 µM;
Sigma-Aldrich; Merck KGaA) and rottlerin (2 µM; Santa Cruz
Biotechnology, Inc.). These factors was added at the indicated
concentration at the same time as cell culture medium containing
serum was exchanged for serum-free cell culture medium and
maintained after the cell monolayer was scratched.
Statistical analysis
A bilateral unpaired Student's t-test assessed
differences between values. The data were analyzed using Excel 2003
(Microsoft Corporation). For datasets containing ≥3 groups,
ordinary one-way ANOVA followed by Tukey's multiple comparison test
were performed (GraphPad Prism v.9.0.2 Software; GraphPad Software,
Inc.). All data were expressed as mean ± standard deviation, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
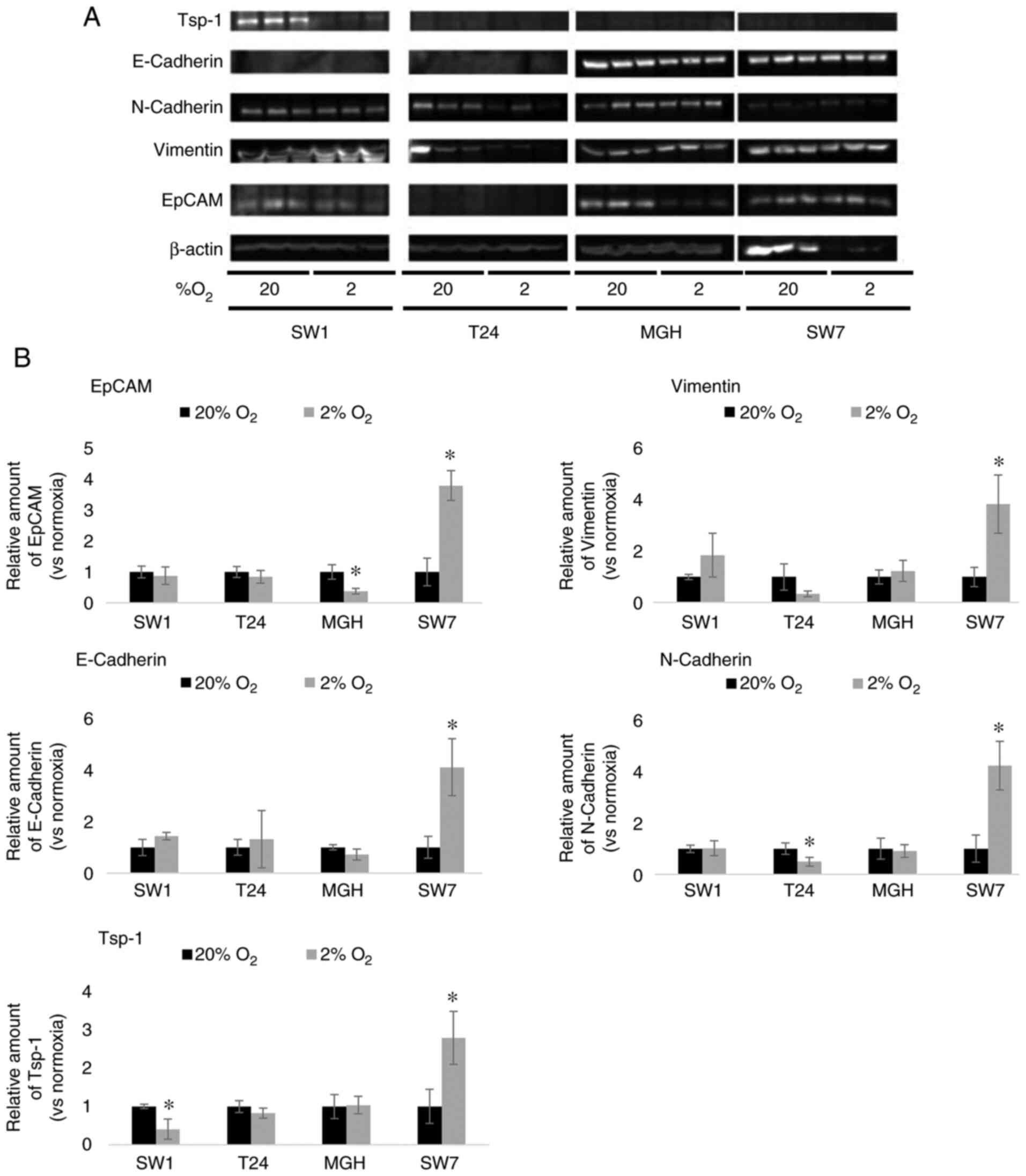
The effect of hypoxia on EMT potential
of bladder cancer cells
When placed in hypoxic cell culture conditions (2%
O2), T24 and SW-780 bladder cancer cell lines did not
present a significant change in epithelial cell adhesion molecule
(EpCAM) expression compared with the cells cultured in normoxic
conditions (20% O2). MGHU-3 bladder cancer cell lines
revealed a significant decrease in EpCAM expression when cultured
in hypoxic cell conditions compared with the cells cultured in
normoxic conditions, whereas it significantly increased in SW-780
cultures exposed to hypoxia (Fig.
1). When placed in hypoxic cell culture conditions, SW-1710,
T24 and MGHU-3 bladder cancer cell lines did not present a
significant change in vimentin expression compared with normoxic
conditions. By contrast, SW-780 cells demonstrated a significant
increase in vimentin expression when cultured in hypoxic cell
conditions compared with the SW-780 cells cultured in normoxic
conditions (Fig. 1). Expression of
E-Cadherin remained unchanged in SW-1710, T24 and MGH-U3 cancer
cell lines cultured in hypoxic conditions compared with normoxic
conditions; whereas it demonstrated a significant increase in
SW-780 cells in hypoxic conditions compared with normoxic
conditions. Nevertheless expression levels of E-Cadherin were very
low, near the limit of detection, in both of the high grade cancer
cell line cultures (T24 and SW-1710). Expression of N-Cadherin
revealed no significant difference in SW-1710 and MGH-U3 cancer
cell lines cultured in hypoxic conditions compared with normoxic
conditions; however, expression was significantly decreased in T24
and significantly increased in SW-780 cancer cell line cultures in
hypoxic conditions compared with the normoxic conditions (Fig. 1).
The effect of hypoxia on
thrombospondin-1 (TSP-1) synthesis by bladder cancer cells
Hypoxia induced a significant reduction of the TSP-1
expression in the SW-1710 grade 3 bladder cancer cell line; by
contrast, the T24 and MGHU-3 cells lines revealed no significant
difference between the normoxic and hypoxic conditions. Grade 1
bladder cancer cell line SW-780 demonstrated a significant increase
of TSP-1 expression in hypoxic conditions compared with the
normoxic cells (Fig. 1).
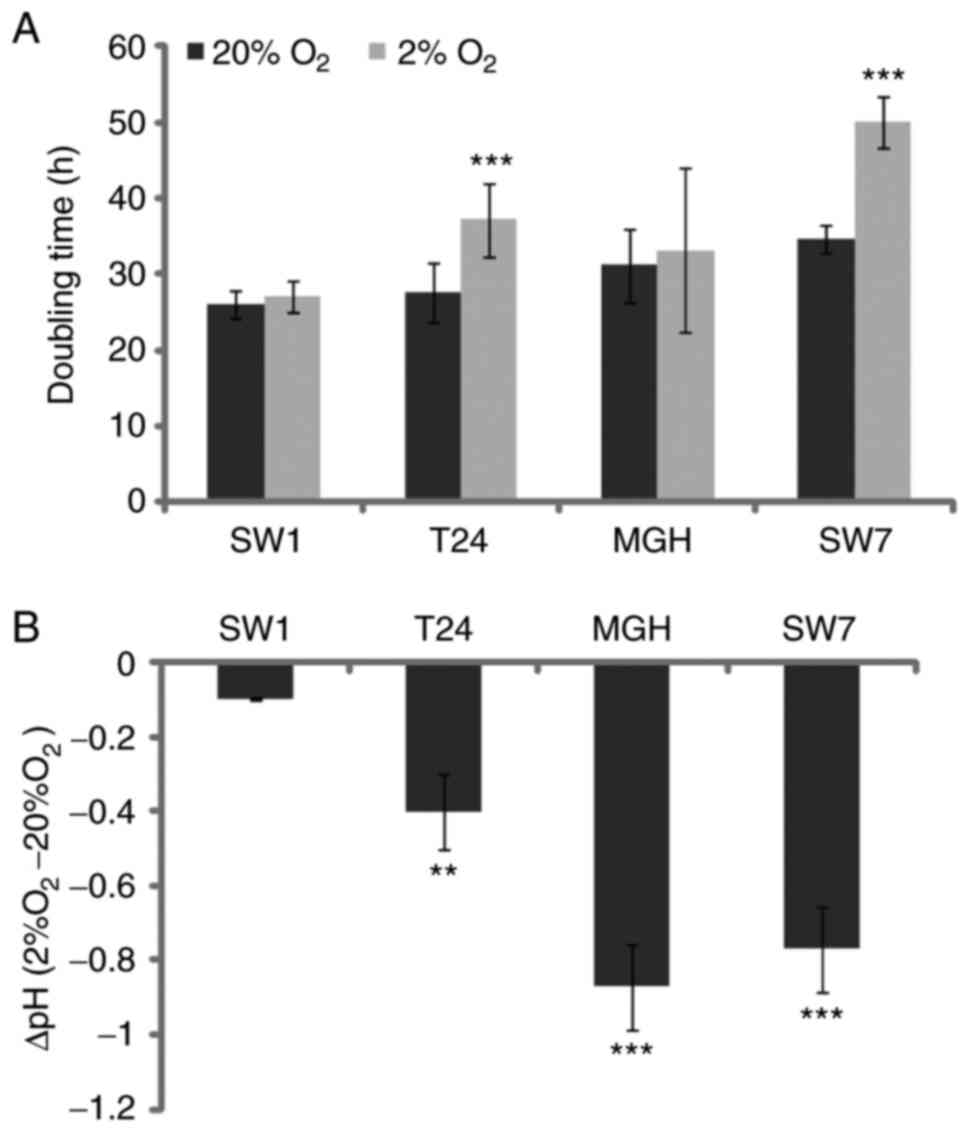
The effect of hypoxia on bladder
cancer cell growth and metabolism
In order to determine the tumor growth capacity
under hypoxic conditions, bladder cancer cell number was determined
on three consecutive days in control atmospheric conditions, 20%
O2, and hypoxic conditions, 2% O2.
Subsequently, doubling times were calculated from the obtained
growth curves (Fig. 2A). Doubling
times were significantly increased for SW-780 and T24 bladder
cancer cells in hypoxic conditions as compared with the control,
indicating that these cells proliferated less rapidly. By contrast,
doubling times remained unaffected or little affected for the two
other bladder cancer cell lines. The pH was measured in 3-day
bladder cancer cell culture supernatants (Fig. 2B). Except for SW-1710, where the pH
remained relatively unchanged, for all three of the other bladder
cancer cell line cultures, the pH was significantly decreased with
the reduction in O2 pressure.
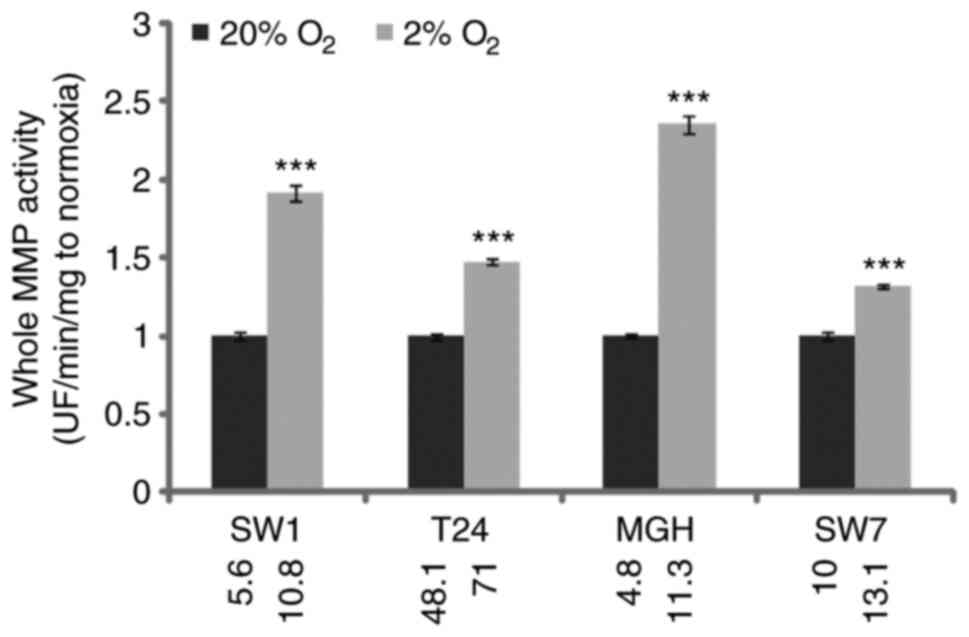
The effect of hypoxia on whole MMP
activity secreted by bladder cancer cells
Next, 3-day bladder cancer cell culture supernatants
were collected and whole MMP activity was determined by cleavage of
a fluorometric substrate (Fig. 3).
Whole MMP activity was significantly increased in hypoxic
conditions in all four cell lines, increasing the potential for the
migration of cancer cells through the ECM to the vascular/lymph
network. Nevertheless, SW-1710 and, notably, MGHU-3 demonstrated
the most significant increases, whereas T24 and SW-780 slightly
increased their potential for ECM degradation.
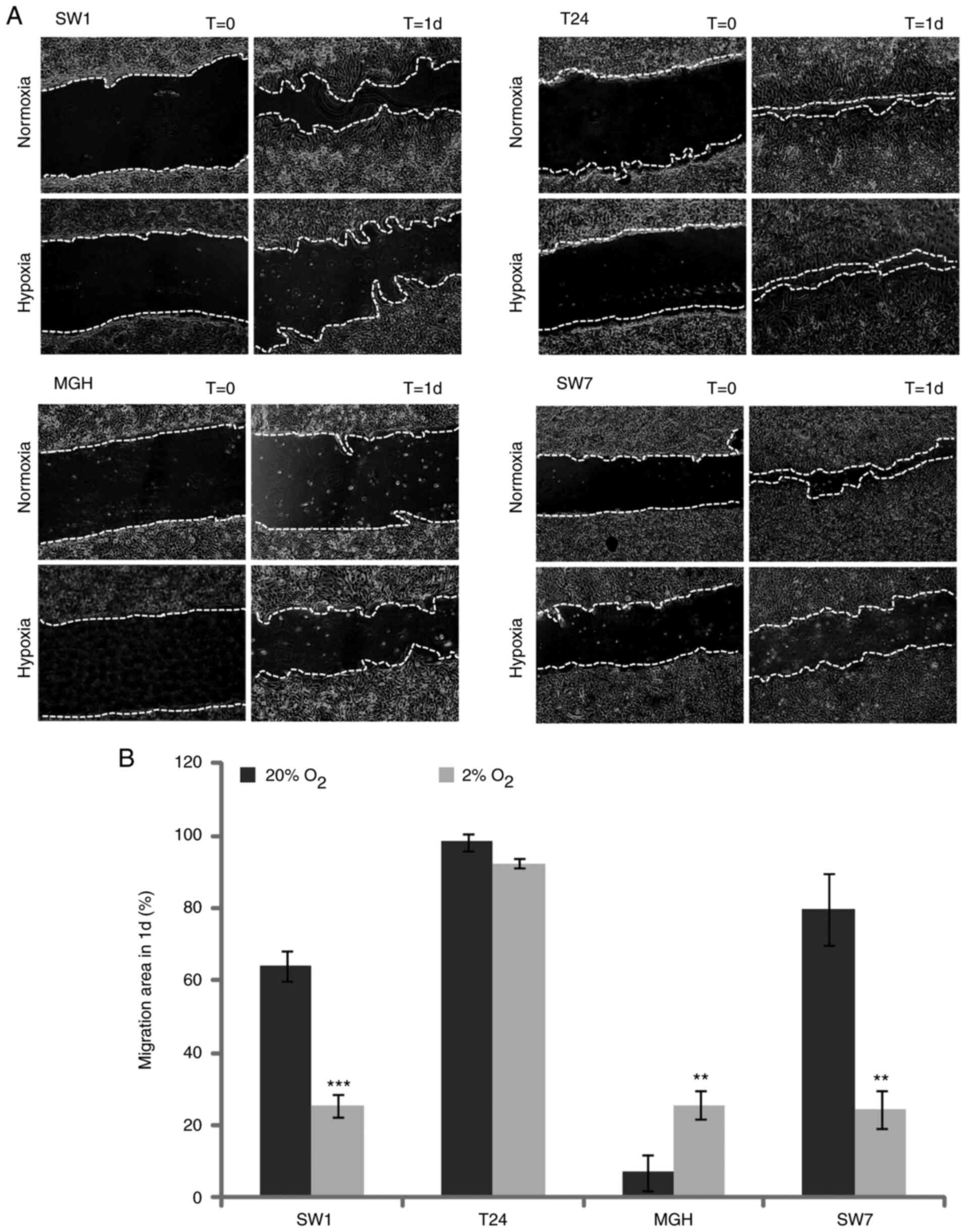
The effect of hypoxia on bladder
cancer cell migration capacities
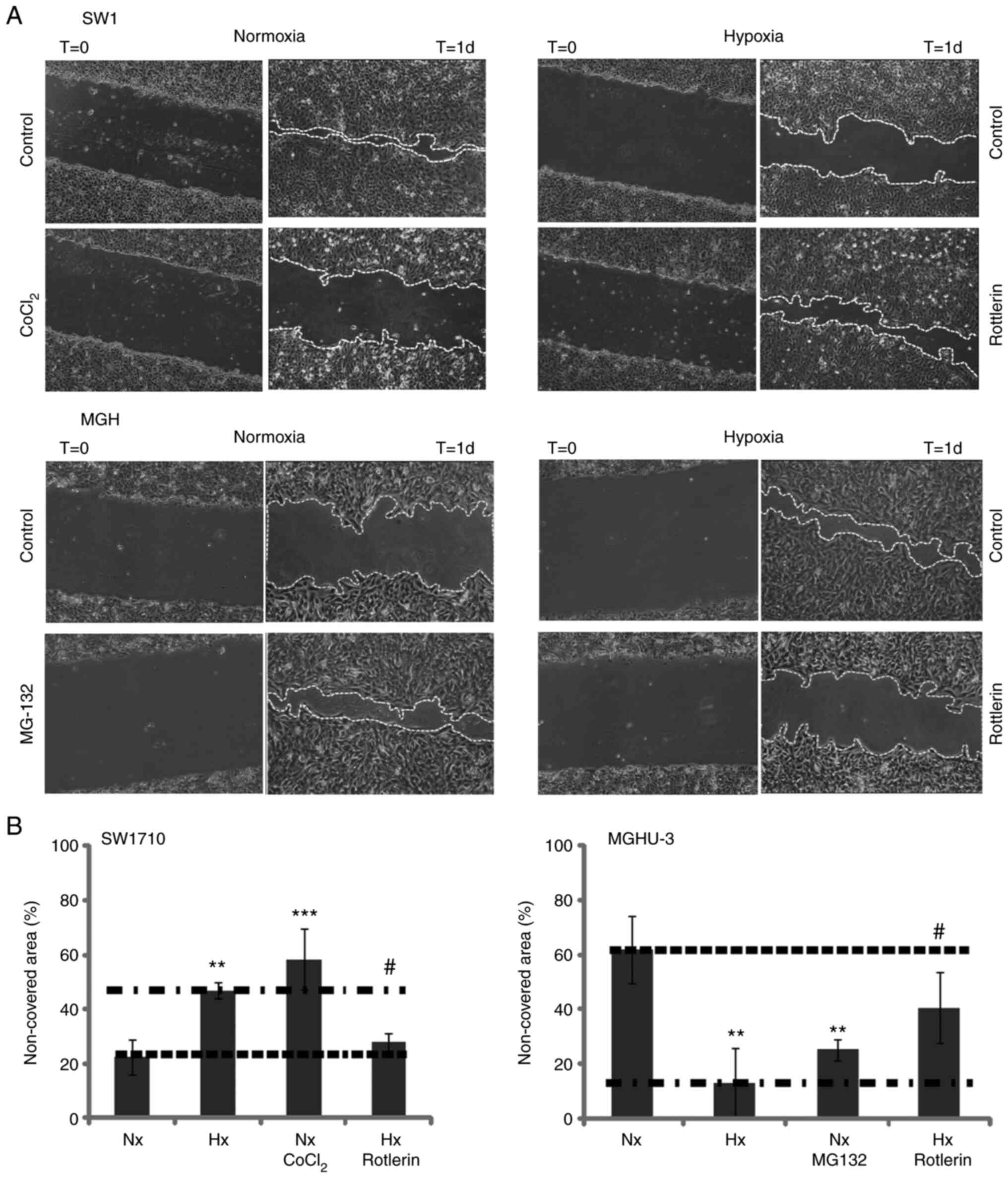
The migration ability of bladder cancer cells was
examined in normoxia (20% O2) and hypoxia (2%
O2). As presented in Fig. 4A
and B, MGHU-3 cells demonstrated a significantly increased
ability to migrate in a hypoxic environment compared with that in a
normoxia environment. By contrast, SW-1710 and SW-780 had a
significantly reduced capacity to migrate in a hypoxic environment,
while T24 migration potential revealed no significant change.
Mimicking or inhibiting hypoxia could be achieved by the use of
chemicals. For SW-1710, ANOVA indicated a P-value <0.0001. The
non-covered area at day 1 after scratching the confluent monolayer
cell culture in normoxia condition was significantly reduced
compared with the hypoxia condition (P=0.0046) and the normoxia +
CoCl2 condition (P=0.0001); however, it was not
significantly different not from the one in hypoxia + rottlerin
group (P=0.9013). The non-covered area at day 1 after scratching
the confluent monolayer cell culture in hypoxia condition was
significantly increased compared with the one in hypoxia +
rottlerin condition (P=0.0251), but not the one in normoxia +
CoCl2 condition (P=0.2684).
For MGHU-3, ANOVA indicated a P-value=0.0006. The
non-covered area at day 1 after scratching the confluent monolayer
cell culture in normoxia condition was significantly increased
compared with the hypoxia condition (P=0.0013) and the normoxia +
MG-132 condition (P=0.0099), but not the one in from hypoxia +
rottlerin condition (P=0.1598). The non-covered area at day 1 after
scratching the confluent monolayer cell culture in hypoxia
condition was significantly decreased compared with the one in
hypoxia + rottlerin condition (P=0.0536), but not from the one in
normoxia + MG-132 condition (P=0.6242). SW-1710 migrated faster in
normoxia compared with in hypoxia, activating hypoxic pathways
slowed SW-1710 migration whereas inhibiting hypoxic pathway
accelerated the migration. Inverse results were found for MGHU-3
(Fig. 5A and B).
The effect of hypoxia on the
resistance of bladder cancer cells to oxidative stress-induced
apoptosis
The resistance of cells to oxidative stress-induced
apoptosis was tested in bladder cancer cell lines in normoxic (20%
O2) or hypoxic (2% O2) cell culture
conditions (Fig. 6) by determining
their viability using a WST-1 viability assay. After ascorbate
treatment, the viability of bladder cancer cells in hypoxia was
significantly reduced compared to the one in normoxia (SW-1710 and
MGHU-3 cells for a concentration of 2 and 4 mM ascorbate, T24 cells
for a concentration of 1 and 2 mM ascorbate). By contrast, after
ascorbate treatment, the viability of the SW-780 cell line in
hypoxia was slightly but significantly increased compared to the
one in normoxic conditions. The discrepancy in the results for
SW-780 should be noted because of its potential impact on
therapies.
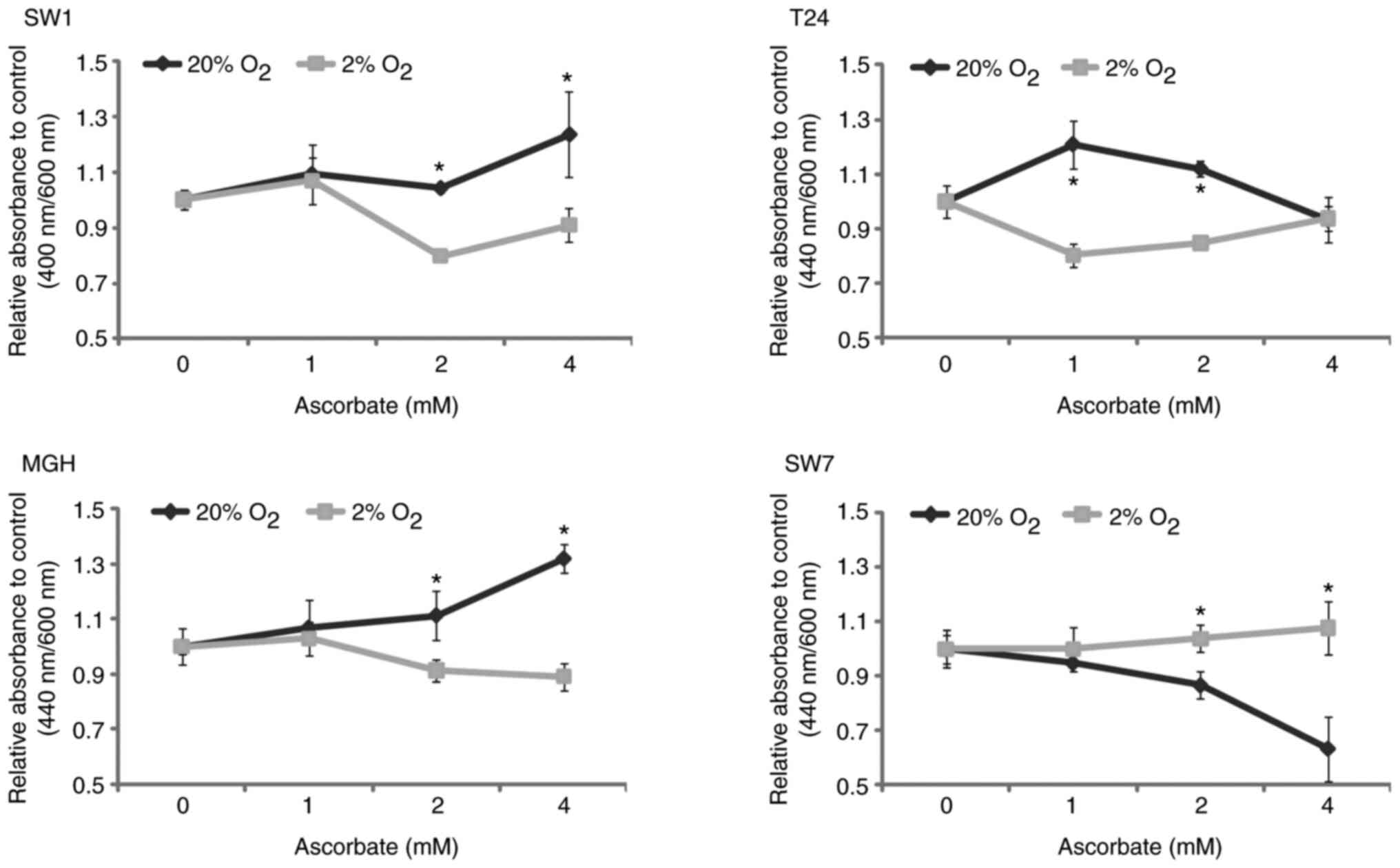 | Figure 6.Resistance to oxidative
stress-induced apoptosis of bladder cancer cells in normoxic or
hypoxic cell culture conditions. After 48 h in normoxic (20%
O2) or hypoxic (2% O2) conditions, ascorbate
was added at a concentration of 0, 1, 2 and 4 mM, and 24 h later
WST-1 was added, and optical density was measured to determine the
number of living cells. N=3, n=8. In the upper panels, the results
are normalized to controls (0 mM ascorbate). Filled lines and
filled squares (□) are for hypoxic cell culture conditions; filled
lines and close diamonds (♦) are for normoxic cell culture
conditions. *P<0.05 vs. normoxic conditions. SW1, SW-1710 cells;
MGH, MGHU-3 cells; SW7, SW-780 cells. |
Discussion
Hypoxia defines a condition where the oxygen
concentration is decreased compared with the physiological
concentration called normoxia. However, in the majority of studies,
hypoxia refers to a lower level of oxygen compared with that of
ambient air (20%). This concentration does not correspond in any
way to a physiological condition because, in the human body, the
oxygen concentrations are much lower (28,29).
What is called hypoxia in the majority of studies is actually
physiological normoxia, and what is called normoxia is hyperoxia
(28,29). A 2% oxygen concentration was selected
in the present study because it is below the concentration found in
the bladder or adjacent tissues (30). In the present study, time of exposure
of bladder cancer cells to hypoxia or normoxia was set at 72 h to
mimic a chronic exposure. Some studies use more prolonged exposure,
but for a technical reason, experiments were terminated after 72 h.
A home-made hypoxic chamber was utilized, and it required that
medium exchange be performed outside the chamber, therefore in
normoxia, and it was challenging to maintain the cells in culture
for >72 h.
It is also clear that in a tumor mass the
concentration of oxygen varies from the center of the tumor, which
could be anoxic or severely hypoxic, and the periphery of the
tumor, which could be at the same O2 concentration of
healthy tissues or even at a higher concentration in the case of an
adequate vascular perfusion (31,32). A
metabolic coupling has been demonstrated in bladder cancer between
these compartments and tumor aggressiveness (33).
EMT is a hallmark of these changes (34). Hypoxia can modify several parameters
such as tumor progression and resistance to therapy (7,35), and
these changes can be irreversible after prolonged exposure,
especially EMT (36). Mechanisms
linking hypoxia and EMT have been extensively described (37–39).
Although it is considered to be a cell adhesion molecule only, the
epithelial cell adhesion molecule EpCAM is overexpressed in a
number of carcinomas (40). Benign
epithelia express EpCAM at a variable but generally lower level
compared with carcinoma cells (41).
EpCAM can modulate cell migration, proliferation and
differentiation, and also prevent cell-cell adhesion (42,43).
Vimentin is a well-known marker of EMT (44,45). In
the present study, SW-1710, T24 and MGHU-3 cells tended to reduce
their EpCAM expression, whereas SW-780 cells tended to increase
theirs. SW-1710, MGHU-3 and SW-780 cells tended to increase their
vimentin expression, whereas T24 cells did not.
Another marker of cancer progression is the
metabolic switch. Cancer cell metabolism affects the immediate
environment, notably by acidifying the culture medium (46–48).
Hypoxia is known to change cell metabolism by up-regulating the
glycolytic enzymes (49). Measuring
the acido-basic equilibrium can, therefore, reflect changes in
metabolism (Warburg effect) (50,51). It
should be noted that the cell number in culture also influences
this parameter. Recently, a distinct metabolomic profile has been
defined for low and high-grade bladder cancer cultured cells, but
the impact of hypoxia has not yet been studied to the best of our
knowledge (52).
Metabolism changes can modify the progression
potential of tumor cells. The relationship between activation of
EMT and modification of the metabolism has been demonstrated in
muscle-invasive bladder cancer via lactate dehydrogenase A (LDH-A);
the enzyme that catalyzes the transformation of pyruvate into
lactate, an acidic component with multiple recognized signaling
function (53). More specifically,
in the case of bladder cancer, cancer progression is inhibited by
the expression of microRNA-200c, which targets LDH-A (54). In the present study, except for
SW-1710 where no modifications were demonstrated between normoxic
and hypoxic conditions, all other cell lines acidified the cell
culture medium faster in hypoxic conditions compared with in
normoxic conditions. Even if the SW-780 and T24 cell lines had
reduced growth, they still increased the acidity of the cell
culture medium, and therefore changes in cell metabolism should be
investigated (55,56).
No clear relationship can be established between the
measure of pH and the activation of the EMT in these bladder cancer
cell lines. It should be noted that the model in the present study
could be improved by the addition of co-culture with fibroblasts,
or even improved more with cancer-associated fibroblasts in order
to investigate the metabolic interplay between bladder cancer cells
and their direct microenvironment (57).
Recently, exposure to hypoxia and cell proliferation
has been associated with bladder cancer through the expression of
long non-coding RNA (58). The
growth rates of the bladder cancer cell lines in the present study
were also impacted by exposure to hypoxic conditions with a
decrease in the proliferation of T24 and SW-780 cell lines and few
changes for SW-1710 and MGHU-3 cell line cultures.
Among ECM components, an important player in
urothelial cancer is TSP-1. Even though an increased expression of
TSP-1 in tumor cells has been associated with an increase or a
decrease in microvessel density, depending on the intracellular
calcium concentration, TSP-1 expression is known to be positively
correlated with cell migration; thus, TSP-1 may play an important
role in tumor aggressiveness (59,60). In
the present study, the expression of TSP-1 was significantly
decreased under hypoxic conditions in SW-1710 high-grade bladder
cancer cell line cultures, whereas this expression was
significantly increased in SW-780 cells or remained unchanged in
low-grade bladder cancer cell line cultures.
To spread in the organism, cancer cells have to
migrate in the epithelium, then across the basal lamina and finally
through the extracellular matrix until they find blood or lymphatic
vessel (61–63). In order to grow and potentially to
escape its original location, a tumor has to degrade the
surrounding ECM (64). This action
is mainly achieved by secreting and activating MMPs. It is also
known that ECM remodeling can play a role in the reorganization of
the stroma in order to facilitate the cancer cell escape (65). In the present study, after exposure
to hypoxic conditions, all bladder cancer cell lines significantly
increased their MMP activities.
After an evaluation of some aspects of the EMT,
proliferation, metabolism and ECM synthesis/degradation balance in
bladder cancer cell line cultures exposed to hypoxia, the present
study aimed to measure their ability to migrate. In contrast to
what was noted for the expression of the EMT markers, SW-1710 and
SW-780 had reduced migration ability under hypoxic conditions,
whereas MGHU-3 increased it and T24 remained unaffected. In order
to confirm the involvement of HIF-1α, several factors were added to
cell cultures, and migration was subsequently evaluated. To mimic
the effects of hypoxia in normoxia, MG-132 and cobalt chloride were
used. On the other hand, to mimic the effects of normoxia in
hypoxia, rottlerin was used in normoxia. HIF-1α is rapidly degraded
in the proteasome after binding with the von Hippel-Lindau factor
(66,67). MG-132 inhibits the degradation of
proteins through the proteasome (68), whereas cobalt chloride stabilizes
HIF-1α by preventing its binding to the von Hippel-Lindau factor
(69). In hypoxia, HIF-α (1, 2 or 3)
forms a transcriptionally active complex with HIF-1β and p300
(70), which activates PKCδ
(71). Rottlerin inhibits PKCδ
(72). These experiments highlighted
the role of HIF-1α in response to hypoxic stress. A previous study
highlighted the link between hypoxia and tumor cell motility
through RhoA and ROCK1 (73).
A number of strategies for limiting tumor growth and
dissemination use oxidative agents such as ascorbic acid (vitamin
C) or menadione (74,75). Even if some studies highlighted that
chronic hypoxia promotes chemotherapeutic agent resistance, such as
etoposide and vincristine (76), it
was hypothesized that cancer cells, which grow in a hypoxic
microenvironment, should be more sensitive to cell death induced by
oxidative agents. Cancer cell lines are adapted to a hypoxic
environment and are thus more sensitive to oxidative agents. This
result is consistent with the literature (74). Future studies will test the
resistance to oxidative stress in the context of low nutrients, as
high glucose levels can affect the response of the cells (77).
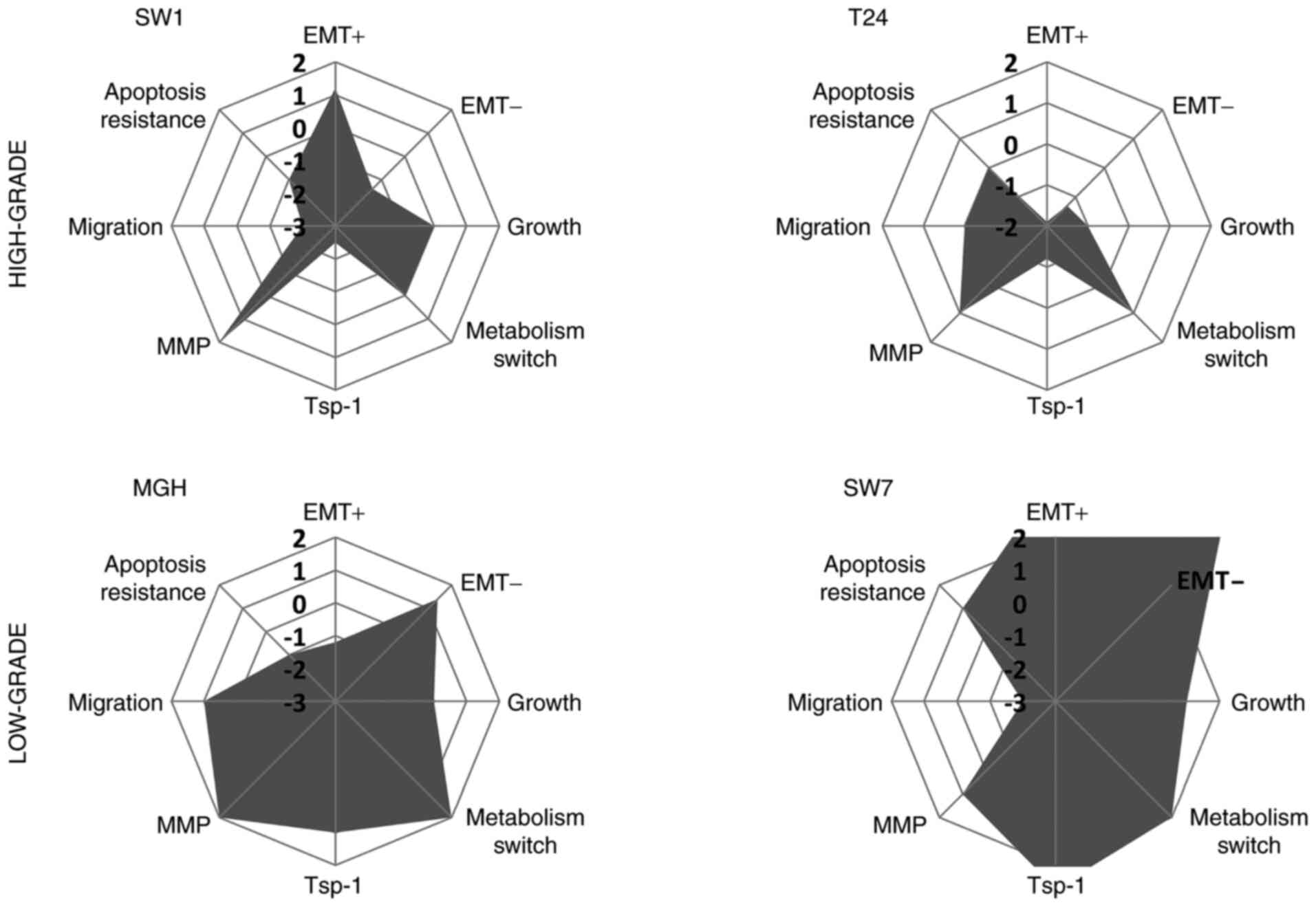
Nevertheless, even if the response of each bladder
cancer cell line seems different to an exposure to hypoxic
conditions in culture, the low-grade bladder cancer cell lines
(MGHU-3 and SW-780) seemed to become more aggressive after low
O2 pressure exposure whereas the high-grade bladder
cancer cell lines (SW-1710 and T24) became less aggressive
(Fig. 7). It should be noted that
every cell line had some increase in pro-aggressive parameters and
some in anti-aggressive elements; what the exact contribution would
be in vivo remains to be determined. A total of eight
parameters were tested, and variations were revealed in the
majority of these when cells were incubated in hypoxic conditions,
as compared with results obtained in normoxia (6, 6, 7 and 8
parameters were modified for SW-1710, T24, MGHU-3 and SW-780 cancer
cell lines respectively). Studies on bladder cancer cell lines have
to consider the microenvironment of the cells, especially hypoxia,
in order to produce data that could be used for clinical
translation. Other elements of the cellular environment also play a
critical role, especially the ECM composition and stiffness, and
the development of new models integrating three-dimensional
cell-cell and cell-matrix contacts (78) could be very helpful.
Acknowledgements
The authors would like to thank Dr Véronique
Laterreur (CRCHU de Québec, Laval University, Québec, Canada at the
time of the study), for the design and adaptation of the hypoxic
chamber from the desiccator cabinets and Dr Cindy J. Hayward (CRCHU
de Québec, Laval University, Québec, Canada) for the revision of
the text.
Funding
This study was supported by grants from the Fonds de la
Recherche en Santé du Québec (Quebec Health Research Fund), the
Canadian Institutes of Health Research (grant no. #258229) and the
CHU de Québec Foundation and the Canadian Urological Association
Scholarship Fund. This study was in part funded by the Quebec Cell,
Tissue and Gene Therapy Network, ThéCell, a thematic network
supported by the Fonds de Recherche du Québec-Santé (Quebec Health
Research Fund).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC designed the experiments, acquired, analyzed and
interpreted the data and drafted and revised the manuscript. EP,
CRG and CC acquired and analyzed the data, and revised the
manuscript. FP and SB designed the experiments and revised the
manuscript. All authors have read and approved the final
manuscript. SC and SB confirm the authenticity of raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feifer AH, Taylor JM, Tarin TV and Herr
HW: Maximizing cure for muscle-invasive bladder cancer: Integration
of surgery and chemotherapy. Eur Urol. 59:978–984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P, Kallinowski F and Okunief P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
5
|
Evans SM, Hahn SM, Magarelli DP and Koch
CJ: Hypoxic heterogeneity in human tumors: EF5 binding,
vasculature, necrosis, and proliferation. Am J Clin Oncol.
24:467–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu L and Hales CA: Long-term exposure to
hypoxia inhibits tumor progression of lung cancer in rats and mice.
BMC Cancer. 11:3312011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh MY, Lemos R Jr, Liu X and Powis G: The
hypoxia-associated factor switches cells from HIF-1α- to
HIF-2α-dependent signaling promoting stem cell characteristics,
aggressive tumor growth and invasion. Cancer Res. 71:4015–4027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Q and Yun Z: Impact of the hypoxic
tumor microenvironment on the regulation of cancer stem cell
characteristics. Cancer Biol Ther. 9:949–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peixoto A, Fernandes E, Gaiteiro C, Lima
L, Azevedo R, Soares J, Cotton S, Parreira B, Neves M, Amaro T, et
al: Hypoxia enhances the malignant nature of bladder cancer cells
and concomitantly antagonizes protein O-glycosylation extension.
Oncotarget. 7:63138–63157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue M, Chen W, Xiang A, Wang R, Chen H,
Pan J, Pang H, An H, Wang X, Hou H and Li X: Hypoxic exosomes
facilitate bladder tumor growth and development through
transferring long non-coding RNA-UCA1. Mol Cancer. 16:1432017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tátrai E, Bartal A, Gacs A, Paku S,
Kenessey I, Garay T, Hegedűs B, Molnár E, Cserepes MT, Hegedűs Z,
et al: Cell type-dependent HIF1 α-mediated effects of hypoxia on
proliferation, migration and metastatic potential of human tumor
cells. Oncotarget. 8:44498–44510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis-molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morandi A, Taddei ML, Chiarugi P and
Giannoni E: Targeting the metabolic reprogramming that controls
epithelial-to-mesenchymal transition in aggressive tumors. Front
Oncol. 7:402017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fitzgerald G, Soro-Arnaiz I and De Bock K:
The Warburg effect in endothelial cells and its potential as an
anti-angiogenic target in cancer. Front Cell Dev Biol. 6:1002018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butturini E, Carcereri de Prati A, Boriero
D and Mariotto S: Tumor dormancy and interplay with hypoxic tumor
microenvironment. Int J Mol Sci. 20:43052019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jing X, Yang F, Shao C, Wei K, Xie M, Shen
H and Shu Y: Role of hypoxia in cancer therapy by regulating the
tumor microenvironment. Mol Cancer. 18:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashrafizadeh M, Hushmandi K, Hashemi M,
Akbari ME, Kubatka P, Raei M, Koklesova L, Shahinozzaman M,
Mohammadinejad R, Najafi M, et al: Role of
microRNA/epithelial-to-mesenchymal transition axis in the
metastasis of bladder cancer. Biomolecules. 10:11592020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakanishi K, Hiroi S, Tominaga S, Aida S,
Kasamatsu H, Matsuyama S, Matsuyama T and Kawai T: Expression of
hypoxia-inducible factor-1alpha protein predicts survival in
patients with transitional cell carcinoma of the upper urinary
tract. Clin Cancer Res. 11:2583–2590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eltz S, Comperat E, Cussenot O and Rouprêt
M: Molecular and histological markers in urothelial carcinomas of
the upper urinary tract. BJU Int. 102:532–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan W, Peng K, Li M, Qin L, Tong Z, Yan J,
Shen B and Yu C: Histone demethylase JMJD1A promotes urinary
bladder cancer progression by enhancing glycolysis through
coactivation of hypoxia inducible factor 1α. Oncogene.
36:3868–3877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reiterer M, Colaço R, Emrouznejad P,
Jensen A, Rundqvist H, Johnson RS and Branco C: Acute and chronic
hypoxia differentially predispose lungs for metastases. Sci Rep.
9:102462019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nobre AR, Entenberg D, Wang Y, Condeelis J
and Aguirre-Ghiso JA: The different routes to metastasis via
hypoxia-regulated programs. Trends Cell Biol. 28:941–956. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Committee on toxicity testing and
assessment of environmental agents, N.R.C., toxicity testing in the
21st century. A Vision and a Strategy The National Academies Press;
2015
|
|
26
|
Lin CW, Lin JC and Prout GR Jr:
Establishment and characterization of four human bladder tumor cell
lines and sublines with different degrees of malignancy. Cancer
Res. 45:5070–5079. 1985.PubMed/NCBI
|
|
27
|
Chabaud S, Saba I, Baratange C, Boiroux B,
Leclerc M, Rousseau A, Bouhout S and Bolduc S: Urothelial cell
expansion and differentiation are improved by exposure to hypoxia.
J Tissue Eng Regen Med. 11:3090–3099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ivanovic Z: Hypoxia or in situ normoxia:
The stem cell paradigm. J Cell Physiol. 219:271–275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keeley TP and Mann GE: Defining
physiological normoxia for improved translation of cell physiology
to animal models and humans. Physiol Rev. 99:161–234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dyson A, Simon F, Seifritz A, Zimmerling
O, Matallo J, Calzia E, Radermacher P and Singer M: Bladder tissue
oxygen tension monitoring in pigs subjected to a range of
cardiorespiratory and pharmacological challenges. Intensive Care
Med. 38:1868–1876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McKeown SR: Defining normoxia, physoxia
and hypoxia in tumours-implications for treatment response. Br J
Radiol. 87:201306762014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abou Khouzam R, Zaarour RF, Brodaczewska
K, Azakir B, Venkatesh GH, Thiery J, Terry S and Chouaib S: The
effect of hypoxia and hypoxia-associated pathways in the regulation
of antitumor response: Friends or foes? Front Immunol.
13:8288752022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Afonso J, Santos LL, Morais A, Amaro T,
Longatto-Filho A and Baltazar F: Metabolic coupling in urothelial
bladder cancer compartments and its correlation to tumor
aggressiveness. Cell Cycle. 15:368–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoo YG, Christensen J, Gu J and Huang LE:
HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal
phenotype for malignant progression. Sci Signal.
4:pt42011.PubMed/NCBI
|
|
37
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu Y, Ning L, Feng J, Yu X, Guan F and Li
X: Dynamic regulation of O-GlcNAcylation and phosphorylation on
STAT3 under hypoxia-induced EMT. Cell Signal. 93:1102772022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv WL, Liu Q, An JH and Song XY:
Scutellarin inhibits hypoxia-induced epithelial-mesenchymal
transition in bladder cancer cells. J Cell Physiol.
234:23169–23175. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Imrich S, Hachmeister M and Gires O: EpCAM
and its potential role in tumor-initiating cells. Cell Adh Migr.
6:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: More than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown TC, Sankpal NV and Gillanders WE:
Functional implications of the dynamic regulation of EpCAM during
epithelial-to-mesenchymal transition. Biomolecules. 11:9562021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fagotto F: EpCAM as modulator of tissue
plasticity. Cells. 9:21282020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Usman S, Waseem NH, Nguyen TKN, Mohsin S,
Jamal A, Teh MT and Waseem A: Vimentin is at the heart of
epithelial mesenchymal transition (EMT) mediated metastasis.
Cancers (Basel). 13:49852021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yun SJ and Kim WJ: Role of the
epithelial-mesenchymal transition in bladder cancer: From prognosis
to therapeutic target. Korean J Urol. 54:645–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
DeBerardinis RJ: Is cancer a disease of
abnormal cellular metabolism? New angles on an old idea. Genet Med.
10:767–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bose S and Le A: Glucose metabolism in
cancer. Adv Exp Med Biol. 1063:3–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pérez-Tomás R and Pérez-Guillén I: Lactate
in the tumor microenvironment: An essential molecule in cancer
progression and treatment. Cancers (Basel). 12:32442020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu L and Simon MC: Regulation of
transcription and translation by hypoxia. Cancer Biol Ther.
3:492–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Potter M, Newport E and Morten KJ: The
Warburg effect: 80 Years on. Biochem Soc Trans. 44:1499–1505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng T, Jäättelä M and Liu B: pH gradient
reversal fuels cancer progression. Int J Biochem Cell Biol.
125:1057962020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rodrigues D, Pinto J, Araújo AM, Jerónimo
C, Henrique R, Bastos ML, Guedes de Pinho P and Carvalho M: GC-MS
metabolomics reveals distinct profiles of low- and high-grade
bladder cancer cultured cells. Metabolites. 9:182019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang F, Ma S, Xue Y, Hou J and Zhang Y:
LDH-A promotes malignant progression via activation of
epithelial-to-mesenchymal transition and conferring stemness in
muscle-invasive bladder cancer. Biochem Biophys Res Commun.
469:985–992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan D, Zheng S, Wang L, Li J, Yang J,
Wang B, Chen X and Zhang X: MiR-200c inhibits bladder cancer
progression by targeting lactate dehydrogenase A. Oncotarget.
8:67663–67669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goto M, Miwa H, Suganuma K, Tsunekawa-Imai
N, Shikami M, Mizutani M, Mizuno S, Hanamura I and Nitta M:
Adaptation of leukemia cells to hypoxic condition through switching
the energy metabolism or avoiding the oxidative stress. BMC Cancer.
14:762014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lamonte G, Tang X, Chen JL, Wu J, Ding CK,
Keenan MM, Sangokoya C, Kung HN, Ilkayeva O, Boros LG, et al:
Acidosis induces reprogramming of cellular metabolism to mitigate
oxidative stress. Cancer Metab. 1:232013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wanandi SI, Ningsih SS, Asikin H, Hosea R
and Neolaka GMG: Metabolic interplay between tumour cells and
cancer-associated fibroblasts (CAFs) under hypoxia versus normoxia.
Malays J Med Sci. 25:7–16. 2018.PubMed/NCBI
|
|
58
|
Xue M, Li X, Li Z and Chen W: Urothelial
carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted
long noncoding RNA that enhances hypoxic bladder cancer cell
proliferation, migration, and invasion. Tumour Biol. 35:6901–6912.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ioachim E, Michael MC, Salmas M, Damala K,
Tsanou E, Michael MM, Malamou-Mitsi V and Stavropoulos NE:
Thrombospondin-1 expression in urothelial carcinoma: Prognostic
significance and association with p53 alterations, tumour
angiogenesis and extracellular matrix components. BMC Cancer.
6:1402006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Firlej V, Mathieu JR, Gilbert C, Lemonnier
L, Nakhlé J, Gallou-Kabani C, Guarmit B, Morin A, Prevarskaya N,
Delongchamps NB and Cabon F: Thrombospondin-1 triggers cell
migration and development of advanced prostate tumors. Cancer Res.
71:7649–7658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeeshan R and Mutahir Z: Cancer
metastasis-tricks of the trade. Bosn J Basic Med Sci. 17:172–182.
2017.PubMed/NCBI
|
|
62
|
Majidpoor J and Mortezaee K: Steps in
metastasis: An updated review. Med Oncol. 38:32021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kanayama H: Matrix metalloproteinases and
bladder cancer. J Med Invest. 48:31–43. 2001.PubMed/NCBI
|
|
65
|
Alfano M, Nebuloni M, Allevi R, Zerbi P,
Longhi E, Lucianò R, Locatelli I, Pecoraro A, Indrieri M, Speziali
C, et al: Linearized texture of three-dimensional extracellular
matrix is mandatory for bladder cancer cell invasion. Sci Rep.
6:361282016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Semenza GL: Hypoxia-inducible factor 1
(HIF-1) pathway. Sci STKE. 2007:cm82007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee DH and Goldberg AL: Selective
inhibitors of the proteasome-dependent and vacuolar pathways of
protein degradation in Saccharomyces cerevisiae. J Biol Chem.
271:27280–24284. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Piret JP, Mottet D, Raes M and Michiels C:
CoCl2, a chemical inducer of hypoxia-inducible factor-1,
and hypoxia reduce apoptotic cell death in hepatoma cell line
HepG2. Ann N Y Acad Sci. 973:443–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Razorenova OV, Finger EC, Colavitti R,
Chernikova SB, Boiko AD, Chan CK, Krieg A, Bedogni B, LaGory E,
Weissman IL, et al: VHL loss in renal cell carcinoma leads to
up-regulation of CUB domain-containing protein 1 to stimulate
PKC{delta}-driven migration. Proc Natl Acad Sci USA. 108:1931–1936.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xia J, Ozaki I, Matsuhashi S, Kuwashiro T,
Takahashi H, Anzai K and Mizuta T: Mechanisms of PKC-mediated
enhancement of HIF-1α activity and its inhibition by vitamin K2 in
hepatocellular carcinoma cells. Int J Mol Sci. 20:10222019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Leong HS and Chambers AF: Hypoxia promotes
tumor cell motility via RhoA and ROCK1 signaling pathways. Proc
Natl Acad Sci USA. 111:887–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gilloteaux J, Jamison JM, Neal DR, Loukas
M, Doberzstyn T and Summers JL: Cell damage and death by
autoschizis in human bladder (RT4) carcinoma cells resulting from
treatment with ascorbate and menadione. Ultrastruct Pathol.
34:140–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hussein D, Estlin EJ, Dive C and Makin GW:
Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent
resistance to etoposide and vincristine in neuroblastoma cells. Mol
Cancer Ther. 5:2241–2250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Poulsen RC, Knowles HJ, Carr AJ and Hulley
PA: Cell differentiation versus cell death: Extracellular glucose
is a key determinant of cell fate following oxidative stress
exposure. Cell Death Dis. 5:e10742014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ringuette Goulet C, Bernard G, Chabaud S,
Couture A, Langlois A, Neveu B, Pouliot F and Bolduc S:
Tissue-engineered human 3D model of bladder cancer for invasion
study and drug discovery. Biomaterials. 145:233–241. 2017.
View Article : Google Scholar : PubMed/NCBI
|