Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive neoplasms, ranking fourth among the causes of
cancer-related deaths in Western countries and Japan (1,2).
Currently, multidisciplinary treatments such as surgery,
chemotherapy, and radiotherapy are used to treat pancreatic cancer,
but the survival outcome has not been significantly improved. In
addition, only a limited number of patients with PDAC may benefit
from new treatment modalities, including immune checkpoint
inhibitors and precision medicine based on genome-wide molecular
alterations. Therefore, it is necessary to seek novel therapeutic
strategies based on improved understanding of the biological and
molecular mechanisms underlying the aggressive progression of
PDAC.
Recently, the focus of cancer research has shifted
to the microenvironment surrounding cancer cells. PDAC typically
consists of a dense stroma comprising various stromal cells and
rich extracellular matrices (ECMs) (3). Hyaluronan (HA), a major component of
the ECM, accumulates to high levels in the microenvironment
surrounding various cancers, including PDAC, and serves an
important role in a variety of cellular processes, including cell
invasion, migration, and proliferation (4–10).
In addition, low-molecular-weight HA (LMW-HA) has been reported to
be more critical for cancer progression in terms of invasion and
metastasis compared to high-molecular weight HA (HMW-HA) (11–14).
In a previous study by the authors it was shown that the
accumulation of LMW-HA is correlated with the motility of PDAC
cells (4). HA, a large linear
glycosaminoglycan weighing up to approximately 107 Da in its naïve
form, is produced by hyaluronan synthase enzymes (HASs) and
degraded into smaller fragments by hyaluronidases (HYALs). In
another previous study, the authors reported that strong expression
of HAS2 (one of the HAS proteins) in PDAC was associated with poor
survival after surgery (15).
Distinct from HA synthesis, HA degradation is implicated in cancer
prognosis. Specifically, the cleavage of large HAs (by HYALs or
other enzymes) to yield smaller HA fragments is also accelerated in
malignant tumors (4,8,11,14).
Notably, in a previous study by the authors, HYAL1 [also referred
to as KIAA1199 and as cell migration-inducing protein (CEMIP)] was
shown to be overexpressed in PDAC (16).
In the present study, focus was on
hyaluronan-binding protein 1 (HABP1), one of the multiple known
hyaluronan-binding proteins. HABP1 originally was designated
globular head receptor for complement component 1q (gC1qR), based
on its characterization as a protein that inhibits C1 activation.
Aberrant expression and/or function of HABP1 has been reported in
neurodegenerative diseases, impaired glucose tolerance, and cancer
(17–26). Notably, HABP1 has been demonstrated
to play an important role in cancer initiation and progression
(27,28). However, there have been (to the
best of our knowledge) few studies on the expression and role of
HABP1 in PDAC (25). In the
present study, the expression, clinicopathological significance,
and biological function of HABP1 in pancreatic cancer were
investigated.
Materials and methods
Patient demographics
This retrospective study included samples from 105
consecutive patients (61 men and 44 women) with PDAC who were
admitted to the Department of Surgery I, School of Medicine,
University of Occupational and Environmental Health (Kitakyushu,
Japan) between 1994 and 2014. The inclusion criteria included
patients i) aged 33–90 years, ii) without other organ metastasis by
preoperative examination, iii) diagnosed as having resectable
tumors, and iv) definitively diagnosed with PDAC by postoperative
pathology. Exclusion criteria included cases with i) preoperative
chemotherapy or radiation therapy, ii) distant metastasis or a
second cancer, iii) multiple organ failure, iv) history of drug
abuse or v) patients who were pregnant. Within one week before
pancreatic surgery, all patients underwent a baseline assessment of
white blood cell count and of serum levels of alanine
aminotransferase, total bilirubin, albumin carcinoembryonic antigen
(CEA), and carbohydrate antigen 19–9 (CA19-9). Additional intra-
and peri-operative data including tumor diameter, surgery time,
blood loss, tumor stage, lymph node (LN) metastasis, and arterial
involvement were collected. PDAC tissues had been fixed, processed,
sectioned at the time of operation. Patients were staged according
to Union for International Cancer Control (UICC) criteria
(8th edition) (29).
Immunohistochemistry (IHC)
The present study used archival tissues obtained
from 105 consecutive patients who underwent surgery at the
Department of Surgery I, School of Medicine, University of
Occupational and Environmental Health (Kitakyushu, Japan). Tissues
were fixed in 10% formalin at room temperature for 24 h and cut to
a 2-µm thickness. After formalin fixation, the tissue was
paraffin-embedded. Written informed consent was obtained from each
patient prior to use of their specimens. The present study was
approved by the Ethics Committee of the School of Medicine,
University of Occupational and Environmental Health (approval no.
H26-118).
Paraffin-embedded sections were dewaxed with xylene
and gradually hydrated. Endogenous peroxidase activity had been
blocked at room temperature by immersing the sections in 0.3%
hydrogen peroxide in methanol for 30 min after antigen retrieval
was performed by autoclaving in 10 mM citrate buffer (pH 6.0) for
10 min. Each section was additionally blocked using 10% normal
rabbit serum (cat. np.424033; Nichirei Biosciences, Inc.). Each
section was incubated in a 1:100 dilution of anti-HABP1 antibody
(monoclonal anti-C1QBP antibody produced in mouse; catalog no.
WH0000708M1; Sigma-Aldrich; Merck KGaA) for 1 day at 4°C, prior to
being treated with secondary antibody and biotin-streptavidin
complex (424033; Nichirei Corporation) for 60 min each at room
temperature. The resultant immunoreactions were visualized with
diaminobenzidine (Dako; Agilent Technologies, Inc.) and the
sections were counterstained with hematoxylin (Wako Pure
Chemical).
As a negative control, immunostaining was performed
~5 times using normal pancreatic tissue 2–5 cm away from the
cancer. In addition, since the positive control of the anti-HABP1
antibody used was the duodenum, a second negative control was
created by immunostaining the duodenum, using a diluted solution
[PBS (pH 7.4) containing 0.1% BSA] excluding the primary antibody.
The total score for the IHC reaction was quantified based on a
staining intensity grade in combination with a score representing
the percentage of positive tumor cells. The first value, the
staining intensity grade, was determined as follows: 0 (no
staining), 1 (weak staining), 2 (moderate staining), and 3 (strong
staining). The second value was determined based on the percentage
(0 to 100%) extent of reactivity, which was scored as follows: 0
(no positive tumor cells), 1 (≤10%), 2 (11–49%) and 3 (≥50%)
(30). Each case was scored
independently by two investigators in a blinded manner. The total
score for each section was calculated as the product of the
staining grade (value of 0–3) and extent of reactivity (0–3),
meaning that the total score ranged from 0–9. Total scores ≤4 were
regarded as negative for expression, and the remainder were
classified as positive for expression. For example, if the staining
intensity was strong (3 points) and the staining area was <10%
(1 point), the final score was 3, and the sample was classified as
negative for expression.
Cell culture and reagents
PDAC cell lines, PANC-1 (CRL-1469; American Type
Culture Collection), and NOR-P1 (TKG 0630; Cell Resource Center for
Biomedical Research, Institute of Development, Aging and Cancer,
Tohoku University) were used, both of which in our laboratory
collection were shown (in the present study) to exhibit strong
HABP1 expression. As other PDAC cell lines, the strains ASPC-1,
Bx-PC3, Capan-1, CFPAC-1 (ASPC-1; CRL-1682, BxPC-3; CRL-1687,
Capan-1; HTB-79, and CFPAC-1; CRL-1918; American Type Culture
Collection), KP-3 (JCRB0178.0; JRCB Cell Bank), MiaPaca-2
(CRL-1420; American Type Culture Collection), SUIT-2 (JCRB01094;
JRCB Cell Bank) and SW-1990 (CRL-2172; American Type Culture
Collection) were used due to their weak expression of HABP1. NOR-P1
is a pancreatic ductal adenocarcinoma cell line established by Sato
et al (31). NOR-P1 was
also used in a previous study (4).
Separately, an immortalized cell line derived from human pancreatic
duct epithelial (HPDE) cells, was also employed; this cell line was
a kind gift from Dr Tsao (University of Toronto, Toronto, Canada).
The PDAC cell lines were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin
and penicillin (all from Life Technologies; Thermo Fisher
Scientific, Inc.). HPDE was maintained in HuMedia-KG2 (Kurabo
Industries, Ltd.). All cell lines were grown at 37°C in a 5%
CO2 incubator.
siRNA knockdown of HABP1
The small interfering RNA (siRNA) used to target
HABP1 (ON-TARGETplus SMARTPool Human HABP1; cat. no.
L-011225-01-0005) and the negative control siRNA (ON-TARGETplus
Control siRNA non-Targeting siRNA #1; cat. no. D-001810-01-05) were
purchased from Horizon; PerkinElmer Inc. HABP1 used a mixture of
four target sequences. The target sequences were as follows (HABP1:
5′-GCGAAAUUAGUGCGGAAAG-3′, 5′-CGCAAGGGCAGAAGGUUGA-3′,
5′-UUUCGUGGUUGAAGUUAUA-3′ and 5′-GAAGUUAGCUUUCAGUCCA-3′. The
non-targeting siRNA (negative control) was
5′-UGGUUUACAUGUCGACUAA-3′. NOR-P1 and PANC-1 were transfected with
100 nM siRNA using DharmaFECT 1 Transfection Reagent (Horizon;
PerkinElmer, Inc.) according to the manufacturer's instructions at
37°C for 48 h. After 48 h of treatment, the cells were used for
further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured cells using the
RNeasy Mini Kit (Qiagen GmbH) according to the manufacturer's
protocol. First-strand cDNA was synthesized from 1.0 µg of total
RNA using the SuperScript® VILO cDNA synthesis Kit and
Master Mix (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Quantitative mRNA expression analysis
of HABP1 and a control housekeeping gene (GAPDH,
encoding glyceraldehyde phosphate dehydrogenase) was performed
using TaqMan® Gene Expression Assays and the
StepOnePlus™ Real-Time PCR System (both from Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
amplification program consisted of 10 min of activation at 95°C,
and 40 cycles of melting at 95°C for 15 sec followed by
annealing/elongation at 60°C for 2 min. The assay IDs for these
genes were as follows: Hs00241825_m1 (HABP1) and
Hs02758991_g1 (GAPDH). The following oligonucleotides were
used for analyses: HABP1 forward, 5′-CTGCACACCGACGGAGACAA-3′ and
reverse, 5′-CATATAAGGCCCAGTCCAAG-3′; GAPDH forward,
5′-CTCCTCCACCTTTGACGCTG-3′ and reverse,
5′-AGGGGAGATTCAGTGTGGTG-3′.
The relative quantification was determined based on
the Cq values, obtained from the reactions for target genes and an
internal control gene in each sample (32).
Cell proliferation assay
PDAC cells (1.0×104/dish) treated with
the siRNA targeting HABP1 or with the negative control siRNA
were incubated for 1, 3 and 5 days at 37°C; cell counts then were
determined using 0.5% trypan blue staining at room temperature for
1 min. The cell number was measured using a LUNA™ automatic cell
counter (Logos Biosystems).
Colony formation assay
Following treatment with siRNA, PDAC cells were
harvested and counted. Consistent numbers of cells (100 cells/dish)
from each group were seeded in dishes. Cells were grown for 14 days
and colonies were fixed at room temperature for 30 min, with the
addition of 1 ml/well 4% neutral formalin solution and stained with
1% aqueous solution at room temperature for 5 min. The number of
colonies on each dish was then counted under a light microscope. A
colony was defined was as a group of >50 cells that was ≥3 mm in
size when stained with crystal violet.
Migration assay
The migratory activity of cells was determined by a
Transwell cell migration assay using cell culture inserts equipped
with a filter membrane containing 8-µm pores (BD Biosciences). The
lower chamber was filled with RPMI-1640 medium containing 10% FBS.
The upper chamber was filled with 2.0×104 cells (for
PANC-1) or 5.0×104 cells (for NOR-P1) in RPMI-1640
medium (without FBS). After 24 h of incubation at 37°C, the cells
remaining on the upper side of the filters were removed. The cells
on the bottom surface of the membrane were stained with hematoxylin
and eosin at room temperature for 15 min. and the number of cells
that had migrated to the bottom surface of the membrane were
counted in five randomly selected fields from each sample using a
light microscope (×200 magnification).
Western blot analysis
The cells were harvested and total protein was
extracted with PRO-PREP protein extraction solution (iNtRON
Biotechnology, Inc.). Total protein was quantified using Pierce™
Microplate BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Each lane was mounted with 10 µl of solution adjusted to a total
protein of 1 µg. Equal amounts of protein per lane were subjected
to electrophoresis on a 12% Mini-PROTEAN Precast Gel (Bio-Rad
Laboratories, Inc.) and transferred to a polyvinylidene fluoride
(PVDF) membrane (ATTO Corporation). Membranes were blocked for 1 h
with 3% bovine serum albumin (BSA; Sigma-Aldrich, Merck KGaA) in
TBST buffer (Tris-buffered saline, pH 7.4, containing 0.1%
Tween-20) at room temperature. Blocked membranes were then
incubated overnight at 4°C with anti-HABP1 antibody at a dilution
of 1:200 (mouse monoclonal anti-C1QBP antibody; WH0000708M1;
Sigma-Aldrich; Merck KGaA) and anti-β-actin at a dilution of
1:5,000 (mouse monoclonal anti-β-actin; 66009-1-Ig; ProteinTech
Group, Inc.), followed by incubation for 1 h at room temperature
with appropriate HRP-conjugated anti-mouse IgG secondary antibodies
(cat. no. SA00001-1; ProteinTech Group, Inc.) at a dilution of
1:4,000. The proteins were visualized using an ECL Western Blotting
Detection System (GE Healthcare).
Statistical analysis
Statistical analyses were performed using SPSS
statistical software (version 25.0; IBM Corp.). Two-tailed
chi-squared tests, Student's t-tests, and Mann-Whitney U tests were
used for group comparisons. In the present study, Student's t-test
was unpaired. Kaplan-Meier survival curves and log-rank tests were
used for survival analysis. Prognostic factors were evaluated by
univariate and multivariate analyses using Cox proportional hazard
regression models. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical analysis and
prognostic relevance of HABP1 levels in PDAC
In the present study, pancreatic cancer tissue from
105 patients, of which 44 were women, was assessed. The median age
was 69 years (range, 33–90 years). PDAC was localized in the
pancreas head in 64 cases and in the pancreas body or tail in 41
cases (Table I). All target
patients underwent R0 resection surgeries.
 | Table I.Comparison of clinicopathological
variables between patients with low HABP1 expression and those with
high HABP1 expression. |
Table I.
Comparison of clinicopathological
variables between patients with low HABP1 expression and those with
high HABP1 expression.
|
| Expression of
HABP1 |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low (n=56) | High (n=49) | P-value |
|---|
| Sex |
|
| 0.559 |
|
Male | 31 | 30 |
|
|
Female | 25 | 19 |
|
| Age, years |
|
| 0.104 |
|
≤65 | 16 | 22 |
|
|
>65 | 40 | 27 |
|
| Location |
|
| 0.559 |
|
Head | 34 | 30 |
|
| Body
and tail | 22 | 19 |
|
| Tumor marker |
|
|
|
|
CEA | 2.6 (1.0-13.6) | 2.8 (1.0-3.7) | 0.565 |
|
CA19-9 | 63.8
(0.6-4610) | 149.1
(3.0-3450) | 0.402 |
| UICC T |
|
| 0.191 |
| 1 | 3 | 3 |
|
| 2 | 7 | 1 |
|
| 3 | 34 | 30 |
|
| 4 | 12 | 15 |
|
| Tumor size
(cm) | 2.6 (0.6-7) | 3.2 (0.6-8) | 0.009a |
| UICC N |
|
| 0.566 |
| 0 | 24 | 16 |
|
| 1 | 25 | 26 |
|
| 2 | 7 | 7 |
|
| UICC M |
|
| 0.533 |
| 0 | 55 | 49 |
|
| 1 | 1 | 0 |
|
| UICC stage |
|
| 0.201 |
| I
(A+B) | 7 | 2 |
|
| II
(A+B) | 32 | 26 |
|
|
III | 16 | 21 |
|
| IV | 1 | 0 |
|
| Vascular
invasion |
|
| 0.497 |
|
Negative | 15 | 10 |
|
|
Positive | 41 | 39 |
|
| Perineural
invasion |
|
| 0.331 |
|
Negative | 31 | 22 |
|
|
Positive | 25 | 27 |
|
| Histological
grade |
|
| 0.509 |
|
High | 4 | 6 |
|
|
Low | 52 | 43 |
|
| Adjuvant
chemotherapy |
|
| 0.672 |
| + | 40 | 32 |
|
| - | 16 | 16 |
|
Immunohistochemical analysis was used to determine
the expression pattern of HABP1 protein in the PDAC tissue samples.
HABP1 expression was negative or only slightly positive (IHC scores
0–4) in normal pancreata, including ductal cells, acinar cells, and
islet cells, whereas HABP1 was highly expressed in some tumor
cells. Staining was detected in the membrane and/or cytoplasm of
the tumor cells (Fig. 1).
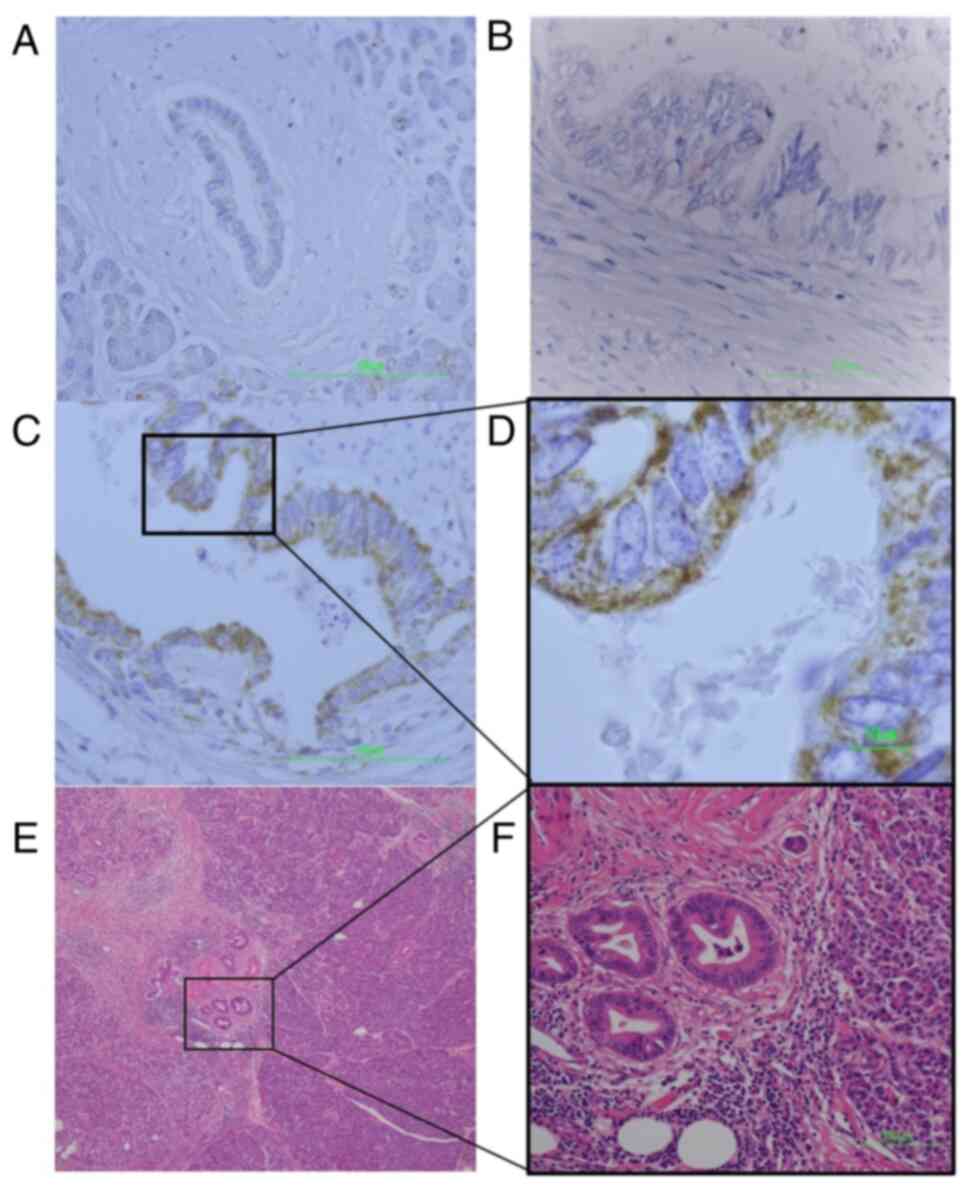 | Figure 1.IHC of PDAC tissue observed by light
microscope. (A) A normal pancreas, including ductal cells
(magnification, ×400; scale bar, 100 µm). Normal pancreas staining
was performed 5 times. (B) PDAC tissue exhibiting weak HABP1
staining (IHC score 1) (magnification, ×400; scale bar, 100 µm).
(C) PDAC tissue exhibiting strong HABP1 staining (IHC score 9)
(magnification, ×400; scale bar, 100 µm). (D) Magnified image of C
(magnification, ×1,000; scale bar, 10 µm). HABP1 staining is
observed in the membrane and/or cytoplasm of the tumor cells. (E)
H&E-stained PDAC tissue. The PDAC portion of the specimen is
located in the center of the micrograph, adjacent to normal
pancreatic tissue (inset image). (F) H&E-stained PDAC tissue
(magnification, ×200; scale bar, 100 µm). The PDAC cells forming
the ductal structure exhibit a large nucleus-cytoplasm ratio and
uneven distribution of nuclei. IHC, immunohistochemistry; PDAC,
pancreatic ductal adenocarcinoma; HABP1, hyaluronan-binding protein
1; H&E, hematoxylin and eosin. |
With regard to the 105 PDAC cases, 49 (46.7%)
exhibited high HABP1 expression, whereas the remaining 56 (53.3%)
exhibited low expression, according to our staining quantification
criteria. Clinicopathological data were compared between the
high-HABP1 expression group and low-HABP1 expression group
(Table I). Analysis using
Student's t-tests revealed that tumor size (tumor diameter) was
significantly larger in the high-HABP1 expression group than in the
low-HABP1 expression group [mean and range, 3.2 (0.6-8) vs. 2.7
(0.6-7) cm; P=0.00883]. There was no significant difference between
the groups in other clinicopathological variables, including age,
sex, tumor location, levels of tumor markers, UICC stage, as well
as other pathological factors, as determined using analysis of
two-tailed chi-squared tests, Student's t-tests, and Mann-Whitney U
tests.
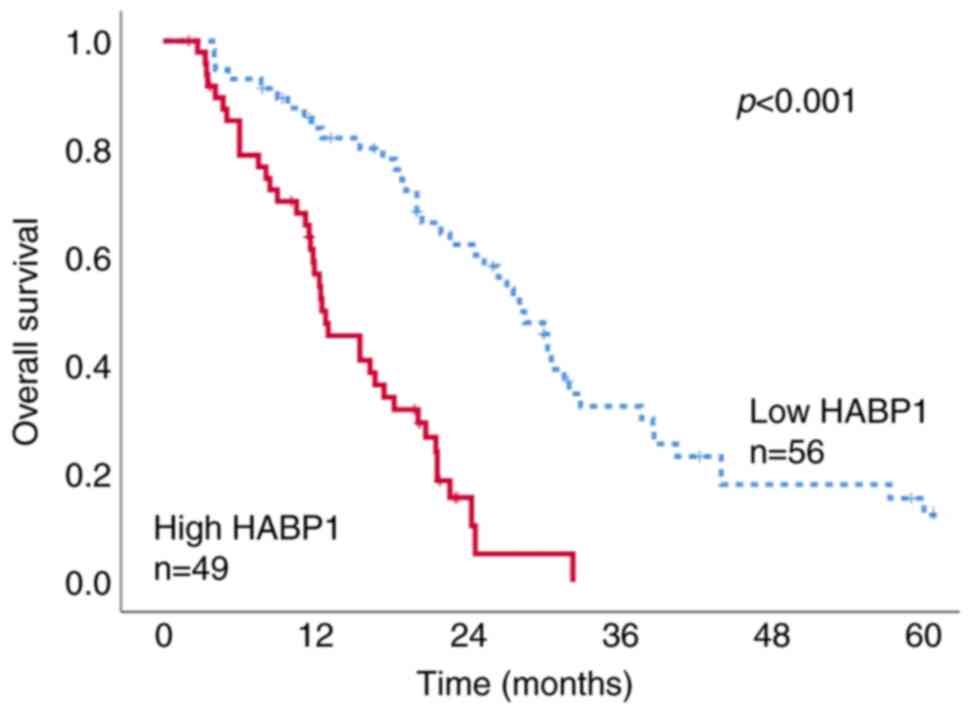
In the present study, the observation period was set
to 5 years after surgery. The median survival time was 18.8 months
(range, 3 to ≥60 months). The survival between the high- and
low-HABP1 expression groups was then compared. The overall survival
was significantly shorter in patients with high HABP1 expression
(median survival time, 12.8 months) than in patients with low HABP1
expression (median survival time, 28.5 months) (log-rank test,
P<0.001) (Kaplan-Meier survival curve; Fig. 2). In the present study, numerous
patients succumbed to cancer metastasis in the HABP1-high
expression group. In the high expression group, 35 out of 49 cases
(71%) were reported as pancreatic cancer-associated deaths. In
contrast, in the HABP1-low expression group, pancreatic
cancer-related deaths were reported in 28 out of 56 (56%) cases
(data not shown).
Prognostic factors were examined using Cox
proportional hazard regression models. Multivariate analysis
revealed high HABP1 expression (P<0.001), preoperative high
CA19-9 levels (P=0.031), histological grade (high/low) (P=0.046),
LN metastasis (P=0.015), and tumor stage (P=0.013) to be
significantly associated with poor prognosis (Table II).
 | Table II.Univariate and multivariate analysis
for factors predicting poor prognosis in patients with PDAC. |
Table II.
Univariate and multivariate analysis
for factors predicting poor prognosis in patients with PDAC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| HABP1
(high/low) | 1.995 | 1.537-2.591 |
<0.001a | 0.106 | 0.048-0.237 |
<0.001a |
| Age | 0.650 | 0.411-1.029 | 0.066 | 1.006 | 0.980-1.034 | 0.637 |
| Sex
(male/female) | 1.153 | 0.734-1.798 | 0.529 | 0.890 | 0.482-1.644 | 0.710 |
| Location
(head/other) | 1.304 | 0.836-2.035 | 0.242 | 0.635 | 0.342-1.180 | 0.151 |
| Preoperative
CEA | 1.039 | 0.997-1.083 | 0.073 | 1.020 | 0.971-1.070 | 0.433 |
| Preoperative CA
19-9 | 1.001 | 1.000-1.001 | 0.036a | 1.001 | 1.000-1.001 | 0.031a |
| Histological grade
(high/low) | 1.196 | 0.610-2.345 | 0.602 | 0.358 | 0.130-0.980 | 0.046a |
| Tumor size | 1.255 | 1.098-1.434 |
<0.001a | 1.165 | 0.932-1.457 | 0.179 |
| Lymph node
metastasis | 1.283 | 0.921-1.788 | 0.140 | 0.387 | 0.573-1.965 | 0.015a |
| UICC stage
(I/II/III/IV) | 1.320 | 0.943-1.846 | 0.106 | 3.716 | 1.315-10.497 | 0.013a |
| Vascular invasion
(P/N) | 1.301 | 0.789-2.145 | 0.302 | 1.084 | 0.530-2.216 | 0.826 |
| Perinural invasion
(P/N) | 1.353 | 0.872-2.099 | 0.178 | 0.842 | 0.484-1.572 | 0.649 |
| Adjvant
chemotherapy (±) | 0.984 | 0.746-1.296 | 0.906 | 0.766 | 0.357-1.642 | 0.493 |
Functional analysis of HABP1 in PDAC
cell lines
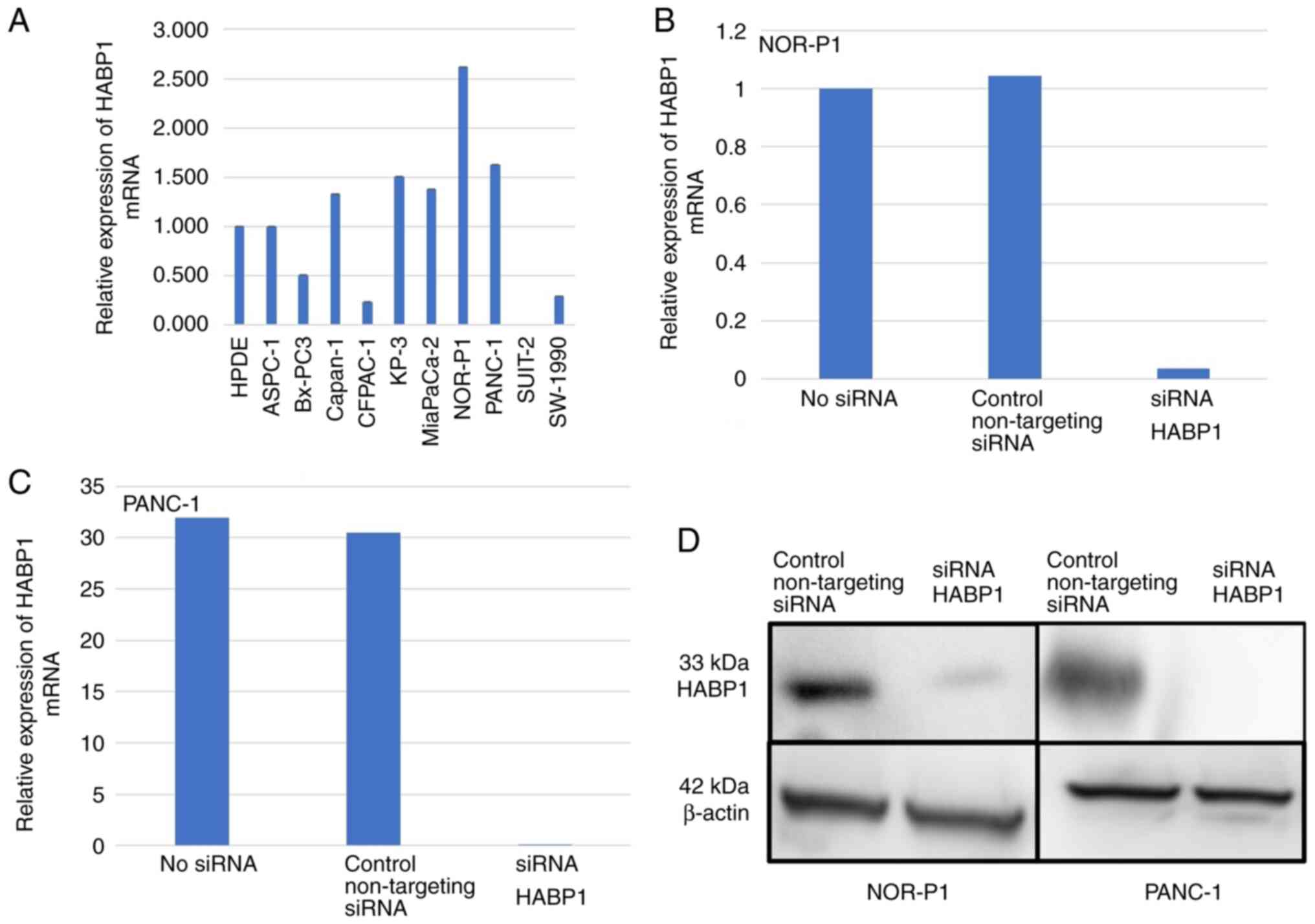
First, the mRNA expression of HABP1 was
investigated in a panel of 10 PDAC cell lines. HABP1 mRNA
was strongly expressed in 5 (50%) out of 10 PDAC cell lines
investigated (compared with the level of expression in a control
cell line, HPDE) (Fig. 3A). With
regard to the cell lines with strong expression, two (NOR-P1 and
PANC-1) were used for our subsequent experiments.
siRNA was used to knockdown HABP1 expression
in NOR-P1 and PANC-1 cells, two of the cell lines with strong
HABP1 mRNA expression. RT-qPCR revealed that transfection
with the siRNA targeting HABP1 (siRNA HABP1) resulted in a
97–99% decrease in HABP1 mRNA levels in these cell lines
(Fig. 3B and C). Western blot
analysis validated the successful knockdown of HABP1
expression at the protein level (Fig.
3D).
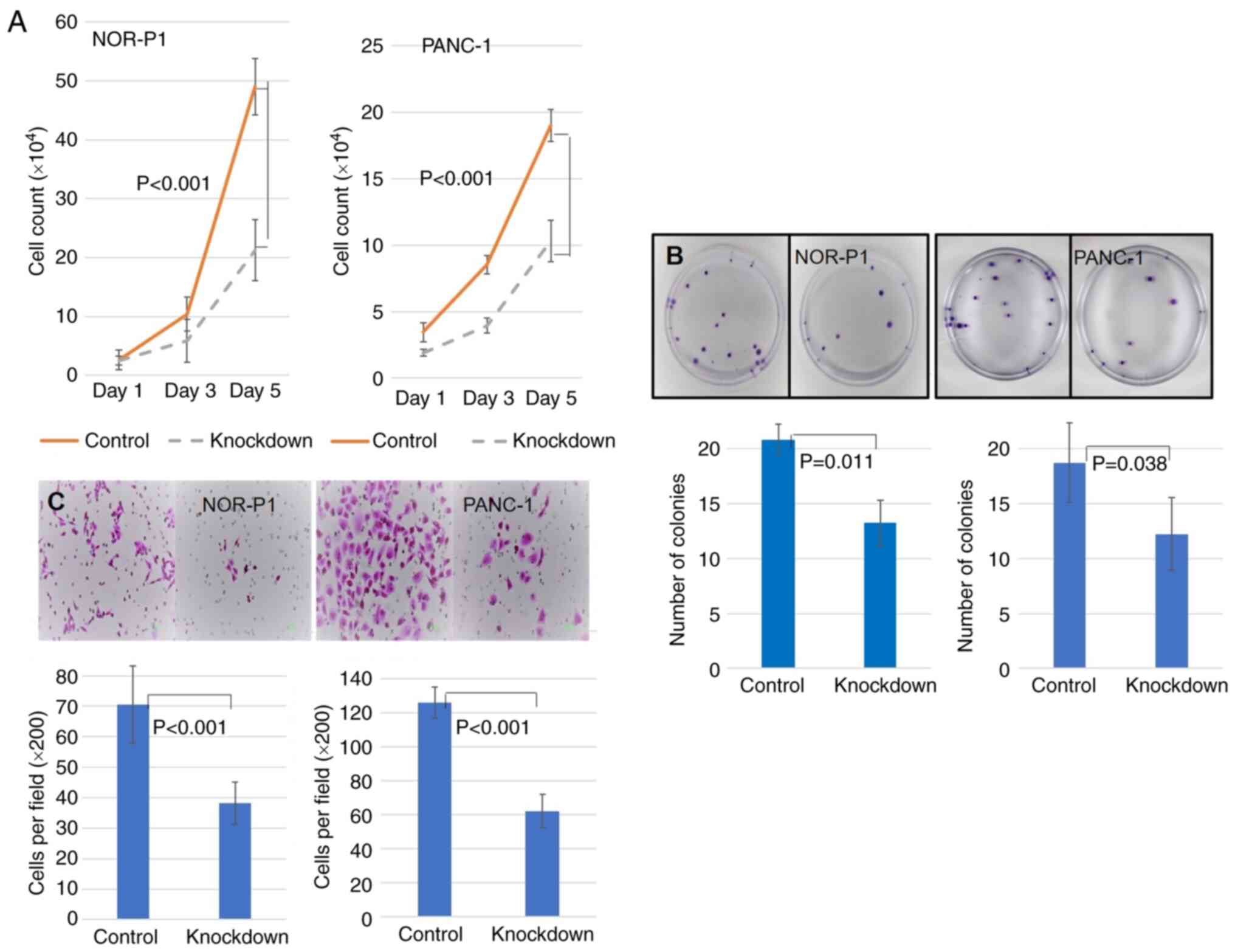
The proliferation, colony formation, and migration
of NOR-P1 and PANC-1 cells with and without knockdown of
HABP1 were next examined; these experiments were expected to
reveal aspects of the biological functions of HABP1 in pancreatic
cancer. First, it was assessed whether HABP1 knockdown
affected PDAC cell proliferation. A cell counting assay revealed
that the knockdown of HABP1 significantly decreased the
proliferation of PDAC cells compared with control cells on both
days 3 and 5, using Student's t-tests (NOR-P1, P<0.001; and
PANC-1, P<0.001; Fig. 4A).
Next, it was revealed that the number of colonies was significantly
decreased in the HABP1-knockdown cells compared with the
control cells for both cell lines (NOR-P1, P=0.011; and PANC-1,
P=0.038; Fig. 4B). Finally, it was
investigated whether HABP1 affects cell migration. The
Transwell migration assay demonstrated that knockdown of
HABP1 significantly inhibited the migration of PDAC cells
compared with the migratory activity of the control for both cell
lines (NOR-P1, P<0.001; and PANC-1, P<0.001; Fig. 4C).
Discussion
In the present study, the expression and functional
significance of HABP1 in PDAC were investigated. The major findings
obtained were as follows: i) HABP1 protein was highly expressed in
49 (46.2%) out of 105 patients with PDAC; ii) the survival of
patients with PDAC in which HABP1 was strongly expressed was
significantly shorter than in those with lower expression of HABP1;
iii) multivariate analysis identified high HABP1 expression as an
independent factor predicting poor prognosis; and iv) knockdown of
HABP1 in PDAC cells resulted in decreased proliferation,
colony formation, and migratory activities. Collectively, these
findings suggest that HABP1 may play a role in aggressive forms of
PDAC.
HABP1 is a multi-functional glycoprotein
ubiquitously expressed in various tissues. This protein has been
shown to be involved in a variety of cellular processes, including
cell motility, senescence, apoptosis, and autophagy (24). Recently, it was revealed that HABP1
overexpression triggers the induction of senescence in fibroblasts
(33). These functions of HABP1
demonstrate the important role of this protein in cancer initiation
and progression. In fact, overexpression of HABP1 in HepG2 cells
was revealed to lead to enhanced cell survival and tumorigenicity
by activating HA-mediated cell survival pathways (27,28).
Similarly, exogenous administration of HABP1 protein enhanced the
migration and tumor growth of a melanoma cell line (34). However, the functional relevance of
HABP1 to PDAC remains unknown. In the present study, it was
demonstrated, for the first time (to the best of our knowledge),
that siRNA knockdown of HABP1 impairs the proliferation,
colony formation, and migration of PDAC cells. These findings
suggest that HABP1 is involved in the progression of PDAC, as well
as in that of other cancer types.
It was also demonstrated that high HABP1 expression
was associated with shorter survival times in patients with PDAC
who underwent surgery. Consistent with the results of the present
study, it recently was reported that high cytoplasmic (but not
nuclear) HABP1 levels were strongly correlated with late tumor
stages, arterial involvement, LN metastasis, CA19-9 levels, and
poor overall survival in patients with PDAC (25). It was also revealed, in the present
study, that high HABP1 expression, as well as LN metastasis, tumor
stage, and CA19-9 levels, were factors indicative of poor
prognosis. The present study further suggested that histological
grade was also an independent factor indicating poor prognosis. In
cell experiments, knockdown of HABP1 suppressed the
malignant behaviors (such as the proliferation and migration
activities) of PDAC cells. These results support the hypothesis
that HABP1 is a prognostic factor for poor outcomes. In the
immunohistochemical staining performed as part of this study, none
of the 105 tested specimens demonstrated nuclear staining with the
anti-HABP1 antibody, in contrast to the results reported by Xie
et al (25). This
difference most likely reflects the use of distinct antibodies.
Nonetheless, the present results as well as those of Xie et
al are in agreement with regard to the observation that the
accumulation of HABP1 in the cytoplasm is an indicator of a poor
prognosis. Since nuclear expression was not invoked as a prognostic
factor in the study by Xie et al, it is proposed that the
expression level of HABP1 in the cytoplasm is the critical
characteristic detected by immunohistochemical staining for this
protein. In other cancer types, including gastric, breast, and
ovarian cancer, increased HABP1 expression levels were associated
with worse patient outcomes (27,29,35–40).
These findings suggest that HABP1 may be a promising prognostic
marker in patients with PDAC and other cancers.
Regarding the therapeutic implications of the
present, it was further inferred that HABP1 may be a promising
therapeutic target for PDAC. Notably, high HABP1 expression was
associated with improved survival of patients with malignant
pleural mesothelioma who had received neoadjuvant or adjuvant
chemotherapy (41). This result
suggests that high HABP1 expression may serve as a biomarker in
predicting the response to chemotherapy. In the present study,
however, the association between high HABP1 expression and response
to chemotherapy was unclear due to the limited number of patients.
Further studies will be required to elucidate the relationship
between HABP1 levels and chemosensitivity in PDAC.
The limitations of the present study were as
follows. First, the study data lacked data on complications of
patients with PDAC. Second, the study was a retrospective, single
center study. Third, the study population was limited, rendering it
difficult to draw a solid conclusion. Fourth, in our cohort of 105
patients with PDAC, some of the established prognostic factors,
including adjuvant chemotherapy, did not demonstrate significant
association with prognosis. Fifth, the number of cell experiments
was not sufficient for statistical analysis in certain experiments.
It is therefore inferred that our results may be biased due to the
small sample size and long study period. Further investigations
with larger and more-recent samples (for example, using tissue
microarrays or data obtained by next-generation sequencing) would
be required to confirm the results of the present study.
In conclusion, it was demonstrated that HABP1
accumulates to high levels in PDAC cells, and the expression of
this protein is associated with prognosis. It was also determined
that HABP1 is involved in the proliferation, colony formation, and
migration of PDAC cells in vitro. These findings suggest
that HABP1 may play a role in the progression of PDAC.
Acknowledgements
The authors would like to thank Ms Ueda (Department
of Surgery I, University of Occupational and Environmental Health,
Kitakyushu, Japan) for providing technical assistance.
Funding
The present research was funded by the University of
Occupational and Environmental Health.
Availability of data and materials
The data that support the findings of this study are
available (in anonymized form) from the corresponding author upon
request.
Authors' contributions
YA conducted the molecular studies and drafted the
manuscript. NS conceived the study, and participated in its design
and coordination, and helped to draft the manuscript. TO, TA, YK
SK, and TN participated in the molecular studies. KH participated
in the design of the study. YA, NS and KH confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study received ethical approval from the
Ethics Committee of the University of Occupational and
Environmental Health (Kitakyushu, Japan; approval no. H26-118). All
patients provided written informed consent prior to specimen
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CEMIP
|
cell migration-inducing and
hyaluronan-binding protein
|
|
ECM
|
extracellular matrix
|
|
gC1qR
|
globular head receptor for complement
component 1q
|
|
HABP1
|
hyaluronan-binding protein 1
|
|
HMW-HA
|
high-molecular-weight hyaluronan
|
|
HA
|
hyaluronan
|
|
HAS
|
hyaluronan synthase
|
|
HPDE
|
human pancreatic duct epithelial
|
|
HYAL
|
hyaluronidase
|
|
IHC
|
immunohistochemistry
|
|
LMW-HA
|
low-molecular-weight hyaluronan
|
|
LN
|
lymph node
|
|
ROS
|
reactive oxygen species
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Center Japan, . Cancer
Registry and Statistics. Cancer Information Service. https://ganjoho.jp/en/index.html1–March. 2022
|
|
3
|
Michl P and Gress TM: Improving drug
delivery to pancreatic cancer: Breaching the stromal fortress by
targeting hyaluronic acid. Gut. 61:1377–1379. 2012. View Article : Google Scholar
|
|
4
|
Cheng XB, Kohi S, Koga A, Hirata K and
Sato N: Hyaluronan stimulates pancreatic cancer cell motility.
Oncotarget. 7:4829–4840. 2016. View Article : Google Scholar
|
|
5
|
Itano N, Zhuo L and Kimata K: Impact of
the hyaluronan-rich tumor microenvironment on cancer initiation and
progression. Cancer Sci. 99:1720–1725. 2008. View Article : Google Scholar
|
|
6
|
Jacobetz MA, Chan DS, Neesse A, Bapiro TE,
Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et
al: Hyaluronan impairs vascular function and drug delivery in a
mouse model of pancreatic cancer. Gut. 62:112–120. 2013. View Article : Google Scholar
|
|
7
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocarcinoma. Cancer Cell. 21:418–429. 2012. View Article : Google Scholar
|
|
8
|
Sato N, Kohi S, Hirata K and Goggins M:
Role of hyaluronan in pancreatic cancer biology and therapy: Once
again in the spotlight. Cancer Sci. 107:569–575. 2016. View Article : Google Scholar
|
|
9
|
Sironen RK, Tammi M, Tammi R, Auvinen PK,
Anttila M and Kosma VM: Hyaluronan in human malignancies. Exp Cell
Res. 317:383–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toole BP, Wight TN and Tammi MI:
Hyaluronan-cell interactions in cancer and vascular disease. J Biol
Chem. 277:4593–4596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du Y, Cao M, Liu Y, He Y, Yang C, Wu M,
Zhang G and Gao F: Low-molecular-weight hyaluronan (LMW-HA)
accelerates lymph node metastasis of melanoma cells by inducing
disruption of lymphatic intercellular adhesion. Oncoimmunology.
5:e12322352016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu M, Tolg C and Turley E: Dissecting the
dual nature of hyaluronan in the tumor microenvironment. Front
Immunol. 10:9472019. View Article : Google Scholar
|
|
13
|
Sugahara KN, Hirata T, Hayasaka H, Stern
R, Murai T and Miyasaka M: Tumor cells enhance their own CD44
cleavage and motility by generating hyaluronan fragments. J Biol
Chem. 281:5861–5868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu M, Cao M, He Y, Liu Y, Yang C, Du Y,
Wang W and Gao F: A novel role of low molecular weight hyaluronan
in breast cancer metastasis. FASEB J. 29:1290–1298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng XB, Sato N, Kohi S and Yamaguchi K:
Prognostic impact of hyaluronan and its regulators in pancreatic
ductal adenocarcinoma. PLoS One. 8:e807652013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kohi S, Sato N, Cheng XB, Koga A and
Hirata K: Increased expression of HYAL1 in pancreatic ductal
adenocarcinoma. Pancreas. 45:1467–1473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chowdhury AR, Ghosh I and Datta K:
Excessive reactive oxygen species induces apoptosis in fibroblasts:
Role of mitochondrially accumulated hyaluronic acid binding protein
1 (HABP1/p32/gC1qR). Exp Cell Res. 314:651–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Souza M and Datta K: A novel
glycoprotein that binds to hyaluronic acid. Biochem Int. 13:79–88.
1986.
|
|
19
|
Li K, Gao B, Li J, Chen H, Li Y, Wei Y,
Gong D, Gao J, Zhang J, Tan W, et al: ZNF32 protects against
oxidative stress-induced apoptosis by modulating C1QBP
transcription. Oncotarget. 6:38107–38126. 2015. View Article : Google Scholar
|
|
20
|
Li Y, Wan OW, Xie W and Chung KK: p32
regulates mitochondrial morphology and dynamics through Parkin.
Neuroscience. 199:346–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Leslie PL, Jin A, Itahana K, Graves
LM and Zhang Y: p32 heterozygosity protects against age- and
diet-induced obesity by increasing energy expenditure. Sci Rep.
7:57542017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Leslie PL, Jin A, Itahana K, Graves
LM and Zhang Y: p32 regulates ER stress and lipid homeostasis by
down-regulating GCS1 expression. FASEB J. 32:3892–3902. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muta T, Kang D, Kitajima S, Fujiwara T and
Hamasaki N: p32 protein, a splicing factor 2-associated protein, is
localized in mitochondrial matrix and is functionally important in
maintaining oxidative phosphorylation. J Biol Chem.
272:24363–24370. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saha P and Datta K: Multi-functional,
multicompartmental hyaluronan-binding protein 1 (HABP1/p32/gC1qR):
Implication in cancer progression and metastasis. Oncotarget.
9:10784–10807. 2018. View Article : Google Scholar
|
|
25
|
Xie ZB, Yao L, Jin C, Zhang YF and Fu DL:
High cytoplasm HABP1 expression as a predictor of poor survival and
late tumor stage in pancreatic ductal adenocarcinoma patients. Eur
J Surg Oncol. 45:207–212. 2019. View Article : Google Scholar
|
|
26
|
Yagi M, Uchiumi T, Takazaki S, Okuno B,
Nomura M, Yoshida S, Kanki T and Kang D: p32/gC1qR is indispensable
for fetal development and mitochondrial translation: Importance of
its RNA-binding ability. Nucleic Acids Res. 40:9717–9737. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaul R, Saha P, Saradhi M, Prasad RL,
Chatterjee S, Ghosh I, Tyagi RK and Datta K: Overexpression of
hyaluronan-binding protein 1 (HABP1/p32/gC1qR) in HepG2 cells leads
to increased hyaluronan synthesis and cell proliferation by
up-regulation of cyclin D1 in AKT-dependent pathway. J Biol Chem.
287:19750–19764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saha P, Ghosh I and Datta K: Increased
hyaluronan levels in HABP1/p32/gC1qR overexpressing HepG2 cells
inhibit autophagic vacuolation regulating tumor potency. PLoS One.
9:e1032082014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th edition.
John Wiley & Sons; 2017
|
|
30
|
Wang J, Song Y, Liu T, Shi Q, Zhong Z, Wei
W, Huang S and Pang D: Elevated expression of HABP1 is a novel
prognostic indicator in triple-negative breast cancers. Tumour
Biol. 36:4793–4799. 2015. View Article : Google Scholar
|
|
31
|
Sato N, Mizumoto K, Beppu K, Maehara N,
Kusumoto M, Nabae T, Morisaki T, Katano M and Tanaka M:
Establishment of a new human pancreatic cancer cell line, NOR-P1,
with high angiogenic activity and metastatic potential. Cancer
Lett. 155:153–161. 2000. View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vikramdeo KS, Saha P, Dutta S, Kumar N,
Roy Chowdhury A, Kumar S, Tyagi RK, Ghosh I and Datta K:
Hyaluronan-binding protein 1 (HABP1) overexpression triggers
induction of senescence in fibroblasts cells. Cell Biol Int.
44:1312–1330. 2020. View Article : Google Scholar
|
|
34
|
Prakash M, Kale S, Ghosh I, Kundu GC and
Datta K: Hyaluronan-binding protein 1 (HABP1/p32/gC1qR) induces
melanoma cell migration and tumor growth by NF-kappaB dependent
MMP-2 activation through integrin α(v)β(3) interaction. Cell
Signal. 23:1563–1577. 2011. View Article : Google Scholar
|
|
35
|
Niu M, Sun S, Zhang G, Zhao Y, Pang D and
Chen Y: Elevated expression of HABP1 is correlated with metastasis
and poor survival in breast cancer patients. Am J Cancer Res.
5:1190–1198. 2015.PubMed/NCBI
|
|
36
|
Yu H, Liu Q, Xin T, Xing L, Dong G, Jiang
Q, Lv Y, Song X, Teng C, Huang D, et al: Elevated expression of
hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP is a novel
indicator for lymph node and peritoneal metastasis of epithelial
ovarian cancer patients. Tumour Biol. 34:3981–3987. 2013.
View Article : Google Scholar
|
|
37
|
Gao H, Yao Q, Lan X, Li S, Wu J, Zeng G
and Xue Y: Elevated HABP1 protein expression correlates with
progression and poor survival in patients with gastric cancer. Onco
Targets Ther. 9:6711–6718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang M, Li N, Liang Y, Liu J, Zhou Y and
Liu C: Hyaluronic acid binding protein 1 overexpression is an
indicator for disease-free survival in cervical cancer. Int J Clin
Oncol. 22:347–352. 2017. View Article : Google Scholar
|
|
39
|
Jiang Y, Wu H, Liu J, Chen Y, Xie J, Zhao
Y and Pang D: Increased breast cancer risk with HABP1/p32/gC1qR
genetic polymorphism rs2285747 and its upregulation in northern
Chinese women. Oncotarget. 8:13932–13941. 2017. View Article : Google Scholar
|
|
40
|
Zhao J, Liu T, Yu G and Wang J:
Overexpression of HABP1 correlated with clinicopathological
characteristics and unfavorable prognosis in endometrial cancer.
Tumour Biol. 36:1299–1306. 2015. View Article : Google Scholar
|
|
41
|
Li X, Eguchi T, Aly RG, Chintala NK, Tan
KS, Zauderer MG, Dembitzer FR, Beasley MB, Ghebrehiwet B,
Adusumilli PS and Peerschke EIB: Globular C1q receptor
(gC1qR/p32/HABP1) is overexpressed in malignant pleural
mesothelioma and is associated with increased survival in surgical
patients treated with chemotherapy. Front Oncol. 9:10422019.
View Article : Google Scholar
|