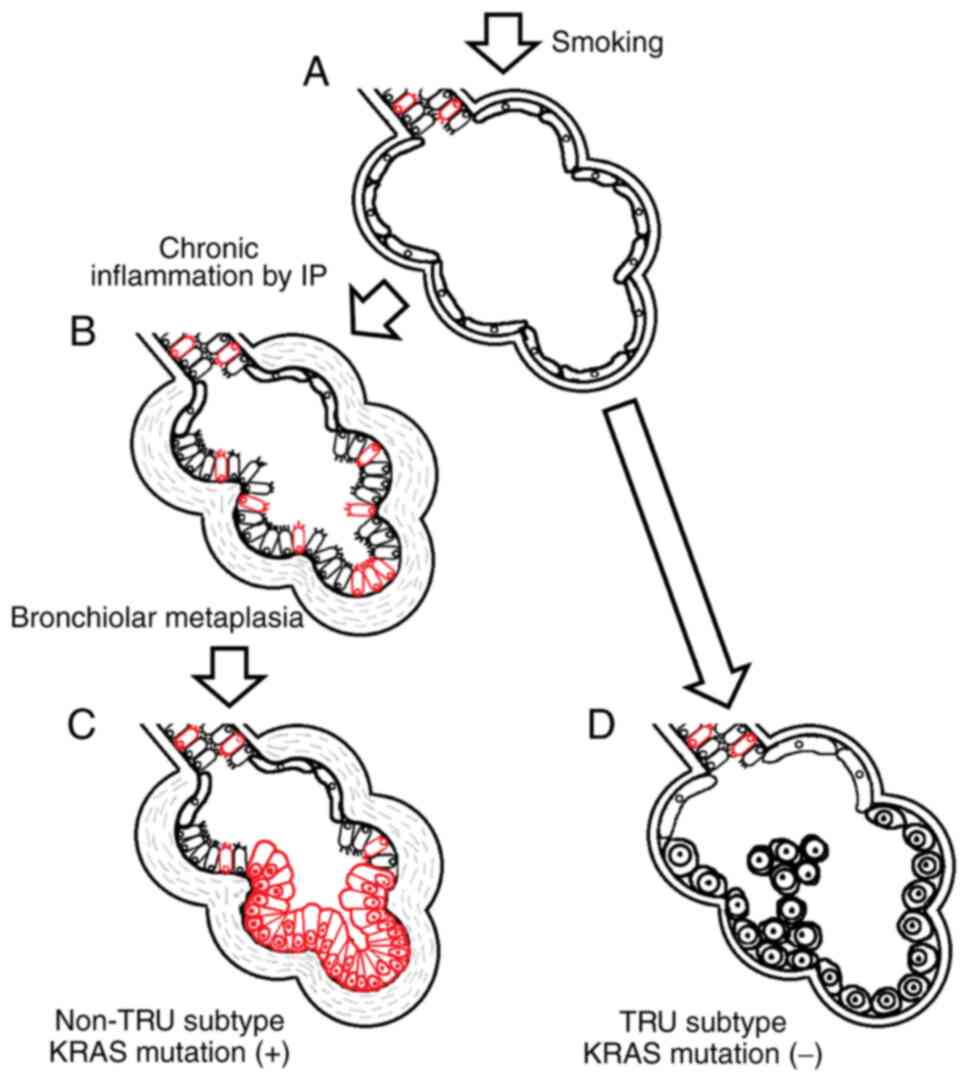
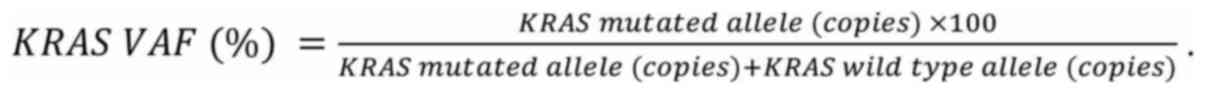Introduction
Interstitial pneumonia (IP) is a group of
inflammatory disorders that affect the alveolar septa. They are
often progressive and intractable, and may cause airspaces to
collapse, leading to honeycombing (1). This may be caused by exposure to
hazardous chemicals, certain medications, and exogenous antigens.
In most cases, the etiology is unknown.
IP is a strong risk factor for lung cancer (14.1%)
(2). The most common histological
type of IP-related lung cancer is adenocarcinoma (3). Our recent studies demonstrated that
IP-related lung adenocarcinomas (LADCs) mostly comprise the
non-terminal respiratory unit (TRU) subtype (4) and that IP-related non-TRU LADCs often
develop from bronchiolar metaplasia lining in honeycomb lesions
(5). These findings suggest that
metaplastic epithelia could be a precursor for IP-related non-TRU
LADC.
Generally, tissue injury could cause DNA damage
(6,7). Repair of honeycomb lesions resulting
from severe chronic lung injury has been described (8). Genetic mutations accumulate in the
metaplastic epithelia lining in honeycomb lesions. Similar to IP,
patients with chronic gastritis (9), hepatitis (10), pancreatitis (11), and inflammatory bowel disease
(12) are also frequently affected
by cancers of the affected organs (13,14).
These observations support the idea that tissue remodeling lesions
could be precursors for cancer.
Kirsten rat sarcoma virus (KRAS) is the most
common oncogene that is mutated in IP-related LADCs (4). In the present study, we examined
metaplastic epithelia lining in honeycomb lesions for the putative
accumulation of KRAS mutations using digital droplet
polymerase chain reaction (ddPCR). This is quantitative and the
most sensitive method for detecting highly infrequent mutations
(~0.01%) (15). The findings
support the hypothesis that metaplastic epithelia could be a
precursor for IP-related LADCs.
Materials and methods
Patients
Patients who underwent surgical lung resection (18
cases of surgical lung biopsy for IP, 16 cases of partial lung
resection for pneumothorax/bulla, and 35 cases of lobectomy for
LADCs) in Kanagawa Prefectural Cardiovascular and Respiratory
Center Hospital (Yokohama, Japan) between January 2014 and December
2020, were included in the analyses.
Tissue samples and DNA extraction
Tissues were fixed with buffered 10% formaldehyde
solution for 48 h and embedded in paraffin wax. Sections were cut
and stained lightly with hematoxylin. Metaplastic epithelia lining
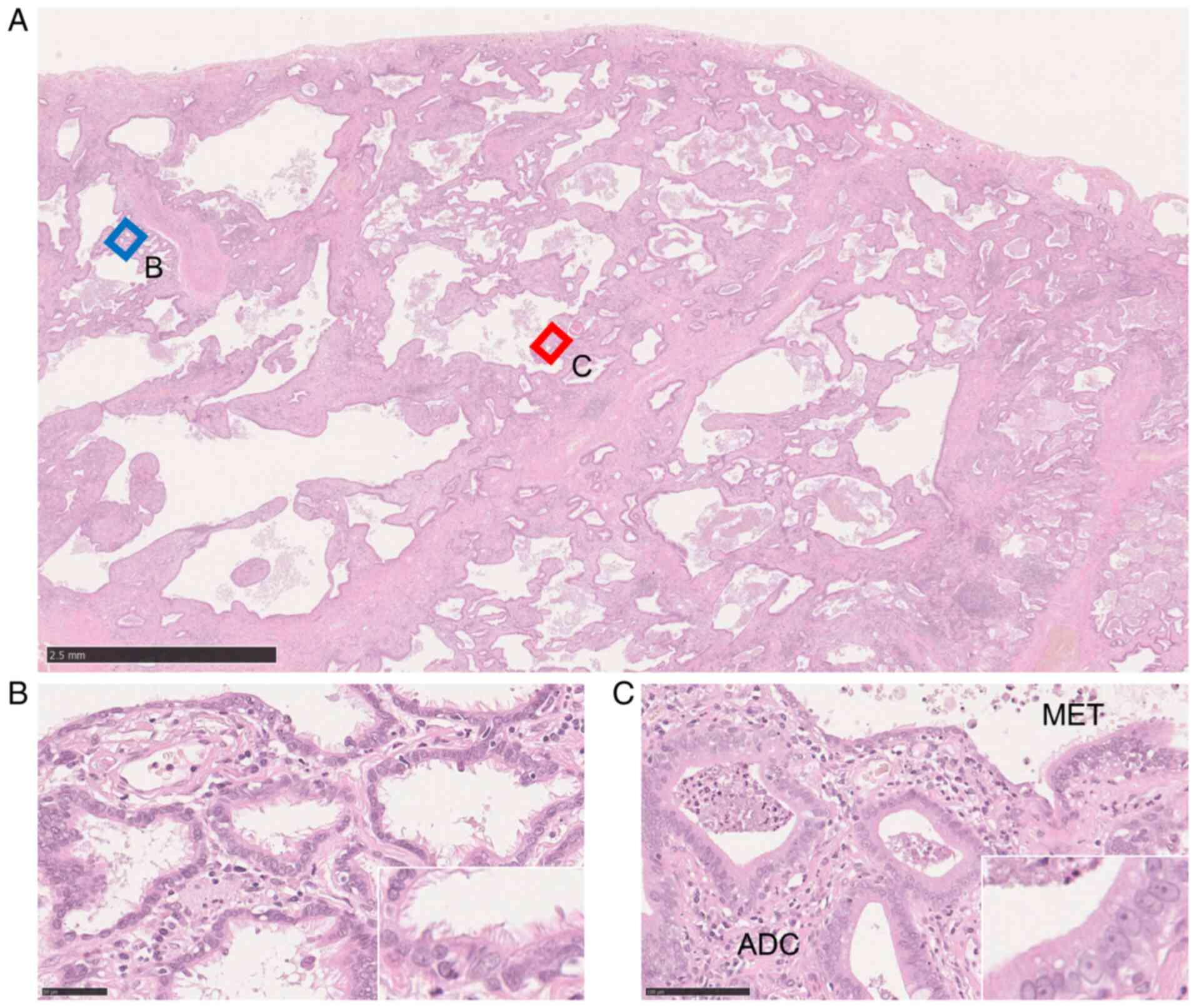
in honeycomb lesions were microscopically collected (Fig. 1) using a PALM microdissection
system (Carl Zeiss). Non-lesioned tissues from patients with LADC
and pneumothorax, and healthy tissue of the lung tissue from
patients with IP were collected by macroscopic dissection. DNA was
extracted using a NucleoSpin® Tissue kit
(Macherey-Nagel) according to the protocol of the manufacturer. For
the successful quantification of infrequent mutations of the
KRAS gene by ddPCR, >1 µg total material (10 ng/µl) was
required.
Quantification of KRAS mutations with
ddPCR
Seven types of KRAS mutations (G12C, G12V,
G12R, G12A, G12S, G12D, and G13D) were detected using the QX200
Droplet Digital PCR system (Bio-Rad). Commercial primers and probes
were used ddPCR KRAS Screening Multiplex Kit (Bio-Rad). Briefly, up
to 20,000 droplets were made from the master mix solution
containing the probe, primers, template DNA, dNTPs, and DNA
polymerase with the Cl000 Touch Thermal Cycler with a 96-Deep Well
Reaction Module (Bio-Rad). PCR was performed using the following
cycling conditions: polymerase activating step (10 min at 95°C),
followed by 40 reaction cycles (30 sec at 95°C for denaturation and
1 min at 55°C for annealing and extension), and DNA polymerase
deactivation step (10 min at 98°C). The PCR products were loaded
onto a QX200™ Droplet Reader (Bio-Rad). The number of droplets
containing mutant and wild-type alleles was counted using the
QuantaSoft v.l.7.4 software (Bio-Rad). The variant allele frequency
(VAF) was calculated as:
Statistical analyses
Differences in KRAS VAFs between the groups
of various parameters, including IP, age, smoking, and sex, were
analyzed using the Mann Whitney U test. Multivariate analyses were
performed using logistic regression analysis. Differences in
KRAS G12V and G12C VAFs between metaplastic epithelia and
non-lesioned bronchiolar epithelia in same patients with IP were
analyzed using the paired t-test. Statistical significance was set
at P<0.05. All analyses were performed using JMP Pro 15.0.0 (SAS
Institute Inc.).
Results
Baseline characteristics of
patients
The baseline characteristics of the 59 patients
examined in this study are shown in Table I. Of the 13 patients in the IP
group, nine suffered from idiopathic pulmonary fibrosis, three from
collagen vascular disease interstitial lung disease (CVD-ILD; two
cases of systemic sclerosis, one case of polymyositis), and one
from non-specific IP (NSIP). One of the five LADCs in the IP group
had a KRAS mutation (G12V). In the non-IP group, 16 patients
had bulla-pneumothorax, and 35 had LADC. Of the 30 LADCs in the
non-IP group, a KRAS mutation (G12C) was present in one
patient.
 | Table I.Baseline characteristics of all the
cases examined. |
Table I.
Baseline characteristics of all the
cases examined.
| Characteristic | IP (n=13) | Non-IP (n=46) |
|---|
| Age, years |
|
|
|
≥65 | 9 | 18 |
|
<65 | 4 | 28 |
| Smoking |
|
|
|
Smoker | 8 | 14 |
|
Non-smoker | 5 | 32 |
| Gender |
|
|
|
Male | 10 | 28 |
|
Female | 3 | 18 |
| Type of IPs |
|
|
|
IPF | 9 | - |
|
NSIP | 1 | - |
|
CVD-ILD | 3 | - |
| LADC |
|
|
| + | 5 | 30 |
| - | 8 | 16 |
| KRAS
mutations in LADCs | 1a | 1b |
Representative histological appearance
of IP-related non-TRU LADC
A non-TRU LADC developed in the IP is shown in
Fig. 1. The LADC cells and
metaplastic epithelia lining in honeycomb lesions were collected
separately by microdissection.
Quantification of KRAS mutations with
ddPCR
Seven types of KRAS mutations (G12C, G12V,
G12R, G12A, G12S, G12D, and G13D) were examined by ddPCR.
Representative results from the IP and non-IP groups are shown in
Fig. 2.
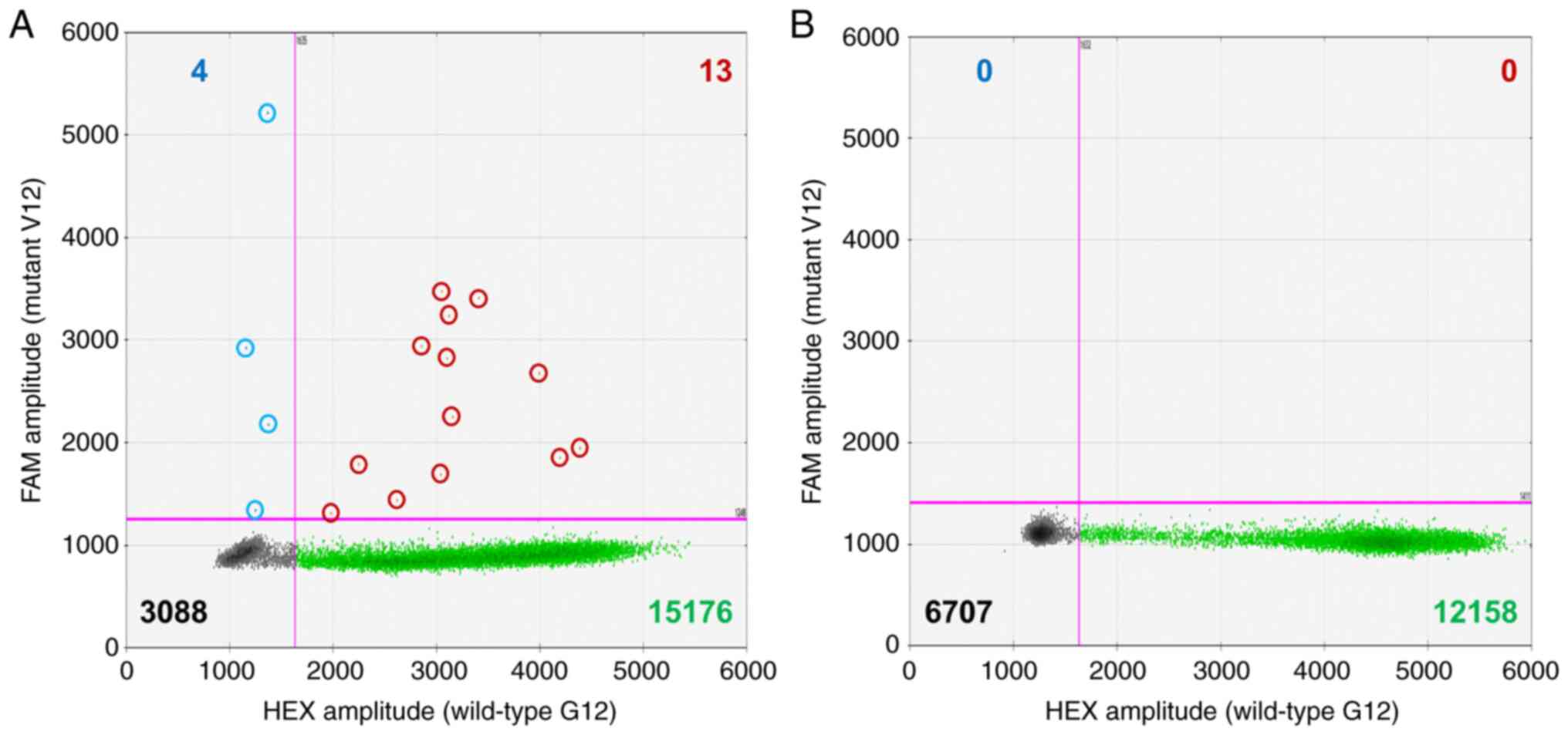 | Figure 2.Representative results of ddPCR from
the KRAS G12V mutations in (A) IP and (B) non-IP cases.
Fluorescent amplitudes from the HEX-labeled-wild-type G12 PCR
product (horizontal axis) and FAM-labeled-mutant V12 PCR product
(vertical axis) are plotted. In both examinations, the number of
droplets is sufficient for analysis (>12,000). (A) In the IP
case, 17 (4+13) mutation droplets (blue and red dots) and 15,189
(13+15,176) wild-type droplets (green dots) were detected.
According to the number and signal levels of the dots, mutant and
wild-type alleles comprised 22 and 41,820 copies, respectively.
Thus, the VAF was 0.052% [22/(22+41,820)]. (B) In contrast, no
mutation- and 12,158 wild-type-droplets (green dots) were detected.
The VAF was 0%. IP, interstitial pneumonia; VAF, variant allele
frequency. |
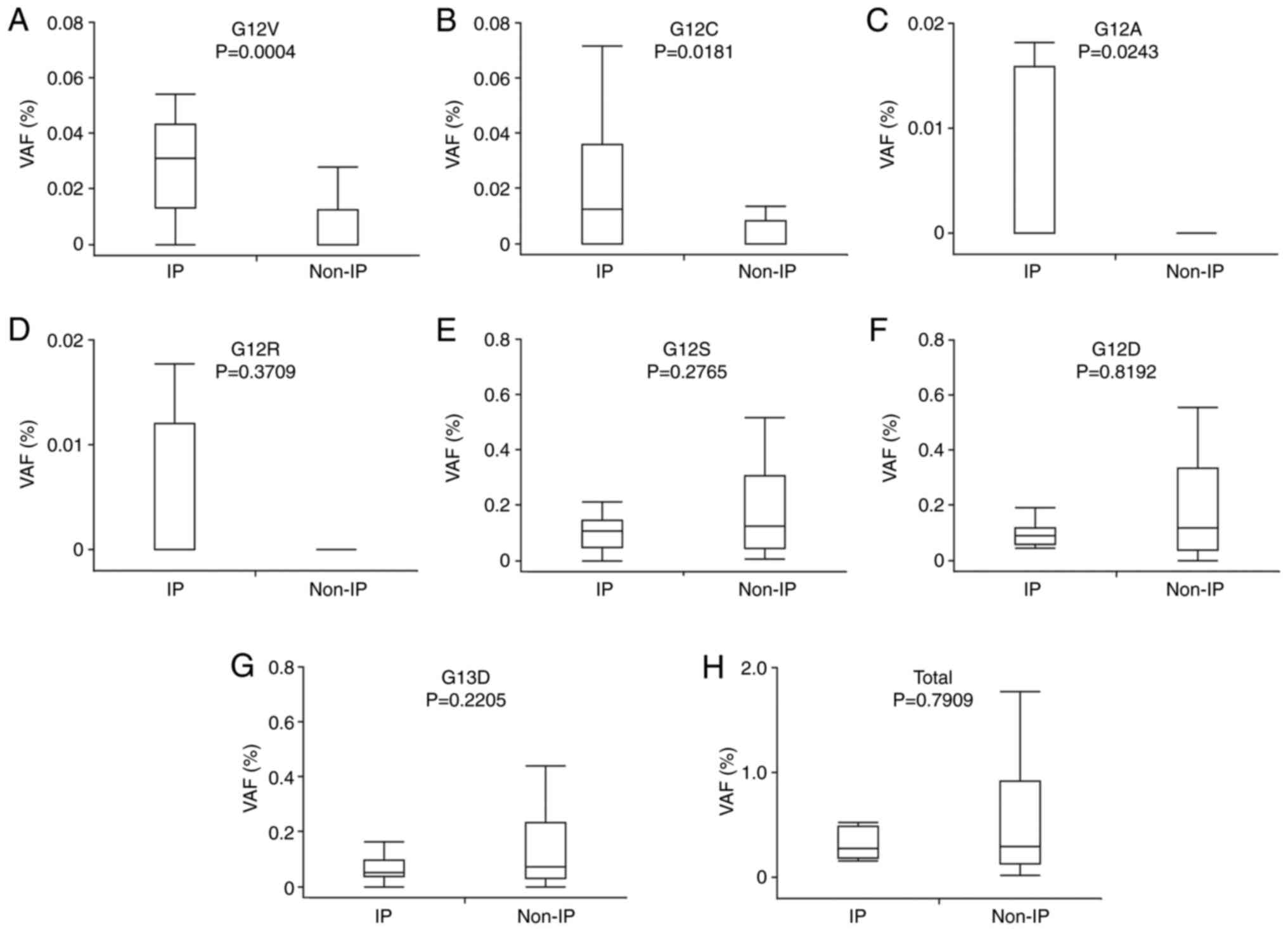
Difference in VAFs of KRAS mutation
between IP and non-IP groups
The IP group showed a significantly higher
prevalence of KRAS mutation VAFs of G12V (P=0.0004), G12C
(P=0.0181), and G12A (P=0.0234) than in the non-IP group (Fig. 3A-C). No significant difference
between the groups was seen in the other VAF types of KRAS
mutations and the total VAFs (Fig.
3D-H). We focused on G12V, G12C, and G12A mutations and
analyzed their relationships with the baseline characteristics.
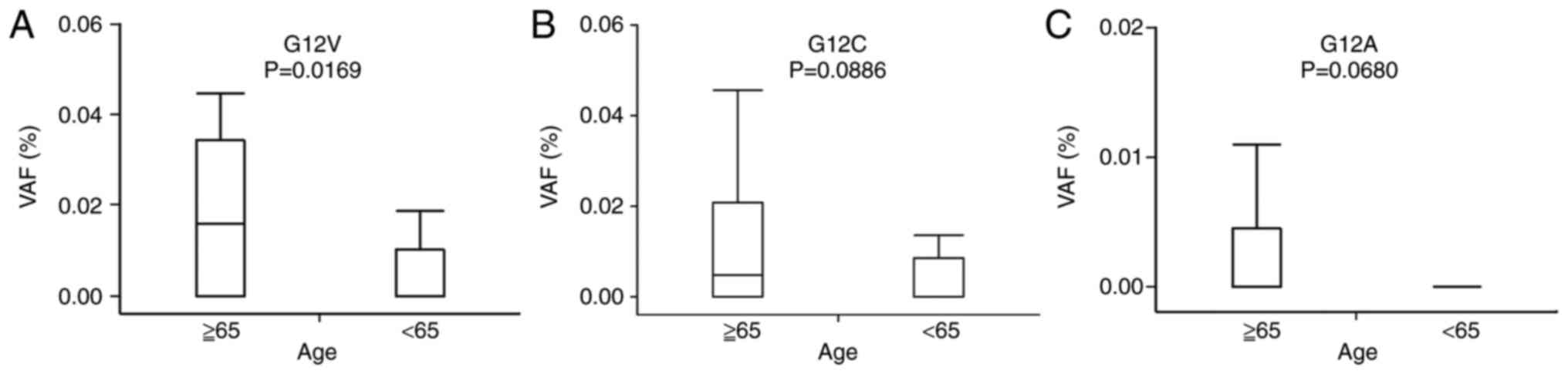
Relationships between VAFs of KRAS
G12V, G12C, and G12A mutations and baseline characteristics
The G12V VAF was significantly higher in elderly
patients (P=0.0169; Fig. 4A).
There was no statistically significant relationship between the
G12C and G12A VAFs and age, although it tended to be higher in
elderly patients (Fig. 4B and C).
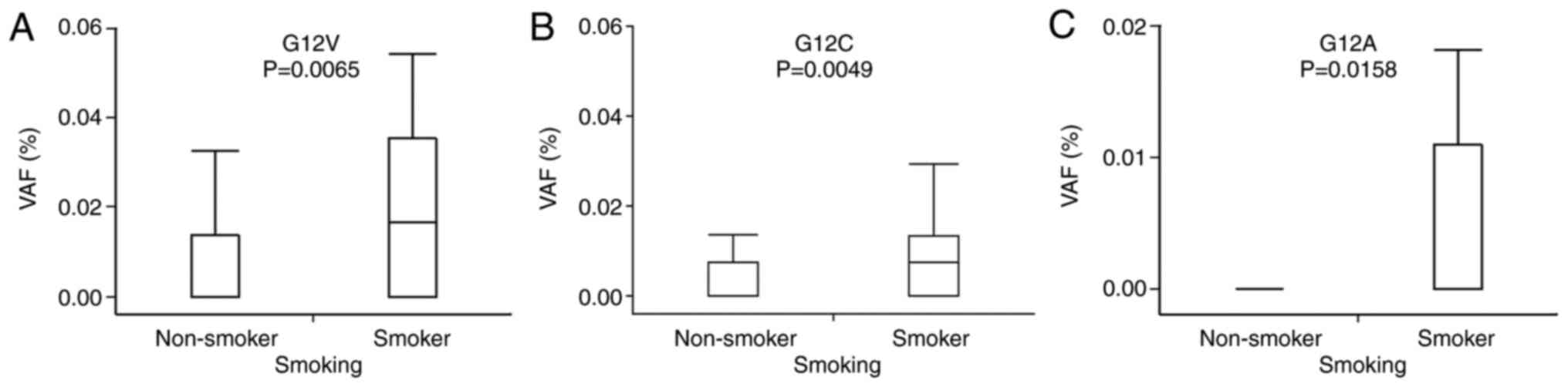
Smokers displayed significantly higher VAFs of G12V (P=0.0065),
G12C (P=0.0049), and G12A (P=0.0158) than non-smokers (Fig. 5). There was no significant
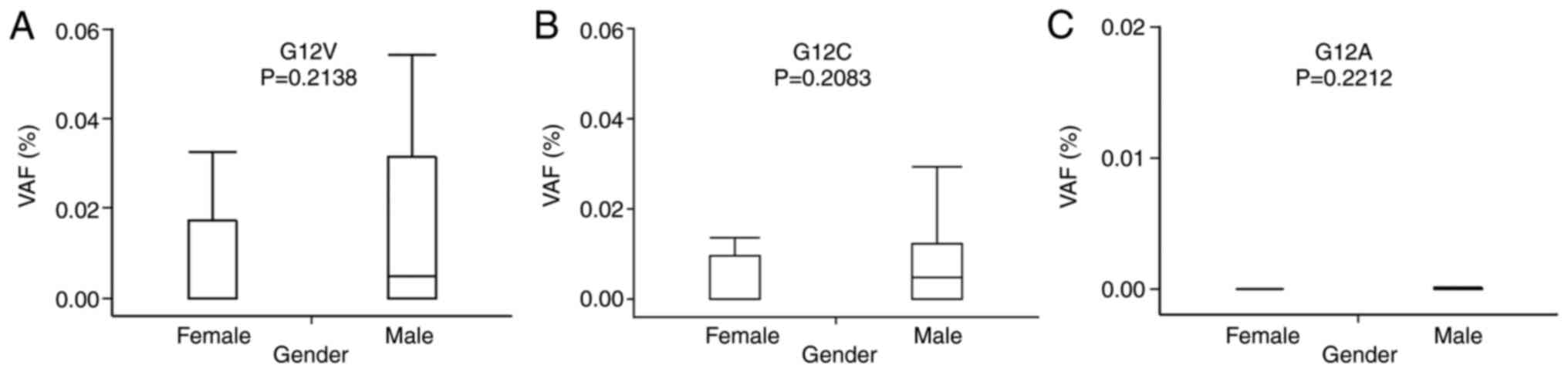
relationship between the G12V, G12C, and G12A VAFs and sex
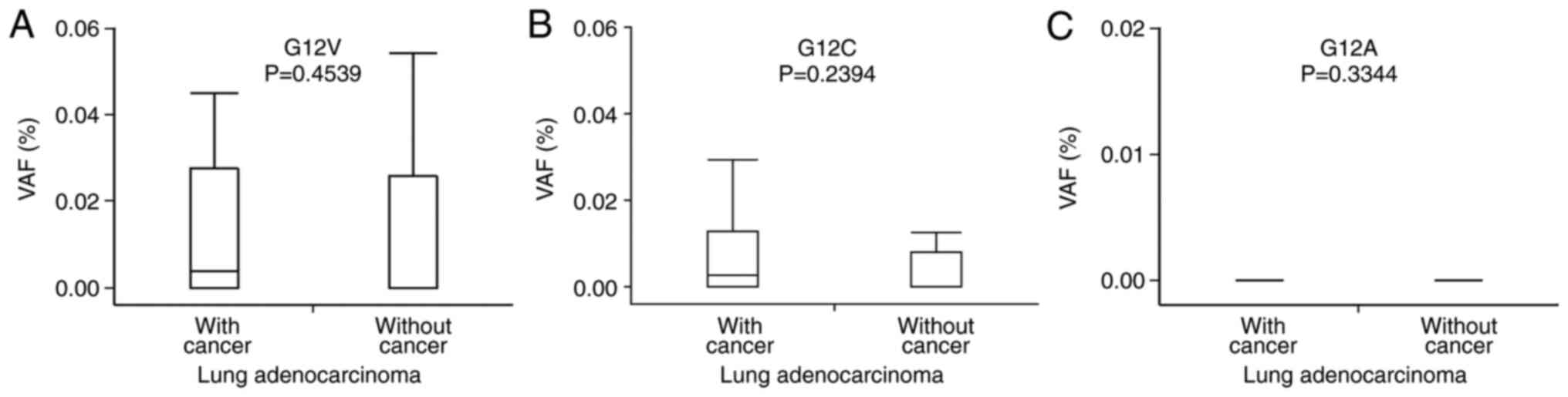
(Fig. 6) or LADC carcinogenesis
(Fig. 7). In addition, there was
no relationship between the VAFs of KRAS mutations and types
of IP or oncogenic mutations in the LADCs (data not shown).
Multivariate analyses to confirm the
independent relationships between VAFs of KRAS mutations and
IP
As described above, age and smoking could be
confounding factors associated with the higher VAFs of G12V, G12C,
and G12A mutations in the IP group. Multivariate analyses with
logistic regression revealed that IP was an exploratory variable
for G12V and G12C VAFs (Table
II), while smoking status and age were not. The IP group
displayed a significantly higher G12V [odds ratio (OR), 7.11; 95%
confidence interval (CI), 1.47-52.6] and G12C [odds ratio (OR),
5.81; 95% confidence interval (CI), 1.33-26.9] VAFs than the non-IP
group (Table II). IP was an
independent factor that caused the accumulation of G12V
mutations.
 | Table II.Multivariate analyses on relationship
between G12V, G12C, and G12A VAFs and IP, smoking, and age. |
Table II.
Multivariate analyses on relationship
between G12V, G12C, and G12A VAFs and IP, smoking, and age.
| VAF | Risk factor | P-value | Odds ratio (95%
CI) |
|---|
| G12V | IP/Non-IP | 0.0133a | 7.11
(1.47-52.6) |
|
|
Smoker/non-smoker | 0.1117 | 2.88
(0.78-10.9) |
|
| Age ≥65/<65 | 0.2640 | 2.06
(0.57-7.39) |
| G12C | IP/Non-IP | 0.0191a | 5.81
(1.33-26.9) |
|
|
Smoker/non-smoker | 0.6537 | 1.44
(0.28-7.11) |
|
| Age ≥65/<65 | 0.3390 | 2.15
(0.44-11.1) |
| G12A | IP/Non-IP | 0.1469 | 3.24
(0.65-15.9) |
|
|
Smoker/non-smoker | 0.2461 | 2.76
(0.49-17.3) |
|
| Age ≥65/<65 | 0.5684 | 1.65
(0.29-10.1) |
Comparison of KRAS G12V VAFs between
metaplastic epithelia and non-lesioned bronchiolar epithelia
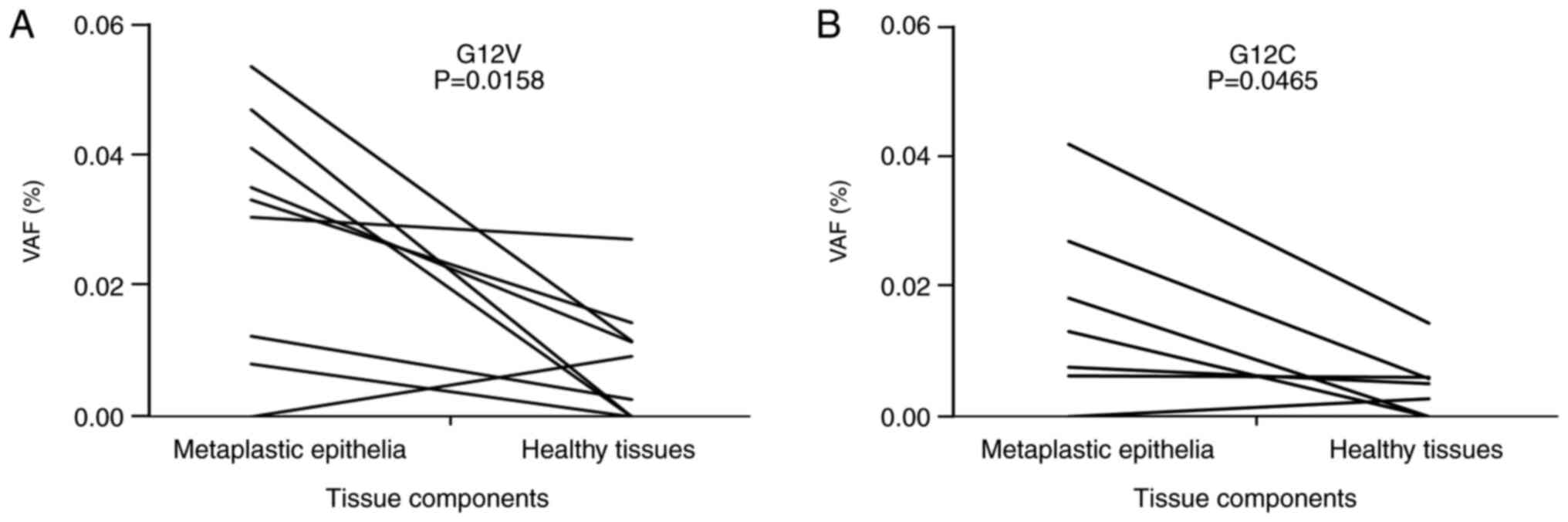
In the 10 IP cases (Table III), metaplastic epithelia and
non-lesioned bronchiolar epithelia were separately collected by
microdissection and examined for KRAS G12V and G12C VAFs.
The metaplastic epithelia showed significantly higher KRAS
G12V (P=0.0153) and G12C (P=0.0465) VAFs than the non-lesioned
bronchiolar epithelia when compared in the same cases (Fig. 8). These observations suggest that
metaplastic epithelia could accumulate more mutations.
 | Table III.Baseline characteristics of the ten
IP cases subjected to the paired analysis. |
Table III.
Baseline characteristics of the ten
IP cases subjected to the paired analysis.
| Characteristic | IP (n=10) |
|---|
| Age |
|
|
≥65 | 6 |
|
<65 | 4 |
| Smoking |
|
|
Smoker | 7 |
|
Non-smoker | 3 |
| Gender |
|
|
Male | 8 |
|
Female | 2 |
| Type of IPs |
|
|
IPF | 7 |
|
CVD-ILD | 2 |
|
Radiation pneumonitis | 1 |
Discussion
Metaplasia is an abnormal regeneration that results
from severe chronic tissue injury and repair. Therefore, it is
conceivable that many genetic mutations that are due to DNA damage
may accumulate in metaplastic cells. Metaplasia is a precancerous
condition in certain types of cancers. For example, gastric
adenocarcinoma develops from gastric intestinal metaplasia
(16), and squamous cell carcinoma
develops from squamous cell metaplasia in different organs, such as
the bronchus (17) and esophagus
(18). Recently, we proposed the
theory that bronchiolar metaplasia can be a precancerous condition
for IP-related LADC, as most LADCs develop from metaplasia-lining
honeycomb lesions (4,5). The present study detected a
significant accumulation of some KRAS mutations (G12V, G12C,
and G12A) in metaplastic epithelia. These findings further support
our theory.
Among the types of KRAS mutations,
KRAS G12V and G12C mutations are common in LADCs, in which
guanine is replaced by thymine. These mutations are caused by
benzo[a]pyrene in cigarette smoke (19). On the other hand, KRAS G12A
mutation, in which guanine is replaced by cytosine, is also common
in LADCs; however, their association with smoking is not understood
(19). Interestingly, multivariate
analyses in this study revealed that G12V and G12C VAFs, but not
G12A VAF, were independently related to IP, suggesting that chronic
tissue injury and repair due to IPs could play potential roles in
the fixation and accumulation of smoking-related KRAS G12V
and G12C mutations. The observations are schematically illustrated
in Fig. 9.
A previous study comprehensively analyzed infrequent
somatic mutations by a deep sequencing method that can detect
mutations with frequencies of 0.1-1.0%. The findings demonstrated
that the mutations accumulated even in non-cancerous esophageal
epithelia. Their mutational burden was significantly more prominent
in elderly individuals and heavy drinkers (20). The study also demonstrated a
positive correlation between the mutational burden and risk of
esophageal cancer, suggesting that tissues bearing a large
mutational burden could be precancerous lesions (20). This finding supports our theory
that metaplastic epithelia lining in honeycomb lesions, in which
higher KRAS VAFs were detected, could be a precursor for
IP-related LADC.
In the present study, there was no statistically
significant relationship between KRAS mutations and VAFs,
and the incidence of LADC. However, the number of patients involved
in the study may have been limited. Therefore, future large-scale
studies are needed to determine such potential relationship.
Another confounding result was that the types of
KRAS mutations and higher VAFs, and those who developed
LADCs were not necessarily consistent (Table SI). Similarly, a previous study on
esophageal epithelia failed to identify any specific mutations in
non-cancerous lesions, which could be drivers in related esophageal
cancers (20). Non-cancerous
lesions consist of heterogeneous cells that may have different
random mutations. Only one or few cells transformed into cancerous
cells, and this may explain the observed discrepancies.
In contrast, adenocarcinoma, squamous cell carcinoma
and small cell carcinoma frequently develop in IP, where
honeycombing lower lobes were preferentially affected (21–23).
Similar to bronchiolar metaplasia, abnormal regeneration such as
squamous cell metaplasia (21) and
neuroendocrine cell hyperplasia (24) are thought to be involved in
carcinogenesis. It would be interesting to investigate the
mutational burden of these lesions.
In summary, metaplastic epithelia lining in
honeycomb lesions showed a greater prevalence of KRAS
mutation VAFs, suggesting that metaplasia could be a precursor for
IP-related LADC. In addition, IP can be a potential cause for the
fixation and accumulation of specific types of KRAS
mutations. The observations are intriguing and deepen the
understanding of the IP-related LADC carcinogenesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by Japan Society for the Promotion of
Science KAKENHI (grant no. JP21K15404).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK and KOk wrote most parts of the manuscript. TB,
HA, HK, TI and TO collected patient information and compiled a
clinical database. TK was responsible for the statistical analyses.
KOk and KOh designed the study and suggested the contents of the
manuscript. KOk, MM, CK and TT examined all the tissue sections and
gave pathological diagnoses. TS, HM, MSu and MSe cut tissue
sections and stained them with hematoxylin and eosin. TK performed
microdissection and dd PCR analyses. All authors read and approved
the final manuscript. TK and KOk confirmed the authenticity of all
the raw data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committees of
Yokohama City University (approval no. A200900001) and the Kanagawa
Cardiovascular and Respiratory Center (approval no. KCRC-20-0020).
Written informed consent was obtained from all patients according
to the principles of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KRAS
|
Kirsten rat sarcoma virus
|
|
PCR
|
polymerase chain reaction
|
|
IP
|
interstitial pneumonia
|
|
LADC
|
lung adenocarcinoma
|
|
VAF
|
variant allele frequency
|
References
|
1
|
Yamaguchi M, Hirai S, Tanaka Y, Sumi T,
Miyajima M, Mishina T, Yamada G, Otsuka M, Hasegawa T, Kojima T, et
al: Fibroblastic foci, covered with alveolar epithelia exhibiting
epithelial-mesenchymal transition, destroy alveolar septa by
disrupting blood flow in idiopathic pulmonary fibrosis. Lab Invest.
97:232–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner-Warwick M, Lebowitz M, Burrows B
and Johnson A: Cryptogenic fibrosing alveolitis and lung cancer.
Thorax. 35:496–499. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Brambilla E, Bruke AP, Marx A
and Nicholson AG: Adenocarcinoma. World Health Organization
Classification of Tumors of the Lung, Pleura, Thymus and Heart.
International Agency for Research on Cancer; Lyon, France: pp.
292015, PubMed/NCBI
|
|
4
|
Kojima Y, Okudela K, Matsumura M, Omori T,
Baba T, Sekine A, Woo T, Umeda S, Takemura T, Mitsui H, et al: The
pathological features of idiopathic interstitial
pneumonia-associated pulmonary adenocarcinomas. Histopathology.
70:568–578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okudela K, Kojima Y, Matsumura M, Arai H,
Umeda S, Tateishi Y, Mitsui H, Suzuki T, Tajiri M, Ogura T and
Ohashi K: Relationship between non-TRU lung adenocarcinomas and
bronchiolar metaplasia-potential implication in their histogenesis.
Histol Histopathol. 33:317–326. 2018.PubMed/NCBI
|
|
6
|
Fujita M, Matsubara N, Matsuda I, Maejima
K, Oosawa A, Yamano T, Fujimoto A, Furuta M, Nakano K, Oku-Sasaki
A, et al: Genomic landscape of colitis-associated cancer indicates
the impact of chronic inflammation and its stratification by
mutations in the Wnt signaling. Oncotarget. 9:969–981. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiraly O, Gong G, Olipitz W, Muthupalani S
and Engelward BP: Inflammation-induced cell proliferation
potentiates DNA damage-induced mutations in vivo. PLOS Genet.
11:e10049012015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leslie KO: Idiopathic pulmonary fibrosis
may be a disease of recurrent, tractional injury to the periphery
of the aging lung: A unifying hypothesis regarding etiology and
pathogenesis. Arch Pathol Lab Med. 136:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parsonnet J, Friedman GD, Vandersteen DP,
Chang Y, Vogelman JH, Orentreich N and Sibley RK: Helicobacter
pylori infection and the risk of gastric carcinoma. N Engl J Med.
325:1127–1131. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beasley RP, Hwang LY, Lin CC and Chien CS:
Hepatocellular carcinoma and hepatitis B virus. A prospective study
of 22 707 men in Taiwan. Lancet. 2:1129–1133. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirao S, Sho M, Kanehiro H, Hisanaga M,
Ikeda N, Tsurui H, Nakajima Y and Nakano H: Pancreatic
adenocarcinoma in a patient with Peutz-Jeghers syndrome: Report of
a case and literature review. Hepatogastroenterology. 47:1159–1161.
2000.PubMed/NCBI
|
|
12
|
Ekbom A, Helmick C, Zack M and Adami HO:
Increased risk of large-bowel cancer in Crohn's disease with
colonic involvement. Lancet. 336:357–359. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farrow B and Evers BM: Inflammation and
the development of pancreatic cancer. Surg Oncol. 10:153–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chui MH, Xing D, Zeppernick F, Wang ZQ,
Hannibal CG, Frederiksen K, Kjaer SK, Cope L, Kurman RJ, Shih IM,
et al: Clinicopathologic and molecular features of paired cases of
metachronous ovarian serous borderline tumor and subsequent serous
carcinoma. Am J Surg Pathol. 43:1462–1472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Correa P and Shiao YH: Phenotypic and
genotypic events in gastric carcinogenesis. Cancer Res. 54 (Suppl
7):S1941–S1943. 1994.
|
|
17
|
Meyer EC and Liebow AA: Relationship of
interstitial pneumonia honeycombing and atypical epithelial
proliferation to cancer of the lung. Cancer. 18:322–351. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singhi AD, Arnold CA, Crowder CD,
Lam-Himlin DM, Voltaggio L and Montgomery EA: Esophageal
leukoplakia or epidermoid metaplasia: A clinicopathological study
of 18 patients. Mod Pathol. 27:38–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tretyakova N, Matter B, Jones R and
Shallop A: Formation of benzo[a]pyrene diol epoxide-DNA adducts at
specific guanines within K-ras and p53 gene sequences: Stable
isotope-labeling mass spectrometry approach. Biochemistry.
41:9535–9544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokoyama A, Kakiuchi N, Yoshizato T,
Nannya Y, Suzuki H, Takeuchi Y, Shiozawa Y, Sato Y, Aoki K, Kim SK,
et al: Age-related remodeling of oesophageal epithelia by mutated
cancer drivers. Nature. 565:312–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hironaka M and Fukayama M: Pulmonary
fibrosis and lung carcinoma: A comparative study of metaplastic
epithelia in honeycombed areas of usual interstitial pneumonia with
or without lung carcinoma. Pathol Int. 49:1060–1066. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wistuba II, Berry J, Behrens C, Maitra A,
Shivapurkar N, Milchgrub S, Mackay B, Minna JD and Gazdar AF:
Molecular changes in the bronchial epithelium of patients with
small cell lung cancer. Clin Cancer Res. 6:2604–2610.
2000.PubMed/NCBI
|
|
23
|
Vancheri C, Failla M, Crimi N and Raghu G:
Idiopathic pulmonary fibrosis: A disease with similarities and
links to cancer biology. Eur Respir J. 35:496–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shyu S, Heath JE and Burke AP:
Neuroendocrine cell proliferations in lungs explanted for fibrotic
interstitial lung disease and emphysema. Pathology. 50:699–702.
2018. View Article : Google Scholar : PubMed/NCBI
|