Introduction
Endometrial cancer (EC) is the most common
gynecological malignancy worldwide. In 2021, 66,570 new cases were
diagnosed in the USA, resulting in 12,940 disease-related deaths
(1). The primary standard
treatment for localized EC is surgery, followed by adjuvant therapy
for high-risk or advanced EC, including chemotherapy and
brachytherapy. The lymph nodes are the first site of extra-uterine
spread in patients with EC; thus, the lymph node status is regarded
as a critical prognostic factor (2). As previously demonstrated, the rate
of pelvic lymph node metastasis (LNM) is 11.5% and the rate of
isolated para-aortic LNM is 1.28% (3); LNM is associated with the tumor
grade, as this has been shown to be 20% in high-grade disease, and
14% in low-grade disease (4).
The use of lymphadenectomy is controversial as it
does not improve the long-term outcomes, such as recurrence-free
survival and overall survival, whereas it increases morbidity and
leads to more severe peri-operative outcomes (5). There is evidence to indicate that a
lymphadenectomy even increases the risk of developing 30-day
complications (6) and the 90-day
risk of venous thromboembolism (7). Of note, lymphadenectomy is not
recommended in patients with low-grade or low-risk EC (8).
Sentinel lymph node mapping (SLNM) is recommended in
EC with uterine-confined malignancy (9). SLNM under ultra-staging increases the
detection of LNM with low false-negative rates. A previous
meta-analysis demonstrated that the sensitivity of SLNM was 96%
(95% CI, 92–98%) for the detection of lymphatic metastases
(10). However, the success rate
of SLNM is affected by several factors, such as the dye tracer, the
injection site and the body mass index of the patient, as adipose
tissues shield the colorimetric signal (11). Moreover, 5% of patients with EC
suffer from para-aortic LNM with a negative pelvic lymph node
status (12), and SLN
ultra-staging is unavailable during surgery; thus, additional
systematic lymphadenectomy and adjuvant therapy are taken into
account for such patients. It is evident that a novel method with
an increased the accuracy for SLNM detection to determine the lymph
node status in patients with EC is required.
Circulating microRNAs (miRNAs/miRs) are considered
stable miRNAs in the serum/plasma. These miRNAs represent potential
biomarkers for evaluating cancer, and a number of circulating
miRNAs indicative of breast cancer have been identified (13). However, there are only a limited
number of studies reporting the utility of potential serum miRNAs
in gynecologic cancer. The authors previously uncovered a
regulatory loop involving TrkB/miR-204-5p that is critical in the
tumorigenesis of EC (14), and
miR-204-5p expression is associated with LNM in patients with EC.
These observations led us to hypothesize that serum miR-204-5p in
patients with EC may have potential for use as an early diagnostic
biomarker combined with SLNM.
Patients and methods
Patients and clinical sample
collection
A total of 52 patients with EC who underwent
hysterectomy with lymph node dissection (sentinel lymph node
mapping first) at the Shanghai General Hospital Affiliated with
Shanghai Jiao Tong University from October, 2018 to November, 2020
were included in the study (Table
I). The stages and histological grades of these tumors were
determined according to the criteria of the Federation
International of Gynecology and Obstetrics (FIGO) surgical staging
system (2009) (15).
 | Table I.Association between serum miR-204-5p
expression and the different clinicopathological features of the
endometrial cancer samples. |
Table I.
Association between serum miR-204-5p
expression and the different clinicopathological features of the
endometrial cancer samples.
|
| Clinical serum
sample |
|---|
|
|
|
|---|
| Variable | n | miR-204-5p
expression | P-value |
|---|
| Total | 52 |
|
|
| Age (years) |
|
|
|
| ≤50 | 11 | 2.53±2.38 | 1.86 |
|
>50 | 41 | 2.67±2.24 |
|
| FIGO stage |
|
|
|
| Stage
I | 37 | 2.34±2.24 | 0.42 |
| Stage
II | 9 | 2.05±1.96 |
|
| Stage
III | 6 | 1.82±1.78 |
|
| Grade
(endometrioid) |
|
|
|
| G1 | 27 | 2.45±2.28 | 0.44 |
| G2 | 18 | 2.12±2.05 |
|
| G3 | 7 | 1.88±1.82 |
|
| Myometrial
invasion |
|
|
|
|
<1/2 | 40 | 2.48±2.32 | 0.12 |
|
≥1/2 | 12 | 1.96±1.85 |
|
| Lymph node
metastasis |
|
|
|
|
Negative | 47 | 2.57±2.36 | <0.001 |
|
Positive | 5 | 0.17±0.03 |
|
All patients with EC underwent laparoscopic staging
surgery with the near-infra-red NOVADAQ Endoscopy system (Stryker)
or robotic (da Vinci® Xi; Intuitive Surgery) staging
surgery with Firefly®. All patients underwent SLNM with
ICG fluorescence detection using NIR/ICG® and
Firefly®, after which bilateral pelvic lymphadenectomy
was performed. In accordance with the National Comprehensive Cancer
Network guidelines, a total of 4 ml of 1.25 mg/ml ICG solution (25
mg ICG mixed with 20 ml distilled water) was injected superficially
(1–3 mm) and deep (1 cm) into the cervix at the 3 o'clock and 9
o'clock positions (1 ml each). At 10–20 min after the injection of
ICG solution, the opening of the retroperitoneum in the left and
right pelvic and paraaortic areas and the development of the
paravesical and pararectal spaces were performed. Fluorescent
uptake was observed through an NIR laparoscopic camera; the first
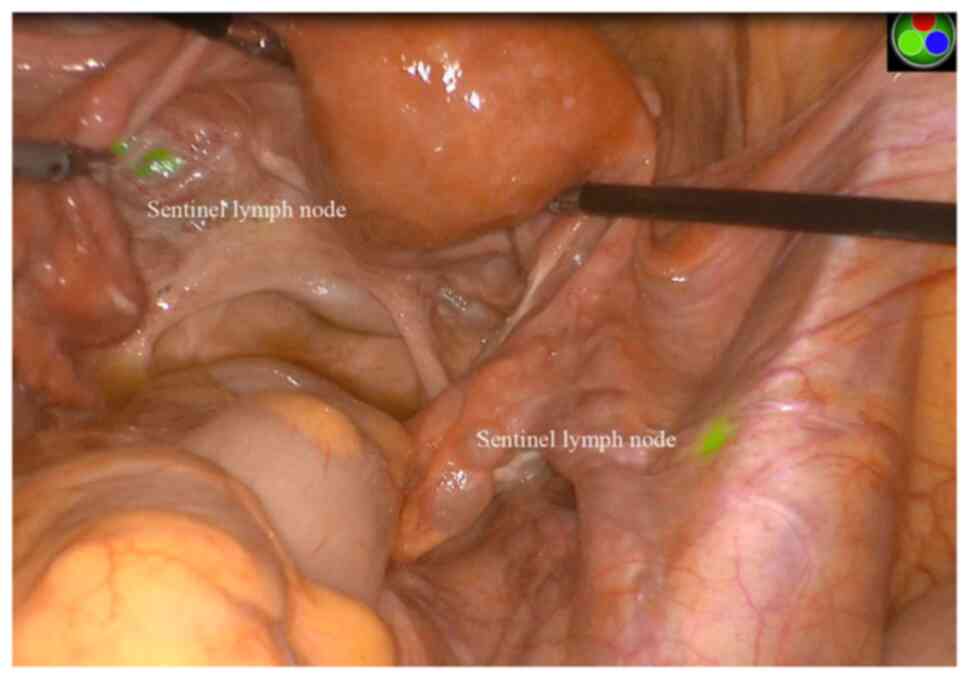
twinkling lymph node was defined as an SLN (Fig. 1). Finally, a conventional
laparoscopic- or robotic-assisted vaginal hysterectomy or
robotic-assisted hysterectomy was performed after sending the
frozen section of the SLN for pathological evaluation.
In addition, 20 serum samples each were obtained
from patients diagnosed with ovarian cysts, 20 patients with myoma,
and 20 serum samples were obtained from patients diagnosed with
endometrial polyps or endometrial hyperplasia. None of the patients
had received hormone therapy, radiotherapy, or chemotherapy prior
to surgery. The resected specimens from patients with EC (4-µm
thick) were stained with hematoxylin and eosin (H&E) at room
temperature (RT) (cat. no. ab245880; Abcam) and in situ
hybridization (ISH) was performed for a histological
examination.
Blood samples were collected from patients with EC
and benign diseases early in the morning. Up to 5 ml fasting venous
blood was collected in a serum separator tube from each
participant. All blood samples were centrifuged at 2,800 × g for 10
min RT within half an hour after collection. The separated
supernatant was then stored in 1.5 ml tubes at −80°C until further
use. The present study was approved by the Ethics Committee of
Shanghai General Hospital (Shanghai, China), and informed consent
was written and obtained from all included patients (no.
2018SQ307-1).
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
As previously described (14), total RNA was extracted from the
serum samples using a miRVana PARIS kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocols. RNA purity and concentration were determined using a
NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). For the
miRNA analysis, a TaqMan microRNA Reverse Transcription kit was
used to reverse transcribe mature miRNA from total RNA. According
to the manufacturer's instructions, qPCR was performed using TaqMan
MicroRNA Assay primers with TaqMan Universal PCR Master Mix and
analyzed with an ABI Prism 7000 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). U6 was used as an
internal reference for the expression of miRNAs (Table II). All reagents were purchased
from Applied Biosystems; Thermo Fisher Scientific, Inc. For all the
experiments, values on the y-axis were equal 2(−ΔCt),
where ΔCt is the difference between gene Ct and normalizer gene Ct.
The data were obtained in triplicate in three independent
experiments.
 | Table II.Primers used for reverse
transcription-quantitative PCR analysis. |
Table II.
Primers used for reverse
transcription-quantitative PCR analysis.
| miRNA | Primer
sequence |
|---|
| miR-204-5p | Forward:
5′-CCCATCGTTAAGCAATGCAT |
|
| GAC-3′ |
|
| Reverse:
5′-GAGGGCCTCCTGATCATTT |
|
| ACC-3′ |
| U6 | Forward:
5′-AGAGCCTGTGGTGTCCG-3′ |
|
| Reverse:
5′-CATCTTCAAAGCACTTCCCT-3′ |
ISH and scoring
As previously described (16), the expression of miRNAs in
paraffin-embedded tissue specimens was determined using an in
situ hybridization kit (MK1030, Wuhan Boster Biological
Technology, Ltd.). Briefly, 6-µm-thick sections of
paraffin-embedded specimens were deparaffinized with xylene and
rehydrated in a series of ethanol. Following proteinase-K
incubation for 15 min at 37°C, the slides were prehybridized in a
hybridization solution at 37°C for 2 h. The tissue sections were
then hybridized with 5′-digoxigenin-labeled (DIG-labeled)
oligonucleotide probe at 37°C overnight. Following stringent washes
with 5X SSC, 1X SSC and 0.2X SSC buffers (cat. no. 11666681001,
Roche), the sections were blocked with DIG blocking buffer at 37°C
for 30 min. An anti-DIG antibody (1:2,000; cat. no. 76907, Abcam)
was applied, and the sections were incubated at 37°C for 1 h. After
washing in a staining solution, the sections were developed by
diaminobenzidine-hydrogen peroxide. Scoring was measured by the
cell cytoplasm staining. The sections were evaluated based on the
percentage of positively stained cells (0–3) and the intensity of
staining (0–3). The score of miRNA expression was then calculated
as a percentage × intensity of the staining. Therefore, score 0
presents negative (−) staining, 1–2 weak positive (+), 3–4
moderately positive (++), and 6–9 strong positive (+++) staining.
Scoring with ‘-’ and ‘+’ was regarded as a lower miR-204-5p
expression, whereas ‘++'and ‘+++’ represented a higher expression
of miR-204-5p.
Statistical analyses
Each experiment was performed at least three times.
Differences between two groups were assessed using the Mann-Whitney
U test, and multiple comparisons between more than two groups were
conducted using the Kruskal-Wallis test (Bonferroni's test). Data
are presented as the mean ± SD. The area under the receiver
operating characteristic curve (AUC) was calculated to assess the
value of serum miR-204-5p in the diagnosis of LNM, and the
sensitivity and specificity were calculated using discriminant
analysis. All P-values are two-sided, and P<0.05 was considered
to indicate a statistically significant difference. All statistical
analyses were performed using SPSS 16.0 software (SPPS, Inc.).
Results
SLNM
After the ICG injection, fluorescent uptake was
observed through an NIR laparoscopic camera; the first twinkling
lymph node was defined as an SLN (Fig.
1). Finally, a conventional laparoscopic- or robotic-assisted
vaginal hysterectomy or robotic-assisted hysterectomy was performed
after sending the frozen section of the SLN for pathological
evaluation.
Downregulation of serum miR-204-5p in
patients with EC and its diagnostic value
To determine the feasibility of miR-204-5p detection
in serum, RT-qPCR was used to detect the levels of serum miR-204-5p
in 52 patients with EC (total SLNM), 20 patients diagnosed with
benign ovarian cysts, 20 patients with myoma, and 20 participants
diagnosed with endometrial polyps or endometrial hyperplasia. The
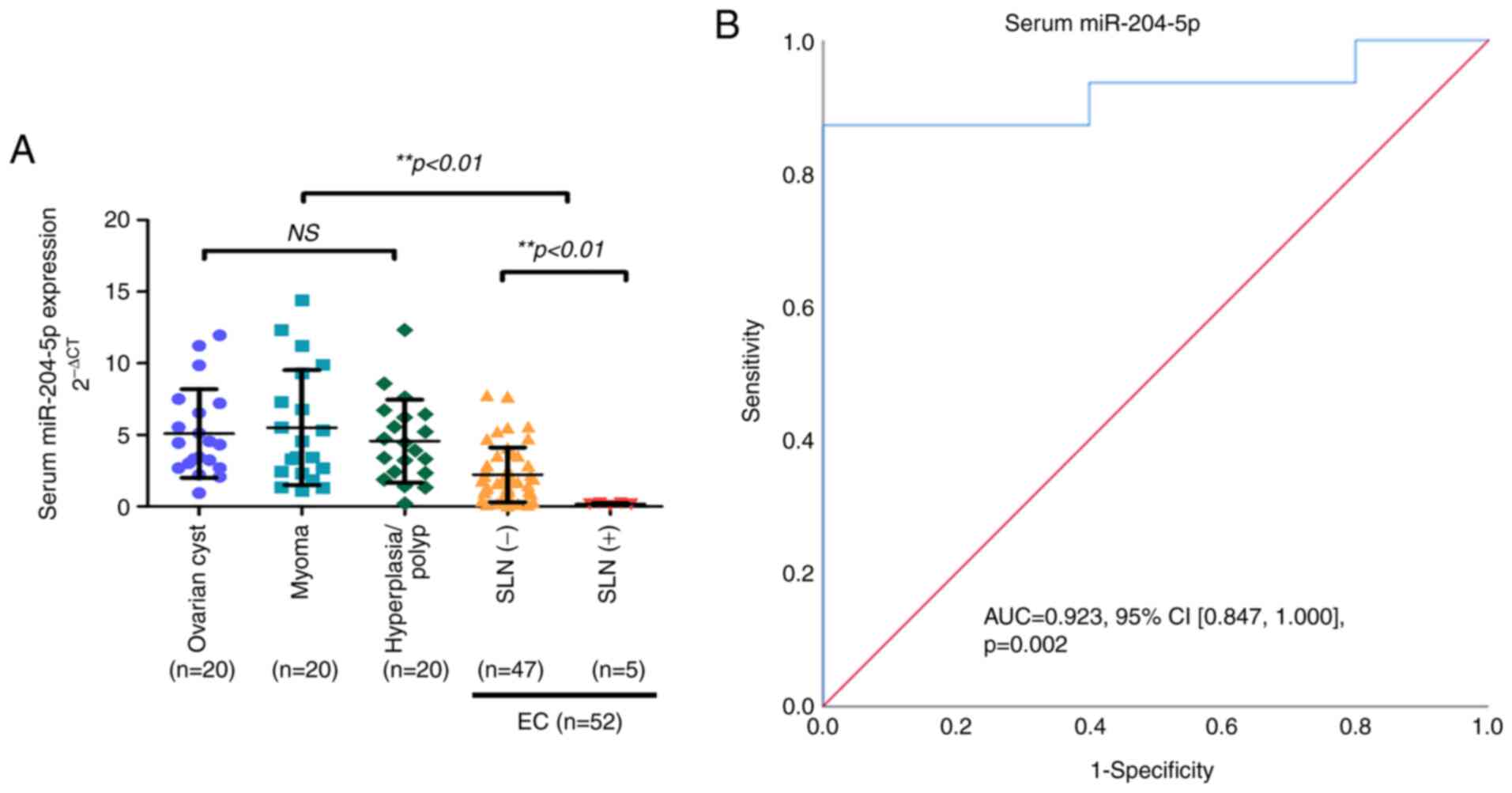
results revealed that serum miR-204-5p expression was evidently
lower in patients with EC than that in the control group patients
(P<0.01; Fig. 2A).
To further examine the clinical implications of
serum miR-204-5p in EC, the association between the serum
miR-204-5p expression levels and the clinicopathological
characteristics of patients with EC was assessed. No statistically
significant associations were found as regards age, FIGO stage,
grade and myometrial invasion (P>0.05; Table I). However, a statistically
significant association was observed between serum miR-204-5p
expression and LNM (P<0.01; Table
I). It was found that the serum miR-204-5p expression was
strongly associated with a positive SLN status, with a sensitivity
of 87.2% and a specificity of 80.0% (AUC, 0.923; 95% CI,
0.847-1.000; P=0.002) (Fig. 2B),
with an optimal cut-off value of 0.253. These results indicate that
the downregulation of serum miR-204-5p in patients with EC has
potential for use as an early diagnostic biomarker combined with
SLNM.
A low miR-204-5p expression is
associated with LNM in patients with SLN(+) EC
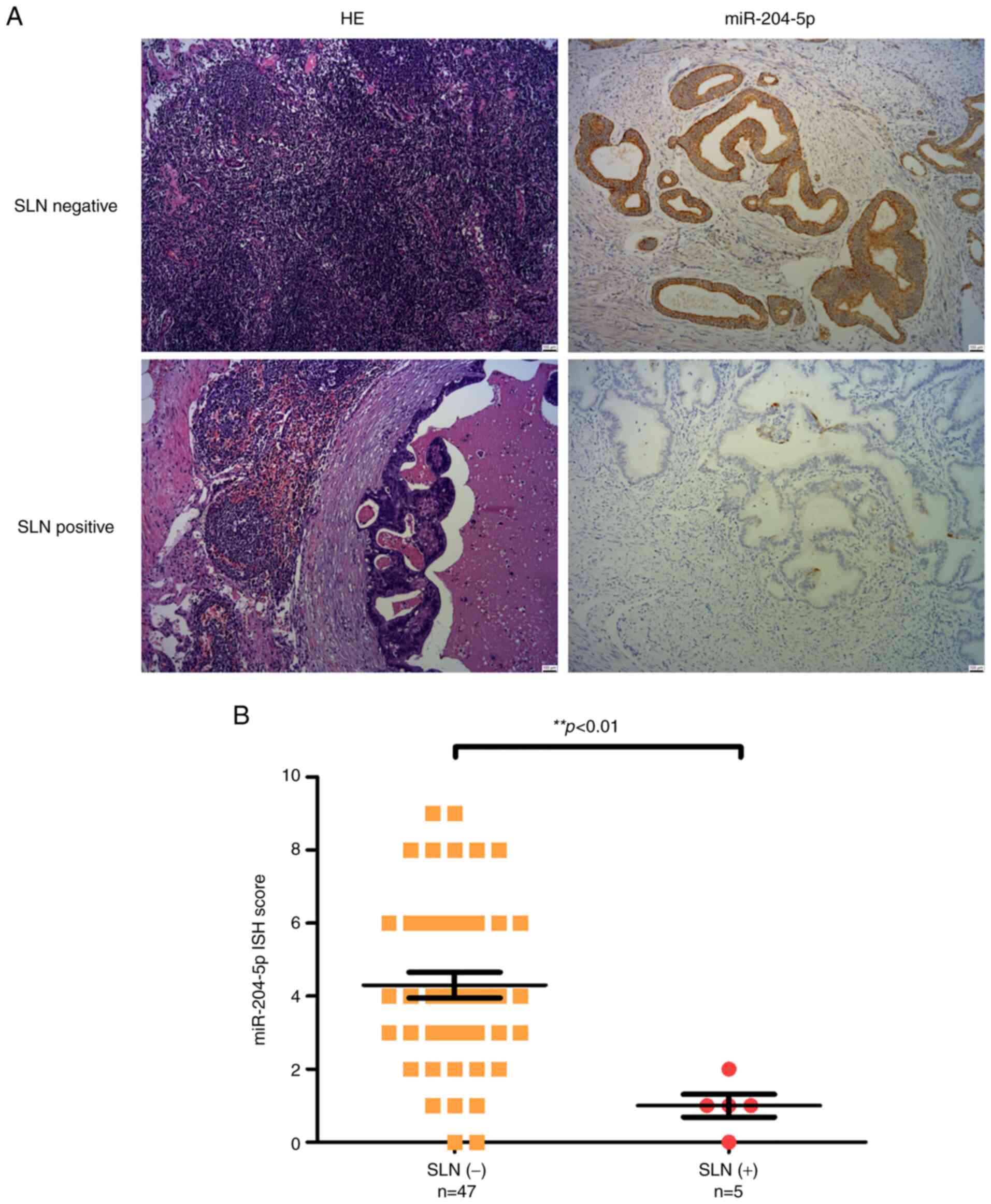
Standard H&E staining of lymph nodes was also
used to reveal metastasis in the frozen sections. The expression of
miR-204-5p in SLN tissue resected from patients with EC was
examined using ISH. In accordance with the serum miR-204-5p level,
it was demonstrated that a lower miR-204-5p expression was
associated with LNM in these SLN(+) EC tissues (Fig. 3A), and the scores of the expression
of miR-204-5p in SLN(+) by ISH were markedly lower than in the
SLN(−) group (P<0.01; Fig.
3B).
Discussion
Sentinel lymph node mapping (SLNM) was first
reported in a lymphangiogram of penile carcinoma in 1977 (17). Currently, SLNM has been applied as
an alternative to lymphadenectomy in numerous malignancies,
including breast cancer and vulvar cancer, and was first applied in
EC in 1996 (18). A tracer is
injected into the corpus uterus or cervix, which flows along the
lymphatic channels, and accumulates in the first station, termed
the SLN, recognized as the first site of extra-uterus metastases.
This technology has certain advantages for treatment, with similar
survival rates and fewer surgical complications compared to
systematic lymphonodectomy. However, certain drawbacks should not
be ignored. For example, as regards the accuracy of intraoperative
frozen sections, researchers have found that 46.58-76.92%
SLN-positive cervical cancer patients were not identified (19,20).
On the one hand, the rate of successful mapping ranges from 23 to
100%, and the difference may be influenced by the experience of
surgeons (10). On the other hand,
as previously demonstrated, there are 1.28% patients with EC
suffering from para-aortic area metastases with negative pelvic
nodes (3). Additionally, for the
tracer selection, the signal of blue dyes is influenced by adipose
tissue, while technetium 99 and tricarbocyanine dye require complex
imaging equipment, and the technologies are difficult to be fully
implemented (21).
Early in 2014, researchers used CK19 mRNA at 250
copies from lymph node tissue during the surgery to predict the
metastases with a sensitivity of 82.4% and a specificity of 99.2%;
however, this method was also based on the dissection of lymph
nodes during the surgery (22).
miRNAs as non-protein coding RNAs are associated with
post-transcriptional regulation, and play roles in a number of
cellular processes, and can induce cancer development. The
expression of miRNAs is tissue-specific; miR-204 has been found to
be highly expressed in renal, eye and pulmonary tissue (23,24),
and promotes pulmonary artery smooth muscle cell proliferation and
resistance to apoptosis by activating the Src/STAT3/NFAT pathway,
leading to the development of pulmonary arterial hypertension
(25). Another study found that
miR-204-5p exhibited a higher expression in mouse mammary glands
than other tissues, stimulating milk lipid secretion by targeting
sirtuin 1 (26). Previous studies
have also demonstrated that miR-204 regulates adipogenesis by
inhibiting the activation of the Wnt/β-catenin signaling pathway
and reducing insulin production by downregulating the insulin
transcription factor, MafA (27,28).
Additionally, miR-204 has been found to be abnormally expressed in
various types of cancer. Zanette et al (29) found that miR-204 was overexpressed
in acute lymphocytic leukemia. However, the level of miR-204 has
been shown to be significantly lower in cancer than in normal
tissue, and to be negatively associated with LNM in gastric and
bladder cancer (30,31). Moreover, there is recent evidence
to indicate that the serum levels of miR-204-5p in patients with
gastric cancer are lower than those in patients with benign
lesions, and indicate that miR-204-5p targets CXCR4 and CXCL12 to
suppress the LNM (32). In
accordance with these findings, the present study demonstrated that
a lower miR-204-5p expression was associated with LNM in these
SLN(+) EC tissues. Previous studies have also revealed the
effective diagnostic value of miRNAs in cancers. Shimomura et
al (33) demonstrated that the
pre-operative combination of serum miR-1246, miR-1307-3p, miR-4634,
miR-6861-5p and miR-6875-5p detected early-stage breast cancer with
a sensitivity of 97.3% and a specificity of 82.9%. The serum levels
of miR-135a have also been shown to distinguish non-small cell lung
cancer tissue from healthy tissue with a specificity and
sensitivity of 83.1 and 81.3%, respectively (34). Another study demonstrated that
miR-204-5p was a potential biomarker for the prediction of the
prevalence of frontotemporal dementia, and the area under the ROC
curve of miR-204-5p was 0.89 (90% CI, 0.79-0.98) (35). The present study found the serum
miR-204-5p level was a more convenient biomarker pre-operation to
predict the status of LNM combined with SLNM in the treatment of
patients with EC, with a sensitivity of 87.2% and a specificity of
80.0% (AUC, 0.923; 95% CI, 0.847-1.000); P=0.002), and the optimal
cut-off value was 0.253. Therefore, serum miR-204-5p levels may be
an efficient biomarker for detecting the status of LNM
pre-operation in patients with EC, with minimal costs.
There were some limitations to the present study.
Firstly, the sample size of the present study was small, and the
number of SLN-positive cases was only five. Secondly, the present
study did not examine the prognostic value of serum miR-204-5p in
patients with EC. Thus, further research is required with larger
sample sizes, and to explore the prognostic value of miR-204-5p in
patients with EC.
In conclusion, the present study demonstrated that
serum miR-204-5p levels were lower in SLN-positive than in
SLN-negative cases, and serum miR-204-5p may be an efficient
biomarker for predicting LNM pre-operation with an AUC of 0.923,
and an optimal cut-off value of 0.253. Thus, serum miR-204-5p
levels may prove to be useful for clinical decision-making for
lymphadenectomy or SLNM in patients with EC.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National Natural Science
Foundation of China (grant no. 81972425), the National Natural
Science Foundation of Shanghai (grant no. 20ZR1444200) and the
project Young Elite of the Shanghai Health System (grant no.
2017YQ063).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, YZ and WB conceived and designed the
experiments. CW, XZ, JL, RX, LD and HX performed the experiments,
and LD and HX analyzed the data. CW, XZ and JL wrote the
manuscript. YZ and WB confirm the authenticity of all the raw data.
All authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai General Hospital (Shanghai, China) on Oct,
2018, and informed consent was obtained from all included patients
(no. 2018SQ307-1); if the participant was <16 years of age, the
informed consent was obtained from the parents. The study was
performed in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buldukoglu OC, Turker A, Usubutun A and
Salman MC: Relationship of lymph node status with survival and
recurrence among women with endometrial cancer. Int J Gynaecol
Obstet. 151:267–271. 2020. View Article : Google Scholar
|
|
3
|
Li Y, Cong P, Wang P, Peng C, Liu M and
Sun G: Risk factors for pelvic lymph node metastasis in endometrial
cancer. Arch Gynecol Obstet. 300:1007–1013. 2019. View Article : Google Scholar
|
|
4
|
Suchetha S, Mathew AP, Rema P and Thomas
S: Pattern of lymph node metastasis in endometrial cancer: A single
institution experience. Indian J Surg Oncol. 12:73–77. 2021.
View Article : Google Scholar
|
|
5
|
Frost JA, Webster KE, Bryant A and
Morrison J: Lymphadenectomy for the management of endometrial
cancer. Cochrane Database Syst Rev. 10:CD0075852017.PubMed/NCBI
|
|
6
|
Accorsi GS, Paiva LL, Schmidt R, Vieira M,
Reis R and Andrade C: Sentinel lymph node mapping vs systematic
lymphadenectomy for endometrial cancer: Surgical morbidity and
lymphatic complications. J Minim Invasive Gynecol. 27:938–945.e2.
2020. View Article : Google Scholar
|
|
7
|
Latif N, Oh J, Brensinger C, Morgan M, Lin
LL, Cory L and Ko EM: Lymphadenectomy is associated with an
increased risk of postoperative venous thromboembolism in early
stage endometrial cancer. Gynecol Oncol. 161:130–134. 2021.
View Article : Google Scholar
|
|
8
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar
|
|
9
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, et al:
Uterine neoplasms, version 1.2018, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 16:170–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith AJB, Fader AN and Tanner EJ:
Sentinel lymph node assessment in endometrial cancer: A systematic
review and meta-analysis. Am J Obstet Gynecol. 216:459–476.e10.
2017. View Article : Google Scholar
|
|
11
|
Tanner EJ, Sinno AK, Stone RL, Levinson
KL, Long KC and Fader AN: Factors associated with successful
bilateral sentinel lymph node mapping in endometrial cancer.
Gynecol Oncol. 138:542–547. 2015. View Article : Google Scholar
|
|
12
|
Kataoka F, Susumu N, Yamagami W, Kuwahata
M, Takigawa A, Nomura H, Takeuchi H, Nakahara T, Kameyama K and
Aoki D: The importance of para-aortic lymph nodes in sentinel lymph
node mapping for endometrial cancer by using hysteroscopic
radio-isotope tracer injection combined with subserosal dye
injection: Prospective study. Gynecol Oncol. 140:400–404. 2016.
View Article : Google Scholar
|
|
13
|
Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C,
Wang X, Luo Z, Wang J, Liu S, et al: MicroRNA-21 promotes breast
cancer proliferation and metastasis by targeting LZTFL1. BMC
Cancer. 19:7382019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao W, Wang HH, Tian FJ, He XY, Qiu MT,
Wang JY, Zhang HJ, Wang LH and Wan XP: A TrkB-STAT3-miR-204-5p
regulatory circuitry controls proliferation and invasion of
endometrial carcinoma cells. Mol Cancer. 12:1552013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar
|
|
16
|
Wang Y, Bao W, Liu Y, Wang S, Xu S, Li X,
Li Y and Wu S: miR-98-5p contributes to cisplatin resistance in
epithelial ovarian cancer by suppressing miR-152 biogenesis via
targeting Dicer1. Cell Death Dis. 9:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cabanas RM: An approach for the treatment
of penile carcinoma. Cancer. 39:456–466. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burke TW, Levenback C, Tornos C, Morris M,
Wharton JT and Gershenson DM: Intraabdominal lymphatic mapping to
direct selective pelvic and paraaortic lymphadenectomy in women
with high-risk endometrial cancer: Results of a pilot study.
Gynecol Oncol. 62:169–173. 1996. View Article : Google Scholar
|
|
19
|
Slama J, Dundr P, Dusek L and Cibula D:
High false negative rate of frozen section examination of sentinel
lymph nodes in patients with cervical cancer. Gynecol Oncol.
129:384–388. 2013. View Article : Google Scholar
|
|
20
|
Sonoda K, Yahata H, Okugawa K, Kaneki E,
Ohgami T, Yasunaga M, Baba S, Oda Y, Honda H and Kato K: Value of
intraoperative cytological and pathological sentinel lymph node
diagnosis in fertility-sparing trachelectomy for early-stage
cervical cancer. Oncology. 94:92–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holloway RW, Ahmad S, Kendrick JE, Bigsby
GE, Brudie LA, Ghurani GB, Stavitzski NM, Gise JL, Ingersoll SB and
Pepe JW: A prospective cohort study comparing colorimetric and
fluorescent imaging for sentinel lymph node mapping in endometrial
cancer. Ann Surg Oncol. 24:1972–1979. 2017. View Article : Google Scholar
|
|
22
|
Nagai T, Niikura H, Okamoto S, Nakabayashi
K, Matoda M, Utsunomiya H, Nagase S, Watanabe M, Takeshima N and
Yaegashi N: A new diagnostic method for rapid detection of lymph
node metastases using a one-step nucleic acid amplification (OSNA)
assay in endometrial cancer. Ann Surg Oncol. 22:980–986. 2015.
View Article : Google Scholar
|
|
23
|
Liu T, Zou XZ, Huang N, Ge XY, Yao MZ, Liu
H, Zhang Z and Hu CP: Down-regulation of miR-204 attenuates
endothelial-mesenchymal transition by enhancing autophagy in
hypoxia-induced pulmonary hypertension. Eur J Pharmacol.
863:1726732019. View Article : Google Scholar
|
|
24
|
Du F, Zhang Y, Xu Q, Teng Y, Tao M, Chen
AF and Jiang R: Preeclampsia serum increases CAV1 expression and
cell permeability of human renal glomerular endothelial cells via
down-regulating miR-199a-5p, miR-199b-5p, miR-204. Placenta.
99:141–151. 2020. View Article : Google Scholar
|
|
25
|
Courboulin A, Paulin R, Giguère NJ,
Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher
S, Côté J, et al: Role for miR-204 in human pulmonary arterial
hypertension. J Exp Med. 208:534–548. 2011. View Article : Google Scholar
|
|
26
|
Zhang M, Cao M, Kong L, Liu J, Wang Y,
Song C, Chen X, Lai M, Fang X, Chen H and Zhang C: miR-204-5p
promotes lipid synthesis in mammary epithelial cells by targeting
SIRT1. Biochem Biophys Res Commun. 533:1490–1496. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He H, Chen K, Wang F, Zhao L, Wan X, Wang
L and Mo Z: miR-204-5p promotes the adipogenic differentiation of
human adipose-derived mesenchymal stem cells by modulating DVL3
expression and suppressing Wnt/β-catenin signaling. Int J Mol Med.
35:1587–1595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu G, Chen J, Jing G and Shalev A:
Thioredoxin-interacting protein regulates insulin transcription
through microRNA-204. Nat Med. 19:1141–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zanette DL, Rivadavia F, Molfetta GA,
Barbuzano FG, Proto-Siqueira R, Silva WA Jr, Falcao RP and Zago MA:
miRNA expression profiles in chronic lymphocytic and acute
lymphocytic leukemia. Braz J Med Biol Res. 40:1435–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan X, Wang S, Liu M, Lu Z, Zhan Y, Wang
W and Xu AM: Histological and pathological assessment of miR-204
and SOX4 levels in gastric cancer patients. Biomed Res Int.
2017:68946752017. View Article : Google Scholar
|
|
31
|
Li Y, Chen R, Li Z, Cheng H, Li X, Li T
and Zhu C: MiR-204 negatively regulates cell growth and metastasis
by targeting ROBO4 in human bladder cancer. Onco Targets Ther.
12:8515–8524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Xing L, Xu H, Wang K, She J, Shi
F, Wu H, Sun Y, Gao J and He S: miR-204-5p suppress lymph node
metastasis via regulating CXCL12 and CXCR4 in gastric cancer. J
Cancer. 11:3199–3206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimomura A, Shiino S, Kawauchi J,
Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S,
Shimizu C, et al: Novel combination of serum microRNA for detecting
breast cancer in the early stage. Cancer Sci. 107:326–334. 2016.
View Article : Google Scholar
|
|
34
|
Zou Y, Jing C, Liu L and Wang T: Serum
microRNA-135a as a diagnostic biomarker in non-small cell lung
cancer. Medicine (Baltimore). 98:e178142019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schneider R, McKeever P, Kim T, Graff C,
van Swieten JC, Karydas A, Boxer A, Rosen H, Miller BL, Laforce R
Jr, et al: Downregulation of exosomal miR-204-5p and miR-632 as a
biomarker for FTD: A GENFI study. J Neurol Neurosurg Psychiatry.
89:851–858. 2018. View Article : Google Scholar : PubMed/NCBI
|