Introduction
Ovarian cancer (OC) is one of the most common
malignancies in the female reproductive system and the leading
cause of cancer-associated mortality worldwide (1). In 2019, it was estimated that there
were ~22,530 new cases and 13,980 moralities in the United States
(2). Despite improvements in the
median survival rates following application of the first-line
therapy, including cytoreductive surgery and combined chemotherapy
or radiotherapy (3,4), the 5-year survival rate of patients
with OC remains low (<40%) in most women diagnosed at advanced
stages (5). Thus, it is important
to understand the molecular pathogenesis of OC for early diagnosis
and to develop innovative therapies.
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNA molecules (18–25 nucleotides in length) that
participate in several biological processes by negatively
regulating gene expression at the post-transcriptional level by
binding to the 3′-untranslated regions (UTRs) of mRNAs (6–8).
Recent studies have reported that aberrant expression of miRNAs is
closely associated with the progression of OC (9,10).
Functionally, miR-665 (11)
promotes, while miR-506-3p (12)
suppresses the proliferation of OC cells by targeting SRC kinase
signaling inhibitor 1 and myotubularin-related protein 6,
respectively. Notably, aberrantly expressed miRNAs have been
reported to act as oncogenes or tumor suppressors (13,14).
Among these, miR-409-5p has recently been demonstrated to play a
key role in the following tumor cells: miR-409 targets
pro-metastatic gene radixin to suppress gastric cancer cell
invasion and metastasis (15),
while miR-409-5p is upregulated and promotes cancer development in
prostate (16) and breast
(17) cancer cells. Notably,
bioinformatics analyses have indicated that miR-409-5p is
downregulated in OC samples (18,19).
However, the functional role of miR-409-5p in OC in vitro
has not yet been investigated.
Discs large-associated protein 5 (DLGAP5, also known
as HURP or KIAA0008), is a member of the DLGAP family, which plays
an important role in spindle assembly (20). Recently, DLGAP5 has been suggested
to be associated with poor prognosis in non-small cell lung cancer
(21,22), anaplastic thyroid carcinoma
(23), glioblastoma (24) and pancreatic carcinoma (25). In vitro analyses have
demonstrated that DLGAP5 knockdown significantly decreases the
migratory and invasive abilities of colorectal cancer cells
(26), as well as the
proliferative ability of hepatocellular carcinoma cells (27,28).
Similarly, DLGAP5 was downregulated by NUSAP1 gene silencing, which
is associated with cell cycle G2/M phase arrest and
inhibition of MCF-7 cell proliferation (29). Based on bioinformatics analysis, it
was hypothesized that miR-409-5p can regulate DLGAP5 expression.
However, the association between miR-409-5p and DLGAP5 in the
progression of OC remains unclear.
The present study aimed to investigate miR-409-5p
expression and determine its association with the
clinicopathological characteristics of patients with OC. In
addition, the role of miR-409-5p on OC cell functions was
investigated, including proliferation, cell cycle progression and
apoptosis. Furthermore, the functional mechanism of miR-409-5p in
regulating the proliferation of OC cells was determined.
Materials and methods
Collection of tissue samples
A total of 39 paired OC tissues and adjacent normal
tissues (at least 4 cm from the tumor edge) were collected from
patients (age range, 25–68 years; mean age, 45.6±6.5 years) via
resection at Mindong Hospital Affiliated to Fujian Medical
University (Fujian, China) between October 2017 and September 2018.
Tissues samples were immediately snap-frozen and stored at −80°C
until subsequent experimentation. Patient characteristics,
including age, tumor size and histological grade are listed in
Table I. All patients were staged
according to the International Federation of Gynecology and
Obstetrics (FIGO) staging system (30) and graded based on the histological
grade (31). The exclusion
criteria included patients which had received chemotherapy,
radiotherapy, hormone therapy or other antitumor therapies. The
inclusion criteria are patients diagnosed with NSCLC and confirmed
not to receive any antitumor therapies. The present study was
approved by the Institutional Ethics Committee of Mindong Hospital
Affiliated to Fujian Medical University (Fujian, China; approval
no. MHFM-39A) and performed in accordance with the Declaration of
Helsinki. Written informed consent was provided by all patients
prior to the study start.
 | Table I.Association between miR-409-5p
expression and the clinicopathological characteristics of patients
with ovarian cancer (n=39). |
Table I.
Association between miR-409-5p
expression and the clinicopathological characteristics of patients
with ovarian cancer (n=39).
|
|
| miR-409-5p
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patients, n | Low (n=25) | High (n=14) |
P-valuea |
|---|
| Age, years |
|
|
| 0.721 |
|
<55 | 29 | 18 | 11 |
|
|
≥55 | 10 | 7 | 3 |
|
| Tumor size, cm |
|
|
| 0.044b |
|
<3 | 25 | 13 | 12 |
|
| ≥3 | 14 | 12 | 2 |
|
| FIGO stage |
|
|
| 0.005c |
| Early
(I–IIA) | 32 | 24 | 8 |
|
|
Advanced (IIB-IV) | 7 | 1 | 6 |
|
| Histological
grade |
|
|
| 0.287 |
|
Low/moderate | 27 | 19 | 8 |
|
|
High | 12 | 6 | 6 |
|
| Lymph node
metastasis |
|
|
| 0.218 |
|
Negative | 31 | 18 | 13 |
|
|
Positive | 8 | 7 | 1 |
|
| Menopause |
|
|
| >0.999 |
|
Pre- | 16 | 10 | 6 |
|
|
Post- | 23 | 15 | 8 |
|
|
Differentiation |
|
|
| >0.999 |
|
Well/moderate | 25 | 16 | 9 |
|
|
Poor | 14 | 9 | 5 |
|
| Depth of
invasion |
|
|
| 0.445 |
|
T1-T3 | 30 | 18 | 12 |
|
| T4 | 9 | 7 | 2 |
|
Cell culture
The OC cell lines, SKOV-3 and OVCAR3, and the
ovarian epithelial cell line, IOSE80, were purchased from the
American Type Culture Collection. Cells were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum (both
purchased from Gibco; Thermo Fisher Scientific, Inc.) at 37°C with
5% CO2.
Cell transfection
Prior to transfection, SKOV-3 and OVCAR3 cells were
seeded into 24-well plates at a density of 2×105
cells/well and cultured until they reached 90% confluence.
Subsequently, miR-409-5p mimics (1.5 µl;
5′-UAAUAGUAAAGGAGGGAAGCAG-3′) or miR-negative control (NC; 20
pmol/µl; 5′-ACUCUAUCUGCACGCUGACUU-3′) (Shanghai GenePharma Co.,
Ltd.) were mixed with 50 µl Opti-MEM medium (Thermo Fisher
Scientific, Inc.), which was further mixed with 1 µl
Lipofectamine® 3000 transfection reagent (Thermo Fisher
Scientific, Inc.) for 10 min at room temperature. OC cells were
transfected for 48 h at room temperature. For rescue experiments,
SKOV-3 cells in the miR-409-5p mimics or miR-NC groups were
transfected with 0.5 µl pcDNA3.1-DLGAP5 or empty pcDNA3.1 plasmid
(Sangon Biotech Co., Ltd.) at 37°C for 48 h. All transfections were
performed for 48 h, followed by subsequent experimentation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissue samples or cell
lines using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) and single-stranded cDNA was synthesized using the TaqMan
MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) or the M-MLV cDNA synthesis kit (Promega
Corporation). The temperature protocol for RT was as follows: 95°C
for 100 sec, 60°C for 60 sec and 65°C for 120 sec. qPCR was
subsequently performed using TaqMan MicroRNA assay kits or SYBR
Premix Ex Taq II (all purchased from Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following thermocycling conditions
were used: Initial denaturation at 95°C for 15 min followed by 40
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30
sec and extension at 72°C for 30 sec. The following primer
sequences were used for qPCR: miR-409-5p forward,
5′-AAGCAAGGTTACCCGCTTTG-3′ and reverse, 5′-AGTCGGGTGTCGGTGCAA-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; DLGAP5 forward,
5′-TTGTGAGGGTTCCTGCTTCG-3′ and reverse, 5′-TTCCTGTGTCGACTGGCAAA-3′;
and GAPDH forward, 5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse,
5′-GCATCGCCCCACTTGATTTT-3′. Relative expression levels of
miR-409-5p and DLGAP5 were calculated using the 2−ΔΔCq
method (32), and normalized to U6
and GAPDH, respectively. All experiments were performed in
triplicate.
Cell Counting Kit-8 (CCK-8) assay
Transfected SKOV-3 and OVCAR3 cells were seeded into
96-well plates at a density of 3,000 cells/well and the CCK-8 assay
(Dojindo Molecular Technologies, Inc.) was performed to assess cell
proliferation. At 0, 24, 48 and 72 h, cells were incubated with 10
µl CCK-8 solution for 1 h and cell proliferation was subsequently
analyzed at a wavelength of 450 nm, using a microplate reader
(SpectraMax M2; Molecular Devices, LLC). Each sample was performed
in triplicate.
Flow cytometry
Transfected SKOV-3 and OVCAR3 cells were harvested
and digested using 0.25% trypsin. Subsequently, single cell
suspension containing 1×106 cells was prepared by
discarding the digestion solution and performing centrifugation at
300 × g for 10 min at 4°C. For analysis of cell cycle distribution,
cells were resuspended in 100 µl RNase A (Sigma-Aldrich; Merck
KGaA) for 30 min at 37°C, fixed with pre-cooled 70% ethanol
overnight at 4°C and stained with 50 µg of RNase-containing
propidium iodide (PI; Sigma-Aldrich; Merck KGaA) solution for 30
min at 4°C in the dark. For cell cycle distribution analysis, the
percentage of cells in the G0/G1, S and
G2/M phases were determine using a flow cytometer (BD
Biosciences).
For apoptosis analysis, SKOV-3 and OVCAR3 cells were
resuspended in 500 µl 1X binding buffer (Sigma-Aldrich; Merck
KGaA), fixed with pre-cooled 70% ethanol overnight at 4°C and
stained with 1 µl Annexin V-FITC (Sigma-Aldrich; Merck KGaA) for 15
min at 4°C in the dark, followed by incubation with 5 µl PI for 15
min at 4°C in the dark. Early and late apoptotic cells were
subsequently analyzed using a flow cytometer (BD Biosciences).
Dual-luciferase reporter assay
TargetScan7.1 (http://www.targetscan.org) was used to predict the
binding sites between miR-409-5p and DLGAP5. The DLGAP5 3′-UTRs
containing wild-type (WT) or mutant (MUT) miR-409-5p binding sites
were synthesized and cloned into pmirGLO Dual-Luciferase vectors
(Promega Corporation), yielding DLGAP5-WT and DLGAP5-MUT,
respectively. Subsequently, 1.5×105 SKOV-3 or OVCAR3
cells were seeded into 12-well plates and co-transfected with 30 nM
miR-409-5p mimics or miR-NC and 300 ng DLGAP5-WT or DLGAP5-MUT for
48 h at 37°C using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.). Following incubation, luciferase activities were
detected using the Dual-Luciferase Reporter Assay kit (Promega
Corporation). Firefly luciferase activity was normalized to
Renilla luciferase activity.
Western blotting
The SKOV-3 and OVCAR3 cells were lysed using RIPA
lysis buffer (Beyotime Institute of Biotechnology), and the lysates
were incubated for 30 min on ice and centrifugated at 300 × g for
10 min at 4°C. Protein samples were quantified using a BCA protein
assay (Beyotime Institute of Biotechnology). Equal amounts of
protein (30 µg per lane) were separated via 12% SDS-PAGE,
transferred onto PVDF membranes and blocked with 5% skimmed milk in
TBS containing 0.1% Tween-20 for 2 h at room temperature. The
membranes were incubated with primary antibodies against DLGAP5
(1:1,000; cat. no. ab70744), CDK1 (1:500; cat. no. ab131450),
Cyclin B1 (1:100; cat. no. ab215436), Bad (1:1,000; cat. no.
ab90435), Bcl-2 (1:500; cat. no. ab196495) and GAPDH (1:1,000; cat.
no. ab245355) (all purchased from Abcam) overnight at 4°C. The
membranes were washed three times with TBST and incubated with
HRP-conjugated secondary antibody (1:5,000; cat. no. ab97051;
Abcam) at room temperature for 2 h. Protein bands were visualized
using enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc.) and quantified using ImageLab_v3.0 software
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ±
standard deviation. An unpaired Student's t-test was used to
compare differences between two groups, while one-way ANOVA
followed by Dunnett's test or Tukey's post hoc test were used to
compare differences between multiple groups. Wilcoxon signed rank
test was used to compare miR-409-5p/DLGAP5 expression levels in
tumor tissues and adjacent normal tissues. Patients were divided
into high (n=14) and low expression groups (n=5), according to the
median miR-409-5p expression value (1.021). Fisher's exact test was
used to assess the association between miR-409-5p expression and
the clinicopathological characteristics of patients with OC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-409-5p expression is significantly
downregulated in OC
To determine the potential role of miR-409-5p in the
progression of OC, RT-qPCR analysis was performed to detect its
expression in 39 histologically diagnosed OC tumor tissues and
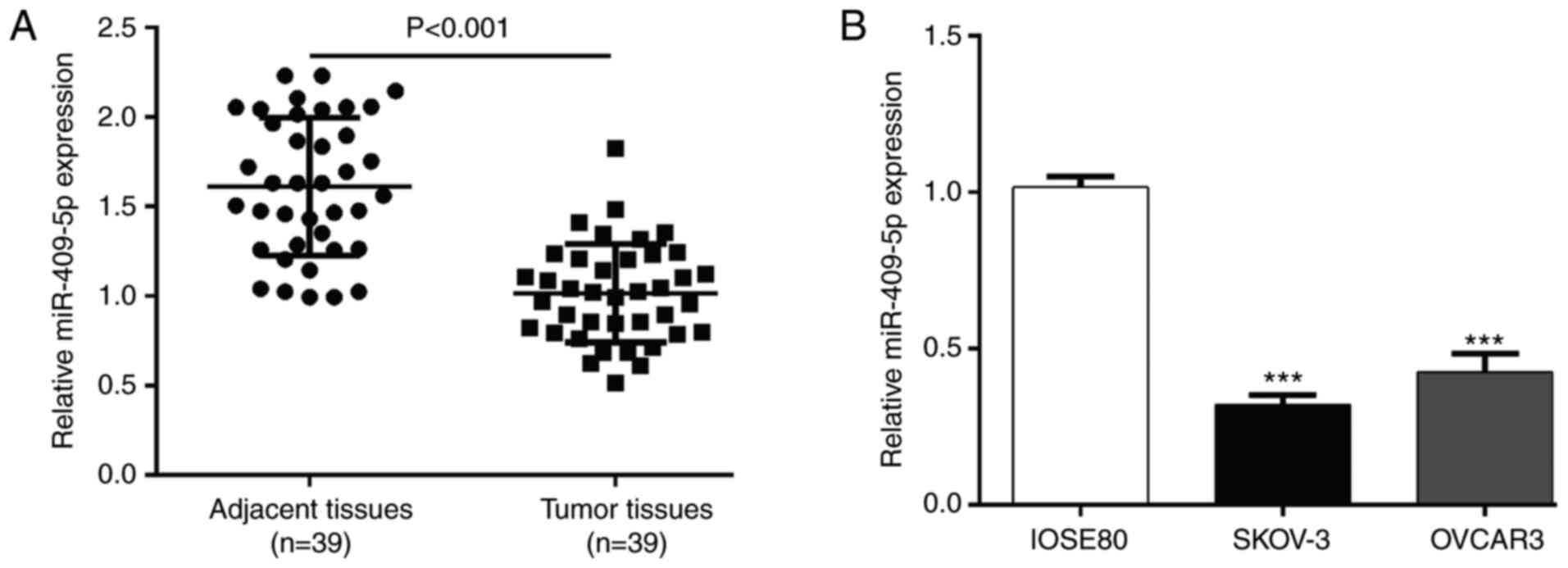
matched adjacent normal tissues. The results demonstrated that
miR-409-5p expression was significantly lower in OC tumor tissues
compared with adjacent normal tissues (P<0.001; Fig. 1A). Similarly, miR-409-5p expression
was significantly lower in the OC cell lines (SKOV-3 and OVCAR3)
compared with normal ovarian epithelial cells (Fig. 1B).
Next, the association between miR-409-5p expression
and the clinicopathological characteristics of patients with OC was
analyzed. As presented in Table I,
miR-409-5p expression was significantly associated with tumor size
(P=0.044) and FIGO stage (P=0.005).
Overexpression of miR-409-5p inhibits
the proliferation of OC cells
Gain-of-function assays were performed to determine
the biological function of miR-409-5p in OC cells in vitro.
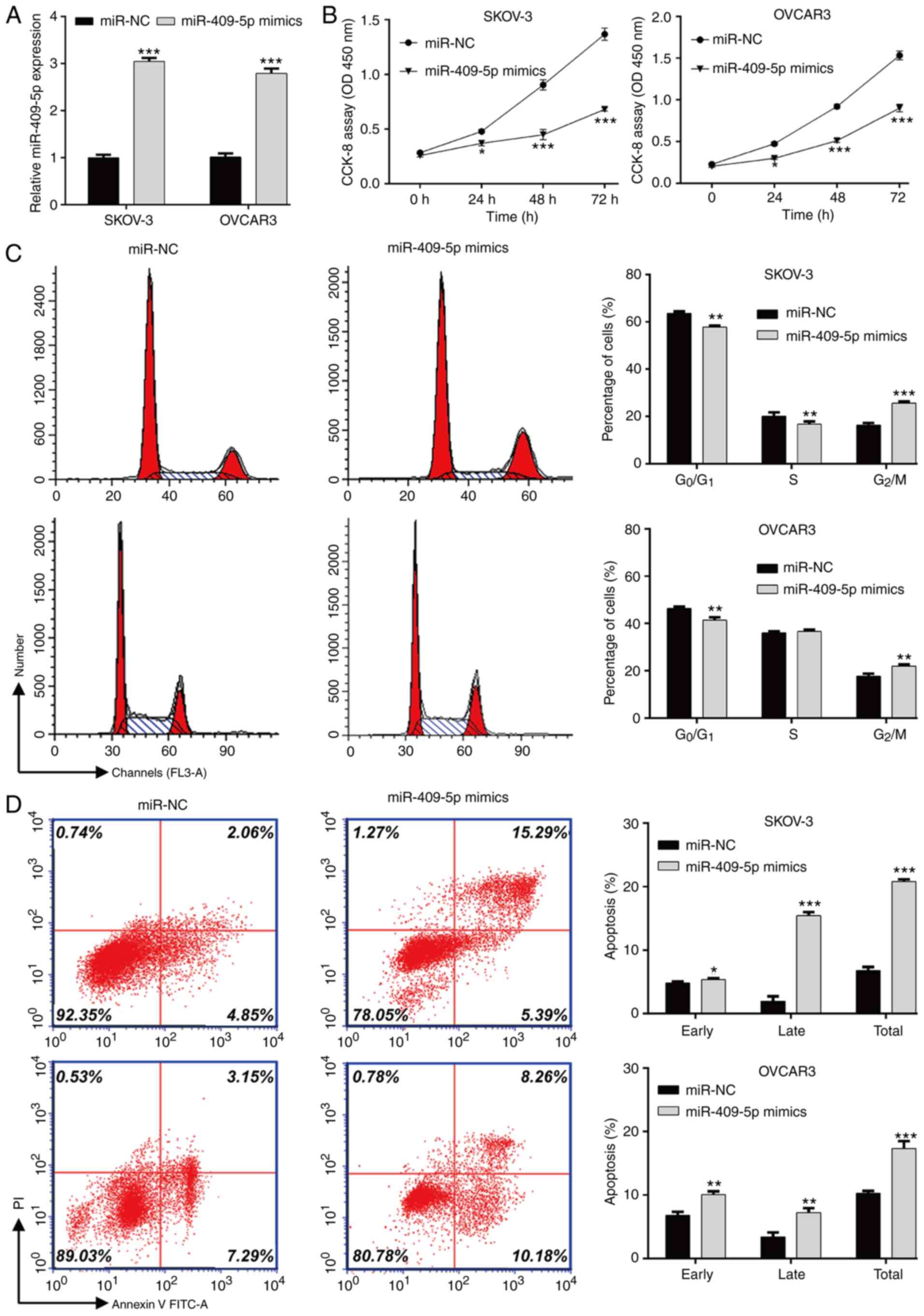
RT-qPCR analysis demonstrated that transfection with miR-409-5p
mimics significantly upregulated miR-409-5p expression in both
SKOV-3 and OVCAR3 cells compared the miR-NC group (Fig. 2A). The results of the CCK-8 assay
indicated that transfection with miR-409-5p mimics significantly
attenuated the proliferation of SKOV-3 and OVCAR3 cells (Fig. 2B). As presented in Fig. 2C, the percentage of SKOV-3
(57.8±0.6 vs. 63.5±0.9) and OVCAR3 (41.4±1.3 vs. 46.3±0.8) cells in
the G0/G1 phase and SKOV-3 cells in the S
phase (16.7±1.2 vs. 20.1±1.6) significantly decreased following
transfection with miR-409-5p mimics compared with the miR-NC group.
Overexpression of miR-409-5p significantly increased the percentage
of SKOV-3 cells in the G2/M phase from 16.4±0.8 to
25.6±0.8 and OVCAR3 cells from 17.7±1.1 to 22.0±0.6. In addition,
overexpression of miR-409-5p mimics significantly elevated early
and late apoptosis in both SKOV-3 and OVCAR3 cells (Fig. 2D). Taken together, these results
suggest that miR-409-5p negatively regulates OC cell proliferation
by affecting cell cycle progression and apoptosis.
DLGAP5 is a direct target of
miR-409-5p
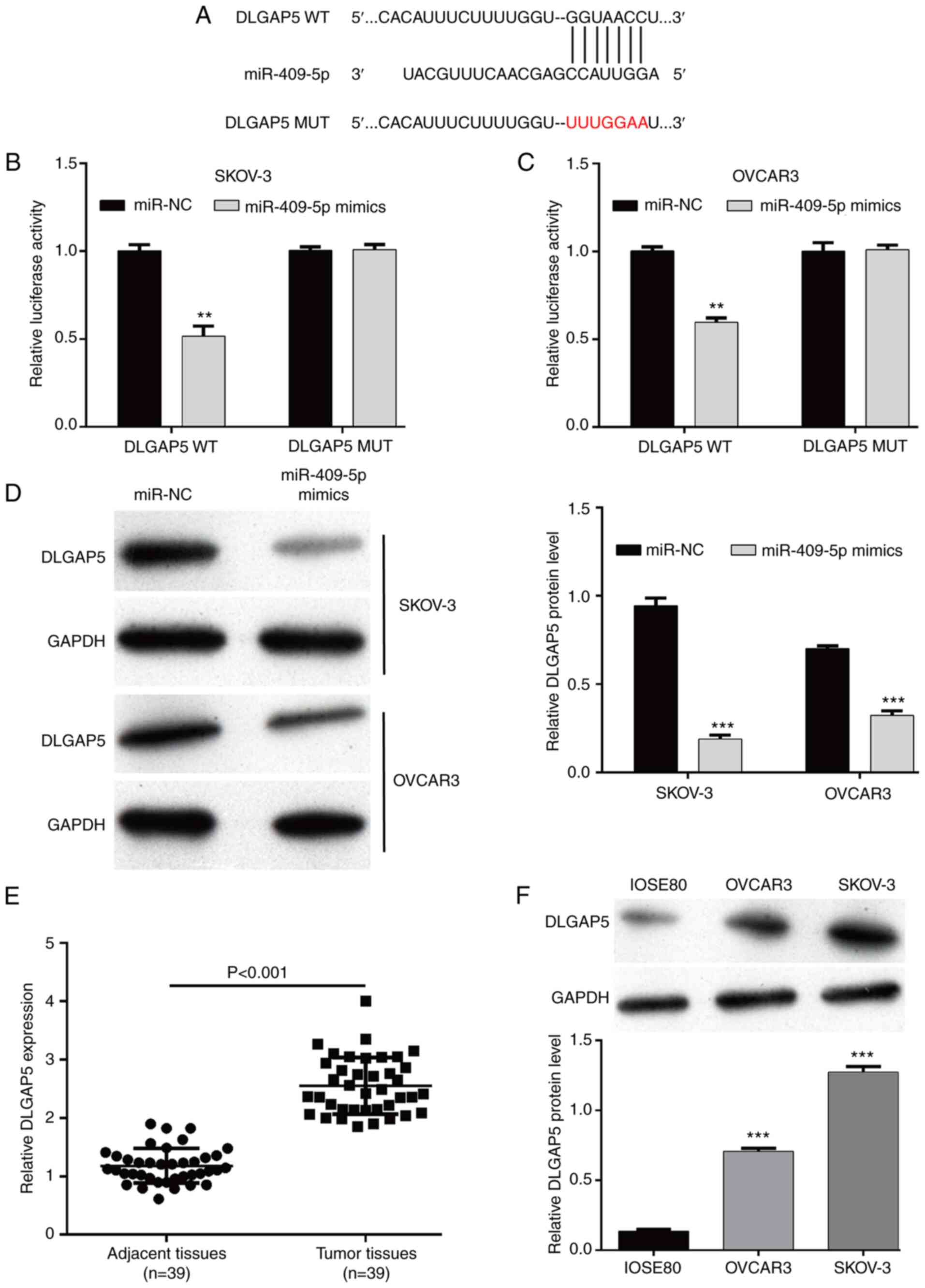
TargetScan software was used to identify the direct
targets of miR-409-5p. Among the predicted targets, DLGAP5, a cell
cycle-related gene (20), was
identified as a potential target of miR-409-5p, and DLGAP5 harbored
a putative miR-409-5p binding site in its 3′-UTR (Fig. 3A). The dual-luciferase reporter
assay was performed in OC cells by constructing DLGAP5 WT or MUT
reporter plasmids. The results demonstrated that luciferase
activity decreased in the DLGAP5 WT groups in SKOV-3 (Fig. 3B) and OVCAR3 (Fig. 3C) cells following transfection with
miR-409-5p. Furthermore, DLGAP5 protein expression significantly
decreased following overexpression of miR-409-5p in both SKOV-3 and
OVCAR3 cells (Fig. 3D). Notably,
DLGAP5 mRNA expression was significantly upregulated in OC tumor
tissues compared with adjacent normal tissues (Fig. 3E). Consistently, DLGAP5 protein
expression was significantly upregulated in the OC cell lines
(OVCAR3 and SKOV-3), compared with normal ovarian IOSE80 epithelial
cells (Fig. 3F). Collectively,
these results suggest that miR-409-5p negatively regulates DLGAP5
expression by binding to its 3′-UTR.
Restoration of DLGAP5 abolishes the
effects of miR-409-5p on OC cells
To further investigate whether DLGAP5 expression is
associated with miR-409-5p regulating OC cell functions,
overexpression of DLGAP5 in SKOV-3 cells was confirmed following
transfection with pcDNA3.1-DLGAP5 (Fig. 4A). Subsequently, co-transfection
with miR-409-5p mimics and pcDNA3.1-DLGAP5 was performed in SKOV-3
cells. As presented in Fig. 4B,
miR-409-5p-induced downregulation of DLGAP5 protein expression was
significantly abolished following co-transfection with miR-409-5p
mimics and pcDNA3.1-DLGAP5. The results of the CCK-8 assay
demonstrated that restoration of DLGAP5 expression in SKOV-3 cells
stably expressing miR-409-5p reversed the inhibitory effect of
miR-409-5p on cell proliferation (Fig.
4C). In addition, G2/M phase arrest (Fig. 4D) and elevated cell apoptosis
(Fig. 4E) induced by miR-409-5p
overexpression was significantly reversed following restoration of
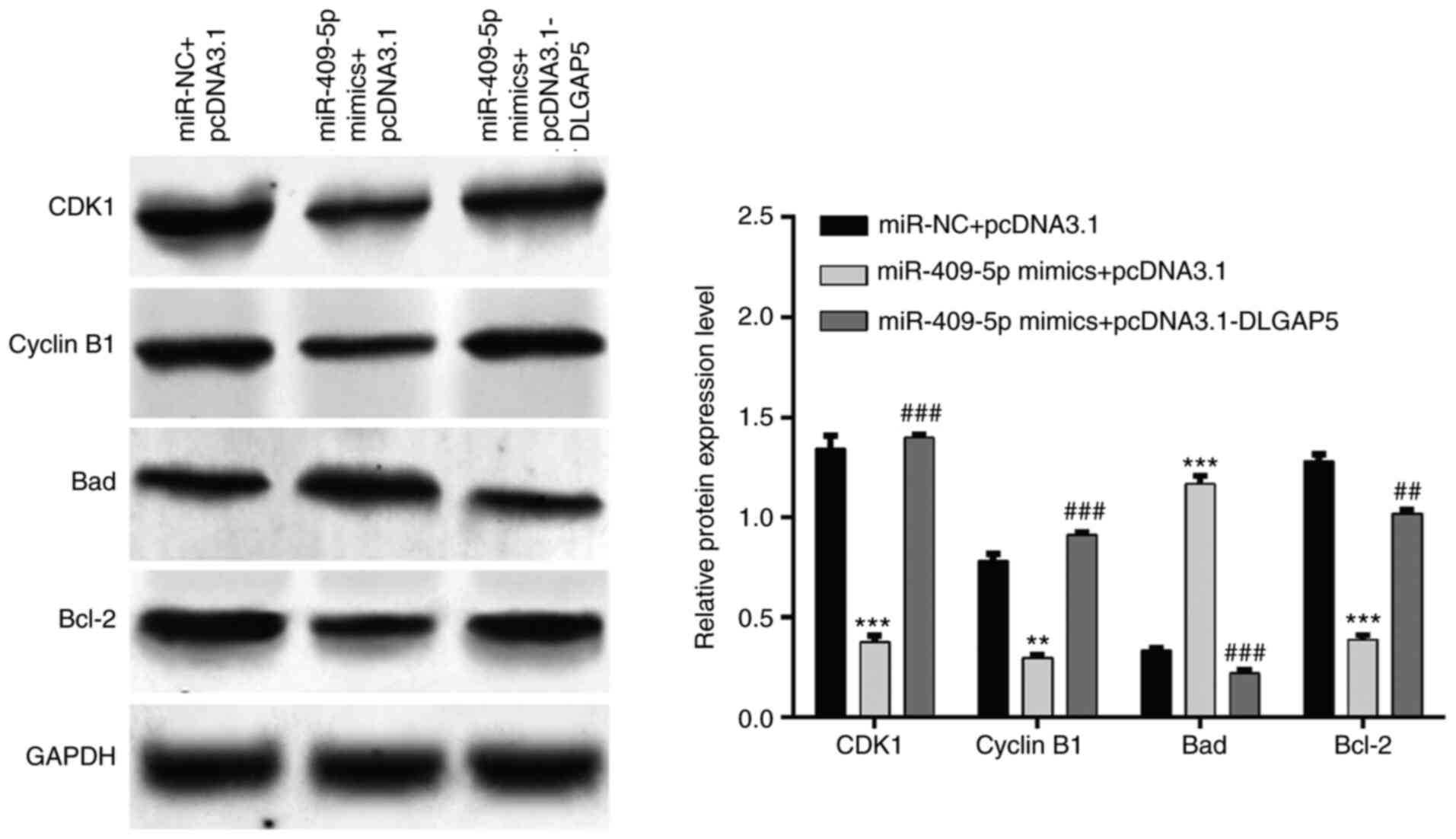
DLGAP5 in SKOV-3 cells. Western blot analysis further demonstrated
that miR-409-5p overexpression induced-downregulation of CDK1,
Cyclin B1 and Bcl-2 and upregulation of Bad were remarkedly
attenuated following restoration of DLGAP5 (Fig. 5). Taken together, these results
suggest that restoration of DLGAP5 expression can reverse the
effects of miR-409-5p on OC cell proliferation, G2/M
phase arrest and apoptosis.
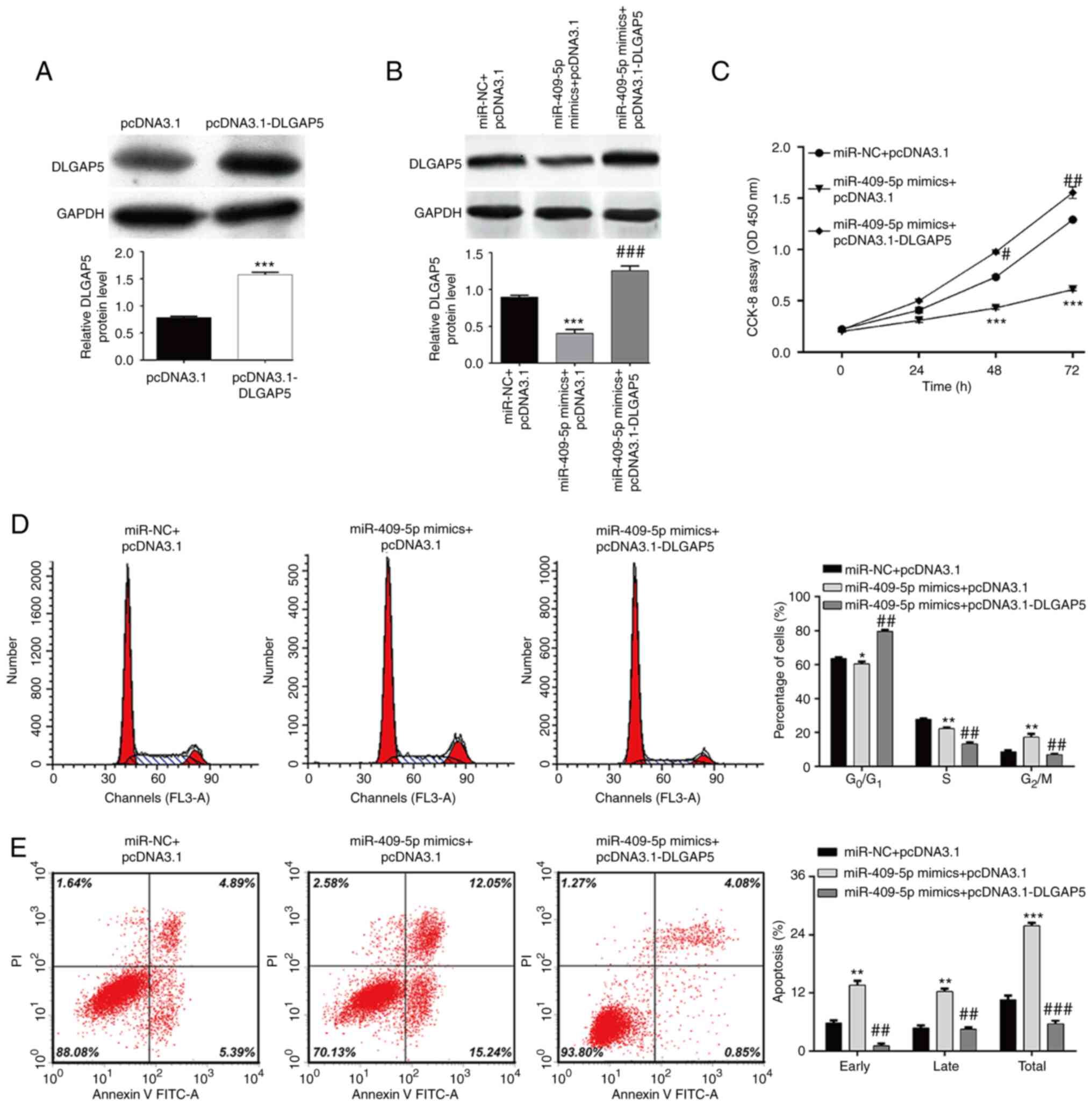 | Figure 4.Restoration of DLGAP5 reverses the
effects of miR-409-5p on ovarian cancer cell proliferation, cell
cycle progression and apoptosis. SKOV-3 cells were transfected with
pcDNA3.1-DLGAP5 alone or together with miR-409-5p mimics. (A and B)
Western blot analysis was performed to detect the DLGAP5 protein
expression in SKOV-3 cells in the different groups. (C) The CCK-8
assay was performed to assess cell proliferation. (D) The
percentage of cells in the G0/G1, S and
G2/M phases were analyzed in SKOV-3 cells in the
different groups via flow cytometry with PI staining. (E) Early and
late apoptotic cells were determined in SKOV-3 cells in the
different groups via flow cytometry with Annexin V/PI staining.
Data are presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01, ***P<0.001 vs. the miR-NC + pcDNA3.1
group; #P<0.05, ##P<0.01,
###P<0.001 vs. the miR-409-5p mimics + pcDNA3.1
group. DLGAP5, discs large-associated protein 5; miR, microRNA; NC,
negative control; PI, propidium iodide; CCK-8, Cell Counting Kit-8;
OD, optical density. |
Discussion
The results of the present study demonstrated that
miR-409-5p expression was significantly downregulated in OC tissues
and cell lines, which was associated with tumor size (P=0.0353) and
FIGO stage (P=0.0024). Consistent with the results of the present
study, downregulated miR-409-3p is associated with TNM stage and
lymph node metastasis in human gastric cancer (15). miR-409-3p expression is also
significantly downregulated in human bladder cancer (33), lung adenocarcinoma (34), colorectal cancer (35), breast cancer (36,37)
and glioma (38). Contrary to the
results of the present study, miR-409-3p/-5p expression is
upregulated in bone metastatic prostate cancer cell lines and human
prostate cancer tissues, with higher Gleason scores (16). In addition, miR-409-5p is
aberrantly upregulated in both breast cancer tumors and cell lines
(17). miR-409-5p functions as a
osteogenesis suppressor by targeting Lrp-8 as a positive effect of
Wnt signaling (39). The
controversial role of miR-409 in different types of cancer may be
due to the different tissues and miRNA maturations.
Overexpression assays in the present study
demonstrated that overexpression of miR-409-5p inhibited OC cell
proliferation, and induced G2/M phase arrest and
apoptosis. It has been speculated that miR-409 plays an essential
role in tumor cell behaviors, including proliferation, cell cycle
distribution and apoptosis. For example, miR-409-3p notably
suppresses cell proliferation and promotes cell apoptosis in
gastric cancer (40), osteosarcoma
(41) and papillary thyroid
carcinoma (42). The suppressive
role of miR-409-3p on tumor cell proliferation has also been
observed in breast cancer (36,43),
glioma (38), tongue squamous cell
carcinoma (44) and osteosarcoma
(45). Orthotopic delivery of
miR-409-3p/-5p appears to be an attractive therapeutic target for
treating bone metastatic prostate cancer by facilitating tumor
growth in the murine prostate gland (16). Yu et al (17) reported that lentiviral
transduction-mediated downregulation of endogenous miR-409-5p
expression suppresses MDA-MB-231 and MCF-7 cell proliferation and
xenograft development.
Currently, Ras suppressor protein has been
identified as a downstream target of miR-409-5p, which is involved
in the development of breast cancer (17). The present study identified DLGAP5
as a novel target of miR-409-5p. Notably, overexpression of DLGAP5
abrogated the effects of miR-409-5p on the proliferation of SKOV-3
cells, G2/M phase arrest and apoptosis, as well as the
regulation of CDK1, Cyclin B1, Bad and Bcl-2 expression levels. The
results of the present study are consistent with previous findings,
suggesting that silencing of DLGAP5 suppresses cell proliferation,
induces G2/M phase arrest and apoptosis in
hepatocellular carcinoma (27,28)
and invasive breast cancer (29).
DLGAP5 can promote spindle formation (46) and the cell cycle progression from M
to G0/G1 phase, which can be modulated by
CDK1/Cyclin B (47). Thus, it is
speculated that miR-409-5p exerts its suppressive effects on OC
cell proliferation via G2/M arrest and apoptosis by
targeting DLGAP5.
Increasing evidence suggest that miRNAs function as
important regulators affecting tumor development and progression
(48,49). Consequently, antagonizing oncogenic
miRNAs or restoration of tumor suppressive miRNAs can represent a
reliable tool for improving cancer therapy, which suggests that
miRNA-based treatment requires a careful choice of the potential
target (50,51).
There are some limitations to the present study.
There were only 2 OC cell lines were used, knockdown of
miR-409-5p/DLGAP5 experiments are also missing, and in vivo
experiments should be performed to confirm the results in future
research.
In conclusion, the results of the present study
demonstrated that overexpression of miR-409-5p downregulated DLGAP5
expression, which suppressed cell proliferation and induced
G2/M phase arrest and apoptosis. These results suggest
that stable overexpression of miR-409-5p may represent a promising
approach to improve the treatment of patients with OC.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the Ningde City Science and
Technology Project (grant no. 20150058).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
XJC designed the present study. All the authors
confirmed the authenticity of all the raw data. WWL and JL
performed the experiments. JFH and ZYC participated in literature
research and collected the data. QYS and FY participated in data
acquisition and data analysis. YF was involved in drafting the
manuscript or revising it critically for important intellectual
content. XY participated in data collection and drawing figures and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Mindong Hospital Affiliated to Fujian Medical
University (Fujian, China; approval no. MHFM-39A) and performed in
accordance with the Declaration of Helsinki. Written informed
consent was provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OC
|
ovarian cancer
|
|
DLGAP5
|
discs large-associated protein 5
|
|
UTR
|
untranslated region
|
|
WT
|
wild-type
|
|
MUT
|
mutant
|
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Staicu CE, Predescu DV, Rusu CM, Radu BM,
Cretoiu D, Suciu N, Crețoiu SM and Voinea SC: Role of microRNAs as
clinical cancer biomarkers for ovarian cancer: A short overview.
Cells. 9:1692020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsujioka H, Yotsumoto F, Hikita S, Ueda T,
Kuroki M and Miyamoto S: Targeting the heparin-binding epidermal
growth factor-like growth factor in ovarian cancer therapy. Curr
Opin Obstet Gynecol. 23:24–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinato DJ, Graham J, Gabra H and Sharma R:
Evolving concepts in the management of drug resistant ovarian
cancer: Dose dense chemotherapy and the reversal of clinical
platinum resistance. Cancer Treat Rev. 39:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu KH: Screening for ovarian cancer in
asymptomatic women. Jama. 319:557–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Ann Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deb B, Uddin A and Chakraborty S: miRNAs
and ovarian cancer: An overview. J Cell Physiol. 233:3846–3854.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mandilaras V, Vernon M, Meryet-Figuiere M,
Karakasis K, Lambert B, Poulain L, Oza A and Denoyelle C: Updates
and current challenges in microRNA research for personalized
medicine in ovarian cancer. Exp Opin Biol Ther. 17:927–943. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou P, Xiong T, Yao L and Yuan J:
MicroRNA-665 promotes the proliferation of ovarian cancer cells by
targeting SRCIN1. Exp Ther Med. 19:1112–1120. 2020.PubMed/NCBI
|
|
12
|
Wang Y, Lei X, Gao C, Xue Y, Li X, Wang H
and Feng Y: miR-506-3p suppresses the proliferation of ovarian
cancer cells by negatively regulating the expression of MTMR6. J
Biosci. 44:1262019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kavitha N, Vijayarathna S, Jothy SL, Oon
CE, Chen Y, Kanwar JR and Sasidharan S: MicroRNAs: Biogenesis,
roles for carcinogenesis and as potential biomarkers for cancer
diagnosis and prognosis. Asian Pac J Cancer Prev. 15:7489–7497.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galasso M, Sandhu SK and Volinia S:
MicroRNA expression signatures in solid malignancies. Cancer J.
18:238–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng B, Liang L, Huang S, Zha R, Liu L,
Jia D, Tian Q, Wang Q, Wang C, Long Z, et al: MicroRNA-409
suppresses tumour cell invasion and metastasis by directly
targeting radixin in gastric cancers. Oncogene. 31:4509–4516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Xing H, Han W, Wang Y, Qi T, Song C,
Xu Z, Li H and Huang Y: MicroRNA-409-5p is upregulated in breast
cancer and its downregulation inhibits cancer development through
downstream target of RSU1. Tumour Biol. 39:10104283177016472017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Lu Z, Unruh AK, Ivan C, Baggerly
KA, Calin GA, Li Z, Bast RC Jr and Le XF: Clinically relevant
microRNAs in ovarian cancer. Mol Cancer Res. 13:393–401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Zhang X, Fu X, Li W, Xing S and
Yang Y: Identification of common differentially-expressed miRNAs in
ovarian cancer cells and their exosomes compared with normal
ovarian surface epithelial cell cells. Oncol Lett. 16:2391–2401.
2018.PubMed/NCBI
|
|
20
|
Koffa MD, Casanova CM, Santarella R,
Köcher T, Wilm M and Mattaj IW: HURP is part of a ran-dependent
complex involved in spindle formation. Curr Biol. 16:743–754. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schneider MA, Christopoulos P, Muley T,
Warth A, Klingmueller U, Thomas M, Herth FJ, Dienemann H, Mueller
NS, Theis F and Meister M: AURKA, DLGAP5, TPX2, KIF11 and CKAP5:
Five specific mitosis-associated genes correlate with poor
prognosis for non-small cell lung cancer patients. Int J Oncol.
50:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi YX, Yin JY, Shen Y, Zhang W, Zhou HH
and Liu ZQ: Genome-scale analysis identifies NEK2, DLGAP5 and ECT2
as promising diagnostic and prognostic biomarkers in human lung
cancer. Sci Rep. 7:80722017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weinberger P, Ponny SR, Xu H, Bai S,
Smallridge R, Copland J and Sharma A: Cell cycle M-phase genes are
highly upregulated in anaplastic thyroid carcinoma. Thyroid.
27:236–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Yang L, Zhang X, Chen R, Chen X,
Tang W and Zhang M: Identification of potential biomarkers in
glioblastoma through bioinformatic analysis and evaluating their
prognostic value. Biomed Res Int. 2019:65815762019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Z, Cheng Y, Jiang Y, Liu S, Zhang M,
Liu J and Zhao Q: Ten hub genes associated with progression and
prognosis of pancreatic carcinoma identified by co-expression
analysis. Int J Biol Sci. 14:124–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Branchi V, Garcia SA, Radhakrishnan P,
Győrffy B, Hissa B, Schneider M, Reißfelder C and Schölch S:
Prognostic value of DLGAP5 in colorectal cancer. Int J Colorectal
Dis. 34:1455–1465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao W, Liu W, Yuan Q, Liu X, Ou Y, He S,
Yuan S, Qin L, Chen Q, Nong K, et al: Silencing of DLGAP5 by siRNA
significantly inhibits the proliferation and invasion of
hepatocellular carcinoma cells. PLoS One. 8:e807892013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo TC, Chang PY, Huang SF, Chou CK and
Chao CCK: Knockdown of HURP inhibits the proliferation of
hepacellular carcinoma cells via downregulation of gankyrin and
accumulation of p53. Biochem Pharmacol. 83:758–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Pan Y, Fu H and Zhang J:
Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell
proliferation and enhances susceptibility to epirubicin in invasive
breast cancer cells by regulating cyclin D kinase (CDK1) and DLGAP5
expression. Med Sci Monit. 24:8553–8564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeppernick F and Meinhold-Heerlein I: The
new FIGO staging system for ovarian, fallopian tube, and primary
peritoneal cancer. Arch Gynecol Obstet. 290:839–842. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosen DG, Yang G, Liu G, Mercado-Uribe I,
Chang B, Xiao XS, Zheng J, Xue FX and Liu J: Ovarian cancer:
Pathology, biology, and disease models. Front Biosci (Landmark Ed).
14:2089–2102. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu
X, Zhu Y, Li S, Zheng X and Xie L: MicroRNA-409-3p inhibits
migration and invasion of bladder cancer cells via targeting c-met.
Mol Cells. 36:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan L, Zhu L, Xu J, Lu B, Yang Y, Liu F
and Wang Z: MicroRNA-409-3p functions as a tumor suppressor in
human lung adenocarcinoma by targeting c-met. Cell Physiol Biochem.
34:1273–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai R, Weng C, Dong H, Li S, Chen G and Xu
Z: MicroRNA-409-3p suppresses colorectal cancer invasion and
metastasis partly by targeting GAB1 expression. Int J Cancer.
137:2310–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Liu Z, Xu H and Yang Q:
miR-409-3p suppresses breast cancer cell growth and invasion by
targeting Akt1. Biochem Biophys Res Commun. 469:189–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao GH, Sun XL, Wu F, Chen WF, Li JQ and
Hu WC: Low expression of miR-409-3p is a prognostic marker for
breast cancer. Eur Rev Med Pharmacol Sci. 20:3825–3829.
2016.PubMed/NCBI
|
|
38
|
Cao Y, Zhang L, Wei M, Jiang X and Jia D:
MicroRNA-409-3p represses glioma cell invasion and proliferation by
targeting high-mobility group nucleosome-binding domain 5. Oncol
Res. 25:1097–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prakash R, John AA and Singh D: miR-409-5p
negatively regulates Wnt/Beta catenin signaling pathway by
targeting Lrp-8. J Cell Physiol. 234:23507–23517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei
M, Ju J, Yu Y, Yan M, et al: MicroRNA-409-3p regulates cell
proliferation and apoptosis by targeting PHF10 in gastric cancer.
Cancer Lett. 320:189–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Hou W, Jia J, Zhao Y and Zhao B:
miR-409-3p regulates cell proliferation and tumor growth by
targeting E74-like factor 2 in osteosarcoma. FEBS Open Bio.
7:348–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao Z, Yang F, Liu Y, Fu K and Jing S:
MicroRNA-409-3p suppresses cell proliferation and cell cycle
progression by targeting cyclin D2 in papillary thyroid carcinoma.
Oncol Lett. 16:5237–5242. 2018.PubMed/NCBI
|
|
43
|
Ma Z, Li Y, Xu J, Ren Q, Yao J and Tian X:
MicroRNA-409-3p regulates cell invasion and metastasis by targeting
ZEB1 in breast cancer. IUBMB Life. 68:394–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen H and Dai J: miR-409-3p suppresses
the proliferation, invasion and migration of tongue squamous cell
carcinoma via targeting RDX. Oncol Lett. 16:543–551.
2018.PubMed/NCBI
|
|
45
|
Wu L, Zhang Y, Huang Z, Gu H, Zhou K, Yin
X and Xu J: MiR-409-3p inhibits cell proliferation and invasion of
osteosarcoma by targeting zinc-finger E-box-binding homeobox-1.
Front Pharmacol. 10:1372019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Santarella RA, Koffa MD, Tittmann P, Gross
H and Hoenger A: HURP wraps microtubule ends with an additional
tubulin sheet that has a novel conformation of tubulin. J Mol Biol.
365:1587–1595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hsu JM, Lee YC, Yu CT and Huang CY: Fbx7
functions in the SCF complex regulating Cdk1-cyclin
B-phosphorylated hepatoma up-regulated protein (HURP) proteolysis
by a proline-rich region. J Biol Chem. 279:32592–32602. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Ann Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ganju A, Khan S, Hafeez BB, Behrman SW,
Yallapu MM, Chauhan SC and Jaggi M: miRNA nanotherapeutics for
cancer. Drug Discov Today. 22:424–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bader AG, Brown D and Winkler M: The
promise of microRNA replacement therapy. Cancer Res. 70:7027–7030.
2010. View Article : Google Scholar : PubMed/NCBI
|