Introduction
Colorectal cancer (CRC), a common digestive tract
cancer, has the third highest incidence and second highest
mortality rates among malignant carcinomas worldwide (1–3).
Although technological advances in surgical resection,
chemotherapy/chemoradiotherapy and immunotherapy have led to
increases in CRC-related survival rate (4–7),
tumor recurrence and metastasis still result in a poor prognosis in
patients with CRC (8,9). Traditional clinicopathological
parameters (i.e., pathological grade and tumor stage), tumor
markers (i.e., carcinoembryonic antigen and defective DNA mismatch
repair) and molecular biomarkers (i.e., adenomatous polyposis coli
and vascular endothelial growth factor) have been used in the
prognostic evaluation of CRC (10–12).
To further improve the management of patients with CRC and
prediction of the risk for relapse, identifying more potential
predictors and therapeutic targets is critical.
Polo-like kinase 4 (PLK4), a serine/threonine
kinase, is a regulator of centriole duplication through its
autophosphorylation (13,14), while its aberrant expression
induces centrosome duplication in cancer cells, and this is
potentially associated with tumor progression (15,16).
Indeed, PLK4 overexpression increases the proliferative and
invasive ability of CRC cells through the Wnt/β-catenin signaling
pathway (17). On the other hand,
inhibiting PLK4 suppresses cell proliferation, migration and
invasion in hepatocellular carcinoma (18). Meanwhile, PLK4 downregulation
induces cell cycle arrest at the G1 phase by activating the
p38/p53/p21 pathway in bladder cancer (19). In addition, the high PLK4
expression not only reflects an advanced cancer stage, but is also
predictive of a poor prognosis in several cancer types, such as
epithelial ovarian cancer, lung cancer and neuroblastoma (20–22).
However, the association between PLK4 and CRC prognosis is not
clear.
In the present study, the PLK4 levels were compared
between CRC tumor and paired adjacent tissues with the objective of
investigating its potential in reflecting clinicopathological
features, as well as its prognostic value in patients with CRC. The
small interfering RNA (siRNA)-mediated downregulation of PLK4 was
also induced to explore the effect of PLK4 on chemosensitivity in
CRC cells.
Materials and methods
Patients
In the present study, 142 patients with CRC (age
ranged 42–80 years) who received surgical resection in our
hospitals between July 2013 and June 2017 were retrospectively
analyzed. The patient data from the hospital database were screened
using the following criteria: i) Diagnosis of CRC based on
pathological examination; ii) adult patients aged >18 years;
iii) tumor-node-metastasis (TNM) stage I–III; iv) surgical
treatment including laparoscopic surgery and radical excision; v)
no neoadjuvant therapy; vi) surgically-removed CRC and
para-carcinoma tissues were retrievable; vii) clinical data
corresponding to surgical specimens of patients were available; and
viii) follow-up records were accessible for survival assessment.
This study was implemented with the approval of the Institutional
Review Board of Xi'an International Medical Center Hospital, and
all patient data were analyzed following anonymization; therefore,
patient informed consent was waived by the Institutional Review
Board.
Acquisition of data and specimens
Clinicopathological data were collected from the
patients' medical records, and survival information, which was used
for the evaluation of overall survival (OS), was obtained from the
follow-up records. Formalin-fixed and paraffin-embedded (FFPE)
specimens (CRC and para-carcinoma tissues) of 142 patients were
collected from the specimen library to assess the protein
expression of PLK4. Only 69 patients had fresh-frozen specimens
stored in liquid nitrogen, which were also collected from the
specimen library to evaluate PLK4 mRNA expression.
Assessment of PLK4 protein
expression
Immunohistochemistry (IHC) was performed to assess
the PLK4 protein expression, as previously reported (23). Briefly, the FFPE specimens were cut
into slices, followed by deparaffinization and rehydration,
followed by H2O2 (Sigma-Aldrich) treatment at
room temperature for 10 min to quench endogenous peroxidases. Next,
heat-induced antigen retrieval was performed. Following blocking,
the slices were incubated with anti-PLK4 antibody (1:5,000;
PA5-80907; Thermo Fisher Scientific, Inc.) at 4°C overnight and
HRP-conjugated goat anti-rabbit IgG H&L secondary antibody
(1:60; 32460; Thermo Fisher Scientific, Inc.) at room temperature
at 1 h. Diaminobenzidine (room temperature for 10 sec;
Sigma-Aldrich) and hematoxylin (room temperature for 5 min;
Sigma-Aldrich) were used for staining and counterstaining,
respectively. Following IHC staining, PLK4 protein expression was
assessed under a microscope (Eclipse Ti-U; Nikon Corporation). The
IHC results were quantified by scoring the staining intensity and
density as previously described (24). The staining intensity was scored as
0 (negative), 1 (weak), 2 (moderate) and 3 (strong), and the
staining density of positive cells was scored as 0 (0%), 1 (1–25%),
2 (26–50%), 3 (51–75%) and 4 (76–100%). The representative images
for each score of staining intensity and density in the IHC
staining in tumor tissues were shown in Fig. S1. The IHC score was calculated by
multiplying the two scores. Two pathologists assessed the IHC score
independently. If the two pathologists gave different IHC scores
for the same specimen, then the mean IHC score of this specimen was
calculated and recorded.
Evaluation of PLK4 mRNA
expression
Reverse transcription-quantitative PCR (RT-qPCR) was
performed in 69 paired CRC and para-carcinoma tissues to determine
PLK4 mRNA expression. Total RNA was extracted using RNeasy Protect
Mini Kit (Qiagen GmbH) and then converted to cDNA (25°C for 3 min,
45°C for 10 min, 85°C for 5 min) using a QuantiNova Reverse
Transcription Kit (Qiagen GmbH). RT-qPCR (95°C for 2 min, then 40
cycles of 95°C for 5 sec and 60°C for 10 sec) was conducted using a
QuantiNova SYBR Green PCR Kit (Qiagen GmbH). The primers used in
this study were designed as previously described (25) and listed as follows: forward primer
for PLK4, 5′-CCTTATCACCTCCTCCTTC-3′; reverse primer for PLK4,
5′-CCAAGTCCTTCATTTGTAACC-3′; forward primer for GAPDH,
5′-ACATCATCCCTGCCTCTAC-3′; reverse primer for GAPDH,
5′-CCTGCTTCACCACCTTCT-3′. PLK4 mRNA expression was analyzed using
the 2−ΔΔCq method (26)
with GAPDH as an internal control.
Chemosensitivity experiment
A further in vitro experiment was conducted
to verify the effect of PLK4 on the chemosensitivity of CRC cells
to 5-fluorouracil (5-FU), since all stage III patients and most
stage II patients received capecitabine monotherapy or XELOX
(capecitabine combined with oxaliplatin) regimen following surgery.
Human colonic epithelial cell (HCoEpic) (2950) was purchased from
ScienCell Research Laboratories, lnc. Human CRC cell lines
including HCT-116 (CBP60028) and LoVo (CBP60032) were purchased
from Nanjing Cobioer Biotechnology Co., Ltd., SW480 (CCL-228) and
HT-29 (HTB-38) were purchased from ATCC. The HCoEpic and SW480
cells were cultured in Leibovitz's L-15 medium (Gibco; Thermo
Fisher Scientific, Inc.) with FBS. The HCT-116 and HT-29 cells were
cultured in McCoy's 5a medium (Gibco; Thermo Fisher Scientific,
Inc.) with FBS. The LoVo cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with FBS. All cells were maintained in a
humidified incubator supplied with 95% air and 5% CO2 at
37°C. The PLK4-siRNA and corresponding negative control (NC) siRNA
were designed by Shanghai GenePharma Co., Ltd. The sequence
(5′->3′) of PLK4 siRNA was: SS, ACACAUAAUUGCUAUCUUCAA; AS,
ACACAUAAUUGCUAUCUUCAA. The sequence (5′->3′) of NC siRNA was:
SS, GAAUUAAUUAAAGAUGGCCCGUUGUACU'; AS,
UCAUCGAAGUUAUAGGGAUACAUUACGUGAUC. PLK4-siRNA (50 pM) and NC-siRNA
(50 pM) were respectively transfected into the HCT-116 and the LoVo
cells using Lipofectamine™ 2000 Transfection Reagent (Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. Following transfection, the
cells in each cell line were categorized as PLK4-siRNA, NC-siRNA
and blank control cells (without transfection). The cells were then
treated with 5-FU (Merck KGaA) at the following concentrations: 0,
1, 2, 4, 8 and 16 µM for 48 h, which was based on previous studies
with some modification (27,28).
Following treatment, Cell Counting Kit-8 reagent (Beyotime
Institute of Biotechnology) was added to the cells, followed by
incubation at 37°C for 2 h. Finally, absorbance was measured at 450
nm using a microplate reader, and cell viability at different
concentrations of 5-FU was calculated. In addition, the 50%
inhibitory concentration (IC50) was calculated using
Probit regression.
Western blot
The protein level of PLK4 in HCoEpic and CRC cells,
as well as nuclear translocation of β-catenin in HCT-116 and LoVo
cells after transfection were determined by western blot. Protein
of the cells was extracted using a nucleoprotein extraction kit
(Sangon Biotech Co., Ltd.) or RIPA reagent (Sangon Biotech Co.,
Ltd.) and quantified using an enhanced BCA protein assay kit
(Beyotime Institute of Biotechnology). Subsequently, the protein
(20 µg) was separated using 4–20% SDS-PAGE, followed by
transferring onto nitrocellulose membranes (Pall Life Sciences).
Then, the membranes were blocked with 5% BSA (Sigma-Aldrich; Merck
KGaA) at room temperature for 1 h, and incubated with primary
antibodies [β-catenin antibody (Cell Signaling Technology, Inc;
8480; 1:1,000), histone H3 antibody (Cell Signaling Technology,
Inc; 4499; 1:2,000), PLK4 antibody (Cell Signaling Technology, Inc;
71033, 1:1,000) and GAPDH (Cell Signaling Technology, Inc; 2118;
1:1,000)] at 4°C overnight, followed by an HRP-linked goat
anti-rabbit IgG antibody (Cell Signaling Technology, Inc; 7074;
1:3,000) at room temperature for 1 h. Then, the brands were
visualized with ECL-PLUS reagents (Thermo Fisher Scientific, Inc.)
and analyzed by ImageJ software (v1.5; NIH).
Statistical analysis
High PLK4 protein expression was assigned an IHC
score of >3, and low PLK4 protein expression an IHC score of ≤3.
The median PLK4 mRNA expression in CRC tissues was used to classify
patients into the high and low PLK4 mRNA expression groups. The IHC
score and mRNA expression of PLK4 were compared between CRC and
para-carcinoma tissues using a paired t-test or Wilcoxon
signed-rank test. The proportion of patients with a high and low
PLK4 protein expression were compared between CRC and
para-carcinoma tissues using the McNamar's test. The association
between the PLK4 expression and tumor characteristics was analyzed
using Kruskal-Wallis followed by Dunn's test. The OS was
illustrated using a Kaplan-Meier curve and analyzed using a
log-rank test. Multivariate Cox's proportional hazard model
regression analysis was performed to identify prognostic factors.
In the in vitro experiment, the PLK4 expression between the
between HCoEpic and CRC cells, the PLK4 expression between groups
after transfection, cell viability and β-catenin expression were
analyzed using one-way ANOVA followed by Dunnett's or Tukey's
multiple comparisons test; IC50 between PLK4-siRNA and
NC-siRNA cells were analyzed using a Student's t-test. P<0.05
was considered to indicate a statistically significant difference.
Data analysis and graphing were conducted using SPSS 22.0 (IBM
Corp.) and GraphPad Prism 7.01 (GraphPad Software Inc.).
Results
Baseline characteristics
A total of 142 patients with CRC were enrolled in
the present study [mean age, 65.3±10.3 years; 55 (38.7%) females
and 87 (61.3%) males]. Of those, 21 (14.8%) had pathological grade
1, 99 (69.7%) patients had grade 2 and 22 (15.5%) patients had
grade 3 CRC. In addition, 3 (2.1%) patients had T1 stage, 15
(10.6%) patients had T2 stage, 122 (85.9%) patients had T3 stage
and 2 (1.4%) patients had T4 stage CRC. A total of 90 (63.4%)
patients had N0 stage, 35 (24.6%) patients had N1 stage and 17
(12.0%) patients had N2 stage CRC. Finally, 18 (12.7%) had TNM
stage I, 72 (50.7%) stage II and 52 (36.6%) stage III CRC. Other
detailed clinical features are shown in Table I.
 | Table I.Clinical features of the
patients. |
Table I.
Clinical features of the
patients.
| Items | CRC patients
(n=142) |
|---|
| Age (years),
mean±SD | 65.3±10.3 |
| Gender, No.
(%) |
|
|
Female | 55 (38.7) |
|
Male | 87 (61.3) |
| Pathological grade,
No. (%) |
|
| Grade
1 | 21 (14.8) |
| Grade
2 | 99 (69.7) |
| Grade
3 | 22 (15.5) |
| Tumor size (cm),
median (IQR) | 4.5 (3.5-5.0) |
| LYN positive, No.
(%) |
|
| No | 90 (63.4) |
|
Yes | 52 (36.6) |
| Number of LYN
positive, median (IQR) | 2.0 (1.0-4.0) |
| T stage, No.
(%) |
|
| T1 | 3 (2.1) |
| T2 | 15 (10.6) |
| T3 | 122 (85.9) |
| T4 | 2 (1.4) |
| N stage, No.
(%) |
|
| N0 | 90 (63.4) |
| N1 | 35 (24.6) |
| N2 | 17 (12.0) |
| TNM stage, No.
(%) |
|
| I | 18 (12.7) |
| II | 72 (50.7) |
|
III | 52 (36.6) |
| Adjuvant
chemotherapy, No. (%) |
|
| No | 40 (28.2) |
|
Yes | 102 (71.8) |
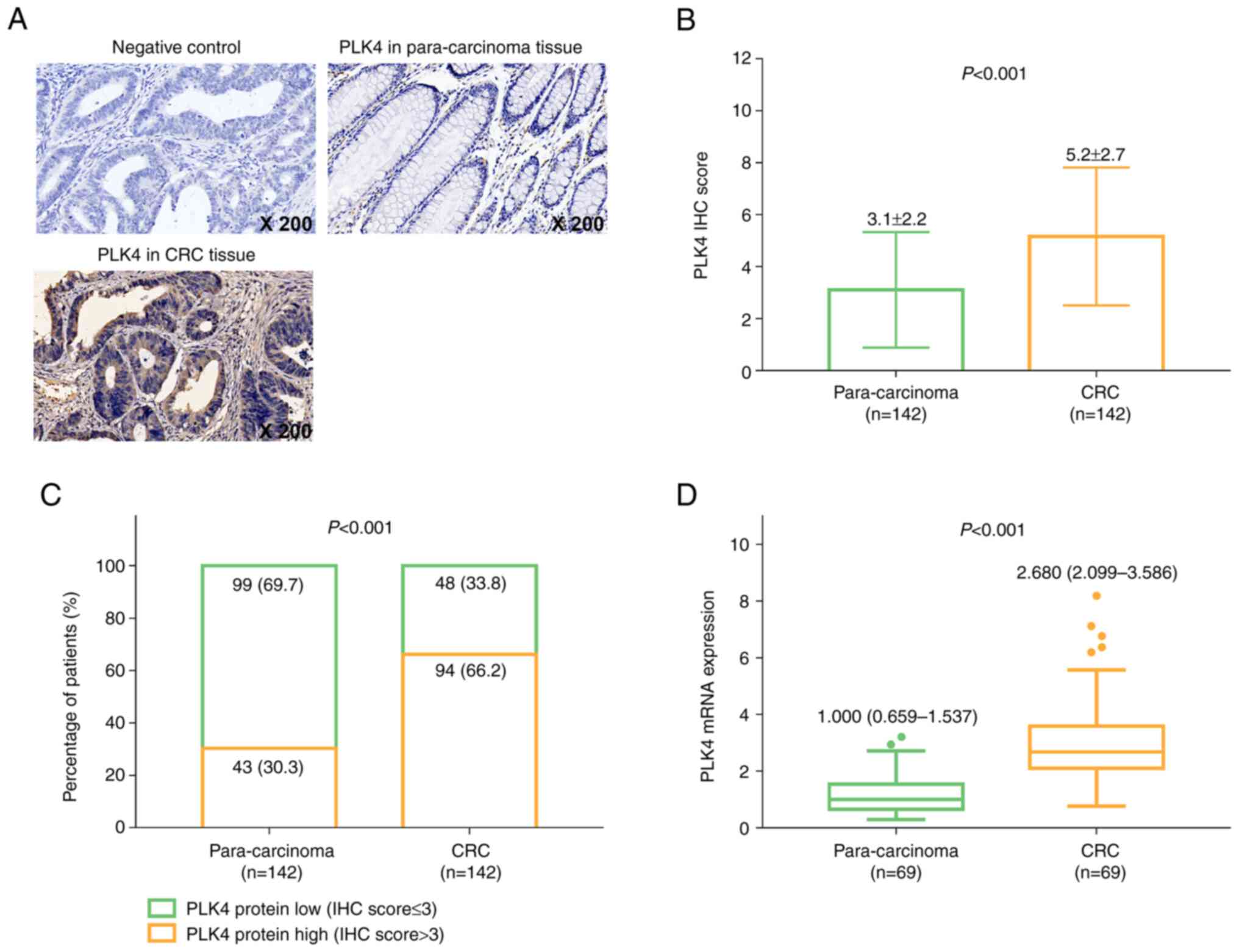
PLK4 is highly expressed in CRC
tissues
The PLK4 expression in the NC, para-carcinoma and
CRC tissues was detected using IHC staining (Fig. 1A). In addition, the IHC score of
PLK4 in CRC tissues was increased compared with that in
para-carcinoma tissues (mean value: 5.2±2.7 vs. 3.1±2.2,
P<0.001; Fig. 1B). In addition,
a PLK4 IHC score of 3 was used as the cut-off value for determining
high and low PLK4 protein expression. Further analysis revealed
that PLK4 protein expression was increased in CRC compared with
para-carcinoma tissues (P<0.001; Fig. 1C). In addition, PLK4 mRNA
expression in CRC tissues was elevated compared with that in
para-carcinoma tissues [median, 2.680 (2.099-3.586) vs. 1.000
(0.659-1.537); P<0.001; Fig.
1D).
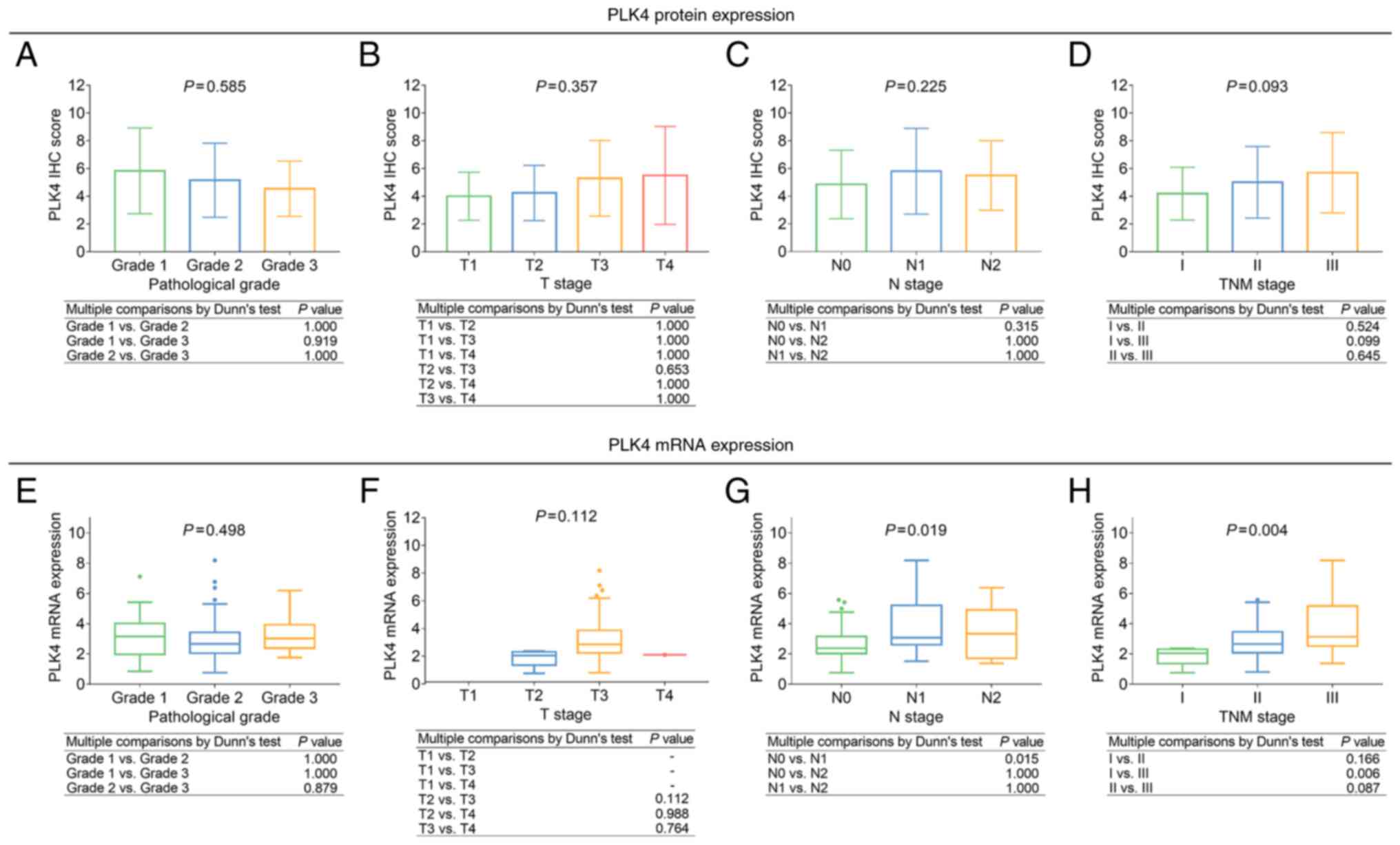
Tumor PLK4 is associated with advanced
tumor properties
Tumor PLK4 protein expression was not associated
with pathological grade, T stage, N stage, or TNM stage (all
P>0.05; Fig. 2A-D). Meanwhile,
the high tumor PLK4 mRNA expression was associated with more
advanced N stage (P=0.019) and TNM stage (P=0.004), but not with
pathological grade or T stage (both P>0.050; Fig. 2E-H).
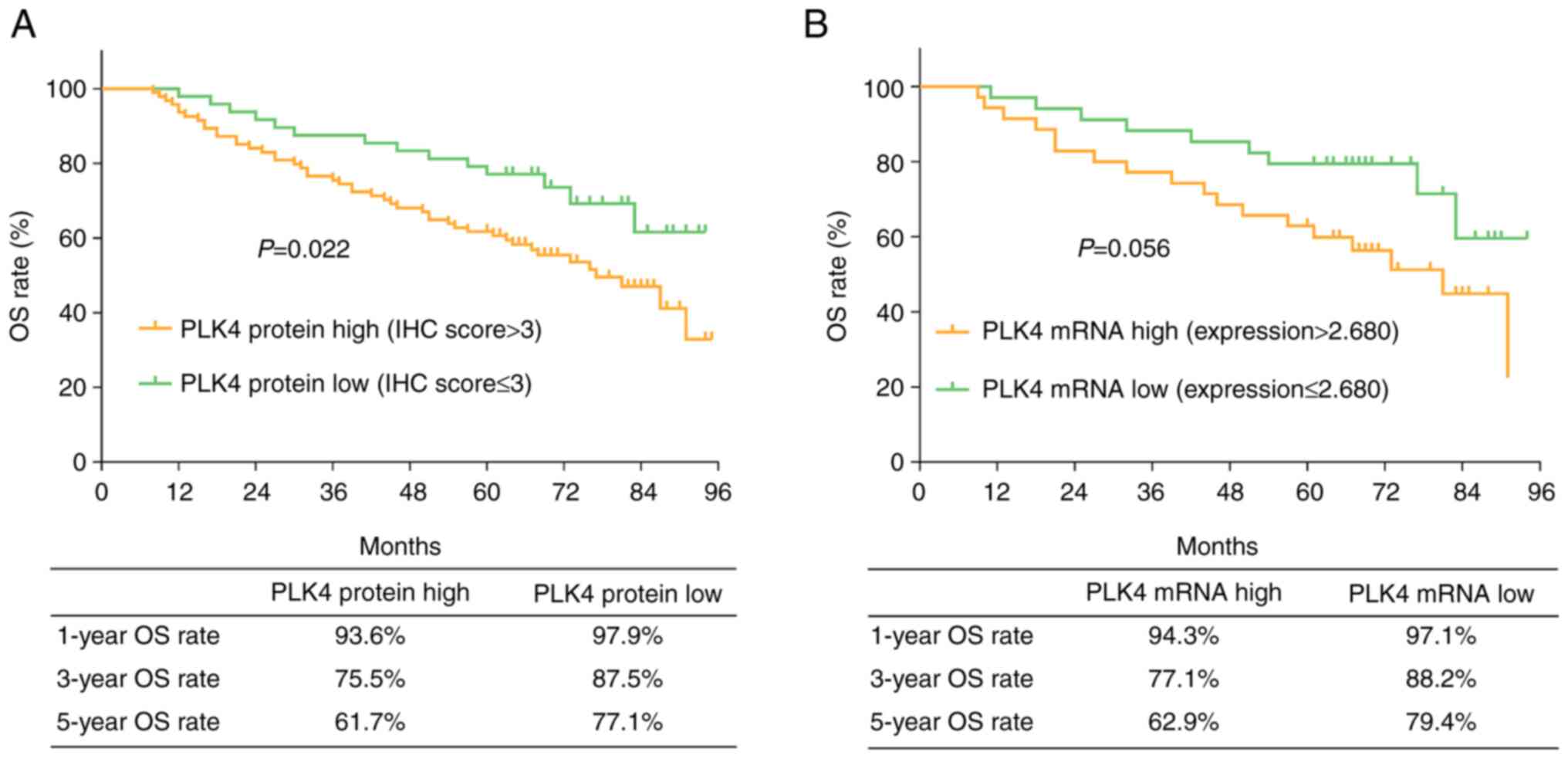
Tumor PLK4 is correlated with poor
prognosis
A high tumor PLK4 protein expression was correlated
with a short OS (P=0.022). The 1-, 3- and 5-year OS rates were
93.6, 75.5 and 61.7%, respectively, in patients with a high PLK4
protein expression, and 97.9, 87.5 and 77.1% in patients with a low
PLK4 protein expression (Fig. 3A).
Tumor PLK4 mRNA expression was not correlated with OS (P=0.056).
The 1-, 3- and 5-year OS rate was 94.3, 77.1 and 62.9% in patients
with a high PLK4 mRNA expression, and 97.1, 88.2 and 79.4% in
patients with a low PLK4 mRNA expression (Fig. 3B).
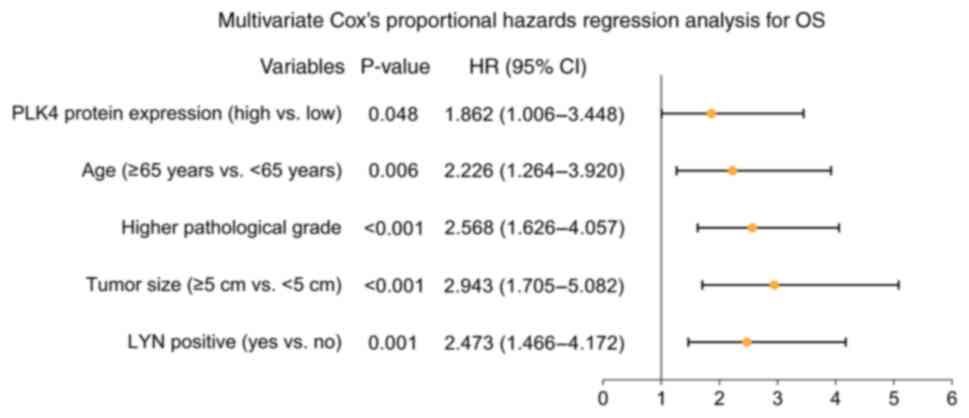
In addition, multivariate Cox's proportional hazards
regression analysis revealed that tumor PLK4 protein expression
(high vs. low, HR=1.862, P=0.048), age (≥65 years vs. <65 years;
HR=2.226, P=0.006), higher pathological grade (HR=2.568,
P<0.001), tumor size (≥5 cm vs. <5 cm; HR=2.943, P<0.001),
LYN positivity (yes vs. no; HR=2.473, P=0.001) were identified as
independent factors for a poor OS (Fig. 4).
PLK4-siRNA improves 5-FU sensitivity
in CRC cells
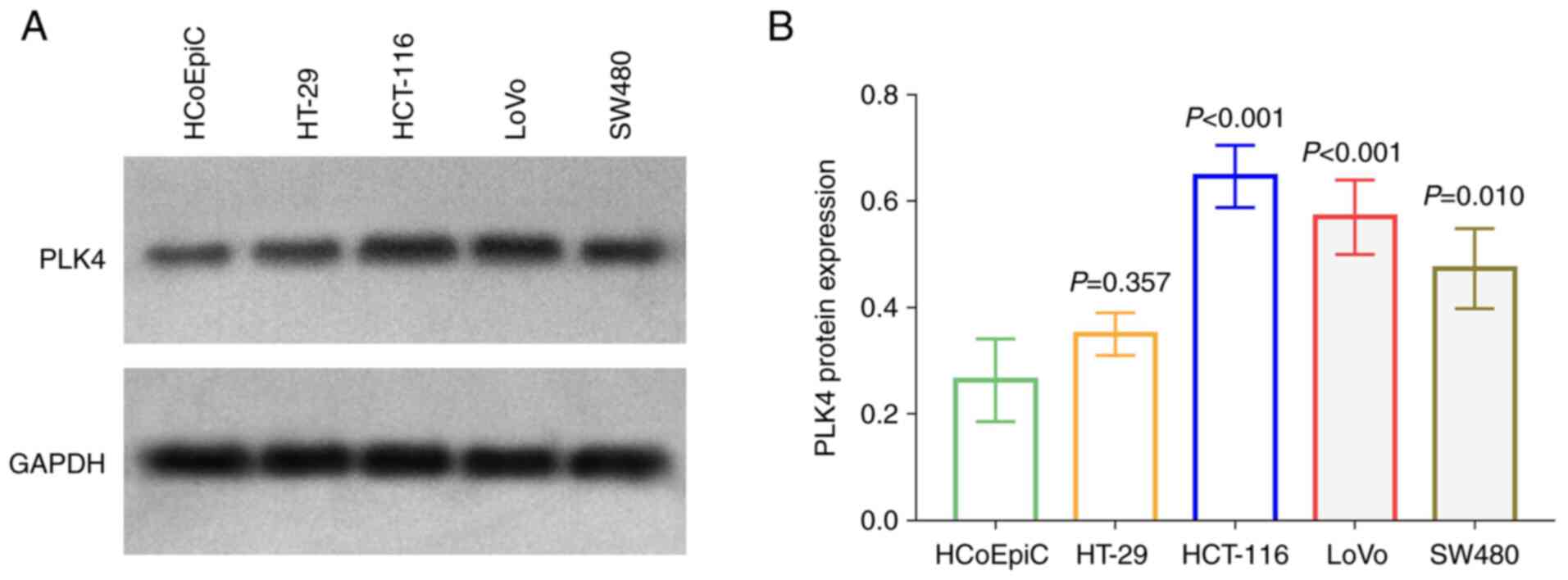
The protein level of PLK4 was increased in HCT-116,
LoVo and SW480 cells compared with HCoEpic (all P<0.05; Fig. 5A and B); since it was increased
more predominantly, the HCT-116 and LoVo cells were chosen for
further experiments. In the HCT-116 cell line, PLK4-siRNA reduced
mRNA level of PLK4 (P<0.001; Fig.
6A) as well as its protein level (P<0.001, Fig. S2A and B); and it markedly reduced
relative cell viability in cells treated with 2–16 µM 5-FU (all
P<0.05). Meanwhile, the IC50 value of 5-FU in
PLK4-siRNA cells was decreased compared with that in NC-siRNA cells
(4.4±0.1 µM vs. 7.6±1.4 µM; P=0.019; Fig. 6B and C). In the LoVo cell line,
PLK4-siRNA also decreased mRNA level of PLK4 (P<0.001; Fig. 6D) as well as its protein level
(P<0.001, Fig. S2A and B).
Besides, a relative decrease in cell viability was observed in
PLK4-siRNA cells compared with NC-siRNA cells treated with 4–16 µM
5-FU (all P<0.05), and the IC50 value of 5-FU in
PLK4-siRNA-transfected cells was reduced compared with that in
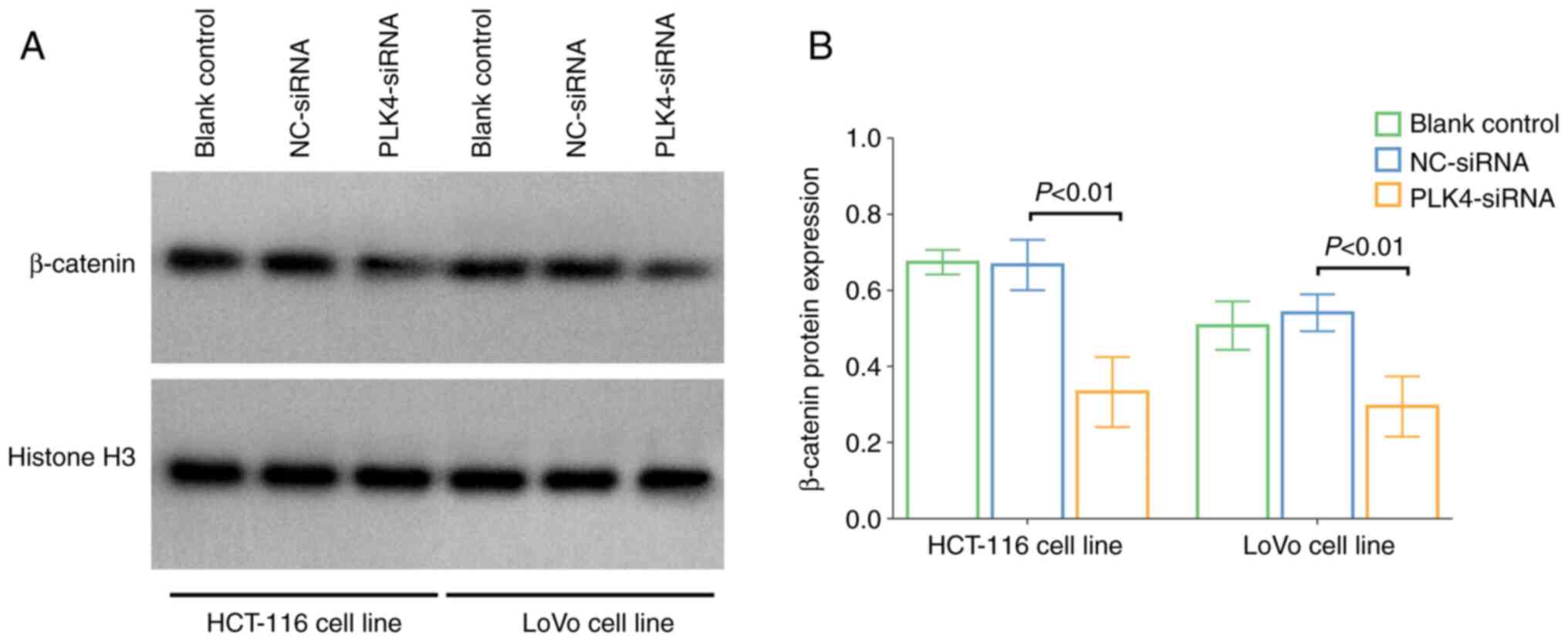
NC-siRNA cells (5.5±0.6 µM vs. 9.9±1.8 µM; P=0.014; Fig. 6E and F). In addition, the nuclear
translocation level of β-catenin was reduced in
PLK4-siRNA-transfected cells was reduced compared with that in
NC-siRNA cells (both P<0.01; Fig.
7A and B).
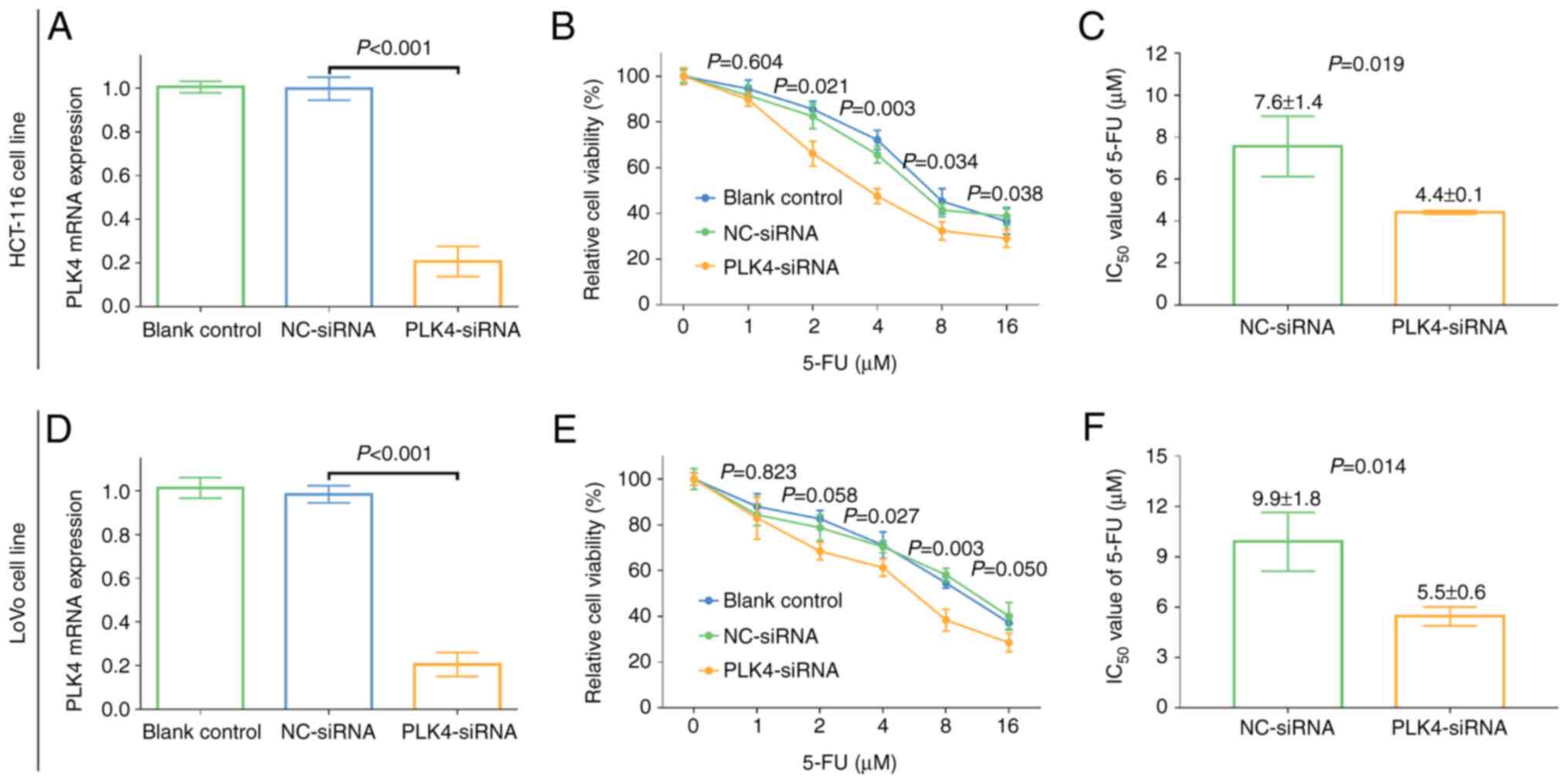 | Figure 6.Effect of PLK4-siRNA on 5-FU
sensitivity in CRC cell lines. (A) Comparison of the mRNA
expression of PLK4 among blank control, NC-siRNA and
PLK4-siRNA-treated HCT-116 cells. One-way ANOVA followed by Tukey's
multiple comparisons test was applied. (B) Comparison of cell
viability among blank control, NC-siRNA and PLK4-siRNA-treated
HCT-116 cells groups. One-way ANOVA followed by Dunnett's multiple
comparisons test was applied. (C) Changes in the IC50
value of 5-FU between NC-siRNA and PLK4-siRNA-treated HCT-116
cells. Student's t-test was applied. (D) Comparison of the mRNA
expression of PLK4 among blank control, NC-siRNA and
PLK4-siRNA-treated LoVo cells. One-way ANOVA followed by Tukey's
multiple comparisons test was applied. (E) Comparison of cell
viability among blank control, NC-siRNA and PLK4-siRNA-treated LoVo
cell groups. One-way ANOVA followed by Dunnett's multiple
comparisons test was applied. (F) Changes in the IC50
value of 5-FU between NC-siRNA and PLK4-siRNA-treated LoVo cells.
Student's t-test was applied. PLK4, polo-like kinase 4; 5-FU,
5-fluorouracil; CRC, colorectal cancer; IC50, 50%
inhibitory concentration; siRNA, short interfering RNA; NC,
negative control. |
Discussion
The PLK family members show distinct effect on
cancer progression, among which PLK1 critically regulates cell
cycles in various malignancies, while PLK4 may have less effect on
this (29). Previous studies have
implied that PLK4 may serve as a potential treatment target in
cancers (30–32). Meanwhile, high PLK4 expression is
associated with poor clinical and pathological features in cancer
patients (23,25,33).
For instance, high PLK4 is associated with LYN metastasis, distant
metastasis or surrounding recurrence in patients with breast cancer
(25). Furthermore, high PLK4
expression is associated with a large tumor size, LYN metastasis
and advanced TNM stage in patients with non-small cell lung cancer
(23). In hepatocellular carcinoma
patients, high PLK4 expression was associated with a more advanced
TNM stage (34). However, the role
of PLK4 in CRC has not been elucidated. In the present study, PLK4
protein and mRNA expression levels were higher in CRC compared with
para-carcinoma tissues, while tumor PLK4 was positively correlated
with TNM stage, which was consistent with the results of previous
studies on other tumors (23,34).
There are several potential reasons for these findings. i) PLK4
reflected the increased proliferation rate of cells, which is a
common characteristic of CRC cells but not of para-carcinoma cells;
thus, PLK4 expression was upregulated in CRC tissues compared with
para-carcinoma tissue. ii) PLK4 upregulation may cause centrosome
amplification, which facilitates tumor progression (16). iii) PLK4 may promote CRC invasion
and metastasis by regulating actin-related protein 2/3-mediated
actin cytoskeleton or Tec kinase phosphorylation (35,36),
and suppress CRC apoptosis through the Ataxia telangiectasia and
Rad3-related-checkpoint kinase 1 signaling pathway (34,37).
Meanwhile, CRC progression, invasion and metastasis may cause
larger tumor size and lymph node metastasis, thus PLK4 was
positively correlated with TNM stage in patients with CRC.
PLK4 is a potential predictor of poor outcomes in
cancer patients (38,39). For instance, upregulated expression
of PLK4 is correlated with worse progression-free survival and OS
in breast cancer patients (25).
Meanwhile, the high PLK4 mRNA expression was associated with a
shorter OS in patients with high-grade glioma (35). Bladder cancer patients with high
PLK4 expression have a lower OS than those with a low PLK4
expression (19). In the present
study, tumor PLK4 protein expression was negatively correlated with
OS, but tumor PLK4 mRNA expression was not. Furthermore, high tumor
PLK4 protein expression was an independent predictive factor for a
shorter OS. The reasons for this may be: i) the high expression of
tumor PLK4 was correlated with a higher TNM stage, indirectly
leading to a poor prognosis in patients with CRC; ii) PLK4 reduced
chemosensitivity through inhibitor of NF-κB kinase subunit ε
(IKBKE) signaling to influence the efficacy of adjuvant
chemotherapy, which can reduce the OS of patients with CRC
(40); iii) PLK4 may promote CRC
stemness and induce epithelial-mesenchymal transition by regulating
the Wnt/β-catenin signaling pathway, increasing the risk of CRC
recurrence (17,41).
It has been confirmed that suppressing PLK4 not only
decreases viability of CRC cells, but also increases
chemosensitivity in several types of cancer (17,39,40).
For instance, PLK4 affects temozolomide (TMZ) sensitivity, while
PLK4 inhibitor could enhance TMZ sensitivity through the
phosphorylation of IKBKE in glioblastoma (40). PLK4 inhibitor increases
conventional chemotherapeutic DNA-damaging agents (doxorubicin or
etoposide) sensitivity in rhabdoid tumors and medulloblastomas
(39). Considering that PLK4 is
associated with a poor prognosis in patients with CRC, the effect
of PLK4-siRNA on 5-FU sensitivity was evaluated. Consistent with
previous studies, PLK4-siRNA enhanced the 5-FU susceptibility in
the HCT-116 and LoVo cell lines. This may have been due to the fact
that the suppression of PLK4 may increase the anti-tumor effect of
5-FU by inhibiting the Wnt/β-catenin signaling pathway, which is a
key pathway causing chemoresistance in several cancer types
(17,42). Furthermore, it was observed that
PLK4 knockdown suppressed the nuclear translocation of β-catenin in
CRC cells, which could explain the effect of PLK4 on
chemosensitivity in CRC cells.
The present study had certain limitations: i) Only
CRC patients with TNM stage I–III were recruited; therefore, our
conclusion is not suitable for TNM stage IV patients. ii) Further
proof on the potential mechanism of PLK4 in CRC progression is
required. iii) The samples size of this study was somewhat
small.
In conclusion, the high expression of tumor PLK4 is
associated with an advanced TNM stage and shorter OS in patients
with CRC. Therefore, targeting PLK4 improves chemosensitivity in
CRC cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and ZD contributed substantially to the
conception and design of the study. LC and WW contributed
substantially to the acquisition, analysis and interpretation of
the data, and was involved in the drafting of the manuscript. ZD
and WW confirm the authenticity of all the raw data. LC and JC
contributed substantially to the interpretation of the data and was
involved in the critical revisions of the manuscript for important
intellectual content. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was conducted with approval from the
Institutional Review Board of Xi'an International Medical Center
Hospital (approval no. 2021013). All patients' data were analyzed
following desensitization and therefore patient' informed consent
was waved by the Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu S, Cao Q, An G, Yan B and Lei L:
Identification of the 3-lncRNA signature as a prognostic biomarker
for colorectal cancer. Int J Mol Sci. 21:93592020. View Article : Google Scholar
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
3
|
Wilkins T, McMechan D and Talukder A:
Colorectal cancer screening and prevention. Am Fam Physician.
97:658–665. 2018.PubMed/NCBI
|
|
4
|
Salibasic M, Pusina S, Bicakcic E, Pasic
A, Gavric I, Kulovic E, Rovcanin A and Beslija S: Colorectal cancer
surgical treatment, our experience. Med Arch. 73:412–414. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koi M and Carethers JM: The colorectal
cancer immune microenvironment and approach to immunotherapies.
Future Oncol. 13:1633–1647. 2017. View Article : Google Scholar
|
|
6
|
Nozawa H, Sonoda H, Ishii H, Emoto S,
Murono K, Kaneko M, Sasaki K, Nishikawa T, Shuno Y, Tanaka T, et
al: Postoperative chemotherapy is associated with prognosis of
stage IV colorectal cancer treated with preoperative
chemotherapy/chemoradiotherapy and curative resection. Int J
Colorectal Dis. 35:177–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang GR, Wang ZW and Jin ZY: Application
and progress of texture analysis in the therapeutic effect
prediction and prognosis of neoadjuvant chemoradiotherapy for
colorectal cancer. Chin Med Sci J. 34:45–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Zhang Y, Zhao W, Deng K, Wang Z,
Yang C, Ma L, Openkova MS, Hou Y and Li K: Metabolomics for
biomarker discovery in the diagnosis, prognosis, survival and
recurrence of colorectal cancer: A systematic review. Oncotarget.
8:35460–35472. 2017. View Article : Google Scholar
|
|
9
|
Jin M and Frankel WL: Lymph node
metastasis in colorectal cancer. Surg Oncol Clin N Am. 27:401–412.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ijsselsteijn R, Jansen JG and de Wind N:
DNA mismatch repair-dependent DNA damage responses and cancer. DNA
Repair (Amst). 93:1029232020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Konishi T, Shimada Y, Hsu M, Tufts L,
Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash
GM, et al: Association of preoperative and postoperative serum
carcinoembryonic antigen and colon cancer outcome. JAMA Oncol.
4:309–315. 2018. View Article : Google Scholar
|
|
12
|
Das V, Kalita J and Pal M: Predictive and
prognostic biomarkers in colorectal cancer: A systematic review of
recent advances and challenges. Biomed Pharmacother. 87:8–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y and Wang X: PLK4: A promising
target for cancer therapy. J Cancer Res Clin Oncol. 145:2413–2422.
2019. View Article : Google Scholar
|
|
14
|
Maniswami RR, Prashanth S, Karanth AV,
Koushik S, Govindaraj H, Mullangi R, Rajagopal S and Jegatheesan
SK: PLK4: A link between centriole biogenesis and cancer. Expert
Opin Ther Targets. 22:59–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Godinho SA, Picone R, Burute M, Dagher R,
Su Y, Leung CT, Polyak K, Brugge JS, Théry M and Pellman D:
Oncogene-like induction of cellular invasion from centrosome
amplification. Nature. 510:167–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DH, Ahn JS, Han HJ, Kim HM, Hwang J,
Lee KH, Cha-Molstad H, Ryoo IJ, Jang JH, Ko SK, et al: Cep131
overexpression promotes centrosome amplification and colon cancer
progression by regulating Plk4 stability. Cell Death Dis.
10:5702019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao Z, Zhang H, Fan P, Huang Q, Dong K,
Qi Y, Song J, Chen L, Liang H, Chen X, et al: High PLK4 expression
promotes tumor progression and induces epithelialmesenchymal
transition by regulating the Wnt/β-catenin signaling pathway in
colorectal cancer. Int J Oncol. 54:479–490. 2019. View Article : Google Scholar
|
|
18
|
Meng L, Zhou Y, Ju S, Han J, Song C, Kong
J, Wu Y, Lu S, Xu J, Yuan W, et al: A cis-eQTL genetic variant in
PLK4 confers high risk of hepatocellular carcinoma. Cancer Med.
8:6476–6484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Sun H, Ma W, Wu K, Peng G, Ou T
and Wu S: Down-regulation of Polo-like kinase 4 (PLK4) induces G1
arrest via activation of the p38/p53/p21 signaling pathway in
bladder cancer. FEBS Open Bio. 11:2631–2646. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Wang H, Yan M, Yang X, Shen R, Ni X,
Chen X, Yang P, Chen M, Lu X, et al: High LIN28A and PLK4
coexpression is associated with poor prognosis in epithelial
ovarian cancer. Mol Med Rep. 18:5327–5336. 2018.PubMed/NCBI
|
|
21
|
Pu JT, Hu Z, Zhang DG, Zhang T, He KM and
Dai TY: MiR-654-3p suppresses non-small cell lung cancer
tumourigenesis by inhibiting PLK4. Onco Targets Ther. 13:7997–8008.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N, Liu FL, Ma TS and Zhang ZZJ:
LncRNA SNHG1 contributes to tumorigenesis and mechanism by
targeting miR-338-3p to regulate PLK4 in human neuroblastoma. Eur
Rev Med Pharmacol Sci. 23:8971–8983. 2019.
|
|
23
|
Zhou Q, Fan G and Dong Y: Polo-like kinase
4 correlates with greater tumor size, lymph node metastasis and
confers poor survival in non-small cell lung cancer. J Clin Lab
Anal. 34:e231522020. View Article : Google Scholar
|
|
24
|
Hu Z, Gu X, Zhong R and Zhong H:
Tumor-infiltrating CD45RO+ memory cells correlate with
favorable prognosis in patients with lung adenocarcinoma. J Thorac
Dis. 10:2089–2099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Dai K, Wang C, Song Y, Gu F, Liu F
and Fu L: Expression of polo-like kinase 4(PLK4) in breast cancer
and its response to taxane-based neoadjuvant chemotherapy. J
Cancer. 7:1125–1132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fohlen A, Bordji K, Assenat E, Gongora C,
Bazille C, Boulonnais J, Naveau M, Breuil C, Pérès EA, Bernaudin M
and Guiu B: Anticancer drugs for intra-arterial treatment of
colorectal cancer liver metastases: In-vitro screening after short
exposure time. Pharmaceuticals (Basel). 14:6392021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Mo R and Zheng L: MicroRNA-490-3p
inhibits migration and chemoresistance of colorectal cancer cells
via targeting TNKS2. World J Surg Oncol. 19:1172021. View Article : Google Scholar
|
|
29
|
Liu X: Targeting polo-like kinases: A
promising therapeutic approach for cancer treatment. Transl Oncol.
8:185–195. 2015. View Article : Google Scholar
|
|
30
|
Parsyan A, Cruickshank J, Hodgson K,
Wakeham D, Pellizzari S, Bhat V and Cescon DW: Anticancer effects
of radiation therapy combined with polo-like kinase 4 (PLK4)
inhibitor CFI-400945 in triple negative breast cancer. Breast.
58:6–9. 2021. View Article : Google Scholar
|
|
31
|
Zhang X, Wei C, Liang H and Han L:
Polo-like kinase 4′s critical role in cancer development and
strategies for Plk4-targeted therapy. Front Oncol. 11:5875542021.
View Article : Google Scholar
|
|
32
|
Garvey DR, Chhabra G, Ndiaye MA and Ahmad
N: Role of polo-like kinase 4 (PLK4) in epithelial cancers and
recent progress in its small molecule targeting for cancer
management. Mol Cancer Ther. 20:632–640. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Zuo J, Wang M, Ma X, Gao K, Bai X,
Wang N, Xie W and Liu H: Pololike kinase 4 promotes tumorigenesis
and induces resistance to radiotherapy in glioblastoma. Oncol Rep.
41:2159–2167. 2019.PubMed/NCBI
|
|
34
|
Bao J, Yu Y, Chen J, He Y, Chen X, Ren Z,
Xue C, Liu L, Hu Q, Li J, et al: MiR-126 negatively regulates PLK-4
to impact the development of hepatocellular carcinoma via ATR/CHEK1
pathway. Cell Death Dis. 9:10452018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kazazian K, Go C, Wu H, Brashavitskaya O,
Xu R, Dennis JW, Gingras AC and Swallow CJ: Plk4 promotes cancer
invasion and metastasis through Arp2/3 complex regulation of the
actin cytoskeleton. Cancer Res. 77:434–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yeung SF, Zhou Y, Zou W, Chan WL and Ching
YP: TEC kinase stabilizes PLK4 to promote liver cancer metastasis.
Cancer Lett. 524:70–81. 2021. View Article : Google Scholar
|
|
37
|
Zhu Y, Liu Z, Qu Y, Zeng J, Yang M, Li X,
Wang Z, Su J, Wang X, Yu L and Wang Y: YLZ-F5, a novel polo-like
kinase 4 inhibitor, inhibits human ovarian cancer cell growth by
inducing apoptosis and mitotic defects. Cancer Chemother Pharmacol.
86:33–43. 2020. View Article : Google Scholar
|
|
38
|
Marina M and Saavedra HI: Nek2 and Plk4:
Prognostic markers, drivers of breast tumorigenesis and drug
resistance. Front Biosci (Landmark Ed). 19:352–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sredni ST, Bailey AW, Suri A, Hashizume R,
He X, Louis N, Gokirmak T, Piper DR, Watterson DM and Tomita T:
Inhibition of polo-like kinase 4 (PLK4): A new therapeutic option
for rhabdoid tumors and pediatric medulloblastoma. Oncotarget.
8:111190–111212. 2017. View Article : Google Scholar
|
|
40
|
Zhang Z, Wang Z, Huang K, Liu Y, Wei C,
Zhou J, Zhang W, Wang Q, Liang H, Zhang A, et al: PLK4 is a
determinant of temozolomide sensitivity through phosphorylation of
IKBKE in glioblastoma. Cancer Lett. 443:91–107. 2019. View Article : Google Scholar
|
|
41
|
Zhang Y, Kang M, Zhang B, Meng F, Song J,
Kaneko H, Shimamoto F and Tang B: m6A
modification-mediated CBX8 induction regulates stemness and
chemosensitivity of colon cancer via upregulation of LGR5. Mol
Cancer. 18:1852019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Miller Z, Musich PR, Thomas AE,
Yao ZQ, Xie Q, Howe PH and Jiang Y: DSTYK promotes metastasis and
chemoresistance via EMT in colorectal cancer. Front Pharmacol.
11:12502020. View Article : Google Scholar
|