Introduction
Adenoid cystic carcinoma (ACC) is a relatively rare
low-grade malignant tumor type, which accounts for ~1% of all head
and neck tumors and 10% of all malignant tumors of salivary gland
origin (1). At present, surgery
and postoperative radiotherapy are the standard treatment
modalities for localized ACC; however, the optimal treatment for
ACC with lung metastasis has remained to be established (2). Use of low-dose radiotherapy (LDRT)
combined with immunotherapy is being gradually used in clinical
settings (3,4). The present study reported on a case
of suboral ACC with lung metastasis who was treated at our
hospital. The pertinent literature on the mechanism and the
advantages of LDRT combined with immunotherapy in patients with
pulmonary metastases was also reviewed. The patient diagnosis and
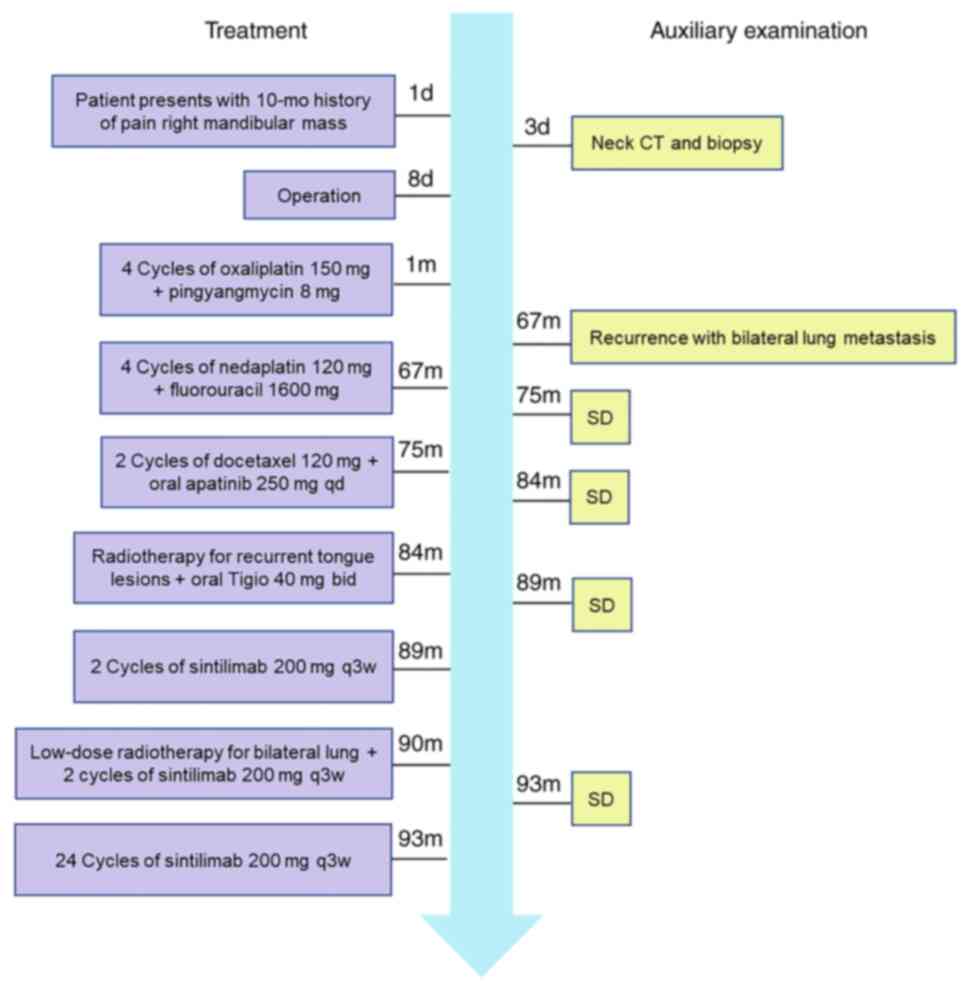
treatment are outlined in a timeline presented in Fig. 1. The present case may provide
insight regarding the treatment of ACC with bilateral lung
metastasis.
Case study
Case presentation
A 55-year-old female presented to the Department of
Oncology, West China Hospital, Sichuan University (Nanchong, China)
due to a painful right mandibular mass in May 2012. There was no
history of chronic disease or family history of adenoid cystic
carcinoma. Physical examination revealed a right mandibular mass
with no signs of inflammation. The mass had a hard texture and its
boundary was poorly demarcated from the surrounding tissues. The
mass measured ~3×3 cm. In addition, a prominent 2-cm right cervical
lymph node was noted.
Diagnostic assessment
The patient did not undergo magnetic resonance
imaging (MRI) due to financial constraints. Contrast-enhanced
computed tomography (CT) revealed a mass in the floor of the right
mouth, denervation atrophy of the right submandibular gland and
signs of lymph node involvement. Biopsy of the mass indicated
adenoid cystic carcinoma (ACC) of the floor of the right side of
the mouth.
Therapeutic intervention
Enlarged resection of the mass plus box-like
resection of the right side of mandible plus dissection of the
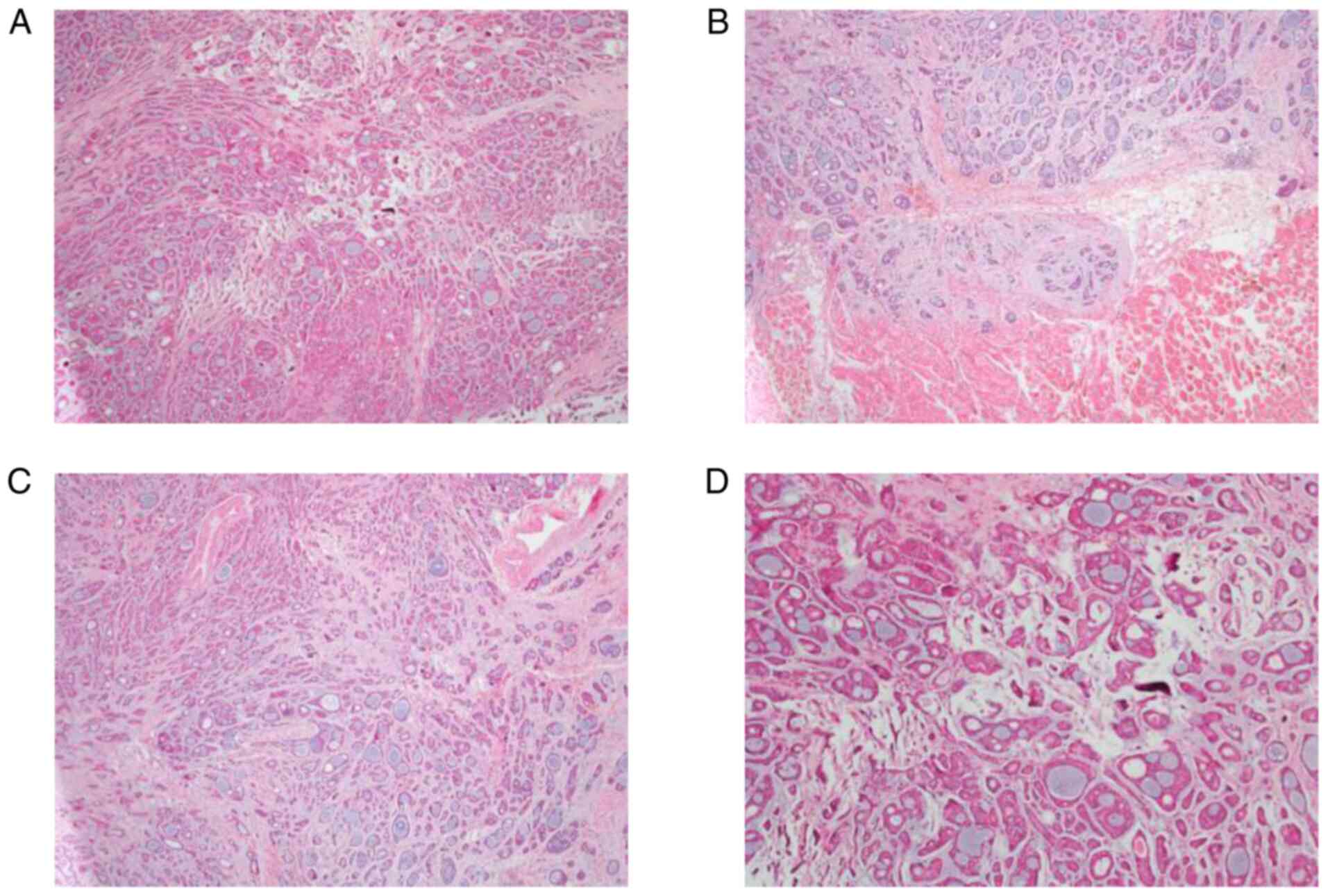
right neck lymph node was performed. Histopathological examination
of the surgical specimen confirmed the diagnosis (Fig. 2). The patient had indications for
post-operative radiotherapy. However, the patient refused
radiotherapy due to various reasons, such as financial constraints,
and was only willing to accept chemotherapy. Post-operatively, the
patient was administered chemotherapy with four cycles of
oxaliplatin 150 mg plus pingyangmycin 8 mg. Subsequently, the
patient underwent regular chemotherapy and follow-up
evaluation.
In October 2018, the patient was re-admitted to the
oncology department of our hospital due to shortness of breath and
dyspnea. Tongue MRI indicated a mass of ~5.3×4.7×4.6 cm on the
right side of the bottom of the mouth. Chest CT revealed multiple
diffuse soft tissue nodules in both lungs. Considering tumor
recurrence with bilateral lung metastasis, four cycles of PF
(nedaplatin 40 mg D1-3 plus fluorouracil 400 mg D1-4) chemotherapy
were administered at our department. On repeat tongue MRI and chest
CT performed in June 2019, the primary lesions and the metastatic
lesions in both lungs were slightly larger than those in October
2018 (stable disease, SD). According to the Response Evaluation
Criteria in Solid Tumours guidelines (5), SD was defined as neither sufficient
shrinkage to qualify for PR nor sufficient increase to qualify for
PD, pertaining to the smallest sum of diameters. PD means at least
a 20% increase in the sum of diameters of target lesions. Clearly,
the lung metastases in the present case did not reach PD. The
patient was administered two cycles of docetaxel 120 mg D1, while
oral apatinib 250 mg qd was administered as antiangiogenic
therapy.
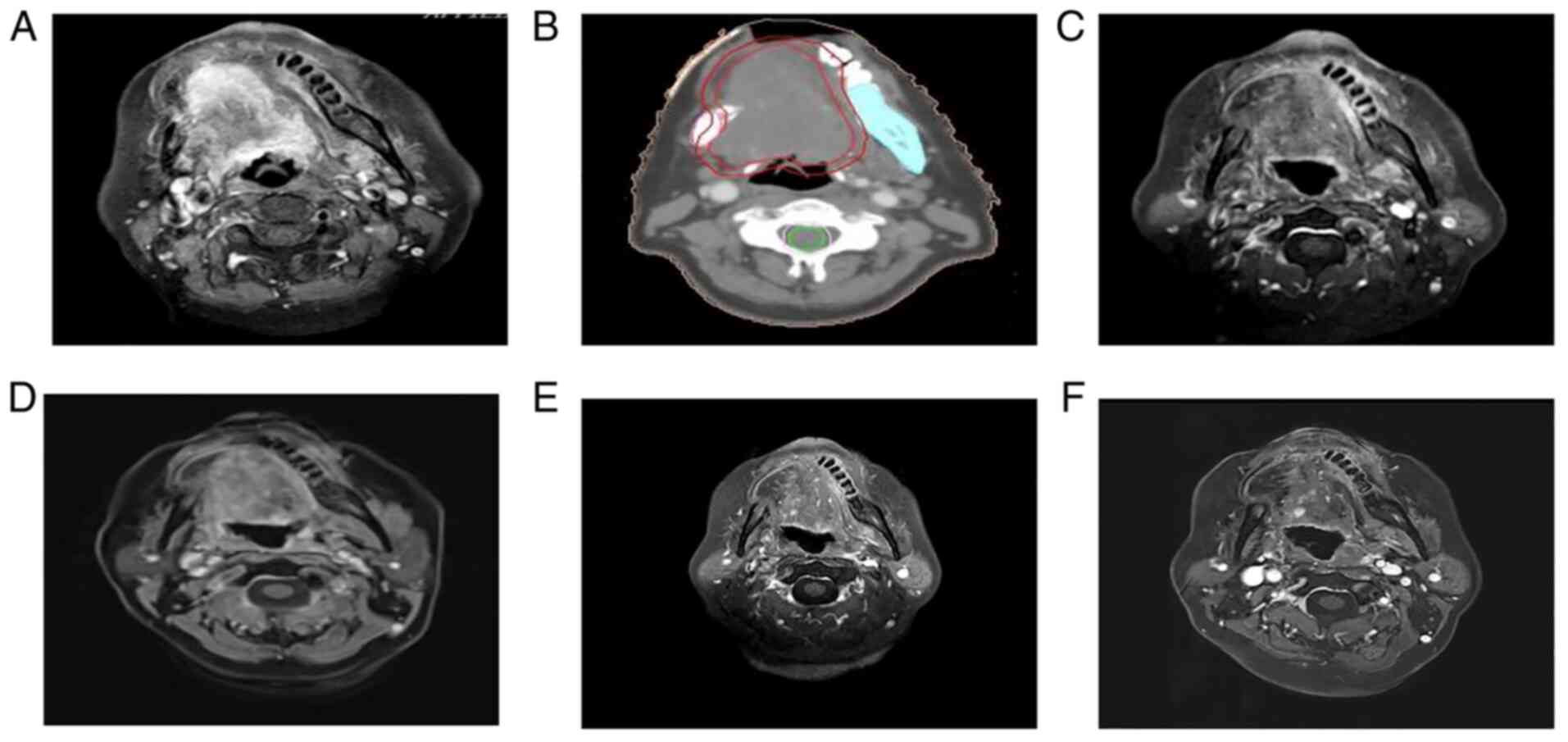
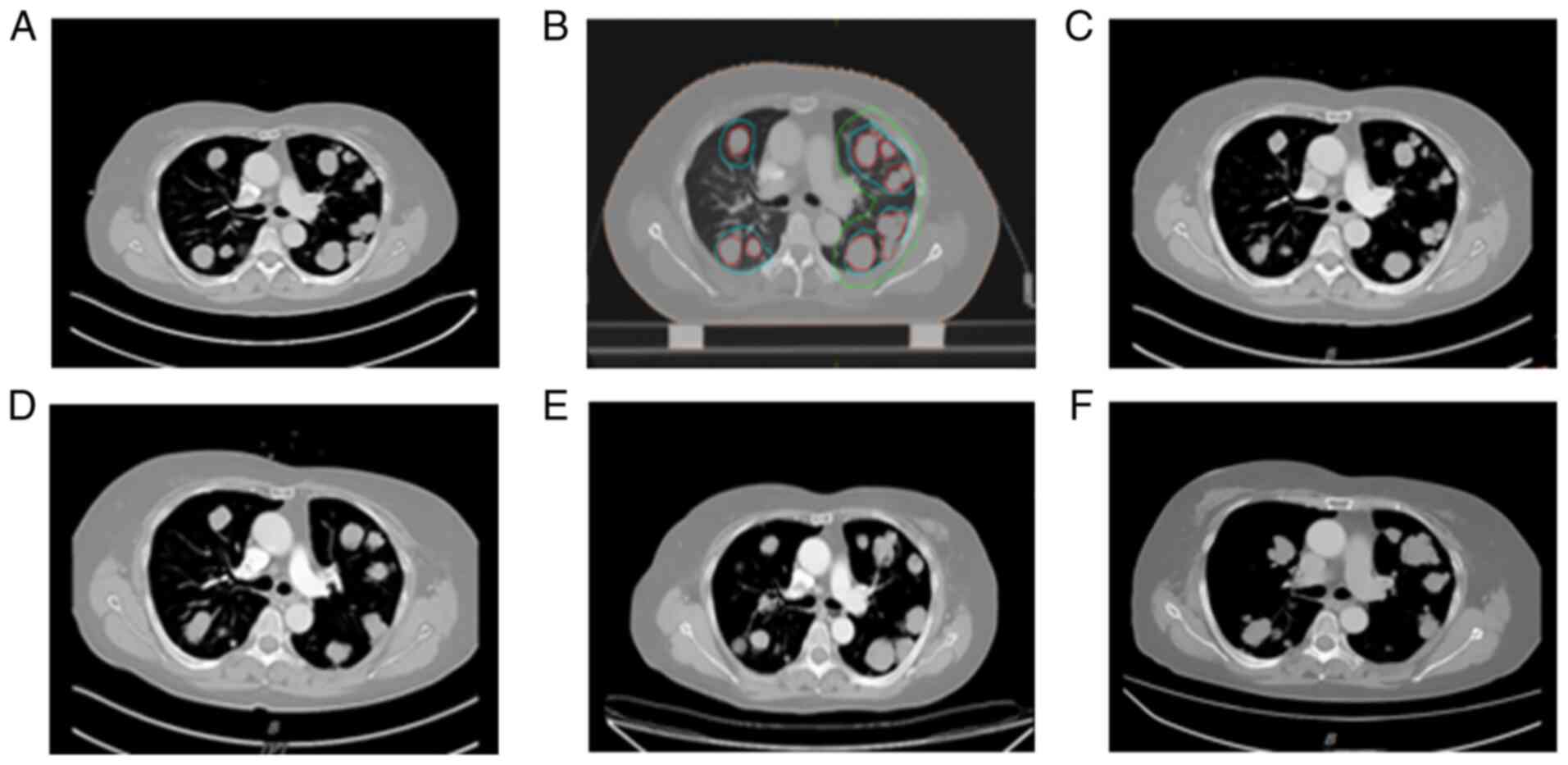
Repeat evaluation in March 2020 indicated a slight
increase in lung metastases, cervical lymph nodes and lesions in
the floor of the mouth (SD) (Figs.
3 and 4). Intensity-modulated
radiation therapy was used for palliative radiotherapy of tongue
tumors in March 2020. The patient was subjected to radiation
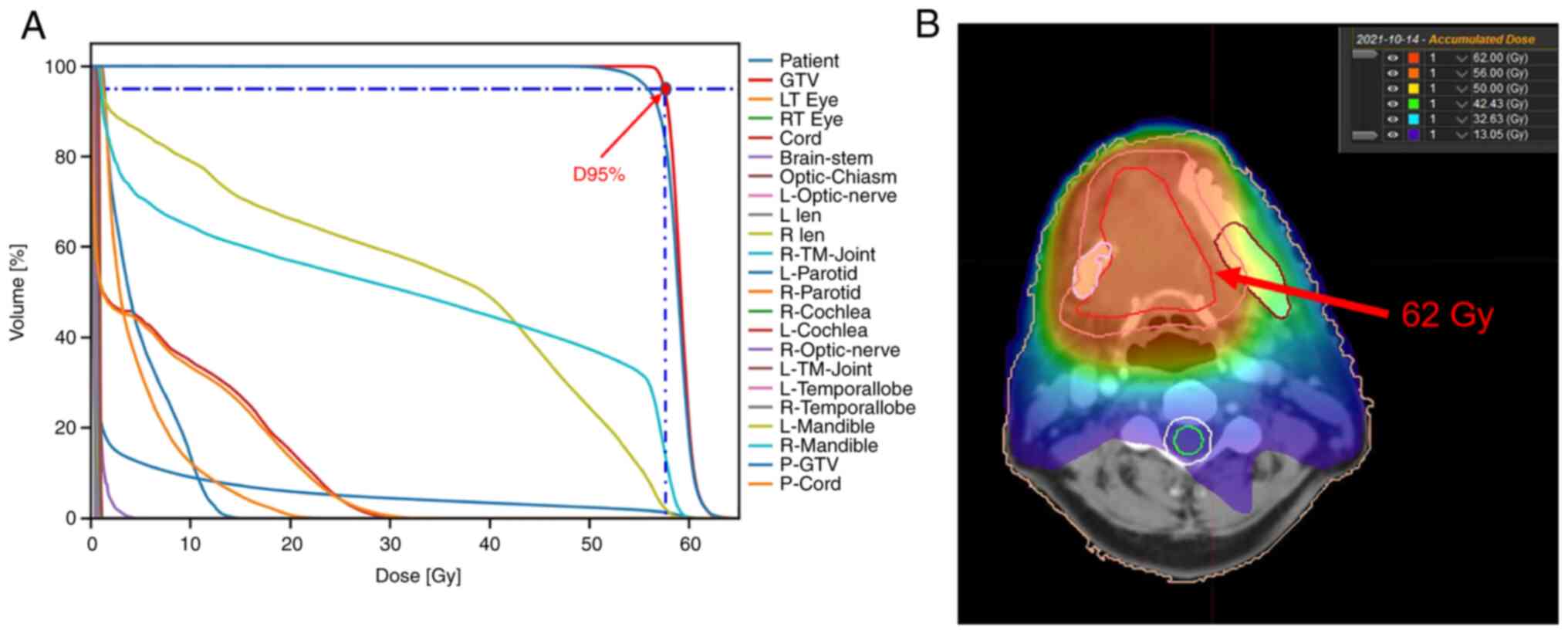
therapy, targeting the gross tumor volume expanding 1 cm outwards
at a dose of 60 Gy/2.0 Gy/30Fx (Fig.
5) and oral Tigio 40 mg twice a day was given as concurrent
chemotherapy. Chest CT examination in August 2020 indicated that
certain lung nodules were larger than those in March 2020
(evaluation: SD). Subsequently, two cycles of sintilimab 200 mg q3w
immunotherapy were administered and lung radiotherapy was initiated
in September 2020. The prescription dose was as follows: Clinical
target volume (CTV1) (right lung metastasis) 2.0 Gy/1Fx; CTV2 (left
lung metastasis) 2.0 Gy/1Fx; two cycles of concomitant sintilimab
200 mg q3w immunotherapy. This led to a significant alleviation of
shortness of breath. After 24 cycles of sintilimab 200 mg q3w
immunotherapy from November 2020 to February 2022, the lung lesions
were reduced in size and the tongue lesions were significantly
smaller than previously (evaluation: SD). At present (February
2022), the patient is in a stable condition and the general
condition is satisfactory. The main symptom is shortness of breath
induced by exercise. Laboratory examination indicated a slight
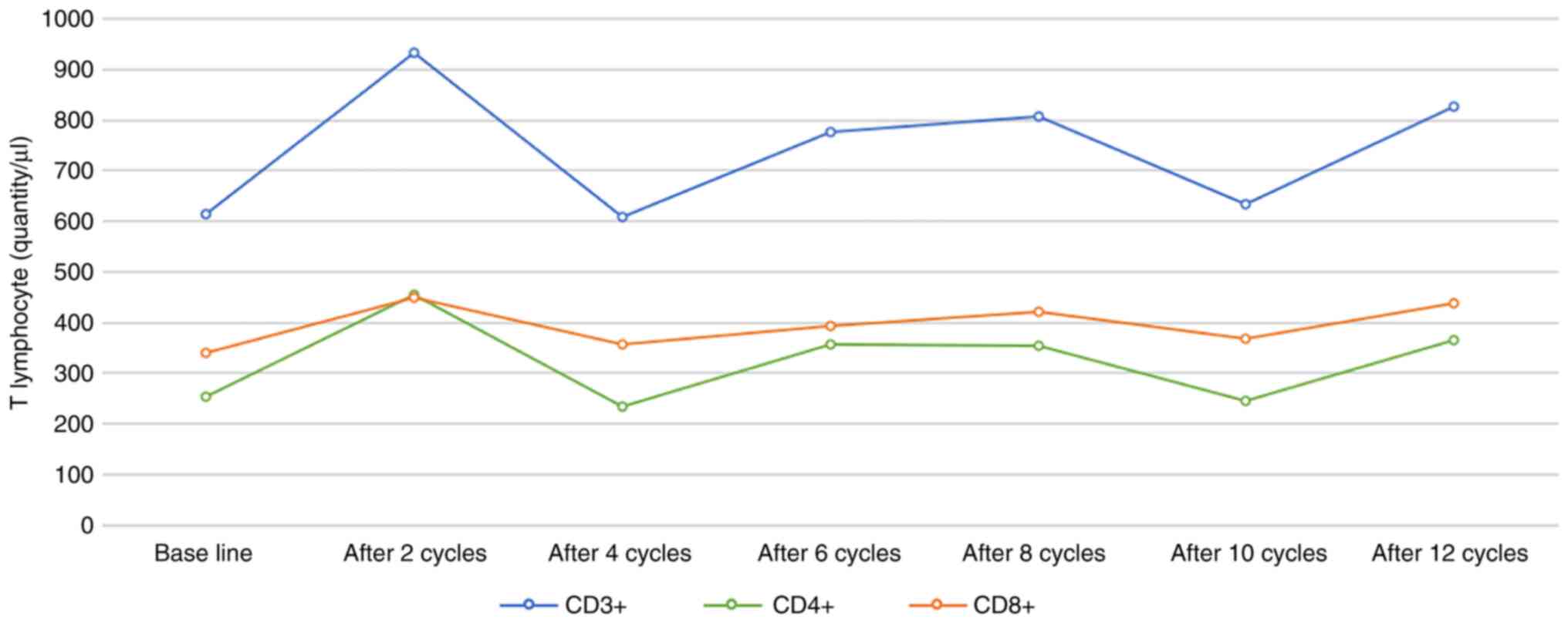
decrease in thyroid function. The counts of T-cell subsets were
measured in the peripheral blood of the patient after chemotherapy
and the temporal trend of its change is presented in Fig. 6. The patient continued treatment
with immunotherapy.
Discussion
ACC typically occurs in salivary gland tissues such
as minor salivary gland, sublingual gland, parotid gland and
submandibular gland (1). The
typical characteristics of ACC are slow growth, no local lymph node
metastasis and a tendency for recurrence and metastasis after
several years (6). The ACC lesion
is usually painless and the clinical course is typically insidious.
Therefore, the condition is frequently ignored by patients in the
early stage of the disease and most cases are typically diagnosed
at an advanced stage. With the progression of the disease, it may
metastasize to sites including the lung, bone, liver, brain and
kidney; of these, lung metastasis accounts for ~70% of metastatic
cases (7). Distant metastasis
usually occurs within 5 years after treatment. Failure to prevent
and control distant metastasis is one of the key determinants of
the low long-term survival rate of patients with ACC (6,8).
Tumor size, peripheral nerve infiltration and local recurrence are
risk factors for lung metastasis (9). In a study of 125 patients with ACC by
Jeong et al (10), the 10-year OS
rates of patients without distant metastasis and those with distant
metastasis were 81.5 and 60.2%, respectively. Due to the biological
characteristics of ACC, surgery is the first-choice treatment for
localized ACC. However, there is no effective treatment for
patients with ACC with lung metastasis. Girelli et al (8) studied 109 patients with lung
metastasis of ACC and observed that surgical treatment of lung
metastasis was effective in patients who had indications for
surgery; the 5- and 10-year survival rate of these patients was
66.8 and 40.5%, respectively. Systemic therapy is the main
treatment for patients with multiple metastases who are not able to
receive surgery or palliative radiotherapy (11).
Local radiotherapy may also have a certain effect on
the unirradiated distant metastatic lesions, a phenomenon referred
to as the remote effect (12).
Radiotherapy directly destroys tumor DNA and endoplasmic reticulum.
Tumor cells release a large quantity of tumor-associated antigens
after injury or death. These antigens are presented to T
lymphocytes, which stimulates their proliferation, inducing a
specific immune response (13).
Furthermore, radiotherapy may promote the expression of tumor
antigens by upregulating the expression of major histocompatibility
complex-I molecules, which improves the ability of antigen therapy
to recognize tumor cells (12). In
addition, radiotherapy may also induce the infiltration of T cells
and neutrophils in the tumor and activate the local immune
inflammatory response, resulting in inhibition of unirradiated
tumor cells (14). The combination
of radiotherapy and immunotherapy helps activate the immune
response and transforms the tumor microenvironment from
immunosuppressive to immunoreactive. Several studies have indicated
that the combination of radiotherapy and immunotherapy improves the
remote effect. The immune response may vary depending on the
radiation doses and radiotherapy regimens. In the study by Yin et
al (15), a 2Gy*1f radiotherapy
regimen was indicated to induce higher infiltration of CD4+ and
CD8+ T cells into tumor tissue than 2Gy*3f and 2Gy*5f regimens.
Shevtsov et al (16) obtained
similar results. They observed that, although LDRT (2Gy*1f) had a
poor killing effect on tumor cells, it may normalize tumor blood
vessels, thus promoting T-cell infiltration into the tumor and
improving the curative effect. LDRT (<1Gy) may induce
differentiation of macrophages into the M2 anti-inflammatory
phenotype, while high-dose radiotherapy (1–10 Gy) may induce their
differentiation into M1 pro-inflammatory phenotype (17). Yin et al (15) explored triple therapy of low-dose
radiotherapy (LDRT) and hypofractionated radiotherapy (HFRT)
combined with immunotherapy for non-small cell lung cancer. It was
indicated that the LDRT of distant metastases significantly
enhances the distant effect of HFRT combined with immunotherapy and
that the triple regimen is well tolerated by the patients (15). The present study also suggested
that LDRT upregulated the expression of genes involved in antigen
presentation and genes that promote tumor T-cell invasion.
In the present case, the previous multi-line therapy
programs, including surgery, chemotherapy, targeted therapy and
radiotherapy for recurrent lesions, failed, and there was an
increase in the number of metastatic lesions in both lungs. At six
years after the treatment, the patient developed extensive
metastasis in both lungs. According to the International Lung
Metastasis staging system, the patient was categorized as stage IV
(unable to be resected completely) with no surgical indication.
Considering the size and the number of pulmonary metastases and the
general condition of the patient, LDRT combined with immunotherapy
was administered, which controlled pulmonary metastases and local
recurrence. Studies have indicated that changes in lymphocyte
subsets may be used to assess the efficacy of immunotherapy and the
prognosis of patients with cancer (18). Immune markers such as natural
killer cells, dendritic cells and T-regulatory cells are not
routinely assessed but would be useful for monitoring the
effectiveness of the treatment. In a bilateral mouse colon tumor
model study (15), the number of
CD8+ T cells was significantly increased after low-dose
radiotherapy plus anti-programmed cell death-1 (PD-1) therapy.
After 1 cycle of anti-PD-1 treatment, the number of peripheral
blood T lymphocytes in the patient of the present study exhibited a
marked increase (CD3+, 933/µl; CD4+, 455/µl; CD8+, 449/µl; and
CD4+/CD8+ ratio, 1.01) as compared with the baseline (CD3+, 613/µl;
CD4+, 254/µl; CD8+, 339/µl; CD4+/CD8+, ratio 0.75), indicating the
effectiveness of the treatment. Subsequently, fluctuations in the
number of immune cells were observed over time, but the levels were
largely above the baseline level. To date, no consensus has been
reached regarding the optimal sequence of radiotherapy and
immunotherapy. Certain researchers suggested that anti-PD-1 therapy
has the best effect when administered within one week after
radiotherapy, but it may also be related to the selection and
efficacy of immunotherapy drugs. This patient of the present study
was treated with immunotherapy combined with sequential
radiotherapy. Repeat examination after 4 months of radiotherapy
indicated a decrease in the size of lung and tongue lesions, which
may reflect the remote effect, indicating that low-dose
radiotherapy may enhance T-lymphocyte infiltration in distant
tumors.
In conclusion, for the present case of ACC with
bilateral lung metastasis, satisfactory results were achieved with
low-dose lung radiotherapy combined with immunotherapy. At present,
there is no standardized treatment for bilateral lung metastasis of
ACC and this approach may be used as a feasible treatment model.
However, further studies are required to determine the optimal
radiotherapy dose and the optimal sequence of radiotherapy and
immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DYL was responsible for the conception, design,
content and writing of the manuscript. XZP made substantial
contributions to acquisition, analysis and interpretation of data
and revising the manuscript critically for important intellectual
content. XXZ and QYS reviewed the pathology findings and made
substantial contributions to conception, design, drafting and
revising the manuscript. GW was responsible for the treatment and
management of the patient and was responsible for acquisition,
analysis and interpretation of the images. DYM made substantial
contributions to conception and design, revising and proofreading
the manuscript and gave final approval of the version to be
published. DYL and XXZ confirm the authenticity of all the raw
data. All authors were involved in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was reviewed and approved by the Ethics
Committee of Affiliated Hospital of North Sichuan Medical College
(Nanchong, China).
Patient consent for publication
Written informed consent was obtained from the
subject for the publication of any potentially identifiable images
or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adenoid cystic carcinoma
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
LDRT
|
low-dose radiotherapy
|
|
HFRT
|
hypofractionated radiotherapy
|
|
SD
|
stable disease
|
|
GTV
|
gross tumor volume
|
|
CTV
|
clinical target volume
|
|
PD-1
|
programmed cell death-1
|
References
|
1
|
Dodd RL and Slevin NJ: Salivary gland
adenoid cystic carcinoma: A review of chemotherapy and molecular
therapies. Oral Oncol. 42:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradley PJ: Adenoid cystic carcinoma
evaluation and management: Progress with optimism! Curr Opin
Otolaryngol Head Neck Surg. 25:147–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel RR, He K, Barsoumian HB, Chang JY,
Tang C, Verma V, Comeaux N, Chun SG, Gandhi S, Truong MT, et al:
High-dose irradiation in combination with non-ablative low-dose
radiation to treat metastatic disease after progression on
immunotherapy: Results of a phase II trial. Radiother Oncol.
162:60–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menon H, Chen D, Ramapriyan R, Verma V,
Barsoumian HB, Cushman TR, Younes AI, Cortez MA, Erasmus JJ, de
Groot P, et al: Influence of low-dose radiation on abscopal
responses in patients receiving high-dose radiation and
immunotherapy. J Immunother Cancer. 7:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–2247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Weert S, Reinhard R, Bloemena E, Buter
J, Witte BI, Vergeer MR and Leemans CR: Differences in patterns of
survival in metastatic adenoid cystic carcinoma of the head and
neck. Head Neck. 39:456–463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seok J, Lee DY, Kim WS, Jeong WJ, Chung
EJ, Jung YH, Kwon SK, Kwon TK, Sung MW and Ahn SH: Lung metastasis
in adenoid cystic carcinoma of the head and neck. Head Neck.
41:3976–3983. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Girelli L, Locati L, Galeone C, Scanagatta
P, Duranti L, Licitra L and Pastorino U: Lung metastasectomy in
adenoid cystic cancer: Is it worth it? Oral Oncol. 65:114–118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma VJ, Gupta A, Yaftian N, Ball D,
Brown R, Barnett S and Antippa P: Low recurrence of lung adenoid
cystic carcinoma with radiotherapy and resection. ANZ J Surg.
89:1051–1055. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong IS, Roh JL, Cho KJ, Choi SH, Nam SY
and Kim SY: Risk factors for survival and distant metastasis in 125
patients with head and neck adenoid cystic carcinoma undergoing
primary surgery. J Cancer Res Clin Oncol. 146:1343–1350. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck-An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Locy H, de Mey S, de Mey W, De Ridder M,
Thielemans K and Maenhout SK: Immunomodulation of the tumor
microenvironment: Turn foe into friend. Front Immunol. 9:29092018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhalla N, Brooker R and Brada M: Combining
immunotherapy and radiotherapy in lung cancer. J Thorac Dis. 10
(Suppl 13):S1447–S1460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theelen WS, de Jong MC and Baas P:
Synergizing systemic responses by combining immunotherapy with
radiotherapy in metastatic non-small cell lung cancer: The
potential of the abscopal effect. Lung Cancer. 142:106–113. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin L, Xue J, Li R, Zhou L, Deng L, Chen
L, Zhang Y, Li Y, Zhang X, Xiu W, et al: Effect of low-dose
radiation therapy on abscopal responses to hypofractionated
radiation therapy and Anti-PD1 in mice and patients with non-small
cell lung cancer. Int J Radiat Oncol Biol Phys. 108:212–224. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shevtsov M, Sato H, Multhoff G and Shibata
A: Novel approaches to improve the efficacy of immuno-radiotherapy.
Front Oncol. 9:1562019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhawan G, Kapoor R, Dhawan R, Singh R,
Monga B, Giordano J and Calabrese EJ: Low dose radiation therapy as
a potential life saving treatment for COVID-19-induced acute
respiratory distress syndrome (ARDS). Radiother Oncol. 147:212–216.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schalper KA, Brown J, Carvajal-Hausdorf D,
McLaughlin J, Velcheti V, Syrigos KN, Herbst RS and Rimm DL:
Objective measurement and clinical significance of TILs in
non-small cell lung cancer. J Natl Cancer Inst. 107:dju4352015.
View Article : Google Scholar : PubMed/NCBI
|