Introduction
BC is one of the most prevalent malignant tumor
types. It affects ~550,000 females each year (1,2).
Approximately 90% of BC cases originate from urothelial cells
(1,3). Smoking has long been recognized as
the most significant risk factor for BC (4). BC is classified into two subtypes
based on the depth of invasion: Non-muscle-invasive BC (NMIBC) and
muscle-invasive BC (MIBC). This classification helps guide
treatment decisions (1).
Currently, NMIBC is frequently treated by adjuvant intravesical
chemotherapy, bacillus Calmette-Guérin (BCG) instillations
(3) and cystectomy in cases of
BCG-unresponsive tumors or high risk of progression and recurrence
(5). Furthermore, patients with
MIBC are routinely treated with radical cystectomy and
neoadjuvant/adjuvant chemotherapy (6). BC is associated with a high risk of
morbidity and mortality worldwide if it is not treated properly
(7). In a population-based study
of 321 patients with BC in Iran's Kurdistan Province (2013–2018),
the 5-year survival rate was 54% (8). In Europe, the 5-year age-standardized
relative survival rate was ~70%, varying amongst specific countries
by an average of 60-80% (2). Given
the disease limitations in terms of treatment options and drug
resistance, it is critical to investigate more effective
therapeutic targets.
Ferroptosis is a type of cell death with distinct
properties and functions associated with physical conditions or
numerous diseases, including cancers (9). Compared with normal cells, cancer
cells require more iron to support their increased rate of
proliferation. This iron dependency makes cancer cells more
vulnerable to iron-catalyzed necrosis (10). Du et al (11) identified that calumenin may affect
the prognosis of BC through ferroptosis based on the analysis of a
The Cancer Genome Atlas (TCGA) dataset of 408 patients with BC
using multi-omics bioinformatics. According to Chen et al
(12), compound 7j, a
quinazolinyl-arylurea derivative, triggered ferroptosis in BC cells
when used at high concentrations and over an extended incubation
time by effectively regulating the SystemXc-/glutathione peroxidase
4 (GPX4)/reactive oxygen species (ROS) and PI3K/Akt/mTOR/ unc-51
like autophagy activating kinase 1 pathways. Guo et al
(13) developed a triple therapy
for inducing ferroptosis in BC cells, which may possess significant
potential for clinical translation. As accumulating evidence
indicates, triggering ferroptosis for cancer therapy may be a
viable option, particularly for the eradication of aggressive
malignancies that are resistant to traditional therapies (14). Previous research has established a
critical role of ferroptosis in BC and four prognostic targets have
been identified (15). However,
additional research is necessary.
In the present study, mRNA expression profiles and
corresponding clinical data of patients with BC were downloaded
from public databases. A multigene signature associated with
prognosis was constructed using genes that were related to
ferroptosis and differentially expressed in the TCGA cohort. In
addition, the results were validated in cohorts from the Gene
Expression Omnibus (GEO) database. Next, the underlying mechanisms
were explored using functional enrichment and phenotypic analysis.
Finally, an analysis of glucose-6-phosphate dehydrogenase (G6PD), a
ferroptosis-related gene (FRG) was performed, which deserves
additional research in BC.
Materials and methods
Data collection and sources
The mRNA expression data and clinical follow-up data
of patients with BC were retrieved from the TCGA database up to
March 2021 (TCGA-BLCA; http://portal.gdc.cancer.gov/) (16). The validation cohort comprised 165
tumor samples obtained from the GEO database (GEO; http://www.ncbi.nlm.nih.gov/geo/) (dataset no.
GSE13507) (17). In addition, the
immunotherapy dataset was downloaded from IMvigor210 (http://research-pub.gene.com/IMvigor210CoreBiologies),
a publicly available data package. The ‘limma’ R package was used
to normalize the gene expression profiles. As all data were
acquired following the relevant guidelines and policies for data
access of the TCGA and GEO, no local ethical approval was required
for this study. The datasets GSE76211 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE76211)
and GSE130598 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE130598)
were used to evaluate the mRNA level of G6PD in BC and adjacent
normal tissue (18,19).
FRGs were identified from previous studies (10,20–22).
All of the FRGs are listed in Table
SI. The immunohistochemistry (IHC) staining of normal and BC
samples was obtained from the human protein atlas (HPA; http://www.proteinatlas.org/), an open-access database
containing multiple protein expression images.
Construction and external validation
of the 6-gene signature
Using the ‘limma’ package in the R software,
differential gene expression between BC and normal tissues was
analyzed using P<0.05 and false discovery rate (FDR)<0.05 as
the screening criteria. Cox-proportional hazard regression was used
to perform univariate survival analysis to identify prognostic
FRGs. The protein-protein interaction network of differentially
expressed genes (DEGs) was analyzed using the Search Tool for the
Retrieval of Interacting Genes and proteins (STRING) database
(http://string-db.org/) (23). The R package ‘glmnet’ was used to
develop a gene-related prognostic model for the least absolute
shrinkage and selection operator (LASSO) Cox regression analysis
(24,25). The normalized expression matrix of
the prognostic DEGs served as independent variables, while the
overall survival (OS) and survival status (alive or dead) of
patients served as response variables in the regression. The
optimal value of the penalty parameter (λ) was determined according
to the minimum 10-fold cross-validations and used to select
significant features for the model. The risk score for each patient
consisted of the normalized expression values of each gene and the
corresponding regression coefficients. It was calculated using the
following formula: risk score=esum (each gene's normalized
expression × each gene's corresponding coefficient). To
analyze the distribution of different groups, principal component
analysis (PCA) was performed using the R package ‘prcomp’ and
t-distributed stochastic neighbor embedding (t-SNE) was performed
using the ‘Rtsne’ R package. For the evaluation of the impact of
various factors on OS, Kaplan-Meier curves were drawn and
differences were assessed using the log-rank test. The optimal
cut-off value for the survival analysis was determined using the R
package ‘survminer’. The R package ‘survivalROC’ was used to depict
time-dependent receiver operating characteristic (ROC) curves.
Functional and gene set enrichment
analysis (GSEA)
Gene Ontology (GO) functional and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway enrichment was performed using
the ‘clusterProfiler’ R package. Gene set variant analysis (GSVA)
was performed using the ‘gsva’ R package. GSEA was performed based
on groups according to their risk score or G6PD expression
(26). Both analyses used the
Hallmark gene set with FDR<0.25 and nominal (NOM) P<0.05 as
the criterion. Single sample (ss)GSEA was carried out using the
‘gsva’ package (27).
Cell lines
A total of three BC cell lines (5637, J82 and T24)
and human normal bladder epithelial cells (SV-HUC-1) were obtained
from the Cell Bank of the Chinese Academy of Sciences. Cells were
cultured under conventional cell culture conditions of 5%
CO2 at 37°C in RPMI 1640 medium (MilliporeSigma) with
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted using an RNA extraction kit
(cat. no. 74004; Qiagen GmbH) and then reverse-transcribed into
cDNA using the FastLane Cell cDNA Kit (cat. no. 215011; Qiagen
GmbH) according to the manufacturer's instructions. The primers
(G6PD forward, 5′-CGAGGCCGTCACCAAGAAC-3′ and reverse,
5′-GTAGTGGTCGATGCGGTAGA-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′) were designed and synthesized in
Tsingke. qPCR was performed using the SYBR Green PCR system (cat.
no. A25776; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The PCR cycling program included a 30
sec incubation step at 95°C and then 40 cycles of 5 sec at 95°C and
30 sec at 60°C. A MyCycler Thermal Cycler (cat. no. 170-9703;
Bio-Rad Laboratories, Inc.) was used for qPCR. GAPDH was used for
normalization and the relative expression levels were calculated
using the 2−∆∆Cq method (28).
Statistical analysis
The Wilcoxon signed-rank test was used to compare
gene expression levels in BC tissues to those in adjacent
non-tumorous tissues. The χ2 test was used to compare
proportions between groups. The ssGSEA scores of different groups
were compared using the Mann-Whitney U test. All reported P-values
were adjusted for multiple comparisons using the Benjamini-Hochberg
method. Univariate and multivariate were performed to determine the
independent prognostic factors. Association analysis was performed
using the R packages ‘ggplot2’ and ‘ggpubr’ and the tests used were
the unpaired t-test (between two groups) and analysis of variance
(among multiple groups). Tukey's post hoc test was applied to
analyze the statistical significance between each of the cancer
cell lines and the normal bladder cell line. All analyses were
conducted using R software (v.3.6.3), Excel (Microsoft; 2016) or
GSEA (v.4.0.1). All P-values were two-tailed and P<0.05 was
considered to indicate statistical significance, unless otherwise
specified.
Results
Data sourcing
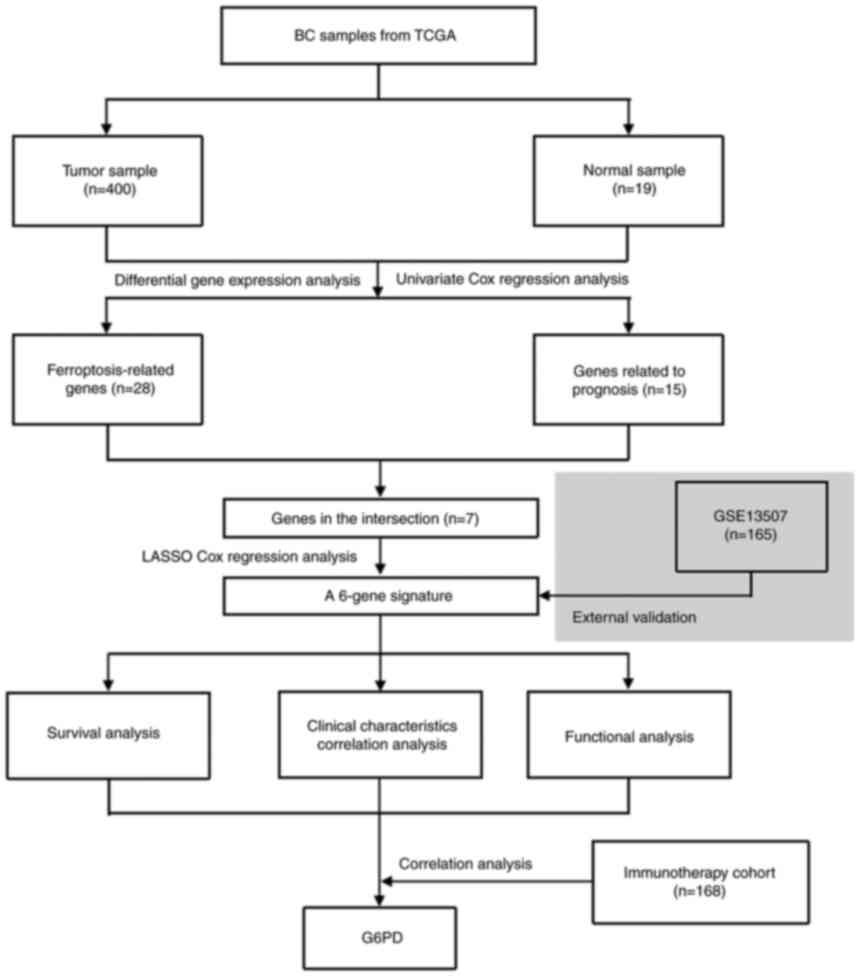
Fig. 1 depicts a
flow chart of the present study. A total of 400 BC samples and 19
adjacent non-tumor samples from the TCGA database, as well as 165
tumor samples from the GEO database, were included in the analysis.
Table I summarizes the detailed
clinical characteristics of the patients with BC. In the TCGA
cohort, the average participant age was 68 (34–89) years and 74.2% of the patients
were male. In the GEO cohort, the mean age was 65 (24–88) years and the proportion of male
patients was 81.8%.
 | Table I.Characteristics of patients used in
this study. |
Table I.
Characteristics of patients used in
this study.
| Item | TCGA cohort
(n=400) | GEO cohort
(n=165) |
|---|
| Age, years | 68 (34–89) | 65 (24–88) |
| Sex |
|
|
|
Female | 103 (25.8) | 30 (18.2) |
|
Male | 297 (74.2) | 135 (81.8) |
| Grade |
|
|
|
Low | 20 (5.0) | 105 (63.6) |
|
High | 377 (94.3) | 60 (36.4) |
|
Unknown | 3 (0.7) | 0 |
| Vascular
invasion |
|
|
|
Yes | - | 62 (37.6) |
| No | - | 103 (62.4) |
| Stage |
|
|
| I | 2 (0.5) | - |
| II | 127 (31.8) | - |
|
III | 138 (34.5) | - |
| IV | 131 (32.7) | - |
|
Unknown | 2 (0.5) | - |
| Overall survival,
months | 48.4
(1.03-136.97) | - |
| Risk, based on the
6-gene signature |
|
|
|
Low | 200 (50.0) | 83 (50.3) |
|
High | 200 (50.0) | 82 (49.7) |
Identification of FRGs in the TCGA
cohort
When tumor and normal samples were compared,
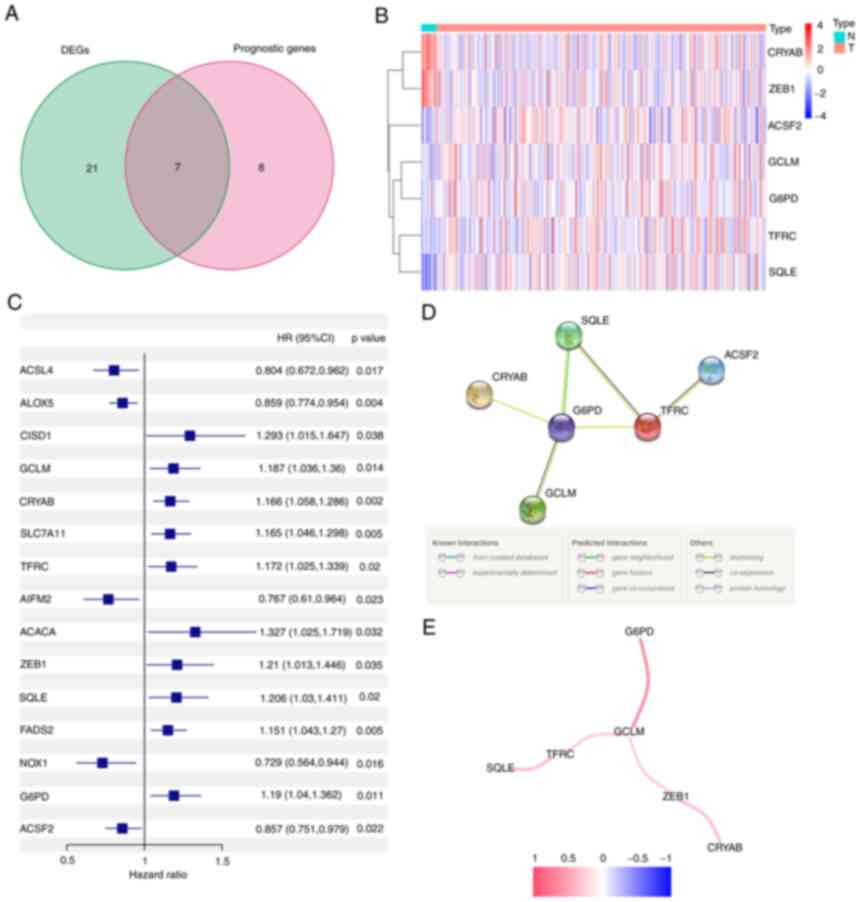
differential expression analysis revealed 28 (46%) significant
DEGs. Univariate Cox regression analysis showed that 15 genes were
associated with prognosis, 7 of which were FRGs (Fig. 2A). The genes in the intersection
included glutamate-cysteine ligase (GCL), crystallin α-B (CRYAB),
transferrin receptor (TFRC), zinc finger E-box binding homeobox 1
(ZEB1), squalene epoxidase (SQLE), G6PD and acyl-CoA synthetase
family member 2 (ACSF2) (ACSF2 was excluded due to the deficiency
of gene expression data in the GEO cohort). Their expression in
normal and tumor tissue is presented in Fig. 2B and C. The interaction network and
correlation are also presented in Fig.
2D and E.
Prior to conducting regression modeling, GSVA was
used to estimate the enrichment of hallmark gene sets in the TCGA
cohort. The results are presented in Fig. S1. The volcano plot and heatmap
displayed the significant enrichment of DNA repair, glycolysis,
unfolded protein response, mammalian target of rapamycin complex 1
(mTORC1) signaling, E2 factor targets and MYC targets in BC
tumors.
Construction of a 6-gene signature and
validation in GEO cohort
To build a model for the prognosis of patients with
BC, the 6 genes listed above were identified using LASSO Cox
regression. A 6-gene signature was successfully established using
the optimal value of λ. This model was formulated as follows: Risk
score = e[0.066 × GCL modifier (GCLM) expression level + 0.157
× CRYAB expression level + 0.109 × TFRC expression level + 0.074 ×
ZEB1 expression level + 0.135 × SQLE expression level + 0.118 ×
G6PD expression level].
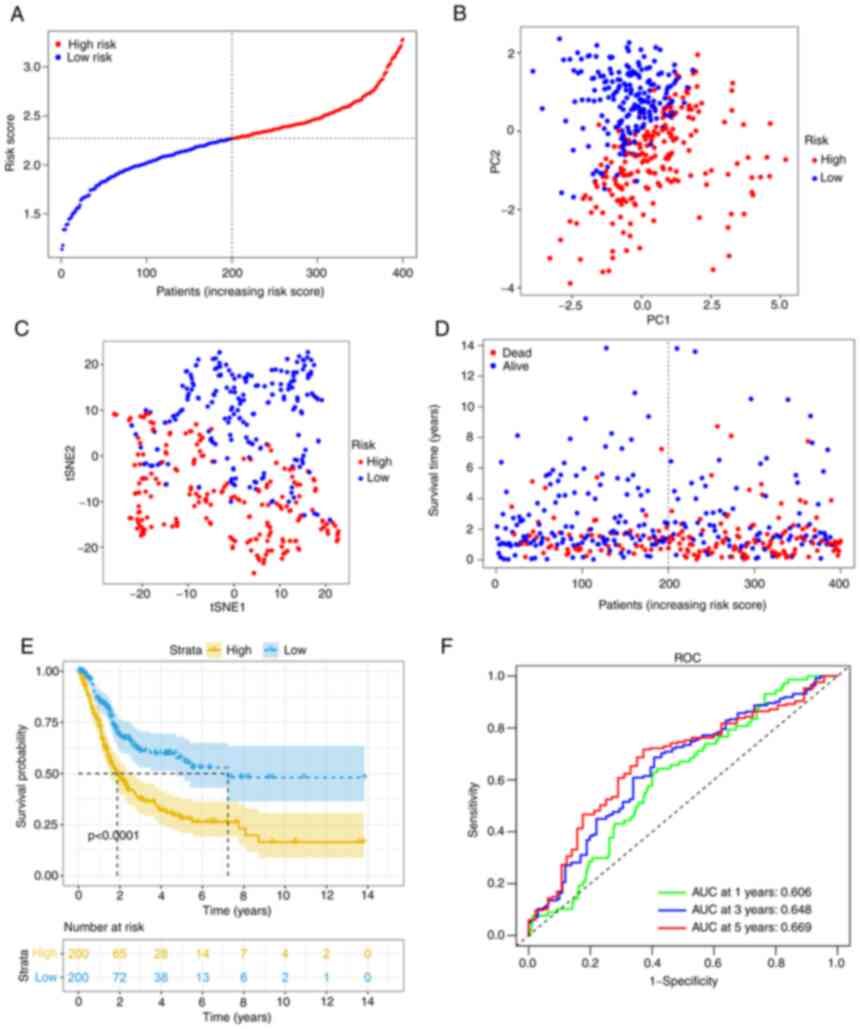
After calculating the median cut-off value in the
TCGA cohort, all the patients were divided into two groups: The
high-risk (n=200) and low-risk (n=200) group (Fig. 3A). Table II presents the characteristics of
patients from the different groups. The high-risk group was
associated with increased age (P<0.01), a higher tumor grade
(P<0.01) and an advanced TNM stage (P<0.01). Fig. 3B and C present the results of the
PCA and t-SNE analysis. The analysis indicated that different risk
groups were distributed in two directions and exhibited a certain
separation. As indicated in Fig.
3D, patients in the high-risk group had a higher probability of
death. Kaplan-Meier survival analysis consistently demonstrated
that patients in the high-risk group had unfavorable OS as compared
with those in the low-risk group (Fig.
3E). The predictive performance of the model was evaluated
using time-dependent ROC curves and the area under the curve (AUC)
reached 0.606 at 1 year, 0.648 at 3 years and 0.669 at 5 years
(Fig. 3F), which was significantly
better than the predictive value of the TNM stage (Fig. S2A).
 | Table II.Distribution of characteristics
between different risk groups. |
Table II.
Distribution of characteristics
between different risk groups.
|
| TCGA cohort | GEO cohort |
|---|
|
|
|
|
|---|
| Characteristic | High risk | Low risk | P-value | High risk | Low risk | P-value |
|---|
| Age, years |
|
|
<0.01a |
|
|
<0.01a |
|
<60 | 29 | 57 |
| 12 | 30 |
|
|
≥60 | 171 | 143 |
| 70 | 53 |
|
| Sex |
|
| 0.30a |
|
| 0.97a |
|
Female | 56 | 47 |
| 15 | 15 |
|
|
Male | 144 | 153 |
| 67 | 68 |
|
| Grade |
|
|
<0.01b |
|
|
<0.01a |
|
High | 197 | 180 |
| 48 | 12 |
|
|
Low | 1 | 19 |
| 34 | 71 |
|
|
Unknown | 2 | 1 |
|
|
|
|
| TMN stage |
|
|
<0.01b |
|
| - |
|
I+II | 37 | 92 |
| - | - |
|
|
III+IV | 162 | 107 |
| - | - |
|
|
Unknown | 1 | 1 |
| - | - |
|
| Muscle
invasion |
|
| - |
|
|
<0.01a |
|
Yes | - | - |
| 47 | 15 |
|
| No | - | - |
| 35 | 68 |
|
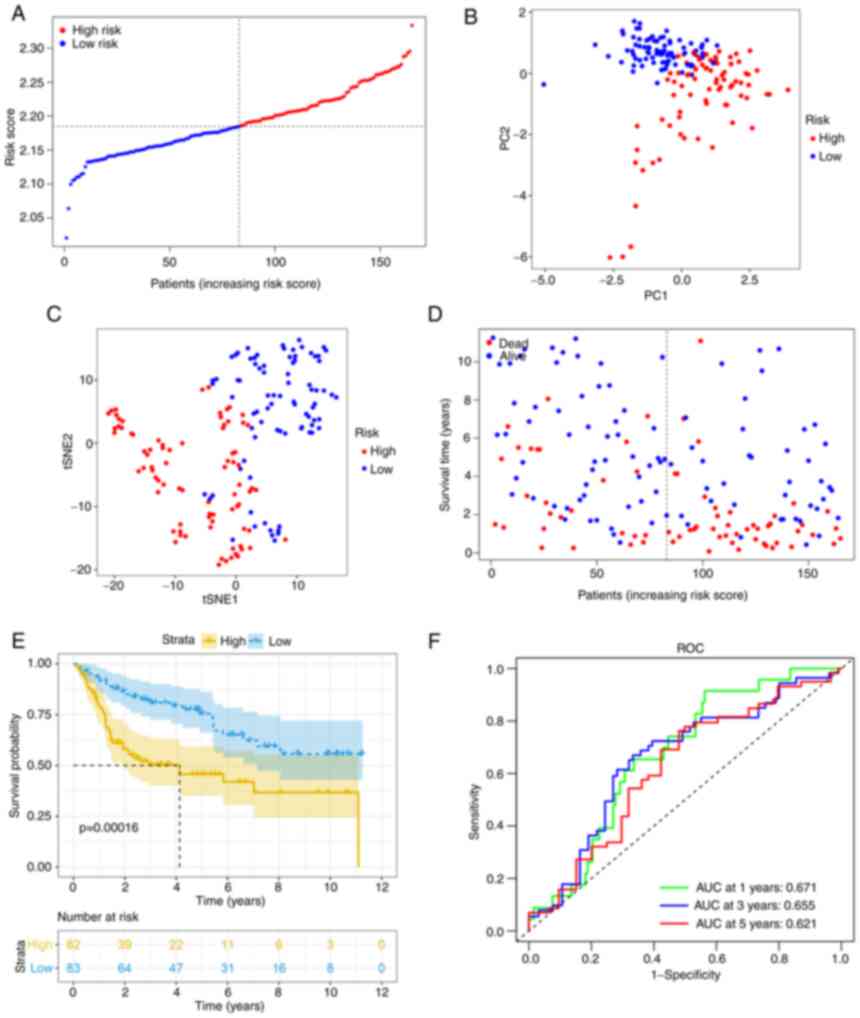
Using the same formula, the risk score of patients
in the GEO cohort was calculated. Similar to the TCGA cohort, they
were divided into high- (n=82) or low-risk (n=83) groups (Fig. 4A). Consistent with the TCGA cohort
data presented above, the high-risk group also demonstrated a
significantly advanced age (P<0.01) and grade (P<0.01). In
addition, the high-risk group had a higher risk of muscle-invasive
disease (Table II). PCA and t-SNE
analysis also indicated that when patients were divided into 2
groups, there was a certain separation between the groups of high
and low risk (Fig. 4B and C).
Again, it appeared that patients assigned to the high-risk group
succumbed earlier and had a shorter OS than those in the low-risk
group (Fig. 4D and E).
Furthermore, the AUC value obtained from the ROC analysis was 0.671
at 1 year, 0.655 at 3 years and 0.621 at 5 years (Fig. 4F).
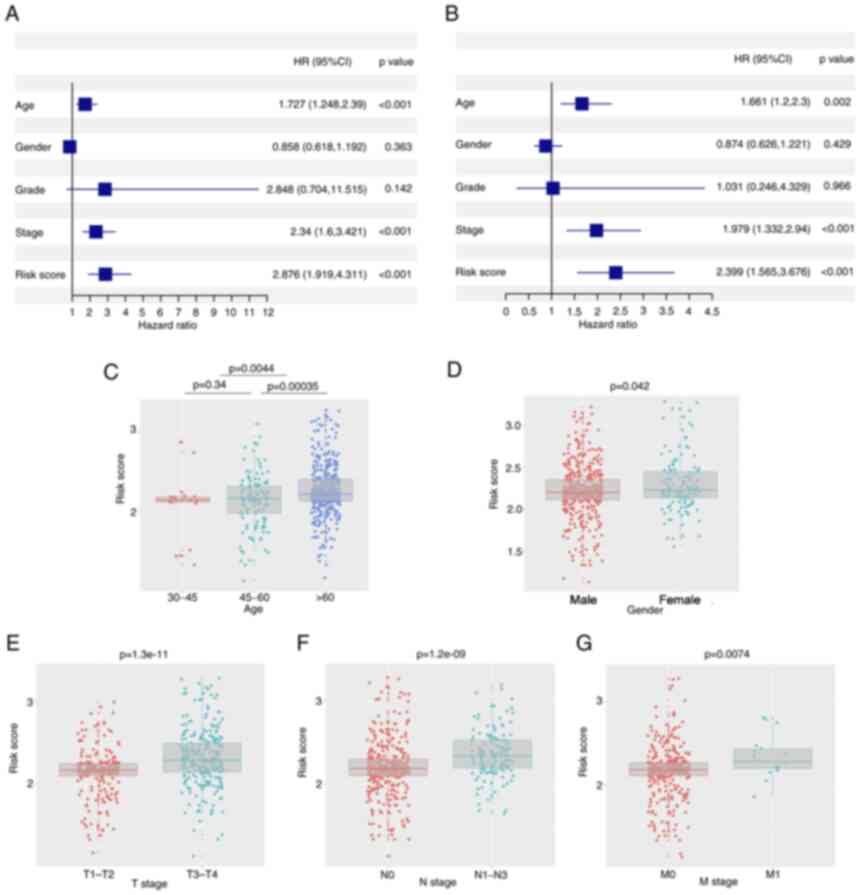
Prognostic value of the risk score and
clinical characteristics
Univariate and multivariate Cox regression analyses
were performed to determine the independent prognostic value of the
model. In the univariate Cox regression analysis, age, stage and
risk score were indicated to have a significant influence on OS
(Fig. 5A). Furthermore, using
multivariate Cox regression analysis, stage and risk score were
identified to have an independent predictive value in OS (Fig. 5B). Furthermore, association
analysis revealed that having a high risk score was associated with
advanced age (>60 years), male sex, tumor infiltration, lymph
node metastasis and distant metastasis in both the TCGA and GEO
cohorts (P<0.05; Fig.
5C-G).
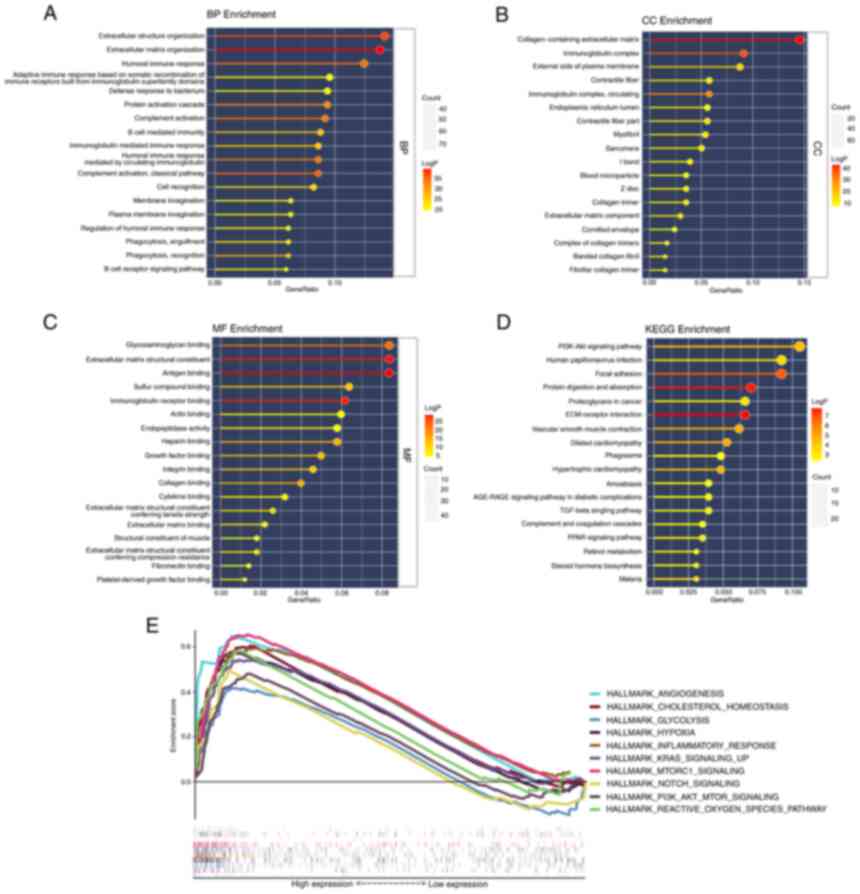
Functional analyses in TCGA
To further elucidate the biological functions and
pathways associated with differential FEGs, GO enrichment and KEGG
pathway analyses were performed. The findings of the GO enrichment
analysis indicated that the differential genes were enriched in
extracellular structure organization and extracellular matrix
organization in the category Biological Process. In addition,
collagen-containing extracellular matrix was markedly enriched in
the category Cellular Component. In the category Molecular
Function, glycosaminoglycan binding, extracellular matrix
structural constituent and antigen-binding were significantly
enriched terms (adjusted P<0.05). Meanwhile, the KEGG pathway
analysis revealed that the PI3K-Akt signaling pathway was
significantly enriched. In addition, certain pathways associated
with immunity were revealed to be enriched (Fig. 6A-D).
The results of the two risk groups using GSEA on the
cancer hallmarks gene sets are presented in Fig. 6E. The pathways with FDR <0.25
and NOM P<0.05 were selected and they are associated with
ferroptosis or bladder cancer risk.
Analysis of immune-related
characteristics in patients with BC
ssGSEA was further used to examine the differences
in immune status between the two risk groups. In the TCGA cohort, B
cells, dendritic cells (DCs), macrophages, neutrophils, follicular
helper T cells (Tfh), Th1 cells, tumor-infiltrating lymphocytes
(TIL) and regulatory T cells (Treg) scored significantly higher in
the high-risk group (Fig. 7A). The
immune function analysis revealed that the high-risk group had
stronger antigen-presenting cell (APC) stimulation, chemokine
receptors (CCR), immune checkpoint, parainflammation, T-cell
inhibition and T-cell stimulation (Fig. 7B). The cells and pathways in
antigen presentation-related processes significantly differed
between the two risk groups. In comparison, in the GEO cohort, DCs,
macrophages, neutrophils, Tfh, Th1 cells, TIL, CCR, immune
checkpoint, parainflammation, T-cell inhibition and T-cell
stimulation demonstrated a similar change (adjusted P<0.01;
Fig. 7C and D).
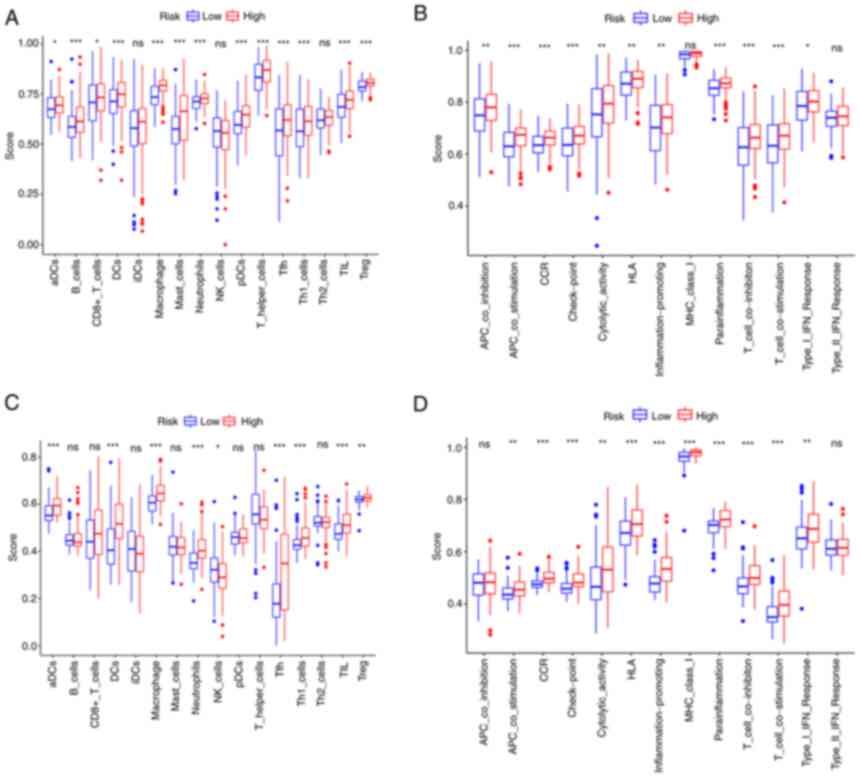 | Figure 7.Single-sample gene set enrichment
analysis results of different risk groups in the (A and B) The
Cancer Genome Atlas cohort: (A) Scores of the 16 immune cells and
(B) 13 immune-related functions. (C and D) Gene Expression Omnibus
cohort: (C) Scores of the 16 immune cells and (D) 13 immune-related
functions. ns, not significant; *P<0.05; **P<0.01;
***P<0.001. DC, dendritic cells; NK, natural killer; Tfh,
follicular helper T cells; Th1, type I T-helper cells; TIL,
tumor-infiltrating lymphocytes; Treg, regulatory T cells; HLA,
human leukocyte antigen; APC, antigen-presenting cell; MHC, major
histocompatibility complex; CCR, chemokine receptors; pDCs,
plasmacytoid DCs; aDCs, activated DCs; iDCs, immature DCs. |
Association between G6PD and
BC-related characteristics
Association analysis between gene expression and
demographic characteristics revealed that G6PD expression was
significantly different between gender-defined subgroups
(P<0.05; Fig. S2B). The
survival analysis of single-gene expression (formula=single gene
expression × corresponding coefficient) indicated that there were
significant differences between two risk groups when CRYAB, G6PD,
GCLM and TFRC expression levels were used to predict patient
prognosis (P<0.05; Fig.
S2C-H). Furthermore, the relationship between each FRG and
patient prognosis was investigated. G6PD was indicated to have a
significant association with prognosis in the univariate Cox
regression analysis (P<0.05; Table
SII).
To further identify the prognostic potential, genes
related to ferroptosis were analyzed in the BC immunotherapy cohort
using IMvigor210 and a significant correlation between G6PD gene
expression and the patients' immunotherapy response was discovered
(Fig. S3A). The ssGSEA of
patients divided by G6PD gene expression in the TCGA cohort
suggested that the immune cell infiltration score was higher and
immune-related processes were stronger in the high-expression
group. However, the type II IFN response of the high-expression
group scored much lower in comparison (Fig. 8A and B). The increase in G6PD
expression followed a similar trend toward advanced age, tumor
infiltration, lymph node metastasis and distant metastasis
(Fig. S4A-F). Further analysis
revealed that immune response genes, including cytotoxic
T-lymphocyte associated protein 4, programmed cell death 1 and
programmed cell death 1 ligand 2 (PD-L2), had higher expression
levels in the high G6PD expression group than in the low G6PD
expression group (Fig. S5).
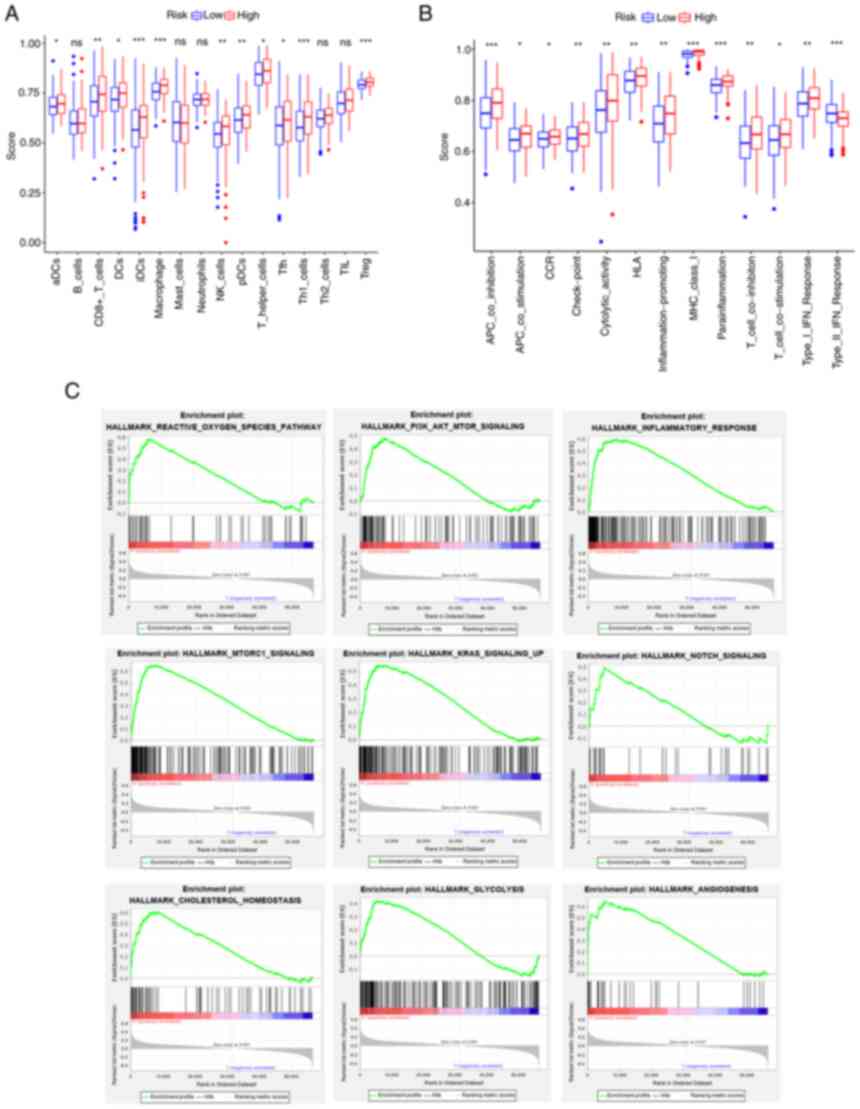 | Figure 8.Results of single-sample GSEA and
GSEA analyses for the high- and low-G6PD groups in The Cancer
Genome Atlas cohort. (A) Differences in immune cell infiltrating
score. (B) Differences in infiltrating score in immune-related
pathways. (C) GSEA results of hallmark gene sets for the high G6PD
expression group. ns, not significant; *P<0.05; **P<0.01;
***P<0.001. GSEA, gene set enrichment analysis; G6PD,
glucose-6-phosphate dehydrogenase. DC, dendritic cells; NK, natural
killer; Tfh, follicular helper T cells; Th1, type I T-helper cells;
TIL, tumor-infiltrating lymphocytes; Treg, regulatory T cells; HLA,
human leukocyte antigen; APC, antigen-presenting cell; MHC, major
histocompatibility complex; CCR, chemokine receptors; pDCs,
plasmacytoid DCs; aDCs, activated DCs; iDCs, immature DCs. |
Furthermore, the GSEA results of the G6PD expression
subgroups in the TCGA cohort are displayed in Fig. 8C. The pathways enriched in the risk
groups were also enriched in the G6PD expression groups
(FDR<0.25, NOM P<0.05).
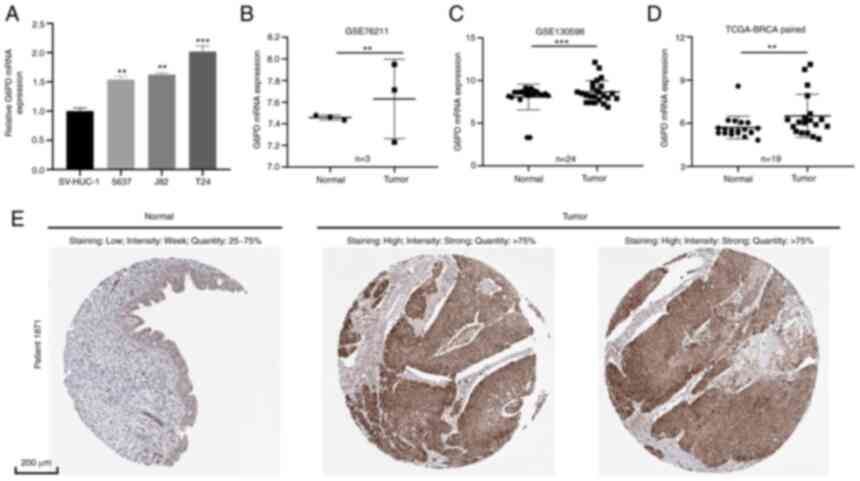
Validation of G6PD expression
level
RT-qPCR was performed to determine the level of G6PD
mRNA expression in BC cell lines and tissue (Fig. 9A and B). The results of the cell
line analysis revealed that BC cell lines (5637, J82 and T24)
expressed more G6PD than the normal cell line SV-HUC-1 (Fig. 9A). Furthermore, it was indicated
that G6PD expression was much higher in BC tissues than in adjacent
tissues using tissue data from GSE76211, GSE130598 and TCGA-BLCA
(Fig. 9B-D; GSE76211: Three paired
BC and normal tissues; GSE76211: 24 paired BC and normal tissues,
TCGA-BLCA: 19 paired BC and normal tissues). In addition, IHC
images obtained from the HPA database indicated that the G6PD
protein level in BC tissue was relatively higher than that in
normal bladder tissue (Fig.
9E).
Discussion
BC is a complex disease associated with a high rate
of morbidity and mortality (7). In
the Kurdistan province of Iran, a population-based study of 321
patients with BC indicated a 5-year survival rate of 54%
(2013–2018) (8). Numerous studies
have characterized DNA and RNA alterations in NMIBC, which is one
of the most frequently mutated human cancer types in terms of
mutation rates, third to lung and skin cancer (29). The identification of common
mutations has resulted in the development of novel therapeutic
approaches, as well as urine and blood surveillance for both early
detection and disease monitoring following treatment (30). In recent years, accumulating
evidence has indicated that the tumor microenvironment has an
important role in BC progression. A study suggested that radiation
caused immunogenic tumor cell death through ferroptosis and
polarized microenvironmental M2 tumor-associated macrophages
(M2-TAMs) to M1-TAMs (31). In BC,
the polarized M2 phenotype of tumor-associated macrophages is
related to tumor grade and stage (32). In the terms of BC treatment, one
study suggested that
methyl-2-cyano-3,11-dioxo-18b-olean-1,12-dien-30-oate exerted its
anti-tumor activity by inducing ROS (33). Sequential administration of
gemcitabine→tamoxifen amplified the cytotoxicity of gemcitabine in
BC cells (34). These studies
provided new directions for BC therapy.
Ferroptosis has a unique role in anti-cancer
therapeutic strategies. Ferroptosis is more lethal to tumor cells
that rely on the suppression of ferroptosis to survive than
apoptosis. At the same time, ferroptosis is also sensitive in
certain special cases, such as tumor cells that are drug-resistant,
aggressive or undergo epithelial-mesenchymal transition (35,36).
In addition, a study conducted by Luan et al (37) identified nine drugs with the
potential to treat BC.
In the present study, 60 FRGs that were selected
from previous studies, 28 DEGs and 15 prognosis-associated genes
were identified. GSVA analysis was used to investigate the
phenotypic characteristics of TCGA samples. The enrichment of
hallmark gene sets in tumor samples was indicated to be strongly
associated with BC oncogenesis and progression. Among them, DNA
repair was previously associated with the occurrence of prostate
cancer (38), while mTORC1 was
related to metastasis in colorectal cancer (39). Subsequently, six genes were
selected to build the prognostic model based on their intersection
with other gene sets. They were all associated with poor prognosis
(hazard ratio >1, P<0.05).
GCLM is a component of the first rate-limiting
enzyme of glutathione synthesis. GCL, which is composed of the
catalytic (C) subunit GCLC and modifier subunit GCLM, is one of the
major determinants of glutathione (GSH) synthesis (40). When GSH-dependent lipid peroxide
repair systems are compromised, the lethal accumulation of
lipid-based ROS results in ferroptosis, which is induced by the
inhibition of cysteine uptake, decreased GSH levels or inactivation
of the lipid repair enzyme GPX4 (41). Genetic loss of GCLM impairs a
tumor's ability to drive malignant transformation. A study by Zhu
et al (42) suggested that
GCLM is an oncogene in esophageal adenocarcinoma. In addition, it
is established that Andrographis treatment is effective in
colorectal cancer and gastric cancer through dysregulating the
expression of genes such as GCLM within the ferroptosis pathway
(43,44). These findings provide evidence to
support the use of GCLM as an adjuvant target treatment in cancers.
Although GCLM has not been described in BC, the present findings
indicate that it may also be a suitable target in BC.
CRYAB is a type of small heat shock protein (HSP),
also known as HspB5. It was first discovered in the lens of the eye
and has since been demonstrated to be ubiquitously expressed
(45). Previous studies have
concentrated on its role in apoptosis, where it acts as a negative
regulator of Bax and caspase-3 (46). HspB1 has been identified as a
negative regulator of ferroptosis-associated cancer cell death
(47). In addition, HspB5 is
associated with redox activity and inflammation. When CRYAB is
expressed, it is possible to observe a decrease in the
mitochondrial membrane potential and GSH. Subsequently, it may
gradually inhibit iron uptake (48,49).
It may also have a significant impact on ferroptosis-mediated
cancer therapy. Increasing evidence suggests that CRYAB is
associated with tumors. Overexpression of CRYAB inhibits BC cell
migration and invasion through the PI3K/AKT and ERK pathways
(50).
TFRC contributes to the ferroptosis process by
collecting extracellular iron and replenishing the intracellular
iron pool (51). This receptor is
a promising target for cancer therapy due to its high expression on
malignant cells, ability to internalize iron and requirement for
cell proliferation. Targeting the TFRC has been demonstrated to be
effective at delivering a wide variety of therapeutic agents and
inducing cytotoxicity in cancer cells both in vitro and
in vivo (52).
SQLE is a rate-limiting enzyme in cholesterol
synthesis (53). Ferroptosis is a
condition caused by the accumulation of iron-dependent lipid
peroxides during cholesterol metabolism. Intracellular cholesterol
promotes tumor formation or growth (54). In the present study, through GSEA
analysis, enrichment of the cholesterol homeostasis pathway was
identified in both high risk score groups and high G6PD expression
groups. SQLE reduction caused by cholesterol accumulation
aggravates colorectal cancer progression by activating the
β-catenin oncogenic pathway and deactivating the p53 tumor
suppressor pathway (55).
Increased SQLE expression promotes cholesteryl ester biosynthesis,
which induces hepatocellular carcinoma cell growth (56).
ZEB1, a central transcriptional component of fat
cell differentiation, directly targets and modulates the expression
of the majority of early and late adipogenic regulators (57). The sensitivity of GPX4 inhibitors
has been reported to be associated with ZEB1 (36). GPX4 has a critical role in the
regulation of ferroptotic cancer cell death (58).
G6PD is a rate-limiting enzyme of the pentose
phosphate pathway, which promotes progression in a variety of
cancer types (59–61). According to a previous study,
patients with BC expressing a high level of G6PD may have a poor
prognosis (62). In addition,
inhibiting G6PD may decrease ROS accumulation and block the AKT
pathway, affecting BC cell proliferation (62).
Ferroptosis in tumors has received increasing
attention in recent years, although associated molecular changes
and mechanisms in BC remain largely elusive. GO and KEGG enrichment
analysis revealed potential mechanisms linking ferroptosis and BC,
including immune-related, redox-related and stromal-related
pathways. The KEGG pathway enrichment analysis revealed a
significant enrichment of the PI3K/AKT/mTOR pathway, which has been
implicated in helping cancer cells evade ferroptosis (63). A previous study on colorectal
cancer suggested that the benzopyran derivative
2-imino-6-methoxy-2H-chromene-3-carbothioamide regulated the
activity of the AMPK/mTOR/p70S6k signaling pathway, which is
related to ferroptosis (64).
However, this pathway warrants further investigation in BC. In
addition, enrichment of numerous immune-related pathways indicated
an association between ferroptosis and tumor immunity. A recent
study by Wang et al (65)
identified that tumor-specific ferroptosis regulators
cysteinyl-tRNA synthetase exhibited a strong association with the
expression of immune checkpoint genes, particularly PD-L1,
demonstrating the influence of ferroptosis on the tumor
microenvironment. Simultaneously, serum PD-L1 levels and the
binding of CD68 and PD-L1 were associated with poor prognosis in
patients with BC (66,67). Thus, ssGSEA was used for further
study. While it has been established that a high infiltration of
certain immune cells, such as macrophages, is associated with a
poor prognosis in patients with BC, the role of others, such as
Tregs, remains controversial (68). In addition, it was observed that
the antigen presentation processes were more pronounced in the
high-risk group. These points warrant further investigation.
G6PD was chosen for further study based on clinical
characteristics analysis and the results of the immunotherapy
cohort analysis. G6PD encodes glucose-6-phosphate dehydrogenase,
which provides NADPH for fatty acid and nucleic acid synthesis. It
has been suggested that G6PD is one of the genes involved in
ferroptosis (69). High levels of
G6PD expression were associated with unfavorable overall survival
in patients with BC (62). Gu
et al (70) reported that
the G6PD-NADPH redox system was related to T cell metabolism. Given
the association between high G6PD expression and immunotherapy
response, as well as the characteristics of the immune
microenvironment, it is indicated that follow-up studies on G6PD in
patients with BC are necessary, while the majority of previous
research has focused on G6PD in malaria.
Simultaneously, it was observed that several
ferroptosis-related pathways, such as angiogenesis, glycolysis and
mTORC1 signaling, were active in both the high-risk and high G6PD
expression groups. A recent study indicated that the
Rag/mTORC1/eukaryotic initiation factor 4E-binding proteins axis
promoted the synthesis of GPX4 protein, which inhibits ferroptosis
(71). As previously reported,
PI3K/AKT/mTOR signaling may also suppress ferroptosis via
lipogenesis (63). Chen et
al (72) demonstrated that
targeting activating transcription factor 4 increased ferroptosis
sensitivity of tumor cells by suppressing angiogenesis.
There are still several limitations to this study.
First, the sample size in the present study was small, and
therefore, the data used for analysis are not comprehensive.
Additional prospective clinical data are required to validate the
reliability of the present data. Finally, it should be emphasized
that the relationship between ferroptosis and BC requires
additional basic experimental validations.
In conclusion, the present study successfully
constructed an FRG signature with prognostic value for BC and
further identified the potential value of G6PD for future research.
This opens up new avenues for targeted therapies in BC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work is supported by the Innovation and Entrepreneurship
Training Program for college students, Education Department of
Jiangsu Province (grant no. KY102J2020058).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The data generated in
the present study may be found under the following URLs: TCGA-BLCA:
https://portal.gdc.cancer.gov/; GEO:
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13507.
Authors' contributions
YTW, HXH and MJJ designed and managed the entire
study. YTW collected all the data. YTW, WCS and YQF analysed the
results. JZT, DZ and QCW performed the experiments. YTW, WCS, QCW
and YQF wrote the manuscript. YTW, JZT and DZ checked and confirmed
the authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richters A, Aben KKH and Kiemeney L: The
global burden of urinary bladder cancer: An update. World J Urol.
38:1895–1904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeGeorge KC, Holt HR and Hodges SC:
Bladder cancer: Diagnosis and Treatment. Am Fam Physician.
96:507–514. 2017.PubMed/NCBI
|
|
4
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF,
Sylvester R, et al: European association of urology guidelines on
non-muscle-invasive bladder cancer (TaT1 and carcinoma in
situ)-2019 update. Eur Urol. 76:639–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang SS, Bochner BH, Chou R, Dreicer R,
Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, et
al: Treatment of non-metastatic muscle-invasive bladder cancer:
AUA/ASCO/ASTRO/SUO guideline. J Urol. 198:552–559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amiri M, Heshmatollah S, Esmaeilnasab N,
Khoubi J, Ghaderi E and Roshani D: Survival rate of patients with
bladder cancer and its related factors in Kurdistan province
(2013–2018): A population-based study. BMC Urol. 20:1952020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du Y, Miao W, Jiang X, Cao J, Wang B, Wang
Y, Yu J, Wang X and Liu H: The epithelial to mesenchymal transition
related gene calumenin is an adverse prognostic factor of bladder
cancer correlated with tumor microenvironment remodeling, gene
mutation, and ferroptosis. Front Oncol. 11:6839512021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JN, Li T, Cheng L, Qin TS, Sun YX,
Chen CT, He YZ, Liu G, Yao D, Wei Y, et al: Synthesis and in vitro
anti-bladder cancer activity evaluation of quinazolinyl-arylurea
derivatives. Eur J Med Chem. 205:1126612020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo P, Wang L, Shang W, Chen J, Chen Z,
Xiong F, Wang Z, Tong Z, Wang K, Yang L, et al: Intravesical in
situ immunostimulatory gel for triple therapy of bladder cancer.
ACS Appl Mater Interfaces. 12:54367–54377. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Ma H, Meng L, Liu X, Lv Z, Zhang Y
and Wang J: Construction and external validation of a
ferroptosis-related gene signature of predictive value for the
overall survival in bladder cancer. Front Mol Biosci. 8:6756512021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Jensen MA and Zenklusen JC: A
practical guide to the cancer genome atlas (TCGA). Methods Mol
Biol. 1418:111–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS,
Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al: Predictive value
of progression-related gene classifier in primary non-muscle
invasive bladder cancer. Mol Cancer. 9:32010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chandrashekar DS, Chakravarthi B, Robinson
AD, Anderson JC, Agarwal S, Balasubramanya SAH, Eich ML, Bajpai AK,
Davuluri S, Guru MS, et al: Therapeutically actionable PAK4 is
amplified, overexpressed, and involved in bladder cancer
progression. Oncogene. 39:4077–4091. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu M, Ge Q, Wang G, Luo Y and Wang X,
Jiang W, Liu X, Wu CL, Xiao Y and Wang X: CIRBP is a novel oncogene
in human bladder cancer inducing expression of HIF-1α. Cell Death
Dis. 9:10462018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon N, Friedman J, Hastie T and
Tibshirani R: Regularization paths for Cox's proportional hazards
model via coordinate descent. J Stat Softw. 39:1–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tibshirani R: The lasso method for
variable selection in the Cox model. Stat Med. 16:385–395. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawrence MS, Stojanov P, Polak P, Kryukov
GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH,
Roberts SA, et al: Mutational heterogeneity in cancer and the
search for new cancer-associated genes. Nature. 499:214–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tran L, Xiao JF, Agarwal N, Duex JE and
Theodorescu D: Advances in bladder cancer biology and therapy. Nat
Rev Cancer. 21:104–121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J,
Huang J, He Q, Wu B, Zhang Z, et al: Irradiated tumor cell-derived
microparticles mediate tumor eradication via cell killing and
immune reprogramming. Sci Adv. 6:eaay97892020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeuchi H, Tanaka M, Tanaka A, Tsunemi A
and Yamamoto H: Predominance of M2-polarized macrophages in bladder
cancer affects angiogenesis, tumor grade and invasiveness. Oncol
Lett. 11:3403–3408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeuchi H, Taoka R, Mmeje CO, Jinesh GG,
Safe S and Kamat AM: CDODA-Me decreases specificity protein
transcription factors and induces apoptosis in bladder cancer cells
through induction of reactive oxygen species. Urol Oncol.
34:337.e11–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi H, Mmeje CO, Jinesh GG, Taoka R
and Kamat AM: Sequential gemcitabine and tamoxifen treatment
enhances apoptosis and blocks transformation in bladder cancer
cells. Oncol Rep. 34:2738–2744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole
D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL,
et al: Drug-tolerant persister cancer cells are vulnerable to GPX4
inhibition. Nature. 551:247–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luan JC, Zeng TY, Zhang QJ, Xia DR, Cong
R, Yao LY, Song LB, Zhou X, Zhou X, Chen X, et al: A novel
signature constructed by ferroptosis-associated genes (FAGs) for
the prediction of prognosis in bladder urothelial carcinoma (BLCA)
and associated with immune infiltration. Cancer Cell Int.
21:4142021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu B, Lu X, Shen H, Yuan X, Wang X, Yin N,
Sun L, Shen P, Hu C, Jiang H and Wang D: Intratumoral heterogeneity
and genetic characteristics of prostate cancer. Int J Cancer.
146:3369–3378. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T and Evers
BM: mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kajarabille N and Latunde-Dada GO:
Programmed cell-death by ferroptosis: Antioxidants as mitigators.
Int J Mol Sci. 20:49682019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu L, Yang F, Wang L, Dong L, Huang Z,
Wang G, Chen G and Li Q: Identification the ferroptosis-related
gene signature in patients with esophageal adenocarcinoma. Cancer
Cell Int. 21:1242021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharma P, Shimura T, Banwait JK and Goel
A: Andrographis-mediated chemosensitization through activation of
ferroptosis and suppression of β-catenin/Wnt-signaling pathways in
colorectal cancer. Carcinogenesis. 41:1385–1394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma R, Shimura T, Yin C, Okugawa Y,
Kitajima T, Koike Y, Okita Y, Ohi M, Uchida K, Goel A, et al:
Antitumor effects of Andrographis via ferroptosis-associated genes
in gastric cancer. Oncol Lett. 22:5232021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Liu J, Wu J, Li W, Chen Z and
Yang L: Progression of the role of CRYAB in signaling pathways and
cancers. Onco Targets Ther. 12:4129–4139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M,
Liu M, Hu XH, Chen P, Yan Q, et al: αA- and αB-crystallins interact
with caspase-3 and Bax to guard mouse lens development. Curr Mol
Med. 12:177–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X,
Wang H, Cao L and Tang D: HSPB1 as a novel regulator of ferroptotic
cancer cell death. Oncogene. 34:5617–5625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Christopher KL, Pedler MG, Shieh B, Ammar
DA, Petrash JM and Mueller NH: Alpha-crystallin-mediated protection
of lens cells against heat and oxidative stress-induced cell death.
Biochim Biophys Acta. 1843:309–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prabhu S, Srinivas V, Ramakrishna T, Raman
B and Rao Ch M: Inhibition of Cu2+-mediated generation of reactive
oxygen species by the small heat shock protein αB-crystallin: The
relative contributions of the N- and C-terminal domains. Free Radic
Biol Med. 51:755–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ruan H, Li Y, Wang X, Sun B, Fang W, Jiang
S and Liang C: CRYAB inhibits migration and invasion of bladder
cancer cells through the PI3K/AKT and ERK pathways. Jpn J Clin
Oncol. 50:254–260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng H, Schorpp K, Jin J, Yozwiak CE,
Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka
JM, et al: Transferrin receptor is a specific ferroptosis marker.
Cell Rep. 30:3411–3423.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Daniels TR, Bernabeu E, Rodríguez JA,
Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera
G and Penichet ML: The transferrin receptor and the targeted
delivery of therapeutic agents against cancer. Biochim Biophys
Acta. 1820:291–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamamoto S and Bloch K: Studies on
squalene epoxidase of rat liver. J Biol Chem. 245:1670–1674. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu H, Zhou S, Tang Q, Xia H and Bi F:
Cholesterol metabolism: New functions and therapeutic approaches in
cancer. Biochim Biophys Acta Rev Cancer. 1874:1883942020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jun SY, Brown AJ, Chua NK, Yoon JY, Lee
JJ, Yang JO, Jang I, Jeon SJ, Choi TI, Kim CH and Kim NS: Reduction
of squalene epoxidase by cholesterol accumulation accelerates
colorectal cancer progression and metastasis. Gastroenterology.
160:1194–1207.e28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu D, Wong CC, Fu L, Chen H, Zhao L, Li
C, Zhou Y, Zhang Y, Xu W, Yang Y, et al: Squalene epoxidase drives
NAFLD-induced hepatocellular carcinoma and is a pharmaceutical
target. Sci Transl Med. 10:eaap98402018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gubelmann C, Schwalie PC, Raghav SK, Röder
E, Delessa T, Kiehlmann E, Waszak SM, Corsinotti A, Udin G,
Holcombe W, et al: Identification of the transcription factor ZEB1
as a central component of the adipogenic gene regulatory network.
Elife. 3:e033462014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu T, Zhang C, Tang Q, Su Y, Li B, Chen L,
Zhang Z, Cai T and Zhu Y: Variant G6PD levels promote tumor cell
proliferation or apoptosis via the STAT3/5 pathway in the human
melanoma xenograft mouse model. BMC Cancer. 13:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang C, Zhang Z, Zhu Y and Qin S:
Glucose-6-phosphate dehydrogenase: A biomarker and potential
therapeutic target for cancer. Anticancer Agents Med Chem.
14:280–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lu M, Lu L, Dong Q, Yu G, Chen J, Qin L,
Wang L, Zhu W and Jia H: Elevated G6PD expression contributes to
migration and invasion of hepatocellular carcinoma cells by
inducing epithelial-mesenchymal transition. Acta Biochim Biophys
Sin (Shanghai). 50:370–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen X, Xu Z, Zhu Z, Chen A, Fu G, Wang Y,
Pan H and Jin B: Modulation of G6PD affects bladder cancer via ROS
accumulation and the AKT pathway in vitro. Int J Oncol.
53:1703–1712. 2018.PubMed/NCBI
|
|
63
|
Yi J, Zhu J, Wu J, Thompson C and Jiang X:
Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang L, Liu W, Liu F, Wang Q, Song M, Yu
Q, Tang K, Teng T, Wu D, Wang X, et al: IMCA induces ferroptosis
mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal
cancer. Oxid Med Cell Longev. 2020:16756132020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Chen S, Ying Y, Ma X, Shen H, Li
J, Wang X, Lin Y, Liu B, Zheng X and Xie L: Comprehensive analysis
of ferroptosis regulators with regard to PD-L1 and immune
infiltration in clear cell renal cell carcinoma. Front Cell Dev
Biol. 9:6761422021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang LR, Zhang N, Chen ST, He J, Liu YH,
Han YQ, Shi XQ, Yang JJ, Mu DY, Fu GH and Gao F: PD-1-positive
tumor-associated macrophages define poor clinical outcomes in
patients with muscle invasive bladder cancer through potential
CD68/PD-1 complex interactions. Front Oncol. 11:6799282021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krafft U, Olah C, Reis H, Kesch C, Darr C,
Grünwald V, Tschirdewahn S, Hadaschik B, Horvath O, Kenessey I, et
al: High serum PD-L1 levels are associated with poor survival in
urothelial cancer patients treated with chemotherapy and immune
checkpoint inhibitor therapy. Cancers (Basel). 13:25482021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
van Wilpe S, Gerretsen E, van der Heijden
A, de Vries I, Gerritsen W and Mehra N: Prognostic and predictive
value of tumor-infiltrating immune cells in urothelial cancer of
the bladder. Cancers (Basel). 12:26922020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu M, Zhou X, Sohn JH, Zhu L, Jie Z, Yang
JY, Zheng X, Xie X, Yang J, Shi Y, et al: NF-κB-inducing kinase
maintains T cell metabolic fitness in antitumor immunity. Nat
Immunol. 22:193–204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang Y, Swanda RV, Nie L, Liu X, Wang C,
Lee H, Lei G, Mao C, Koppula P, Cheng W, et al: mTORC1 couples
cyst(e)ine availability with GPX4 protein synthesis and ferroptosis
regulation. Nat Commun. 12:15892021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen D, Fan Z, Rauh M, Buchfelder M,
Eyupoglu IY and Savaskan N: ATF4 promotes angiogenesis and neuronal
cell death and confers ferroptosis in a xCT-dependent manner.
Oncogene. 36:5593–5608. 2017. View Article : Google Scholar : PubMed/NCBI
|