Introduction
Cervical cancer is one of the most common types of
cancer among female patients worldwide, with ~530,000 new cases and
>274,000 deaths each year (1,2).
Although the morbidity and mortality rates of patients with
cervical cancer have decreased in most parts of the world over the
past few decades, due to the improvement of the average
socio-economic levels and the reduced risk of persistent high-risk
human papillomavirus infection, cervical cancer remains the most
commonly diagnosed type of cancer in low-and middle-income
countries, mainly in sub-Saharan Africa (2,3).
Particularly in low-income countries, the incidence and mortality
rates of cervical cancer are markedly higher, and it is a major
cause of cancer-related mortality among female cancer patients
(1,4).
Cervical cancer has not been reported to be
sensitive to chemotherapeutic drugs in the conventional view.
Therefore, surgery and radiotherapy are usually selected for
treatment. However, with recent advancements being made in
research, numerous experimental results and clinical practices have
confirmed that surgery and radiotherapy cannot completely control
or eliminate the occurrence and metastasis of cervical cancer
(5). In terms of chemotherapy,
cisplatin-based chemotherapy is the most commonly applied regimen;
however, cisplatin-based chemotherapy as the main treatment of
metastatic cervical cancer does not significantly improve survival
(6,7). Therefore, there is an urgent need for
improved treatments and more effective therapeutic targets for the
treatment of cervical cancer.
Traditional Chinese herbal medicines and their
active ingredients have been widely used in clinical practice, due
to their reduced toxicity and high efficacy in tumour therapy
(8,9). Therefore, the anticancer efficacy of
traditional Chinese medicine and its extracts has become a hot
topic of research. Among these medicines, shikonin is a
naphthoquinone pigment, extracted from the traditional medicinal
herb, Lithospermum erythrorhizon, which has been reported to
exhibit extensive biological activities, particularly anticancer
activity (10). It has been
demonstrated in a previously published study that the cancer
inhibitory effects of shikonin may occur through various
mechanisms, including inhibition of cell proliferation and
migration, induction of apoptosis and autophagy, and inhibition of
glycolysis and metabolism (11).
Additionally, it has been revealed that shikonin may block PI3K/Akt
and ERK-mediated epithelial-mesenchymal transition (EMT) pathways
by inhibiting c-Met, thus preventing HCC827 lung cancer cell
migration and invasion and inhibiting the proliferation of HCC827
cells by acting on the EMT transition and HGF (12). Shikonin-containing liposomes have
also been revealed to inhibit angiogenesis and induce the
downregulation of VEGF gene expression in the human umbilical vein
endothelial cells (HUVECs), ultimately inhibiting angiogenesis
(13). Other studies have revealed
that shikonin may inhibit DNA methyltransferase 1 expression,
decrease PTEN gene methylation and increase PTEN protein
expression, thereby inhibiting the migration of TPC-1 cells from
thyroid cancers (14). However,
limited reports have been released on the effects of shikonin on
cervical cancer. Previous studies have only focused on the
inhibitory effects of shikonin on EMT (15) and the activation of caspase-3
(16) in cervical cancer; thus,
the complete mechanisms remain to be explored in depth.
Epidermal growth factor receptor (EGFR) is one of
the most frequently overexpressed, amplified and mutated genes in
human cancers (17). EGFR
regulates tumour proliferation, invasion, apoptosis and
angiogenesis through multiple signalling pathways, including PI3K,
RAS/RAR/MEK1/ERK1/2 and Janus kinase (JAK)/STAT, in cervical and
other cancers (18–20). The focal adhesion kinase
(FAK)-mediated signalling pathway, which is dependent on receptor
tyrosine kinase (PTK) activity, plays a pivotal role in regulating
the occurrence and development of tumours (21). FAK may be phosphorylated after
binding to some signalling and cytoskeleton molecules to transmit
signals from the extracellular matrix or deliver signals from
soluble bioactive factors (22).
As it has been reviewed Zhou et al (23) FAK may play an indispensable role in
tumourigenesis, by consistently promoting proliferation and
survival signals.
Of note, it has been revealed that salinomycin, an
antitumour drug, may increase cell stiffness and F-actin formation
in hepatocellular carcinoma stem cells via the FAK-ERK1/2 pathway,
in order to attenuate hepatocellular carcinoma stem cell motility
(24). FAK has also been
demonstrated to induce inflammatory factor expression, which may
suppress antitumour immunity in the microenvironment and ultimately
lead to tumour immune escape (25). Those previously published findings
may suggest that EGFR and FAK could be potential targets for cancer
therapy.
In the present study, it was revealed that shikonin
inhibits cervical cancer cell proliferation and migration, probably
by inhibiting the EGF-mediated phosphorylation signalling pathway
of FAK/AKT/glycogen synthase kinase 3β (GSK3β).
Materials and methods
Reagents
Shikonin was acquired from MedChemExpress (cat. no.
HY-N0822, batch 39014, purity >99.80%). PF-562271 (FAK selective
inhibitor) (cat. no. HY-10459) was purchased from MedChemExpress.
EGF (cat. no. 236-EG-01 M) was purchased from Merck KGaA. HeLa and
SiHa cells were pre-treated with PF-562271 (10 µM) for 60 min
followed by co-treatment with EGF (10 ng/ml) for 60 min. Reagents
used for cell culture, including DMEM and foetal bovine serum
(FBS), as well as penicillin and streptomycin, were purchased from
Gibco; Thermo Fisher Scientific, Inc. Finally, 2X M5 HiPer SYBR
Premix EsTaq (with Tli RNaseH) (cat. no. MF787-01) was purchased
from Beijing Jumei Biotechnology Co., Ltd.
Cells and cell culture
HeLa and SiHa human cervical cancer cells (iCell
Bioscience Inc.) were cultured in Dulbecco's modified Eagle's
medium (DMEM) containing 4.5 g/l glucose supplemented with 10%
foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cells
were maintained at 37°C in an incubator with 5% CO2.
When the degree of cell confluence reached 85–90%, the cells were
sub-cultured. All cell lines were found to be free of mycoplasma
(data not shown).
Measurement of cell viability
Cell viability assays were performed, using the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Inc.) as previously
described (26). Briefly, cells
were seeded in culture medium in 96-well plates (2.0×104
cells/ml; 100 µl). The wells without cells served as the blank
control. Following attachment, the cells were serum-starved
overnight and incubated at 37°C with various concentrations of
shikonin for 24, 48 and 72 h. The concentration range (1–4 µM) was
selected according to previous studies, which have revealed that
shikonin can effectively inhibit cell proliferation at this range
in oesophageal cancer (27,28).
Subsequently, 10 µl CCK-8 solution were added to each well. The
optical density (OD) at 450 nm was assayed by spectrophotometer
(type 1530; Thermo Fisher Scientific, Inc.) after cell incubation
at 37°C for 2 h. The IC50 values of shikonin on cell viability were
calculated by non-linear regression, using GraphPad Software Prism
7.0 (GraphPad Software, Inc.) (29).
Western blot analysis
Cells were cultured in serum-free DMEM medium
overnight for shikonin treatment. The concentration of shikonin
used was 2.5 µM, according to the concentration-related experiments
and previously published studies (27,30).
Following treatment, all cells were incubated with cell lysis
buffer (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) containing protease inhibitors (cat. no. 04 693 132 001;
Roche Diagnostics GmbH) and phosphatase inhibitors (cat. no. 04 906
837 001; Roche Diagnostics GmbH) at 4°C for 10 min. The protein
concentration was measured using the BCA protein assay kit (cat.
no. P0010; Beyotime Institute of Biotechnology). Equal amounts of
protein (10 µg/lane) were loaded and separated by 10% SDS-PAGE.
Proteins were transferred to PVDF membranes (cat. no. IPVH00010;
EMD Millipore) and blocked in 5% non-fat dry milk for 2 h, at room
temperature. Subsequently, the membranes were incubated overnight
at 4°C with the following primary antibodies: Anti-phospho-FAK
(Tyr397) (cat. no. 8556; 1:2,000), anti-FAK (cat. no. 71433;
1:2,000), anti-phospho-AKT (Ser473) (cat. no. 4060; 1:2,000),
anti-AKT (cat. no. 4685; 1:2,000), β-actin (cat. no. 4970; rabbit
anti-human; 1:5,000), anti-phospho-GSK3β (Ser9) (cat. no. 9323;
1:2,000) and anti-GSK3β (cat. no. 9315; 1:2,000). The membranes
were then incubated with HRP-conjugated anti-rabbit IgG secondary
antibody (cat. no. 7074; goat anti-rabbit; 1:4,000) for 2 h at room
temperature. All primary and secondary antibodies were bought from
Cell Signaling Technology, Inc. After washing the membrane with
TBST (0.1% Tween-20), enhanced chemiluminescence reagent
(Pierce™ ECL Western Blotting Substrate; cat. no. 32106;
Thermo Fisher Scientific, Inc.) was added. ImageJ software (version
1.47t; National Institutes of Health) was used to analyse the grey
value of each target band, which indicated the expression level of
the target protein as compared with that of β-actin.
Wound healing assay
The wound healing assay was performed to measure the
effects of shikonin on cell migration according to previous studies
(31,32). Briefly, the cells were seeded in a
6-well plate at a concentration of 4.5×105 cells/well
and were grown until reaching 90% confluency. The bottoms of the
6-well plates were marked, and the wounds were scratched vertically
with a 200 µl pipette tip. After washing three times with
phosphate-buffered saline (PBS; cat. no. KGB5001; KeyGEN BioTECH,
Inc.), the cells were treated with shikonin and incubated at 37°C
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 2% FBS (Gibco;
Thermo Fisher Scientific, Inc.) for 24 and 48 h. The representative
scrape lines for each set were photographed using a phase-contrast
microscope (DMi1; Leica Microsystems) at 0, 24 and 48 h.
Immunofluorescence confocal
microscopy
The immunofluorescence assay was performed as
previously described (26).
Briefly, 6×104 cells per well were seeded on coverslips
in 12-well plates for 24 h. Following drug treatment, the cells
were fixed with 4% paraformaldehyde for 20 min at room temperature,
permeabilized with 0.3% Triton X-100 (cat. no. P0096; Beyotime
Institute of Biotechnology) in PBS for 15 min, and blocked in PBST
(0.1% Tween-20) containing 5% bovine serum albumin. Thereafter, the
cells were incubated with anti-human rabbit Ki67 antibody (cat. no.
27309-1-AP; 1:200, ProteinTech, Group, Inc.) overnight at 4°C. The
cells were then washed three times with ice-cold PBS, followed by
incubation with the anti-rabbit secondary antibody [Goat
Anti-Rabbit IgG H&L (Alexa Fluor® 594); cat. no.
ab150080; 1:300; Abcam] for 60 min at room temperature. DAPI (cat.
no. AR1177; Wuhan Boster Biological Technology, Ltd.) was used for
nuclear staining at room temperature for 10 min. Finally, images
were captured with a fluorescence microscope (IX83; Olympus
Corporation) and quantified with Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.).
Colony formation assay
In total, 200 HeLa and SiHa cells per well seeded
into 6-well plates were cultured for 5–7 days until small colonies
could be clearly observed. Cells were treated with various
concentrations (0, 2.5 and 3.5 µM) of shikonin in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) for 8–12 days. Subsequently, cells
were fixed with 4% formaldehyde (Sigma-Aldrich; Merck KGaA) for 15
min. The colonies were stained with 0.2% crystal violet solution
(Sigma-Aldrich; Merck KGaA) in 10% ethanol for 10 min. Excess stain
was removed by washing repeatedly with PBS. All procedures were
conducted at room temperature. Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.) was used for quantification.
Reverse transcription-quantitative PCR
(RT-qPCR)
The RT-qPCR assay was performed as previously
described (33,34). Briefly, total cellular RNA was
extracted using TRIzol® reagent (cat. no. 15596018;
Invitrogen; Thermo Fisher Scientific, Inc.). According to the
manufacturer's instructions, total RNA was reverse transcribed into
cDNA using the Thermo Scientific™ RevertAid™
First Strand cDNA Synthesis kit (cat. no. K1621; Thermo Fisher
Scientific, Inc.) in a thermal cycler (Mastercycler®
Nexus; cat. no. 6331000076; Eppendorf) at the following
temperatures: 40°C for 60 min and 70°C for 5 min. The PCR primer
sequences are presented in Table
I. qPCR was performed using 2X M5 HiPer SYBR Premix EsTaq
(Beijing Jumei Biotechnology Co., Ltd.; cat. no. MF787-01), the
20-µl PCR system contained 2 µl cDNA, 10 µl 2X M5 HiPer SYBR Premix
EsTaq (with Tli RNaseH), 0.4 µl ROX Reference Dye II, 0.4 µl 10 µM
Primer 1, 0.4 µl 10 µM Primer 2 and 6.8 µl ddH2O. The
qPCR amplification was performed using the ViiA7 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
thermocycler conditions: 95°C for 30 sec, 95°C for 3 sec and 60°C
for 30 sec for 40 cycles. The relative gene expression levels were
calculated using the 2−ΔΔCq method and normalized to
GAPDH (35). At least three
independent experiments were conducted, and samples were evaluated
in triplicate in each experiment.
 | Table I.Primer pair sequences. |
Table I.
Primer pair sequences.
| Gene name | Primer sequences
(5′-3′) |
|---|
| MTA1 | Forward:
5′-CATCAGAGGCCAACCTTTTCG-3′ |
|
| Reverse:
5′-GCACGTATCTGTCGGTGGTC-3′ |
| TGFβ1 | Forward:
5′-ACTCTCTGACTTCCGCGTTC-3′ |
|
| Reverse:
5′-CACTTGCCCAGCAATAGGTTTAT-3′ |
| VEGF | Forward:
5′-CTGGGCTGTTCTCGCTTCG-3′ |
|
| Reverse:
5′-CTCTCCTCTTCCTTCTCTTCTTCC-3′ |
| GAPDH | Forward:
5′-ACAACTTTGGTATCGTGGAAGG-3′ |
|
| Reverse:
5′-GCCATCACGCCACAGTTTC-3′ |
Statistical analysis
Where indicated, data are represented as the mean ±
SEM and were analysed by using GraphPad Software Prism 7.0
(GraphPad Software, Inc.). Comparisons were made using one/two-way
ANOVA with Bonferroni's multiple comparison tests (when more than
two groups were compared). P<0.05 was considered to indicate a
statistically significant difference.
Results
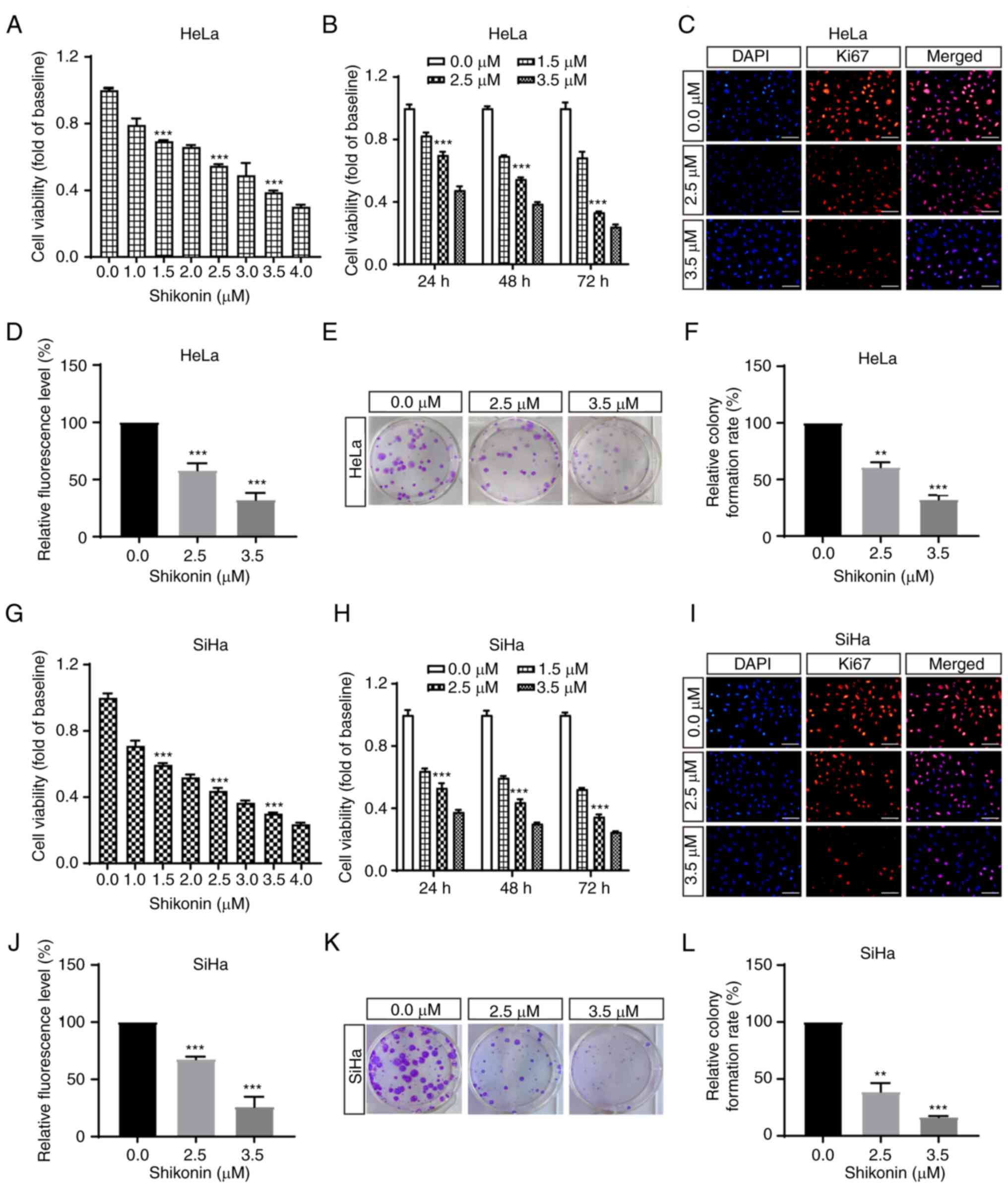
Shikonin inhibits the growth of
cervical cancer cells
To detect the effect of shikonin on the cell growth
of cervical cancer cells, various concentrations of shikonin were
added to the HeLa and SiHa cervical cancer cell lines. Following
incubation for 48 h, cell viability was assessed using a CCK-8
assay. The results revealed that shikonin inhibited HeLa and SiHa
cell proliferation in a concentration-dependent manner, with IC50
values of ~2.9 µM in HeLa cells and 2.2 µM in SiHa cells (Fig. 1A and G). To further examine the
effects of different treatment times on the cell proliferation,
HeLa and SiHa cells were treated with Shikonin for 24, 48 and 72 h
at concentrations of 0, 1.5, 2.5 and 3.5 µM. The results
demonstrated that the inhibitory effect of shikonin on cell
proliferation became increasingly obvious with prolonged
administration time (Fig. 1B and
H). Taken together, these results suggest that shikonin
markedly inhibits the proliferation of cervical cancer cells in a
concentration- and time-dependent manner.
Ki67 is a nuclear antigen related to cell
proliferation and it is a critical biomarker for tumour cell
proliferation (36). In the
present study, HeLa and SiHa cells were then treated with shikonin
for 48 h and the expression of Ki67 was detected using
immunofluorescence assay. As depicted in Fig. 1C and I, shikonin treatment markedly
decreased the number and fluorescence intensity of Ki67-positive
cells. The results indicated that shikonin exerted a significant
inhibitory effect on the proliferation of HeLa and SiHa cervical
cancer cells (Fig. 1C, D, I and
J).
To further confirm the aforementioned findings, a
colony formation assay was then performed, in order to evaluate
cell proliferation. The results revealed that shikonin
significantly inhibited the size of HeLa and SiHa cervical cancer
cell colonies. By increasing the drug concentration, the spaces
between cells became larger, and the shape of the clonogenic bodies
became increasingly smaller (Fig. 1E,
F, K and L).
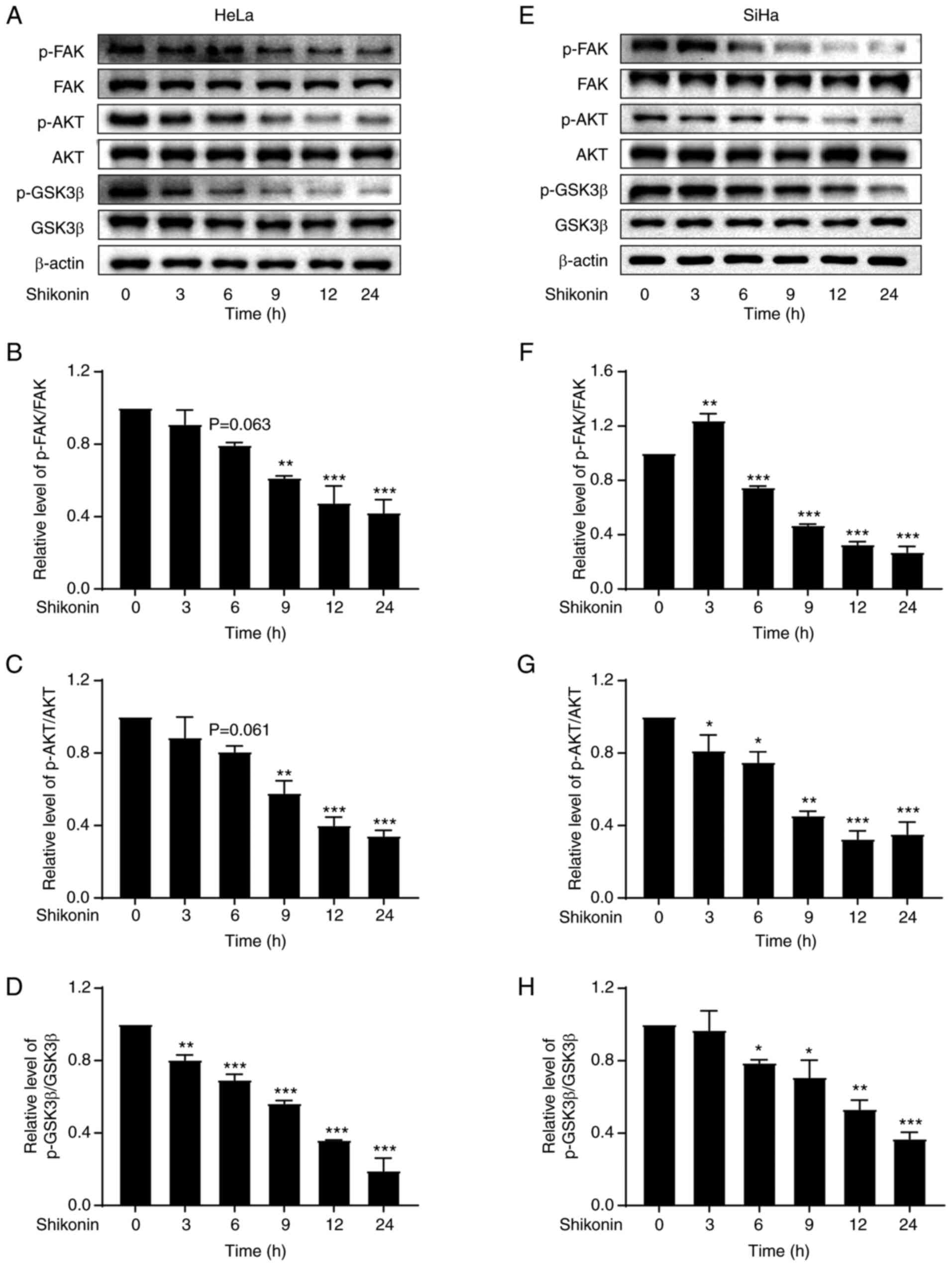
Shikonin reduces the phosphorylation
levels of FAK, AKT and GSK3β in cervical cancer cells
FAK is a key regulator of growth factor receptor and
integrin-mediated signalling, regulating the basic processes of
cancer cell proliferation, migration, invasion and apoptosis
through its kinase activity and scaffolding function (37). Similarly, AKT and GSK3β also play a
vital role in the growth of tumour cells (38,39).
To evaluate whether FAK, AKT and GSK3β are involved in the effects
of shikonin, the FAK, AKT and GSK3β phosphorylation levels in
cervical cancer cells were measured, following shikonin treatment.
It was observed that shikonin significantly inhibited FAK, AKT and
GSK3β phosphorylation without any changes in FAK, AKT and GSK3β
total protein expression (Fig. 2),
suggesting that shikonin inhibits the proliferation of cervical
cancer cells through FAK/AKT/GSK3β signalling.
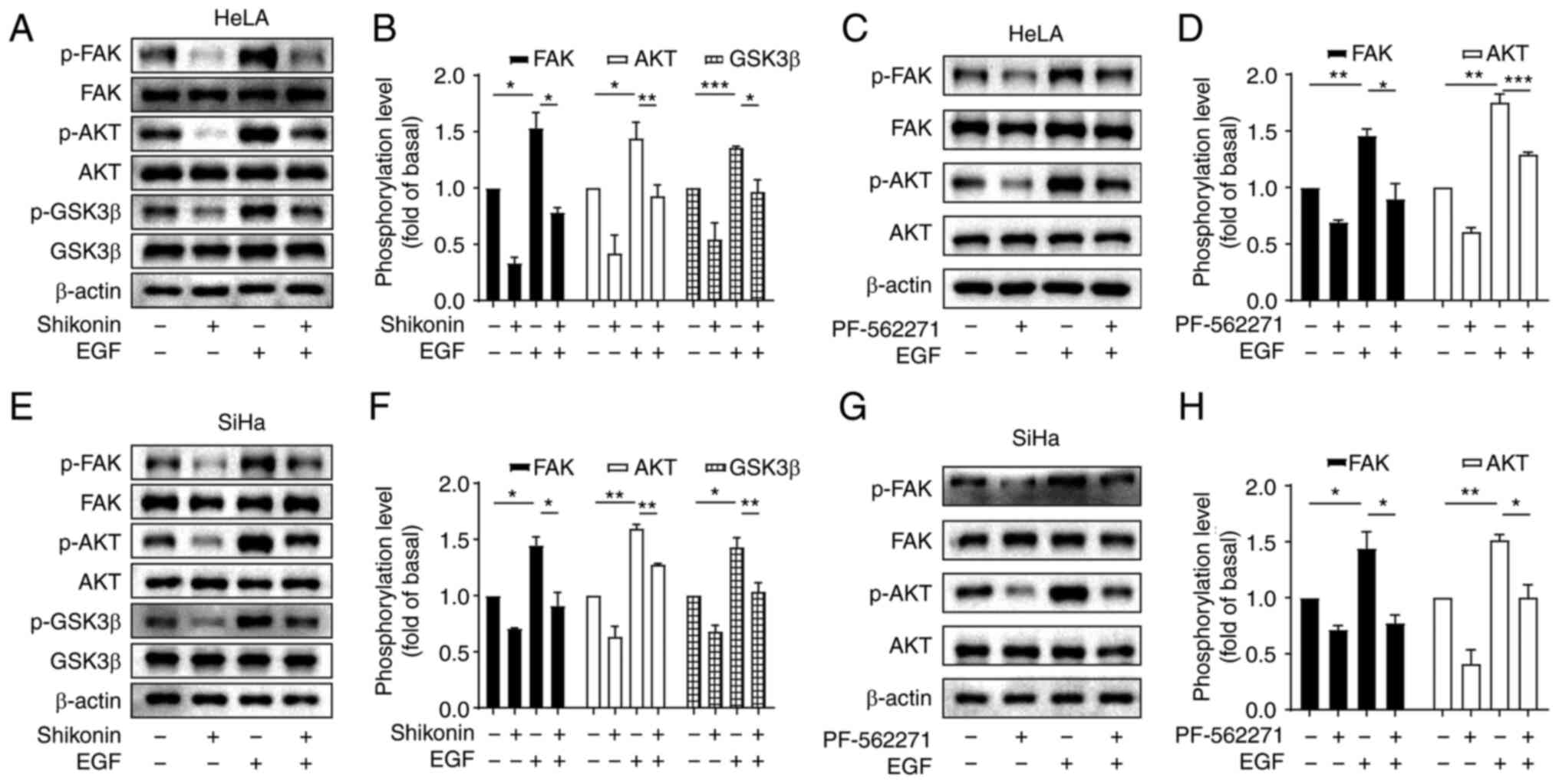
Shikonin blocks the EGF-induced
FAK/AKT signalling pathway in cervical cancer cells
Since EGF/EGFR signalling is overactivated in
multiple human types of cancer (18), it contributes to tumour
proliferation, invasion, apoptosis and angiogenesis. Subsequently,
in the present study, the effects of shikonin on EGF-induced FAK,
Akt and GSK3β phosphorylation were explored. The cells were
pre-treated with shikonin and then stimulated with EGF. As
demonstrated in Fig. 3A, EGF
upregulated the phosphorylation of FAK, AKT and GSK3β, while
shikonin pre-treatment significantly blocked the EGF-induced FAK,
AKT and GSK3β phosphorylation (Fig.
3A, B, E and F). Subsequently, to determine whether AKT serves
as downstream signalling target of FAK, the effect of PF-562271, a
selective inhibitor of FAK, on EGF-induced AKT phosphorylation in
HeLa and SiHa cells was detected. All cells were pre-treated with
PF-562271 (10 µM) for 60 min and then the cells were treated with
EGF (10 ng/ml) for 60 min. It was revealed that PF-562271 markedly
inhibited the basal and EGF-induced AKT phosphorylation levels
(Fig. 3C, D, G and H), indicating
that AKT is a downstream factor of FAK. Therefore, it was
hypothesized that the inhibitory effect of shikonin may be also
mediated through the FAK/AKT/GSK3β pathway.
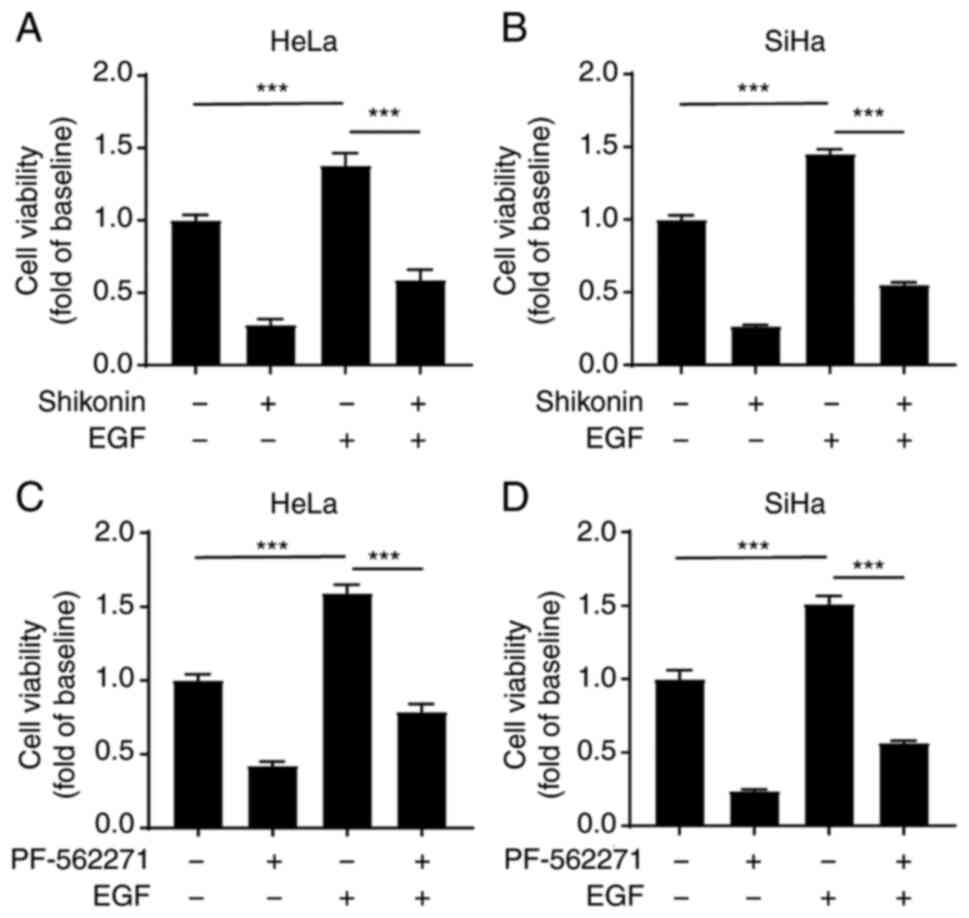
Shikonin inhibits the EGFR-induced
proliferation of cervical cancer cells through the FAK/AKT/GSK3β
pathway
To further validate the results, the present study
then whether shikonin inhibits the proliferation of cervical cancer
cells induced by EGF. The cells were pre-treated with shikonin and
then stimulated with EGF. The results revealed that EGF
significantly promoted the proliferation of cervical cancer cells,
while shikonin significantly inhibited this effect (Fig. 4A and B). Consistent with the
results of western blot analysis, the blocking of FAK using
PF-562271 also markedly inhibited the EGF-induced proliferation of
cervical cancer cells (Fig. 4C and
D). Taken together, these results indicated that shikonin
inhibited the proliferation of cervical cancer cells via the
FAK/AKT/GSK3β signalling pathway.
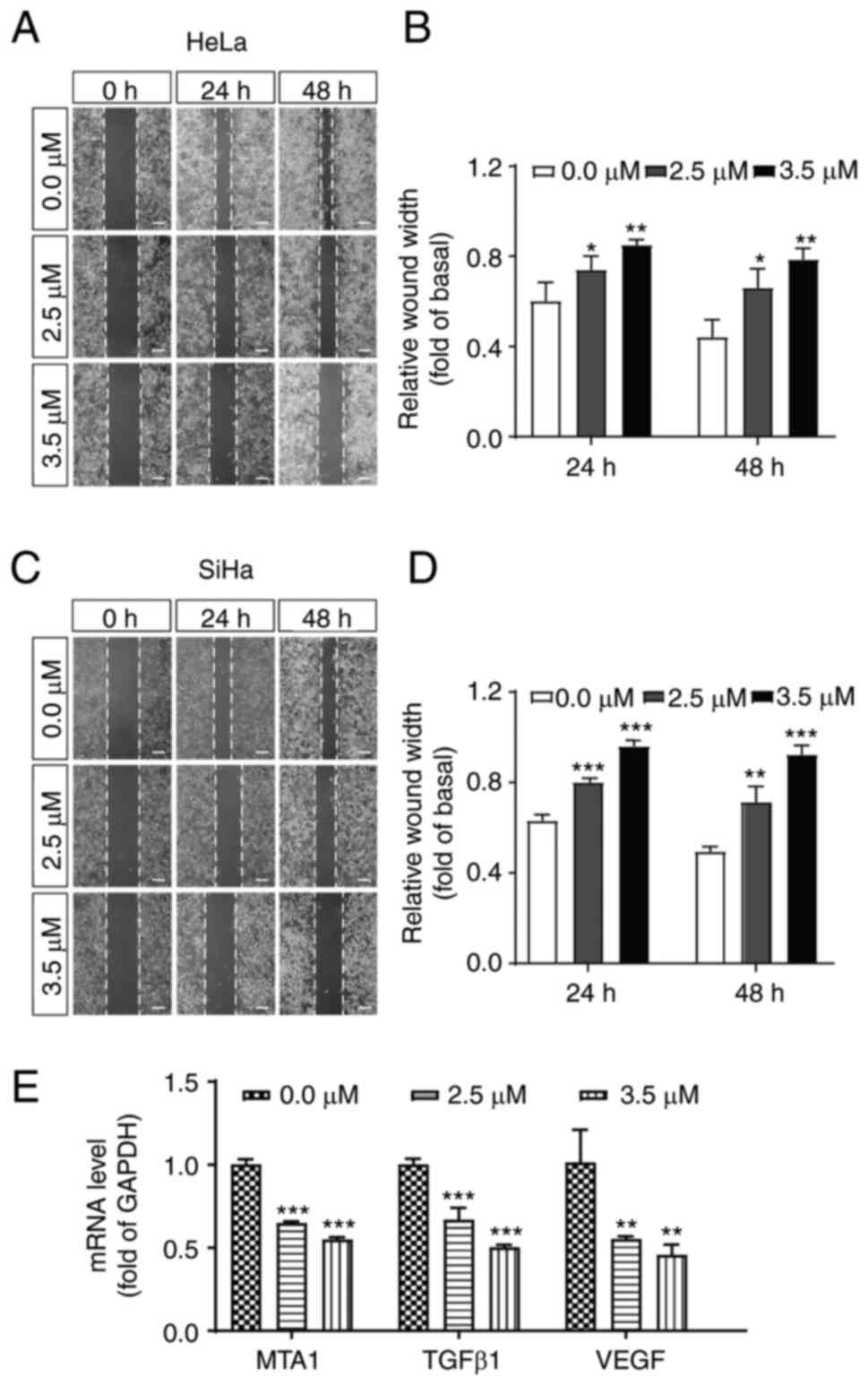
Shikonin inhibits cell migration and
decreases the expression level of related proteins
Cell migration plays an essential role in tumour
progression. Thus, to determine whether shikonin has any effect on
the migration of cervical cancer cells, a wound healing assay was
then performed to evaluate the effects of shikonin on HeLa and SiHa
cell metastatic capability. Notably, shikonin significantly
inhibited the migration of cervical cancer cells (Fig. 5A-D). Numerous studies have
demonstrated that metastasis-associated 1 (MTA1) (40,41),
TGFβ1 (42) and VEGF (43) play crucial roles in the migration
of cervical cancer cells. Thus, the present study then detected the
transcription levels of these genes. As shown in Fig. 5E, shikonin markedly inhibited the
expression of MTA1, TGFβ1 and VEGF in HeLa cells (Fig. 5E), further confirming the
inhibitory effects of shikonin on cervical cancer cells.
Discussion
A recent review article revealed that shikonin
exerts a significant inhibitory effect on the occurrence of
numerous tumours (44); however,
its effects and mechanisms of action in cervical cancer remain
largely unknown. In the present study, it was revealed that
shikonin inhibited the proliferation of HeLa and SiHa cervical
cancer cells, and this effect may be mediated through the
downregulation of FAK/AKT/GSK3β phosphorylation. In addition,
shikonin significantly inhibited the migration of cervical cancer
cells and reduced the transcriptional levels of the MTA1, TGFβ1 and
VEGF genes.
Shikonin has been reported to exert antitumour
effects on a variety of tumours, such as lung, breast,
gastrointestinal and pancreatic cancer (44). In the present study, it was
demonstrated that shikonin inhibited the viability of HeLa and SiHa
cervical cancer cells in a concentration- and time-dependent
manner. This was further supported by evidence that shikonin
inhibited the expression of Ki67, a widely used biomarker for cell
proliferation of tumour cells. In addition, the colony formation
assay also revealed that the number of colonies was significantly
reduced following shikonin treatment. All these findings by
different assays consistently underline that shikonin may exert a
significant inhibitory effect on the proliferation of HeLa and SiHa
cervical cancer cells. One of the limitations of the present study
was that the effect of shikonin on a non-cancerous cervical cell
line was not examined. Notably, a recent study indicated that
shikonin may exert a general anticancer effect and a relatively
less prominent inhibitory effect on normal cells (29). Furthermore, a clinical trial study
reported that the shikonin mixture was safe and effective in the
treatment of patients with late-stage lung cancer who were
unsuitable for radiotherapy, chemotherapy and surgery; more
importantly, shikonin treatment had no harmful effects on
peripheral system, heart, kidney and liver and even increased the
body weight and appetite of the patients (45). Another limitation of the present
study is that although this study was mainly focused on the effects
of shikonin on cell proliferation, whether shikonin exerts its
anti-proliferative effect on cervical cancer due to cell cycle
arrest, death and/or apoptosis remains unknown. Of note, it has
previously been reported that shikonin inhibits the proliferation
of AGS human stomach carcinoma cells by inducing apoptotic cell
death (46). Shikonin has been
revealed to arrest the cell cycle in the G2/M phase, inhibit cell
growth and induce cell death, which collectively contributes to the
growth inhibitory effects of shikonin in various cancer cell lines
originating from lung, breast, pancreas, osteosarcoma, while its
inhibitory effects on normal cells are more limited in comparison
(29). Thus, the effects of
shikonin on cell cycle progression and the apoptosis of cervical
cancer require further investigation.
FAK is an important regulatory molecule in growth
factor receptors and integral protein-mediated signalling pathways
(47). In multiple types of
tumours, the expression of activated FAK has been reported to be
significantly increased (48). The
overexpression and hyperphosphorylation of FAK have been revealed
to promote cancer cell proliferation, motility and survival
(49). Of note, in the present
study, it was observed that shikonin inhibited FAK phosphorylation
levels in cervical cancer cells. AKT is a serine/threonine protein
kinase that plays a crucial role in regulating cell survival and
apoptosis, and studies have revealed that AKT activation can affect
cell growth, proliferation, migration, apoptosis and angiogenesis
of tumour cells by regulating many downstream proteins (50). In addition, several studies have
demonstrated that the PI3K/AKT/GSK3β signalling pathway may
regulate epithelial-mesenchymal transition in numerous tumours,
thus participating in tumour invasion and metastasis (51–53).
However, the FAK/AKT/GSK3β signalling pathway has not, to the best
of our knowledge, been previously studied in cervical
carcinogenesis. In the present study, it was demonstrated that
shikonin significantly inhibited the phosphorylation levels of FAK,
AKT and GSK3β in cervical cancer cells, which is a different
mechanism in comparison to the signalling pathways reported
concerning other tumour types. For example, Tang et al
(27) demonstrated that shikonin
inhibited the proliferation of oesophageal cancer cells, which is
associated with the HIF1α/PKM signalling pathway. Shan et al
(54) revealed that shikonin
suppress human leukemia NB4 cell proliferation and induce apoptosis
by regulating MAPKs and c-Myc. Pan et al (55) reported that shikonin blocked human
lung adenocarcinoma development through the IL-6/STAT3 signalling
pathway. The mechanisms by which shikonin functions to block the
phosphorylation of FAK/AKT remain largely unknown. Previous studies
have revealed that the FAK/AKT axis is activated by receptor
tyrosine kinases, including EGFR and IGF-1R. However, there are few
reports on the exact molecular mechanisms through which these
receptors regulate FAK phosphorylation in tumours, and previous
studies have reported that these receptors may regulate the FAK/AKT
pathway through Src and NF-KB phosphorylation (56,57).
EGFR is an important tyrosine kinase receptor in
tumourigenesis and has been reported to play a crucial role in
tumour cell proliferation, migration and apoptosis (58). In the present study, to further
investigate the underlying mechanisms through which shikonin
inhibits the proliferation of cervical cancer cells, the effects of
shikonin on the EGFR-induced signalling pathway were first
detected. EGF significantly increased the phosphorylation levels of
FAK and AKT, which were markedly decreased by shikonin treatment.
However, compared to shikonin alone, EGF still increased the
phosphorylation level of FAK and AKT in the presence of shikonin,
indicating shikonin may not completely block EGF-induced activation
of downstream signalling cascades. There are two possible
explanations for these findings. On the one hand, the concentration
of shikonin may markedly low; thus, it can only partially block the
EGF-induced effect. Firstly, EGF may also induce FAK and AKT
phosphorylation through other signalling cascades that are
insensitive to shikonin. It is worth noting that since the
mechanisms through which shikonin blocks FAK/AKT phosphorylation
and affect the phosphorylation and total expression levels of EGFR
remain unknown, the exact mechanisms involved need to be further
investigated.
Notably, the EGF-induced increase in AKT
phosphorylation could be significantly inhibited by the
FAK-specific blocker, PF-562271. Therefore, it can be inferred that
AKT may be the downstream signalling molecule of FAK. In addition,
cervical cancer cells were treated with EGF in the absence or
presence of PF-562271 and it was observed that PF-562271 inhibited
the EGF-induced increase in FAK and AKT phosphorylation levels.
Taken together with the CCK-8 assay findings, demonstrating that
PF-562271 significantly inhibited EGF-induced cell proliferation,
it was speculated that the inhibition of FAK phosphorylation may
cause a decrease in downstream AKT phosphorylation, in turn
weakening the proliferation of cervical cancer cells. Thus, these
findings indicated that shikonin-induced inhibition of cervical
carcinogenesis may be mediated through the FAK/AKT/GSK3β
phosphorylation signalling pathway.
Another important finding of the present study is
that shikonin also suppressed the cell migration of cervical cancer
cells, as evaluated using wound healing assay. Moreover, shikonin
inhibited the expression of migration-related genes, including
MTA1, TGFβ1 and VEGF, suggesting that shikonin may inhibit the
proliferation of tumour cells by affecting downstream related genes
after inhibiting the phosphorylation of FAK and AKT. In addition,
in the future, the authors aim to further validate the tumour
suppressive effects in mouse models with subcutaneous tumours or
in situ cervical cancer in vivo. The authors also aim
to use mouse tumour tissues for protein, RNA and
immunohistochemical staining evaluation, in order to detect the
expression of p-FAK, p-AKT and Ki67 proteins to further elucidate
the molecular mechanisms involved.
In conclusion, it was observed that shikonin
significantly inhibited the proliferation and migration of cervical
cancer cells. It was also clarified that shikonin inhibited the
proliferation of cervical cancer cells, possibly by regulating the
EFG-mediated phosphorylation of the FAK/AKT/GSK3βsignalling
pathway. The findings of the present study suggest that shikonin
may be a potential drug for use in the clinical treatment of
cervical cancer, and provide a theoretical basis for the
development of novel therapeutic drugs in the clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81760505 and
32160184), the Scientific Project of Jiangxi Province (grant nos.
20192BCB23008, 20181ACG70003, 20202BBGL73011 and 2018BAB215018) and
the Scientific Research Project of Jiangxi Provincial
Administration of Traditional Chinese Medicine (grant no.
2021Z008).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NL, PH and ZX designed the study. ZX and LH
conducted the experiments. TZ, YL, FF and XW analysed most of the
experimental data. WC, LL and YZ analysed the western blot analysis
data. ZX and PH wrote the manuscript. All authors have read and
approved the final manuscript. NL and PH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pilleron S, Cabasag CJ, Ferlay J, Bray F,
Luciani S, Almonte M and Piñeros M: Cervical cancer burden in Latin
America and the Caribbean: Where are we? Int J Cancer.
147:1638–1648. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynaecol
Obstet. 143 (Suppl 2):S22–S36. 2018. View Article : Google Scholar
|
|
5
|
Fernandez-Retana J, Lasa-Gonsebatt F,
Lopez-Urrutia E, Coronel-Martínez J, Cantu De Leon D,
Jacobo-Herrera N, Peralta-Zaragoza O, Perez-Montiel D,
Reynoso-Noveron N, Vazquez-Romo R and Perez-Plasencia C: Transcript
profiling distinguishes complete treatment responders with locally
advanced cervical cancer. Transl Oncol. 8:77–84. 2015. View Article : Google Scholar
|
|
6
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A gynecologic oncology
group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar
|
|
7
|
Rajkumar T, Vijayalakshmi N, Sabitha K,
Shirley S, Selvaluxmy G, Bose MV and Nambaru L: A 7 gene expression
score predicts for radiation response in cancer cervix. BMC Cancer.
9:3652009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Yang S, Wang K, Lu J, Bao X, Wang
R, Qiu Y, Wang T and Yu H: Cellular senescence and cancer: Focusing
on traditional Chinese medicine and natural products. Cell Prolif.
53:e128942020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao YH, Li CI, Lin CC, Lin JG, Chiang JH
and Li TC: Traditional Chinese medicine as adjunctive therapy
improves the long-term survival of lung cancer patients. J Cancer
Res Clin. 143:2425–2435. 2017. View Article : Google Scholar
|
|
10
|
Wang F, Yao X, Zhang Y and Tang J:
Synthesis, biological function and evaluation of shikonin in cancer
therapy. Fitoterapia. 134:329–339. 2019. View Article : Google Scholar
|
|
11
|
Boulos JC, Rahama M, Hegazy MF and Efferth
T: Shikonin derivatives for cancer prevention and therapy. Cancer
Lett. 459:248–267. 2019. View Article : Google Scholar
|
|
12
|
Hsieh YS, Liao CH, Chen WS, Pai JT and
Weng MS: Shikonin inhibited migration and invasion of human lung
cancer cells via suppression of c-met-mediated
epithelial-to-mesenchymal transition. J Cell Biochem.
118:4639–4651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia H, Tang C, Gui H, Wang X, Qi J, Wang X
and Yang Y: Preparation, cellular uptake and angiogenic suppression
of shikonin-containing liposomes in vitro and in vivo. Biosci Rep.
33:e000202013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Sun B, Huang Z, Zhao DW and Zeng
Q: Shikonin inhibites migration and invasion of thyroid cancer
cells by downregulating DNMT1. Med Sci Monit. 24:661–670. 2018.
View Article : Google Scholar
|
|
15
|
Tang Q, Liu L, Zhang H, Xiao J and Hann
SS: Regulations of miR-183-5p and snail-mediated shikonin-reduced
epithelial-mesenchymal transition in cervical cancer cells. Drug
Des Devel Ther. 14:577–589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Z, Wu LJ, Li LH, Tashiro SI, Onodera S
and Ikejima T: Shikonin regulates HeLa cell death via caspase-3
activation and blockage of DNA synthesis. J Asian Nat Prod Res.
6:155–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wykosky J, Fenton T, Furnari F and Cavenee
WK: Therapeutic targeting of epidermal growth factor receptor in
human cancer: Successes and limitations. Chin J Cancer. 30:5–12.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yarden Y: The EGFR family and its ligands
in human cancer. Signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37 (Suppl 4):S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Q, Wang N, Ren L, Tian J, Yang S and
Cheng H: miR-125a-5p post-transcriptionally suppresses GALNT7 to
inhibit proliferation and invasion in cervical cancer cells via the
EGFR/PI3K/AKT pathway. Cancer Cell Int. 20:1172020. View Article : Google Scholar
|
|
20
|
Muthusami S, Sabanayagam R, Periyasamy L,
Muruganantham B and Park WY: A review on the role of epidermal
growth factor signaling in the development, progression and
treatment of cervical cancer. Int J Biol Macromol. 194:179–187.
2022. View Article : Google Scholar
|
|
21
|
Cance WG, Kurenova E, Marlowe T and
Golubovskaya V: Disrupting the scaffold to improve focal adhesion
kinase-targeted cancer therapeutics. Sci Signal. 6:pe102013.
View Article : Google Scholar
|
|
22
|
Yoon H, Dehart JP, Murphy JM and Lim STS:
Understanding the roles of FAK in cancer: Inhibitors, genetic
models, and new insights. J Histochem Cytochem. 63:114–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Yi Q and Tang L: The roles of
nuclear focal adhesion kinase (FAK) on cancer: A focused review. J
Exp Clin Cancer Res. 38:2502019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun J, Luo Q, Liu L, Yang X, Zhu S and
Song G: Salinomycin attenuates liver cancer stem cell motility by
enhancing cell stiffness and increasing F-actin formation via the
FAK-ERK1/2 signalling pathway. Toxicology. 384:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rogovskii VS: The linkage between
inflammation and immune tolerance: Interfering with inflammation in
cancer. Curr Cancer Drug Targets. 17:325–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu YG, Lv YX, Guo CY, Xiao ZM, Jiang QG,
Kuang H, Zhang WH and Hu P: Harmine inhibits the proliferation and
migration of glioblastoma cells via the FAK/AKT pathway. Life Sci.
270:1191122021. View Article : Google Scholar
|
|
27
|
Tang JC, Zhao J, Long F, Chen JY, Mu B,
Jiang Z, Ren Y and Yang J: Efficacy of shikonin against esophageal
cancer cells and its possible mechanisms in vitro and in vivo. J
Cancer. 9:32–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du W, Hao X, Yuan Z, Wang Y, Zhang X and
Liu J: Shikonin potentiates paclitaxel antitumor efficacy in
esophageal cancer cells via the apoptotic pathway. Oncol Lett.
18:3195–3201. 2019.
|
|
29
|
Wang F, Mayca Pozo F, Tian D, Geng X, Yao
X, Zhang Y and Tang J: Shikonin inhibits cancer through P21
upregulation and apoptosis induction. Front Pharmacol. 11:8612020.
View Article : Google Scholar
|
|
30
|
Bao C, Liu T, Qian L, Xiao C, Zhou X, Ai
H, Wang J, Fan W and Pan J: Shikonin inhibits migration and
invasion of triple-negative breast cancer cells by suppressing
epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. J
Cancer. 12:76–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hussein HAM, Walker LR and Akula SM: KSHV
gB associated RGD interactions promote attachment of cells by
inhibiting the potential migratory signals induced by the
disintegrin-like domain. BMC Cancer. 16:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Luo Y, Zhong R, Law PTY, Boon SS,
Chen Z, Wong CH and Chan PKS: Role of polycyclic aromatic
hydrocarbons as a co-factor in human papillomavirus-mediated
carcinogenesis. BMC Cancer. 19:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He JJ, Zhang WH, Liu SL, Chen YF, Liao CX,
Shen QQ and Hu P: Activation of β-adrenergic receptor promotes
cellular proliferation in human glioblastoma. Oncol Lett.
14:3846–3852. 2017. View Article : Google Scholar
|
|
34
|
Hu P, He J, Liu S, Wang M, Pan B and Zhang
W: β2-adrenergic receptor activation promotes the proliferation of
A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol Rep.
36:1757–1763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar
|
|
37
|
Peng X and Guan JL: Focal adhesion kinase:
From in vitro studies to functional analyses in vivo. Curr Protein
Pept Sci. 12:52–67. 2011. View Article : Google Scholar
|
|
38
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mancinelli R, Carpino G, Petrungaro S,
Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E and
Giampietri C: Multifaceted roles of GSK-3 in cancer and
autophagy-related diseases. Oxid Med Cell Longev. 2017:46294952017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rao Y, Wang H, Fan L and Chen G: Silencing
MTA1 by RNAi reverses adhesion, migration and invasiveness of
cervical cancer cells (SiHa) via altered expression of p53, and
E-cadherin/β-catenin complex. J Huazhong Univ Sci Technolog Med
Sci. 31:1–9. 2011. View Article : Google Scholar
|
|
41
|
Guo N, Shen G, Zhang Y, Moustafa AA, Ge D
and You Z: Interleukin-17 promotes migration and invasion of human
cancer cells through upregulation of MTA1 expression. Front Oncol.
9:5462019. View Article : Google Scholar
|
|
42
|
Zhang L, Zhou J, Qin X, Huang H and Nie C:
Astragaloside IV inhibits the invasion and metastasis of SiHa
cervical cancer cells via the TGF-β1-mediated PI3K and MAPK
pathways. Oncol Rep. 41:2975–2986. 2019.PubMed/NCBI
|
|
43
|
Chen L, Wu YY, Liu P, Wang J, Wang G, Qin
J, Zhou J and Zhu J: Down-regulation of HPV18 E6, E7, or VEGF
expression attenuates malignant biological behavior of human
cervical cancer cells. Med Oncol. 28 (Suppl 1):S528–S539. 2011.
View Article : Google Scholar
|
|
44
|
Guo C, He J, Song X, Tan L, Wang M, Jiang
P, Li Y, Cao Z and Peng C: Pharmacological properties and
derivatives of shikonin-a review in recent years. Pharmacol Res.
149:1044632019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo XP, Zhang XY and Zhang SD: Clinical
trial on the effects of shikonin mixture on later stage lung
cancer. Zhong Xi Yi Jie He Za Zhi. 11:598–599. 5801991.(In
Chinese).
|
|
46
|
Ko H, Kim SJ, Shim SH, Chang H and Ha CH:
Shikonin induces apoptotic cell death via regulation of p53 and
Nrf2 in AGS human stomach carcinoma cells. Biomol Ther (Seoul).
24:501–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brami-Cherrier K, Gervasi N, Arsenieva D,
Walkiewicz K, Boutterin MC, Ortega A, Leonard PG, Seantier B, Gasmi
L, Bouceba T, et al: FAK dimerization controls its kinase-dependent
functions at focal adhesions. EMBO J. 33:356–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Watanabe N, Takaoka M, Sakurama K, Tomono
Y, Hatakeyama S, Ohmori O, Motoki T, Shirakawa Y, Yamatsuji T,
Haisa M, et al: Dual tyrosine kinase inhibitor for focal adhesion
kinase and insulin-like growth factor-I receptor exhibits
anticancer effect in esophageal adenocarcinoma in vitro and in
vivo. Clin Cancer Res. 14:4631–4639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang J and Hochwald SN: The role of FAK
in tumor metabolism and therapy. Pharmacol Ther. 142:154–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar
|
|
51
|
Zhang X, Jiang G, Sun M, Zhou H, Miao Y,
Liang M, Wang E and Zhang Y: Cytosolic THUMPD1 promotes breast
cancer cells invasion and metastasis via the AKT-GSK3-snail
pathway. Oncotarget. 8:13357–13366. 2017. View Article : Google Scholar
|
|
52
|
Shi X, Yang J and Wei G: Ginsenoside
20(S)-Rh2 exerts anti-cancer activity through the Akt/GSK3β
signaling pathway in human cervical cancer cells. Mol Med Rep.
17:4811–4816. 2018.PubMed/NCBI
|
|
53
|
Dai J, Qian C, Su M, Chen M and Chen J:
Gastrokine-2 suppresses epithelial mesenchymal transition through
PI3K/AKT/GSK3β signaling in gastric cancer. Tumour Biol.
37:12403–12410. 2016. View Article : Google Scholar
|
|
54
|
Shan ZL, Zhong L, Xiao CL, Gan LG, Xu T,
Song H, Yang R, Li L and Liu BZ: Shikonin suppresses proliferation
and induces apoptosis in human leukemia NB4 cells through
modulation of MAPKs and c-Myc. Mol Med Rep. 16:3055–3060. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pan T, Zhang F, Li F, Gao X, Li Z, Li X
and Ren X: Shikonin blocks human lung adenocarcinoma cell migration
and invasion in the inflammatory microenvironment via the
IL-6/STAT3 signaling pathway. Oncol Rep. 44:1049–1063. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen Y, Hu X and Yang S: Clinical
significance of focal adhesion kinase (FAK) in cervical cancer
progression and metastasis. Int J Clin Exp Pathol. 13:2586–2592.
2020.
|
|
57
|
Chuang HH, Zhen YY, Tsai YC, Chuang CH,
Hsiao M, Huang MS and Yang CJ: FAK in cancer: From mechanisms to
therapeutic strategies. Int J Mol Sci. 23:17262022. View Article : Google Scholar
|
|
58
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar
|