Introduction
Osteosarcoma (OS), a malignant tumor originating
from the mesenchymal tissue, commonly occurs in children and
adolescents (1–3). OS may only be accompanied by local
pain and swelling. However, pulmonary metastasis from OS is likely
to occur at an early stage of the disease and develops rapidly
(4). With surgical procedures and
the application of chemotherapy, the 5-year survival rate has
improved significantly (5).
However, further improvement of therapeutics of OS has been
limited, especially for patients with lung metastasis (6). Therefore, identifying novel
therapeutic methods and strategies for the treatment of OS and its
development is of importance.
Anesthetic agents may serve an essential role in
tumor relapse and metastasis as they are administered at the moment
of greatest risk of transmission, which is the surgical removal of
the tumor (7). Recently, numerous
studies have reported that the type of anesthetic agent used could
influence the prognosis of patients with cancer undergoing surgery
for cancer treatment (8,9). Propofol, a common intravenous
anesthetic, is commonly used for anesthesia prior to tumor
resection (10). Emerging evidence
suggests that propofol can inhibit the growth and metastasis of
tumor cells (11,12). Propofol can enhance the anti-tumor
effect of chemotherapeutic drugs or certain small molecular
compounds (13). Furthermore,
in vivo animal models have demonstrated that propofol
suppresses tumor growth and metastasis (14). It has also been demonstrated in
clinical trials that propofol is associated with better survival
rates in patients with cancer following surgery (15,16).
Therefore, it is important to study the effects of propofol on
numerous types of cancer and its potential underlying
mechanisms.
Previous studies have reported that propofol is
involved in tumor development via modulation of the expression of
key RNAs, such as microRNAs (miRs) and long non-coding RNAs
(lncRNAs), and the activation of several signaling pathways,
including the hypoxia-inducible factor-1α, MAPK, NF-κB and nuclear
factor E2-related factor-2 signaling pathways (17–19).
Moreover, it has been demonstrated that propofol affects the degree
of host immunosuppression and modulates immune function (20). It has also been reported that
propofol has a regulatory effect on the proliferation, invasion and
apoptosis of OS cells (21,22).
However, the specific molecular mechanisms underlying the effect of
propofol on OS remain unclear.
Autophagy is an evolutionarily conserved catabolic
process that maintains cellular homeostasis via degrading
long-lived and damaged proteins or organelles in cells (23). Emerging evidence has suggested that
autophagy can inhibit the malignant development of OS (24). Furthermore, a previous study has
reported that propofol can benefit organs and tissues in cancer by
modulating autophagy, which is an evolutionarily conserved
catabolic process that maintains cellular homeostasis by degrading
long-lived proteins and damaged cellular proteins or organelles
(25).
Therefore, the aim of the present study was to
investigate whether propofol inhibited the development of OS via
inducing cell autophagy. Furthermore, whether the adenosine
monophosphate-activated protein kinase (AMPK)/FOXO1 signaling
pathway was involved in the regulation of autophagy in OS was also
explored.
Materials and methods
Cell culture and treatment
The human OS U2OS cell line was purchased from the
American Type Culture Collection. Cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and
100 U/ml penicillin at 37°C in a humidified atmosphere with 5%
CO2. U2OS cells were treated with different
concentrations (0, 2.5, 5 and 10 µg/ml) of propofol
(MilliporeSigma) at 37°C for 48 h (26–28).
Untreated cells were used as the control. To reveal the underlying
molecular mechanism of propofol, U2OS cells were also co-treated
with 10 µg/ml propofol with or without 20 µM compound C
(MedChemExpress), an antagonist of AMPK, with or without 50 nM
rapamycin (RAP; Abcam), an autophagy agonist, for 24 h at 37°C.
Cell counting kit 8 (CCK-8) assay
U2OS cells under different treatment conditions were
cultured in DMEM with 10% FBS at 37°C for 24 h. Subsequently, 10 µl
CCK-8 solution (Dojindo Laboratories, Inc.) was added into each
well and the cells (5×103 cells/well) were incubated for
2 h. The absorbance of each well was assessed at a wavelength of
450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). A
total of six parallel wells were used for each group.
Colony formation assay
U2OS cells under different treatment conditions were
seeded into 6-well plates (500 cells/well) and incubated in DMEM
with 10% bovine calf serum (Thermo Fisher Scientific, Inc.) at
37°C. The medium was replaced with fresh complete culture medium
every 3 days, and the cells were incubated for 2 weeks. The cells
were fixed with 4% paraformaldehyde for 20 min at room temperature
and stained with 0.1% crystal violet solution for 30 min at room
temperature. A cell group containing >50 cells was identified as
a colony. The number of visible colonies was counted manually using
a light microscope (Olympus Corporation). All experiments were
repeated three times.
TUNEL assay
Cell apoptosis was assessed using the TUNEL assay.
An in situ Cell Death Detection Kit (Roche Diagnostics GmbH)
was used according to the manufacturer's instructions. Briefly,
U2OS cells (2×105 cells/well) in a 24-well plate under
different treatment conditions were fixed with 4% paraformaldehyde
at room temperature for 30 min and incubated with proteinase K for
15 min at 37°C. Subsequently, the cells were incubated with 3%
H2O2 for 15 min at room temperature and were
then treated using the in situ Cell Death Detection Kit for
60 min at 37°C. Following incubation, cells were co-labeled with 1
µg/ml DAPI working solution for 10 min in the dark. Slides were
mounted using glycerol. Apoptotic cells in six randomly selected
fields of view were imaged using a fluorescence microscope (Leica
Microsystems GmbH) and quantified by ImageJ Software (version 6.0;
National Institutes of Health).
Wound healing assay
U2OS cell migration ability was assessed using a
wound healing assay. Briefly, U2OS cells under different treatment
conditions were seeded into a 6-well plate (1×105
cells/well) and cultured in serum-free DMEM until they reached 90%
confluency. Subsequently, a straight scratch was made on the cell
monolayer using a 20-µl pipette tip followed by washing with
serum-free medium three times. Following incubation for 24 h, the
cell migration rate was calculated using the following formula:
(wound width at 0 h-wound width at 24 h)/wound width at 0 h × 100.
Images were captured by a light Nikon ECLIPSE E100 microscope and
the gap distance was quantitatively evaluated using ImageJ software
(version 1.8.0 172; National Institutes of Health).
Transwell assay
Cell invasion ability was assessed using the
Transwell assay. The Transwell chambers (Costar; Corning, Inc.)
were first coated with 0.1 ml Matrigel (BD Biosciences) at 37°C for
1 h. U2OS cells under different treatment conditions were suspended
in serum-free DMEM at a final concentration of 5×105
cells/ml and seeded into the upper chamber, whereas the lower
chamber was supplemented with DMEM containing 5% FBS. Following
incubation for 24 h at 37°C, cells on the lower surface of the
membrane were fixed with 100% methanol at room temperature for 20
min and stained with 0.1% crystal violet for 15 min at room
temperature. Invaded cells were counted using an inverted light
microscope and analyzed using ImageJ software (version 1.8.0 172;
National Institutes of Health).
Western blotting
Total protein was extracted from U2OS cells under
different treatment conditions using RIPA buffer (Auragene; Hunan
Aijia Biotechnology Co., Ltd.) and protein concentration was
quantified using the BCA method (Thermo Fisher Scientific, Inc.).
Total protein (50 µg/lane) was separated using SDS-PAGE on a 10%
gel and separated proteins were then electrotransferred onto PVDF
membranes. Following blocking with 5% non-fat milk for 1 h at room
temperature, the membranes were incubated at 4°C overnight with the
following primary antibodies against: LC3II/I (cat. no. 14600-1-AP;
1:1,000; ProteinTech Group, Inc.), Beclin1 (cat. no. ab207612;
1:2,000; Abcam), p62 (cat. no. ab207305; 1:1,000; Abcam),
phosphorylated (p)-AMPK (cat. no. ab133448; 1:1,000; Abcam), AMPK
(cat. no. ab32047; 1:1,000; Abcam), p-FOXO1 (cat. no. ab131339;
1:1,000; Abcam), FOXO1 (cat. no. ab179450; 1:1,000; Abcam), Bcl-2
(cat. no. ab32124; 1;1,000; Abcam), Bax (cat. no. ab32503; 1:1,000;
Abcam), cleaved caspase-3 (cat. no. ab32042; 1:500; Abcam),
caspase-3 (cat. no. ab32351; 1:5,000; Abcam), N-cadherin (cat. no.
ab76011; 1:5,000; Abcam), Vimentin (cat. no. ab92547; 1:1,000;
Abcam), E-cadherin (cat. no. ab40772; 1:10,000; Abcam) and GAPDH
(cat. no. ab9485; 1:2,500; Abcam). Following washing three times
with TBS-0.1% Tween 20, the membranes were incubated with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (cat. no. 7074; 1:1,000; Cell Signaling Technology,
Inc.) for 1 h at room temperature. Separated proteins were
visualized using an enhanced chemiluminescence detection system
(Amersham; Cytiva). ImageJ software (version 1.8.0 172; National
Institutes of Health) was used to analyze the images. The data were
normalized to GAPDH to obtain the integral optical density
values.
Statistical analysis
All experiments were repeated three times. All
statistical analysis was performed using SPSS 22.0 software (IBM
Corp.). All data are presented as the mean ± SD. The differences
among multiple groups were analyzed using one-way ANOVA followed by
Bonferroni's multiple comparison post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Propofol attenuates U2OS cell
viability and promotes cell autophagy
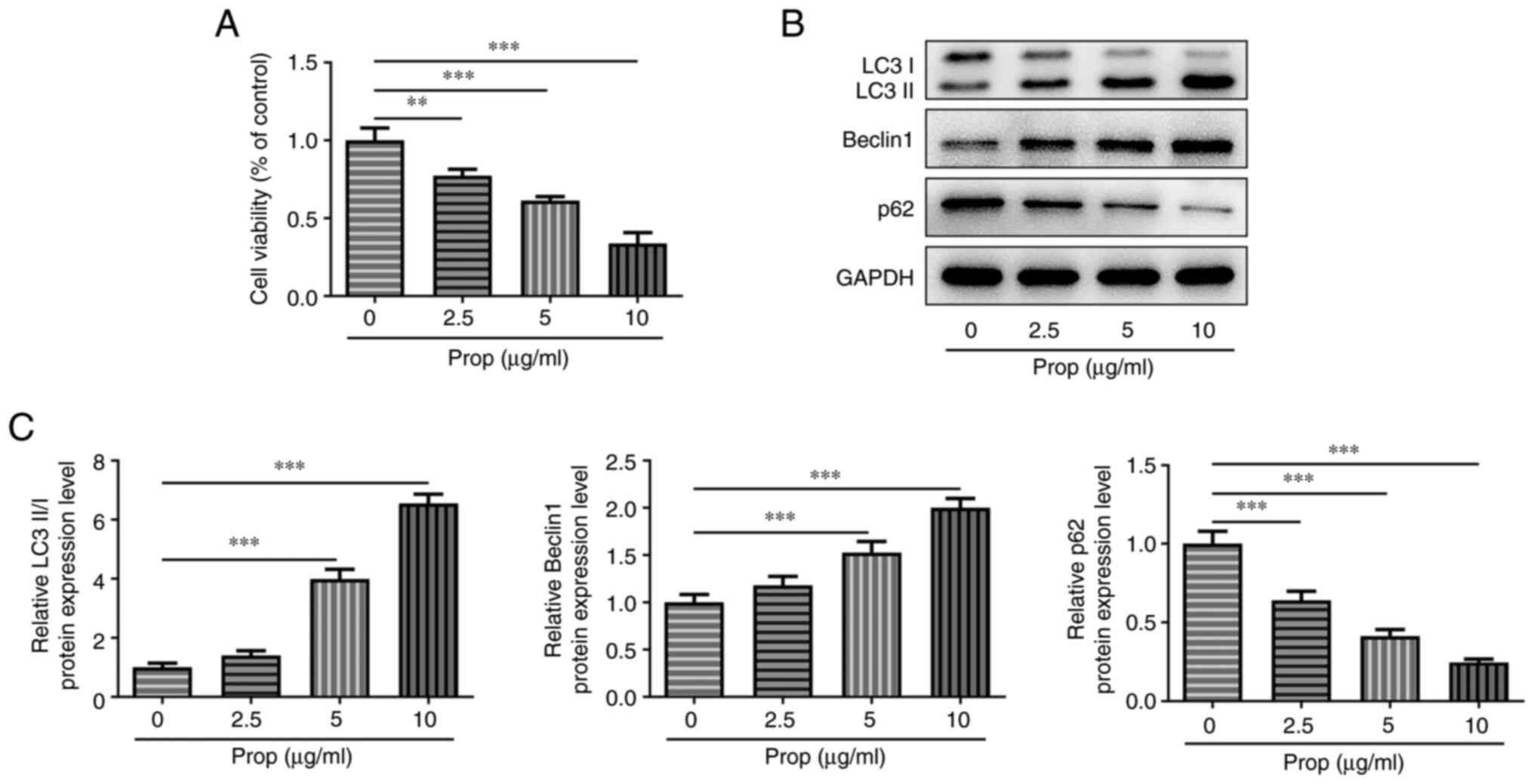
To explore the effects of propofol on OS cells, the
present study first investigated the effect of different doses of
propofol on U2OS cell viability. The results demonstrated that
compared with untreated cells, U2OS cell viability was
significantly reduced in a dose-dependent manner following cell
treatment with different doses of propofol (Fig. 1A). Furthermore, treatment with
propofol significantly upregulated LC3II/I and Beclin1 protein
expression levels at 5 and 10 µg/ml and significantly downregulated
p62 protein expression levels at all doses, compared with the
untreated control. These results therefore indicated that propofol
may promote U2OS cell autophagy (Fig.
1B and C).
Propofol promotes U2OS cell autophagy
via dose-dependent activation of the AMPK/FOXO1 signaling
pathway
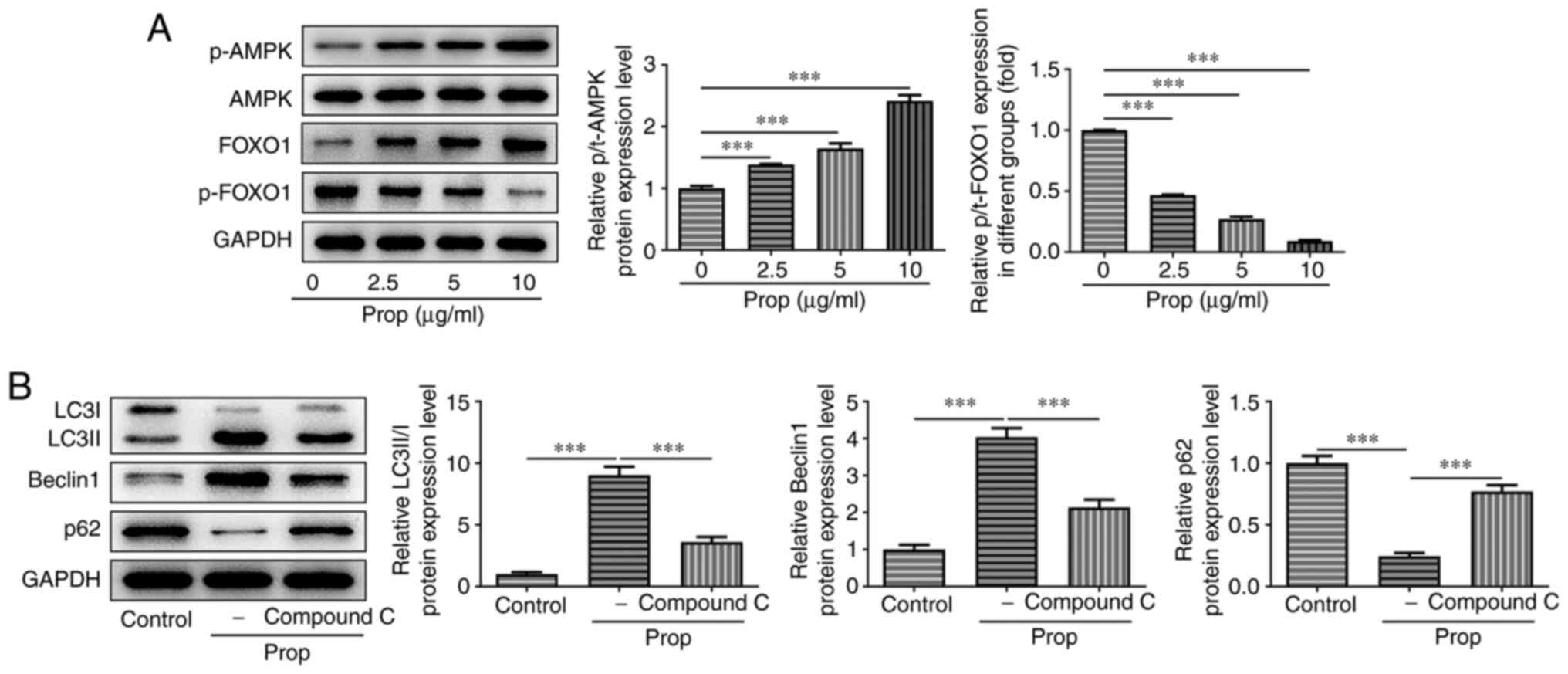
Subsequently, the present study aimed to investigate
how propofol affected U2OS cell autophagy. Western blotting
demonstrated that treatment with propofol significantly increased
the protein expression levels of p-AMPK in a dose-dependent manner
compared with the untreated control, whereas the total levels of
AMPK remained unchanged. Furthermore, compared with the untreated
control, the protein expression levels of total FOXO1 were
significantly increased in a dose-dependent manner, whereas those
of p-FOXO1 were significantly reduced following cell treatment with
increasing concentrations of propofol (Fig. 2A). Cells were also treated with 20
µM compound C, an AMPK/FOXO1 signaling pathway inhibitor. The
results demonstrated that the protein expression levels of LC3II/I
and Beclin1 were significantly reduced, whereas those of p62 were
significantly elevated in cells co-treated with propofol and
compound C, compared with cells treated only with propofol
(Fig. 2B). These results suggested
that the AMPK/FOXO1 signaling pathway may be involved in
propofol-induced autophagy in U2OS cells.
Propofol attenuates cell proliferation
and promotes apoptosis via AMPK/FOXO1 signaling pathway-induced
autophagy in U2OS cells
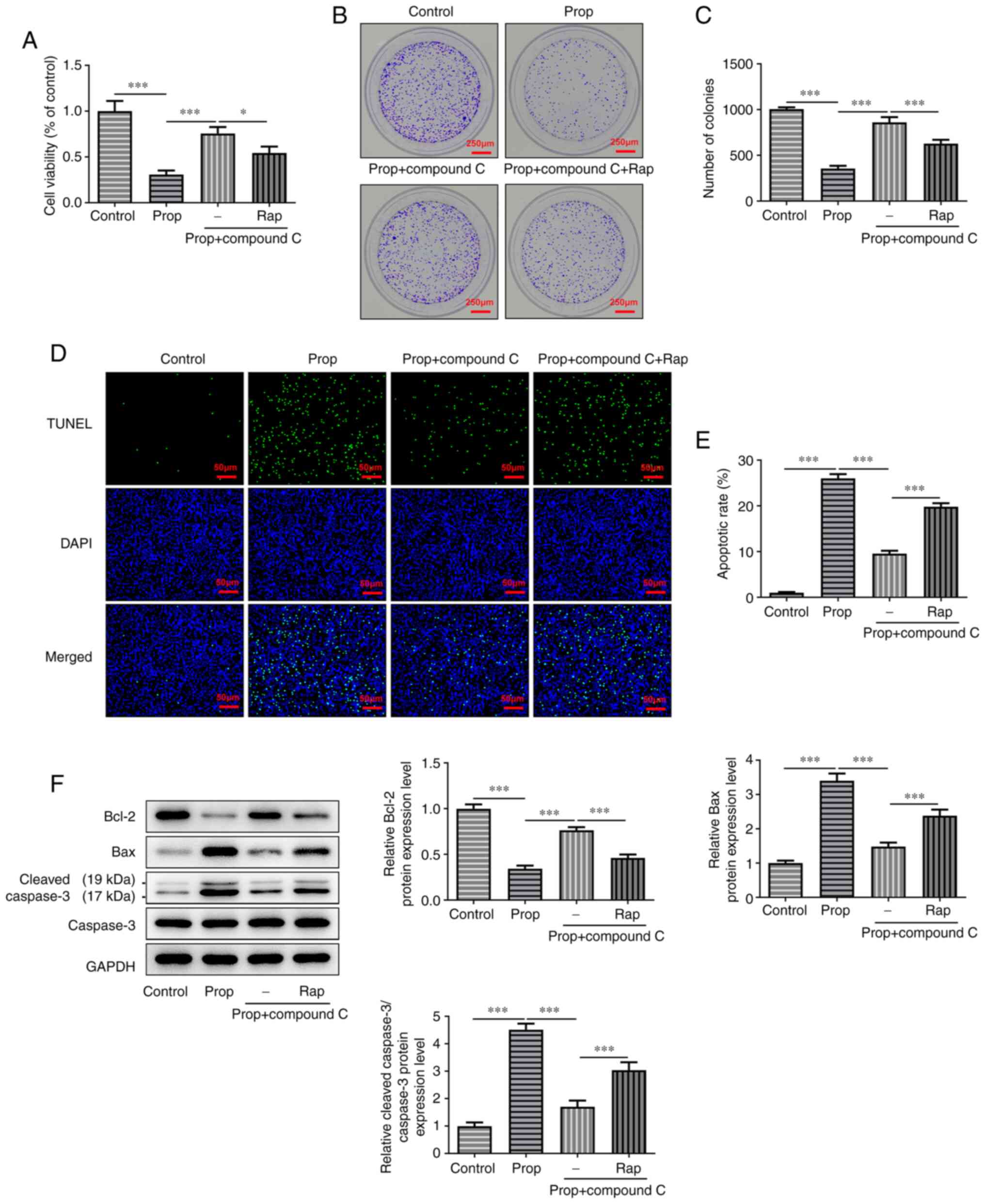
To explore the effects of propofol-induced autophagy
on cell proliferation and apoptosis, U2OS cells were treated with
50 nM RAP. The results of the CCK-8 assay demonstrated that
co-treatment of U2OS cells with propofol and compound C
significantly promoted cell viability compared with cells treated
with propofol only. However, RAP significantly reversed the effects
of compound C on cell viability compared with the propofol +
compound C group (Fig. 3A).
Furthermore, the colony formation assay demonstrated that
co-treatment of propofol and compound C significantly increased the
number of cell colonies compared with the propofol group. However,
the additional treatment with RAP significantly reversed this
increase in U2OS cell colonies compared with the propofol +
compound C group (Fig. 3B and C).
The results of the TUNEL assay demonstrated that cell apoptosis was
significantly increased in propofol-treated cells compared with the
control group. However, cell co-treatment with compound C
significantly attenuated propofol-induced cell apoptosis compared
with the propofol only group, whereas RAP significantly reversed
the inhibitory effect of compound C on U2OS cell apoptosis compared
with the propofol + compound C group (Fig. 3D and E). Furthermore, cell
treatment with compound C significantly promoted Bcl-2 protein
expression levels and significantly downregulated the protein
expression levels of Bax and cleaved caspase-3 compared with the
propofol only group. However, the protein expression levels of the
aforementioned proteins were significantly reversed following cell
treatment with RAP compared with the propofol + compound C group
(Fig. 3F). In summary, propofol
hindered the occurrence of OS via modulation of AMPK/FOXO1
signaling pathway-induced autophagy.
Propofol suppresses U2OS cell
migration, invasion and the epithelial-mesenchymal transition (EMT)
via autophagy through the activation of the AMPK/FOXO1 signaling
pathway
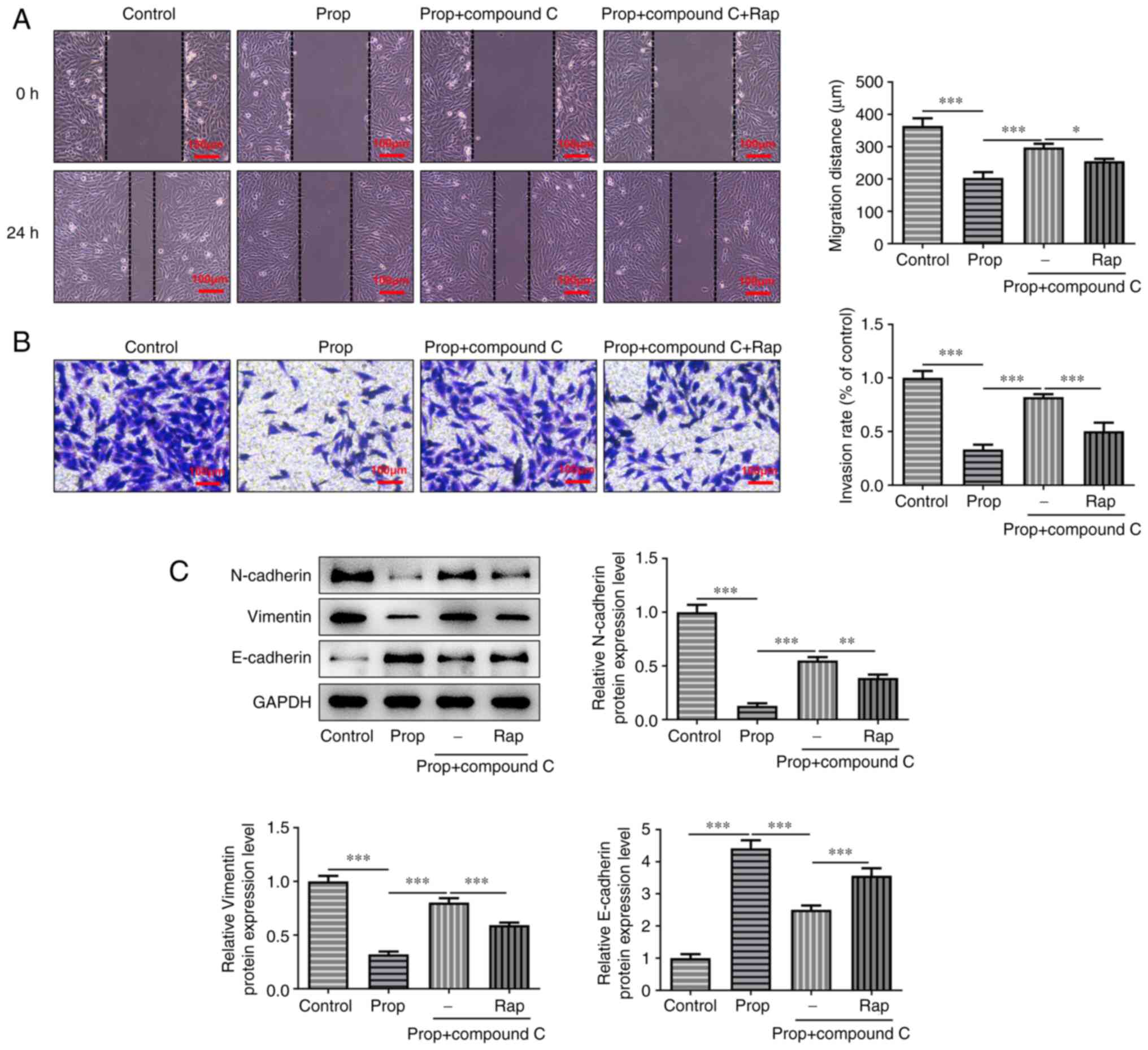
Subsequently, the effects of propofol-induced
autophagy on cell migration, invasion and the EMT were assessed.
The results demonstrated that propofol significantly inhibited cell
migration and invasion compared with the untreated control group,
which was significantly reversed by cell treatment with compound C
(Fig. 4A and B). The effect of
compound C on enhancing cell invasion was markedly abrogated and on
enhancing cell migration was slightly reduced following cell
treatment with RAP, compared with the propofol + compound C group.
Furthermore, western blotting demonstrated that the protein
expression levels of N-cadherin and Vimentin were significantly
reduced, whereas those of E-cadherin were significantly enhanced by
propofol treatment compared with the control group. However,
inhibition of the AMPK/FOXO1 signaling pathway using compound C
significantly reversed these results compared with the propofol
only group. Furthermore, cell treatment with RAP resulted in a
significant inverse effect on the protein expression levels of
N-cadherin, Vimentin and E-cadherin compared with the propofol +
compound C group. In conclusion, propofol impeded the progression
of OS via modulation of AMPK/FOXO1 signaling pathway-induced
autophagy.
Discussion
OS is an aggressive type of malignant tumor that
often occurs in young individuals (29). OS is characterized by a high
metastatic rate and drug resistance, which therefore leads to a
high mortality rate (30). The
present study investigated the effect of propofol on human OS cells
and its potential underlying molecular mechanism. The results
demonstrated that propofol significantly inhibited cell viability,
migration, invasion and the EMT, and significantly promoted U2OS
cell apoptosis. Furthermore, the results demonstrated that
AMPK/FOXO1-mediated autophagy may be involved in the mechanism of
propofol on OS, which therefore suggested that propofol may
potentially serve a crucial role in OS progression via
AMPK/FOXO1-mediated autophagy.
Propofol is an intravenous anesthetic that is
commonly used in clinical practice and it has been reported to
inhibit the development of several types of cancer, including
breast cancer, gastric cancer and colon cancer (12,31,32).
Ye et al (28) demonstrated
that propofol attenuates the proliferation and invasion and
promotes the apoptosis of OS cells via the upregulation of miR-143.
Furthermore, Xu et al (21)
reported that propofol could downregulate TGF-β1 expression, which
results in the inhibition of the proliferation and invasion of OS
cells. In the present study, propofol significantly reduced U2OS
cell viability in a dose-dependent manner. Moreover, TUNEL assays
and western blotting demonstrated that propofol significantly
promoted cell apoptosis and modulated the protein expression levels
of Bcl-2, Bax and cleaved caspase-3, respectively. Wound healing
and Transwell assays demonstrated that propofol significantly
reduced the migration and invasion abilities and suppressed the EMT
process in U2OS cells, which was consistent with previous studies
(21,28).
Autophagy can function as an important process that
can inhibit the malignant development of OS (33). A previous study reported that
propofol serves a beneficial role in organs and tissues via the
regulation of autophagy (34).
Propofol also regulates cancer cell autophagy (35). For example, Zhang et al
(36) demonstrated that propofol
could improve gastric cancer cell sensitivity to cisplatin via
lncRNA metastasis-associated lung adenocarcinoma transcript
1/miR-30e/autophagy related 5 axis-mediated autophagy. Furthermore,
Wang et al (35) reported
that propofol inhibits cell proliferation and the cell cycle but
promotes cell apoptosis during hepatocarcinogenesis via the
activation of AMPK and the induction of autophagy, both in
vivo and in vitro. In the present study, the protein
expression levels of autophagy-related proteins were detected via
western blotting. The results demonstrated that propofol may have
promoted autophagy via the significant upregulation of LC3II/I and
Beclin1 and the downregulation of p62 protein expression levels.
However, transmission electron microscopy and GFP-LC3 fluorescence
assays may better locate autophagy-related proteins and therefore
these methods will be used to investigate the effect of propofol on
cell autophagy in future work.
Emerging evidence has suggested that inhibiting the
FOXO1/tumor suppressor candidate 7 axis via regulating the
AKT/GSK-3β signal transduction pathway can decrease the
proliferation, migration and invasion abilities of OS cells
(22). Huang et al
(22) reported that propofol could
increase the transcriptional activity of FOXO1. FOXO1 serves a key
role in autophagy in cancer cells (37,38).
A previous study demonstrated that trichostatin A induced cell
autophagy via activating the transcriptional activity of FOXO1 in
OS (39). Furthermore, Chen et
al (40) demonstrated that
propofol could attenuate HeLa cell growth via impairing autophagic
flux via AMPK activation and calcium-regulated endoplasmic
reticulum stress. The present study demonstrated that autophagy may
be promoted in OS cell treatment with propofol via the activation
of the AMPK/FOXO1 signaling pathway. Furthermore, propofol
potentially enhanced AMPK/FOXO1 pathway-mediated autophagy to
inhibit OS cell proliferation and metastasis and promote cell
apoptosis. It should be noted that animal and clinical trials are
more complex and uncontrollable than cell experiments. Therefore
the dose of propofol used in clinical trials does not apply to the
doses used in the present study. Furthermore, there may be other
signaling pathways that are regulated by propofol in OS, which will
be investigated in future work.
To the best of our knowledge the present study was
the first to provide evidence that propofol potentially regulated
OS progression via AMPK/FOXO1 pathway-mediated autophagy. This has
therefore provided a novel insight into the potential effect of
propofol in the treatment of OS.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
Ningbo City, China (grant no. 2021J257).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LD and BJ designed the study, drafted and revised
the manuscript. LD, SL and XL performed the experiments, searched
the literature and analyzed the data. BJ guided the experiments.
All authors read and approved the final manuscript. LD and BJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaton BR, Schwarz R, Vatner R, Yeh B,
Claude L, Indelicato DJ and Laack N: Osteosarcoma. Pediatr Blood
Cancer. 68 (Suppl 2):e283522021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corre I, Verrecchia F, Crenn V, Redini F
and Trichet V: The Osteosarcoma microenvironment: A complex but
targetable ecosystem. Cells. 9:9762020. View Article : Google Scholar
|
|
4
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kager L, Tamamyan G and Bielack S: Novel
insights and therapeutic interventions for pediatric osteosarcoma.
Future Oncol. 13:357–368. 2017. View Article : Google Scholar
|
|
6
|
Wang S, Zhong L, Li Y, Xiao D, Zhang R,
Liao D, Lv D, Wang X, Wang J, Xie X, et al: Up-regulation of PCOLCE
by TWIST1 promotes metastasis in Osteosarcoma. Theranostics.
9:4342–4353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurosawa S: Anesthesia in patients with
cancer disorders. Curr Opin Anaesthesiol. 25:376–384. 2012.
View Article : Google Scholar
|
|
8
|
Guerrero Orriach JL, Raigon Ponferrada A,
Malo Manso A, Herrera Imbroda B, Escalona Belmonte JJ, Ramirez
Aliaga M, Ramirez Fernandez A, Diaz Crespo J, Soriano Perez AM,
Fontaneda Heredia A, et al: Anesthesia in combination with propofol
increases disease-free survival in bladder cancer patients who
undergo radical tumor cystectomy as compared to inhalational
anesthetics and opiate-based analgesia. Oncology. 98:161–167.
2020.
. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ,
Kim HR, Lee EH and Choi IC: Impact of anesthetic agents on overall
and recurrence-free survival in patients undergoing esophageal
cancer surgery: A retrospective observational study. Sci Rep.
7:140202017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahinovic MM, Struys MMRF and Absalom AR:
Clinical pharmacokinetics and pharmacodynamics of propofol. Clin
Pharmacokinet. 57:1539–1558. 2018. View Article : Google Scholar
|
|
11
|
Xu Y, Pan S, Jiang W, Xue F and Zhu X:
Effects of propofol on the development of cancer in humans. Cell
Prolif. 53:e128672020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li R, Liu H, Dilger JP and Lin J: Effect
of propofol on breast cancer cell, the immune system, and patient
outcome. BMC Anesthesiol. 18:772018. View Article : Google Scholar
|
|
13
|
Li H, Lu Y, Pang Y, Li M, Cheng X and Chen
J: Propofol enhances the cisplatin-induced apoptosis on cervical
cancer cells via EGFR/JAK2/STAT3 pathway. Biomed Pharmacother.
86:324–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu W, Zheng J, Bie S, Kang L, Mao Q, Liu
W, Guo J, Lu J and Xia R: Propofol inhibits Wnt signaling and
exerts anticancer activity in glioma cells. Oncol Lett. 16:402–408.
2018.
|
|
15
|
Huang YH, Wu ZF, Lee MS, Lou YS, Wu KL,
Cheng KI and Lai HC: Propofol-based total intravenous anesthesia is
associated with better survival than desflurane anesthesia in
glioblastoma surgery. PLoS One. 16:e02556272021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cata JP and Forget P: Paravertebral block
with propofol anaesthesia does not improve survival compared with
sevoflurane anaesthesia for breast cancer surgery: Independent
discussion of a randomised controlled trial. Br J Anaesth.
124:19–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farooqi AA, Adylova A, Sabitaliyevich UY,
Attar R, Sohail MI and Yilmaz S: Recent updates on true potential
of an anesthetic agent as a regulator of cell signaling pathways
and non-coding RNAs in different cancers: Focusing on the brighter
side of propofol. Gene. 737:1444522020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng X, Li C, Yu W, Liu S, Cong Y, Fan G
and Qi S: Propofol attenuates hypoxia-induced inflammation in BV2
microglia by inhibiting oxidative stress and NF-κB/Hif-1α
signaling. Biomed Res Int. 2020:89787042020. View Article : Google Scholar
|
|
19
|
Yan HJ, Qi GQ and Ma Y: Effect of propofol
on myocardial ischemia-reperfusion injury through MAPK/ERK pathway.
Eur Rev Med Pharmacol Sci. 23:11051–11061. 2019.
|
|
20
|
Gao X, Mi Y, Guo N, Luan J, Xu H, Hu Z,
Wang N, Zhang D, Gou X and Xu L: The mechanism of propofol in
cancer development: An updated review. Asia Pac J Clin Oncol.
16:e3–e11. 2020. View Article : Google Scholar
|
|
21
|
Xu YB, Jiang W, Zhao FR, Li G, Du QH,
Zhang MY and Guo XG: Propofol suppresses invasion and induces
apoptosis of osteosarcoma cell in vitro via downregulation of
TGF-β1 expression. Eur Rev Med Pharmacol Sci. 20:1430–1435.
2016.
|
|
22
|
Huang X, Liu J and Xie H: Propofol
suppresses osteosarcoma cell function by regulating FOXO1/TUSC7. J
Pharm Pharmacol. 73:720–725. 2021. View Article : Google Scholar
|
|
23
|
Yun CW and Lee SH: The roles of autophagy
in cancer. Int J Mol Sci. 19:34662018. View Article : Google Scholar
|
|
24
|
Lin YC, Chen HY, Hsieh CP, Huang YF and
Chang IL: Betulin inhibits mTOR and induces autophagy to promote
apoptosis in human osteosarcoma cell lines. Environ Toxicol.
35:879–887. 2020. View Article : Google Scholar
|
|
25
|
Guo YN and Ma X: The effects of propofol
on autophagy. DNA Cell Biol. 39:197–209. 2020. View Article : Google Scholar
|
|
26
|
Li F, Li F and Chen W: Propofol inhibits
cell proliferation, migration, and invasion via
mir-410-3p/transforming growth factor-β receptor type 2 (TGFBR2)
Axis in glioma. Med Sci Monit. 26:e9195232020.
|
|
27
|
Sun H and Gao D: Propofol suppresses
growth, migration and invasion of A549 cells by down-regulation of
miR-372. BMC Cancer. 18:12522018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lilienthal I and Herold N: Targeting
molecular mechanisms underlying treatment efficacy and resistance
in osteosarcoma: A review of current and future strategies. Int J
Mol Sci. 21:68852020. View Article : Google Scholar
|
|
31
|
Zheng X, Wang Y, Dong L, Zhao S, Wang L,
Chen H, Xu Y and Wang G: Effects of propofol-based total
intravenous anesthesia on gastric cancer: A retrospective study.
Onco Targets Ther. 11:1141–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS,
Lin KT, Lou YS, Lin C, Chang YC and Lai HC: Propofol-based total
intravenous anesthesia is associated with better survival than
desflurane anesthesia in colon cancer surgery. Anesthesiology.
129:932–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu X, Liu JM, Song HH, Yang QK, Ying H,
Tong WL, Zhou Y and Liu ZL: Aurora-B knockdown inhibits
osteosarcoma metastasis by inducing autophagy via the mTOR/ULK1
pathway. Cancer Cell Int. 20:5752020. View Article : Google Scholar
|
|
34
|
Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M
and Cai H: Propofol inhibited autophagy through
Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron
injury. Mol Med. 24:582018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Xu B, Zhou J and Wu X: Propofol
activates AMPK to inhibit the growth of HepG2 cells in vitro and
hepatocarcinogenesis in xenograft mouse tumor models by inducing
autophagy. J Gastrointest Oncol. 11:1322–1332. 2020. View Article : Google Scholar
|
|
36
|
Zhang YF, Li CS, Zhou Y and Lu XH:
Propofol facilitates cisplatin sensitivity via lncRNA
MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric
cancer. Life Sci. 244:1172802020. View Article : Google Scholar
|
|
37
|
Zhao Y, Yang J, Liao W, Liu X, Zhang H,
Wang S, Wang D, Feng J, Yu L and Zhu WG: Cytosolic FoxO1 is
essential for the induction of autophagy and tumour suppressor
activity. Nat Cell Biol. 12:665–675. 2010. View Article : Google Scholar
|
|
38
|
Zhao Y, Li X, Cai MY, Ma K, Yang J, Zhou
J, Fu W, Wei FZ, Wang L, Xie D and Zhu WG: XBP-1u suppresses
autophagy by promoting the degradation of FoxO1 in cancer cells.
Cell Res. 23:491–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai Y, Chen Y, Chen X, Jiang J, Wang X,
Wang L, Wang J, Zhang J and Gao L: Trichostatin A activates FOXO1
and induces autophagy in osteosarcoma. Arch Med Sci. 15:204–213.
2019. View Article : Google Scholar
|
|
40
|
Chen X, Li K and Zhao G: Propofol Inhibits
HeLa cells by impairing autophagic flux via AMP-Activated protein
kinase (AMPK) activation and endoplasmic reticulum stress regulated
by calcium. Med Sci Monit. 24:2339–2349. 2018. View Article : Google Scholar
|