Introduction
The prevalence of excess body weight and obesity is
rapidly increasing. Recent studies have shown that the number of
obese adults has reached more than 600 million worldwide in 2014
(1). Excess body weight and
obesity are well-known risk factors for cardiovascular diseases and
diabetes (2,3). Additionally, several cohort studies
have shown that excess body weight and obesity are associated with
cancer development. Colorectal cancer is the third most common
cancer worldwide, and approximately 8% of colorectal cancer cases
can be attributed to obesity (4,5).
Thus, obesity-associated colorectal cancer imposes serious
challenges to healthcare systems globally. A previous meta-analysis
revealed that higher body mass index (BMI) was a significant risk
factor for colorectal adenomas (6); another large cohort study suggested
that higher BMI was an independent risk factor for colorectal
adenoma incidence and adenoma recurrence (7).
The intestinal mucosa is composed of crypts and
finger-like protrusions (villi). The crypt bottoms contain rapidly
proliferating intestinal stem cells and transit-amplifying
progenitor cells. These cells differentiate into enterocytes,
goblet cells, or enteroendocrine cells, and migrate as clonal
lineages to the villi within 4–5 days, where they are shed into the
lumen (8). The intestinal stem
cell differentiation, also known as crypt-villus axis, is strictly
regulated, and dysregulation of this process is considered to play
a crucial role in colon carcinogenesis (8,9).
Ephrin receptors and ligands are essential in the crypt-villus axis
regulation and have been, therefore, implicated in colorectal
cancer.
Ephrin receptors are the largest family of receptor
tyrosine kinases (RTKs). Their ligands, ephrins, are
membrane-anchored proteins and are divided into two subclasses:
type-A ephrins, and type-B ephrins. EphA receptors (EphA1-EphA10)
bind type-A ephrins, while EphB receptors (EphB1-EphB6) bind type-B
ephrins. Eph/ephrin signalling regulates tissue and organ
patterning, in addition to controlling cell survival during normal
and neoplastic development (10).
Moreover, EphB/ephrin-B signalling plays a critical role in the
turnover of the intestinal mucosa by controlling the cell
positioning along the intestinal crypt (8,9).
Abnormal ephrin expression has been implicated in a variety of
human cancers, including colorectal cancer (11–13).
Recent studies have suggested that ephrins may also play a
tumour-suppressive role in some cancers, highlighting the complex
role of Ephs in oncogenesis (9,14–16).
Previous studies have demonstrated that colorectal cancer
development and progression were suppressed by the repulsive
interactions between ephrin-B1-positive normal epithelial cells and
EphB-positive tumour cells (9,14,15,17).
Interestingly, a few studies have suggested an
association between ephrins and obesity. It has been shown that
obesity-related inflammatory cytokines induce the downregulation of
ephrin-B1 in adipose cells (18),
suggesting that obesity not only promotes the secretion of
cytokines but also influences the structure of the gastrointestinal
mucosa through cytokine secretion. As the majority of studies have
focused on the relationship between cytokine secretion and
obesity-related colorectal carcinogenesis, little is known about
colonic mucosal changes that happen during obesity-related
colorectal cancer development and progression. Considering the key
role of crypt-villus axis dysregulation in colorectal
carcinogenesis, investigating the association between
obesity-related colorectal cancer development and alterations in
the crypt-villus axis is of great importance.
The aim of this study was to elucidate the
association between obesity-associated colorectal cancer and
alterations in the crypt-villus axis induced by Eph/ephrin
signalling, which will allow for the establishment of preventive
measures for obesity-associated cancer.
Materials and methods
Mouse experiments
Fifteen male C57BL/6JJcl mice (C57BL mice) and
fifteen male KK-Ay/TAJcl mice (KKAy mice) were purchased
from Nippon CLEA (Tokyo, Japan). KK mice are polygenic mouse models
of type 2 diabetes mellitus; leptin and its receptor are intact in
these animals (19). The genes
responsible for the inherited characters of KK are unknown. KKAy
mice were obtained by cross-mating KK mice with C57BL mice with
type 2 diabetes mellitus but normal leptin and leptin receptor
levels and 6J-Ay carrying the Agouti yellow (Ay) gene. The latter
mice exhibit severe hyperphagia, impaired glucose tolerance, and
hyperlipidaemia; such mice are often used in studies on metabolic
disease (20). Five-week-old male
C57BL and KKAy mice were intraperitoneally injected with a single
dose of 10 mg/kg (approximately 200 µg/mouse) azoxymethane (AOM)
(Sigma-Aldrich, St. Louis, MO) weekly for 6 weeks. Two groups of
mice were culled at the 20th week (Fig. 1A). Euthanasia involved cervical
dislocation; death was confirmed by cardiac and respiratory arrest.
All animal procedures were performed in accordance with our
institutional guidelines and were approved by the ethics committee
of Keio University School of Medicine Ethics Committee (approval
no. 20140001).
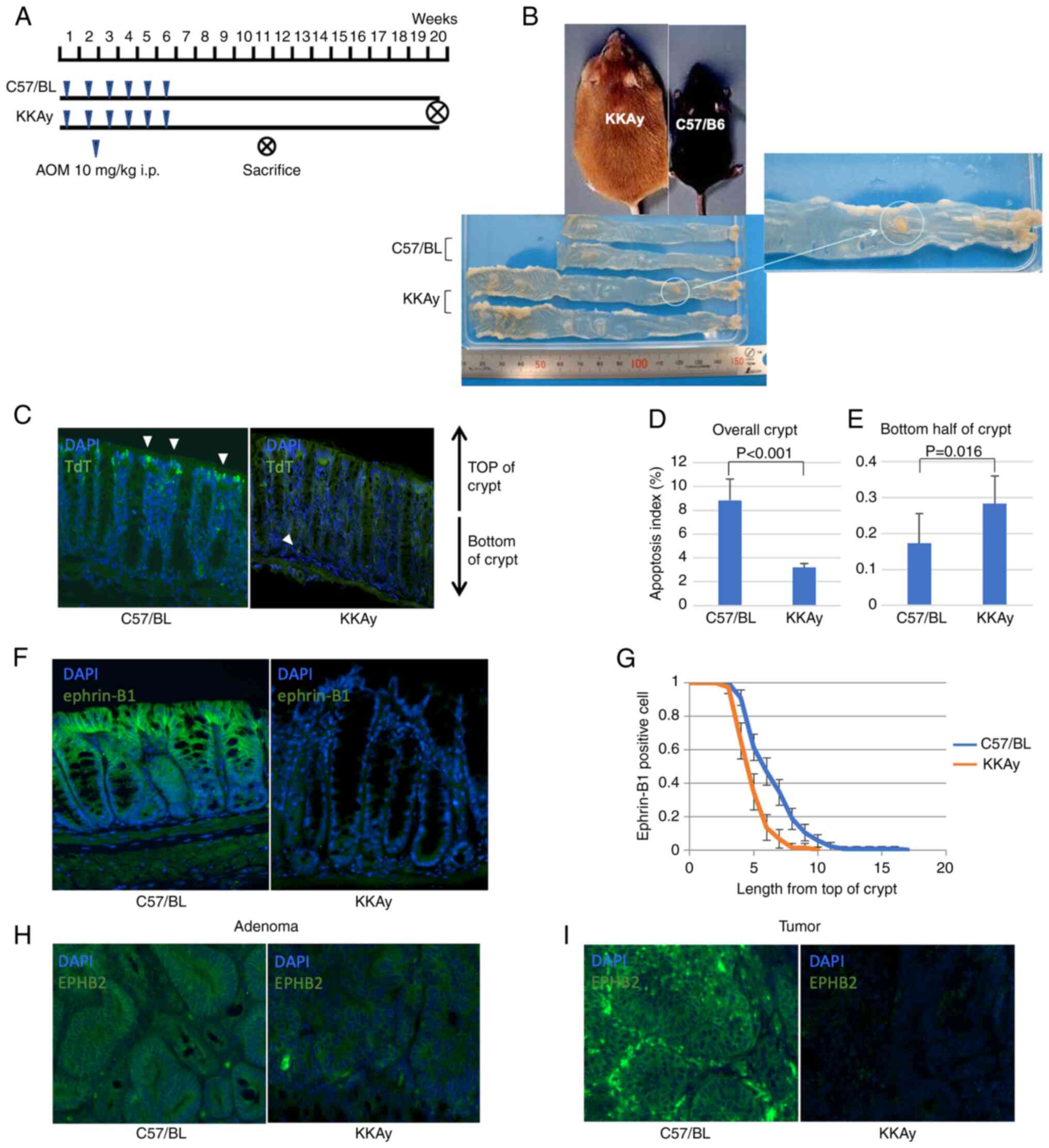 | Figure 1.TdT-mediated dUTP nick-end labelling,
and expression analysis of ephrin-B1 and EphB2 in murine colon. (A)
Procedure of the azoxymethane-induced obesity-associated cancer
model. (B) Macroscopic images of colons of C57/BL and KKAy mice.
(C) Representative fluorescent TUNEL images of normal mucosa from
C57/BL and KKAy mice. White arrows indicate apoptotic cells.
Magnification, ×200. (D) Apoptosis index in the whole crypt of
C57/BL and KKAy mice. (E) Apoptosis index in the bottom half of the
crypt of C57/BL and KKAy mice. (F) Representative
immunohistochemistry images of the normal, ephrin-B1 stained mucosa
of C57/BL and KKAy mice. Magnification, ×200. (G) Analysis of
ephrin-B1-positive cell position in the crypt of C57/BL and KKAy
mice. (H) Representative immunohistochemistry images of C57/BL and
KKAy mouse adenoma after EphB2 staining. Magnification, ×200. (I)
Representative immunohistochemistry images of C57/BL and KKAy mouse
tumours after staining for EphB2. Magnification, ×200. Error bars
indicate 95% confidence intervals. TdT, terminal deoxynucleotidyl
transferase; dUTP, deoxyuridine triphosphate; C57BL, C57BL/6JJcl
mice; KKAy, C57BL/6JJcl-derived KK-Ay/TAJcl mice. |
Patients and clinical samples
The expression levels of Ephrin receptors and
ligands were analysed in a panel of human benign and colorectal
cancer (CRC) specimens resected from colorectal cancer patients.
Consecutive patients who underwent surgical resection of stages
I–IV CRC at Keio University Hospital between September 2015 and
March 2016 were enrolled. The study included patients with
pathologically confirmed American Joint Committee on Cancer (AJCC)
stage I–IV CRC who underwent resection of the primary lesions. The
study excluded patients with ulcerative colitis-associated cancer,
familial adenomatous polyposis, limited resection (such as
trans-anal resection), or unavailable data. The clinical
characteristics and pathological findings of the patients,
including sex, BMI, degree of tumour differentiation, and AJCC
stage (TNM stage), were collected from medical records.
Certificated pathologists assessed the degree of tumour
differentiation in colorectal cancer according to AJCC guidelines
(21). Patients with a BMI higher
than 25 kg/m2 were defined as obese. Fifty primary CRCs
from patients who underwent colectomy as primary treatment, as well
as paired normal colon mucosa samples were collected during the
operation at Keio University Hospital (Tokyo, Japan) between
September 2015 and March 2016. Patient consent, including for the
use of tumour tissue, was obtained using the opt-out method. The
study was approved by the Keio University School of Medicine Ethics
Committee (approval number: 20140001).
TdT-mediated dUTP nick-end labelling
(TUNEL) staining
For the TUNEL staining, tissue slides were
deparaffinised, rehydrated, and digested with 1 µg/ml proteinase K
for 10 min at 37°C. Next, TUNEL staining was performed using the
ApopTag® Fluorescein In Situ Apoptosis Detection
Kit (Merck Millipore, Darmstadt, Germany) according to the
manufacturer's instructions. Then, the samples were mounted with
DAPI Vectashield (Vector Laboratories, Burlingame, CA, USA). The
frequency of apoptosis was expressed as the apoptotic index, which
was calculated as the apoptotic cell count in a crypt divided with
the total cell count of the crypt.
Immunohistochemistry (IHC)
Paraffin-embedded sections (5m) were deparaffinised
with xylene and rehydrated with a graded series of ethanol.
Sections were incubated with 2.0% hydrogen peroxide for 30 min to
block endogenous peroxidase. Antigen retrieval was carried out by
boiling in 10 mM sodium citrate buffer. The following antibodies
were used: goat anti-EphB2 (1:200; R&D systems, Minneapolis,
MN, USA), goat anti-ephrinB1 (1:400; R&D systems), rat
anti-Macrophage (1:30; BMA Biomedicals, Rheinstrasse, Switzerland),
goat anti-monocyte chemoattractant protein-1 (MCP-1) (1:100;
R&D Systems), and rat anti-diphosphorylated extracellular
signal-regulated kinase 1/2 (ERK1/2) (1:100; Sigma-Aldrich, St.
Louis, MO, USA). The sections were stained with haematoxylin-eosin
(HE) and observed under a light microscope. For fluorescent IHC,
samples were stained for ephrin-B1- and EphB2-GFP and mounted using
DAPI Vectashield.
To assess the immunoreactivity, the sections were
scored in terms of their proportion (score 0,-10%; 1, 10–50%; 2,
50–80%; 3, >80%) and intensity (score 0: none; 1, weak; 2,
moderate; 3, strong) as previously reported (22); the scoring was performed by two
investigators (Y.S. and T.T.) who were blinded to the
clinicopathological factors. The total score was calculated by
multiplying the two scores.
Statistical analyses
Continuous variables were expressed as averages and
standard error. Associations between Eph-ephrin expression and
other variables were analysed using Pearson's chi-squared test,
Fisher's exact probability test, the Mann-Whitney U test or
Kruskal-Wallis test followed by Dunn's post hoc test. All analyses
were two-sided, and P-values <0.05 were considered statistically
significant. Statistical analyses were performed using SPSS ver. 23
(IBM Corp, Armonk, NY, USA).
Results
Obesity and colorectal
carcinogenesis
The incidence of colorectal cancer in KKAy mice and
C57BL mice was assessed using an AOM-induced colon carcinogenesis
mouse model. Fig. 1B shows colonic
macroscopic images of both mouse strains. KKAy mice were heavier
and had longer intestines than C57BL mice. Even though only two of
15 C57BL mice developed colorectal tumours, tumourigenesis occurred
in all KKAy mice (P<0.001, Mann-Whitney U test). The average
tumour diameter was larger in KKAy mice compared with C57BL mice
(largest tumour diameter: 0.47±1.36 mm in C57BL vs. 7.40±5.70 mm in
KKAy mice; P<0.001, Mann-Whitney U test; Table I). These results suggested that
obesity enhanced CRC development.
 | Table I.Comparison of azoxymethane-induced
colon carcinogenesis in KKAy mice and C57BL mice. |
Table I.
Comparison of azoxymethane-induced
colon carcinogenesis in KKAy mice and C57BL mice.
| Parameter | C57/BL | KKAy | P-value |
|---|
| Number of mice | 15 | 10 |
|
| Number of mice with
tumours | 2 | 10 | <0.001 |
| Number of tumours
per a mouse | 0.13±0.35 | 8.40±3.06 | <0.001 |
| Largest tumour
diameter (mm) | 0.47±1.36 | 7.40±5.70 | <0.001 |
Dysregulation of apoptosis in obese
mice
To assess the influence of cell turnover
dysregulation in the gastrointestinal mucosa, the frequency and
localisation of apoptotic cells in colon mucosa were compared
between C57BL mice and KKAy mice (Fig.
1C). Generally, intestinal cell apoptosis occurs on the luminal
side of the crypt, resulting in mucosal shedding. Apoptosis in
normal colon mucosa is significantly suppressed in KKAy mice; the
apoptotic index in C57BL mice was 8.8%, while in KKAy it was only
3.2% (P<0.001, Mann-Whitney U test; Fig. 1D). Interestingly, apoptotic cell
death was more frequently observed at the base of the crypt in KKAy
mice than in C57BL mice (apoptotic index in the bottom half of the
crypt: 0.17% in C57BL vs. 0.28% in KKAy, P=0.016, Mann-Whitney U
test; Fig. 1E), which suggested
the dysregulation of cell turnover in KKAy mice.
Expression of EphB2 and ephrin-B1 in
murine colon
Typically, ephrin-B1 is strongly expressed in cells
at the top of the crypt, while its expression is very low at the
bottom of the crypt. The expression patterns of EphB2 and ephrin-B1
in the intestinal mucosa of C57BL and KKAy mice were compared, and
it was found that ephrin-B1 was expressed in lower levels in KKAy
mice than in C57BL mice (Fig. 1F).
Additionally, ephrin-B1 positive cells were frequently observed in
the crypts of C57BL mice (Fig.
1G). Although EphB2 expression was detected at the bottom of
the crypts in C57BL and KKAy mice, it was expressed at lower levels
in KKAy mice compared with C57/BL mice. Interestingly, in KKAy
mice, EphB2 was expressed at lower levels in adenomas, and its
expression was not detectable in cancer (Fig. 1H and I). In contrast, in C57BL
mice, EphB2 was expressed in normal mucosa, adenoma and cancer.
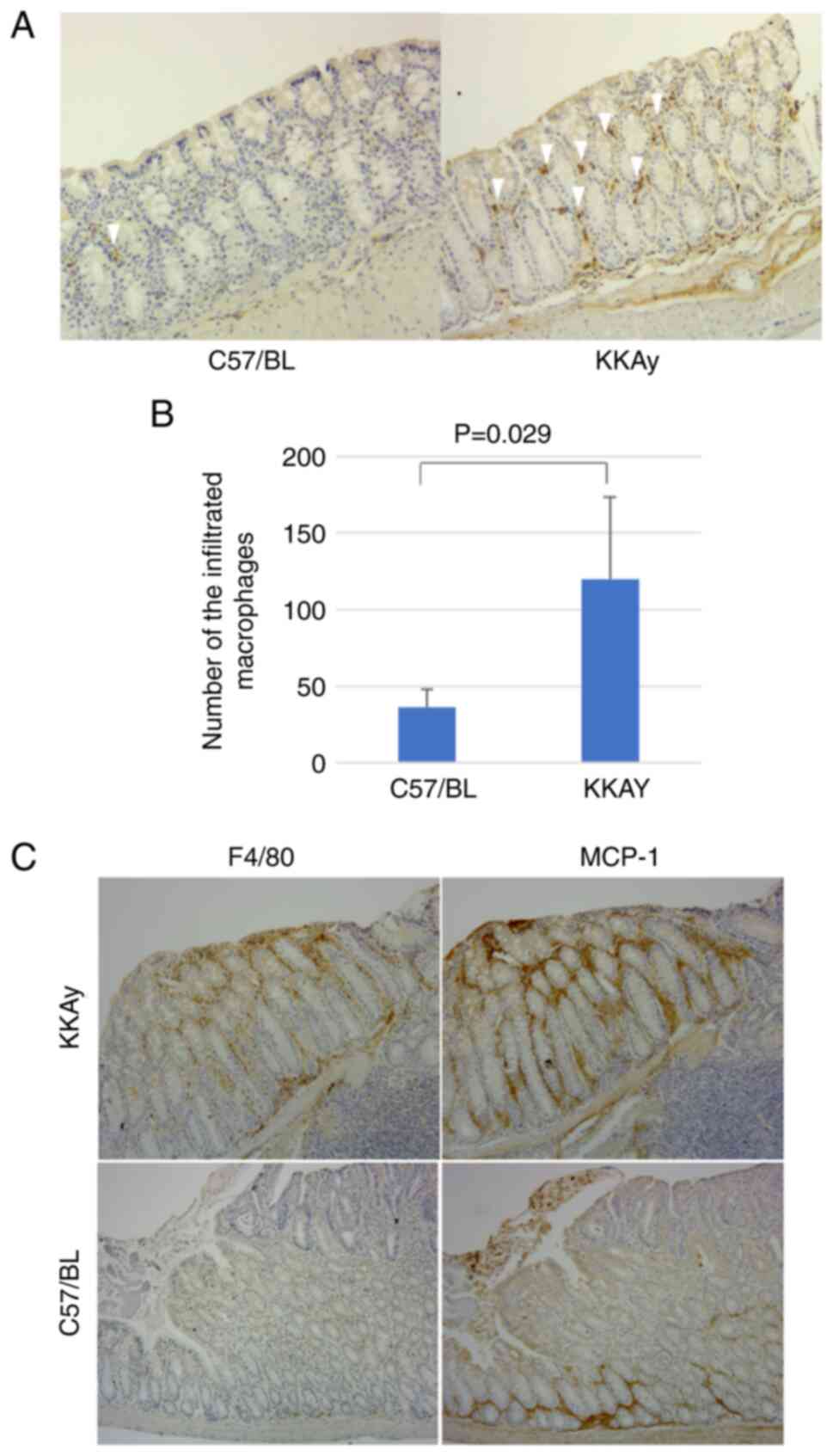
Expression of MCP-1 and macrophage
migration in murine colon
In normal colon mucosa, the number of macrophages in
C57/BL mice is significantly lower than in KKAy mice (36.3±11.9 in
C57/BL vs. 120.0±54.8 in KKAy, P=0.029, Mann-Whitney U test;
Fig. 2A and B). To investigate the
relationship between inflammation and alterations in the
crypt-villus axis, the expression of MCP-1 was evaluated. A
previous study reported that MCP-1 downregulated ephrin-B1
expression in adipose cells (18).
Initially, our hypothesis was that MCP-1 expression is regulated by
ephrin-B1 in the intestinal epithelium. Ephrin-B1 promotes
macrophage migration in the lamina propria of the murine colon.
Fig. 2C shows MCP-1 and F4/80
expression in the same region in consecutive slices. MCP-1
expression was elevated around the tumour and adenoma in both
C57/BL and KKAy mice. The distribution of infiltrated macrophages
corresponded to the MCP-1 expression pattern in KKAy mice; however,
C57/BL mice had a lower number of infiltrated macrophages, and
their distribution did not correspond to the MCP-1 expression
pattern. These results suggest that obesity accelerated the
inflammation of colon mucosa through MCP-1 upregulation and
subsequent macrophage infiltration.
ERK1/2 expression in the murine
colon
After determining the EphB2/ephrin-B1 and MCP-1
expression status and macrophage infiltration, MAPK/ERK pathway
activity was measured in the colons of KKAy and C57BL mice by IHC.
ERK activation triggered inflammatory cytokine secretion by adipose
tissue, which was followed by ephrin-B1 downregulation. This
upregulated MCP-1 via ERK1/2 activation, in turn promoting
macrophage recruitment (23). The
colon of KKAy mice showed significantly more ERK1/2 expression in
the upper half of crypts than that of C57BL mice (Fig. S1A). The ERK1/2 immunoreactive
score (IRS) was significantly higher in KKAy than C57BL mice (7.11
vs. 2.83, P<0.001) (Fig.
S1B).
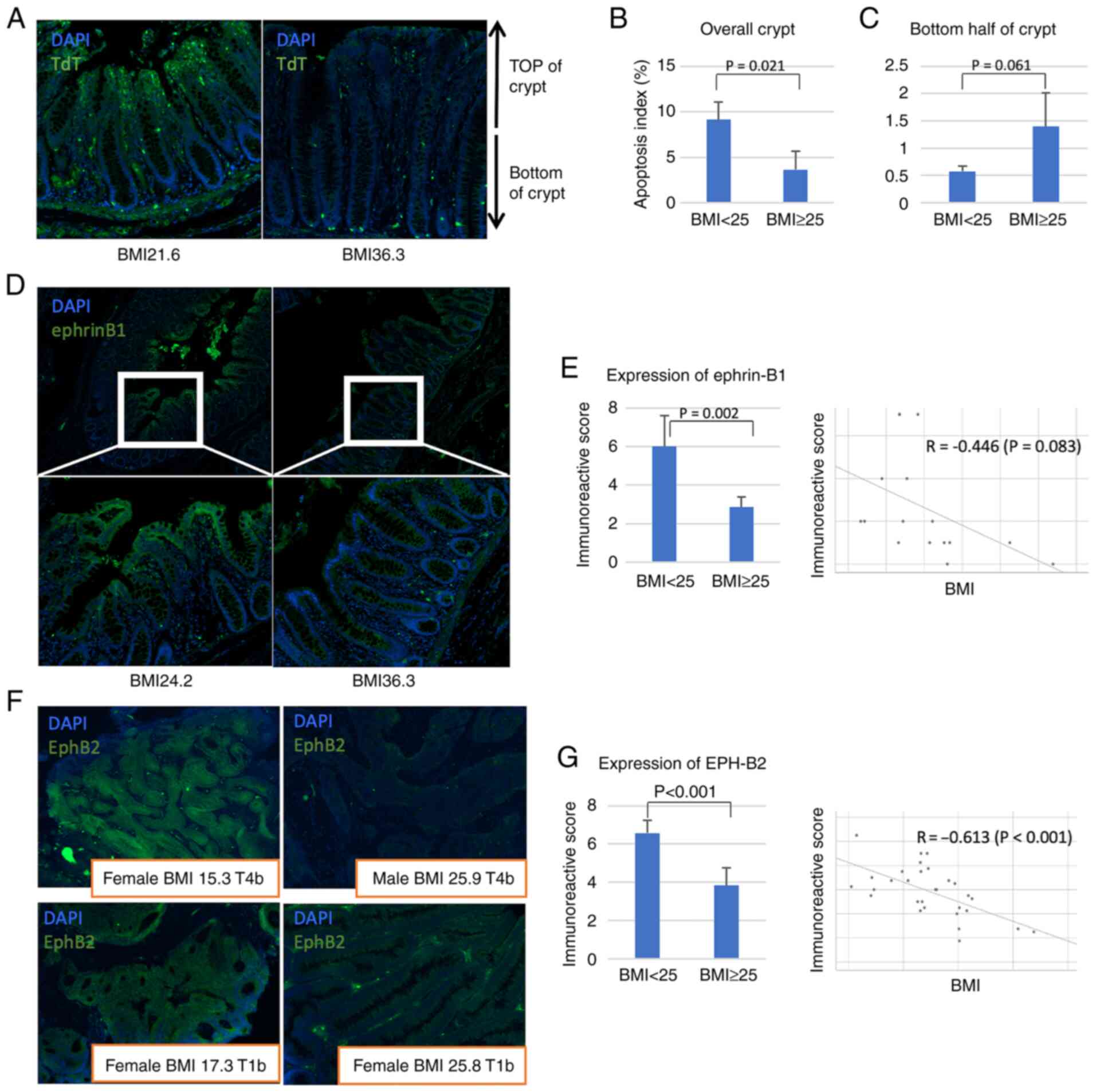
Expression of EphB2 and ephrin-B1 in
human colon
Next, the results obtained from the obesity mouse
model were validated in human samples. The patient characteristics
are detailed in the Table II.
TUNEL assay revealed that the number of apoptotic cells in normal
mucosa was significantly lower in patients with BMI >25 compared
with patients with BMI<25 (Fig.
3A). Furthermore, the apoptotic cells in the mucosa of obese
patients were more frequently found in the base of the crypt,
similar to the mouse model (Fig. 3B
and C).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Characteristic | BMI <25 | BMI >25 |
|---|
| Tumour samples,
n | 32 | 16 |
| Age, years ±
SD | 65.7±11.5 | 64.1±11.4 |
| Sex, n |
|
|
|
Male | 17 | 9 |
|
Female | 15 | 7 |
| Location, n |
|
|
|
Colon | 26 | 11 |
|
Rectum | 6 | 5 |
| T stage, n |
|
|
|
T1,2 | 7 | 5 |
|
T3,4 | 25 | 11 |
Ephrin-B1 was expressed at lower levels in the
normal colon mucosa of patients with a high BMI (Fig. 3D); the IRS of ephrin-B1 was
significantly lower in patients with a high BMI than that in
patients with a low BMI (6.00 in BMI <25 vs. 2.86 in BMI ≥25,
P=0.002, Mann-Whitney U test; Fig.
3E). On the other hand, EphB2 was expressed at higher levels in
tumours of patients with a lower BMI (Fig. 3F). Moreover, the IRS of EphB2 was
significantly higher in patients with a lower BMI (6.58 in BMI
<25 vs. 3.83 in BMI ≥25, P<0.001, Mann-Whitney U test;
Fig. 3G). These results suggest
that obesity results in dysregulation of EphB2 and ephrin-B1
expression, promoting obesity-related cancer development and
progression.
The EphB2 level was analysed according to the degree
of tumour differentiation (poorly differentiated, p/d;
well-differentiated, w/d; and moderately differentiated, m/d) to
evaluate the association between tumour malignancy and EphB2.
However, there was no significant difference in the degree of
differentiation among w/d, m/d, and p/d tumours (IRS=6.0, 5.5, and
6.25 in the w/d, m/d, and p/d groups, respectively, P=0.896,
Kruskal-Wallis test) (Fig.
S2).
Discussion
The association between obesity and colorectal
carcinogenesis has been demonstrated by numerous epidemiological
studies. Our findings demonstrate that obesity and obesity-induced
inflammation promote colon cancer development and progression. Our
findings also suggest that repulsive Eph-ephrin interactions play a
critical role in obesity-associated colorectal cancer. In obesity,
the ephrin-B1 expression in normal colon mucosa is downregulated,
leading to decreased cell apoptosis and carcinogenesis. The
obesity-induced secretion of inflammatory cytokines, including
MCP-1, drive carcinogenesis by downregulating ephrin-B1 expression.
Obesity also results in EphB2 downregulation, leading to the more
rapid cancer development and progression observed in obese mice and
humans. Our study also offers new insight into how obesity promotes
colorectal tumourigenesis and enhances cancer progression.
It was found that ephrin-B1 expression at the top of
the crypt was downregulated in obesity and that the dysregulated
EphB2/ephrin-B1 signalling may disrupt cell apoptosis and
carcinogenesis. Previous studies have suggested a correlation
between Eph-ephrin signalling and colorectal cancer. In the colon
of ApcMin/+ mice, Eph-B expressing tumour cells
expanded laterally, forming additional crypt structures that
replaced the normal epithelium, and resulted in the apparent
compartmentalisation of Eph-B-positive tumour cells and
ephrin-B-positive normal cells (9,24,25).
Furthermore, Cortina et al demonstrated that in
ephrin-B-deficient mice, Apc-mutant cells repopulated the
normal mucosa due to the lack of repulsive interaction between
Eph-B and ephrin-B (15). As a
result, the progression of adenomas was suppressed by Eph-ephrin
repulsive signals. In obesity, reduction in ephrin-B1 levels may
lead to apoptosis inhibition in the crypt-villus axis and
subsequent development and progression of adenoma. Our
immunohistological findings support the premise that obesity
promotes adenoma and carcinoma development by the disruption of
Eph-ephrin signalling.
Eph-ephrin signalling also plays a critical role in
the progression of colorectal cancer. The current study
demonstrated that KKAy mice had larger tumours than C57BL/6 mice
and that Eph downregulation is a crucial step in the progression of
obesity-associated colorectal cancer. Clevers and Batlle suggested
that Eph-B expression was disrupted in a subset of tumour cells and
that, in the absence of Eph-B, tumour progression was accelerated,
resulting in the development of highly aggressive colorectal
adenocarcinomas (14). EphB2
downregulation has been associated with poor prognosis in various
human cancers, including colorectal cancer (26,27).
It has been reported that mutations in repetitive sequences in the
exon 17 of EphB2 are frequent in colon adenomas and colorectal
carcinomas. In addition, hypermethylation of EphB2 promoter is
frequently found in colorectal cancer patients (28). Another study has shown that DNA
methylation is an important mechanism regulating gene expression in
mice receiving a high fat diet (29). Taken together, these studies
suggest that obesity results in EphB2 downregulation in colorectal
cancer by promoting the methylation of its promoter. The
association between obesity and EphB2 mutations should be analysed
in future studies.
The relationship between Eph-ephrin signalling and
obesity-associated carcinogenesis was also analysed. Decreased
expression of ephrin-B1 associated with increased macrophage
infiltration in the colon mucosa. Mori et al suggested a
positive feedback regulation between inflammation, MCP-1, and
ephrin-B1 (18). Obesity induces
the secretion of inflammatory cytokines in adipose tissue, such as
MCP-1 and tumour necrosis factor-α (TNF-α) via activation of the
ERK, nuclear factor-κB (NFκB), and c-Jun N-terminal kinase (JNK)
pathways, which contribute to ephrin-B1 downregulation. The
disruption in ephrin-B1 expression results in MCP-1 upregulation
via TNF-α-mediated ERK1/2 activation, which in turn promotes the
recruitment of macrophages; macrophages further promote ephrin-B1
downregulation. This network is believed to also occur in the colon
mucosa of obese individuals. In addition, previous studies
suggested that mitogen-activated protein kinase/ERK (MAPK/ERK)
signalling occurs downstream of ephrin-EphB signalling and that Eph
signals suppress the ERK activity (30,31).
These reports supports our results. The dysregulation of ephrin
signals and activation of MAPK/ERK pathway can lead to the
exacerbation of inflammation and carcinogenesis in the intestinal
mucosa (23,31). However, evidence supporting an
association between obesity, ephrin-Eph signalling, MAPK/ERK
pathway and colorectal cancer development and progression is still
lacking. Further studies are therefore needed.
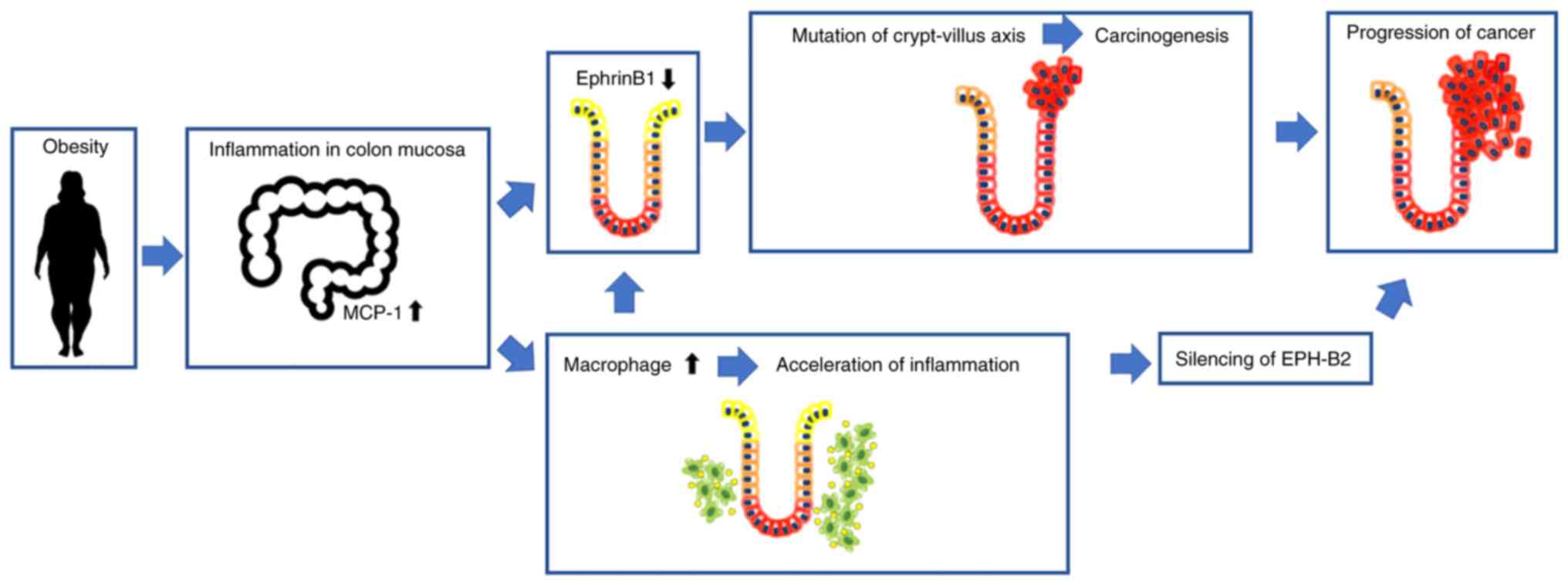
According to findings of this study, in normal colon
mucosa, repulsive interactions occur between Eph-B2-positive cells
at the bottom of the crypt and ephrin-B1-positive cells at the top
of the crypt, and these interactions regulate the position of cells
in the crypt (Fig. 4). However,
obesity-induced inflammation results in ephrin-B1 downregulation at
the top of the crypt, disrupting the repulsive EphB2/ephrin-B1
interaction and promoting the migration of mutation-harbouring
cells at the bottom of the crypt; cells in the crypt evade
apoptosis and eventually grow into adenoma. The expression of EphB2
is also downregulated by undetermined factors; hence, the repulsive
Eph-ephrin interaction is fully lost, leading to obesity-associated
colorectal cancer progression. A better understanding of the
mechanism underlying obesity-associated cancer development and
progression will allow for the discovery of therapeutic approaches.
Anti-inflammatory therapy targeting macrophage infiltration or
inhibiting the silencing of EphB2 may offer novel approaches for
inhibiting obesity-associated colorectal cancer development and
progression.
There were several limitations to this study. First,
the obesity mouse model that was used may not fully reflect the
acquired obesity. The KKAy mouse strain was developed by
introducing the Ay mutation into the inbred KK strain of native
Japanese mice (20), which leads
to the development of congenital obesity. In addition, there are
several confounding factors in the relationship between obesity and
cancer development, such as diabetes, diet and the level of
physical exercise. Although KKAy mice have been widely used in
mouse model of obesity (32–34)
further assessment using another obesity model mice, e.g.
leptin-deficient ob/ob mouse or leptin receptor deficient db/db
mouse, can validate our results. Second, obesity is only one of
many lifestyle-related diseases; this study analysed obesity
independently of other conditions. Since excess weight and obesity
are closely linked with hypertension, hyperglycaemia, and
hyperlipidaemia, which are collectively referred to as metabolic
syndrome, it is important also to consider the effect of metabolic
syndrome in relation to cancer as a whole. Third, MCP-1 expression
and macrophage infiltration in human mucosa were not evaluated;
therefore, further studies are needed to validate the present data.
Fourth, downstream pathway markers activated by ephrinB1/EphB2
signal are not fully evaluated in this study. Further analysis such
as an evaluation of phosphorylated transducer and activator of
transcription-3 is needed to validate our findings. Finally, our
investigations focused on EphB2 and ephrin-B1 expression in
colorectal cancer; however, other ephrins, including Eph-B3 and
Eph-B4, have also been implicated in colorectal carcinogenesis
(10,12,16,35).
A comprehensive analysis of the role of the ephrin family in
obesity-associated colorectal cancer should be conducted.
In conclusion, the findings of this study suggest
that obesity-induced inflammation results in the disruption in
Eph-ephrin signalling and the crypt-villus axis, promoting
oncogenesis. This study highlights the importance of Eph-ephrin
signalling in obesity-associated colorectal cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, KO, RS and YK designed the study. YS and KO
analyzed the data and wrote the manuscript. YS, KO and RS designed
the experiments. HH, MT and TT helped with the experiments. YS, KO
and YK confirm the authenticity of all the raw data. All authors
reviewed the draft and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Keio University School of Medicine Ethics Committee (approval no.
20140001). The study was performed in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization, . Obesity and
Overweight Fact. http://www.who.int/mediacentre/factsheets/fs311/en/January
5–2022
|
|
2
|
Gunter MJ and Leitzmann MF: Obesity and
colorectal cancer: Epidemiology, mechanisms and candidate genes. J
Nutr Biochem. 17:145–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Pandeya N, Byrnes G, Renehan
PAG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit
R, et al: Global burden of cancer attributable to high body-mass
index in 2012: A population-based study. Lancet Oncol. 16:36–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andersson TM, Weiderpass E, Engholm G,
Lund AQ, Olafsdottir E, Pukkala E, Stenbeck M and Storm H:
Avoidable cancer cases in the nordic countries-the impact of
overweight and obesity. Eur J Cancer. 79:106–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otani T, Iwasaki M and Inoue M; Shoichiro
Tsugane for the Japan Public Health Center-based Prospective Study
Group, : Body mass index, body height, and subsequent risk of
colorectal cancer in middle-aged and elderly Japanese men and
women: Japan public health center-based prospective study. Cancer
Causes Control. 16:839–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okabayashi K, Ashrafian H, Hasegawa H, Yoo
JH, Patel VM, Harling L, Rowland SP, Ali M, Kitagawa Y, Darzi A and
Athanasiou T: Body mass index category as a risk factor for
colorectal adenomas: A systematic review and meta-analysis. Am J
Gastroenterol. 107:1175–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitahara CM, Berndt SI, de Gonzalez AB,
Coleman HG, Schoen RE, Hayes RB and Huang WY: Prospective
investigation of body mass index, colorectal adenoma, and
colorectal cancer in the prostate, lung, colorectal, and ovarian
cancer screening trial. J Clin Oncol. 31:2450–2459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermeulen L and Snippert HJ: Stem cell
dynamics in homeostasis and cancer of the intestine. Nat Rev
Cancer. 14:468–480. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Batlle E, Henderson JT, Beghtel H, van den
Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering
M, Pawson T and Clevers H: Beta-catenin and TCF mediate cell
positioning in the intestinal epithelium by controlling the
expression of EphB/ephrinB. Cell. 111:251–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyd AW, Bartlett PF and Lackmann M:
Therapeutic targeting of EPH receptors and their ligands. Nat Rev
Drug Discov. 13:39–62. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chukkapalli S, Amessou M, Dilly AK, Dekhil
H, Zhao J, Liu Q, Bejna A, Thomas RD, Bandyopadhyay S, Bismar TA,
et al: Role of the EphB2 receptor in autophagy, apoptosis and
invasion in human breast cancer cells. Exp Cell Res. 320:233–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv J, Xia Q, Wang J, Shen Q, Zhang J and
Zhou X: EphB4 promotes the proliferation, invasion, and
angiogenesis of human colorectal cancer. Exp Mol Pathol.
100:402–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oweida A, Bhatia S, Hirsch K, Calame D,
Griego A, Keysar S, Pitts T, Sharma J, Eckhardt G, Jimeno A, et al:
Ephrin-B2 overexpression predicts for poor prognosis and response
to therapy in solid tumors. Mol Carcinog. 56:1189–1196. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clevers H and Batlle E: EphB/EphrinB
receptors and Wnt signaling in colorectal cancer. Cancer Res.
66:2–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cortina C, Palomo-Ponce S, Iglesias M,
Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N,
Gallego L, Jonkheer S, et al: EphB-ephrin-B interactions suppress
colorectal cancer progression by compartmentalizing tumor cells.
Nat Genet. 39:1376–1383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herath NI and Boyd AW: The role of Eph
receptors and ephrin ligands in colorectal cancer. Int J Cancer.
126:2003–2011. 2010.PubMed/NCBI
|
|
17
|
Guo DL, Zhang J, Yuen ST, Tsui WY, Chan
AS, Ho C, Ji J, Leung SY and Chen X: Reduced expression of EphB2
that parallels invasion and metastasis in colorectal tumours.
Carcinogenesis. 27:454–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori T, Maeda N, Inoue K, Sekimoto R,
Tsushima Y, Matsuda K, Yamaoka M, Suganami T, Nishizawa H, Ogawa Y,
et al: A novel role for adipose ephrin-B1 in inflammatory response.
PLoS One. 8:e761992013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gohda T, Tanimoto M, Kaneko S, Shibata T,
Funabiki K, Horikoshi S and Tomino Y: Minor gene effect of leptin
receptor variant on the body weight in KK/Ta mice. Diabetes Obes
Metab. 8:581–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura M and Yamada K: Studies on a
diabetic (KK) strain of the mouse. Diabetologia. 3:212–221. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang YP, Wu XH, Shi B, Wu WX and Yin GR:
Expression of chemokine CXCL12 and its receptor CXCR4 in human
epithelial ovarian cancer: An independent prognostic factor for
tumor progression. Gynecol Oncol. 103:226–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi T, Shiraishi A, Murata J,
Matsubara S, Nakaoka S, Kirimoto S and Osawa M: Muscarinic receptor
M3 contributes to intestinal stem cell maintenance via
EphB/ephrin-B signaling. Life Sci Alliance. 4:e2020009622021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oshima H, Oshima M, Kobayashi M, Tsutsumi
M and Taketo MM: Morphological and molecular processes of polyp
formation in Apc(delta716) knockout mice. Cancer Res. 57:1644–1649.
1997.PubMed/NCBI
|
|
25
|
Shih IM, Wang TL, Traverso G, Romans K,
Hamilton SR, Ben-Sasson S, Kinzler KW and Vogelstein B: Top-down
morphogenesis of colorectal tumors. Proc Natl Acad Sci USA.
98:2640–2645. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lugli A, Spichtin H, Maurer R, Mirlacher
M, Kiefer J, Huusko P, Azorsa D, Terracciano L, Sauter G,
Kallioniemi OP, et al: EphB2 expression across 138 human tumor
types in a tissue microarray: High levels of expression in
gastrointestinal cancers. Clin Cancer Res. 11:6450–6458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jubb AM, Zhong F, Bheddah S, Grabsch HI,
Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P and Koeppen H:
EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res.
11:5181–5187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alazzouzi H, Davalos V, Kokko A, Domingo
E, Woerner SM, Wilson AJ, Konrad L, Laiho P, Espin E, Armengol M,
et al: Mechanisms of inactivation of the receptor tyrosine kinase
EPHB2 in colorectal tumors. Cancer Res. 65:10170–10173. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang P, Chu T, Dedousis N, Mantell BS,
Sipula I, Li L, Bunce KD, Shaw PA, Katz LS, Zhu J, et al: DNA
methylation alters transcriptional rates of differentially
expressed genes and contributes to pathophysiology in mice fed a
high fat diet. Mol Metab. 6:327–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao Z, Carrasco R, Kinneer K, Sabol D,
Jallal B, Coats S and Tice DA: EphB4 promotes or suppresses
Ras/MEK/ERK pathway in a context-dependent manner: Implications for
EphB4 as a cancer target. Cancer Biol Ther. 13:630–637. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu F, Song Y, Wu Y, Huang Y, Zhong Q,
Zhang Y, Fan Z and Xu C: The protective role of Ephrin-B2/EphB4
signaling in osteogenic differentiation under inflammatory
environment. Exp Cell Res. 400:1125052021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Azushima K, Tamura K, Wakui H, Maeda A,
Ohsawa M, Uneda K, Kobayashi R, Kanaoka T, Dejima T, Fujikawa T, et
al: Bofu-tsu-shosan, an oriental herbal medicine, exerts a
combinatorial favorable metabolic modulation including
antihypertensive effect on a mouse model of human metabolic
disorders with visceral obesity. PLoS One. 8:e755602013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heaberlin JR, Ma Y, Zhang J, Ahuja SS,
Lindsey ML and Halade GV: Obese and diabetic KKAy mice show
increased mortality but improved cardiac function following
myocardial infarction. Cardiovasc Pathol. 22:481–487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nonogaki K, Hazama M and Satoh N:
Liraglutide suppresses obesity and hyperglycemia associated with
increases in hepatic fibroblast growth factor 21 production in KKAy
mice. Biomed Res Int. 2014:7519302014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Surawska H, Ma PC and Salgia R: The role
of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev.
15:419–433. 2004. View Article : Google Scholar : PubMed/NCBI
|