Introduction
Giant cell tumor of bone (GCTB) is an intermediate
bone tumor occurring in the sacrum or other vertebral bones, as
well as the epiphysis of long bones, including the distal femur,
proximal tibia, distal radius, and proximal humerus (1–3).
Moreover, GCTB is relatively rare, accounting for about 3–5% of all
primary bone tumors and is most common in patients 20–40 years of
age (1–3). GCTB is composed of two types of
cells: multinucleated giant cells expressing receptor activator of
nuclear factor-kB (RANK) and neoplastic mononuclear stromal cells
expressing RANK ligand (RANKL) (4). The interactions between these two
cell types induce bone destruction.
Treatment of GCTB often includes curettage and
adjuvants such as argon, phenol, alcohol, or
polymethylmethacrylate. A bone graft is used to overcome the bone
defect. Other procedures, such as radiotherapy (RT) and
embolization may be employed in cases where surgery is not
possible. However, it is locally aggressive, and recurrence is
observed in 15–50% of cases, usually within 3 years after treatment
(1–3). Some clinical trials have shown that
denosumab, a monoclonal antibody inhibitor of RANKL, is effective
in patients with recurrent or unresectable giant cell tumors,
although recent studies have demonstrated that it may increase the
risk of recurrence (5–7).
Rarely, GCTB undergoes transformation into a
malignant tumor, becoming primary or secondary malignant GCTB
(SMGCTB) (8–11). Primary malignant GCTB (PMGCTB) is
defined as a lesion in which a high-grade sarcoma component appears
simultaneously next to the conventional GCTB component at the time
of first presentation. SMGCTB is defined as a lesion in which a
high-grade sarcoma component occurs at the site of previously
treated GCTB. Most MGCTBs are secondary and occur after RT,
multiple local recurrences after surgery for GCTB, or late local
recurrence (8). The incidence of
MGCTB, PMGCTB, and SMGCTB was reported to be 1.1–11.3, 0.5–9.7, and
1.3–5%, respectively among GCTB patients (8). Moreover, because most cases of SMGCTB
occur post-RT, they should be classified as radiation-induced
sarcomas. True spontaneous SMGCTB following surgery without prior
RT treatment is extremely uncommon with an incidence rate of
0.5-1.5% among GCTB cases and has been reported in less than 150
cases (9–31). Furthermore, recent studies have
reported the occurrence of SMGCTB following denosumab treatment,
with a total of less than 20 cases (6,7,32–40).
Notably, p53 has been proposed to be a cause of
SMGCTB development, and it has been reported that p53 is
overexpressed in these tumors (41,42).
However, there are no reports on continuous changes in p53
expression at the time of primary cancer, recurrence, malignant
change, or metastasis.
Surgical resection is the definitive management for
resectable SMGCTB (8). However,
while resection with wide margins is an achievable goal for SMGCTB
located in the limbs, it is more challenging for sacrum or
vertebral lesions due to their anatomical complexity (28). Furthermore, chemotherapy is
performed for advanced SMGCTB, but only limited data is available
regarding the role of chemotherapy in SMGCTB due to its rarity
(8). Clinical outcomes of SMGCTB
are poor, with a distant metastasis rate of 33–80%, a 5-year
disease-free survival rate of 32%, and a 5-year overall survival
rate of 40% (8,16,20,30,31).
However, these reports include patients treated with various
modalities, including RT in most cases, and only a few reports have
assessed the clinical outcomes of SMGCTB (30,31).
Moreover, evaluation of clinical cancer genomic profiling and the
effects of molecular targeted therapy and proton ion therapy have
not been reported.
Therefore, we investigated the clinicopathologic and
histologic features of SMGCTB in patients not previously treated
with RT. We specifically asked the following questions: i) How
about the rate of SMGCTB in patients treated with surgery or
denosumab? ii) What are the changes of the immunohistochemical
features and expression of p53 and Ki67, including primary,
recurrence, malignant change, and metastasis? iii) What is the role
of clinical cancer genomic profiling and heavy iron treatment and
molecular targeted therapy? Furthermore, we reviewed the clinical
features of SMGCTB in patients not previously treated with RT and
the clinical effects of denosumab.
Patients and methods
Patients
We retrospectively evaluated the medical records of
75 patients with pathologically proven GCTB treated at Okayama
University Hospital (Okayama, Japan) between March 1986 and August
2020. The inclusion criterion was a pathologically proven diagnosis
of sarcoma. Patients excluded were those followed up for less than
one year after surgery, patients with PMGCTB, or treated in other
institution. Additionally, 48 patients underwent curettage, and 19
patients underwent resection. None of the patients received RT. We
examined the rate of SMGCTB in these patients.
Imaging
Plain X-ray, computed tomography (CT), and magnetic
resonance (MR) imaging (MRI) were utilized for initial examination
in all cases. CT (Discovery CT750 HD, GE) images, obtained at 120
kV and with a slice thickness of 5 mm, were viewed in the axial,
sagittal, and coronal planes. Results of MRI (MEGNETOM Prisma,
Siemens) consisted of T1-weighted images, and T2-weighted images
were obtained in the axial, sagittal, and coronal planes. In two
cases of SMGCTB, we utilized 2-deoxy-2[18F]
fluoro-D-glucose positron emission tomography-computed tomography
(FDG PET-CT) (Biograph 16; Siemens Medical Solution USA, Knoxville,
TN, USA) at a diagnostic imaging center adjacent to our
institution. After fasting for at least 5 h, the patients received
an intravenous injection of 3.7 MBq/kg 18F-FDG. PET image
acquisition was started 90 min after injection of 18F-FDG, with the
patient in a relaxed supine position. First, a total-body low-dose
CT scan for the calculation of attenuation correction was
performed, using a standardized protocol involving 120 kV, auto mA
mode, rotation time of 0.5 sec, pitch of 0.8, section thickness of
3 mm, and scan field from the head to the mid-thigh level.
Thereafter, PET imaging consisting of 6–8 bed positions with 2.4
min per position over the same region was performed. The PET images
were reconstructed with an ordered-subset expectation maximization
iterative reconstruction algorithm. Integrated, co-registered
PET/CT images were obtained using a workstation that enables image
fusion and analysis (syngo. via; Siemens Medical Solution USA).
Pathologic findings of SMGCTB
Preparation for histologic
evaluation
All cases were immediately fixed in 4%
paraformaldehyde for 12 h then decalcified in 10%
ethylenediaminetetraacetic acid at 4°C for 14 days. The tissue was
routinely embedded in paraffin, and five thick serial sections were
prepared. The sections were subjected to hematoxylin-eosin (HE) and
immunohistochemistry (IHC) staining.
Immunohistochemistry
Following antigen retrieval in a cooker heating for
1 or 8 min in 0.01 M Dako Target Retrieval Solution (pH 9; cat. no.
S2367; Agilent Technologies, Inc. USA), 5-µm sections were blocked
with 10% normal serum (Vector Laboratories, Inc. USA) for 20 min at
room temperature and incubated with primary antibodies, including
ki67 (cat. no. A0047; 1:50; DAKO, USA), p53 (M7001; 1:50; DAKO,
USA) overnight at 4°C. Signals were enhanced using the
avidin-biotin complex method (Vector Lab, Burlingame, CA, USA).
Color development was performed using 3,3′-diaminobenzidine
(Histofine, Nichirei, Tokyo, Japan), and the staining results were
observed with an optical microscope (BX53, Olympus, Tokyo,
Japan).
Quantification and statistical
analysis
To compare the tissue characteristics throughout
each time of GCTB progression in the same patient, cell counting
was performed in each area. After counterstaining with hematoxylin,
the sections were examined microscopically at ×400 magnification.
Five areas were chosen randomly in each sample, one hundred cells
were counted in each area, and the percentage of positive cells was
calculated and compared among the groups. Counting was performed by
a pathologist specializing in tumor evaluation. All statistical
analyses were conducted using GraphPad Prism 9.1.1. Repeated
measures ANOVA followed by Sidak's multiple comparison post hoc
test was used to compare differences among more than two groups
where necessary. Differences were considered significant at
P<0.05. Data are presented as mean ± SD.
Clinical cancer genomic profiling
One patient (Case 3; patient with stage IIA sacral
MGCTB) underwent clinical cancer genomic profiling using the
FoundationOne Medicine (Cambridge, MA, USA; http://www.foundationmedicine.com/) platform to
identify potential targetable molecular aberrations. The
FoundationOne®CDx assay is the first and only
comprehensive companion diagnostic for all solid tumors approved by
the Ministry of Health, Labor, and Welfare in Japan (43). This assay evaluates 309 genes
involved in substitutions, insertions/deletions, copy number (CN)
alterations, as well as introns of 36 genes related to
rearrangements. Genomic DNA was isolated from the formalin-fixed
paraffin-embedded tissue samples (excised specimen of the sacrum),
and its purity and concentration were determined.
Brief literature review
We performed a literature review to identify other
published cases and describe the clinical characteristics of
SMGCTB. Searches through the PubMed database were conducted through
December 2021, identifying the reports published till April 2021.
Keywords employed in the search process included: giant cell tumor,
malignancy, and secondary malignant giant cell tumor. The
literature search was further limited to articles published in
English. We also searched ‘related articles’ of included studies
suggested by PubMed.
Results
The frequency of SMGCTB
Eighteen patients experienced local recurrence, and
twenty-two patients had received denosumab among 75 patients.
Moreover, we investigated SMGCTB in patients without prior RT
treatment and detected three patients (4%) with SMGCTB. The patient
characteristics are summarized in Table I. Two of the three cases of SMGCTB
occurred after surgery and one of the cases occurred after
denosumab treatment. There were two men and one woman. The tumors
were located in the distal ulna (case 1), distal femur (case 2),
and sacrum (case 3). The follow-up periods were 12, 4, and 13
years. Moreover, for initial treatment of GCTB, the patient with
the tumor in the distal ulna underwent tumor resection, the patient
with the tumor in the distal femur underwent curettage and
artificial bone graft, and the patient with the tumor in the sacrum
underwent conservative embolization followed by denosumab and
resection.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case | Gender | Age at
Presentation, years | Location | Campanacci
classification | Initial
treatment | Time to SMGCTB,
years | Treatment | Recurrence | Metastases | Outcome |
|---|
| 1 | Male | 61 | Ulna | 3 | Surgery | 7 | Surgery | + | Lung and bone | Dead |
| 2 | Male | 23 | Femur | 2 | Surgery | 3 | Surgery and
chemotherapy | - | Lung | Dead |
| 3 | Female | 23 | Sacrum | 3 | Embolization | 10 | Carbonion
radio-therapy | - | Lung and heart | Alive |
Pathologic finding
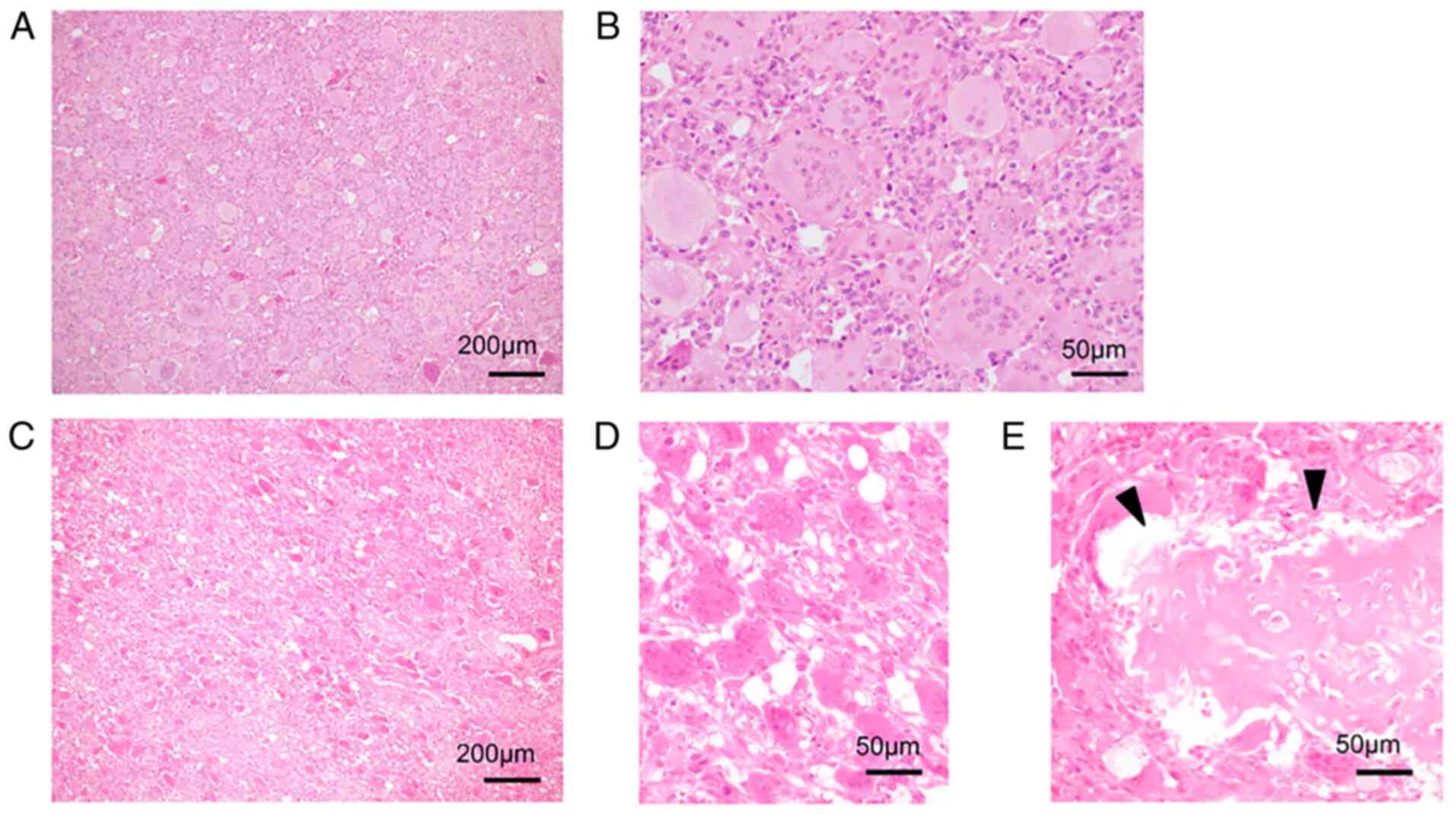
Histologic findings of primary and
recurrent lesion of conventional GCTB
In all cases, the primary tumors were mainly
composed of a proliferation of ovular or short, spindle-shaped
stromal cells with evenly scattered, osteoclast-type giant cells,
diagnosed as conventional GCTB. The tissue pattern was monogenous,
and no necrosis was observed. Additionally, mitotic figures were
not found in the tumor cells (Fig. 1A
and B). The recurrent lesions had a similar pattern to that of
the primary specimen (Fig. 1C).
Moreover, the multinuclear giant cells were sparsely spread around
the ovoid/round or spindle mononuclear stromal cells (Fig. 1D). Additionally, woven bone was
observed and was considered to be the remaining artificial bone
(Fig. 1E). Finally, no necrotic
area or atypical findings were observed.
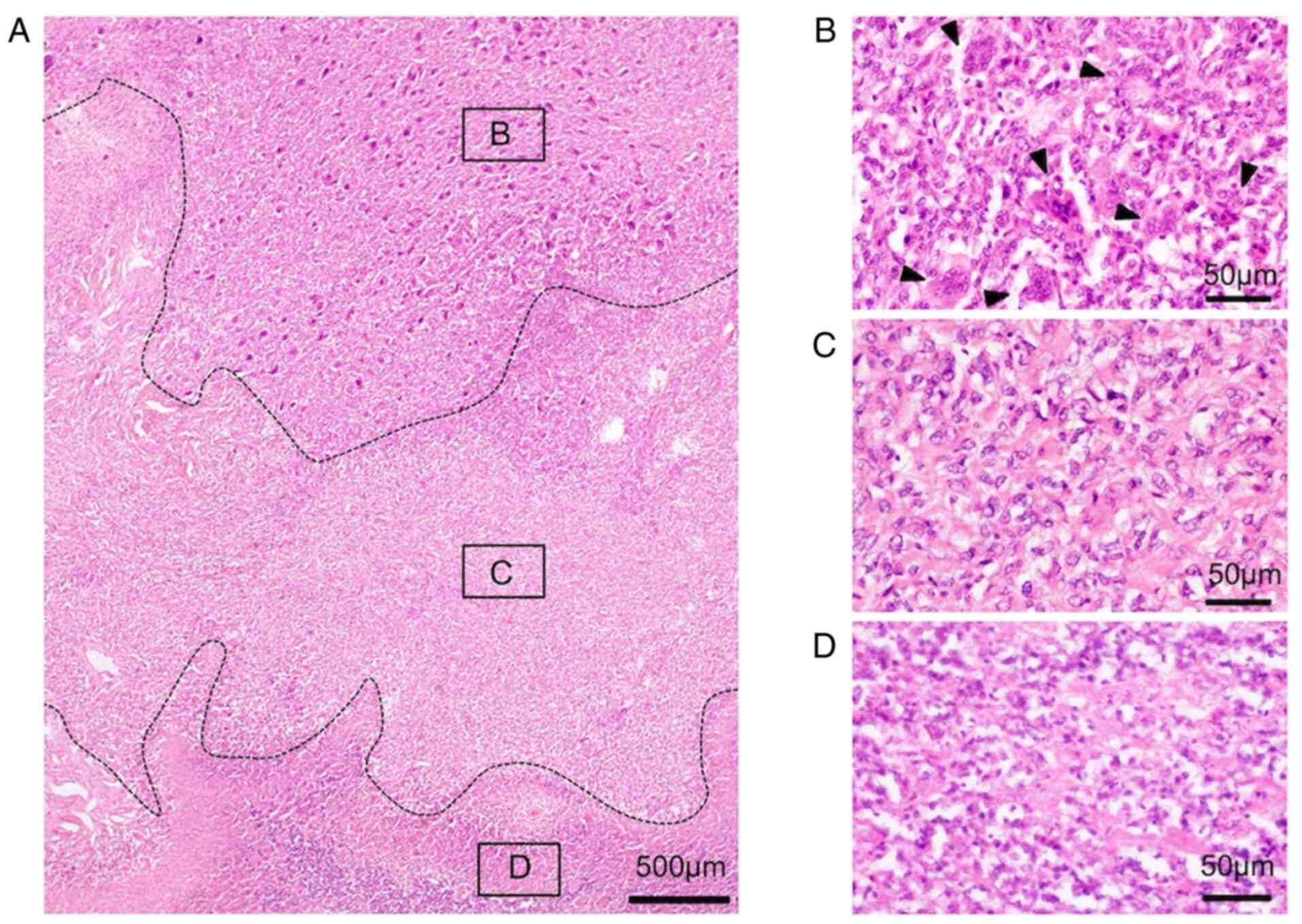
Histologic findings of lesion of
malignant transformation
In all cases, the lesions of malignant
transformation were composed of two components (Fig. 2A). One was a conventional GCTB
component (Fig. 2B), and the other
was a malignant component (Fig.
2C). In conventional GCTB component is composed of a
proliferation of mononuclear, oval, or short, spindle-shaped
stromal cells with evenly scattered, multi-nucleolus giant cells.
Mitotic figures were not found in these tumor cells (Fig. 2B). In the fibrosarcoma component,
there were interlacing fascicular pattern of proliferation of
spindle-shaped tumor cells in a collagen background (Fig. 2C). Tumor cells were highly atypical
and had hyperchromatic nuclei and atypical mitosis could also be
found. Giant cells were banished. A wide necrotic section was
observed in the fibrosarcomatous area, where it was possible to
find cells that had more eosinophilic cytoplasm with irregular
distribution, or flocculation of chromatin and fragmentation, or
disappearance of nuclear (Fig.
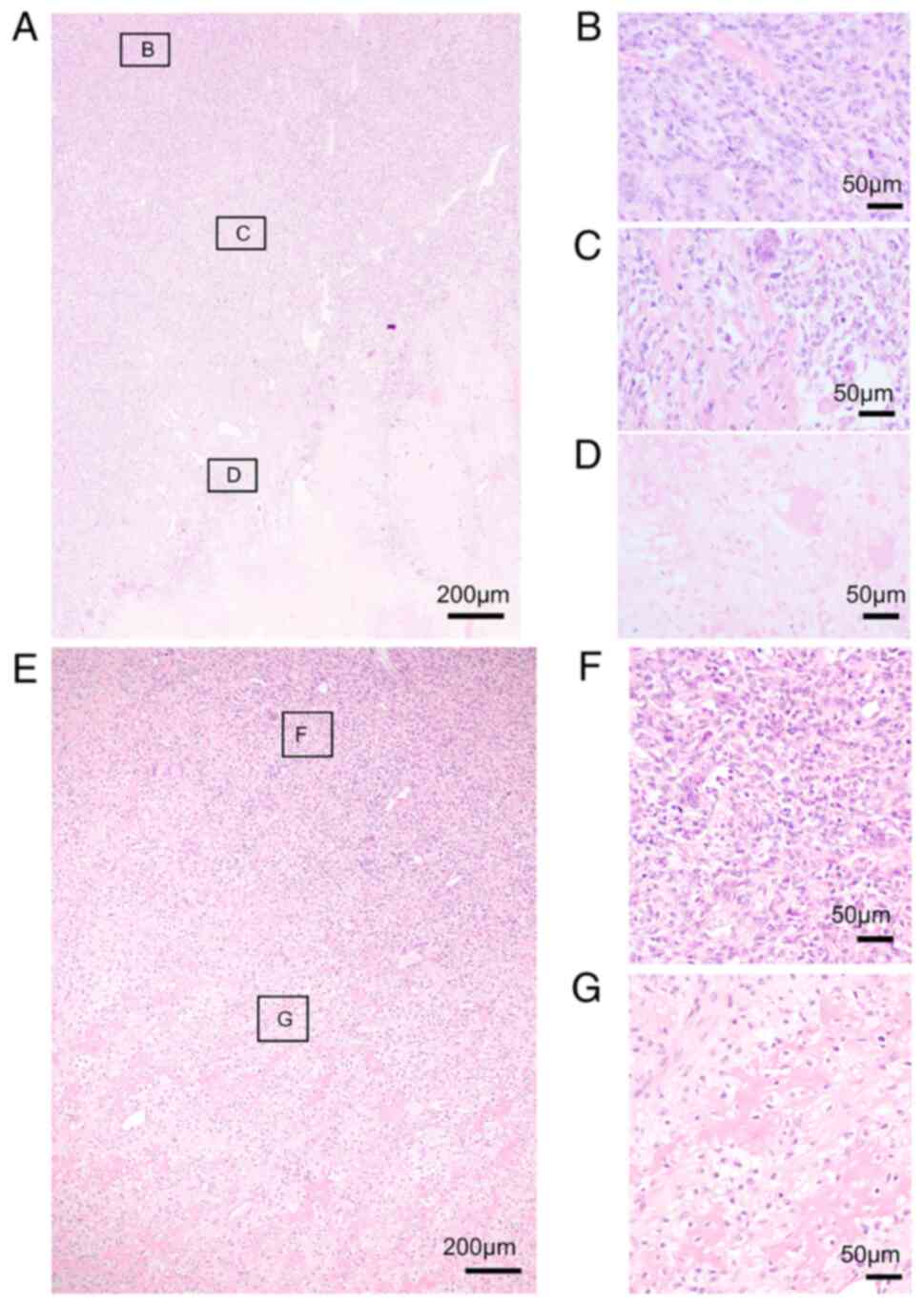
2D). In the recurrent SMGCTB, fibrosarcomatous tissue was
observed similar to that of the first specimen of malignant
transformation (Fig. 3A and B). In
addition, osteoid formation was observed in some areas (Fig. 3C). These findings were indicative
of osteosarcoma. In the osteosarcoma component, the cells were
highly anaplastic and pleomorphic with large hyperchromatic nuclei.
Moreover, wide necrosis was observed in the specimen of recurrence
of SMGCTB (Fig. 3D). No benign
components were found. The specimens from lung metastasis were
similar to those of recurrent SMGCTB (Fig. 3E) displaying a combination of
fibrosarcomatous tissue (Fig. 3F)
and osteosarcomatous tissue (Fig.
3G).
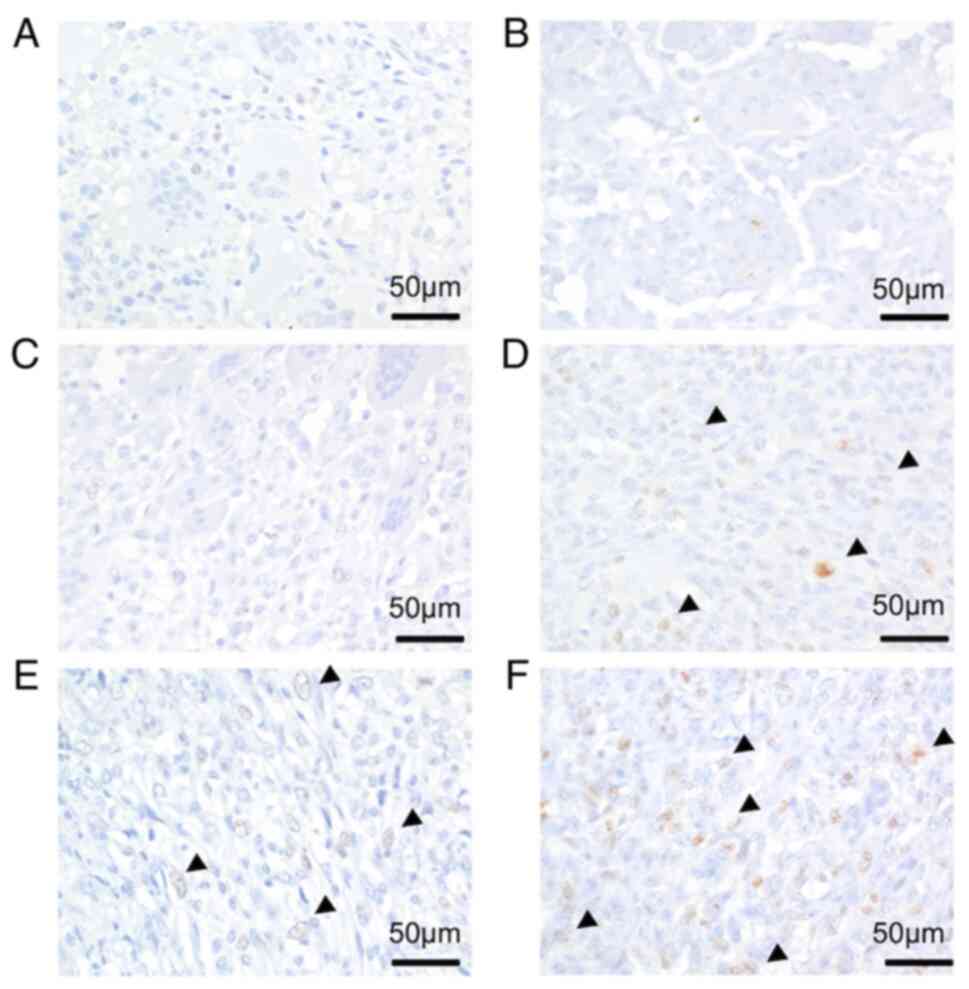
Immunohistochemical evaluation
To investigate the changes of malignant
transformation within the same tumor, we performed Ki67 and p53
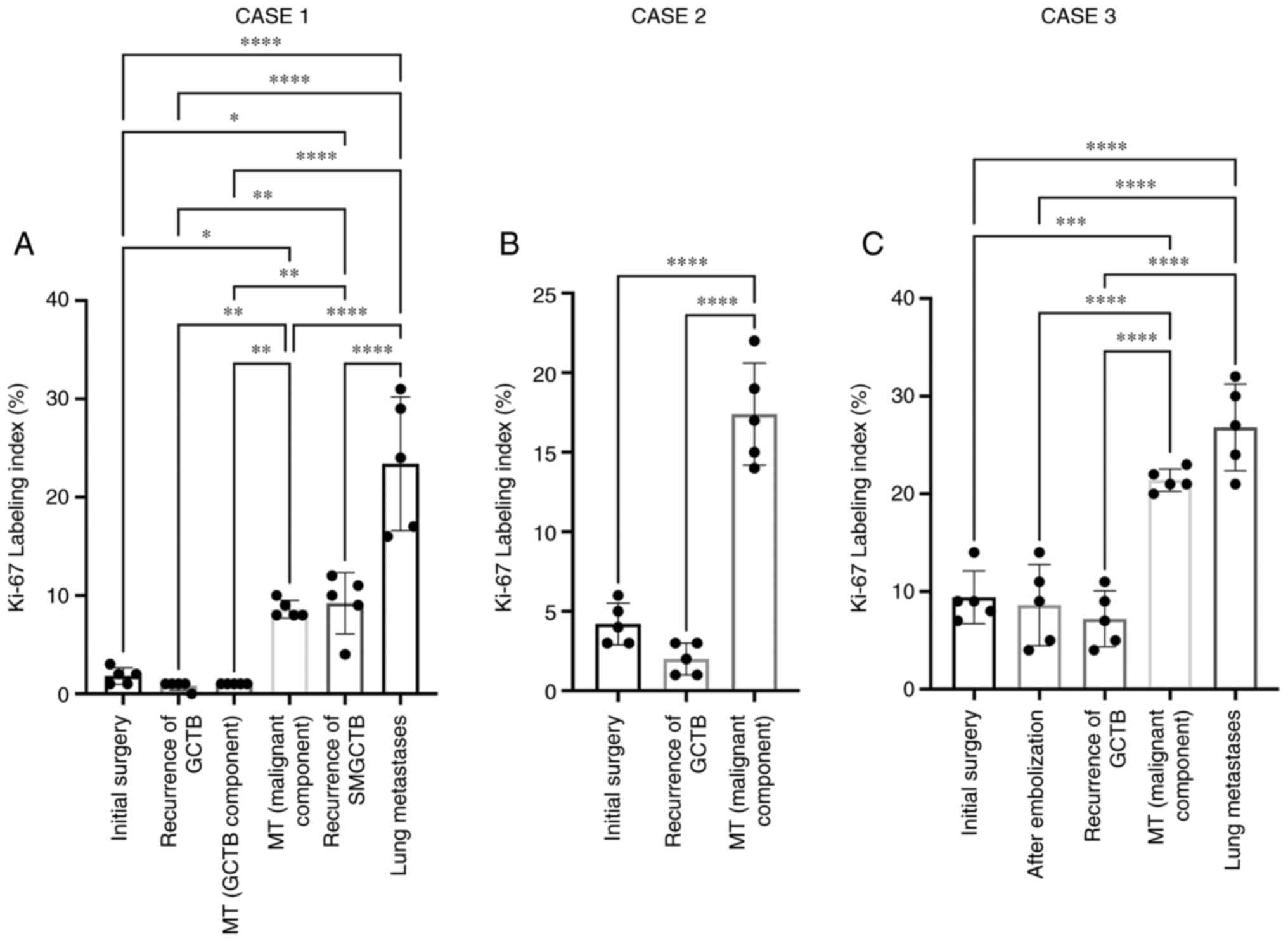
immunostaining. The Ki67 labeling index increased according to
malignant transformation. Histologically, the Ki67 labeling index
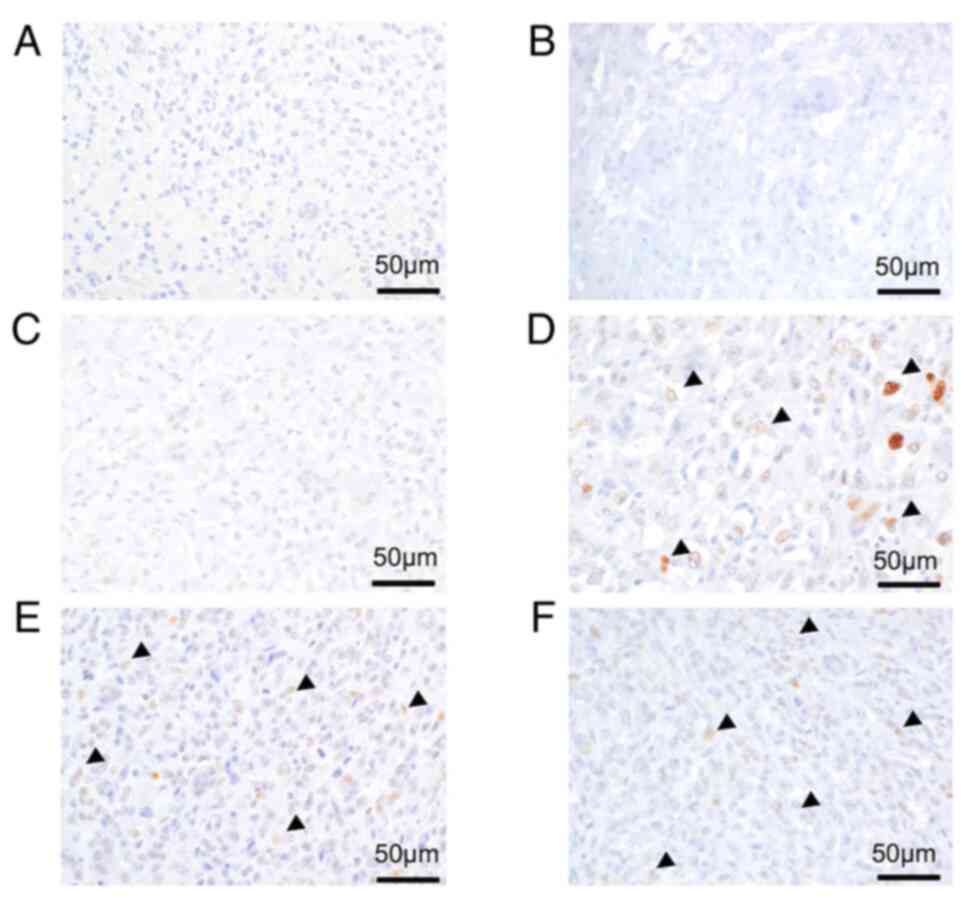
was low in primary and recurrent conventional GCTB (Fig. 4A and B) and the conventional GCTB
component of SMGCTB (Fig. 4C).
Meanwhile, it increased in the malignant component of SMGCTB
(Fig. 4D) and recurrent and
metastatic lesions (Fig. 4E and
F). For example, the benign and malignant areas of case 1
displayed significantly different Ki67 labeling indices (Fig. 5). In the remaining two cases of
SMGCTB, the index also increased significantly according to
malignant changes (Fig. 5; Case 2
and 3, Fig. S1).
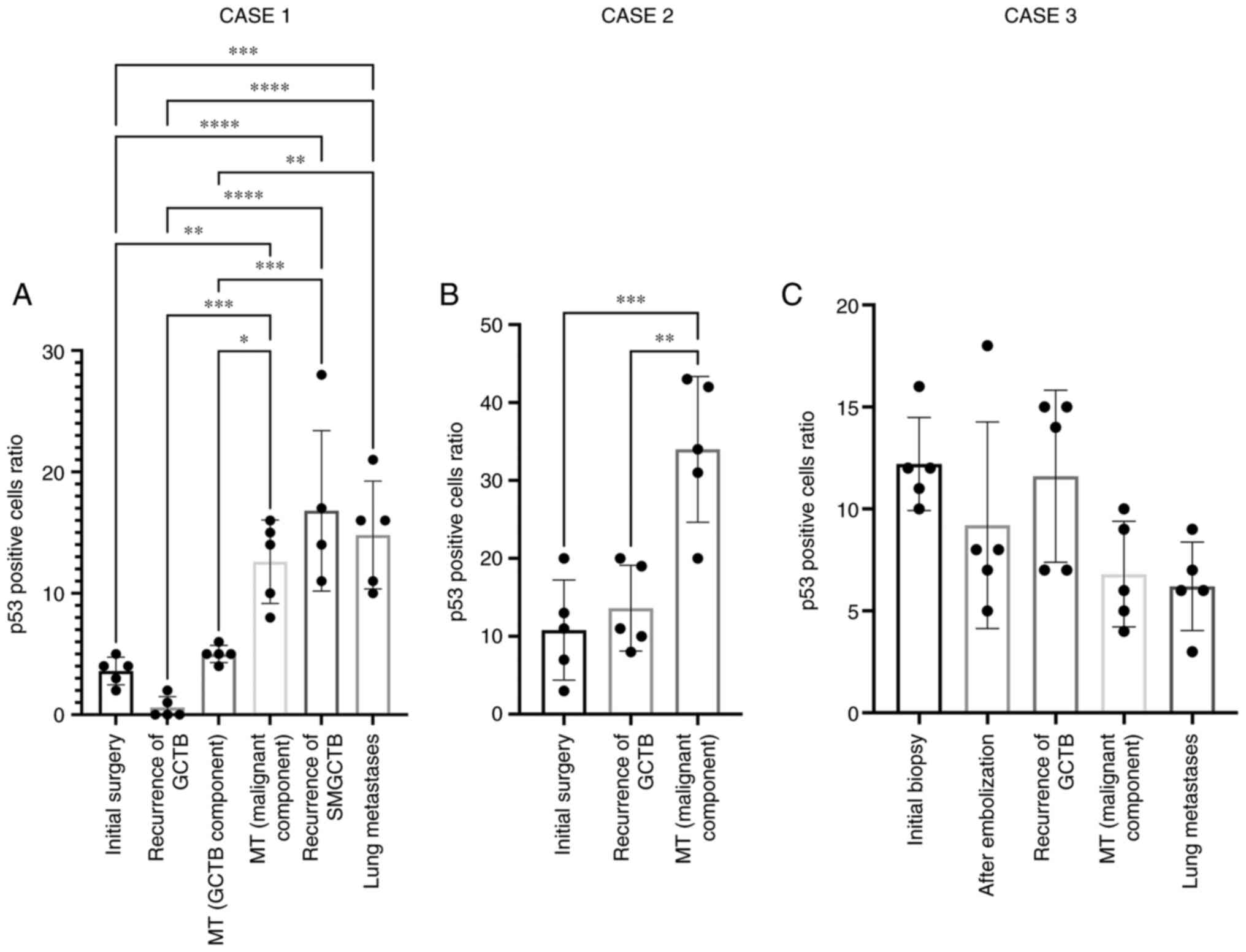
In case 1, the p53 expression level increased with
malignant transformation. Specifically, p53 expression was very low
in primary and recurrent (Fig. 6A and
B) conventional GCTB. However, p53 expression level increased
at malignant area of third surgery specimen of case 1 (Fig. 6D), although it was low in
conventional GCT area in the same specimen (Fig. 6C). Interestingly, p53 expression
pattern changed according to malignant transformation in the same
specimen. And high expression levels were maintained in recurrent
and metastatic lesions (Fig. 6E and
F) of SMGCTB. Additionally, cell count quantification revealed
that p53 expression levels were significantly different before and
after malignant transformation (Fig.
7). Of particular importance was the significant change of p53
expression in MT (GCTB component) and MT (malignant component) in
case 1, even though they were in the same sample. And after
malignant transformation in case 2, p53 expression levels were
significantly increased (Fig.
S2A-C). However, no significant change in p53 expression was
observed in patient 3 who received denosumab treatment (Fig. S2D-H).
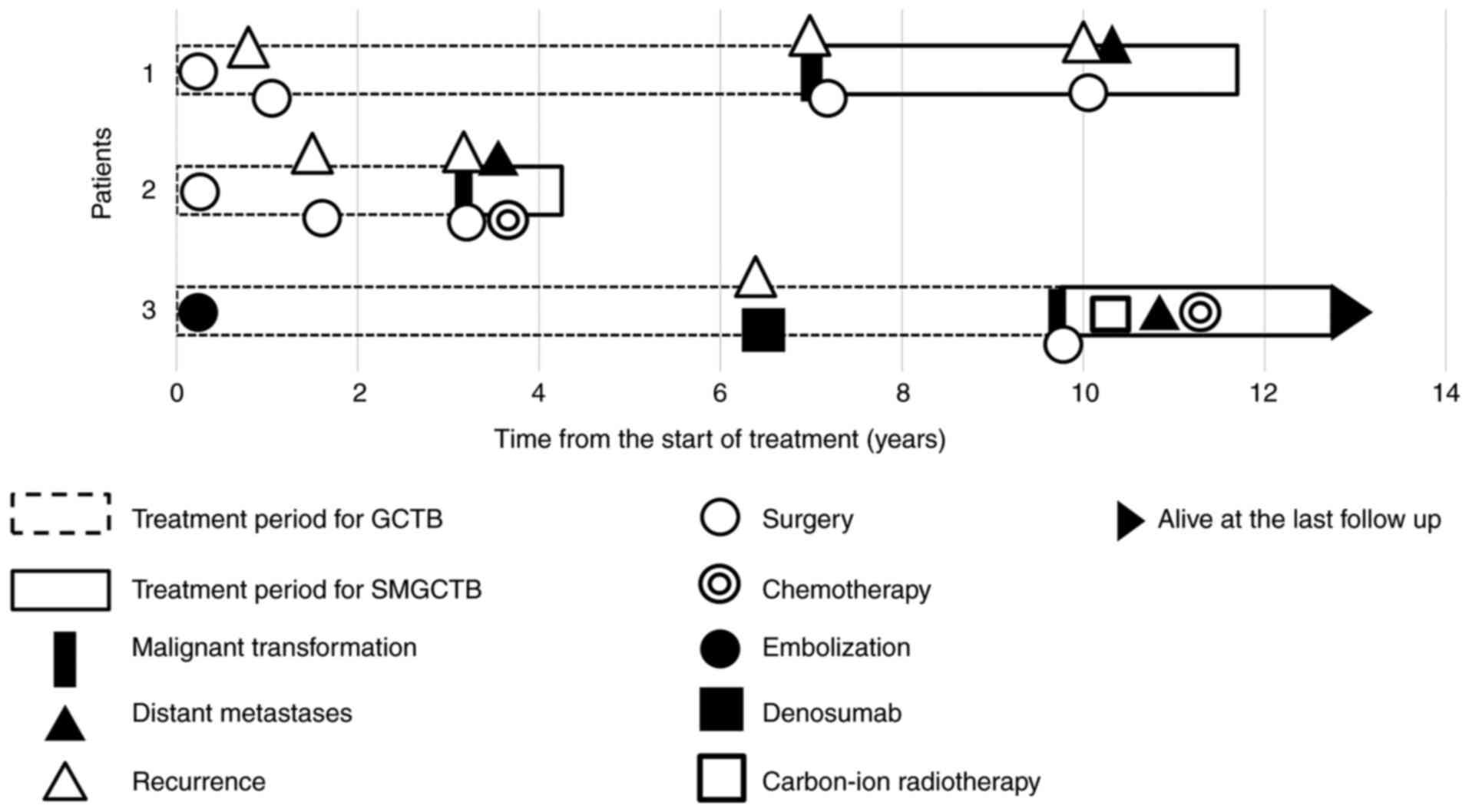
Survival
All three patients with SMGCTB experienced local
recurrence after 1, 1.5, or 36 months (Fig. 8). All patients developed distant
metastasis to the lungs. Two patients also exhibited bone
metastasis, and one patient developed heart metastasis. Two
patients died 13 and 54 months after being found to have malignant
transformation. One patient was alive at the last follow-up 3 years
later.
Case report
Case 1
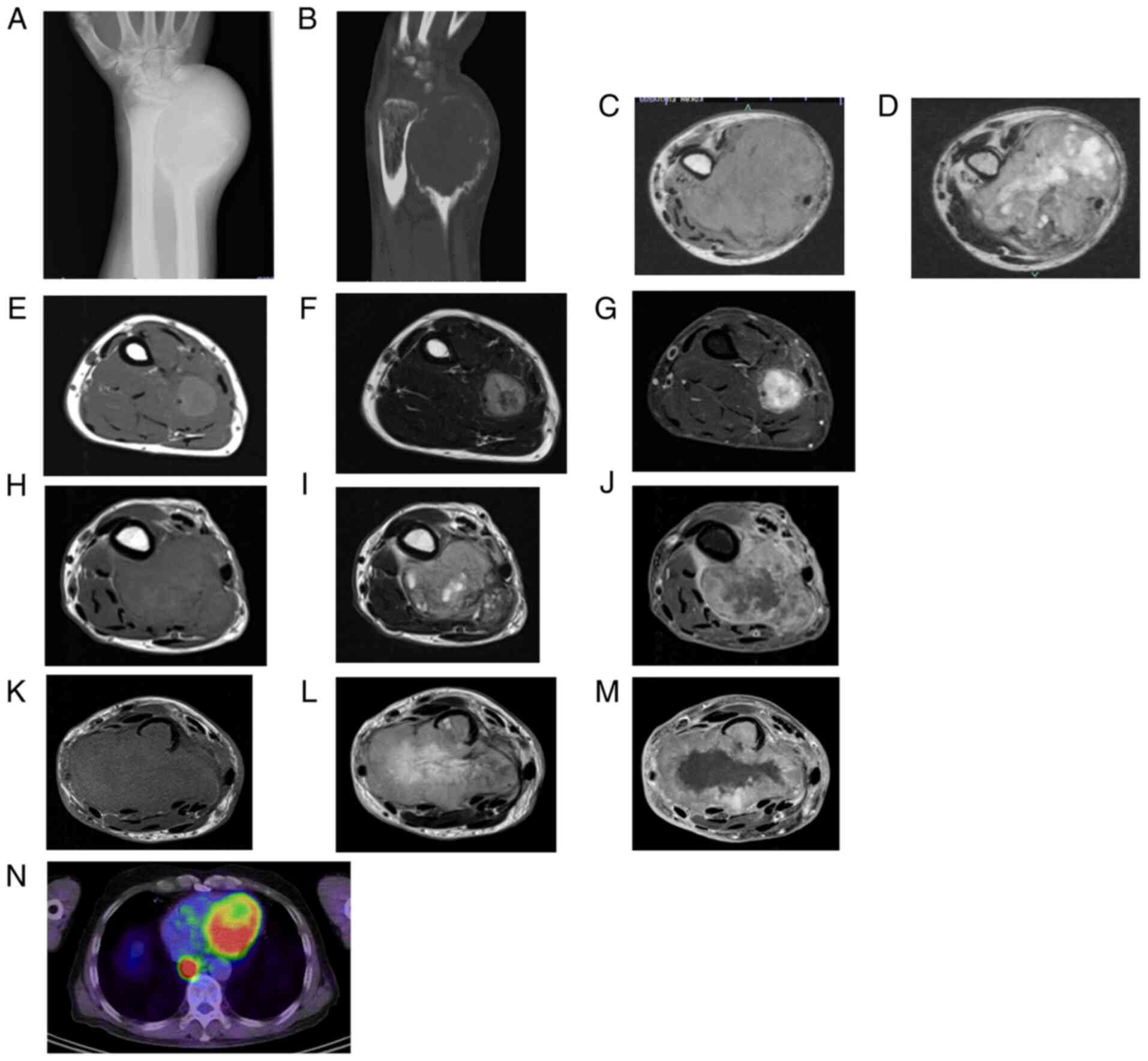
The patient was a 61-year-old man with GCTB in the
left distal ulna. At first presentation, plain radiographs
demonstrated a lytic lesion in the distal ulna (Fig. 9A). CT revealed an expanded,
thinned, and partially discontinuous cortex (Fig. 9B). MRI revealed a large lesion of
the distal ulna with low intensity on T1-weighted images (Fig. 9C) and high intensity on T2-weighted
images (Fig. 9D). An open biopsy
revealed conventional GCTB. The tumor was marginally resected.
Eleven months post-operation, local recurrence was observed in the
forearm. MRI revealed a mass with low intensity on T1-weighted
images (Fig. 9E) and high
intensity on T2-weighted images (Fig.
9F) and uniform enhancement on gadolinium-enhanced images
(Fig. 9G). The lesion was resected
and histologically determined to be conventional GCTB. Seven years
after the primary surgery, MRI revealed a large mass in the left
forearm with low intensity on T1-weighted images (Fig. 9H) and high intensity on T2-weighted
images (Fig. 9I) and heterogeneous
enhancement on gadolinium-enhanced images (Fig. 9J). The lesion was resected and
histologically determined to be malignant transformation of GCTB.
Ten years after the primary surgery, MRI revealed the same findings
as those from 7 years post-operation (Fig. 9K-M). Forearm amputation was
performed, and the lesion was histologically diagnosed as recurrent
SMGCTB. Subsequent FDG PET-CT demonstrated a nodular opacity with
high FDG uptake in the lung, suggesting metastasis (Fig. 9N). The patient underwent
metastasectomy of the lung metastasis. After 4 months, chest CT
revealed multiple nodular opacities in both lungs, suggesting
metastasis. The patient refused treatment and died 11 months
later.
Case 2
The patient was a 25-year-old man with GCTB in the
left distal femur. Open biopsy revealed conventional GCTB, and
curettage and bone grafting were performed. At the 18-month
follow-up, local recurrence was revealed. Curettage and bone
grafting were performed, and the lesion was histologically
determined to be conventional GCTB. Three years after the primary
surgery, CT revealed a lytic lesion at the site of operation. The
lesion was curetted and revealed to be SMGCTB. Subsequent chest CT
revealed multiple lung metastasis. The patient underwent below-knee
amputation. He also received adjuvant chemotherapy with high-dose
methotrexate (10 g/m2), cisplatin (120
mg/m2), adriamycin (60 mg/m2), and ifosfamide
(14 g/m2). However, this treatment had no effect and the
patient died 13 months after the development of SMGCTB.
Case 3
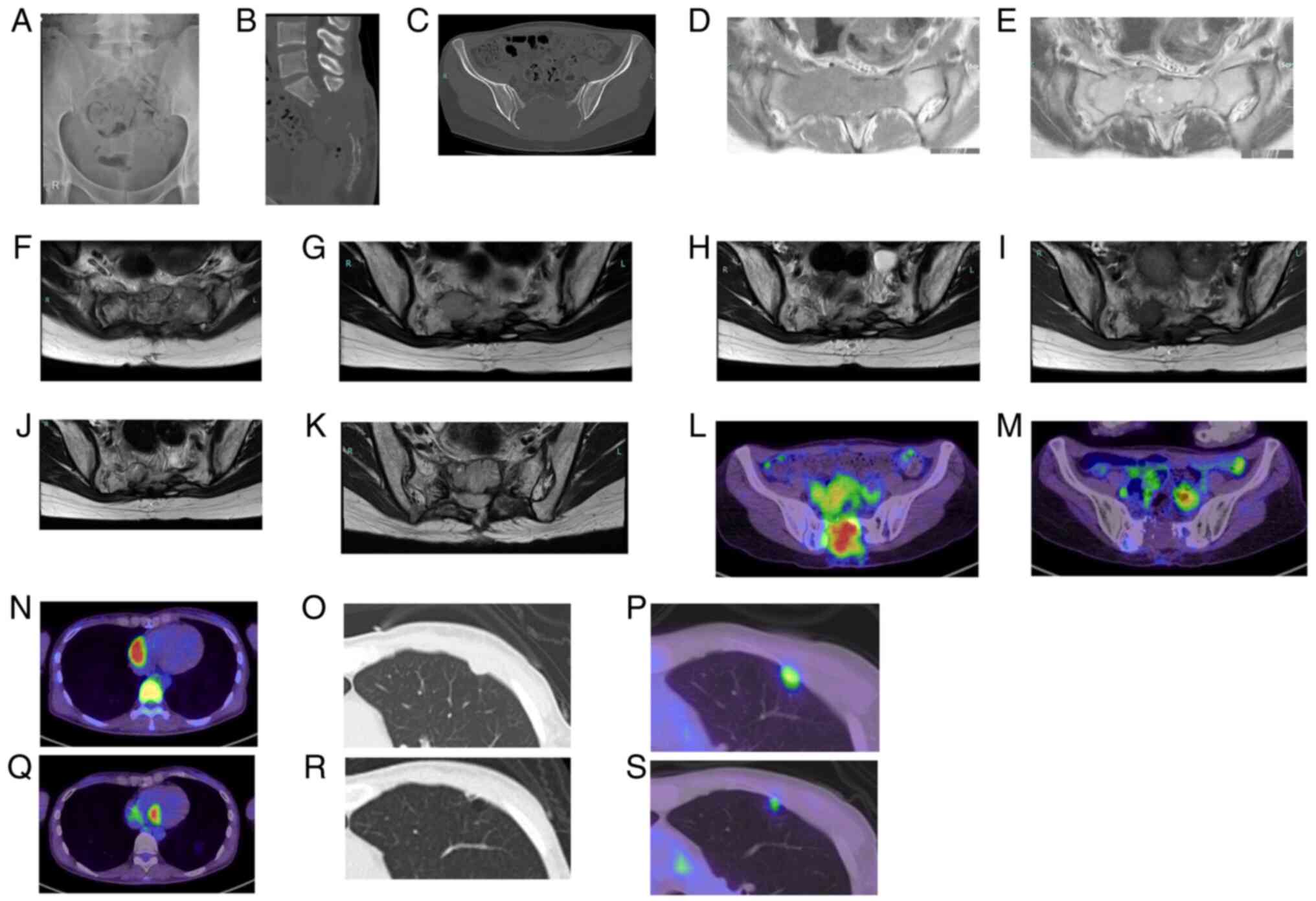
A 24-year-old woman was referred to our institution
for further examination after undergoing minimal curettage for
conventional GCTB at another hospital. Radiographs (Fig. 10A) and CT (Fig. 10B and C) showed a large mass in
the sacrum displaying cortical destruction and extensive soft
tissue involvement. MRI revealed a large lesion in the sacrum with
low intensity on T1-weighted images and high intensity on
T2-weighted images (Fig. 10D and
E). Intra-arterial embolization was performed using femoral
access to selectively embolize the main arteries feeding the tumor.
Post-embolization, the patient experienced no recurrence, with
intermittent complaints of pain. MRI revealed tumor shrinkage
(Fig. 10F). Additionally, the
tumor showed stable disease throughout the next 6 years. However,
6.5 years after the initial embolization, follow-up MRI revealed
enlargement of the soft tissue mass adjacent to the sacrum, which
caused suspicion of recurrence (Fig.
10G). CT-guided biopsy confirmed the presence of recurrent
benign GCTB. The patient was started on subcutaneous denosumab (120
mg monthly). A reduction in tumor size was observed, and imaging
over the next 2 years showed stable disease (Fig. 10H). However, a follow-up MRI scan
revealed enlargement of the soft tissue mass adjacent to the sacrum
with low intensity on T1-weighted images and high intensity on
T2-weighted images (Fig. 10I and
J), suggestive of regrowth. CT-guided biopsy confirmed the
presence of benign GCTB. Denosumab therapy was discontinued and the
lesion gradually enlarged and excised, revealing malignant
transformation to osteosarcoma. Recurrence was noted 1.5 months
later (Fig. 10K). PET-CT
demonstrated high FDG uptake in the tumor (SUVmax, 7.0) (Fig. 10L). Carbon-ion RT was performed,
and local control had been obtained for >3 years at the time of
last follow-up (Fig. 10M). Nine
months after the diagnosis of malignant transformation, CT revealed
multiple lung metastasis. Subsequent PET-CT demonstrated a mass in
the right atrium (Fig. 10N) and
nodular opacities in bilateral lungs through high FDG uptake
(Fig. 10O and P), suggesting
metastasis. She underwent stereotactic radiosurgery of the right
atrium and received pazopanib (800 mg). The treatment was
effective, and tumor shrinkage and decreased FDG uptake were
observed in the metastatic lesions of right atrium (Fig. 10Q) and lung (Fig. 10R and S). She received clinical
cancer genomic profiling utilizing the excised specimen of the
sacrum and the following CN alterations were detected:
CDKN2A (CN=0), CDKN2B (CN=0), and MTAP (CN=0).
The microsatellite instability status was stable, and the tumor
mutation burden score was calculated to be 1.26. One lung
metastasis regrowth was excised. The patient continued pazopanib
treatment, and the other lesions were found to have remained stable
at the last follow-up (3 years after the development of malignant
transformation).
Discussion
The occurrence rate of SMGCTB after surgery among
GCTB patients without prior RT treatment was reported to be
0.5-1.5% (8,18). We showed an occurrence rate of 4%.
We performed a brief literature review using the PubMed database to
identify other published cases and describe the clinical
characteristics of SMGCTB (8–31).
Less than 150 cases were included, and the clinical characteristics
of these cases are summarized in Table SI. Furthermore, Picci et al
reported that malignant transformation develops at a median of 22
years (range: 7–28) after primary surgery (24). Moreover, Bertoni et al
reported a median latent period of 19 years (range: 7–28) (20). However, Liu et al reported a
shorter median latent period of only 5.1 years (range: 0.5-25.6)
(30). Additionally, Tsukamoto
et al reported a longer interval from the last surgery to
local recurrence with malignant transformation (median 15.2 years)
than from the last surgery to conventional GCTB (median 1.3 years)
(31). They also reported that
multivariate analysis showed local recurrence to be an independent
risk factor for unfavorable malignant transformation. In line with
these studies, two of the three patients in the current study
experienced recurrence of conventional GCTB, and the interval from
the start of primary treatment to the development of malignant
transformation was 3 and 7 years in these two patients. Therefore,
tumor specimens should be carefully inspected for malignant
transformation in patients with late recurrences and a history of
multiple recurrences.
Recently, several reports have described cases of
malignant transformation of GCTB during or after denosumab therapy
(6,7). We performed a brief literature review
using the PubMed database to identify other published cases and
describe the clinical characteristics of SMGCTB treated with
denosumab. Less than 20 cases were included, and the clinical
characteristics of these cases are summarized in Table SII (6,7,18–23).
Almost all of these patients developed SMGCTB 6 months to 4 years
after starting denosumab therapy. In a phase II study evaluating
the clinical benefits of monthly denosumab treatment in 37 patients
with recurrent or unresectable GCTB, Thomas et al reported
two patients who developed malignant transformation: one patient
during denosumab treatment and the other 8 months after
discontinuing denosumab (6).
Chawla et al also reported five cases (1%) of SMGCTB in a
phase II study showing the clinical benefits of denosumab treatment
in 532 patients with GCTB (7). In
the current study, 1 of 22 patients (4.5%) treated with denosumab
developed SMGCTB. GCTB is characterized by stromal cells expressing
RANKL and osteoclast-like giant cells expressing RANK (4,5).
Denosumab binds to RANKL, substantially reducing or eliminating
osteoclast-like giant cells (4,5).
RANKL also plays an important role in lymphocyte differentiation
and upregulates nuclear factor IB, a transcription factor that
reduces susceptibility to nuclear oncogenes. Thus, inhibition of
RANKL could increase the risk of malignancy through
immunosuppression and increase susceptibility to nuclear oncogenes
(32). However, patients treated
with denosumab therapy likely have a history of multiple
recurrences and long-term treatments, which can lead to a higher
baseline risk for malignant transformation. Thus, additional
controlled studies and long-term follow-up are needed before
drawing definitive conclusions regarding the direct correlation
between denosumab and malignant transformation.
Histologically, we found that lesions of malignant
transformation were constructed by two components: the conventional
GCTB component and the malignant component with features of
osteosarcoma. This finding indicates that primary GCTB replaces the
malignant component after transformation over time. Additionally,
we found that the Ki67 labeling index increased according to
malignant transformation. The Ki67 labeling index was low in
primary and recurrent conventional GCTB and in the conventional
GCTB component of SMGCTB. Meanwhile, the Ki67 index increased in
all malignant areas, especially metastatic lesions. Ki67 is a
nuclear protein found in proliferating cells, and its index is
usually high in aggressive tumors; thus, it is regarded as a poor
prognostic factor (44). In this
study, we found that Ki67 labeling index increased with each event,
such as malignant transformation and metastasis, suggesting that
the tumor acquires a greater ability to proliferate.
Recently, several studies had been revealed the
possible mechanism of malignant transformation of GCTB without
previous RT (18,41–44).
In these studies, mutations or LOH of p53 as well as p53
overexpression were found in malignant cases, though no p53
mutation nor overexpression was shown in the primary GCT (18,41–44).
Okubo et al investigated the p53 mutations and
expression of p53 in samples of SMGCTB and conventional GCTB in two
patients. They found mutations of p53 and p53 overexpression
in both patients with SMGCTB who received curettage for primary
GCTB. However, no p53 mutation nor overexpression was shown in the
primary GCT (42). Similarly, Oda
et al reported a case of SMGCTB in which point mutation of
p53 and p53 nuclear accumulation was observed in the
atypical stromal cells of the SMGCTB, whereas no p53
mutation and no p53 nuclear accumulation was observed in stromal
cells in the primary GCT (18).
More recently, Ishihara et al performed next generation
sequencing (NGS) and immunohistochemical analysis of SMGCTB. NGS of
two SMGCTB revealed pathogenic mutations in TP53 and several other
genes in both patients (45).
Furthermore, three of four SMGCTB were immunohistochemically
positive for p53. These results suggested that p53
alteration may play an important role in the malignant progression
of GCTB. Interestingly, we found that p53 expression significantly
increased when lesions underwent malignant transformation in
patients who underwent curettage and bone graft. On the other hand,
p53 expression was low in the primary and recurrent samples.
However, high expression of p53 was also maintained in metastatic
lesions. Numerous cases have been reported the secondary malignancy
of bone and soft tissue tumor associated with mutation of p53
(46). Furthermore, the
association between p53 mutations and tumorigenesis has been widely
investigated in many malignancies (47,48).
Molecular mechanisms of mutant p53 include; i) Mutant p53 interacts
with DNA directly using mutant p53 binding elements or other
regions on the DNA to regulate transcription. ii) Mutant p53
enhances transcription by forming a complex with transcription
factors that can include transcriptional cofactors and other
proteins. iii) Mutant p53 decreases transcription by binding
transcription factors and/or transcriptional cofactors and other
proteins, sometimes preventing their binding to DNA. iv) Mutant p53
interacts with other proteins, not directly involved in
transcriptional regulation, and enhances or blocks their function
(47,48). It has been found that p53 may play
a role in the malignant transformation of GCTB in patients who
undergo curettage. However, p53 overexpression was not observed in
the patient who received denosumab treatment. In this patient, we
performed clinical cancer genomic profiling using the
FoundationOne®CDx assay containing 309 genes to identify
potential driver genes of SMGCTB specific for denosumab treatment.
We detected copy number alterations in CDKN2A (CN=0),
CDKN2B (CN=0), and MTAP (CN=0). CDKN2A encodes
two unrelated tumor suppressor proteins, p16INK4a and p14ARF,
whereas CDKN2B encodes the tumor suppressor protein,
p15INK4b (49). Both p15INK4b and
p16INK4a inhibit CDK4 and CDK6, thereby maintaining the
growth-suppressive activity of the Rb tumor suppressor. Therefore,
the loss of p15INK4b and p16INK4a leads to dysregulation of the
CDK4/6-cyclin-Rb pathway and loss of cell cycle control (49). Moreover, the tumor suppressive
functions of p14ARF involve stabilization and activation of p53 via
MDM2 inhibition. The loss of CDKN2A and CDKN2B can
lead to tumorigenesis through uncontrolled proliferation. Finally,
the mechanism of malignant transformation induced by denosumab can
be different from that of due to surgery. The correlation between
denosumab and malignant transformation should be investigated in
additional controlled studies with long-term follow-up.
There are numerous reports that 91–95% of GCTB
harbour pathogenic H3F3A mutation, which may be a driver for
tumorgenesis of GCTB (50,51). Gong L reported that H3F3A
mutations was found in 95% of GCTB, including glycine 34 to
tryptophan (G34W, 91%), glycine 34 to leucine (G34L, 2%), glycine
34 to valine (G34V, 1%), and glycine 34 to arginine (G34R, 1%) by
DNA sequencing analysis (50).
H3F3A mutation is characteristic of GCTB and shown to be
useful for the diagnosis of GCTB and differential diagnosis from
other bone tumors (50,51). Recently, several studies revealed
the absence of H3F3A G34W mutation in malignant giant cell
tumor of bone, though it was present in the associated giant cell
tumor tissues (38,52). Yoshida et al investigated
H3F3A G34W mutation in seven SMGCTB following surgery for
conventional GCTB using a combination of immunohistochemical and
molecular methods (Sanger sequencing and pyrosequencing or next
generation sequencing) (52). They
found that 5 of 7 patients had absence of H3F3A G34W
mutation in malignant giant cell tumor of bone, though it was
present in the associated giant cell tumor tissues. Potential
interpretations for this discordant mutation status include: i)
incidental coexistence of two genetically distinct independent
tumors; ii) clonal replacement, with a minor population of
preexisting H3F3A G34-wild-type clone in giant cell tumor of bone
outgrowing an H3F3A-mutant clone; and iii) loss of H3F3A mutation
during linear clonal evolution. Hasenfratz et al analysed
the samples of 2 patients with H3F3A-mutated GCTBs before and after
denosumab treatment by histomorphology, immunohistochemistry, and
next generation panel sequencing (38). The initial GCTB in the biopsy and
in the recurrence was H3F3A-mutated, while the sarcoma was negative
for this mutation as shown by sequencing and immunohistochemical
staining. Sequencing revealed a persisting H3F3A mutation in one
patient while the other lost the H3F3A mutation after malignant
transformation. They speculated that one explanation is a
transformation of the H3F3A-negative mononuclear cells residing in
the tumor after denosumab treatment. In this study, we did not find
H3F3A G34W mutation in SMGCTB by cancer genomic profiling.
Although we did not investigate the genomic profiling of the
primary GCTB lesion, H3F3A G34W mutation in this patient can
be lost during progression to malignant transformation.
Although surgery is the standard of care for extreme
SMGCTB, its issue is limited for patients with SMGCTB of the spine
or sacrum due to the complex anatomical structures and neurological
dysfunction associated with surgery to these locations. Yin et
al reported that three out of six SMGCTB cases demonstrated
local recurrence in the spine (28). Postoperative RT was performed in
two cases; both patients experienced recurrence, denying the
effectiveness of RT (28). In our
literature review, four cases utilized RT, however, no information
regarding its effectiveness was reported (11,16,19,31).
Recently, carbon-ion RT has been proven to be effective in patients
with unresectable sarcoma of the sacrum (53). Additionally, we utilized carbon-ion
RT in the patient with SMGCTB in the sacrum and achieved good and
prolonged local control for >3 years. In this patient (case 3),
conventional RT also proved to be effective for metastatic lesions.
Thus, the role of RT, including carbon-ion RT for sacral SMGCTB
should be further investigated.
Clinical outcomes of SMGCTB are poor, with local
recurrence rates of 20–50% and distant metastasis rates of 22–80%.
Liu et al reported that in a total of 20 cases of SMGCTB,
local recurrence occurred in 4 patients (20%), and 16 patients
(80%) developed metastasis [lung (all cases), brain (1 case), and
bone (2 cases)] (30). They also
reported that 14 patients died before the last follow-up, with a
5-year OS rate of 40%. In our review, most patients with metastasis
had poor survival and died within 12 months. In this study, we also
identified that all patients developed distant metastasis,
including to the lung and bone, and two out of three patients died
before the last follow-up.
There is no consensus regarding optimal treatment
for SMGCTB due to its low incidence. Current treatment strategies
include surgery alone or surgery combined with chemotherapy and RT.
Moreover, Liu et al reported that local recurrence occurred
in 7 of 9 cases with inadequate margins and in 5 of 24 cases with
adequate margins (P=0.006) in patients with PMGCTB and SMGCTB
(30). Thus, resection should be
performed with wide margins. However, patients have often
previously undergone multiple surgeries with curettage and bone
grafting, which leads to difficulty in limb sparing. In some
studies of various series of SMGCTB cases, the rate of amputation
was as high as 33–66% (16,24,30,31).
Our two patients with extremity SMGCTB also underwent amputation
for local control.
There are limited data regarding the role of
systemic treatment in patients with SMGCTB (15,17).
The role of chemotherapy for SMGCTB is controversial, and there is
still insufficient evidence of survival benefits (15,17).
Some authors used high-dose methotrexate, cisplatin, doxorubicin
(MAP), and ifosfamide, and other used cisplatin and doxorubicin
(AP) regimen similar to those used to treat osteosarcoma (15,17,18).
Based on our review, we could not conclude the utility of
chemotherapy because there is very little information about the
regimen and its effectiveness against SMGCTB; only two case reports
have described the effect of chemotherapy (15,17).
First, Hefti et al utilized an MAP regimen for a patient
with SMGCTB in the tibia with lung metastasis, but the patient
experienced progressive disease and died 9 months later (15). Second, Mori et al utilized
four cycles of an AP regimen for localized SMGCTB in the tibia
preoperatively, which resulted in shrinkage of the extraskeletal
mass and ossification (17). The
patient later underwent resection and prosthetic replacement and
was disease-free at the last follow-up. In the current study, we
used chemotherapy in two patients who had distant metastasis. One
patient received MAP and ifosfamide. However, the treatment had no
effect and the patient died 13 months after SMGCTB development. The
other patient received pazopanib, a multi-kinase inhibitor, which
was effective, and the patient was alive for >3 years after the
development of SMGCTB. Although the histology of SMGCTB is similar
to that of osteosarcoma, the effect of the MAP regimen, which is
the standard chemotherapy for osteosarcoma, is limited in SMGCTB.
Therefore, a new regimen for SMGCTB should be developed.
This study has several limitations. First, there was
a small sample size of only three patients. However, this
limitation is common in the study of patients with SMGCTB, because
it is an extremely rare malignancy. Second, we did not provide the
figure of Western blotting. Since the GCTB and SMGCTB were bone
tumors, we performed decalcification procedures. Decalcification
methods employed hydrochloric acid to dissolve the calcium salts,
which also might damage the protein of the samples. Then, we found
that no band was detectable by samples from the formalin-fixed
paraffin-embedded tissues. Another limitation is the inherent bias
in the choice of treatment. Since standard treatment has not been
established, we believe that multicenter studies will be necessary
in the future.
In conclusion, high expression of p53 was found in
SMGCTB, but not in conventional GCTB, and can be associated with
tumorigenesis. The correlation between denosumab and malignant
transformation should be investigated in additional controlled
studies and long-term follow-up. The clinical behavior of SMGCTB is
extremely aggressive, resulting in metastasis in all patients. New
emerging treatments using molecular targeted therapy and carbon-ion
RT should be further investigated to improve the clinical outcomes
of SMGCTB.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
EN, HoK, HI and TO designed the study, and
collected and analyzed data. EN and TK confirm the authenticity of
all the raw data. TK, TF, HaK, TI and MF analyzed data. EN, TK and
TF treated the patients presented in this manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective chart review study involving
human participants was conducted in accordance with the ethical
standards of the institutional and national research committee and
with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The Human Investigation Committee
(IRB) of Okayama University Hospital approved this study (approval
no. K 2103-040).
Patient consent for publication
Written informed consent was obtained from each
participant included in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basu Mallick A and Chawla SP: Giant cell
tumor of bone: An update. Curr Oncol Rep. 23:512021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Errani C, Tsukamoto S, Ciani G and Donati
DM: Present day controversies and consensus in curettage for giant
cell tumor of bone. J Clin Orthop Trauma. 10:1015–1020. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsukamoto S, Mavrogenis AF, Kido A and
Errani C: Current concepts in the treatment of giant cell tumors of
bone. Cancers (Basel). 13:36472021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta A, Durocher-Allen L, Popovic S,
Tozer R, Yao X and Ghert M: The role of denosumab for surgical
outcomes in patients with giant cell tumour of bone: A systematic
review. Curr Oncol. 28:1302–1313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Gao J, Gao Y, Lin N, Zheng M and Ye
Z: Denosumab in giant cell tumor of bone: Current status and
pitfalls. Front Oncol. 10:5806052020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chawla S, Blay JY, Rutkowski P, Le Cesne
A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger
L, et al: Denosumab in patients with giant-cell tumour of bone: A
multicentre, open-label, phase 2 study. Lancet Oncol. 20:1719–1729.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palmerini E, Picci P, Reichardt P and
Downey G: Malignancy in giant cell tumor of bone: A review of the
literature. Technol Cancer Res Treat. 18:15330338198400002019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy WR and Ackerman LV: Benign and
malignant giant-cell tumors of bone; a clinical-pathological
evaluation of thirty-one cases. Cancer. 9:317–339. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mnaymneh WA, Dudley HR and Mnaymneh LG:
Giant-cell tumor of bone: An analysis and follow-up study of the
forty-one cases observed at the Massachusetts general hospital
between 1925 and 1960. J Bone Joint Surg Am. 46:63–75. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dahlin DC, Cupps RE and Johnson EW:
Giant-cell tumor: A study of 195 cases. Cancer. 25:1061–1070. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sundaram M, Martin AS and Tayob AA: Case
report 182: Osteosarcoma arising in giant cell tumor of tibia.
Skeletal Radiol. 7:282–285. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rock MG, Sim FH, Unni KK, Witrak GA,
Frassica FJ, Schray MF, Beabout JW and Dahlin DC: Secondary
malignant giant-cell tumor of bone. Clinicopathological assessment
of nineteen patients. J Bone Joint Surg Am. 68:1073–1079. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gitelis S, Wang JW, Quast M, Schajowicz F
and Templeton A: Recurrence of a giant-cell tumor with malignant
transformation to a fibrosarcoma twenty-five years after primary
treatment. A case report. J Bone Joint Surg Am. 71:757–761. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hefti FL, Gächter A, Remagen W and
Nidecker A: Recurrent giant-cell tumor with metaplasia and
malignant change, not associated with radiotherapy. A case report.
J Bone Joint Surg Am. 74:930–934. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anract P, De Pinieux G, Cottias P,
Pouillart P, Forest M and Tomeno B: Malignant giant-cell tumours of
bone. Clinico-pathological types and prognosis: A review of 29
cases. Int Orthop. 22:19–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori Y, Tsuchiya H, Karita M, Nonomura A,
Nojima T and Tomita K: Malignant transformation of a giant cell
tumor 25 years after initial treatment. Clin Orthop Relat Res.
381:185–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oda Y, Sakamoto A, Saito T, Matsuda S,
Tanaka K, Iwamoto Y and Tsuneyoshi M: Secondary malignant
giant-cell tumour of bone: Molecular abnormalities of p53 and H-ras
gene correlated with malignant transformation. Histopathology.
39:629–637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marui T, Yamamoto T, Yoshihara H, Kurosaka
M, Mizuno K and Akamatsu T: De novo malignant transformation of
giant cell tumor of bone. Skeletal Radiol. 30:104–108. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertoni F, Bacchini P and Staals EL:
Malignancy in giant cell tumor of bone. Cancer. 97:2520–2529. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashimoto K, Hatori M, Hosaka M, Watanabe
M, Hasegawa T and Kokubun S: Osteosarcoma arising from giant cell
tumor of bone ten years after primary surgery: A case report and
review of the literature. Tohoku J Exp Med. 208:157–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machinami R, Nishida K, Ishida T,
Matsumoto S, Kuroda K, Kobayashi M, Takeuchi K and Ishikawa Y:
Carcinosarcomatous malignancy, osteosarcoma and squamous cell
carcinoma, in giant cell tumor of the right distal femur. Pathol
Res Pract. 204:583–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller IJ, Blank A, Yin SM, McNickle A,
Gray R and Gitelis S: A case of recurrent giant cell tumor of bone
with malignant transformation and benign pulmonary metastases.
Diagn Pathol. 5:622010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Picci P, Sieberova G, Alberghini M,
Balladelli A, Vanel D, Hogendoorn PC and Mercuri M: Late sarcoma
development after curettage and bone grafting of benign bone
tumors. Eur J Radiol. 77:19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kadowaki M, Yamamoto S and Uchio Y: Late
malignant transformation of giant cell tumor of bone 41 years after
primary surgery. Orthopedics. 35:e1566–e1570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muramatsu K, Ihara K, Miyoshi T, Kawakami
Y, Nakashima D and Taguchi T: Late development of malignant fibrous
histiocytoma at the site of a giant cell tumour 38 years after
initial surgery. Acta Orthop Belg. 78:279–284. 2012.PubMed/NCBI
|
|
27
|
Li J, Zhu Y and Wei Y: Fibrosarcoma
development 15 years after curettage and bone grafting of giant
cell tumor of bone. Orthopedics. 37:e512–e516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin H, Cheng M, Li B, Li B, Wang P, Meng
T, Wang J, Zhou W, Yan W and Xiao J: Treatment and outcome of
malignant giant cell tumor in the spine. J Neurooncol. 124:275–281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takesako H, Osaka E, Yoshida Y, Sugitani M
and Tokuhashi Y: Secondary malignant giant cell tumor of bone due
to malignant transformation 40 years after surgery without
radiation therapy, presenting as fever of unknown origin: A case
report. J Med Case Rep. 10:472016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu W, Chan CM, Gong L, Bui MM, Han G,
Letson GD, Yang Y and Niu X: Malignancy in giant cell tumor of bone
in the extremities. J Bone Oncol. 26:1003342020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsukamoto S, Righi A, Mavrogenis AF,
Akahane M, Honoki K, Tanaka Y, Donati DM and Errani C: Late local
recurrence of bone giant cell tumors associated with an increased
risk for malignant transformation. Cancers (Basel). 13:36442021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alaqaili SI, Abduljabbar AM, Altaho AJ,
Khan AA and Alherabi JA: Malignant sarcomatous transformation of
benign giant cell tumor of bone after treatment with denosumab
therapy: A literature review of reported cases. Cureus.
10:e37922018.PubMed/NCBI
|
|
33
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase II trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broehm CJ, Garbrecht EL, Wood J and
Bocklage T: Two cases of sarcoma arising in giant cell tumor of
bone treated with denosumab. Case Rep Med. 2015:7671982015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aponte-Tinao LA, Piuzzi NS, Roitman P and
Farfalli GL: A high-grade sarcoma arising in a patient with
recurrent benign giant cell tumor of the proximal tibia while
receiving treatment with denosumab. Clin Orthop Relat Res.
473:3050–3055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park A, Cipriano CA, Hill K, Kyriakos M
and McDonald DJ: Malignant transformation of a giant cell tumor of
bone treated with denosumab: A case report. JBJS Case Connect.
6:e782016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsukamoto S, Righi A, Vanel D, Honoki K,
Donati DM and Errani C: Development of high-grade osteosarcoma in a
patient with recurrent giant cell tumor of the ischium while
receiving treatment with denosumab. Jpn J Clin Oncol. 47:1090–1096.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hasenfratz M, Mellert K, Marienfeld R, von
Baer A, Schultheiss M, Roitman PD, Aponte-Tinao LA, Lehner B,
Möller P, Mechtersheimer G and Barth TFE: Profiling of three
H3F3A-mutated and denosumab-treated giant cell tumors of bone
points to diverging pathways during progression and malignant
transformation. Sci Rep. 11:57092021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yung D, Asano N, Hirozane T, Yamaguchi S,
Mori T, Susa M, Okita H, Morioka H, Horiuchi K and Nakayama R:
Malignant transformation of metastatic giant cell tumor of bone in
a patient undergoing denosumab treatment: A case report. J Orthop
Sci. S0949-2658(21)00222-0. 2021.(Epub ahead of print). View Article : Google Scholar
|
|
40
|
Palmerini E, Seeger LL, Gambarotti M,
Righi A, Reichardt P, Bukata S, Blay JY, Dai T, Jandial D and Picci
P: Malignancy in giant cell tumor of bone: Analysis of an
open-label phase 2 study of denosumab. BMC Cancer. 21:892021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong L, Liu W, Sun X, Sajdik C, Tian X,
Niu X and Huang X: Histological and clinical characteristics of
malignant giant cell tumor of bone. Virchows Arch. 460:327–334.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okubo T, Saito T, Mitomi H, Takagi T,
Torigoe T, Suehara Y, Kaneko K and Yao T: p53 mutations may be
involved in malignant transformation of giant cell tumor of bone
through interaction with GPX1. Virchows Archiv. 463:67–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bieg-Bourne CC, Millis SZ, Piccioni DE,
Fanta PT, Goldberg ME, Chmielecki J, Parker BA and Kurzrock R:
Next-Generation sequencing in the clinical setting clarifies
patient characteristics and potential actionability. Cancer Res.
77:6313–6320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishihara S, Yamamoto H, Iwasaki T, Toda Y,
Yamamoto T, Yoshimoto M, Ito Y, Susuki Y, Kawaguchi K, Kinoshita I,
et al: Histological and immunohistochemical features and genetic
alterations in the malignant progression of giant cell tumor of
bone: A possible association with TP53 mutation and loss of H3K27
trimethylation. Mod Pathol. 35:640–648. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Movahedinia S, Shooshtarizadeh T and
Mostafavi H: Secondary malignant transformation of giant cell tumor
of bone: Is it a fate? Iran J Pathol. 14:165–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miranda Alcalde B, Villa Alcázar M,
Martínez Romera I and López Ibor B: The importance of Li-Fraumeni
syndrome, a hereditary cancer predisposition disorder. Arch Argent
Pediatr. 119:e11–e17. 2021.Mantovani F, Collavin L and Del Sal G:
Mutant p53 as a guardian of the cancer cell. Cell Death Differ 26,
199–212, 2019. PubMed/NCBI
|
|
48
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
González-Gil C, Ribera J, Ribera JM and
Genescà E: The Yin and Yang-like clinical implications of the
CDKN2A/ARF/CDKN2B gene cluster in acute lymphoblastic leukemia.
Genes (Basel). 12:792021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gong L, Bui MM, Zhang W, Sun X, Zhang M
and Yi D: H3F3A G34 mutation DNA sequencing and G34W
immunohistochemistry analysis in 366 cases of giant cell tumors of
bone and other bone tumors. Histol Histopathol. 36:61–68.
2021.PubMed/NCBI
|
|
51
|
Behjati S, Tarpey PS, Presneau N, Scheipl
S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, et
al: Distinct H3F3A and H3F3B driver mutations define
chondroblastoma and giant cell tumor of bone. Nat Genet.
45:1479–1482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yoshida KI, Nakano Y, Honda-Kitahara M,
Wakai S, Motoi T, Ogura K, Sano N, Shibata T, Okuma T, Iwata S, et
al: Absence of H3F3A mutation in a subset of malignant giant cell
tumor of bone. Mod Pathol. 32:1751–1761. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Imai R, Kamada T and Araki N; Working
Group for Bone, . Soft Tissue Sarcomas: Carbon ion radiation
therapy for unresectable sacral chordoma: An analysis of 188 cases.
Int J Radiat Oncol Biol Phys. 95:322–327. 2016. View Article : Google Scholar : PubMed/NCBI
|