Introduction
Invasive breast carcinoma (IBC) is the most common
neoplasia that affects women worldwide and is one of the leading
causes of cancer-related deaths in this gender (1). In Portugal, about 7,000 new cases of
female IBC occur annually, along with 1,800 deaths from the disease
(2).
It is widely established that classic features such
as TNM status (tumour size, lymph node involvement, distant
metastasis) and histological grade have a great influence on the
clinical course, prognosis and treatment strategies for IBC. Other
risk factors related to disease outcome have been reported, and
among them, the patient's age at diagnosis (3–6).
Despite being an uncommon disease (1), IBC diagnosed at a young age, when
compared to older patients, seems to have a more aggressive
biological behaviour. Indeed, younger patients show in general
tumours more likely to be of higher grade and detected at a more
advanced disease stage, consequently leading to a worse prognosis
(7–9). Conversely, most IBCs in the elderly
are low-grade and estrogen receptors-positive, consistent with more
favourable tumour biology and evolution (10).
In our institution, DNA ploidy and S-phase fraction
(SPF), measured by flow cytometry, are usually assessed, whenever
possible, in IBC patients to improve the panel of prognostic
factors. We have shown that these parameters provide significant
prognostic information that is biologically relevant and clinically
useful for the management of patients with IBC (11–13).
Although potentially related to IBC age-specific differences, the
impact of DNA flow cytometry on both extremes of the age spectrum
has not yet been fully investigated.
The present study aims to evaluate significant
differences in histopathological and molecular characteristics
between two subgroups of young (≤45 years) vs. older (≥75 years)
IBC patients, with emphasis on DNA flow cytometry data. We also
sought to analyse the influence of age on patients' survival, as
well as whether the age itself is suitable as a prognostic factor,
independently of the common clinicopathological parameters.
Materials and methods
Clinicopathological data
The whole series consisted of 219 patients (≤45
years: 103 patients; ≥75 years: 116 patients) with primary IBC,
diagnosed and treated at the Portuguese Institute of Oncology of
Lisbon between January 1992 and December 2017. The present cohort
was retrieved from a larger dataset that encompasses DNA flow
cytometry information. Beyond the pre-established selective option
concerning the age, patients' eligibility criteria included the
availability of unfixed fresh/frozen samples for DNA flow cytometry
and complete follow-up information. Moreover, patients had no
metastatic disease at the time of diagnosis and have not received
any type of neoadjuvant treatment. The local institutional ethical
committee approved the study. The histological type and
pathological staging were evaluated according to WHO classification
(14). Tumour differentiation was
assessed using the Nottingham grading system (15). Table
I shows the different forms of treatment in the two subgroups
of IBC patients. A higher proportion of young patients underwent
breast-conserving surgery, radiation therapy and chemotherapy,
while older patients were submitted mainly to mastectomy and
hormonal therapy. Only one patient in each group was treated with
trastuzumab.
 | Table I.Treatment modalities among young (≤45
years) and older (≥75 years) IBC patients in the series. |
Table I.
Treatment modalities among young (≤45
years) and older (≥75 years) IBC patients in the series.
| Variables | Young patients (≤45
years), n (%) | Older patients (≥75
years), n (%) | P-valuea |
|---|
| Type of
treatment |
|
|
|
|
Mastectomy | 62 (60.2) | 87 (75.0) | 0.028 |
|
Breast-conserving surgery | 40 (38.8) | 27 (23.3) | 0.019 |
|
Radiation therapy | 65 (63.1) | 54 (46.5) | 0.020 |
|
Chemotherapy | 79 (76.7) | 16 (13.8) | <0.001 |
|
Hormonal therapy | 41 (39.8) | 69 (59.5) | 0.006 |
|
Trastuzumab | 1 (0.97) | 1 (0.86) | NS |
Immunohistochemistry (IHC)
analyses
Estrogen receptors (ER) and progesterone receptors
(PR) were re-evaluated in the whole series by IHC analysis on
paraffin-embedded material using the peroxidase-indirect-polymer
with Ventana ultraview universal DAB detection kit ref 760–500
(Ventana Medical Systems, Inc., Tucson, USA). The primary
antibodies used were Ventana anti-estrogen receptor ref 790–4324
(SP1), and Ventana anti-progesterone receptor ref 790–2223 (1E2).
The assessment was unavailable in two and nine cases for ER and PR,
respectively. The results were recorded as the percentage of
positively stained (cut-off ≥1%) neoplastic cell nuclei (Figs. S1 and S2).
Ki67 index was re-assessed in the whole series on
paraffin-embedded material, using Ventana optiview DAB IHC
detection kit ref 760–700. The primary antibody used was anti-Ki67
(30–9) ref 790–4286, with antigen retrieval of
40 min CC1 94°C, and 28 min antibody incubation. The assessment was
unavailable in 66 cases, due mostly to missing paraffin blocks. A
pre-defined 20% cut-off point distinguished low (<20%) from high
(≥20%) proliferative tumours (16)
(Fig. S3).
Human epidermal growth factor receptor 2 (HER2)
expression was first determined by standardised IHC technique
(Ventana Pathway anti-HER-2/neu, clone 4B5, ref 790–2991) as a
screening test, and by silver in situ hybridization (SISH) (Ventana
Silver ISH plus Ventana Red ISH ref 760–512 + ref 760–516, and
Ventana Her2 dual ISH dna probe cocktail ref 800–6043) in all IHC
equivocal (2+) cases (17,18). HER2 positive status was defined by
protein overexpression (3+) (Fig.
S4) or gene amplification.
Surrogate IHC molecular classification was based on
ER and PR expression, Ki67 proliferation index and HER2 status,
including Luminal A, Luminal B, HER2 positive, Triple-negative, and
Triple-positive tumours.
DNA flow cytometry
Flow cytometric analysis was performed on
fresh/frozen representative tumour samples according to a technique
described previously (11,19). Briefly, the tissue samples were
mechanically disaggregated using scalpel blades in cold
phosphate-buffered saline (PBS). For DNA staining, the nuclei were
incubated with propidium iodide (Sigma) 50 µg/ml in Tris-Mg
Cl2 buffer, for one hour in the dark at 4°C, treated
with RNase (Sigma), 1 mg/ml in PBS, and 0.05% Nonidet P40 (Sigma).
Usually, a minimum of 20.000 nuclei were acquired and recorded on a
single parameter, 256 channel integrated fluorescence histogram.
The Multicycle software program (Phoenix Flow Systems, San Diego,
CA, USA) was used for cell cycle analysis of DNA histograms. Mixed
non-malignant diploid cells from the same tumour sample analysed
were used as the internal reference standard. Regarding DNA ploidy
pattern, IBCs were classified as diploid vs. aneuploid. Tumours
were considered diploid when the DNA index (DI) obtained was 1.0
(range, 0.95-1.05). The aneuploid tumours were further
subclassified into several categories based on DI: hypodiploid (DI:
<0.95), hyperdiploid (DI: >1.05 and <1.9), tetraploid (DI:
1.9-2.1), hypertetraploid (DI: >2.1), and multiploid (if more
than one aneuploid peak was observed). The SPF was determined from
the histogram according to a polynomial model, as the percentage of
cells in the S-phase of the cycle (20). Forty-nine (22.4%) of 219 tumours
could not be reliably evaluated for SPF, because of the high amount
of background debris (>20%), the overlap of cell populations, or
the presence of hypertetraploid or multiploid tumours. The median
SPF value (5.65%) in the whole series was used to discriminate
between low vs. high SPF proliferative tumours.
Statistical analysis
The statistical differences between
histopathological and molecular characteristics within the two
subgroups of young (≤45 years) and older (≥75 years) patients with
IBC were evaluated using the two proportion Z test. Chi-squared
test with Yates correction and Fisher's exact test were used for
assessing differences between treatment modalities and DNA
aneuploidy subcategories, respectively. The two-sample Mann-Whitney
U test for equality of medians was used for assessing differences
between continuous variables (21). Survival analyses were performed
using the Kaplan-Meier estimation, and the differences between
survival curves were evaluated by the log-rank test. All tests with
a P-value of less than 5% were considered statistically
significant.
Results
The mean and median values for age in the subgroup
of young patients were 39.1 and 40 years (range, 19–45 years)
respectively, while the corresponding values in the subgroup of
older patients were 78.9 and 78 years (range, 75–91 years).
The median follow-up of the study was 90 months,
ranging from two to 252 months. At the end of follow-up time, 74
patients had shown disease recurrences (48 in the younger and 26 in
the older subgroup) and 61 patients had died of the disease (37 in
the younger and 24 in the older subgroup).
Table II shows the
differences in histopathological and molecular characteristics
between the young (≤45 years) vs. older (≥75 years) patients'
subgroups. Compared to the subgroup of older patients, young
patients showed a higher frequency of IBC of no special type (NST),
higher axillary lymph node involvement, more advanced disease
staging and higher SPF tumour proliferative activity. The median
SPF value (7.1%; range, 1.5-23.7%) in the subgroup of young
patients was significantly higher than the corresponding value
(4.5%; range, 0.7-26.4%) among older patients (P=0.017;
Mann-Whitney U test) (Figs. S5
and S6). Concerning DNA ploidy
pattern (Table III), no
significant difference was observed between the broad dichotomy
diploidy vs. aneuploidy in both subgroups, although older patients
presented a higher incidence of hypertetraploid tumours (P=0.016;
Fisher's exact test). Young patients had a higher frequency of
hypodiploid and hyperdiploid tumours, but without reaching
statistical significance. No statistical differences were found for
the histological grade, tumour size, Ki67 index, estrogen
receptors, progesterone receptors, and HER2 status. There was a
trend (P=0.058) toward a lower incidence of Luminal A and higher
incidence of Luminal B tumours in the young patients' subgroup.
 | Table II.Differences in histopathological and
molecular characteristics between young (≤45 years) vs. older (≥75
years) IBC patients. |
Table II.
Differences in histopathological and
molecular characteristics between young (≤45 years) vs. older (≥75
years) IBC patients.
|
Characteristics | Young patients (≤45
years), n (%) | Older patients (≥75
years), n (%) |
P-valuea |
|---|
| Histological
type |
|
| 0.023 |
|
Invasive carcinomas of
NST | 94 (91.3) | 92 (79.3) |
|
|
Other | 9 (8.7) | 24 (21.7) |
|
| Grade of
differentiation |
|
| NS |
| G1 | 20 (20.0) | 28 (24.8) |
|
| G2 | 52 (52.0) | 59 (52.2) |
|
| G3 | 28 (28.0) | 26 (23.0) |
|
| Tumour size |
|
| NS |
|
pT1 | 35 (35.3) | 38 (33.9) |
|
|
pT2 | 57 (57.6) | 66 (58.9) |
|
|
PT3 | 7 (7.1) | 8 (7.2) |
|
| Lymph node
status |
|
| <0.001 |
|
Negative | 44 (44.0) | 76 (66.7) |
|
|
Positive (≤ 3) | 37 (37.0) | 34 (29.8) |
|
|
Positive (> 3) | 19 (19.0) | 4 (3.5) |
|
| Disease
staging |
|
| 0.021 |
| Stage I
+ Stage IIA | 54 (55.1) | 80 (71.4) |
|
| Stage
IIB + Stage III | 44 (44.9) | 32 (28.6) |
|
| DNA ploidy |
|
| NS |
|
Diploid | 45 (43.7) | 57 (49.1) |
|
|
Aneuploid | 58 (56.3) | 59 (50.9) |
|
| S-Phase
fraction |
|
| 0.021 |
|
Low | 34 (40.5) | 51 (59.3) |
|
|
High | 50 (59.5) | 35 (40.7) |
|
| Ki67 index |
|
| NS |
|
Low | 39 (60.0) | 53 (60.2) |
|
|
High | 26 (40.0) | 35 (39.8) |
|
| Estrogen
receptors |
|
| NS |
|
Positive | 84 (81.6) | 86 (75.4) |
|
|
Negative | 19 (18.4) | 28 (24.6) |
|
| Progesterone
receptors |
|
| NS |
|
Positive | 66 (67.3) | 67 (61.5) |
|
|
Negative | 32 (32.7) | 42 (38.5) |
|
| HER2 status |
|
| NS |
|
Negative | 72 (83.7) | 89 (84.8) |
|
|
Positive | 14 (16.3) | 16 (15.2) |
|
| Molecular
subtyping |
|
| 0.058 |
| Luminal
A | 26 (37.1) | 43 (47.2) |
|
| Luminal
B | 24 (34.3) | 18 (19.8) |
|
| HER2
positive | 3 (4.3) | 8 (8.8) |
|
|
Triple-negative | 10 (14.3) | 19 (20.9) |
|
|
Triple-positive | 7 (10.0) | 3 (3.3) |
|
| Death from
disease |
|
| 0.030 |
| No | 66 (64.1) | 87 (78.4) |
|
|
Yes | 37 (35.9) | 24 (21.6) |
|
| Disease
recurrence |
|
| <0.001 |
| No | 55 (53.4) | 85 (76.6) |
|
|
Yes | 48 (46.6) | 26 (23.4) |
|
 | Table III.DNA ploidy pattern in the subgroups
of young (≤45 years) vs. older (≥75 years) IBC patients. |
Table III.
DNA ploidy pattern in the subgroups
of young (≤45 years) vs. older (≥75 years) IBC patients.
| Variables | Young patients (≤45
years), n (%) | Older patients (≥75
years), n (%) |
P-valuea |
|---|
| Diploidy | 45 (43.7) | 57 (49.1) | NS |
| Aneuploidy | 58 (56.3) | 59 (50.9) | - |
|
Hypodiploidy | 2 (3.4) | 0 (0) | NS |
|
Hyperdiploidy | 46 (79.3) | 39 (66.1) | NS |
|
Tetraploidy | 4 (6.9) | 6 (10.2) | NS |
|
Hypertetraploidy | 2 (3.4) | 11 (18.6) | 0.016 |
|
Multiploidy | 4 (6.9) | 3 (5.1) | NS |
Concerning the clinical outcome, statistically
significant differences were found in the whole series, occurring
more recurrences (46.6% vs. 22.4%; P<0.001) and deaths from
disease (35.9% vs. 20.7%; P=0.030) among young patients (Table II). The finding was also observed
among patients with IBC of NST (n=186), where young patients showed
more recurrences (46.8% vs. 22.8%; P<0.001) and deaths from
disease (35.1% vs. 20.7%; P=0.028) than older ones. Table IV illustrates the Kaplan-Meier
5/10-year survival estimates between both patients' subgroups.
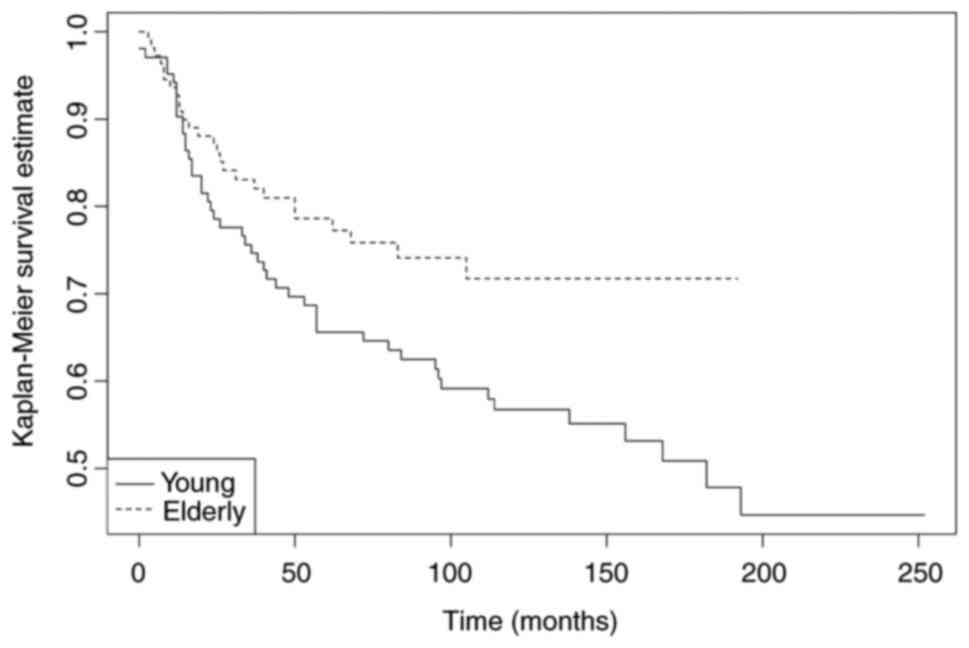
There is a statistically significant difference in DFS (Fig. 1) between young vs. older IBC
patients, with the younger patients showing a less favourable
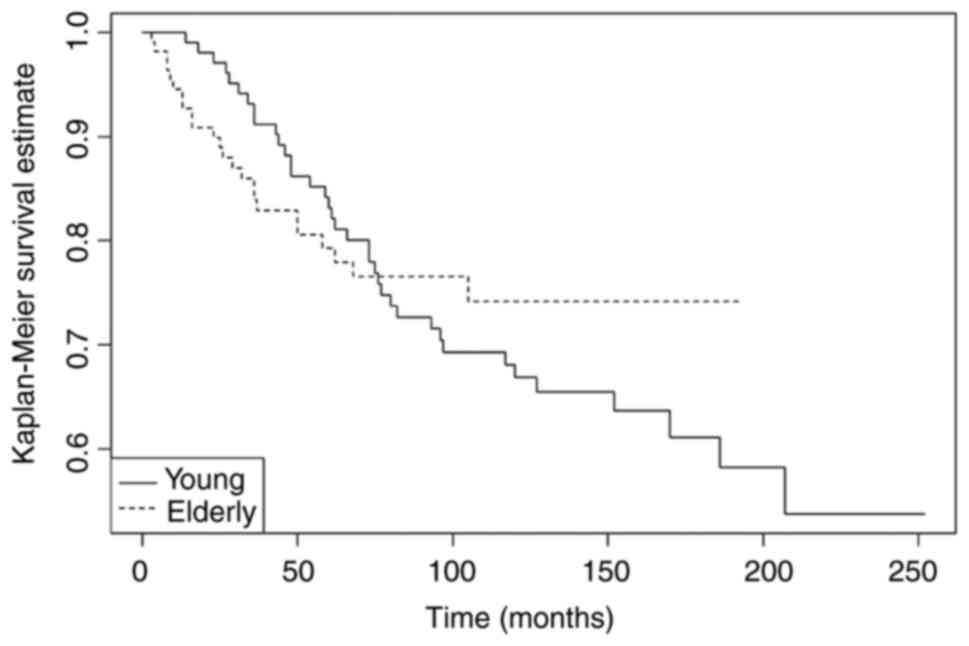
clinical course. For OS, no significant differences were found.
Nevertheless, an overlap of OS curves (Fig. 2) up to the first six years since
diagnosis was observed, followed by worse long-term clinical
evolution in the young patients' subgroup.
 | Table IV.Kaplan-Meier estimates for survival
between young (≤45 years) vs. older (≥75 years) IBC patients. |
Table IV.
Kaplan-Meier estimates for survival
between young (≤45 years) vs. older (≥75 years) IBC patients.
| Variable | 5/10-year DFS
(%) |
P-valuea | 5/10-year OS
(%) |
P-valuea |
|---|
|
|
| 0.04 |
| NS |
| Young patients (≤45
years) | 65.6/56.8 |
| 83.2/66.9 |
|
| Older patients (≥75
years) | 78.6/71.7 |
| 79.3/74.2 |
|
Discussion
IBC is a very heterogeneous disease, including
distinct molecular subtypes (22,23).
However, other patient-related factors, such as age at diagnosis,
may affect outcome and influence prognosis. Our present study
sought to determine the impact of age (extremes of age) on
patients' survival in two subgroups of young (≤45 years) vs. older
(≥75 years) IBC patients, also assessing significant differences in
histopathological and molecular features, with special focus on DNA
flow cytometry, that could distinguish both groups of patients.
Our data confirm the view that younger patients have
more aggressive disease, with an increased risk of recurrences and
shorter disease-free survival (Fig.
1). Furthermore, they appear to show a worse long-term overall
survival, mainly after the first six years since diagnosis
(Fig. 2). Overall, the findings
are clinically relevant because they indicate that age itself may
influence prognosis, and thus potentially, the treatment strategies
and management of patients with IBC. In this context, Beadle et
al (4) reported that the risk
of recurrence after early-stage IBC decreases with age, and is
relatively high in young women, for whom maximizing loco-regional
therapy should be a priority. Zavagno et al (24), in their study of 1226 IBC patients,
analysing the influence of age and menopausal status on
pathological features, also showed that the youngest (≤40 years)
patients had the worst prognostic pattern, which improves as age
increases and is the best in patients ≥75 years of age. However, in
a prospective cohort of 594 women with early IBC, Karihtala et
al (25) reported comparable
survival rates between the age groups of <41 years, 41–69 years,
and ≥71 years.
In our study, after initially observing the distinct
clinical outcome between both subgroups, we sought to investigate
further, which were the possible causes. To achieve this task, we
hypothesized that tumours arising in young vs. older IBC patients
could have differences in histopathological and molecular
characteristics, which was confirmed. The main prognostic factors
that differentiate young patients from older ones were higher
axillary lymph node involvement, more advanced disease stage, and
higher SPF proliferative activity. These different key aspects of
tumour biology, associated with intrinsic aggressive behaviour
(high SPF) and advanced stage, could be considered as constituting
a specific phenotype that may explain the distinct prognosis
between the two patients' subgroups. On the one hand, it is known
that mutations usually accumulate in the various genes that control
cell proliferation, accelerating cell division rates or inhibiting
normal controls of the system, such as the cell cycle arrest or
apoptosis. The challenge for research would be to identify those
mutations that are responsible for the higher proliferative
activity, as measured by SPF, in young IBC patients. Indeed,
younger age at diagnosis has been associated with higher expression
of gene signatures related to proliferation (26), but it requires further elucidation
in future studies. Furthermore, Anders et al (27), using genomic expression analysis,
identified 367 biologically relevant gene sets that could
differentiate IBCs of young (≤45 years) patients from those of
older (≥65 years) ones, suggesting that age-specific IBC may be
considered a distinct clinical/molecular entity. Zingh et al
(28) also showed that
significantly fewer women with >70 years presented positive
lymph nodes as compared to younger patients. On the other hand,
beyond the fact that young women are not routinely included in
screening programs (29), a low
index of suspicion and a delayed diagnosis could have an impact as
compared to older counterparts for a later stage presentation
(30).
Regarding surrogate molecular subtyping, we found a
trend toward young IBC patients presenting a lower incidence of
Luminal A and a higher incidence of Luminal B tumours, which might
be related to their clinical aggressiveness. Partridge et al
(8), studying the effect of age on
survival by molecular subtype, concluded that young age seems to be
particularly prognostic in patients with Luminal IBCs. In this
specific Luminal subtype, Sheridan et al (31) showed even that young age is an
independent prognostic factor for poor outcome. In their
comprehensive review (32), van
Herck et al reported that older age is associated, beyond a
higher incidence of Luminal tumours, with fewer Triple-negative and
HER2-positive subtypes than a younger age. On the contrary, we
found no differences in the incidence of Triple-negative and
HER2-positive tumours between both groups of patients, which may be
explained by missing data and a relatively small sampling size.
Nevertheless, it should be noted that other authors (8), like us, have shown that young age is
not a predictor of outcome in women with Triple-negative and
HER2-positive subtype tumours.
Concerning the histological type, IBCs of NST were
more prevalent among the subgroup of younger patients, similarly to
data of Azim et al and Wang et al studies (26,33).
Interestingly, within this more common type of IBC, we found that
younger patients had also a worse prognosis. The K-M survival
estimation was not performed in other histological types (n=33)
because it seems superfluous, due to the small number of cases and
weak statistical strength. However, contrarily to some studies
(7,8,34–36),
we could not find significant differences in other classic
prognostic features, such as histological grade, tumour size,
hormonal receptors, and HER2 status between both patients'
subgroups.
Little attention has been paid to the association
between the patient's age and tumour cell proliferation in IBC.
This was the main reason to perform a thorough analysis of DNA flow
cytometry parameters, ploidy and SPF, in our study. Of note, the
striking finding is that, as compared to older patients, younger
patients have shown tumours with statistically significant higher
SPF, since high tumour proliferative rates are usually associated
with more aggressive biological behaviour and adverse clinical
outcome. However, contrarily to others (37), no differences related to the Ki67
index were observed, which corroborates some data disagreement,
previously reported by our group (38), between the two cell proliferation
markers. A higher SPF reflects alterations in DNA replication,
leading to genomic instability, which has been associated with the
development of lymph node metastases and worse disease outcomes
(39,40). A significant difference in SPF
between young vs. older IBC patients may be a specific indicator of
the underlying molecular mechanisms that could differentiate the
two age groups. Unfortunately, technical difficulties to assess SPF
are well known, being its usefulness limited in clinical practice
by a lack of inter-laboratory standardization, which includes the
definition of cut-off thresholds that could reliably discriminate
low vs. high SPF proliferative tumours.
Regarding the DNA ploidy pattern, no significant
differences were found related to the broad dichotomy diploid vs.
aneuploid tumours between both subgroups. Nevertheless, further
aneuploidy subcategories analysis allowed observing interesting
differences. Young patients showed mostly hyperdiploid tumours, an
aneuploid category that, following an intermediate stage of
tetraploidization (41,42), has been associated with a worse
prognosis in IBC. On the other hand, older patients presented a
significantly higher proportion of hypertetraploid tumours, a
finding that has been considered as a mirror of sequential
molecular alterations during life (43), and thus, more prevalent in older
patients, although not necessarily related to poor prognosis.
In conclusion, our present data strongly suggest
that IBCs in women of different ages could be considered different
diseases or clinical entities. To support the view, the finding of
distinct clinical evolution and survival, when comparing both
subgroups of young (≤45 years) vs. older (≥75 years) IBC patients.
Some histopathological and molecular characteristics, namely SPF,
axillary lymph node status, and disease staging, appear as the main
factors implicated in the distinction. Therefore, the age of
patients, at least at the extremes of the spectrum, seems to be
itself a relevant prognostic factor. Because it is an area largely
unexplored, further studies are warranted to investigate underlying
genomic, transcriptomic, and epigenetic alterations that may
differentiate IBCs among patients of different ages.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study is part of the research project ‘Implicações
prognósticas e terapêuticas da análise por citometria de fluxo DNA
nos subtipos moleculares de cancro da mama avaliados por
imunohistoquímica’, supported by a grant from ‘Projectos de
Investigação Básica/Translacional-2015’, of the Portuguese
Institute of Oncology of Lisbon (grant no. UIC/909). Giovani L.
Silva was partially funded by Fundação para a Ciência e a
Tecnologia (FCT-Portugal; grant no. UIDB/00006/2020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AEP and SA contributed to the study conception and
design, data analysis and interpretation, and wrote the manuscript.
JM and TP performed the immunohistochemistry and SISH analyses. GLS
performed the statistical analyses. AEP and SA confirm the
authenticity of all the raw data. All authors have read, reviewed,
discussed and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in this study were in
accordance with the ethical standards and approved by the
institutional ethical research committee of the Portuguese
Institute of Oncology of Lisbon (ref. no. UIC/909). For this type
of retrospective study, informed consent is not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Organisation for Economic Cooperation and
Development (OECD)/European Union, Health at a Glance, . Europe
2020: State of Health in the EU Cycle. OECD Publishing; Paris:
2020
|
|
3
|
Gabriel CA and Domchek SM: Breast cancer
in young women. Breast Cancer Res. 12:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beadle BM, Woodward WA and Buchholz TA:
The impact of age on outcome in early-stage breast cancer. Semin
Radiat Oncol. 21:26–34. 2011. View Article : Google Scholar
|
|
5
|
Liedtke C, Rody A, Gluz O, Baumann K,
Beyer D, Kohls EB, Lausen K, Hanker L, Holtrich U, Becker S and
Karn T: The prognostic impact of age in different molecular
subtypes of breast cancer. Breast Cancer Res Treat. 152:667–673.
2015. View Article : Google Scholar
|
|
6
|
Zhong W, Tan L, Jiang WG, Chen K, You N,
Sanders AJ, Liang G, Liu Z, Ling Y and Gong C: Effect of younger
age on survival outcomes in T1N0M0 breast cancer: A propensity
score matching analysis. J Surg Oncol. 119:1039–1046. 2019.
View Article : Google Scholar
|
|
7
|
Keegan TH, DeRouen MC, Press DJ, Kurian AW
and Clarke CA: Occurrence of breast cancer subtypes in adolescent
and young adult women. Breast Cancer Res. 14:R552012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Partridge AH, Hughes ME, Warner ET,
Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC,
Winer EP, et al: Subtype-dependent relationship between young age
at diagnosis and breast cancer survival. J Clin Oncol.
34:3308–3314. 2016. View Article : Google Scholar
|
|
9
|
Paluch-Shimon S, Pagani O, Partridge AH,
Abulkhair O, Cardoso MJ, Dent RA, Gelmon K, Gentilini O, Harbeck N,
Margulies A, et al: ESO-ESMO 3rd international consensus guidelines
for breast cancer in young women (BCY3). Breast. 35:203–217. 2017.
View Article : Google Scholar
|
|
10
|
Diab SG, Elledge RM and Clark GM: Tumor
characteristics and clinical outcome of elderly women with breast
cancer. J Natl Cancer Inst. 92:550–556. 2000. View Article : Google Scholar
|
|
11
|
Pinto AE, André S and Soares J: Short term
significance of DNA ploidy and cell proliferation in breast
carcinoma: A multivariate analysis of prognostic markers in a
series of 308 patients. J Clin Pathol. 52:604–611. 1999. View Article : Google Scholar
|
|
12
|
Pinto AE, Pereira T, Santos M, Branco M,
Dias A, Silva GL, Ferreira MC and André S: DNA ploidy is an
independent predictor of survival in breast invasive ductal
carcinoma: A long-term multivariate analysis of 393 patients. Ann
Surg Oncol. 20:1530–1537. 2013. View Article : Google Scholar
|
|
13
|
Pinto AE, Pereira T, Silva GL and André S:
Prognostic relevance of DNA flow cytometry in breast cancer
revisited: The 25-year experience of the Portuguese institute of
oncology of Lisbon. Oncol Lett. 13:2027–2033. 2017. View Article : Google Scholar
|
|
14
|
WHO Classification of Tumours Editorial
Board, . Breast Tumours. WHO Classification of Tumour Series. 5th
edition. Vol 2. International Agency for Research on Cancer; Lyon:
2019
|
|
15
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the st gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar
|
|
17
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
american pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar
|
|
18
|
Ahn S, Woo JW, Lee K and Park SY: HER2
status in breast cancer: Changes in guidelines and complicating
factors for interpretation. J Pathol Transl Med. 54:34–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deitch AD, Law H and White RD: A stable
propidium iodide staining procedure for flow cytometry. J Histochem
Cytochem. 30:967–972. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dean PN and Jett JH: Mathematical analysis
of DNA distributions derived from flow microfluorometry. J Cell
Biol. 60:523–527. 1974. View Article : Google Scholar
|
|
21
|
Corder GW and Foreman DI: Nonparametric
statistics: A step-by-step approach. 2nd edition. Wiley; Hoboken,
NJ: 2014
|
|
22
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Desmedt C, Haibe-Kains B, Wirapati P,
Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M and
Sotiriou C: Biological processes associated with breast cancer
clinical outcome depend on the molecular subtypes. Clin Cancer Res.
14:5158–5165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zavagno G, Meggiolaro F, Pluchinotta A,
Bozza F, Favretti F, Marconato R, Geraci G, Nistri R, Fontana P,
Sorrentino P, et al: Influence of age and menopausal status on
pathologic and biologic features of breast cancer. Breast.
9:320–328. 2000. View Article : Google Scholar
|
|
25
|
Karihtala P, Jääskeläinen A, Roininen N
and Jukkola A: Real-world, single-centre prospective data of age at
breast cancer onset: Focus on survival and reproductive history.
BMJ Open. 11:e0417062021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azim HA Jr, Nguyen B, Brohée S, Zoppoli G
and Sotiriou C: Genomic aberrations in young and elderly breast
cancer patients. BMC Med. 13:2662015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anders CK, Hsu DS, Broadwater G, Acharya
CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, et
al: Young age at diagnosis correlates with worse prognosis and
defines a subset of breast cancers with shared patterns of gene
expression. J Clin Oncol. 26:3324–3330. 2008. View Article : Google Scholar
|
|
28
|
Zingh R, Hellman S and Heimann R: The
natural history of breast carcinoma in the elderly: Implications
for screening and treatment. Cancer. 100:1807–1813. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avci O, Tacar SY, Seber ES and Yetisyigit
T: Breast cancer in young and very young women; Is age related to
outcome? J Cancer Res Ther. 17:1322–1327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Assi HA, Khoury KE, Dbouk H, Khalil LE,
Mouhieddine TH and El Saghir NS: Epidemiology and prognosis of
breast cancer in young women. J Thorac Dis. 5:S2–S8.
2013.PubMed/NCBI
|
|
31
|
Sheridan W, Scott T, Caroline S, Yvonne Z,
Vanessa B, David V, Karen G and Stephen C: Breast cancer in young
women: have the prognostic implications of breast cancer subtypes
changed over time? Breast Cancer Res Treat. 147:617–629. 2014.
View Article : Google Scholar
|
|
32
|
Van Herck Y, Feyaerts A, Alibhai S,
Papamichael D, Decoster L, Lambrechts Y, Pinchuk M, Bechter O,
Herrera-Caceres J, Bibeau F, et al: Is cancer biology different in
older patients? Lancet Healthy Longev. 2:e663–e677. 2021.
View Article : Google Scholar
|
|
33
|
Wang MX, Ren JT, Tang LY and Ren ZF:
Molecular features in young vs elderly breast cancer patients and
the impacts on survival disparities by age at diagnosis. Cancer
Med. 7:3269–3277. 2018. View Article : Google Scholar
|
|
34
|
Collins LC, Marotti JD, Gelber S, Cole K,
Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP
and Partridge AH: Pathologic features and molecular phenotype by
patient age in a large cohort of young women with breast cancer.
Breast Cancer Res Treat. 131:1061–1066. 2012. View Article : Google Scholar
|
|
35
|
Lodi M, Scheer L, Reix N, Heitz D, Carin
AJ, Thiébaut N, Neuberger K, Tomasetto C and Mathelin C: Breast
cancer in elderly women and altered clinico-pathological
characteristics: A systematic review. Breast Cancer Res Treat.
166:657–668. 2017. View Article : Google Scholar
|
|
36
|
Kim HJ, Kim S, Freedman RA and Partridge
AH: The impact of young age at diagnosis (age <40 years) on
prognosis varies by breast cancer subtype: A U S. SEER database
analysis. Breast. 61:77–83. 2022. View Article : Google Scholar
|
|
37
|
Massafra R, Bove S, La Forgia D, Comes MC,
Didonna V, Gatta G, Giotta F, Latorre A, Nardone A, Palmiotti G, et
al: An invasive disease event-free survival analysis to investigate
Ki67 role with respect to breast cancer patients' age: A
retrospective cohort study. Cancers (Basel). 14:22152022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pinto AE, André S, Pereira T, Nóbrega S
and Soares J: Prognostic comparative study of S-phase fraction and
Ki67 index in breast carcinoma. J Clin Pathol. 54:543–549. 2001.
View Article : Google Scholar
|
|
39
|
Callaghan KA, Becker TE, Ellsworth DL,
Hooke JA, Ellsworth RE and Shriver CD: Genomic instability and the
development of metastatic lymph node tumors. Ann Surg Oncol.
14:3125–3132. 2007. View Article : Google Scholar
|
|
40
|
Lischka A, Doberstein N, Freitag-Wolf S,
Koçak A, Gemoll T, Heselmeyer-Haddad K, Ried T, Auer G and
Habermann JK: Genome instability profiles predict disease outcome
in a cohort of 4,003 breast cancer patients. Clin Cancer Res.
26:4606–4615. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shackney SE, Smith CA, Miller BW, Burholt
DR, Murtha K, Giles HR, Ketterer DM and Pollice AA: Model for the
genetic evolution of human solid tumors. Cancer Res. 49:3344–3354.
1989.PubMed/NCBI
|
|
42
|
Sennerstam RB and Strömberg JO: Young
breast cancer patients aged <40 years and tumor DNA ploidy
progression. Anal Quant Cytopathol Histopathol. 39:57–68. 2017.
|
|
43
|
Chatsirisupachai K, Lesluyes T, Paraoan L,
Van Loo P and de Magalhães JP: An integrative analysis of the
age-associated multi-omic landscape across cancers. Nat Commun.
12:23452021. View Article : Google Scholar : PubMed/NCBI
|