Introduction
Androgen receptor axis-targeted (ARAT) agents are
available for metastatic prostate cancer patients, and several
randomized trials established that the use of ARAT agents improves
the prognosis for patients with metastatic, castration-resistant
prostate cancer (mCRPC) (1–4). The
use of ARAT agents is clearly more efficacious in terms of survival
improvement when compared to using androgen-deprivation therapy
(ADT) alone; this holds true not only in patients with mCRPC but
also those with metastatic castration-sensitive prostate cancer
(mCSPC) (5–7). This finding has dramatically changed
the treatment and clinical outlook of metastatic prostate cancer
patients, especially those with mCSPC, heralding the arrival of the
‘ARAT era’ in prostate cancer therapy.
In Japan, the combination therapy of bicalutamide
and ADT, which is referred to as ‘combined androgen blockade
(CAB)’, has been widely used to treat metastatic prostate cancer
patients due to the observation of long-term efficacy (8). The 2012 Prostate Cancer Guidelines of
the Japanese Urological Association recommend alternative
antiandrogen therapy with flutamide as second-line treatment for
prostate cancer patients who have failed CAB; this regimen has
often been used to treat CRPC patients (9). In addition, other oral agents (e.g.,
estramustine phosphate (EMP), ethinylestradiol, low-dose
glucocorticoid therapy) are also effective in patients with CRPC
(10–12), and conventional hormone therapy
using these therapeutic agents was more common in Japan than in
other countries owing to the characteristics of patients on the
Japanese PREVAIL trial (13).
ARAT agents have been available for patients with
mCRPC since 2014 and for those with mCSPC since 2019. Several
studies have reported the clinical benefit of using ARAT agents in
patients with mCSPC compared to CAB (14–18).
However, few studies have compared the therapeutic outcome of
conventional hormonal therapy, which is commonly performed in
Japan, and the therapeutic outcome of using ARAT agents in Japanese
patients with mCRPC (19). In
order to prove the effectiveness of ARAT agents for Japanese
patients, we considered it very important to compare the outcome of
treatment using ARAT agents with the outcome of conventional
hormonal therapy.
We therefore designed this study to comparing the
clinical outcomes after conventional hormone therapy with those
after ARAT for the Japanese patients with mCRPC.
Materials and methods
Patient selection
One hundred ten male patients who underwent hormonal
therapy for the treatment of mCRPC in the Shiga University of
Medical Science Hospital from July 2007 to December 2020 were
evaluated. All cases were pathologically diagnosed as prostatic
adenocarcinoma and already had distant metastases at diagnosis.
Radiographic examinations, including CT scan and bone scintigraphy,
were performed for all cases. Classification of tumor volume was
determined according to the CHAARTED criteria, which was defined as
the presence of visceral metastases or ≥4 bone lesions with one or
more beyond the vertebral bodies and pelvis (20). Prostate-specific antigen (PSA)
progression was determined using the criteria defined by the
Prostate Cancer Working Group 2 (PCWG2) as an increase of 25% or
greater and an absolute increase of 2 ng/ml or more from the PSA
nadir that was confirmed by a second value obtained three or more
weeks later (21).
Definition of each therapy group
All patients had received hormonal therapy with ADT
alone or CAB using bicalutamide (80 mg/day) as first-line
treatment. As a subsequent therapy, the patients treated with only
conventional drugs [EMP (560 mg/day), ethinylestradiol (1.5
mg/day), prednisolone (10 mg/day), and flutamide (375 mg/day)] were
defined as the ‘conventional’ group. The cases who were
administered ARAT agents [enzalutamide (160 mg/day), abiraterone
acetate (1000 mg/day), or both] with/without conventional drugs
were defined as the ‘ARAT era’ group. Chemotherapy [docetaxel (70
mg/m2 every 3 weeks) and cabazitaxel (20
mg/m2 every 3 weeks)] was performed as appropriate at
the discretion of the attending physician in both groups.
We compared OS in the ARAT era group to the
conventional group. We also investigated risk factors that affect
the OS of mCRPC patients. This retrospective observational study
was approved by the internal ethical committee of Shiga University
of Medical Science (approval number R2018-186).
Statistical analysis
The analysis of patient characteristics between the
two groups was performed using the Mann-Whitney U-test and Fisher's
exact test. Kaplan-Meier curves were prepared and analyzed using
the log-rank test to evaluate the rate of OS. The factors affecting
OS were examined using the Cox-proportional hazard model.
Statistical analyses were performed using SPSS Statistics version
22 software (IBM, Armonk, NY, USA) and EZR software which is based
on R and R commander (22). A
P<0.05 denoted a statistically significant difference.
Results
Patient characteristics
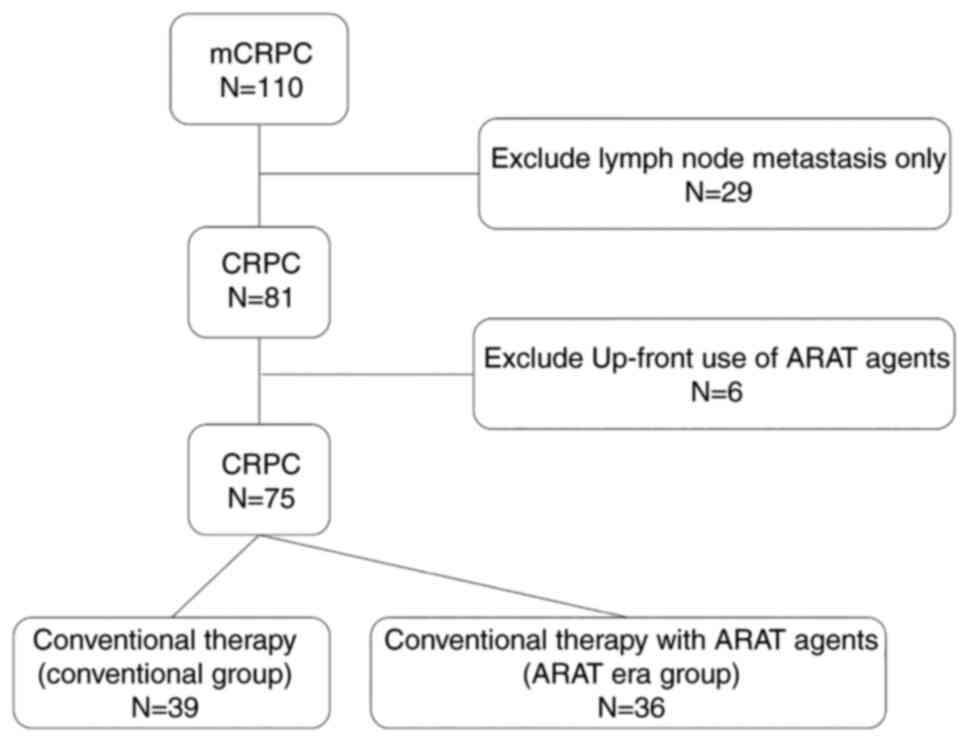
There were 110 cases of mCRPC patients who were
treated with hormonal therapy. Thirty-five cases were ineligible
for our study, as they had only local lymph node metastases (n=29)
or were cases with up-front use of ARAT agents (n=6). Thirty-nine
patients constituted the conventional group, and 36 patients
constituted the ARAT era group (Fig.
1). In the ARAT era group, ARAT agents were used as a
second-line therapy in fourteen patients, and as a third-line
therapy in the remaining 22 patients.
Patient characteristics in the conventional and ARAT
era groups are shown in Table I.
The median follow-up period for all cases was 49.4 months
(9.0-175.1 months). There were no significant differences between
the two groups with regard to initial PSA value, Gleason grade at
the time of biopsy, the state of organ metastasis, tumor volume,
the PSA nadir value, and time to PSA nadir. In the ARAT era group,
the age at diagnosis was significantly younger than in the
conventional group. Time to CRPC was shorter in the conventional
group than in the ARAT era group.
 | Table I.Characteristics of patients in the
Conventional and ARAT era groups. |
Table I.
Characteristics of patients in the
Conventional and ARAT era groups.
| Variable | Conventional
(N=39) | ARAT era (N=36) | P-value |
|---|
| Median age,
years | 73.0 (59.0-88.0) | 68.0 (53.0-86.0) | 0.037 |
| Median initial PSA,
ng/ml | 355.0
(8.5-7225.3) | 415.5
(6.6-3262.0) | 0.907 |
| Gleason grade, n
(%) |
|
| 0.161 |
| 1 | 1 (2.6) |
|
|
| 2 | 2 (5.1) |
|
|
| 3 | 6 (15.4) | 4 (11.1) |
|
| 4 | 10 (25.6) | 13 (36.1) |
|
| 5 | 12 (30.8) | 17 (47.2) |
|
|
Unknown | 8 (20.5) | 2 (5.6) |
|
| Metastasesa, n (%) |
|
|
|
| Lung | 5 (12.8) | 9 (25.0) | 0.242 |
|
Liver | 3 (7.7) | 1 (2.8) | 0.615 |
| Bone | 34 (87.2) | 33 (91.7) | >0.999 |
| CHAARTED, n (%) |
|
| 0.297 |
| Low | 12 (30.8) | 8 (22.2) |
|
| High | 22 (56.4) | 27 (75.0) |
|
| Value of PSA nadir,
ng/ml | 0.6 (0.0-231.6) | 1.3 (0.0-144.2) | 0.206 |
| Time to PSA nadir,
months | 8.2 (1.8-41.5) | 6.3 (0.6-29.4) | 0.259 |
| Time to CRPC,
months | 16.4 (2.8-64.0) | 9.7 (1.4-88.4) | 0.027 |
Comparison of OS between ARAT era
group and conventional group
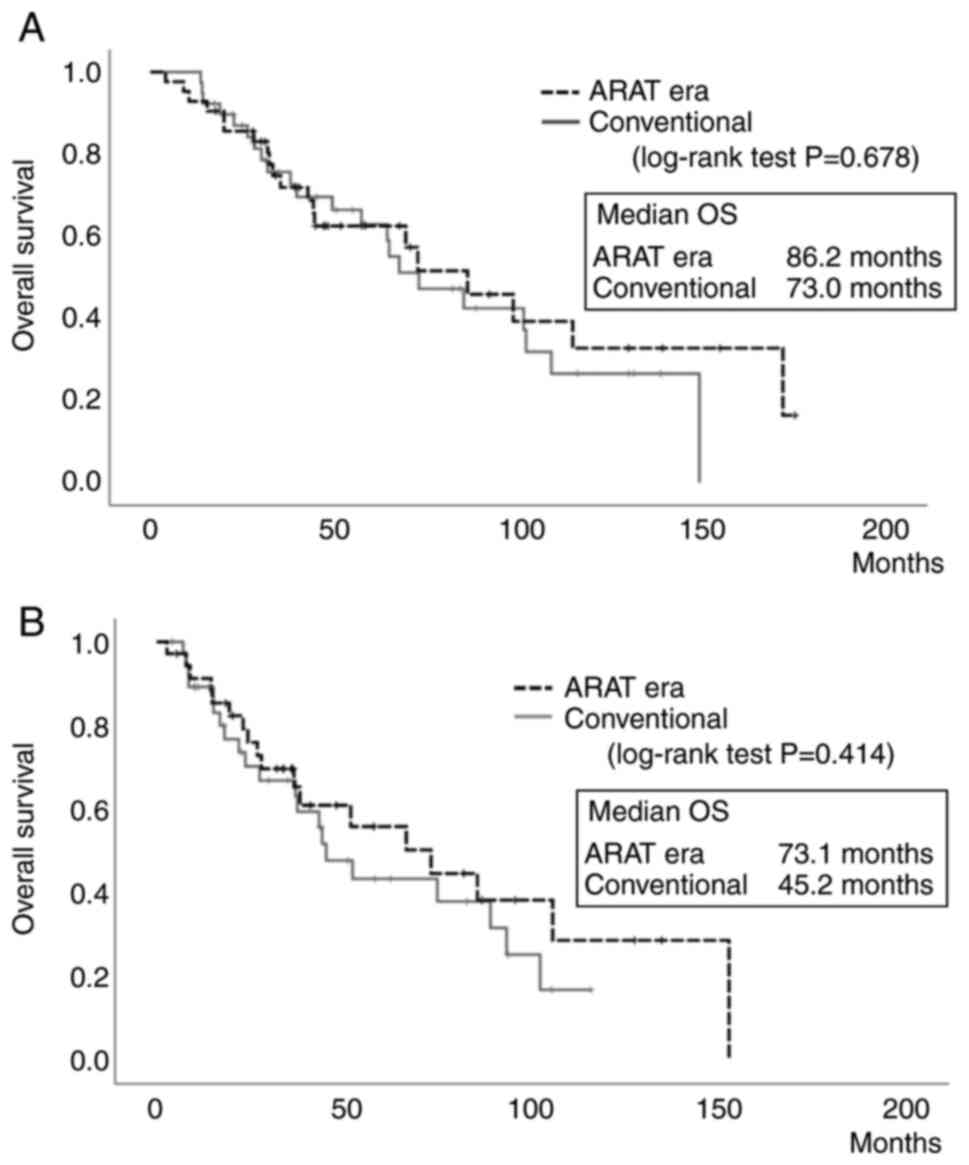
The median OS from the initial treatment was 86.2
months in the ARAT era group and 73.0 months in the conventional
group. Although OS tended to be slightly longer in the ARAT era
group, there was no significant difference among these groups
(P=0.678, Fig. 2A). The median OS
from the time of progression to CRPC was 73.1 months in the ARAT
era group and 45.2 months in the conventional group. Again,
although OS also tended to be slightly longer in the ARAT era
group, the difference between groups was not significant (P=0.414,
Fig. 2B).
Agents used in subsequent therapeutic
regimens in each group
Several treatments were performed for PSA recurrence
in each group, and proportion of patients receiving each treatment
is shown in Table II. Flutamide
was administered to 87.2 and 58.3% of patients, 48.7 and 19.4% of
the patients were treated with EMP or ethinylestradiol, while 28.2
and 8.3% of patients were treated with prednisolone in the
conventional group and the ARAT era group, respectively.
Chemotherapy was also administered to about half of all patients;
48.7 and 52.8% of the patients were treated with docetaxel in the
group of conventional therapy and ARAT era, respectively. Although
no-one in the conventional group was treated with cabazitaxel,
36.1% of the patients in the ARAT era group received this drug.
 | Table II.Agents used in subsequent therapeutic
regimens. |
Table II.
Agents used in subsequent therapeutic
regimens.
| Agents | Conventional
(N=39) | ARAT era
(N=36) | Total (N=75) |
|---|
| Endocrine therapy,
n (%) |
|
|
|
|
Flutamide | 34 (87.2) | 21 (58.3) | 55 (73.3) |
| EMP or
ethinylestradiol | 19 (48.7) | 7 (19.4) | 26 (34.7) |
|
Prednisolone | 11 (28.2) | 3 (8.3) | 14 (18.7) |
| Antineoplastic
agents, n (%) |
|
|
|
|
Docetaxel | 19 (48.7) | 19 (52.8) | 38 (50.7) |
|
Cabazitaxel | 0 (0.0) | 13 (36.1) | 13 (17.3) |
Prognostic factors for OS
We performed univariate and multivariate analysis of
prognostic factors for overall survival in patients with metastatic
prostate cancer and it was shown in Table III. The factors affecting OS were
examined using the Cox-proportional hazard model. In this analysis,
treatment of using ARAT agents was not an independent factor
associated with improved OS. The mortality risk was significantly
lower in cases with a higher initial PSA value (>361 ng/ml), a
lower value of PSA nadir (<1.0 ng/ml), a longer time to PSA
nadir (<6.7 months), and a longer time to CRPC in univariate
analysis. In the multivariate analysis, mortality was significantly
higher in cases with a higher initial PSA value, a lower of PSA
nadir value, and a longer time to CRPC.
 | Table III.Univariate and multivariate analysis
of prognostic factors for overall survival in patients with
metastatic prostate cancer. |
Table III.
Univariate and multivariate analysis
of prognostic factors for overall survival in patients with
metastatic prostate cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age at diagnosis
(≥72 years vs. <72 years) | 1.29
(0.68-2.45) | 0.431 |
|
|
| Initial PSA (≥361
ng/ml vs. <361 ng/ml) | 0.52
(0.28-0.99) | 0.047 | 0.22
(0.10-0.49) | <0.001 |
| Gleason grade (≥4
vs. <4) | 0.67
(0.30-1.49) | 0.333 |
|
|
| Existence of
visceral metastasis (yes vs. no) | 1.54
(0.76-3.12) | 0.230 | 1.80
(0.83-3.80) | 0.131 |
| CHAARTED (high vs.
low) | 0.89
(0.44-1.79) | 0.756 |
|
|
| PSA nadir (≥1.0
ng/ml vs. <1.0 ng/ml) | 2.13
(1.12-4.02) | 0.020 | 2.72
(1.27-5.83) | 0.010 |
| Time to PSA nadir
(≥6.7 vs. <6.7 months) | 0.24
(0.11-0.49) | <0.001 | 0.28
(0.10-0.77) | 0.014 |
| Time to CRPC (≥12
vs. <12 months) | 0.28
(0.14-0.56) | <0.001 | 0.58
(0.20-1.67) | 0.317 |
| ARAT treatment (yes
vs. no) | 0.87
(0.46-1.63) | 0.679 |
|
|
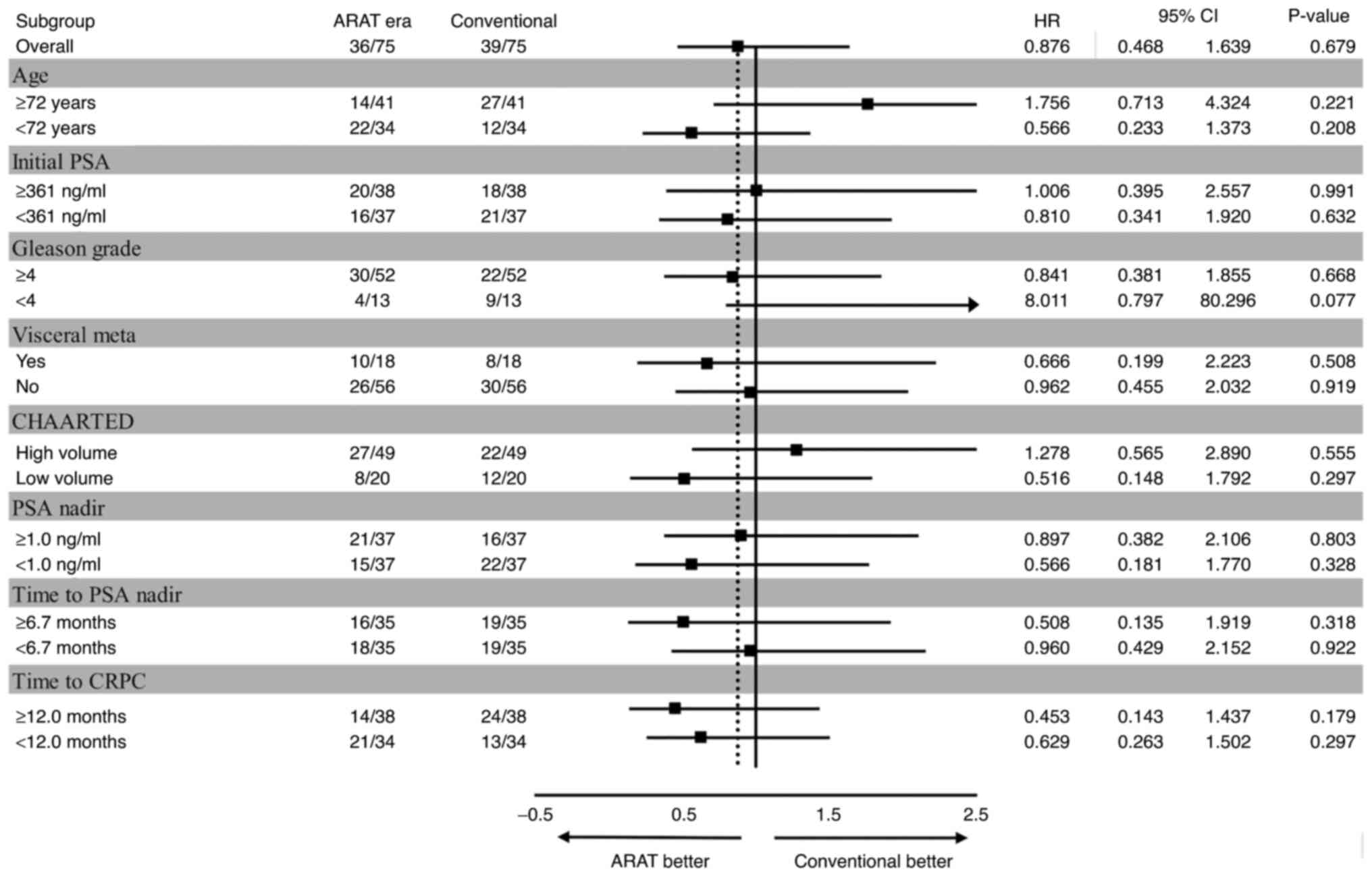
Subgroup analysis revealed that the outlook was
better for individuals in the conventional group who were older,
had a lower Gleason grade or a higher tumor volume (Fig. 3).
Discussion
In this study, we did not observe a significant
difference in OS for mCRPC patients in the ARAT era group vs. those
in the conventional group. However, one study has reported the
effectiveness of using ARAT agents as second-line therapy for mCRPC
patients. Uemura et al (19) compared the therapeutic outcomes
after treatment with either enzalutamide or flutamide for Japanese
patients with CRPC; they found that enzalutamide significantly
extended the time to PSA failure for both first-line and
second-line treatment. Recently, Chowdhury et al (23) have suggested that the time to PSA
failure of second-line treatment, so-called progression free
survival 2 (PFS2), can be used as a predictor of OS, and that PFS2
can be used to measure long-term clinical benefit when OS cannot be
assessed. For this reason, we expected a significantly extended OS
in the ARAT era cohort of our study; however, this was not the
case. One explanation for this is that patients were started on
ARAT agents too late after developing CRPC to benefit from their
effects. Our study included many cases in which ARAT agents were
used after treatment with flutamide, EMP, and steroids.
Additionally, the ARAT agents were generally used later in our
study than in the study performed by Uemura et al (19). Furthermore, the LATITUDE study
reported that OS of high-risk mCSPC patients was improved when
combinations of ARAT agents were used from the time of the initial
hormonal therapy (24).
Considering the result of those studies, we suggest that the
therapeutic effect of ARAT agents is maximized if they are started
at an earlier stage of the treatment. Conversely, ARAT treatment
has a weak therapeutic effect if started after onset of CRPC.
In the present study, we showed that PSA nadir and
initial PSA values are prognostic factors for metastatic prostate
cancer. Hamano et al (25)
examined the data of 321 Japanese patients who received hormonal
therapy for metastatic prostate cancer, and reported that PSA nadir
>0.64 ng/ml and time to PSA nadir <7 M are poor prognostic
factors in Japanese patients. PSA nadir ≥1.0 ng/ml and time to PSA
nadir <6.7 M were identified as poor prognostic factors in the
current study. We conclude that the prognosis is good in cases
where the PSA nadir value is low and when there is a prolonged time
to PSA failure (i.e., in cases where initial hormonal therapy is
effective). On the other hand, an initial PSA value ≥361 ng/ml was
identified as a good prognostic factor for OS in our current study.
Yamada et al (26) reported
that Japanese patients who received hormonal therapy and had a high
PSA developed CRPC more rapidly, but responded well to AWS and AA
alternation therapy, and the OS rate did not change. From this
result, we infer those cases with high initial PSA value respond
well to hormonal therapy, and high initial PSA value is a good
prognostic factor.
In our subgroup analysis, the therapeutic effect of
conventional hormonal therapy was better than that of ARAT agents
in the older patients, and in those with high tumor volume, and
high Gleason grades. In addition, we considered that the benefit of
adding ARAT agents with conventional hormonal therapy may be small
even in cases where the PSA nadir is high or the time to PSA nadir
is short (i.e., patients who are likely to be refractory to
hormonal therapy). However, this does not mean that the use of ARAT
agents early in the initial treatment is futile. Regarding
high-volume cases, Narita et al (27) reported that upfront use of
abiraterone significantly improved OS when compared to ADT/CAB
treatment. Considering these results, we recommend that ARAT
reagents should be used from the time of initial hormonal therapy
to obtain the maximum therapeutic effect. The problems associated
with the use of ARAT agents are related to side effects specific to
this class of therapeutics, as well as the increased costs. The
results obtained in this study may provide useful to physicians who
are considering whether to use ARAT agents at the beginning of
treatment or instead add them to conventional hormonal therapy.
The present study has a few limitations. Due to its
retrospective nature, there are several differences in patient
characteristics, and the number of examined patients is relatively
small. Although there are limitations as mentioned above, we
consider that this result accurately reflects the actual clinical
practice in Japan. Thus, when considering the therapy of metastatic
prostate cancer in Japanese patients, we are convinced that this
result from the present study is sufficiently informative and
significant. We are planning further investigate this topic using
an increased number of cases in future research.
In conclusion, although ARAT agents appeared to
prolong the survival of patients with metastatic prostate cancer,
this effect did not reach the level of statistical significance. We
infer that ARAT agents have only a minimal impact on survival
outcome when they are used in later lines of treatment for mCRPC.
Therefore, we suggest that upfront therapy using ARAT agents at the
time of the initial hormone therapy is essential, and that this can
have a significantly positive effect on survival in mCSPC
patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AW, MiN, MaN, TK, SKu, TY, KJ, AK and SKa
substantially contributed to the study design and
conceptualization. AW, TK and TY performed the acquisition of data
for the work. SKu, KJ and MaN contributed to data analysis and
interpretation. AW, SKa, MiN and AK substantially contributed to
the manuscript drafting. TY, MiN and AK provided expertise and
feedback. AW and SKa confirm the authenticity of all the raw data.
All authors critically reviewed and revised the manuscript draft.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Shiga University of Medical Science (Otsu,
Japan; approval number R2018-186). The informed consent was
obtained in the form of opt-out on the website of Shiga University
of Medical Science Hospital (Otsu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARAT
|
androgen receptor-axis targeted
|
|
mCRPC
|
metastatic castration-resistant
prostate cancer
|
|
ADT
|
androgen-deprivation therapy
|
|
mCSPC
|
metastatic castration-sensitive
prostate cancer
|
|
CAB
|
combined androgen blockade
|
|
EMP
|
estramustine phosphate
|
|
OS
|
overall survival
|
|
PSA
|
prostate-specific antigen
|
|
PFS2
|
progression free survival 2
|
References
|
1
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis ID, Martin AJ, Stockler MR, Begbie
S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath
LG, et al: Enzalutamide with standard first-line therapy in
metastatic prostate cancer. N Engl J Med. 381:121–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akaza H, Hinotsu S, Usami M, Arai Y,
Kanetake H, Naito S and Hirao Y: Combined androgen blockade with
bicalutamide for advanced prostate cancer: Long-term follow-up of a
phase 3, double-blind, randomized study for survival. Cancer.
115:3437–3445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okegawa T, Nutahara K and Higashihara E:
Alternative antiandrogen therapy in patients with
castration-resistant prostate cancer: A single-center experience.
Int J Urol. 17:950–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano D, Minei S, Kishimoto Y, Yamaguchi
K, Hachiya T, Yoshida T, Yoshikawa T, Endoh M, Yamanaka Y, Yamamoto
T, et al: Prospective study of estramustine phosphate for hormone
refractory prostate cancer patients following androgen deprivation
therapy. Urol Int. 75:43–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Izumi K, Kadono Y, Shima T, Konaka H,
Mizokami A, Koh E and Namiki M: Ethinylestradiol improves
prostate-specific antigen levels in pretreated castration-resistant
prostate cancer patients. Anticancer Res. 30:5201–5205.
2010.PubMed/NCBI
|
|
12
|
Nishimura K, Nonomura N, Satoh E, Harada
Y, Nakayama M, Tokizane T, Fukui T, Ono Y, Inoue H, Shin M, et al:
Potential mechanism for the effects of dexamethasone on growth of
androgen-independent prostate cancer. J Natl Cancer Inst.
93:1739–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura G, Yonese J, Fukagai T, Kamba T,
Nishimura K, Nozawa M, Mansbach H, Theeuwes A, Beer TM, Tombal B,
et al: Enzalutamide in Japanese patients with chemotherapy-naive,
metastatic castration-resistant prostate cancer: A post-hoc
analysis of the placebo-controlled PREVAIL trial. Int J Urol.
23:395–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaishampayan UN, Heilbrun LK, Monk P III,
Tejwani S, Sonpavde G, Hwang C, Smith D, Jasti P, Dobson K, Dickow
B, et al: Clinical efficacy of enzalutamide vs bicalutamide
combined with androgen deprivation therapy in men with metastatic
hormone-sensitive prostate cancer: A randomized clinical trial.
JAMA Netw Open. 4:e20346332021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shore ND, Chowdhury S, Villers A, Klotz L,
Siemens DR, Phung D, van Os S, Hasabou N, Wang F, Bhattacharya S,
et al: Efficacy and safety of enzalutamide versus bicalutamide for
patients with metastatic prostate cancer (TERRAIN): A randomised,
double-blind, phase 2 study. Lancet Oncol. 17:153–163. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueda T, Shiraishi T, Ito S, Ohashi M,
Matsugasumi T, Yamada Y, Fujihara A, Hongo F, Okihara K and Ukimura
O: Abiraterone acetate versus bicalutamide in combination with
gonadotropin releasing hormone antagonist therapy for high risk
metastatic hormone sensitive prostate cancer. Sci Rep.
11:100942021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Paller CJ, Hong H, De Felice A,
Alexander GC and Brawley O: Comparison of systemic treatments for
metastatic castration-sensitive prostate cancer: A systematic
review and network meta-analysis. JAMA Oncol. 7:412–420. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagisawa T, Kimura T, Mori K, Suzuki H,
Sano T, Otsuka T, Iwamoto Y, Fukuokaya W, Miyajima K, Enei Y, et
al: Abiraterone acetate versus nonsteroidal antiandrogen with
androgen deprivation therapy for high-risk metastatic
hormone-sensitive prostate cancer. Prostate. 82:3–12. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemura H, Kobayashi K, Yokomizo A, Hinotsu
S, Horie S, Kakehi Y, Naito S, Nonomura N, Ogawa O, Oya M, et al:
Enzalutamide + androgen deprivation therapy (ADT) versus flutamide
+ ADT in Japanese men with castration-resistant prostate cancer:
AFTERCAB study. BJUI Compass. 3:26–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the Prostate Cancer Clinical
Trials Working Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chowdhury S, Mainwaring P, Zhang L, Mundle
S, Pollozi E, Gray A and Wildgust M: Systematic review and
meta-analysis of correlation of progression-free survival-2 and
overall survival in solid tumors. Front Oncol. 10:13492020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki H, Shin T, Fukasawa S, Hashine K,
Kitani S, Ohtake N, Shibayama K, Tran N, Mundle S, Fizazi K, et al:
Efficacy and safety of abiraterone acetate plus prednisone in
Japanese patients with newly diagnosed, metastatic hormone-naive
prostate cancer: Final subgroup analysis of LATITUDE, a randomized,
double-blind, placebo-controlled, phase 3 study. Jpn J Clin Oncol.
50:810–820. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamano I, Hatakeyama S, Narita S,
Takahashi M, Sakurai T, Kawamura S, Hoshi S, Ishida M, Kawaguchi T,
Ishidoya S, et al: Impact of nadir PSA level and time to nadir
during initial androgen deprivation therapy on prognosis in
patients with metastatic castration-resistant prostate cancer.
World J Urol. 37:2365–2373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada Y, Sakamoto S, Amiya Y, Sasaki M,
Shima T, Komiya A, Suzuki N, Akakura K, Ichikawa T and Nakatsu H:
Treatment strategy for metastatic prostate cancer with extremely
high PSA level: Reconsidering the value of vintage therapy. Asian J
Androl. 20:432–437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Narita S, Kimura T, Hatakeyama S, Hata K,
Yanagisawa T, Maita S, Chiba S, Sato H, Kashima S, Koizumi A, et
al: Real-world survival outcomes of adding docetaxel or abiraterone
in patients with high-volume metastatic castration-sensitive
prostate cancer: Historically controlled, propensity score matched
comparison with androgen deprivation therapy. World J Urol.
40:1135–1141. 2022. View Article : Google Scholar : PubMed/NCBI
|