Introduction
Esophageal cancer is the seventh most common cancer
worldwide (1). In Japan, more than
85% of esophageal cancers are esophageal squamous cell carcinoma
(ESCC) (2). Neoadjuvant therapy
including chemotherapy and/or radiation therapy is recommended for
advanced esophageal cancer to improve patient prognosis by
downstaging tumors (3–6) and controlling local and distant
micrometastasis (7,8). Despite advances in neoadjuvant
therapy, surgical technique and patient selection, the 5-year
recurrence-free survival (RFS) rate after neoadjuvant therapy
followed by esophagectomy is approximately 35–55% (9,10).
In Japan, the Guidelines for Diagnosis and Treatment of Carcinoma
of the Esophagus 2017 (11,12)
recommend neoadjuvant chemotherapy (NAC), not neoadjuvant
chemoradiotherapy (CRT), as preoperative therapy for advanced ESCC
before esophagectomy. In addition, adjuvant therapy in cases with
NAC is not recommended after surgery (9). However, the impact of surgery after
NAC on recurrence is limited in ESCC patients (9,13).
Several risk factors for recurrence (e.g., clinical
or pathological lymph node metastasis, lymphovascular invasion
(LVI), perineural invasion, and extracapsular invasion) have been
identified in cases with neoadjuvant CRT (14–16).
In such analysis of ESCC patients who underwent neoadjuvant CRT and
esophagectomy, LVI was indicated as an independent risk factor for
recurrence (14–16). Advanced stage indicators including
T and N in pathological diagnosis are also risk factors for
recurrence of ESCC following curative resection with or without
neoadjuvant therapy (17–21). However, the risk factors for
recurrence after esophagectomy with NAC alone for ESCC have not
been clarified so far.
The present study aimed to identify the post-NAC
specific recurrence factors for ESCC. We analyzed the
clinicopathological factors focusing on patients treated with NAC
alone followed by esophagectomy.
Materials and methods
Patients
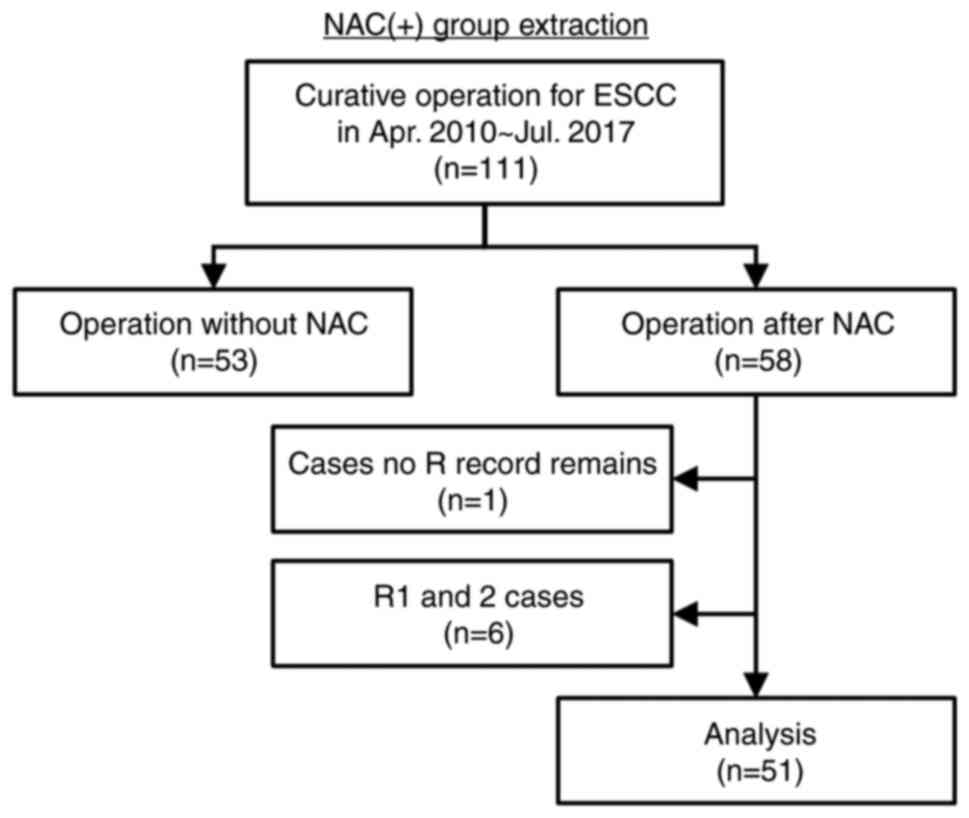
We retrospectively reviewed the records of 111
consecutive patients who underwent curative operation for ESCC at
the Department of Surgery and Oncology, Kyushu University Hospital
between April 2010 and July 2017 (Fig.
1). The tumor staging was classified according to the Japanese
Classification of Esophageal Cancer, 11th Edition (22,23).
There are no big differences in the definitions of the T and M
categories between the Japanese staging system and the staging
system of the Union for International Cancer Control (UICC)
(24), but there is a big
difference in N category. The UICC system defines the N category
according to the number of metastatic lymph nodes, while the
Japanese system determines the N category on the basis of the
location of the main tumor and the metastatic lymph nodes.
Demographic, clinical, surgical, pathological, postoperative, and
survival data were collected from the prospectively entered
clinical database of the department. Only patients identified as
ESCC on record were included. Patients who did not receive NAC or
patients with no record of residual tumor (R) and who had R were
excluded. After the application of these criteria, 51 patients were
extracted as the NAC (+) group. We included an NAC (−) group
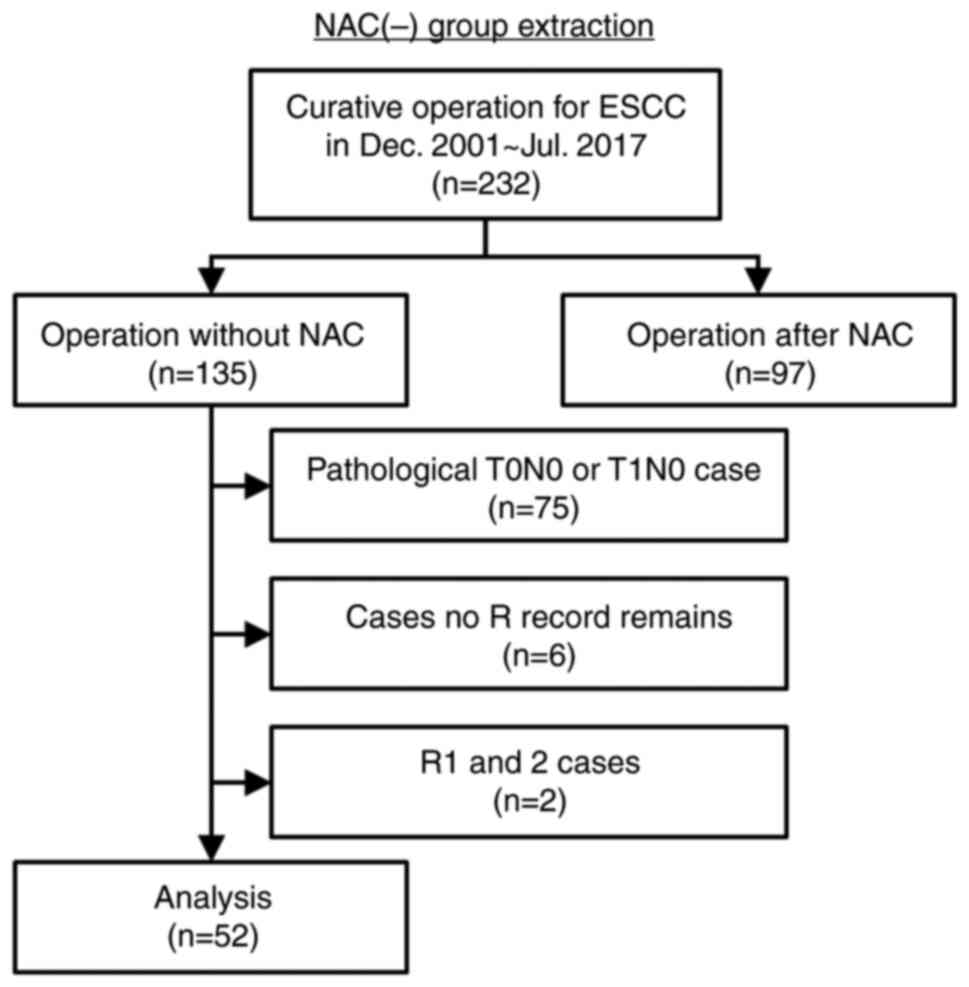
(Fig. 2) and compared this data
with the NAC (+) group. We retrospectively reviewed the records of
232 consecutive patients who underwent curative operation for ESCC
between December 2001 and July 2017. Among the patients without
NAC, pathological T0N0 or T1N0 cases were excluded to align the
background of both groups. Patients with no R record and patients
with R were also excluded. After application of the criteria, 52
patients were extracted as the NAC (−) group.
This study was approved by the institutional review
board of the Kyushu University Hospital (approval no. 22002-00) and
written informed consent was waived owing to the retrospective
analysis of the study.
Diagnosis and treatment
Clinical diagnosis was determined by barium swallow,
esophagogastroduodenoscopy (EGD), endoscopic ultrasound,
contrast-enhanced computed tomography (CT), and whole-body positron
emission tomography (PET). Clinical stage II and III patients
underwent NAC followed by esophagectomy.
The NAC regimen consisted of 80 mg/m2 of
cisplatin administered intravenously on day 1 followed by
continuous intravenous infusion of 800 mg/m2
5-fluorouracil on days 1 through 5. Most patients were administered
the medication for two cycles every 4 weeks. NAC was discontinued
if the patient experienced any issues (e.g., side effects such as
severe allergy, febrile neutropenia, and renal function disorder).
At approximately 4 weeks after the last round of NAC, the patients
underwent surgery. To assess medical operability, cardiac and
pulmonary functions were evaluated by electrocardiography,
echocardiography, and pulmonary function tests. All patients had an
American Society of Anesthesiologists physical status of I or II.
The operation for ESCC in this study was a subtotal esophagectomy
with three-field regional lymph node dissection (3-FL) regardless
of the use of thoracoscope and laparoscope.
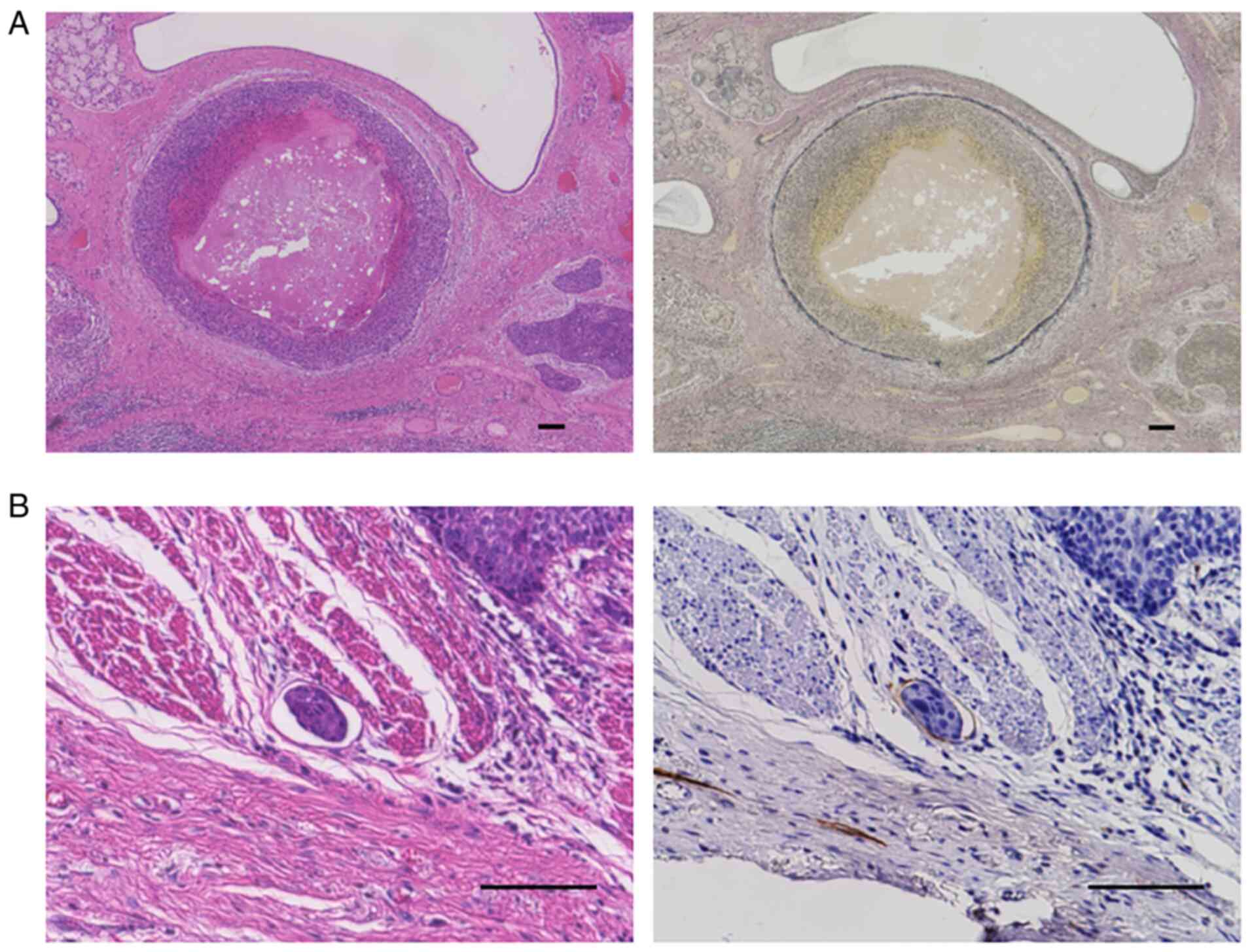
All surgically resected specimens were stained using
hematoxylin-eosin (HE). When the findings of HE-stained sections
were not sufficient to identify venous invasion (V) and lymphatic
invasion (LY), Elastica van Gieson (EVG) and D2-40 (413451, 1:5
dilution, Nichirei Biosciences Inc.) staining were performed for
the diagnosis (Fig. 3). An
automated immunohistochemistry system BOND-III (13B2X10268B30001,
Leica Biosystems) was used for D2-40. According to the protocols of
this system, the sections were incubated with BOND Epitope
Retrieval Solution 2 (AR9640, Leica Biosystems) at 100°C for 10
min, 3% H2O2 at room temperature for 5 min, D2-40 at room
temperature for 15 min, and BOND Polymer Refine Detection (DS9800,
Leica Biosystems) including DAB. The stained specimens were
confirmed by two or more pathologists, and the postoperative
diagnosis was determined according to the Japanese classification
(22,23).
Follow-up examinations were performed for 5 years
after operation using tumor marker measurements every 3 months,
contrast-enhanced CT every 6 months, and EGD every year.
Statistical analyses
Statistical analysis was performed using
JMP® 15 (SAS Institute Inc.). The 3-year RFS was
calculated from the date of the operation to the date of
recurrence, and patients without recurrence 3 years after operation
were censored at that time. Overall survival (OS) was calculated
from the date of the operation to the date of death. Patients who
were lost to follow-up were also censored at the date of last
contact. Univariate analysis for 3-year RFS and OS were estimated
with the Kaplan-Meier method and compared using the log-rank test.
The variables with P<0.05 in univariate analysis were included
in the multivariate models. Multivariate analysis for 3-year RFS
was performed using a Cox proportional hazards model. Student's
t-test and ANOVA were used for the comparison of continuous
variables. Pearson's χ2 test was used to compare
categorical variables. The threshold for significance was
P<0.05.
Results
Patient characteristics
A total of 51 patients were eligible for inclusion
in the NAC (+) group (Fig. 1).
Table I shows the detailed
clinical and pathological characteristics of the included patients.
Japanese guidelines indicate that NAC should be performed for only
stage II and III patients (11,12).
However, post-NAC preoperative diagnosis included 3 (6%) stage I
patients. Furthermore, 2 (4%) patients were stage 0, 4 (8%)
patients were stage I, and 3 (6%) patients were stage IV in
pathological diagnosis. Among the 51 total patients, 15 (29%)
patients had LY.
 | Table I.Characteristics of patients in the
NAC (+) group (n=51). |
Table I.
Characteristics of patients in the
NAC (+) group (n=51).
|
Characteristics | Number of
patients |
|---|
| Age, years |
|
| Median
(range) | 64 (44–79) |
| Sex [n (%)] |
|
|
Male/Female | 41 (80)/10
(20) |
| Location [n
(%)] |
|
|
Ce/Ut/Mt/Lt/Ae | 2 (4)/2 (4)/30 (59)/16 (31)/1 (2) |
| Post-NAC diagnosis
[n (%)] |
|
| T |
|
|
1a/1b/2/3 | 1 (2)/15 (29)/15 (29)/20 (40) |
| N |
|
|
0/1/2/3 | 14 (27)/13 (25)/15 (29)/9 (18) |
|
Stage |
|
|
I/II/III | 3 (6)/24 (47)/24 (47) |
| Operative time,
min |
|
| Median
(range) | 615 (340–935) |
| Blood loss, g |
|
| Median
(range) | 100 (21–524) |
| Pathological
diagnosis [n (%)] |
|
| T |
|
|
0/1a/1b/2/3 | 1 (2)/4 (8)/15 (29)/10 (20)/21 (41) |
| N |
|
|
0/1/2/3/4 | 13 (25)/7 (14)/24 (47)/5 (10)/2 (4) |
|
Stage |
|
|
0/I/II/III/IV | 2 (4)/4 (8)/20 (40)/23 (45)/2 (4) |
| V |
|
|
(−)/(+) | 42 (82)/9 (18) |
| LY |
|
|
(−)/(+) | 36 (71)/15
(29) |
Survival analysis in the NAC (+)
group
Kaplan-Meier analysis showed that lymph node
metastasis in pathological diagnosis (pN) (P=0.0290) and LY
(P=0.0031) were significantly associated with 3-year RFS in
patients who underwent NAC (Table
II). The Kaplan-Meier curves of RFS according to the status of
postoperative diagnosis are shown in Fig. 4. There was no significant
difference in OS between LY-positive and -negative patients during
the 3-year observation period (P=0.0747) (Fig. S1). In the multivariate analysis,
the independent factors significantly associated with 3-year RFS in
patients with esophagectomy after NAC were only LY (hazard ratio:
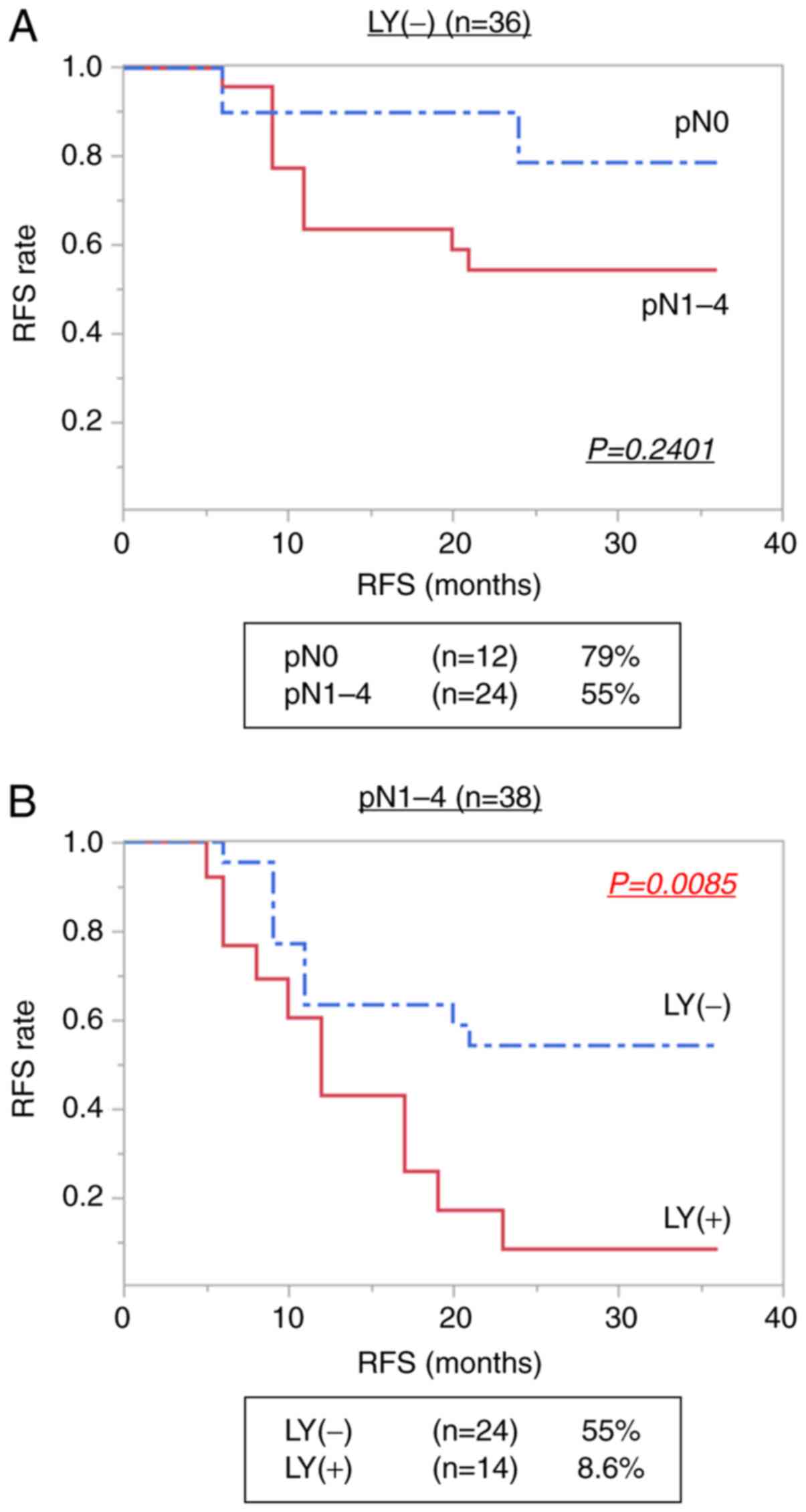
2.761; 95% CI: 1.86-6.43, P=0.018) (Table III). In the patients without LY
(n=36), there was no significant difference in 3-year RFS according
to the presence and absence of pN (P=0.2401) (Fig. 5A). However, in patients with pN
(n=38), a significant increase in the recurrence rate was observed
in patients with LY compared with those without LY (P=0.0085)
(Fig. 5B).
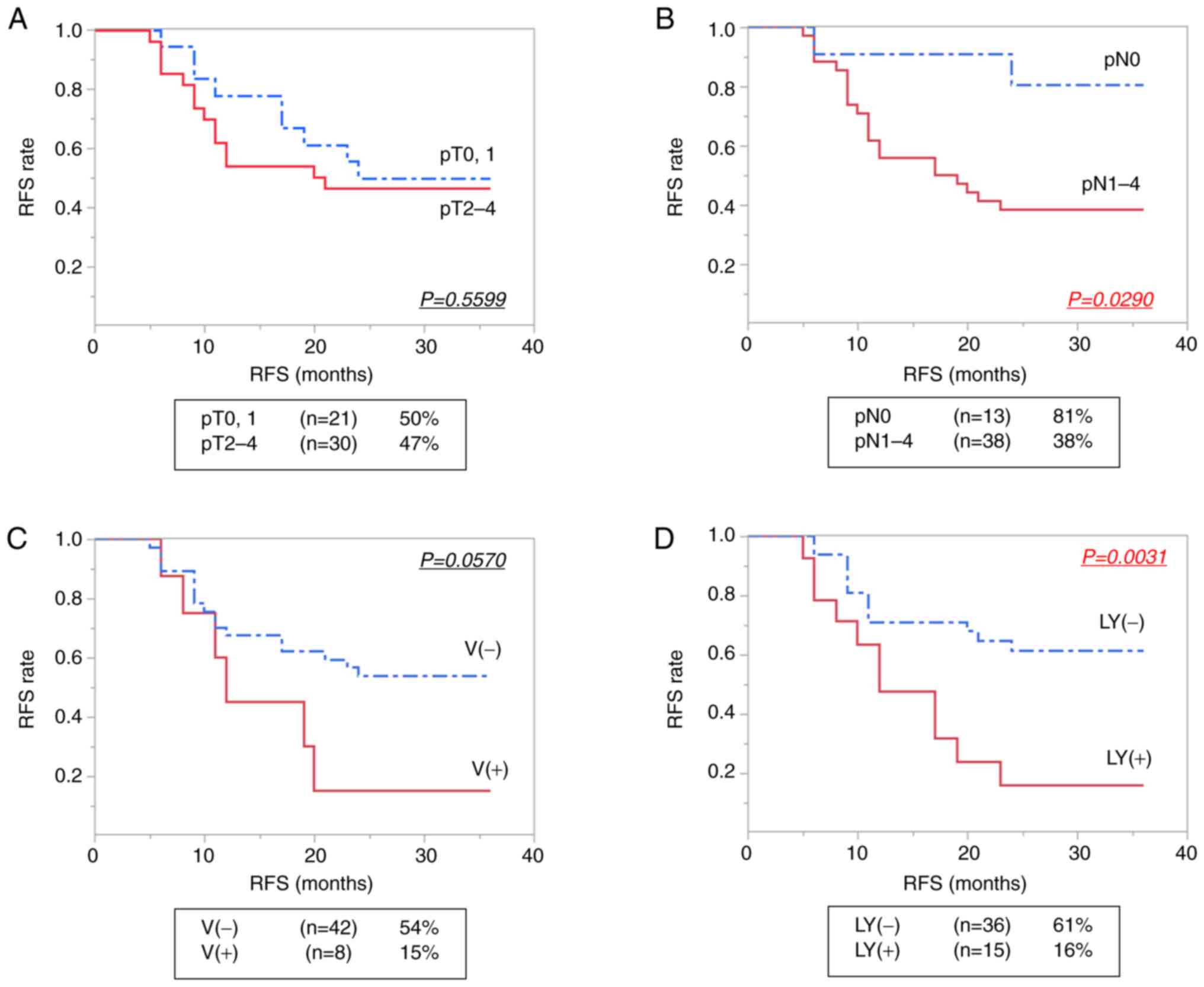 | Figure 4.Kaplan-Meier estimates of 3-year RFS
in NAC (+) patients. (A) pT0, 1 and pT2-4 subgroups, (B) pN0 and
pN1-4 subgroups, and according to the presence of (C) V and (D) LY.
The 3-year RFS rate is shown as a percentage in the square. All
factors were diagnosed based on the Japanese Classification of
Esophageal Cancer (11th Edition) edited by the Japan Esophageal
Society (22,23). RFS, recurrence-free survival; NAC,
neoadjuvant chemotherapy; pT, depth of tumor invasion; pN, grading
of lymph node metastasis; V, venous invasion; LY, lymphatic
invasion. |
 | Table II.Univariate analysis for 3-year RFS of
NAC (+) group (n=51). |
Table II.
Univariate analysis for 3-year RFS of
NAC (+) group (n=51).
|
Characteristics | n (%) | Mean RFS
(months) | 3-year RFS rate
(%) | P-value |
|---|
| Total | 51 (100) | 18 | 48 |
|
| Age, years |
|
|
| 0.5750 |
|
≤64 | 27 (53) | 18 | 42 |
|
|
>64 | 24 (47) | 11 | 55 |
|
| Sex |
|
|
| 0.2373 |
|
Male | 41 (80) | 19 | 50 |
|
|
Female | 10 (20) | 12 | 38 |
|
| Location |
|
|
| 0.4116 |
| Ce, Ut,
Mt | 34 (67) | 18 | 42 |
|
| Lt,
Ae | 17 (33) | 14 | 60 |
|
| Post-NAC
diagnosis |
|
|
|
|
| T |
|
|
| 0.2185 |
|
T0, 1 | 16 (31) | 21 | 57 |
|
|
T2-4 | 35 (69) | 15 | 44 |
|
| N |
|
|
| 0.6029 |
|
N0 | 14 (27) | 16 | 42 |
|
|
N1-4 | 37 (73) | 18 | 50 |
|
| Operative time,
min |
|
|
| 0.1703 |
|
≤600 | 23 (45) | 11 | 64 |
|
|
>600 | 28 (55) | 18 | 36 |
|
| Blood loss, g |
|
|
| 0.2593 |
|
≤100 | 27 (53) | 17 | 40 |
|
|
>100 | 24 (47) | 19 | 59 |
|
| Pathological
diagnosis |
|
|
|
|
| T |
|
|
| 0.5599 |
|
T0, 1 | 21 (41) | 20 | 50 |
|
|
T2-4 | 30 (59) | 15 | 47 |
|
| N |
|
|
| 0.0290 |
|
N0 | 13 (25) | 22 | 81 |
|
|
N1-4 | 38 (75) | 16 | 38 |
|
| V |
|
|
| 0.0570 |
|
(−) | 42 (82) | 19 | 54 |
|
|
(+) | 8 (18) | 14 | 15 |
|
| LY |
|
|
| 0.0031 |
|
(−) | 36 (71) | 19 | 61 |
|
|
(+) | 15 (29) | 14 | 16 |
|
 | Table III.Multivariate analysis for 3-year RFS
of the NAC (+) group. |
Table III.
Multivariate analysis for 3-year RFS
of the NAC (+) group.
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| pN (pN1-4 vs.
pN0) | 3.567 | 0.819-15.5 | 0.090 |
| LY [LY (+) vs. LY
(−)] | 2.761 | 1.86-6.43 | 0.018 |
Comparison of characteristics between
NAC (−) and (+) groups
We established the NAC (−) group (n=52) (Fig. 2) and compared the characteristics
of patients in the NAC (−) and (+) groups (Table IV). The NAC (+) group included
significantly more advanced cases in clinical N (P<0.0001), and
stage (P<0.0001) than the NAC (−) group. In contrast,
pathological results showed that there were significantly fewer LY
(+) cases in the NAC (+) group than those in the NAC (−) group
(P=0.0158). No significant difference in pN was observed between
patients with or without NAC (P=0.0680).
 | Table IV.Comparison of characteristics between
the NAC (−) and (+) groups. |
Table IV.
Comparison of characteristics between
the NAC (−) and (+) groups.
|
Characteristics | NAC(−) (n=52) | NAC(+) (n=51) | P-value |
|---|
| Age, years |
|
|
|
| Median
(range) | 69 (34–83) | 64 (44–79) | 0.2243 |
| Sex |
|
|
|
|
Male/Female | 46/6 | 41/10 | 0.2583 |
| Location |
|
|
|
|
Ce/Ut/Mt/Lt/Ae | 2/6/29/11/3 | 2/2/30/16/1 | 0.4138 |
| Clinical or
post-NAC diagnosis |
|
|
|
| T |
|
|
|
|
1a/1b/2/3 | 1/23/17/8 | 1/15/15/20 | 0.0747 |
|
1/2, 3 | 24/25 | 16/35 | 0.0724 |
| N |
|
|
|
|
0/1/2/3 | 37/7/5/0 | 14/13/15/9 | <0.0001 |
|
0/1-3 | 37/12 | 14/37 | <0.0001 |
|
Stage |
|
|
|
|
I/II/III | 21/20/8 | 3/24/24 | <0.0001 |
| Operative time,
min |
|
|
|
| Median
(range) | 596 (293–984) | 615 (340–935) | 0.8074 |
| Blood loss, g |
|
|
|
| Median
(range) | 215 (60–1370) | 100 (21–524) | <0.0001 |
| Pathological
diagnosis |
|
|
|
| T |
|
|
|
|
0/1a/1b/2/3/4a | 0/6/18/9/19 | 1/4/15/10/21 | 0.7696 |
|
0, 1/2-4 | 24/28 | 21/30 | 0.6106 |
| N |
|
|
|
|
0/1/2/3/4 | 6/21/19/5/1 | 13/7/24/5/2 | 0.0330 |
|
0/1-4 | 6/46 | 13/38 | 0.0680 |
|
Stage |
|
|
|
|
0/I/II/III/IV | 0/1/29/21/1 | 1/3/16/29/2 | 0.1180 |
| V |
|
|
|
|
(−)/(+) | 39/13 | 42/8 | 0.2611 |
| LY |
|
|
|
|
(−)/(+) | 24/27 | 36/15 | 0.0158 |
Discussion
This study investigated the clinicopathological
factors of patients treated with curative operation for ESCC after
NAC, with the aim of identifying NAC-specific recurrence factors.
Previous studies showed that R is a well-known recurrence factor
regardless of NAC (25–28), and thus we excluded cases with R
from this analysis. The results showed that LY in pathological
examination were significantly associated with recurrence. Our
results also showed that LY was a significant recurrence factor
among patients with pN, although the presence of pN was not
significantly correlated with the recurrence rate among patients
without LY.
Previous studies have shown that LVI is an
independent risk factor for recurrence after preoperative CRT and
esophagectomy in patients with ESCC (14–16).
Yoshida et al (29)
reported that V was an independent risk factor for early recurrence
within 6 months of resectable advanced ESCC following NAC, and
Zhang et al (30)
demonstrated that simultaneous LY and V were significantly
correlated with postoperative recurrence for ESCC without
neoadjuvant or adjuvant therapy. However, no report has examined
the clinical significance of LY as distinguished from V in cases of
NAC only rather than neoadjuvant CRT. This is the first study that
has focused on LY, and our results show that LY is an independent
recurrence factor in patients treated with esophagectomy after NAC
alone.
Previous studies have also shown that advanced stage
indicators including T and N in pathological examination are risk
factors for the recurrence of ESCC following curative resection
(17–21). Wang et al (18) reported that patients with pN had a
much higher recurrence rate than patients without pN. However, in
the present multivariate analysis, pN was not an independent risk
factor for recurrence. Furthermore, the presence or absence of pN
was not significantly related to recurrence among the patients
without LY. In contrast, LY was significantly associated with the
recurrence rate in patients with pN. The fact that pN was not a
significant factor for recurrence in our study may be related to
the surgical method specific to Japan. In Japan, 3-FL is the
standard method for lymph node dissection in esophageal cancer
operation, in line with the esophageal cancer practice guidelines
of Japan (11,12). The widespread use of 3-FL in Japan
is due to the rapid increase in the number of laparoscopic
esophagectomy for esophageal cancer in recent years (31). Meta-analyses and studies comparing
3-FL and 2-FL reported a tendency for a better prognosis of the
3-FL group (32–38). Ye et al (35) reported that 3-FL provides a better
5-year survival rate than 2-FL for thoracic esophageal cancer with
lymph node metastasis. In the present study, pN was not a risk
factor in NAC cases, indicating that 3-FL possibly decreased the
significance of lymph node metastasis in the risk of recurrence.
Taken together, the present data suggests that remnant LY, rather
than remnant lymph node metastasis, is a critical risk factor for
recurrence in patients who underwent esophagectomy with 3-FL after
NAC.
The main purpose of neoadjuvant therapy is to
downstage the primary tumor to facilitate complete resection
(3–6) and to reduce micrometastasis that
cause local or systemic recurrence (7,8).
Pathological tumor regression and the number of involved lymph
nodes have been reported to be significantly associated with the
prognosis of the patients who have received neoadjuvant CRT for
esophageal cancer (6,39–44).
However, the prognostic impact of pathological LY status in
patients with esophageal cancer who have undergone NAC has not been
fully investigated. In the present study, LY was significantly less
frequent in patients with NAC than in patients without NAC. These
data suggest that NAC contributes to regulate LY, which is a type
of microinvasion, and remnant LY after NAC may reflect the limited
control of micrometastasis in patients with NAC.
It is sometimes difficult to identify LVI and to
distinguish LY from V based only on the findings of HE-stained
sections (45).
Immunohistochemistry with D2-40 and EVG staining has been reported
to be useful for evaluate LVI (46). In the present study, we identified
LY and V using D2-40 and EVG staining, respectively, when the
findings of HE-stained sections were not sufficient to identify LY
and V. Therefore, our results may differ from reports that only
evaluated cases by HE staining. For further examination, all cases
should be subjected to stain with D2-40 and EVG to evaluate LVI
accurately.
This study has several limitations. First, it was a
retrospective study of a small number of patients that was
conducted at a single institution. Selection bias was also present
in the extraction of the NAC (−) group. Moreover, we did not
establish criteria for NAC dosage reduction during the study
period. The administration and dosage of NAC were ultimately
decided by the attending physicians depending on the patient's
condition and/or willingness, and thus the NAC (+) group in our
study included patients with both full-dose and lowered-dose NAC.
Therefore, further prospective multi-institutional studies with
larger populations are required to assess the true impact of
remnant LY for ESCC patients with NAC following esophagectomy. In
addition, the preoperative diagnosis may differ between our report
and reports from other countries because imaging examinations
including endoscopic ultrasound and PET are frequently used in
Japan.
We found that the presence of LY in pathological
examination was an independent risk factor for recurrence of ESCC
after esophagectomy with 3-FL following NAC. The present data also
showed that patients with NAC had significantly less LY than those
without NAC, although the NAC group included more advanced cases in
clinical diagnosis than the non-NAC group. These data suggest that
the remnant LY after NAC reflects the insufficient control of
micrometastasis. Therefore, adjuvant treatment after surgery may be
desirable in cases with remnant LY after NAC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI (grant nos. JP19H03732,
JP20K17621 and JP21K19530).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO, KO and MN contributed to the conception and
design of work. JK and YO contributed to the pathological
examination. SO and KO confirm the authenticity of all the raw
data. SO, KO, KS, TM, JK, KT, MS, KoN, YM, NI, KiN, YO and MN
contributed to the data analysis and interpretation, read and
approved of the final version to be published, agreed to be
accountable for all aspects of the work and ensured that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was performed in line with the principles
of the Declaration of Helsinki. Approval for this study was
obtained from the institutional review board of the Kyushu
University Hospital (approval no. 22002-00). The requirement of
written informed consent to participate was waived in accordance
with the standards of the institutional review board, and an
opt-out method was used at our institution due to the retrospective
analysis of de-identified data.
Patient consent for publication
Informed consent for publication was obtained from
all individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
RFS
|
recurrence-free survival
|
|
NAC
|
neoadjuvant chemotherapy
|
|
CRT
|
chemoradiotherapy
|
|
LVI
|
lymphovascular invasion
|
|
UICC
|
Union for International Cancer
Control
|
|
R
|
residual tumor
|
|
EGD
|
esophagogastroduodenoscopy
|
|
CT
|
contrast-enhanced computed
tomography
|
|
PET
|
positron emission tomography
|
|
3-FL
|
three-field regional lymph node
dissection
|
|
HE
|
hematoxylin-eosin
|
|
V
|
venous invasion
|
|
LY
|
lymphatic invasion
|
|
EVG
|
Elastica van Gieson
|
|
pN
|
pathological lymph node metastasis
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe M, Tachimori Y, Oyama T, Toh Y,
Matsubara H, Ueno M, Kono K, Uno T, Ishihara R, Muro K, et al:
Comprehensive registry of esophageal cancer in Japan, 2013.
Esophagus. 18:1–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackham AU, Yue B, Almhanna K, Saeed N,
Fontaine JP, Hoffe S, Shridhar R, Frakes J, Coppola D and Pimiento
JM: The prognostic value of residual nodal disease following
neoadjuvant chemoradiation for esophageal cancer in patients with
complete primary tumor response. J Surg Oncol. 112:597–602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamarajah SK, Navidi M, Wahed S, Immanuel
A, Hayes N, Griffin SM and Phillips AW: Significance of neoadjuvant
downstaging in carcinoma of esophagus and gastroesophageal
junction. Ann Surg Oncol. 27:3182–3192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bollschweiler E, Hölscher AH, Metzger R,
Besch S, Mönig SP, Baldus SE and Drebber U: Prognostic significance
of a new grading system of lymph node morphology after neoadjuvant
radiochemotherapy for esophageal cancer. Ann Thorac Surg.
92:2020–2027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berger AC, Farma J, Scott WJ, Freedman G,
Weiner L, Cheng JD, Wang H and Goldberg M: Complete response to
neoadjuvant chemoradiotherapy in esophageal carcinoma is associated
with significantly improved survival. J Clin Oncol. 23:4330–4337.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuyama J, Doki Y, Yasuda T, Miyata H,
Fujiwara Y, Takiguchi S, Yamasaki M, Makari Y, Matsuura N, Mano M
and Monden M: The effect of neoadjuvant chemotherapy on lymph node
micrometastases in squamous cell carcinomas of the thoracic
esophagus. Surgery. 141:570–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hiraki Y, Kimura Y, Imano M, Kato H, Iwama
M, Shiraishi O, Yasuda A, Shinkai M, Makino T, Motoori M, et al:
Controlling lymph node micrometastases by neoadjuvant chemotherapy
affects the prognosis in advanced esophageal squamous cell
carcinoma. Surg Today. 51:118–126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mariette C, Dahan L, Mornex F, Maillard E,
Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
stage I and II esophageal cancer: Final analysis of randomized
controlled phase III trial FFCD 9901. J Clin Oncol. 32:2416–2422.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
esophageal society: Part 1. Esophagus. 16:1–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
esophageal society: Part 2. Esophagus. 16:25–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamasaki M, Yasuda T, Yano M, Hirao M,
Kobayashi K, Fujitani K, Tamura S, Kimura Y, Miyata H, Motoori M,
et al: Multicenter randomized phase II study of cisplatin and
fluorouracil plus docetaxel (DCF) compared with cisplatin and
fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for
resectable esophageal squamous cell carcinoma (OGSG1003). Ann
Oncol. 28:116–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu PK, Chien LI, Wang LC and Chou TY;
Taipei Veterans General Hospital Esophageal Cancer Panel, :
Lymphovascular invasion and extracapsular invasion are risk factors
for distant recurrence after preoperative chemoradiotherapy and
oesophagectomy in patients with oesophageal squamous cell
carcinoma. Eur J Cardiothorac Surg. 51:1188–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu CC, Hsu PK, Chien LI, Liu WC, Huang CS,
Hsieh CC, Hsu HS and Wu YC: Prognostic histological factors in
patients with esophageal squamous cell carcinoma after preoperative
chemoradiation followed by surgery. BMC Cancer. 17:622017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagarde SM, Phillips AW, Navidi M, Disep
B, Immanuel A and Griffin SM: The presence of lymphovascular and
perineural infiltration after neoadjuvant therapy and
oesophagectomy identifies patients at high risk for recurrence. Br
J Cancer. 113:1427–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee PC, Mirza FM, Port JL, Stiles BM, Paul
S, Christos P and Altorki NK: Predictors of recurrence and
disease-free survival in patients with completely resected
esophageal carcinoma. J Thorac Cardiovasc Surg. 141:1196–1206.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Jia Y, Wang J, Wang X, Bao C, Song
Q, Tan B and Cheng Y: Prognostic significance of lymph node ratio
in esophageal cancer. Tumour Biol. 36:2335–2341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon DH, Jeon JH, Yang HC, Kim YI, Lee JY,
Kim MS, Lee JM and Lee GK: Intramural metastasis as a risk factor
for recurrence in esophageal squamous cell carcinoma. Ann Thorac
Surg. 106:249–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan M, Urooj N, Syed AA, Khattak S, Kazmi
A, Ashraf MI and Batool S: Prognostic factors for recurrence in
esophageal cancer patients treated with neoadjuvant therapy and
surgery: A single-institution analysis. Cureus.
12:e81082020.PubMed/NCBI
|
|
21
|
Kang CH, Hwang Y, Lee HJ, Park IK and Kim
YT: Risk factors for local recurrence and optimal length of
esophagectomy in esophageal squamous cell carcinoma. Ann Thorac
Surg. 102:1074–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Japan Esophageal Society: Japanese
classification of esophageal cancer, 11th edition: Part I.
Esophagus. 14:1–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Japan Esophageal Society: Japanese
classification of esophageal cancer, 11th edition: Part II and III.
Esophagus. 14:37–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brierley J, Gospodarowicz MD and Wittekind
CT: TNM Classification of Malignant Tumours. 8th Edition. Wiley;
Oxford: pp. 57–62. 2017
|
|
25
|
Markar SR, Gronnier C, Duhamel A, Pasquer
A, Théreaux J, Chalret du Rieu M, Lefevre JH, Turner K, Luc G and
Mariette C; FREGAT Working Group-FRENCH-AFC, . Significance of
microscopically incomplete resection margin after esophagectomy for
esophageal cancer. Ann Surg. 263:712–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hulshoff JB, Faiz Z, Karrenbeld A,
Kats-Ugurlu G, Burgerhof JG, Smit JK and Plukker JT: Prognostic
value of the circumferential resection margin in esophageal cancer
patients after neoadjuvant chemoradiotherapy. Ann Surg Oncol. 22
(Suppl 3):S1301–S1309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gilbert S, Martel AB, Seely AJ, Maziak DE,
Shamji FM, Sundaresan SR and Villeneuve PJ: Prognostic significance
of a positive radial margin after esophageal cancer resection. J
Thorac Cardiovasc Surg. 149:548–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schlick CJR, Khorfan R, Odell DD, Merkow
RP and Bentrem DJ: Margin positivity in resectable esophageal
cancer: Are there modifiable risk factors? Ann Surg Oncol.
27:1496–1507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshida N, Baba Y, Shigaki H, Harada K,
Iwatsuki M, Sakamoto Y, Miyamoto Y, Kurashige J, Kosumi K, Tokunaga
R, et al: Risk factors of early recurrence within 6 months after
esophagectomy following neoadjuvant chemotherapy for resectable
advanced esophageal squamous cell carcinoma. Int J Clin Oncol.
21:1071–1078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Chen X, Wang S, Fan J and Lu L:
Poorer prognosis associated with simultaneous lymphatic and
vascular invasion in patients with squamous carcinoma of the
thoracic oesophagus. Eur J Cardiothorac Surg. 52:378–384. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inomata M, Shiroshita H, Uchida H, Bandoh
T, Akira S, Yamaguchi S, Kurokawa Y, Seki Y, Eguchi S, Wada N, et
al: Current status of endoscopic surgery in Japan: The 14th
national survey of endoscopic surgery by the Japan society for
endoscopic surgery. Asian J Endosc Surg. 13:7–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Udagawa H: Past, present, and future of
three-field lymphadenectomy for thoracic esophageal cancer. Ann
Gastroenterol Surg. 4:324–330. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuda S, Takeuchi H, Kawakubo H and
Kitagawa Y: Three-field lymph node dissection in esophageal cancer
surgery. J Thorac Dis. 9 (Suppl 8):S731–S740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang QX, Chen LQ, Hu WP, Deng HY, Yuan Y
and Cai J: Three-field lymph node dissection in treating the
esophageal cancer. J Thorac Dis. 8:E1136–E1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye T, Sun Y, Zhang Y, Zhang Y and Chen H:
Three-field or two-field resection for thoracic esophageal cancer:
A meta-analysis. Ann Thorac Surg. 96:1933–1941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma GW, Situ DR, Ma QL, Long H, Zhang LJ,
Lin P and Rong TH: Three-field vs two-field lymph node dissection
for esophageal cancer: A meta-analysis. World J Gastroenterol.
20:18022–18030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altorki N, Kent M, Ferrara C and Port J:
Three-field lymph node dissection for squamous cell and
adenocarcinoma of the esophagus. Ann Surg. 236:177–183. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lerut T, Nafteux P, Moons J, Coosemans W,
Decker G, De Leyn P, Van Raemdonck D and Ectors N: Three-field
lymphadenectomy for carcinoma of the esophagus and gastroesophageal
junction in 174 R0 resections: Impact on staging, disease-free
survival, and outcome: A plea for adaptation of TNM classification
in upper-half esophageal carcinoma. Ann Surg. 240:962–974. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reynolds JV, Muldoon C, Hollywood D, Ravi
N, Rowley S, O'Byrne K, Kennedy J and Murphy TJ: Long-term outcomes
following neoadjuvant chemoradiotherapy for esophageal cancer. Ann
Surg. 245:707–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mariette C, Piessen G, Briez N and
Triboulet JP: The number of metastatic lymph nodes and the ratio
between metastatic and examined lymph nodes are independent
prognostic factors in esophageal cancer regardless of neoadjuvant
chemoradiation or lymphadenectomy extent. Ann Surg. 247:365–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koen Talsma A, Shapiro J, Looman CW, van
Hagen P, Steyerberg EW, van der Gaast A, van Berge Henegouwen MI,
Wijnhoven BP, van Lanschot JJ; CROSS Study Group, ; et al: Lymph
node retrieval during esophagectomy with and without neoadjuvant
chemoradiotherapy: Prognostic and therapeutic impact on survival.
Ann Surg. 260:786–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chirieac LR, Swisher SG, Ajani JA, Komaki
RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR and Wu TT:
Posttherapy pathologic stage predicts survival in patients with
esophageal carcinoma receiving preoperative chemoradiation. Cancer.
103:1347–1355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schneider PM, Baldus SE, Metzger R, Kocher
M, Bongartz R, Bollschweiler E, Schaefer H, Thiele J, Dienes HP,
Mueller RP and Hoelscher AH: Histomorphologic tumor regression and
lymph node metastases determine prognosis following neoadjuvant
radiochemotherapy for esophageal cancer: Implications for response
classification. Ann Surg. 242:684–692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyata H, Tanaka K, Makino T, Yamasaki M,
Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Morii
E, et al: The impact of pathological tumor regression and nodal
status on survival and systemic disease in patients undergoing
neoadjuvant chemotherapy for esophageal squamous cell carcinoma.
Ann Surg Oncol. 25:2409–2417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mohammed RA, Martin SG, Gill MS, Green AR,
Paish EC and Ellis IO: Improved methods of detection of
lymphovascular invasion demonstrate that it is the predominant
method of vascular invasion in breast cancer and has important
clinical consequences. Am J Surg Pathol. 31:1825–1833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu J, Li H, Zhou P, Cai T, Tang Z, Wang
Y, Cui Y, Sun Y and Wang X: Reevaluation of lymphovascular invasion
in gastric cancer using endothelial markers D2-40 and EVG: Enhanced
detection, better predictor of lymph node metastasis and biological
aggressiveness. J Surg Oncol. 123:1736–1741. 2021. View Article : Google Scholar : PubMed/NCBI
|