Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequently occurring malignant tumors, which accounts for >80%
of liver cancer cases (1,2). HCC can be generally subclassified as
primary and secondary malignant HCC, and the former type has a poor
prognosis, with a particularly high mortality rate in China
(1,2). The current treatment options for HCC
include surgical resection, chemotherapy, radiation therapy and
gene therapy (3). However, despite
the rapid progress in technology yielding new candidate diagnostic
and therapeutic approaches, the curative rate for HCC remains poor
with an estimated 5-year survival rate of only 12% (3). To overcome this challenge, new
treatment strategies are needed, thus necessitating improved
understanding of HCC progression.
Tumor-treating fields (TTFields) are low-intensity,
intermediate-frequency alternating electric fields, which can act
on rapidly dividing glioma and other cancer cells, especially
during the metaphase, anaphase and telophase stages of mitotic cell
division (4). With the application
of an alternating electric field, charged molecules within the
cancerous cells start to oscillate along with the rotation of the
dipoles (5). Under the alternating
electric field, molecules with a high electrical dipole moment such
as tubulin dimers and septins are forced to align with the
direction of the alternating electric fields. Since these molecules
are the polymers generated in a mitotic cell during metaphase
(6,7), the TTFields disrupt the microtubule
spindle formation and the localization of septin fibers, leading to
mitotic catastrophe and ultimately mitotic cell death (8). Nevertheless, a high number of cells
can still pass through this stage and enter the subsequent anaphase
and telophase stages, in which the mitotic cell assumes an
hourglass shape through the formation of a cleavage furrow in the
center to facilitate the formation of daughter cells. An
alternative electric field disrupts this new shape, causing the
polarized components to move toward the furrow (dielectrophoresis),
ultimately obstructing the entire process of mitosis (8–10).
Therefore, the application of TTFields can lead to death or to the
formation of abnormal dividing cells with an unusual number of
chromosomes (9,10).
Preclinical studies have demonstrated increased
sensitivity to chemotherapy in combination with TTFields, both in
human glioblastoma (GBM) cell lines and in animal tumor models
(9–12). In addition, a synergistic effect
was observed between TTFields and radiotherapy, which may benefit
patients with GBM (13,14).
Clinical trials have also shown that patients with
recurrent GBM can benefit from TTFields alone, as this treatment
prolonged their overall survival without complications (15). Furthermore, the common TTFields
side effects were not observed, except for medical device site
reaction headache and muscle twitching, and the main side effect
for TTFields is mild to moderate skin irritation underneath the
transducer arrays. Further, common TTFields side effects did not
show except included medical device site reaction, headache and
muscle twitching (16). However,
TTFields technology has evolved in recent years to achieve improved
results, leading to its approval by the US Food and Drug
Administration. Currently, TTFields are regarded as an alternative
to the standard treatment for patients with recurrent GBM
designated as National Comprehensive Cancer Network category 1 and
has also received a Communauté Européenne certification mark in
Europe (17).
The preclinical and clinical data of the effects of
TTFields are currently mainly available for GBM and are being
studied for other cancer types. Therefore, in the present study,
the effects of TTFields were evaluated in liver cancer cells for
their ability to inhibit proliferation.
Materials and methods
Experimental setup of the electric
fields
TTFields were generated using a pair of insulated
wires connected to a functional generator and a high-voltage
amplifier, which generated sine-wave signals ranging from 0 to 800
V (18). The setup resulted in an
applied electric field intensity and frequency of 0.9 V/cm and 150
kHz, respectively. A field intensity of 1.0 V/cm was used due of
its use in clinical settings. For irradiation, cells were plated in
100-mm dishes and incubated at 37°C in a humidified atmosphere
containing 5% CO2 until they reached 70–80%
confluence.
Cell culture
The human hepatocarcinoma Hep3B cells were obtained
from the Korean Cell Line Bank (KCLB; cat. no. 88064) and were
cultured in DMEM supplemented with 10% heat-inactivated FBS (both
Gibco; Thermo Fisher Scientific, Inc.), 0.1 mM non-essential amino
acids, glutamine, HEPES and antibiotics at 37°C in a 5%
CO2 humidified incubator. The human HepG2 hepatoblastoma
cell line was obtained from the KCLB (cat. no. 88065) and grown in
RPMI-1640 medium supplemented with 10% FBS, glutamine, HEPES and
antibiotics at 37°C in a 5% CO2 humidified
incubator.
Cell viability assay
Liver cancer cells were treated with TTFields (1.0
V/cm; 150 kHz), 5 µM sorafenib (Selleck Chemicals) or a combination
of both for 24 h, and cell viability was determined using a Trypan
blue exclusion assay. An equal volume of Trypan blue reagent was
added to the cell suspension, and the percentage of viable cells
was evaluated under a light microscope (Olympus CK40; Olympus
Corporation). The assays were performed in triplicate.
Colony-forming assay
Hep3B and HepG2 cells (500–1,000) were seeded into
6-well plates in triplicate and treated with TTFields (1.0 V/cm;
150 kHz), sorafenib (5 µM) or both concurrently for 72 h. After
10–14 days, colonies were fixed with 100% methanol for 30 min and
stained with 0.4% crystal violet (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's instructions (11). The plating efficiency (PE)
represents the percentage of seeded cells that grew into colonies
under the specific culture conditions of a given cell line. The
survival fraction was calculated as follows: Survival
fraction=colonies counted/(cells seeded × PE/100). Colonies are
counted using imaging analysis software.
Cell death detection
Cell death was analyzed in the Hep3B and HepG2 cell
lines 72 h after concurrent treatment with TTFields (1.0 V/cm; 150
kHz) and 5 µM sorafenib using a Cell Death Detection ELISA kit
(Roche Diagnostics GmbH). Cells were treated, harvested and stained
with cell death detection reagent according to the manufacturer's
protocol (19). The absorbance was
then measured using a Multiskan EX plate reader (Thermo Fisher
Scientific, Inc.) at 450 nm.
Caspase3 activity assay
Caspase-3 activity was analyzed in the Hep3B and
HepG2 cell lines 72 h after concurrent treatment with TTFields (1.0
V/cm; 150 kHz) and 5 µM sorafenib using a Caspase-Glo 3/7 assay
detection kit (cat. no. G8091; Promega Corporation). The assay is
based on spectrophotometric detection of the chromophore
p-nitroanilide (pNA) after cleavage from the labeled
substrates of DEVD-pNA (for caspase-3). The pNA light
emission was quantified using a Multiskan EX plate reader (Thermo
Fisher Scientific, Inc.) at 405 nm. Comparison of the pNA
absorbance of apoptotic and control samples allows determination of
the fold increase in caspase activity.
Reactive oxygen species (ROS)
detection
ROS generation was analyzed in the Hep3B and HepG2
cell lines 6 h after treatment with TTFields (1.0 V/cm; 150 kHz)
and 5 µM sorafenib. The cells were cultured, harvested at the
indicated times, according to the manufacturer's protocol (20) and ROS levels were then measured
using a Multiskan EX plate reader (Thermo Fisher Scientific, Inc.)
at 450 nm (20). ROS was monitored
using the fluorescent ROS indicator,
C2′,7′-dichlorodihydrofluorescein diacetate (5 µM; Molecular
Probes). N-acetyl cysteine (NAC) was obtained from Sigma-Aldrich
(Merck KGaA), and Hep3B and HepG2 cell lines were subsequently
treated with NAC (10 mM) and the indicated concentration of
sorafenib or TTFields. The production of ROS was estimated using
FACS analysis with DCFDA staining. The data were acquired using a
FACSort™ flow cytometer with CellQuest™ software (version 7.5.3;
both from BD Biosciences).
Three-dimensional (3D) culture
system
Hep3B and HepG2 cells were seeded in 96-well plates
at a density of 1×104 cells/well. In the 3D culture
model, the 96-well plates were pre-coated with 40 µl
Matrigel® basement membrane, then incubated at 37°C for
30 min. The cells were plated on the gel in an appropriate medium
(10% heat-inactivated FBS, 0.1 mM non-essential amino acids,
glutamine, HEPES and 1% (v/v) penicillin-streptomycin
(Gibco®, Life Technologies), and the wells were examined
using a light microscope (Olympus CK40; Olympus Corporation) after
a duration of 10 days (21).
Transwell chamber assay
The migration and invasion of liver cancer cells
were measured using Transwell chambers according to the
manufacturer's protocol and as described previously (21). Briefly, the cells were seeded onto
the membrane of the upper chamber at a concentration of
4×105 cells/ml in 150 µl serum-free medium and were
either left untreated or treated with TTFields for 24 h. The medium
in the lower chamber contained 10% (v/v) FBS as a source of
chemoattractants. For the invasion assay, cells that passed through
the Matrigel®-coated membrane (coating time, 30 min at
37°C) were stained with Cell Stain Solution containing Crystal
violet (MilliporeSigma) for 30 min, and for the migration assay,
cells that passed through the gelatin-coated membrane were stained
as previously described and examined after 24-h incubation. The
wells were evaluated under a light microscope (Olympus CK40;
Olympus Corporation).
Western blot analysis
Total proteins from liver cancer cells were
extracted in RIPA buffer [50 mM Tris-Cl (pH 7.4), 1% NP-40, 150 mM
NaCl and 1 mM EDTA] supplemented with protease inhibitors (1 mM
PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin and 1 mM
Na3VO4) and quantified using the Bradford
method. Protein samples (30 µg) were separated using SDS-PAGE on an
11% gel and subsequently transferred to a nitrocellulose membrane.
After blocking non-specific antibody binding sites using 5% skim
milk diluted in 1X TBS with 0.1% Tween-20 for 1 h at room
temperature, the membrane was incubated overnight at 4°C with
primary antibodies against poly (ADP-ribose) polymerase (1:1,000;
PARP; cat. no. 9542), cleaved PARP (1:1,000; cat. no. 9541),
caspase-3 (1:1,000; cat. no. 9662), cleaved caspase-3 (1:1,000;
cat. no. 9664) and MMP9 (1:1,000; cat. no. 3852), all purchased
from Cell Signaling Technology, Inc. Anti-β-actin (1:200; cat. no.
sc-47778) was purchased from Santa Cruz Biotechnology, Inc. After
incubation with the following peroxidase-conjugated secondary
antibodies: Mouse anti-rabbit IgG-HRP (1:5,000; cat. no. sc-2357;
Santa Cruz Biotechnology, Inc.) and goat anti-mouse IgG-HRP
(1:5,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) at 37°C
for 1 h, the protein bands were visualized using enhanced
chemiluminescence reagent (GE Healthcare Life Sciences) and
detected using the Amersham Imager 680 (version, 2.0; GE Healthcare
Life Sciences).
Statistical analysis
Statistical significance was determined using
two-way ANOVA followed by tukey's post hoc test. Values represent
the mean of three experimental repeats ± standard deviation. Data
analysis was performed using the GraphPad Prism 6 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of TTFields on liver cancer
cell proliferation
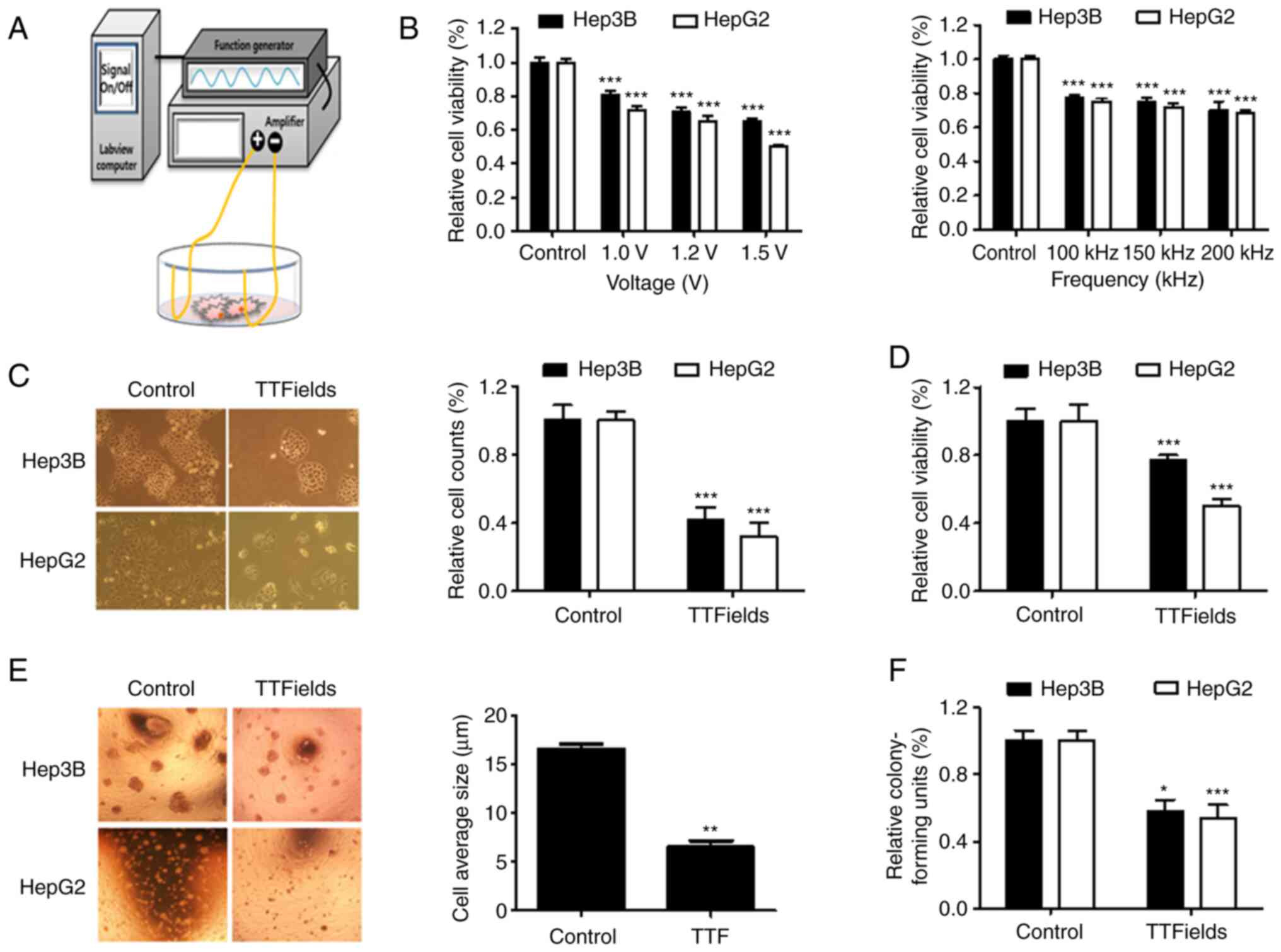
The TTFields setup was constructed using insulated
wires connected to a generator and an amplifier to generate a
sine-wave signal ranging from 0 to 800 V (Fig. 1A). To determine the optimal
TTFields voltage and frequency, Hep3B and HepG2 cells were
subjected to various conditions (Voltage, 0, 1.0, 1.2 and 1.5 V/cm;
frequency, 0, 100, 150 and 200 kHz) for 48 h (Fig. 1B). The two liver cancer cell lines
exhibited a voltage-dependent reduction in cell viability (~20% at
1.0 V/cm; 150 kHz). To evaluate TTFields-induced cytotoxicity and a
cell viability assay was performed using Hep3B and HepG2 cells.
Application of TTFields for 48 h resulted in a significant
reduction in the proliferation of Hep3B and HepG2 cells, as
determined by the Trypan blue (Fig.
1C) and MTT assays (Fig. 1D).
Furthermore, the colonies in untreated 3D cultures (size, 17 µm)
were significantly larger than those formed by TTFields-treated
cells (size, 6 µm; Fig. 1E). The
survival fraction showed a clonogenic efficiency with a reduction
of 42% in Hep3B cells and of 46% in HepG2 cells following treatment
(Fig. 1F). Collectively, these
findings suggest that TTFields can inhibit the proliferation of
liver cancer cells.
TTFields enhances the apoptosis of
liver cancer cells
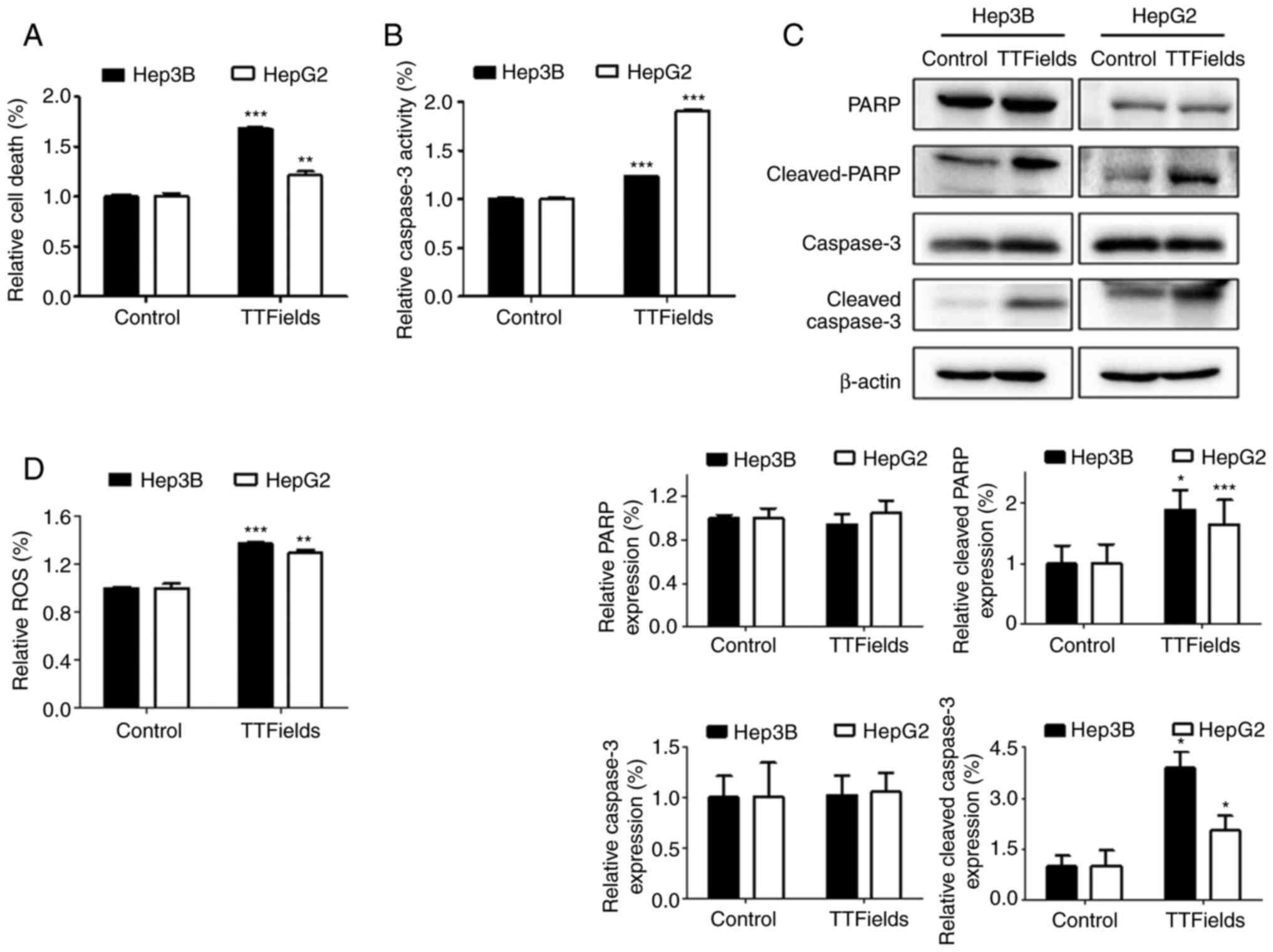
To investigate whether the apoptosis of liver cancer
cells was induced by the TTFields, early apoptosis was assessed
using a cell death detection kit. Exposure to TTFields for 72 h
significantly increased the proportion of apoptotic cells in both
liver cancer cell lines (Fig. 2A).
It was also hypothesized that enhanced TTFields-induced
cytotoxicity resulted from the activation of the chief executioners
of cell death, caspase-3 and PARP fragmentation (22). The results demonstrated increased
caspase-3 activity and PARP cleavage in response to TTFields
compared with the control group (Fig.
2B and C). In addition, the production of ROS significantly
increased in both cells lines following TTFields application
(Fig. 2D). These results indicate
that ROS generated by the TTFields treatment increases
intracellular caspase signaling leading to apoptosis.
TTFields suppresses cell migration and
invasion
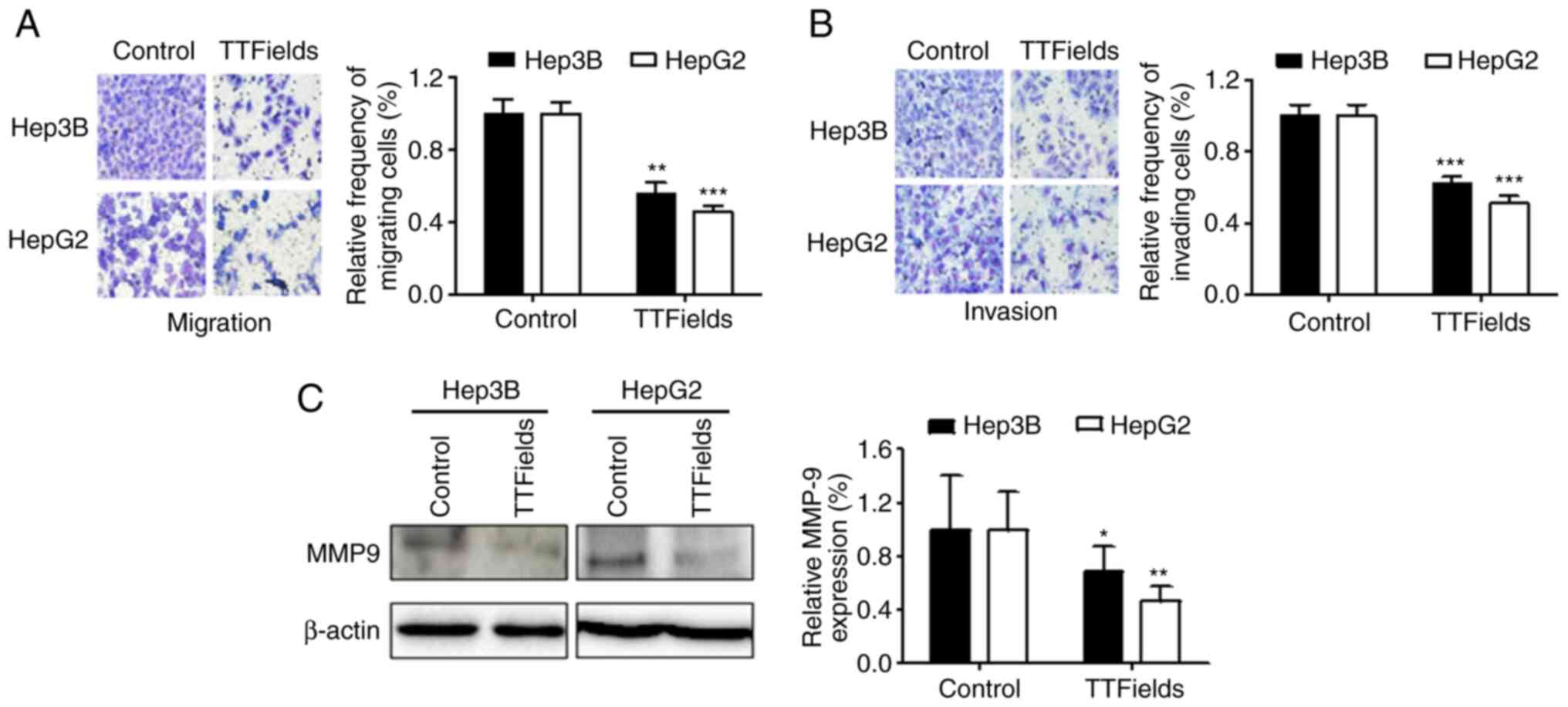
TTFields treatment has been shown to significantly
inhibit tumor cell migration and invasion (21). Therefore, the effect of TTFields on
the invasive and migratory capacities of liver cancer cells was
examined using Transwell chamber assays. The results demonstrated
that TTFields significantly inhibited cell migration compared with
the control group (Fig. 3A).
Similarly, TTFields treatment inhibited the invasion of both liver
cancer cell lines (Fig. 3B).
Notably, TTFields also downregulated the expression of MMP9 in
liver cancer cells (Fig. 3C).
Sorafenib sensitizes liver cancer
cells to TTFields
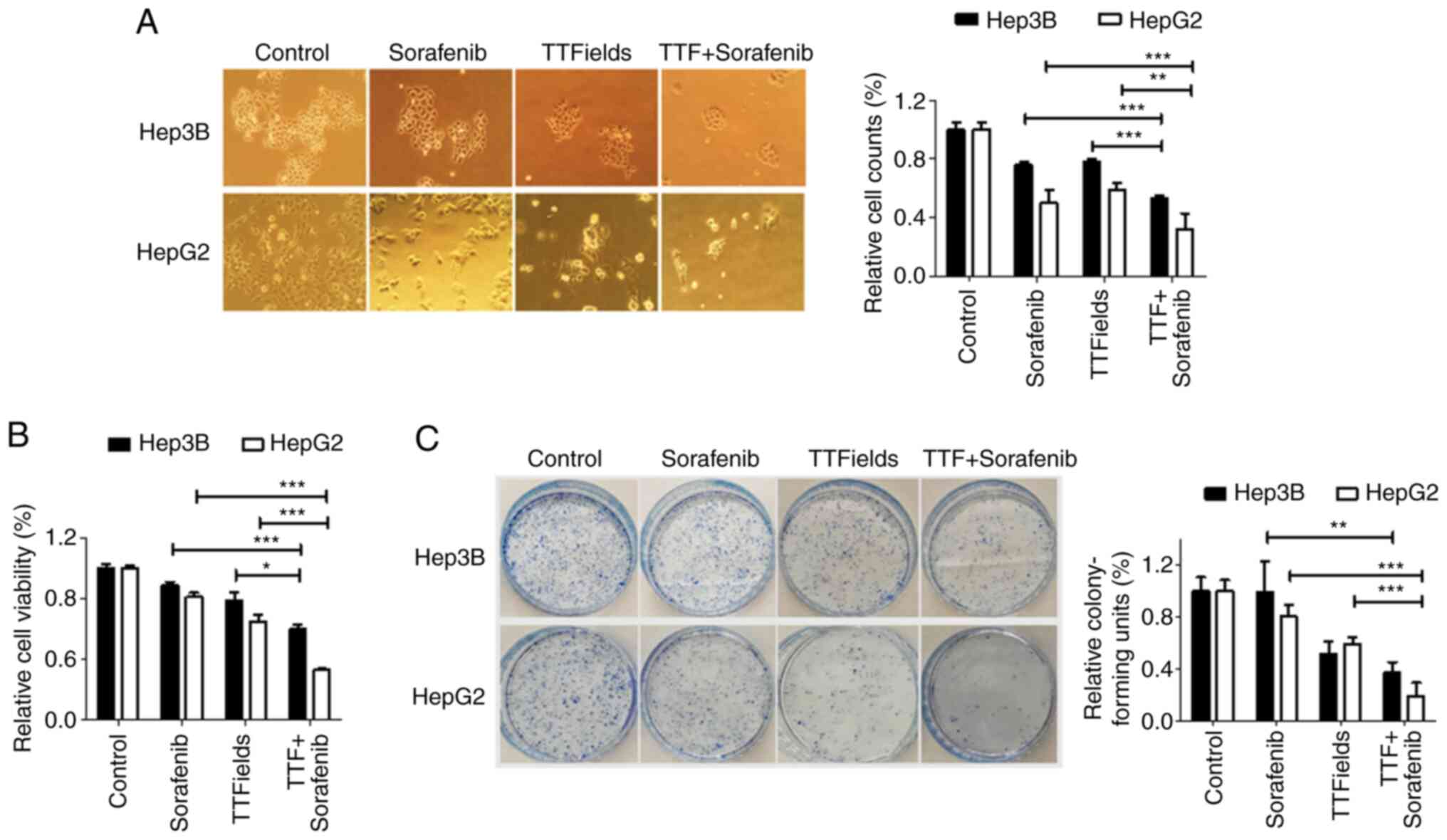
TTFields were applied to Hep3B and HepG2 cells in
combination with the multi-kinase inhibitor sorafenib (Fig. 4A). In previous studies, to evaluate
the effects of sorafenib on TTFields-induced cytotoxicity, a 5-µM
sorafenib concentration was used, which resulted in 25% growth
inhibition after a 48-h exposure in each experiment (23,24).
In the present study, after 48 h, the combination of sorafenib and
TTFields resulted in a significantly greater antitumor effect on
Hep3B and HepG2 cells than either treatment alone, as evidenced by
the Trypan blue exclusion assay and the MTT assay (Fig. 4A and B). Moreover, in the colony
formation assay, the relative colony forming units were further
decreased in cells treated with TTFields and sorafenib than those
in cells receiving either of these treatments alone (Fig. 4C). These results indicated that
sorafenib sensitized liver cancer cells to TTFields in
vitro.
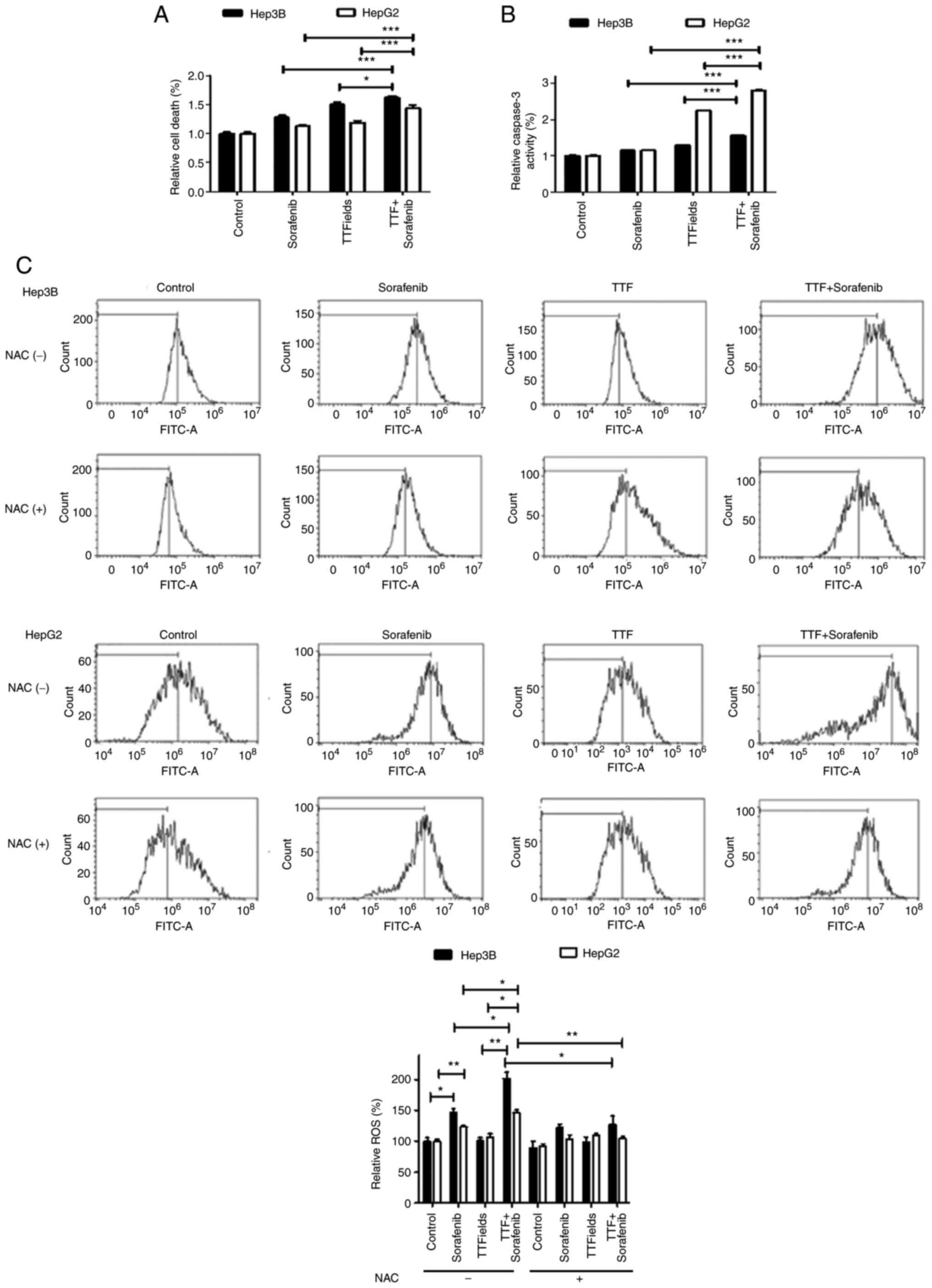
Sorafenib enhances TTFields-induced
apoptosis
To investigate whether sorafenib and TTFields could
induce apoptosis, early apoptosis was assessed using cell death
kit. In both liver cancer cell lines, 48 h of exposure to sorafenib
and TTFields resulted in a significant increase in the proportion
of early apoptotic cells (Fig.
5A). Apoptotic cell death significantly increased following
combined treatment. To examine the underlying pathway, the activity
of caspase 3 was measured, in order to determine whether increased
activity of this protein mediates the cytotoxicity of the combined
therapy. The results demonstrated a significant increase following
combined treatment compared with sorafenib alone (Fig. 5B). ROS production following
TTFields application alone or with sorafenib was also evaluated.
ROS production significantly increased following combined treatment
compared with single treatments (Fig.
5C), which may explain the increased apoptotic rate following
combined treatment. These data were further confirmed by incubating
the cells with NAC, a ROS scavenger, which abolished the release of
ROS following combination treatment in both liver cancer cell
lines.
Discussion
Combination therapy, consisting of maximal safe
surgical resection followed by combined chemo-radiotherapy and
adjuvant temozolomide, currently represents the standard of care
for patients with different forms of cancer. Developing an
understanding of the TTFields-oriented approach requires
familiarity with two concepts. Firstly, electric fields can be
uniform (i.e., an electric field that remains unchanged at all
points) or non-uniform (i.e., tends to vary in direction
(divergent/convergent) and magnitude). Secondly, it is possible for
an electric field to remain in an unchanging field, wherein the
constant state of source charge allows a test charge to converge
unidirectionally (4,5,6,7). To
inhibit the growth of cancerous cells, both their proliferation and
apoptosis need to be considered. In the present study, the findings
of western blot analysis and ROS assays suggested that, in
comparison with monotherapy, TTFields combined with sorafenib
demonstrated greater anti-proliferative and apoptosis effects on
liver cancer cells. Moreover, Transwell migration and invasion
assays demonstrated that TTFields combined with sorafenib inhibited
liver cancer cell invasion and metastasis synergistically.
Preclinical studies have demonstrated that the
optimal anti-proliferative effect of TTFields on isolated cancer
cells is dependent on the frequency of the electric fields specific
to the source of the isolated tumor cells (9,11,12,25).
In clinical settings, TTFields was applied at 200 kHz for GBM,
which was the frequency demonstrating the greatest decrease in
glioma cell proliferation in vitro (5,7).
Similarly, TTFields showed the greatest inhibitory effect at up to
150 kHz in non-small cell lung cancer in vitro (26). Clinical studies are currently
underway for the use of TTFields in the treatment of brain
metastases from lung (150 kHz), non-small cell lung (150 kHz),
ovarian (200 kHz) and pancreatic (150 kHz) cancer, as well as
mesothelioma (150 kHz) (27,28).
Based on this previous work, the TTFields used in the present study
were set at 150 kHz to inhibit the growth of liver cancer cell
lines in vitro, and the results provided evidence supporting
the potential use of TTFields treatment in liver cancer.
HCC is associated with multiple genetic aberrations,
demonstrating the involvement of various signaling pathways in its
initiation and progression. Patients suffering from HCC at advanced
stages or those who have unresectable tumors typically receive
treatment with sorafenib, which is a multi-kinase inhibitor
(29). However, sorafenib can only
improve the survival of patients by ~3 months (30,31),
indicating that monotherapy is not sufficient to improve the
outcome of patients with HCC. Therefore, a combination therapy that
can simultaneously target multiple pathways to prevent the invasion
and proliferation of HCC is urgently needed.
The present study demonstrated that TTFields induced
apoptosis in vitro, which should be further explored in a
xenograft model in vivo. Sorafenib treatment was reported to
decrease the expression levels of phosphorylated (p)-Akt, PI3K,
p-mTOR and p-p70S6K, and to inhibit the PI3K/Akt/mTOR signaling
pathway in HCC cells (32,33). The present results demonstrated
that TTFields and sorafenib combination treatment could inhibit
liver cancer cell proliferation and invasion, suggesting that this
treatment could prevent metastasis by synergistically enhancing
apoptosis. As an alternative approach to inhibit tumor growth, it
is important to establish whether TTFields-induced autophagy is
related to the viability of cancer cells or to their sensitivity to
apoptosis.
Moreover, undiscovered and potentially confounding
synergistic properties may be present with a multitude of other
novel or repurposed agents. This is evident through preliminary
reports of TTFields with bevacizumab, as well as TTFields combined
with triflouropromazine, an approved antipsychotic drug (34). Triflouropromazine has been
identified to inhibit mitotic slippage when used in combination
with TTFields (34,35). This is particularly notable, as the
treatment appeared to decrease cell counts by up to 14% when used
in combination, thus suggesting improved efficacy for the treatment
of liver cancer (36). Cells
receiving the combined treatment increased in size by up to 35%,
suggesting decreased clonogenic potential in these cells (37). TTFields have also been combined
with withaferin A, a steroidal lactone originating from the winter
cherry plant, Withania somnifera. Additionally, withaferin A
has been previously shown to have efficacy against glioma cell
lines in vitro and in murine orthotopic GBM models (38). As seen in other combinational
therapeutic strategies with TTFields, greater efficacy was achieved
in treating liver cancer and other types of cancer when combining
TTFields with withaferin A (39).
The anti-microtubular class of mitotic inhibitors can be divided
into microtubular-stabilizing and microtubular-destabilizing
agents. In combination with TTFields, these agents have
demonstrated antitumor activity in a variety of cancer types, such
as liver, breast and ovarian cancer (40).
In conclusion, the present findings suggest that the
combination of TTFields and other agents, especially sorafenib,
promotes the apoptosis while inhibiting the proliferation and
invasion of liver cancer cells in vitro. These results offer
a potential strategy for using this combination chemotherapeutic
treatment in patients with liver cancer in a clinical setting.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Catholic University
of Daegu (2020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ, WSL and EHK designed the study, the experimental
setup and wrote the manuscript. YJ, WSL, SS, JYK and JKK performed
the experiments. YJ, WSL, EHK, JYK and JKK analyzed and confirmed
the data. YJ, WSL and EHK confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Inokawa Y, Inaoka K, Sonohara F, Hayashi
M, Kanda M and Nomoto S: Molecular alterations in the
carcinogenesis and progression of hepatocellular carcinoma: Tumor
factors and background liver factors. Oncol Lett. 12:3662–3668.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez-Rodriguez CM and
Gutierrez-Garcia ML: Prevention of hepatocellular carcinoma in
patients with chronic hepatitis B. World J Gastrointest Pharmacol
Ther. 5:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buendia MA and Neuveut C: Hepatocellular
carcinoma. Cold Spring Harb Perspect Med. 5:a0214442015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pless M and Weinberg U: Tumor treating
fields: Concept, evidence and future. Expert Opin Investig Drugs.
20:1099–1106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mun EJ, Babiker HM, Weinberg U, Kirson ED
and Von Hoff DD: Tumor-treating fields: A fourth modality in cancer
treatment. Clin Cancer Res. 24:266–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gera N, Yang A, Holtzman TS, Lee SX, Wong
ET and Swanson KD: Tumor treating fields perturb the localization
of septins and cause aberrant mitotic exit. PLoS One.
10:e01252692015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirson ED, Gurvich Z, Schneiderman R,
Dekel E, Itzhaki A, Wasserman Y, Schatzberger R and Palti Y:
Disruption of cancer cell replication by alternating electric
fields. Cancer Res. 64:3288–3295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durand DM and Bikson M: Suppression and
control of epileptiform activity by electrical stimulation: A
review. Proc IEEE. 89:1065–1082. 2001. View
Article : Google Scholar
|
|
9
|
Kirson ED, Dbaly V, Tovarys F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giladi M, Schneiderman RS, Voloshin T,
Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A,
et al: Mitotic spindle disruption by alternating electric fields
leads to improper chromosome segregation and mitotic catastrophe in
cancer cells. Sci Rep. 5:180462015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jo Y, Kim EH, Sai S, Kim JS, Cho JM, Kim
H, Baek JH, Kim JY, Hwang SG and Yoon M: Functional biological
activity of sorafenib as a tumor-treating field sensitizer for
glioblastoma therapy. Int J Mol Sci. 19:36842018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirson ED, Schneiderman RS, Dbaly V,
Tovarys F, Vymazal J, Itzhaki A, Mordechovich D, Gurvich Z, Shmueli
E, Goldsher D, et al: Chemotherapeutic treatment efficacy and
sensitivity are increased by adjuvant alternating electric fields
(TTFields). BMC Med Phys. 9:12009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giladi M, Munster M, Schneiderman RS,
Voloshin T, Porat Y, Blat R, Zielinska-Chomej K, Hååg P, Bomzon Z,
Kirson ED, et al: Tumor treating fields (TTFields) delay DNA damage
repair following radiation treatment of glioma cells. Radiat Oncol.
12:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim EH, Kim YH, Song HS, Jeong YK, Lee JY,
Sung J, Yoo SH and Yoon M: Biological effect of an alternating
electric field on cell proliferation and synergistic antimitotic
effect in combination with ionizing radiation. Oncotarget.
7:62267–62279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Bonis P, Doglietto F, Anile C, Pompucci
A and Mangiola A: Electric fields for the treatment of
glioblastoma. Expert Rev Neurother. 12:1181–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo Y, Hwang SG, Jin YB, Sung J, Jeong YK,
Baek JH, Cho JM, Kim EH and Yoon M: Selective toxicity of tumor
treating fields to melanoma: An in vitro and in vivo study. Cell
Death Discov. 4:462018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nabors LB, Ammirati M, Bierman PJ, Brem H,
Butowski N, Chamberlain MC, DeAngelis LM, Fenstermaker RA, Friedman
A, Gilbert MR, et al: Central nervous system cancers. J Natl Compr
Canc Netw. 11:1114–1151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong H, Sung J, Oh SI, Jeong S, Koh EK,
Hong S and Yoon M: Inhibition of brain tumor cell proliferation by
alternating electric fields. Appl Phys Lett. 105:2037032014.
View Article : Google Scholar
|
|
19
|
Liu C, Zhu Y, Lou W, Cui Y, Evans CP and
Gao AC: Inhibition of constitutively active Stat3 reverses
enzalutamide resistance in LNCaP derivative prostate cancer cells.
Prostate. 74:201–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji WO, Lee MH, Kim GH and Kim EH:
Quantitation of the ROS production in plasma and radiation
treatments of biotargets. Sci Rep. 9:198372019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim EH, Song HS, Yoo SH and Yoon M: Tumor
treating fields inhibit glioblastoma cell migration, invasion and
angiogenesis. Oncotarget. 7:65125–65136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang F, Lau SS and Monks TJ: A dual role
for poly(ADP-ribose) polymerase-1 during caspase-dependent
apoptosis. Toxicol Sci. 128:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen KF, Tai WT, Liu TH, Huang HP, Lin YC,
Shiau CW, Li PK, Chen PJ and Cheng AL: Sorafenib overcomes TRAIL
resistance of hepatocellular carcinoma cells through the inhibition
of STAT3. Clin Cancer Res. 16:5189–5199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rangwala F, Williams KP, Smith GR, Thomas
Z, Allensworth JL, Lyerly HK, Diehl AM, Morse MA and Devi GR:
Differential effects of arsenic trioxide on chemosensitization in
human hepatic tumor and stellate cell lines. BMC Cancer.
12:4022012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benson L: Tumor treating fields
technology: Alternating electric field therapy for the treatment of
solid tumors. Semin Oncol Nurs. 34:137–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pless M, Droege C, von Moos R, Salzberg M
and Betticher D: A phase I/II trial of tumor treating fields
(TTFields) therapy in combination with pemetrexed for advanced
non-small cell lung cancer. Lung Cancer. 81:445–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rivera F, Benavides M, Gallego J,
Guillen-Ponce C, Lopez-Martin J and Küng M: Tumor treating fields
in combination with gemcitabine or gemcitabine plus nab-paclitaxel
in pancreatic cancer: Results of the PANOVA phase 2 study.
Pancreatology. 19:64–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ceresoli GL, Aerts JG, Dziadziuszko R,
Ramlau R, Cedres S, van Meerbeeck JP, Mencoboni M, Planchard D,
Chella A, Crinò L, et al: Tumour treating fields in combination
with pemetrexed and cisplatin or carboplatin as first-line
treatment for unresectable malignant pleural mesothelioma
(STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol.
20:1702–1709. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Copur MS: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:24982008.PubMed/NCBI
|
|
31
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Wang F, Jiang Y, Xu S, Lu F, Wang
W and Sun X and Sun X: Migration of retinal pigment epithelial
cells is EGFR/PI3K/AKT dependent. Front Biosci (Schol Ed).
5:661–671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Porat Y, Giladi M, Schneiderman R, Munster
M, Blatt R, Weinberg U, Kirson E and Palti Y:
ET-47Triflouropromazine, an approved antipsychotic drug, enhances
tumor treating fields treatment efficacy in vitro. Neuro Oncol. 16
(Suppl 5):v892014. View Article : Google Scholar
|
|
35
|
Riffell JL, Zimmerman C, Khong A, McHardy
LM and Roberge M: Effects of chemical manipulation of mitotic
arrest and slippage on cancer cell survival and proliferation. Cell
Cycle. 8:3025–3038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J,
Finn RS, et al: Hepatocellular carcinoma. Nat Rev Dis Primers.
7:62021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneiderman RS, Giladi M, Porat Y,
Munster M, Weinberg U, Kirson ED and Palti Y: Overcoming cell size
escape from tumor treating fields using a varying frequency
treatment paradigm in vitro. J Clin Oncol. 31:e22134. 2013.
View Article : Google Scholar
|
|
38
|
Chang E, Pohling C, Natarajan A, Witney
TH, Kaur J, Xu L, Gowrishankar G, D'Souza AL, Murty S, Schick S, et
al: AshwaMAX and Withaferin A inhibits gliomas in cellular and
murine orthotopic models. J Neurooncol. 126:253–264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lavie D, Glotter E and Shvo Y:
Constituents of Withania somnifera Dun. III. The side chain of
withaferin A*, 1. J Org Chem. 30:1774–1778. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Branter J, Basu S and Smith S: Tumour
treating fields in a combinational therapeutic approach.
Oncotarget. 9:36631–36644. 2018. View Article : Google Scholar : PubMed/NCBI
|