Introduction
Meningiomas most frequently occur as slow-growing
intracranial tumors and typically present with the symptoms of
headaches, dizziness, seizures or the gradual progression of
neurological deficits; however, their acute presentation with
spontaneous hemorrhage appears to be a rare event (1–3).
Meningioma with hemorrhagic onset usually refers to the significant
intracranial hemorrhage stemming from a meningioma and results in a
series of clinical symptoms, such as severe headaches, nausea and
vomiting, epilepsy, hemiparesis or disturbance of consciousness
(2,3). According to previously reported
cases, the incidence ranges from 0.5-2.4% (3). Although rare, intracranial hemorrhage
stemming from meningioma could have a detrimental effect on
outcomes and may even be a life-threatening event due to acutely
increased intracranial pressure (2–4).
Cheng and Lin (5) reported that
the mortality rate associated with hemorrhagic meningiomas was as
high as 38.5% in the CT era and 77.8% in the era before CT. Boŝnjak
et al (2) reported overall
mortality and morbidity rates of 21.1 and 32.6%, respectively, for
hemorrhagic meningiomas in 2005. Therefore, early diagnosis and
correct treatment are the key factors to improve patient
outcomes.
While no difficulty is encountered in the diagnosis
of a hemorrhage in a previously known brain tumor, hemorrhagic
onset as the initial presentation of a brain tumor may pose
diagnostic problems (6).
Meningiomas with hemorrhagic onset can manifest in several ways and
some of the clinical features of such a condition have been
characterized; however, previous studies regarding this condition
have been limited to single-case reports or small case series
(2,4,7–12).
The cases in the literature were reported according to the site of
hemorrhage such as the subarachnoid space (8), subdural space (7,9),
peritumoral space (4), brain
parenchyma (2,10) or intratumor region (11,12).
However, this description of tumoral bleeding cannot reflect the
association between the meningioma and the hemorrhage, and it also
has no practical significance for clinical diagnosis and does not
significantly contribute to guiding treatment. Furthermore, the
previous studies explored the results without considering the
impact of different hemorrhagic types. In the present study, a new
classification system of hemorrhage associated with meningiomas
that is based on a retrospective analysis of 19 cases is proposed
and its clinical significance is explored.
Materials and methods
Patient selection
Following study approval by the Institutional Ethics
Board of the Affiliated Li Hui Li Hospital of Ningbo University
(Ningbo, China), a retrospective study was performed on the
patients who underwent craniotomy for meningioma resection between
July 2008 and March 2021 at the Department of Neurosurgery, The
Affiliated Li Hui Li Hospital of Ningbo University (Ningbo, China).
All methods were performed in accordance with the relevant
guidelines and regulations. The inclusion criteria were set as
follows: i) Patients meeting the diagnostic criteria of the 2016
World Health Organization (WHO) classification of meningiomas; ii)
patients who underwent craniotomy for tumor resection in The
Affiliated Li Hui Li Hospital of Ningbo University (Ningbo, China);
iii) patients with complete clinical information and radiological
data; iv) patients being diagnosed for the first time; v) follow-up
data being available for ≥6 months. The exclusion criteria were as
follows: i) An age of <18 years; ii) patients on a regimen of
anticoagulant or antiplatelet therapy; iii) patients with serious
diseases associated with the heart, lungs, kidneys, endocrine
system and blood; iv) patients who had previously undergone
preoperative adjuvant therapies such chemotherapy or radiotherapy;
and v) hemorrhage distant from the tumor site. The patients
enrolled in the study were divided into two groups according to
whether or not tumor-associated hemorrhage was recorded. The
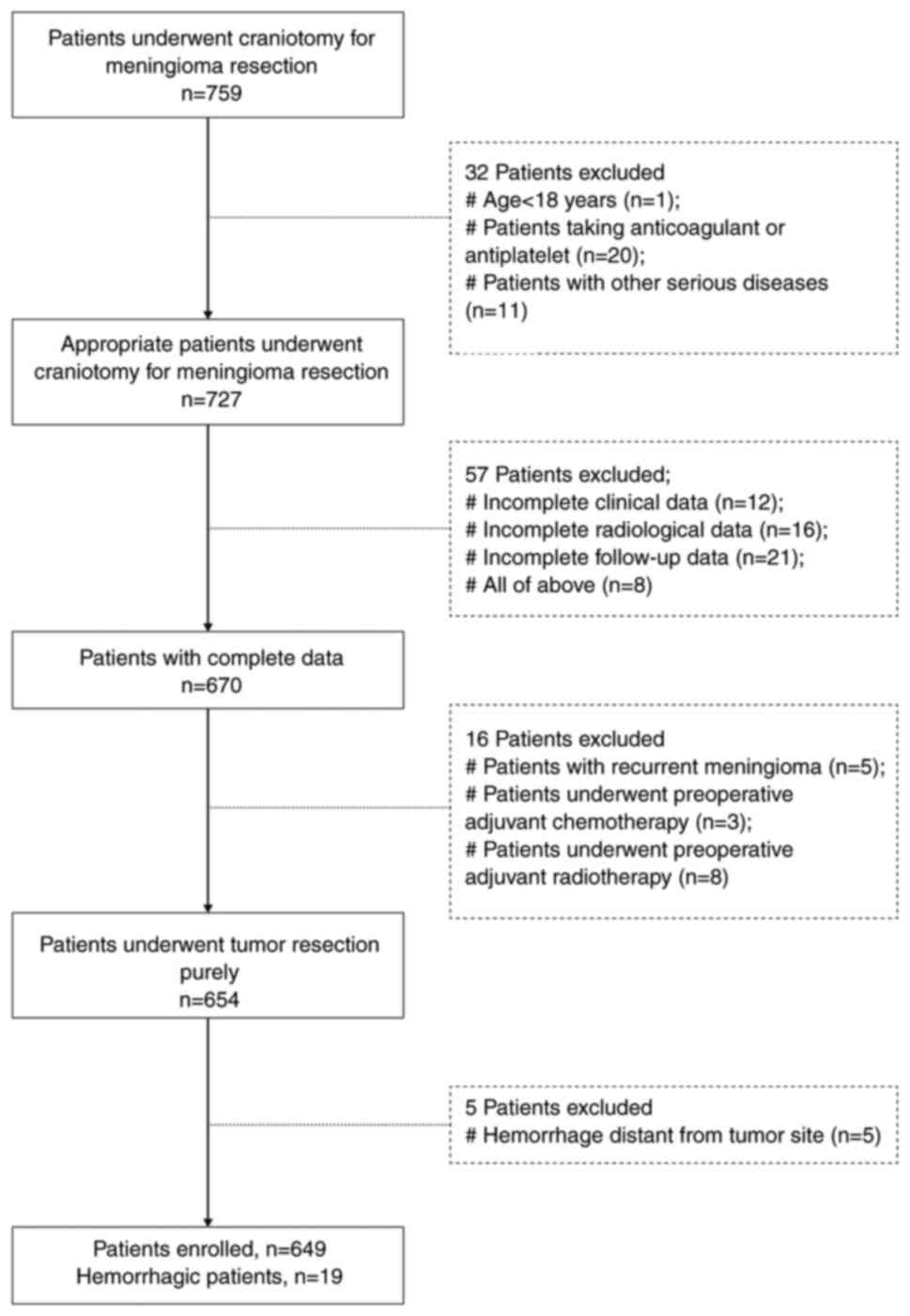
patient flowchart is summarized in Fig. 1.
Data collection
This retrospective study enrolled a total of 649
consecutive patients with meningiomas who underwent craniotomy for
tumor resection at the Affiliated Li Hui Li Hospital of Ningbo
University (Ningbo, China), and 19 of these 649 patients presented
with acute spontaneous hemorrhage stemming from a meningioma. A
total of 20 cases with non-hemorrhagic meningiomas were randomly
selected from the 630 patients in the same period and served as a
comparison group (group 4). The clinical data, including age, sex,
history of hypertension and diabetes, onset symptoms, Simpson
resection grade (1) and
histological variants, were extracted from the medical records.
Laboratory variables, such as international normalized ratio,
prothrombin time and activated partial thromboplastin time, were
retrieved from the hospital database. The pre- and post-operative
computed tomography (CT), magnetic resonance imaging (MRI) and/or
CT angiography (CTA) data were examined via picture archiving and
communication system (PACS).
Radiological evaluation
Radiological outcomes were analyzed by CT scans, MRI
and/or CTA. The radiological features, including tumor location,
size, signal intensity, contrast enhancement, tumor margins and
hemorrhagic site, were analyzed independently by two neurosurgeons.
Tumor size was determined by measuring the greatest diameter of the
enhanced tumor on the preoperative CT or the contrast enhancement
of T1-weighted MRI. The signal intensities of tumors on T2-weighted
MRI were categorized as hypointense, isointense or hyperintense
relative to that of the cortical gray matter on the same MRI.
Peritumoral brain edema (PTBE) was also evaluated on T2-weighted
MRI, and the extent of PTBE was assessed by calculation of the
edema index (EI). The EI was calculated in this study according to
the methodology previously described (13).
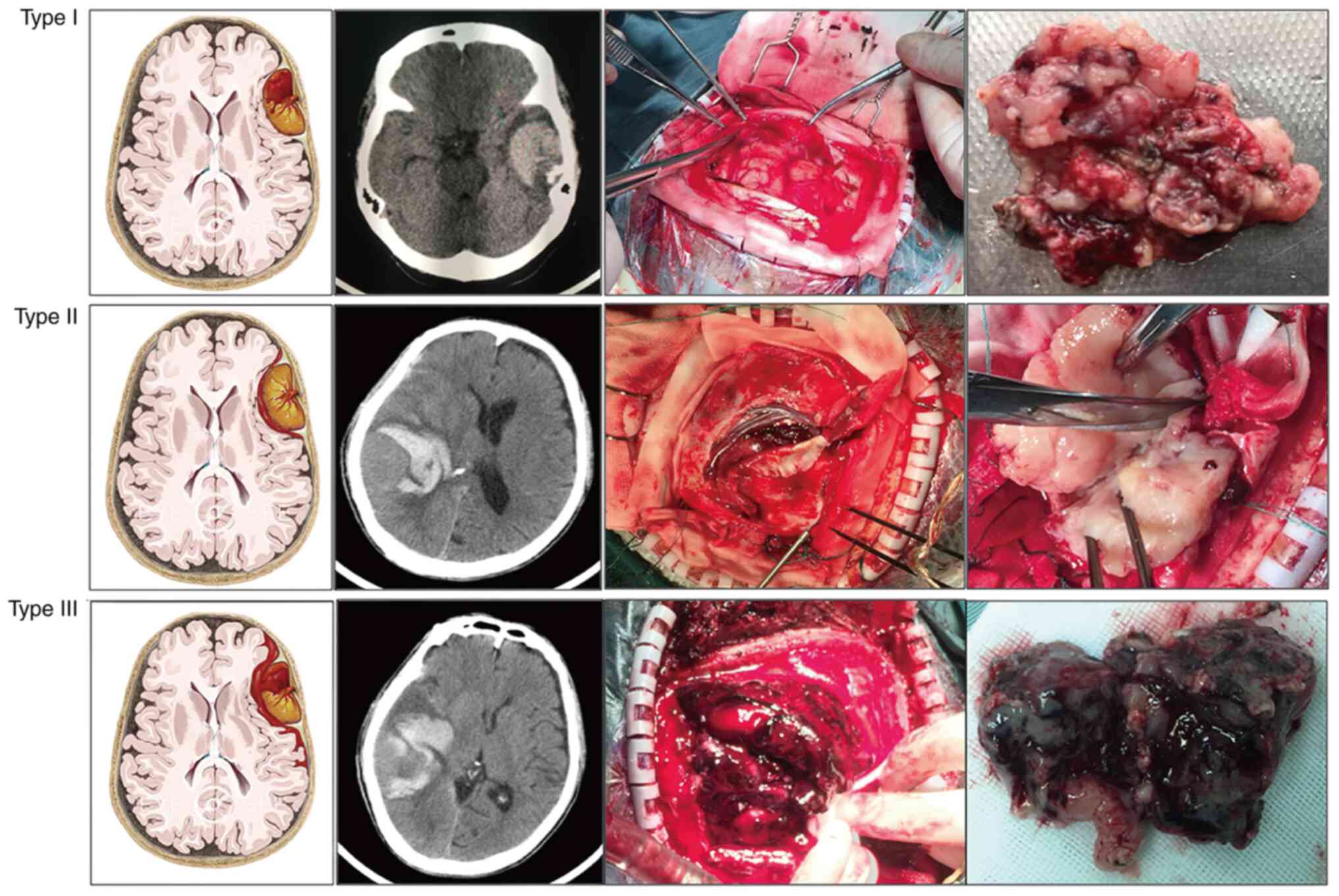
In order to further reflect the association between
meningioma and hemorrhage, a new bleeding classification system of
meningiomas was proposed and the 19 cases were correspondingly
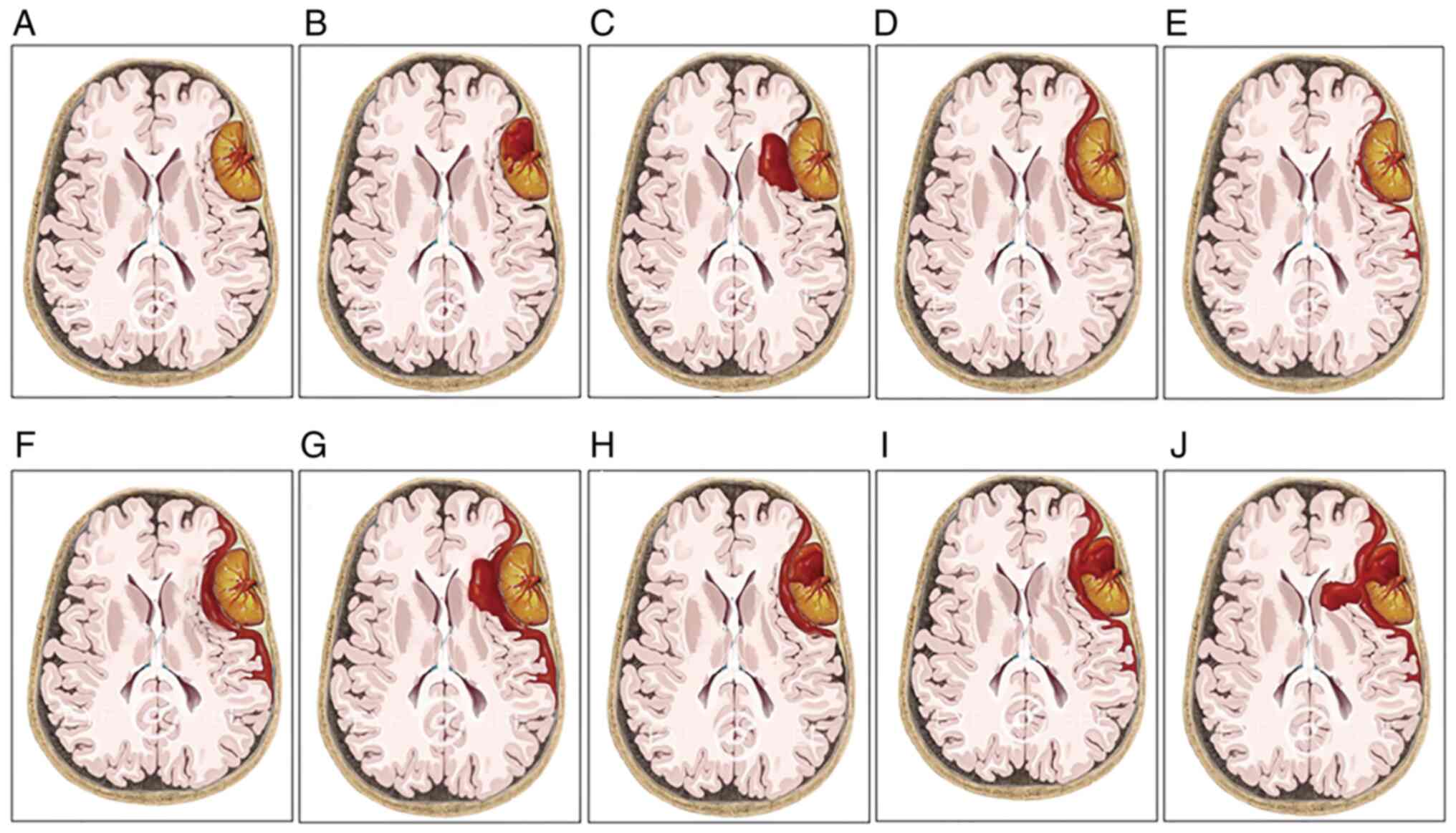
divided into three subgroups (Fig.
2): Type I bleeding was defined as the hemorrhage being purely
found in the tumor (group 1); type II bleeding referred to a purely
extratumoral hemorrhage, and the hemorrhage may be found in the
peritumoral, subdural, subarachnoid or/and intracerebral spaces
(group 2); and type III bleeding manifested as both intra-and
extratumoral hemorrhages (group 3).
Treatment and prognostic
assessment
Hematoma evacuation and a macroscopically complete
resection of the tumor were planned for completion in one stage for
all patients. The treatment strategy was performed according to the
clinical status of the patients and their radiological results. The
Simpson resection grade classification was used to evaluate the
extent of resection on the basis of the surgical reports and the
postoperative MRI within 72 h of surgery. Pathological sections of
these tumors were reviewed by two independent pathologists under
light microscopy, and the diagnosis was reconfirmed according to
the WHO Classification of Tumors of the Central Nervous System 2016
(1). Follow-up data were collected
from clinical visits and telephone interviews, with a mean
follow-up time of 48.8 months [standard deviation (SD), 35.13;
range, 3–125 months]. The postoperative status of the patient was
investigated by physical examination and enhanced MRI. Radiological
and functional assessments were usually performed preoperatively,
at discharge, at 6 months postoperatively and annually thereafter.
According to the Glasgow Outcome Scale (GOS; 1,2), clinical
outcomes were graded from GOS 1 to 5; a good outcome was classified
as GOS 4 and 5, and a poor outcome as GOS 1 to 3.
Statistical analysis
All continuous data are expressed as the mean ± SD.
Statistical analysis was performed with the SAS system (version
8.1; SAS Institute, Inc.), while the statistical images were drawn
using R software (version 3.5.3; R core team). Patient age, EI and
tumor size among the four groups were compared using one-way
analysis of variance followed by the Tukey-Kramer post-hoc test.
Qualitative data, including sex ratio, the occurrence rate of
hyperintensity on T2-weighted images, the occurrence rate of PTBE
and the proportion of WHO Grade I tumors, were compared using
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features
Of the 649 meningioma patients treated, 19 (2.9%)
patients presented with hemorrhagic onset. These 19 cases had
complete medical records and were included in this study. The
clinical data for these cases are summarized in Table I. There were 9 males and 10
females, yielding a male-to-female ratio of 0.9:1. There was no
significant difference in the clinical factor of sex ratio among
these groups (P>0.05; Table
II). The confirmed age at diagnosis ranged from 19 to 79 years
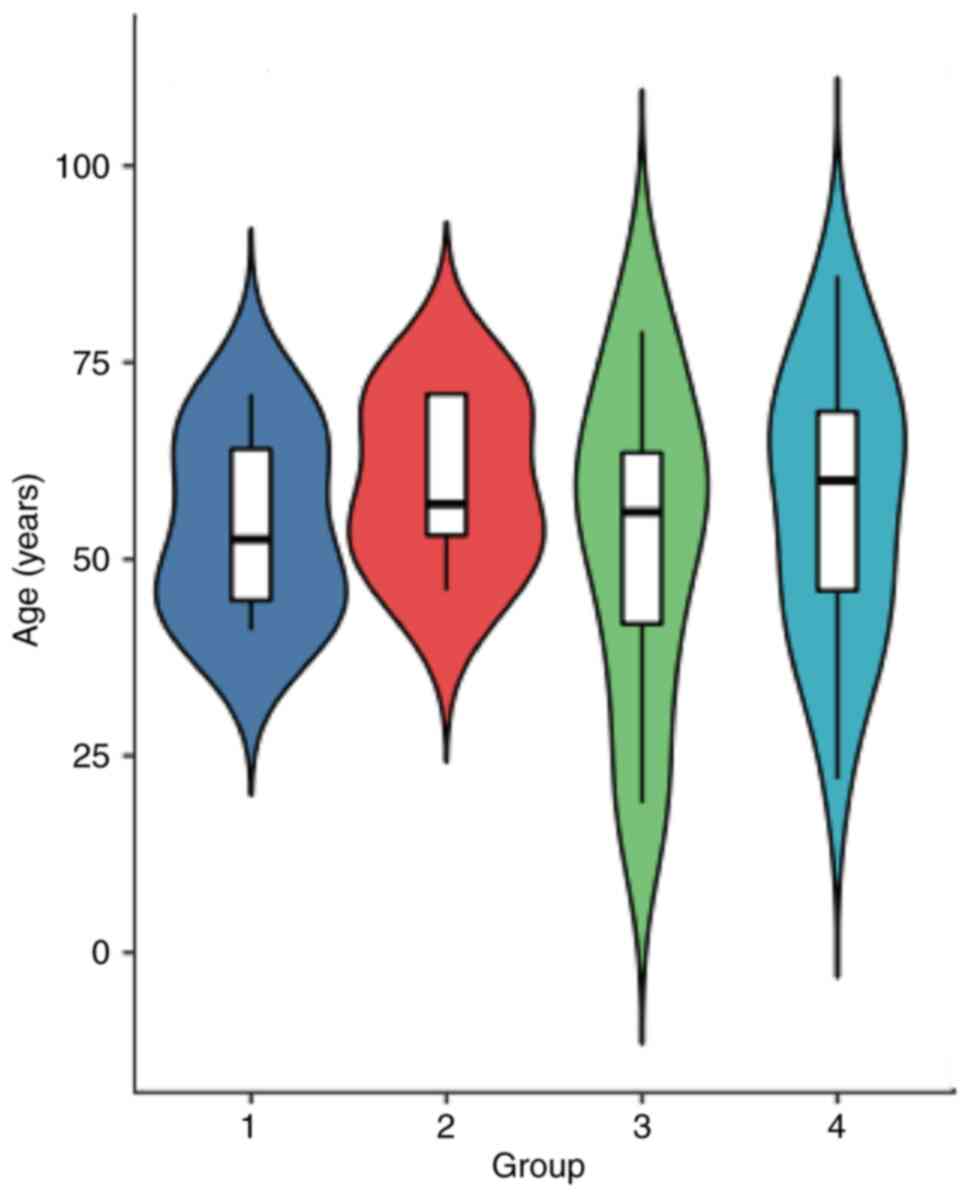
old, and the mean age was 55±14.6 years old. The mean age exhibited
no statistical difference among the four groups (Fig. 3; F=0.2401; P>0.05). All the
patients experienced a stroke-like episode characterized by the
sudden onset of acute headache, nausea and vomiting, vertigo,
epileptic seizures, hemiparesis and/or altered consciousness. The
above hemorrhagic symptoms were the first clinical presentations
for 14 patients (73.7%). In total, 5 patients (26.3%) had initial
symptoms such as mild headache, dizziness, mental disturbance or
limb weakness before bleeding events, and the hemorrhagic event
aggravated the initial symptoms or resulted in new symptoms. In
addition, no patients with evidence of bleeding tendency and no
other predisposing factors for hemorrhage were found.
 | Table I.Clinical features of 19meningiomas
with hemorrhagic onset. |
Table I.
Clinical features of 19meningiomas
with hemorrhagic onset.
| Case no. | Age, years | Sex | Symptoms | Tumor location | Bleeding type | Tumor subtype | Tumor size, mm | Resection
grade | Signal
intensity | Outcome |
|---|
| 1 | 59 | F |
Headache/hemiparesis/aphasia | Convexity | I | Fibrous | 65 | I | Isointense |
Alive/hemiparesis/no tumor recurrence |
| 2 | 45 | M |
Headache/nausea/vomiting | Parasagital | I | Atypical | 39 | II | Hyperintense | Alive/tumor
recurrence |
| 3 | 41 | M |
Headache/nausea | Convexity | I | Meningothelial | 22 | I | Hyperintense | Alive/no tumor
recurrence |
| 4 | 41 | M |
Headache/hemiparesis | Convexity | I | Meningothelial | 73 | I | Hyperintense | Alive/no tumor
recurrence |
| 5 | 46 | F | Headache | Parasagital | I | Microcystic | 31 | III | Hyperintense | Alive/no tumor
recurrence |
| 6 | 56 | F |
Headache/epilepsy | Convexity | I | Atypical | 25 | I | Isointense | Alive/no tumor
recurrence |
| 7 | 71 | F |
Headache/nausea | Middle skull
base | I | Angiomatous | 53 | IV | Hyperintense | Alive/no tumor
progression |
| 8 | 63 | M |
Headache/vertigo/facial paralysis | Cerebellopontine
angle | I | Fibrous | 25 | II | Hypointense | Alive/facial
paralysis/no tumor recurrence |
| 9 | 57 | F |
Headache/nausea | Convexity | II | Fibrous | 43 | I | Isointense | Alive/no tumor
recurrence |
| 10 | 71 | M |
Headache/drowsiness/hemiparesis | Convexity | II | Malignant | 57 | I | Hyperintense |
Alive/hemiparesis/no tumor recurrence |
| 11 | 46 | F | Headache | Parasagital | II | Angiomatous | 21 | II | Hyperintense | Alive/no tumor
recurrence |
| 12 | 71 | M | Coma/epilepsy | Anterior skull
base | II | Atypical | 68 | III | / |
Alive/epilepsy/tumor recurrence |
| 13 | 53 | F |
Headache/nausea/vomiting | Parafalx | II | Psammomatous | 51 | I | Hypointense | Alive/no tumor
recurrence |
| 14 | 19 | M |
Headache/drowsiness | Convexity | III | Meningothelial | 45 | I | Hyperintense | Alive/no tumor
recurrence |
| 15 | 79 | M | Coma | Parasagital | III | Malignant | 68 | I | / | Died |
| 16 | 51 | F |
Headache/drowsiness/hemidysesthesia | Convexity | III | Transitional | 52 | I | Hyperintense | Alive/no tumor
recurrence |
| 17 | 53 | F |
Headache/drowsy/hemiparesis | Convexity | III | Angiomatous | 55 | I | Hyperintense | Alive/no tumor
recurrence |
| 18 | 64 | M |
Headache/hemiparesis | Parasagital | III | Fibrous | 41 | II | Isointense | Alive/no tumor
recurrence |
| 19 | 39 | F |
Headache/nausea/vomiting | Parafalx | III | Secretory | 31 | I | Hyperintense | Alive/no tumor
recurrence |
 | Table II.Differences in sex ratio, the
occurrence rate of hyperintensity on T2-weighted images, the
occurrence rate of PTBE and the proportion of WHO grade I tumors
among the four study groups. |
Table II.
Differences in sex ratio, the
occurrence rate of hyperintensity on T2-weighted images, the
occurrence rate of PTBE and the proportion of WHO grade I tumors
among the four study groups.
|
| Hemorrhagic
groups |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | Group 1 (n=8) | Group 2 (n=5) | Group 3 (n=6) | Group
4a (n=20) | P-value |
|---|
| Sex |
|
|
|
| 0.3429 |
| Male | 4 | 2 | 3 | 7 |
|
| Female | 4 | 3 | 3 | 13 |
|
| Intensity on
T2-weighted images |
|
|
|
| 0.5333 |
| Hyperintensity | 5 | 2 | 4 | 12 |
|
| Isointensity and
hypointensity | 3 | 2 | 1 | 8 |
|
| PTBE |
|
|
|
| 0.0244 |
| Occurrence | 4 | 4 | 5 | 8 |
|
| None | 4 | 0 | 0 | 12 |
|
| WHO grade |
|
|
|
| 0.7781 |
| I | 6 | 3 | 5 | 16 |
|
| II and III | 2 | 2 | 1 | 4 |
|
Radiological characteristics
All 19 patients had preoperative CT scans, 17
patients had preoperative MRI and 7 patients had preoperative CTA
according to the PACS database. Preoperative MRI was not performed
in 2 cases with respective type II and type III bleeding due to
severe symptoms. All patients underwent postoperative CT within 24
hand MRI scans within 72 h of surgery. Preoperative CT and MRI
generally gave evidence of well-defined, dense, contoured
extra-axial masses displacing the adjacent brain and acute or
subacute hemorrhage associated with masses. Abnormal blood vessels,
such as aneurysms or arteriovenous malformations, were not detected
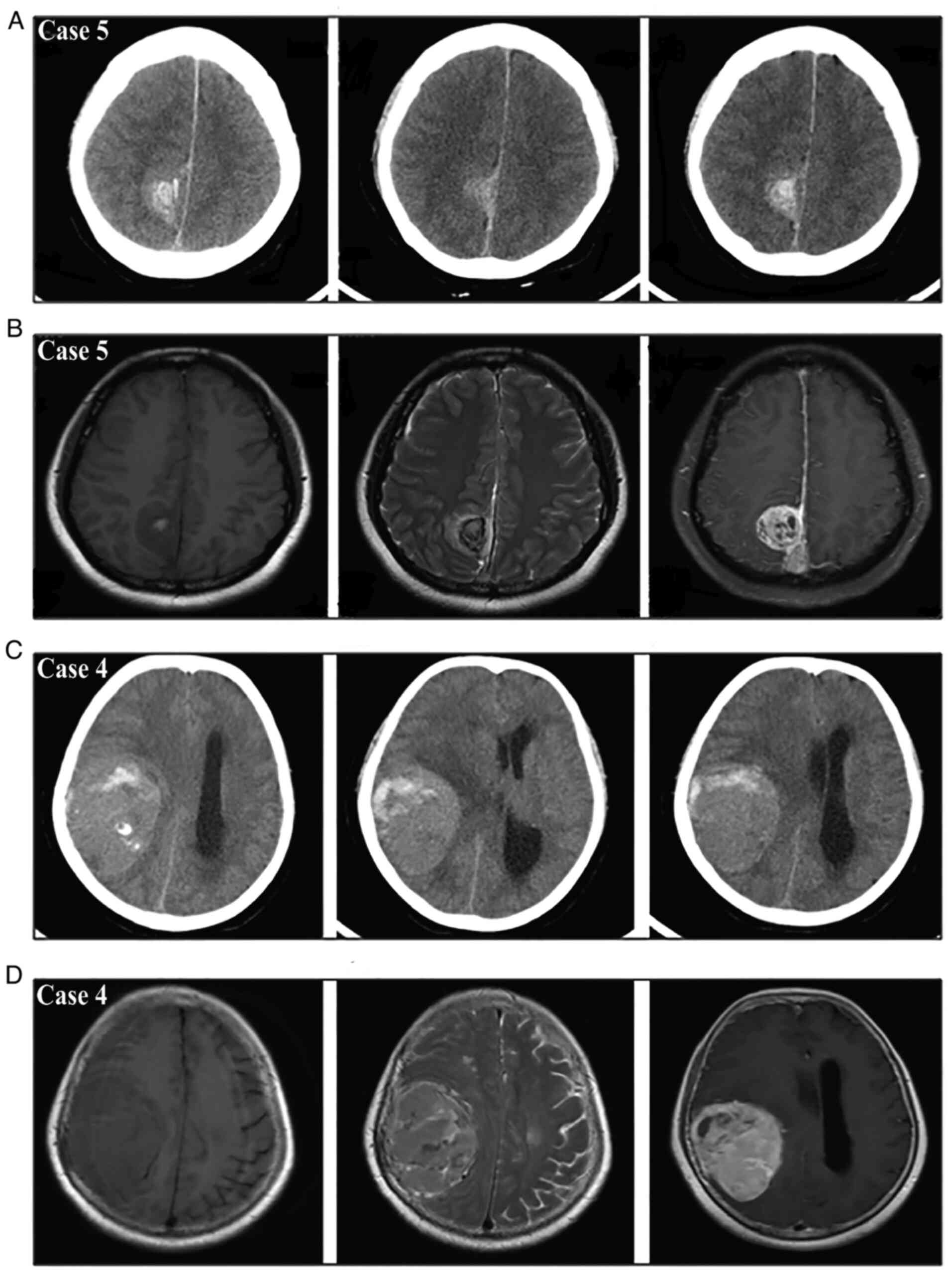
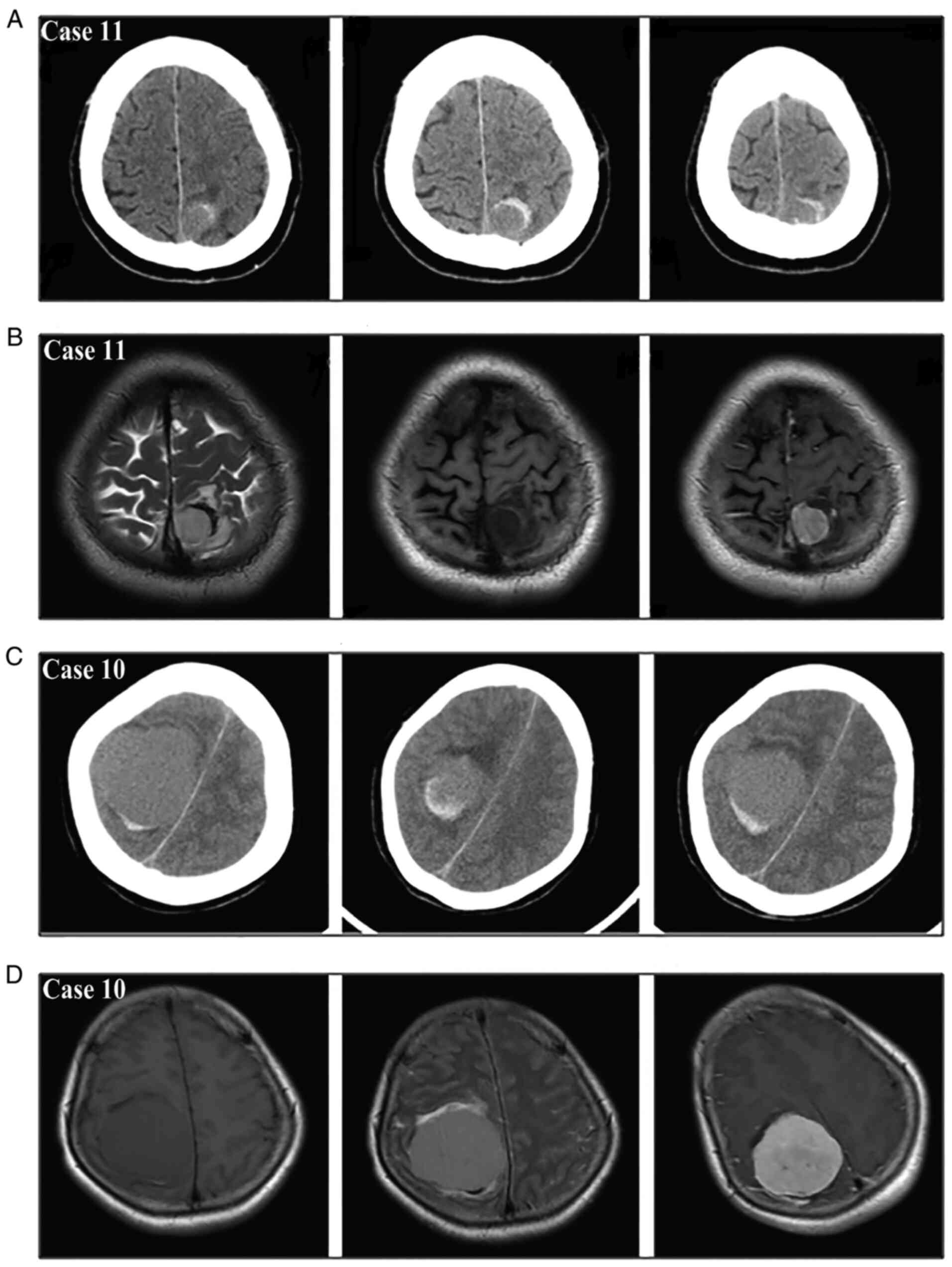
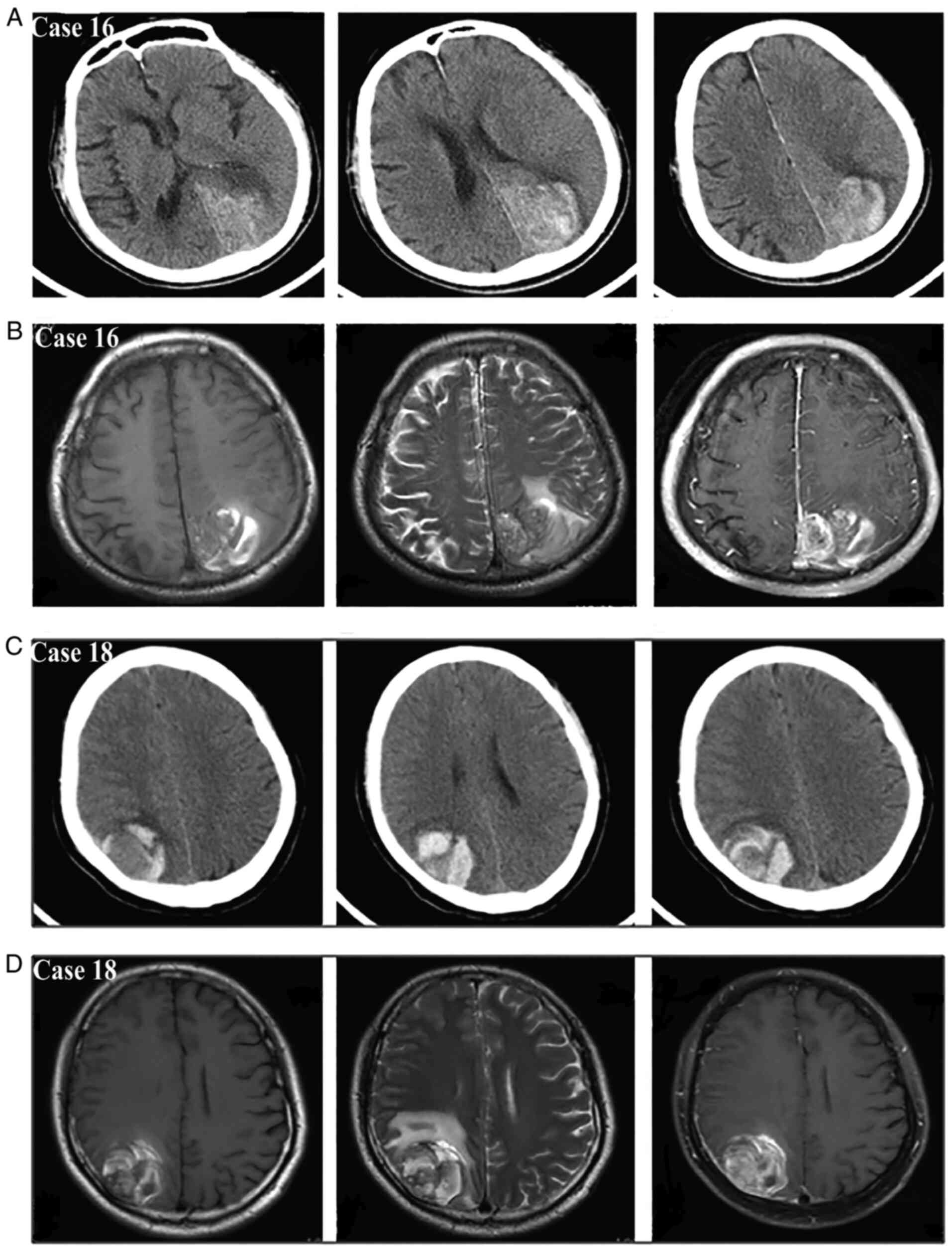
on CTA. According to the new bleeding classification, 8 masses
manifested with type I bleeding (Fig.
4), 5 masses presented with type II bleeding (Fig. 5) and 6 masses exhibited type III
bleeding (Fig. 6). Moreover, the
type I bleeding could break through the tumor and result in type
III bleeding.
On MRI with gadolinium enhancement, the masses with
a dural base showed moderate to strong enhancement except for the
portion representing the hemorrhage. All cases in group 2 showed
homogenous enhancement, while the other cases showed heterogeneous
enhancement. A total of 4 masses in group 3 (80%), 5 masses in
group 1 (62.5%) and 2 masses in group 2 (50%) presented as
hyperintense on T2-weighted images. Although the occurrence rate of
hyperintensity on T2-weighted images was not significantly
different among the four groups (P>0.05; Table II), the patients in group 3 had
the highest rate.
All masses in groups 2 and 3 showed PTBE on
T2-weighted images; 4 masses in group 1 (50.0%) showed PTBE on
T2-weighted images, and the occurrence rate was significantly
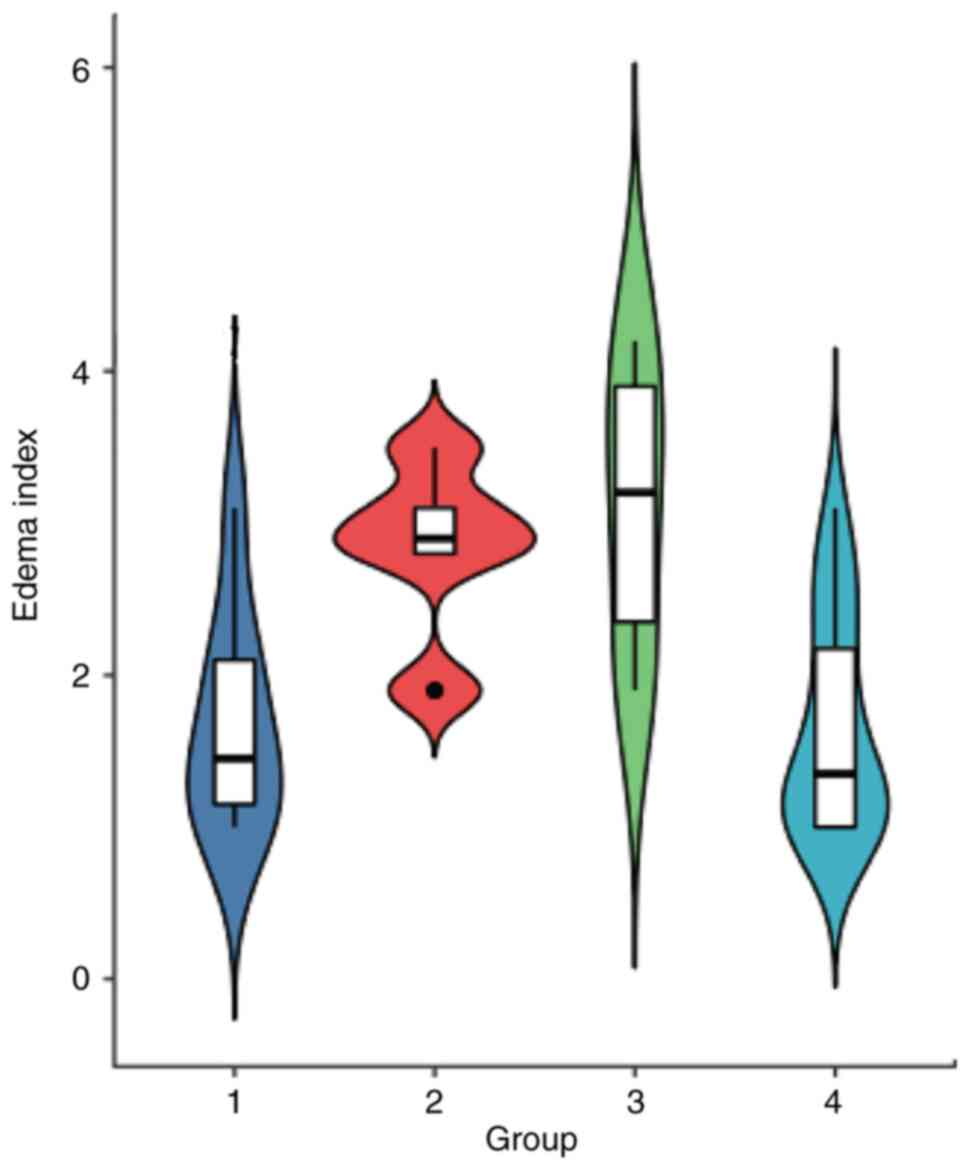
different among the four groups (P<0.05; Table II). The EI was 3.12±0.97 in group
3 and 2.84±0.59 in group 2, which were both significantly higher
than those in groups 1 and 4 (P<0.05; Fig. 7). The EI was not significantly
different between groups 3 and 2 (P>0.05); there was also no
statistical difference between groups 1 and 4 (P>0.05). The
hemorrhagic masses were located at the convexity in 9 patients
(47.4%), in the parasagittal areas in 5 patients (26.3%), in the
parafalx in 2 patients (10.5%), at the skull base in 2 patients
(10.5%) and in the cerebellopontine angle in 1 patient (5.3%); the
convexity and parasagittal areas were the two most common sites for
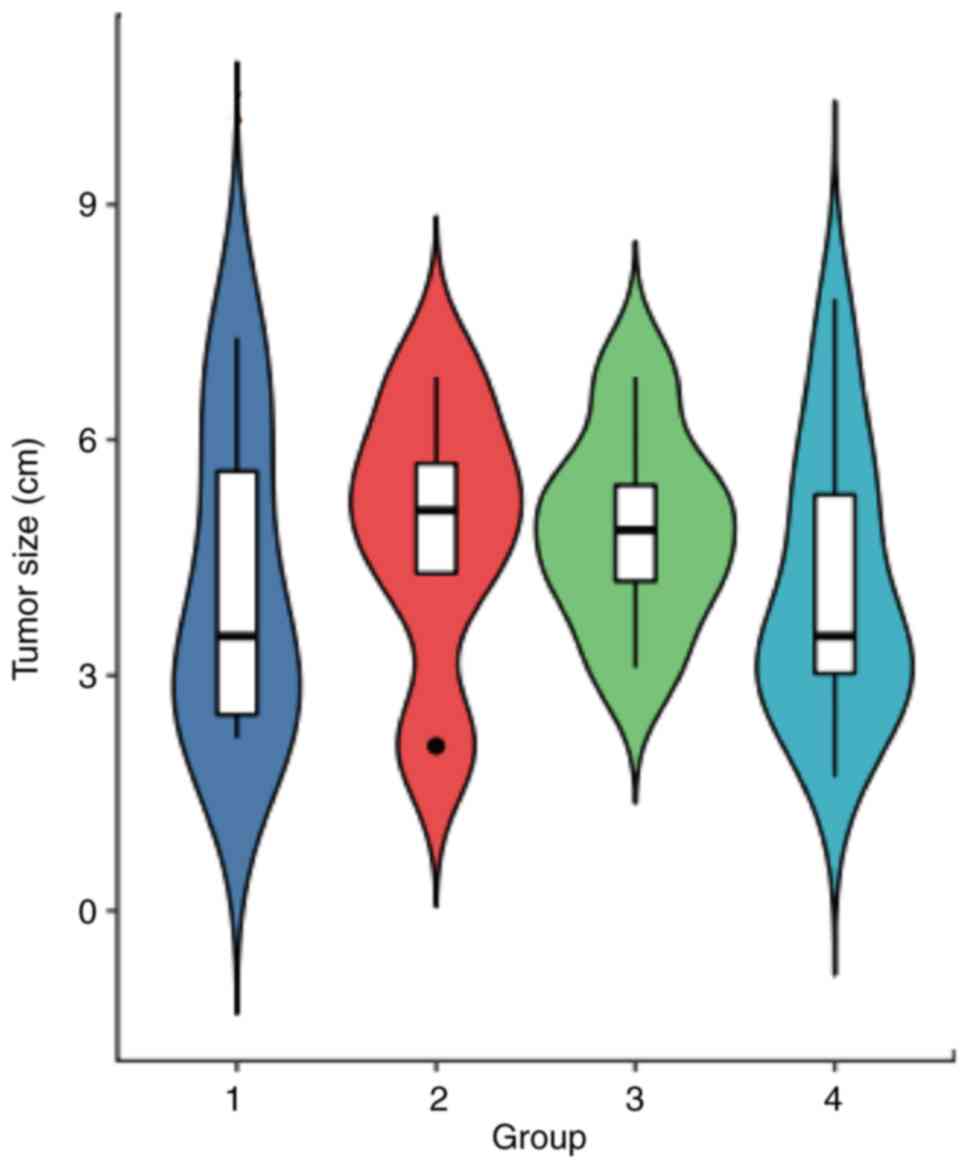
hemorrhagic meningiomas. The tumor size ranged from 2.1 to 7.3 cm
(mean, 4.6±1.7 cm), with 10 tumors (52.6%) <5.0 cm and 9 tumors
(47.4%) >5.0 cm in diameter. There was no statistical difference
with respect to the tumor size among the four groups (F=0.4163;
P>0.05; Fig. 8).
Intraoperative findings
The symptoms in most patients of group 1 (7/8 cases;
87.5%) were mild and their clinical statuses were stable, so
selective surgeries were performed following adequate preoperative
evaluations. The symptoms of the patients in group 2 were sometimes
mild (3/5 cases; 60.0%) and sometimes moderate to severe (2/5
cases; 40.0%), so emergency or early surgeries were chosen
according to the clinical status of the patient and their
radiological results. Almost all patients (5/6 cases; 83.3%) in
group 3 had moderate to severe symptoms, and emergency surgery was
performed in these patients after the necessary examinations.
Hematoma evacuation and a macroscopically complete
resection of the tumor were performed in one stage for all but 1
patient. During the operation, a solid extra parenchymal tumor
attached to the dura and tumor-associated hemorrhage were found in
every case; the surface of the brain contacting the tumor was
intact in 12 cases and invaded by the tumor in 7 cases (2 in
group1; 2 in group 2 and 3 in group 3). All tumors in group 3
(100.0%), 6 cases (75.0%) in group 1 and 2 cases (40.0%) in group 2
had a soft consistency. Of the masses in group 1, 8 had only
intratumoral hemorrhage and necrotic tumor tissue was usually
found. In group 2, 5 masses were only surrounded by extratumoral
hemorrhage involving peritumoral, subarachnoid, subdural and/or
intracerebral hemorrhage. The remaining 6 masses in group 3
included both intratumoral hemorrhage and extratumoral hemorrhage,
and normal vessels with invasion by the tumor were found in 2
masses. Furthermore, tumor rupture was found in group 3 tumors and
the intratumoral hemorrhage was connected with extratumoral
hemorrhage. These operative results were consistent with the
corresponding radiological presentations and the new bleeding
classification (Fig. 9). Simpson
classification of the hemorrhagic masses resulted in 63.2% grade I
(n=12), 21.1% grade II (n=4), 10.5% grade III (n=2) and 5.3% grade
IV (n=1) classifications.
Pathological and surgical
outcomes
The diagnosis of meningioma was established
according to standard histopathological criteria following the WHO
system. The identified tumors were subtyped as fibrous (n=4),
meningothelial (n=3), atypical (n=3), angiomatous (n=2), malignant
(n=2), microcystic (n=2), transitional (n=1), psammomatous (n=1)
and secretory (n=1). Correspondingly, 14 cases (73.7%) were WHO
Grade I tumors, 3 cases (15.8%) were WHO Grade II tumors and 2
cases (10.5%) were WHO Grade III tumors. In group 4, 16 cases
(80.0%) were WHO Grade I tumors, 2 cases (10.0%) were WHO Grade II
tumors and 2 cases (10.0%) were WHO Grade III tumors. There was no
significant difference in the proportion of WHO Grade I tumors
between the hemorrhagic group and the control group (P>0.05;
Table II). Although a statistical
analysis was not performed among the four groups in this study due
to the limited numbers of cases in the subgroups, the
aforementioned different pathological subtypes and WHO Grade tumors
were almost evenly distributed in each group. Endothelial
hyperplasia, thin-walled dilated vessels, venous obstruction and
tumor infarction were observed more frequently on microscopic
examination in patients in groups 1 and 3. Radiotherapy was
administered as an adjuvant treatment in 3 patients (15.8%) after
initial surgery for 2 malignant cases and 1 case of grade IV
resection. The postoperative recoveries of these patients were all
uneventful; 1 patient died from a cardiovascular event 7 years
after surgery and the other patients are currently alive. However,
the tumors in 2 patients recurred within a follow-up period of 2
and 5 years respectively, and these individuals underwent a second
surgery.
Discussion
Malignant brain tumors, including glioblastoma,
metastatic brain tumors and melanoma, may occasionally lead to
intracranial hemorrhage (14).
However, spontaneous hemorrhage as an initial presentation for
meningioma, even though reported, is less frequent, although they
are usually vascular tumors (11).
As this condition is rare, the determination of causative factors
for the hemorrhage is difficult, and it is not as easy to
understand the mechanisms of spontaneous hemorrhage (11,15,16).
Cases of spontaneous hemorrhage in meningioma have been
sporadically reported, however, and a number of clinical features
of the condition have been characterized (4–7,10,11,16–18).
Although CT is the reference standard for the
detection of hemorrhage, meningioma with intracranial hemorrhage is
not always easy to distinguish on CT, and multi-model radiological
examinations are needed (11).
Brain CT and MRI usually display dense, well-defined, contoured
extra-axial masses that displace the adjacent brain along with
acute or subacute hemorrhage (3,6). The
site of the hematoma does not typically exhibit hypertensive
intracerebral bleeding, and it is usually away from the center of
the cerebral parenchyma (3).
Masses with a dural base show moderate to strong enhancement on MRI
with gadolinium enhancement, with the exception of the region
representing the hemorrhage. Previous reports have provided some
information on the signal intensity on MRI and have indicated
hyperintensity on T2-weighted MRI as a risk factor of meningioma
bleeding (16,18); Specifically, Niiro et al
(16) found that all hemorrhagic
meningiomas were remarkably hyperintense on T2-weighted MRI in
their study and Lin and Chen (18)
reported 1 case with the same above radiological feature. Comparing
type II bleeding, this finding of hyperintensity on T2-weighted MRI
was found more frequently in the patients with type I and type III
bleeding in the present study. While the implication of this
hyperintensity has not been fully established, some cases with
intratumoral hemorrhage may be attributed to the soft consistency
of the tumor (10,16,19).
In type III bleeding cases, it is hypothesized that the hemorrhage
may have begun within the tumor and then progressed, resulting in
blood in the peritumoral areas and even encroachment of the blood
in the brain parenchyma (10).
A previous study found that an increased tendency
for bleeding was associated with two age groups (<30 years and
>70 years), convexity and intraventricular locations, and
fibrous meningiomas (2); however,
the most frequent localizations were the convexity and parasagittal
areas in the present study, and the pathological outcomes of these
tumors were in line with the histopathological distribution of
meningiomas in general (1).
Although the patients with type II and type III bleeding had
significant PTBE comparing with the control group in the present
study, and PTBE might be an indicator for certain bleeding types,
PTBE could be as a result of the tumor hemorrhage. The exact
mechanism of the hemorrhage from meningiomas is not fully
understood; however, several pathological mechanisms have been
considered in the explanation of this rare condition. The proposed
mechanisms include weakened blood vessel rupture, endothelial
proliferation and resultant vascular occlusion, direct tumor cell
invasion of the vasculature, bioactive substance accumulation in
the tumor, concomitant vascular malformation or aneurysm,
stretching and rupture of subdural bridging veins, venous
compression induced by tumor growth and associated with peritumoral
edema, and infarction due to rapid tumor growth (2–6,8–11,16–18).
In addition, two specific types of blood vessels, differentiated
and undifferentiated vessels, were found in our prior study;
undifferentiated blood vessels contribute to a fragile tumor
vasculature, which a precipitating event may disrupt, thus
resulting in a spontaneous hemorrhage (11).
The aforementioned proposed mechanisms were mainly
based on reported cases. The majority of these were recorded
according to the site of hemorrhage, such as the cases of
subarachnoid hemorrhage, intracerebral hematoma, intratumoral
hemorrhage or subdural hematoma (7–12,18–20).
This description cannot reflect the association between meningioma
and hemorrhage; moreover, it contributes little to guiding the
clinical diagnosis and treatment. Correlating prior reports of such
cases with the present cases, a new bleeding classification of
meningiomas was proposed in the present study on the basis of the
anatomical relationship between meningioma and hematoma. This
distinct type of hemorrhage from meningioma was separated into
three bleeding patterns: Purely intratumoral hemorrhage, purely
extratumoral hemorrhage, and combined intratumoral and extratumoral
hemorrhage. According to the intraoperative findings of tumor
fragmentation and intratumoral hemorrhage continuous with
extratumoral hemorrhage in group 3 tumors, it could be inferred
that this type of combined hemorrhage arose from intratumoral
bleeding with extension into the surrounding intracranial
spaces.
Apart from showing the direct relationship between
meningioma and hemorrhage, this new bleeding classification makes
it easier to understand the possible mechanism of meningioma
hemorrhage. For example, subdural bridging veins may stretch and
rupture, which may explain the purely extratumoral hemorrhage
involved in subdural hematoma and subarachnoid hemorrhage cases
(20). The rupture of weakened or
undifferentiated blood vessels is typically associated with a
purely intratumoral hemorrhage (11,18).
The extratumoral hemorrhage should be secondary to intratumoral
bleeding in the third type of hemorrhage (10,11).
Infarction and necrosis owing to rapid growth of the tumor could
explain the third type of hemorrhage (10,21).
Traumatic head injury is unlikely to be a causative factor in cases
with purely intratumoral hemorrhage (11,22).
In syncytial meningiomas, the bleeding is likely associated with
the release of intratumoral vasoactive substances, such as
histamine, which could induce vasodilatation and result in the
purely intratumoral hemorrhage (23,24).
Venous hypertension due to tumor compression of the surrounding
veins may also lead to the purely extratumoral hemorrhage (13,25).
In addition, cerebral edema and venous obstruction could cause
infarction, eventual rupture of the peritumoral vessel and then
induce the purely extratumoral hemorrhage (13). Nevertheless, any one of the
aforementioned mechanisms alone cannot explain the various bleeding
patterns and the mechanism might vary in these different bleeding
patterns.
Emergency or early one-stage total removal of the
hemorrhagic meningioma and hematoma is the main treatment of choice
(2–4,11).
In general, the risks of meningioma hemorrhage usually vary with
the amount of bleeding, the location of bleeding, the size of tumor
or the location of tumor. There is no difference in the therapeutic
method for the different bleeding patterns; however, this bleeding
classification system could offer some implications for the
treatment strategy. According to the review of prior cases reported
in the literature (4–8,11,17–21,25,26)
and the cases in the present study, the symptoms in most patients
with the first bleeding type were usually mild and their clinical
statuses were generally stable, and early or selective surgery
could be performed following adequate preoperative evaluations. The
symptoms in patients with the second bleeding type were at times
mild and in other cases moderate to severe, so emergency or early
surgery should be chosen according to the clinical status of the
patient and their corresponding radiological results. Almost all
patients with the third bleeding type had moderate to severe
symptoms, and these patients usually need emergency surgery after
necessary examinations. Therefore, the impact on patients of
meningioma hemorrhage is closely correlated with hemorrhagic type.
Recognizing these facts and consequent treatment changes may result
in an improvement of patient outcome.
The main limitation of this study, especially
regarding the bleeding subgroups, is the small sample size and lack
of integrative data analysis among subgroups. Although the data
were preliminary and the bleeding mechanisms were also not proven
in the present study, the study highlighted the new bleeding
classification of meningiomas based on the anatomical relationship
between meningioma and hematoma. Moreover, sharing our opinion and
typical radiographic images may help improve awareness of this
specific condition. Future research will focus on the issue of
collecting more cases to explore the findings in this study.
In summary, although hemorrhagic meningiomas are
fairly uncommon, they represent a distinct clinical entity. The
following three bleeding patterns were proposed in the present
study: Purely intratumoral hemorrhage, purely extratumoral
hemorrhage, and combined intratumoral and extratumoral hemorrhage.
Patients with different bleeding patterns may exhibit different
clinical features and radiological outcomes. This bleeding
classification makes it easier to understand the possible
hemorrhagic mechanisms and aid in the early diagnosis of this
condition and in the selection of a treatment strategy.
Acknowledgements
Not applicable.
Funding
The clinical data collection in this study was supported by a
grant from the Natural Science Foundation of Ningbo (grant no.
2019A610283) and NINGBO Medical and Health Leading Academic
Discipline Project (grant no. 2022-F04). The figure design and case
follow-up in this study were supported by grants from the Ningbo
Municipal Bureau of Science and Technology (grant nos. 2021S172 and
2014C50089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZRX, HCW and YLT, interpreted the data prepared the
images and wrote the manuscript. HCW contributed to the conception
and drafted the manuscript. SWL and BDW contributed to the
acquisition and analysis of the data and figure design. MSC and HCW
designed the work, revised and edited the manuscript for important
intellectual content. HCW, BDW and MSC confirm the authenticity of
all raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval of the retrospective study was obtained
from the Research Ethics Committee of the Li Hui Li Hospital of
Ningbo University (Ningbo, China). Written informed consent was
obtained from all the participants.
Patient consent for publication
All patients provided the consent for publication of
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldbrunner R, Minniti G, Preusser M,
Jenkinson M, Sallabanda K, Houdart E, Deimling A, Stavrinou P,
Lefranc F, Lund-Johansen M, et al: EANO guidelines for the
diagnosis and treatment of meningiomas. Lancet Oncol. 17:e383–e912.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boŝnjak R, Derham C, Popović M and Ravnik
J: Spontaneous intracranial meningioma bleeding:
Clinicopathological features and outcome. J Neurosurg. 103:473–484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pereira BJ, de Almeida AN, Paiva WS, de
Aguiar PH, Teixeira MJ and Marie SK: Assessment of hemorrhagic
onset on meningiomas: Systematic review. Clin Neurol Neurosurg.
199:1061752020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kouyialis AT, Stranjalis G, Analyti R,
Boviatsis EJ, Korfias S and Sakas DE: Peritumouralhaematoma and
meningioma: A common tumour with an uncommon presentation. J Clin
Neurosci. 11:906–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng MH and Lin JW: Intracranial
meningioma with intratumoral hemorrhage. J Formos Med Assoc.
96:116–120. 1997.PubMed/NCBI
|
|
6
|
Huang RB, Chen LJ, Su SY, Wu XJ, Zheng YG,
Wang HP and Liu Y: Misdiagnosis and delay of diagnosis in
hemorrhagic meningioma: A case series and review of the literature.
World Neurosurg. 155:e836–e846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aloraidi A, Abbas M, Fallatah B,
Alkhaibary A, Ahmed ME and Alassiri AH: Meningioma presenting with
spontaneous subdural hematoma: A report of two cases and literature
review. World Neurosurg. 127:150–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pressman E, Penn D and Patel NJ:
Intracranial hemorrhage from meningioma: 2 novel risk factors.
World Neurosurg. 135:217–221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hambra DV, Danilo de P, Alessandro R, Sara
M and Juan GR: Meningioma associated with acute subdural hematoma:
A review of the literature. Surg Neurol Int. 5 (Suppl
12):S469–S471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han S, Yang Y, Yang Z, Liu N, Qi X, Yan C
and Yu C: Continuous progression of hemorrhage of sphenoid ridge
meningioma causing cerebral hernia: A case report and literature
review. Oncol Lett. 20:785–793. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HC, Wang BD, Chen MS, Li SW, Chen H,
Xu W and Zhang JM: An underlying pathological mechanism of
meningiomas with intratumoral hemorrhage: Undifferentiated
microvessels. World Neurosurg. 94:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krisht KM, Altay T and Couldwell WT:
Myxoid meningioma: A rare metaplastic meningioma variant in a
patient presenting with intratumoral hemorrhage: Case report. J
Neurosurg. 116:861–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bitzer M, Topka H, Morgalla M, Friese S,
Wöckel L and Voigt K: Tumor-related venous obstruction and
development of peritumoral brain edema in meningiomas.
Neurosurgery. 42:724–728. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schrader B, Barth H, Lang EW, Buhl R, Hugo
HH, Biederer J and Mehdorn HM: Spontaneous intracranial haematomas
caused by neoplasms. Acta Neurochir (Wien). 142:979–985. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DG, Park CK, Paek SH, Choe GY, Gwak
HS, Yoo H and Jung HW: Meningioma manifesting intracerebral
haemorrhage: A possible mechanism of haemorrhage. Acta Neurochir
(Wien). 142:165–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niiro M, Ishimaru K, Hirano H, Yunoue S
and Kuratsu J: Clinico-pathological study of meningiomas with
haemorrhagic onset. Acta Neurochir (Wien). 145:767–772. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jr SS, Shuangshoti S and Vajragupt L:
Meningiomas associated with hemorrhage: A report of two cases with
a review of the literature. Neuropathology. 19:150–160. 1999.
View Article : Google Scholar
|
|
18
|
Lin RH and Shen CC: Meningioma with purely
intratumoral hemorrhage mimicked intracerebral hemorrhage: Case
report and literature review. J Med Sci. 36:1582016. View Article : Google Scholar
|
|
19
|
Yamaguchi N, Kawase T, Sagoh M, Ohira T,
Shiga H and Toya S: Prediction of consistency of meningiomas with
preoperative magnetic resonance imaging. Surg Neurol. 48:579–583.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Gwak HS, Hong EK, Bang CW, Lee SH
and Yoo H: A case of benign meningioma presented with subdural
hemorrhage. Brain Tumor Res Treat. 3:30–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasagawa Y, Tachibana O, Iida T and Iizuka
H: Oncocytic meningioma presenting with intratumoral hemorrhage. J
Clin Neurosci. 20:1622–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dang DD, Mugge L, Awan O, Dang J and
Shenai M: Spontaneous or traumatic intratumoral hemorrhage? A rare
presentation of parafalcine meningioma. Cureus.
12:e114862020.PubMed/NCBI
|
|
23
|
Agazzi S, Burkhardt K and Rilliet B: Acute
haemorrhagic presentation of an intracranial meningioma. J Clin
Neurosci. 6:242–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Motegi H, Kobayashi H, Terasaka S, Ishii
N, Ito M, Shimbo D and Houkin K: Hemorrhagic onset of rhabdoid
meningioma after initiating treatment for infertility. Brain Tumor
Patholo. 29:240–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lefranc F, Nagy N, Dewitte O, Balériaux D
and Brotchi J: Intracranial meningiomas revealed by non-traumatic
subdural haematomas: A series of four cases. Acta Neurochir (Wien).
143:977–983. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mangubat EZ and Byrne RW: Major
Intratumoral hemorrhage of a petroclival atypical meningioma: Case
report and review of literature. Skull Base. 20:469–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|