Introduction
Pancreatic cancer (PC) is one of the most aggressive
and lethal types of cancer worldwide, where it is the seventh
leading cause of cancer-associated mortality in both sexes
(1). At present, there is a lack
of effective screening tools for early-stage PC due to the lack of
typical early symptoms (2). The
majority of patients with PC present already with locally advanced
(30–35%) or metastatic (50–55%) disease at diagnosis and are
therefore not eligible for curative surgery, leading to poor
clinical outcomes (3). This poor
prognosis resulted in the similar number of deaths (466,003) to the
number of diagnosed cases (495,774) across 185 countries in 2020
(2). In addition, patients with PC
have the lowest survival rates among all cancer types, with only
~4% surviving beyond 5 years (4).
The histology of PC is characterized by its aggressiveness, leading
to early infiltration and a high propensity for systemic
dissemination (5). The most common
site of PC metastasis is the liver (40–50%) (6), with other commonly affected organs
include the lungs, bones, adrenal glands, stomach lying adjacent to
the pancreas, duodenum, transverse colon and left kidney (7). Compared with the aforementioned
target organs, the ovary is an uncommon site for PC metastasis
(5). Secondary ovarian
malignancies account for 10–25% of all ovarian tumors (8), where the mechanism of dissemination
may be through implantation, lymphatic and/or hematological
metastasis (5).
The present report describes a case of PC diagnosed
by pancreatic puncture, with ovarian metastasis subsequently
confirmed by surgery. The patient responded favorably to
combination immunotherapy treatment [including use of a programmed
cell death protein-1 (PD-1) inhibitor, nab-paclitaxel and
gemcitabine]. Therefore, further exploration of anti-PD-1 therapy
for PC treatment is warranted in the future.
Case report
A 42-year-old woman presented with increased levels
of serum tumor markers, including carbohydrate antigen (CA)19-9
(3,793 U/ml; normal range, 0–27 U/ml) and carcinoembryonic antigen
(CEA; 58.6 ng/ml; normal range, 0–4.7 ng/ml), during a routine
physical examination in June 2020, with no obvious symptoms.
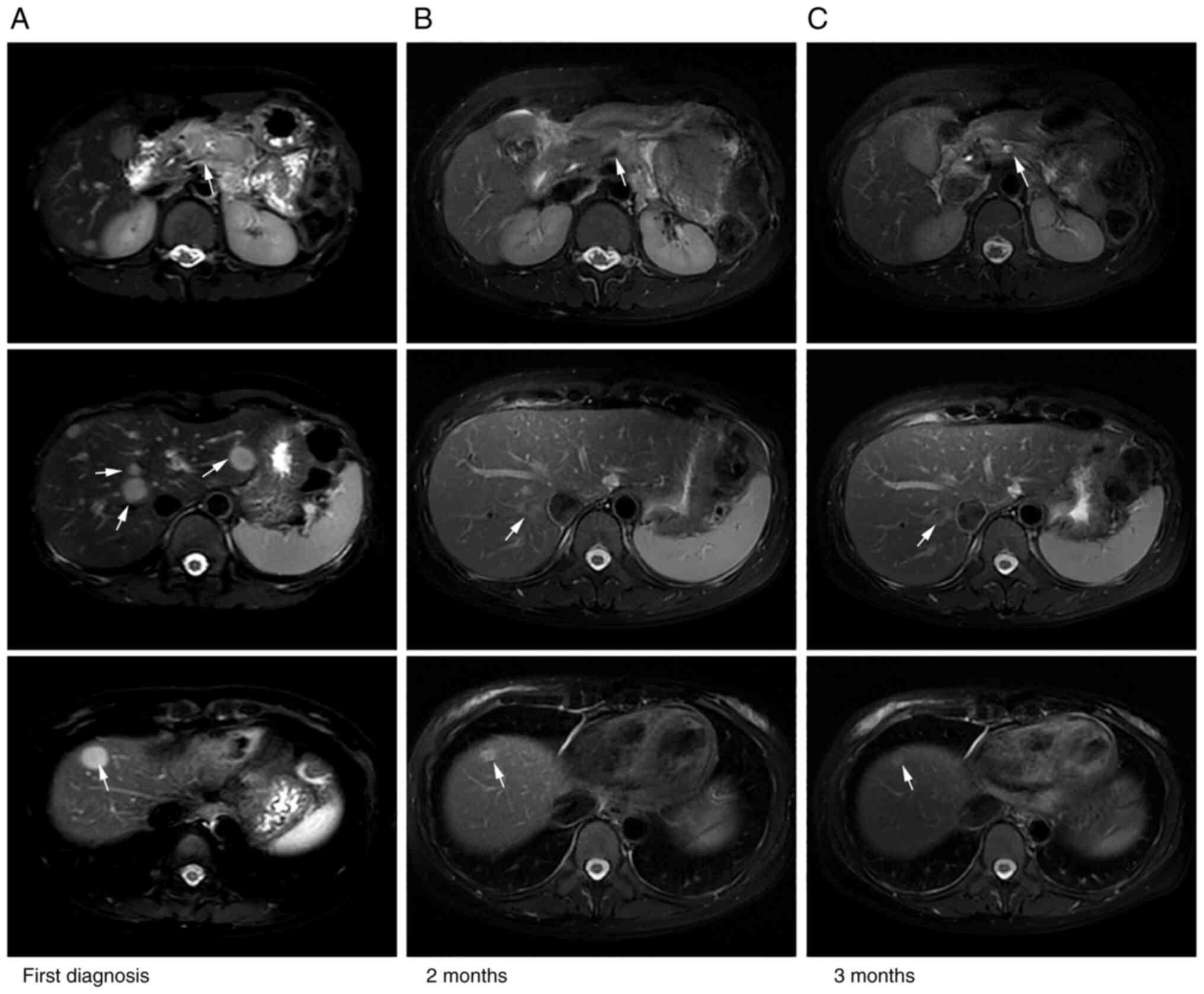
Enhanced MRI in Renji Hospital (Shanghai, China) 5 days later
revealed a mass (49×26 mm) in the pancreatic body and multiple
hepatic lesions (Fig. 1A). Next,
MRI-guided biopsy of the pancreatic tumor was performed, following
which somatic adenocarcinoma of the pancreas was diagnosed. The
staging was cT3NxM1, stage IV according to the 8th edition of the
American Joint Committee on Cancer for Pancreas and Hepatobiliary
Cancers (9). Therefore, the
patient was eligible to take part in a single-arm clinical trial
assessing the combination of doublet chemotherapy [nab-paclitaxel
and gemcitabine (AG)] and the novel PD-1 inhibitor camrelizumab
(formerly SHR-1210) for the first-line treatment of metastatic
pancreatic carcinoma (ClinicalTrials.gov identifier: NCT04181645; Table I). Therefore, the patient was
prescribed first-line immunotherapy treatment of AG + anti-PD-1
immunotherapy 2 weeks after admission for six cycles. The regimen
included nab-paclitaxel (Abraxane; 125 mg/m2) and
gemcitabine (1,000 mg/m2) on days 1 and 8, along with
camrelizumab (200 mg) on day 1 every 3 weeks. Repeat imaging
assessment after two and four cycles of this combination treatment
revealed a significant reduction in the size of the pancreatic and
liver lesions (Fig. 1B). In
addition, subsequent MRI scans (Fig.
1C) showed further shrinkage of the tumor and partial response
(PR) was concluded using the modified Response Evaluation Criteria
in Solid Tumors assessment criteria (Fig. 1) (10). The tumor markers CA19-9 and CEA
were restored to the normal ranges. According to the Common
Terminology Criteria for Adverse Events (version 4.03) (11), adverse events occurred, which
included grade 1–2 rash, edema of the bilateral ankles and eyelids,
knee pain, hyperhidrosis and grade 1 anemia. However, no
treatment-related grade 3 or 4 adverse events were observed. The
patient was next administered six cycles of first-line anti-PD-1
immune maintenance therapy (200 mg camrelizumab on day 1 every 3
weeks) 4 months after admission. After two cycles, a continuous PR
was obtained. After the fourth cycle, the pancreatic mass increased
slightly in size, and the PR of the liver lesions persisted. The
toxicity parameter of transient hyperthyroidism appeared during
this period. Afterwards, hypothyroidism was detected, which was
controlled by supplementation with levothyroxine sodium tablets
(100 µg every day). The progression-free survival (PFS) time from
this first-line therapy was 9 months.
 | Table I.Ongoing clinical trials of
camrelizumab (formerly SHR-1210) in pancreatic cancer treatment in
China. |
Table I.
Ongoing clinical trials of
camrelizumab (formerly SHR-1210) in pancreatic cancer treatment in
China.
| ClinicalTrials.gov
identifier | Study phase | Trial arm | Condition | Subjects, n | Study status | Location |
|---|
| NCT04181645 | NA | SHR-1210 +
gemcitabine + paclitaxel-albumin | Pancreatic cancer
stage IV | 20 | Recruiting | Renji Hospital,
Shanghai, China |
| NCT04498689 | II | Camrelizumab + nab
paclitaxel + gemcitabine injection | Metastatic
pancreatic cancer | 117 | Recruiting | Fudan University,
Shanghai, China |
| NCT04420130 | NA | Camrelizumab +
chemotherapy + ablation | Pancreatic cancer;
liver metastasis | 34 | Not yet
recruiting | Harbin Medical
University, Harbin, China |
| NCT04415385 | II | Camrelizumab +
apatinib | Pancreatic
cancer | 48 | Recruiting | Zhejiang Cancer
Hospital, Hangzhou, China |
| NCT04674956 | III | Camrelizumab +
paclitaxel (albumin-bound) and gemcitabine vs. placebo + paclitaxel
(albumin-bound) and gemcitabine | Pancreatic cancer
stage IV; pancreatic metastatic cancer | 401 | Not yet
recruiting | Renji Hospital,
Shanghai, China |
| NCT05218889 | II | Surufatinib +
camrelizumab + nab paclitaxel + S-1 vs. nab paclitaxel +
gemcitabine | Pancreatic
cancer | 68 | Not yet
recruiting | Chinese People's
Liberation Army General Hospital, Beijing, China |
| NCT04723030 | II | Carleilizumab +
apathy mesylate + radiotherapy + paclitaxel (albumin-bound) | Locally advanced
pancreatic cancer | 30 | Not yet
recruiting | Peking University
Cancer Hospital and Institute, Beijing, China |
| NCT04932187 | I | Camrelizumab +
capecitabine | Hepatobiliary,
pancreatic and other gastrointestinal carcinoma (non-stomach,
non-esophageal) | 20 | Recruiting | Ruijin Hospital,
Shanghai, China |
At 10 months post-diagnosis, enhanced CT of the low
abdomen demonstrated a large irregular mass (28 mm in diameter)
with scatted dot enhancement in the bilateral adnexa area. Since
the new mass was suspected to be metastatic, another PET-CT was
performed. The size of the pancreatic body mass was similar to the
previous result (9 months ago) but showed a markedly decreased
fluorodeoxyglucose (FDG) uptake. According to the sizes of the
hepatic metastatic lesions and the hilar and para-aortic lymphatic
metastases, a PR was achieved and the FDG uptake was low. By
contrast, the newly discovered bilateral adnexal mass showed
increased FDG uptake, suggesting that it may be prone to tumor
metastasis. However, primary ovarian carcinoma should not be ruled
out either. The multidisciplinary therapy (MDT) team suggested a
right adnexectomy to confirm the origin of the mass pathologically.
After 10 days, a right adnexectomy was successfully performed using
single-port laparoscopy. The right ovary was enlarged by ~5 cm,
whereas the right oviduct and left adnexa appeared normal. H&E
and immunohistochemical staining were subsequently performed.
The tissue samples derived from resected and core
needle biopsy specimens were fixed in 10% formalin at room
temperature for 24 h, paraffin embedded and subjected to
histological or immunohistochemical analysis. Sections (4-µm thick)
were heated at 58°C for 2 h and then deparaffinized in xylene and
hydrated with a series of graded alcohols, including anhydrous
ethanol for 5 min, 95% ethanol for 2 min, 90% ethanol for 2 min,
80% ethanol for 2 min and 70% ethanol for 2 min. H&E staining
was then used for histological analysis. Hematoxylin staining was
performed for 5 min at room temperature and eosin staining for 1
min at room temperature.
For immunohistochemistry, after 3 min of blocking at
room temperature with the blocking reagent (working fluid; cat. no.
DM841; Dako; Agilent Technologies, Inc.), antigen recovery was
performed by heating and immersing the slides in citrate buffer
(0.01 M, pH 9.0) in a microwave oven (121°C) for 10 min twice.
Endogenous peroxidase activity was blocked using 3% hydrogen
peroxide for 30 min at 20°C and the sections were incubated with
primary antibodies against Ki-67 (1:100; cat. no. MIB-1; Dako;
Agilent Technologies, Inc.), tumor protein 53 (p53; working fluid;
cat. no. MAB-0674; Fuzhou Maixin Biotech Co., Ltd.), cytokeratin
(CK)7 (1:50; cat. no. OV-TL12/30; Dako; Agilent Technologies,
Inc.), vimentin (VIM; working fluid; cat. no. MAB-0735; Fuzhou
Maixin Biotech Co., Ltd.), estrogen receptor [ER; working fluid;
cat. no. 790-4325; Roche Diagnostics (Shanghai) Co., Ltd.],
progesterone receptor [PR; working fluid; cat. no. 790-4296; Roche
Diagnostics (Shanghai) Co., Ltd.], Wilm's tumor-1 (WT-1; working
fluid; cat. no. MAB-0678; Fuzhou Maixin Biotech Co., Ltd.),
hepatocyte nuclear factor 1 homeobox B (HNF-1B; working fluid; cat.
no. ZA-0129; Origene Technologies, Inc.), CA125 (working fluid;
cat. no. MAB-0007; Fuzhou Maixin Biotech Co., Ltd.), p16 (working
fluid; cat. no. MAB-0673; Fuzhou Maixin Biotech Co., Ltd.), CK20
(1:80; cat. no. M7019; Dako; Agilent Technologies, Inc.),
caudal-related homeobox transcription factor 2 (CDX2; working
fluid; cat. no. RMA-0631; Fuzhou Maixin Biotech Co., Ltd.), special
AT-rich binding protein 2 (SATB2; working fluid; cat. no. RMA-0750;
Fuzhou Maixin Biotech Co., Ltd.), post-meiotic segregation
increased 2 (PMS2; working fluid; cat. no. IR087; Dako; Agilent
Technologies, Inc.), mut L homolog 1 (MLH1; working fluid; cat. no.
IR079; Dako; Agilent Technologies, Inc.), mut S homolog (MSH)2
(working fluid; cat. no. IR085; Dako; Agilent Technologies, Inc.),
MSH6 (working fluid; cat. no. IR086; Dako; Agilent Technologies,
Inc.), programmed death ligand 1 (PD-L1; working fluid; cat. no.
M3666; Dako; Agilent Technologies, Inc.), paired-box gene (PAX)-2
(working fluid; cat. no. RMA-0816; Fuzhou Maixin Biotech Co.,
Ltd.), PAX-8 (working fluid; cat. no. RMA-0817; Fuzhou Maixin
Biotech Co., Ltd.), mucin 1 (MUC1; 1:50; cat. no. MRQ-17; Aimeijie
Technology Co., Ltd.) at 4°C overnight. Subsequently, the sections
were washed with PBS three times for 2 min and incubated with a
biotinylated anti-mouse/rabbit secondary antibody (1:500; cat. no.
D0486 and D0487, conjugated to alkaline phosphatase; Dako; Agilent
Technologies, Inc.) at 37°C for 15 min. The signals was detected
using a 3,3′diaminobenzidine kit (Dako; Agilent Technologies,
Inc.). Finally, the sections were counterstained with hematoxylin
at room temperature for between 3 sec and 5 min. The positively
stained cells were then counted and imaged under a light microscope
(Olympus BX43; Olympus Corporation) with ×100 and ×400
magnification. The negative control was conducted by replacing the
primary antibody with 0.1% bovine sum albumin (cat. no. BAH62-0100;
AmyJet Scientific, Inc.) or PBS. Cells with brown granule staining
of the membrane/cytoplasm/nucleus were considered positive. For
Ki-67 and p53, percentages of labeled positive cells within the
investigated cell population are stated.
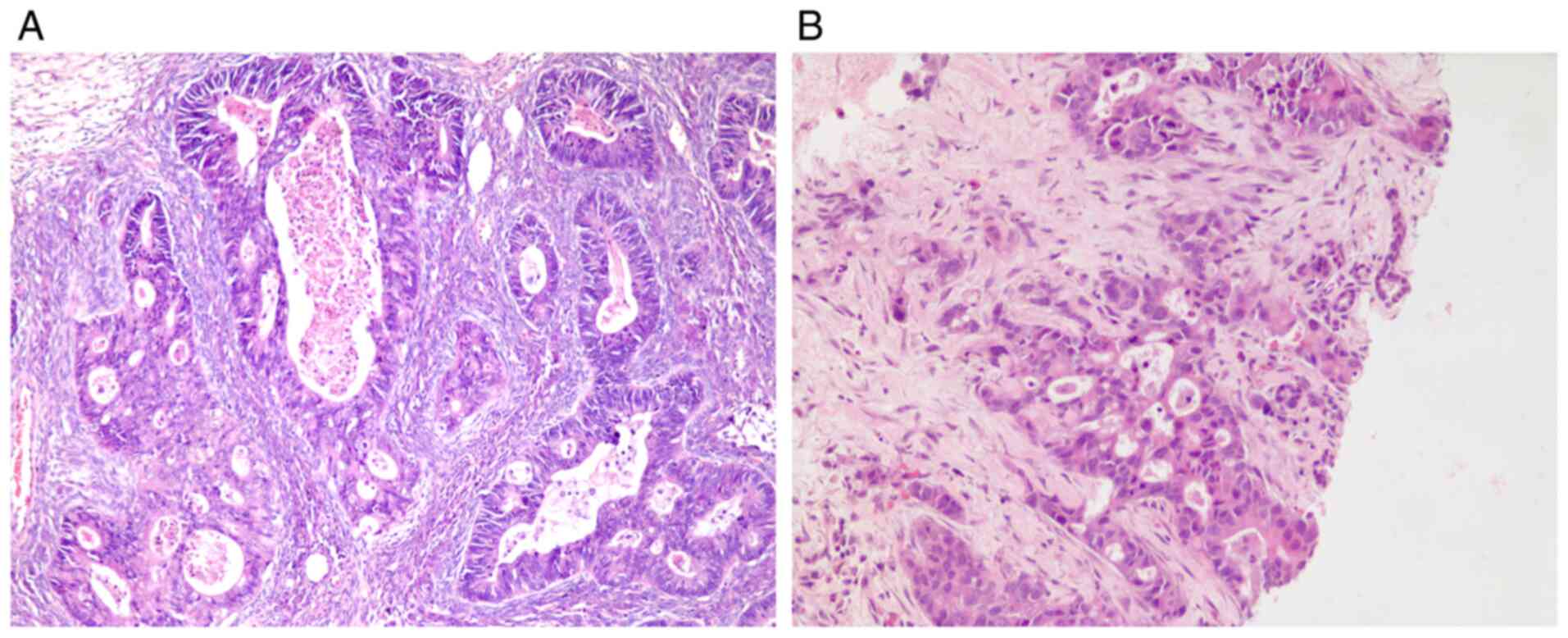
The results revealed a right ovarian adenocarcinoma
(maximum 8 cm in diameter; Fig. 2)
and the serosa of the right oviduct was congested due to tumor
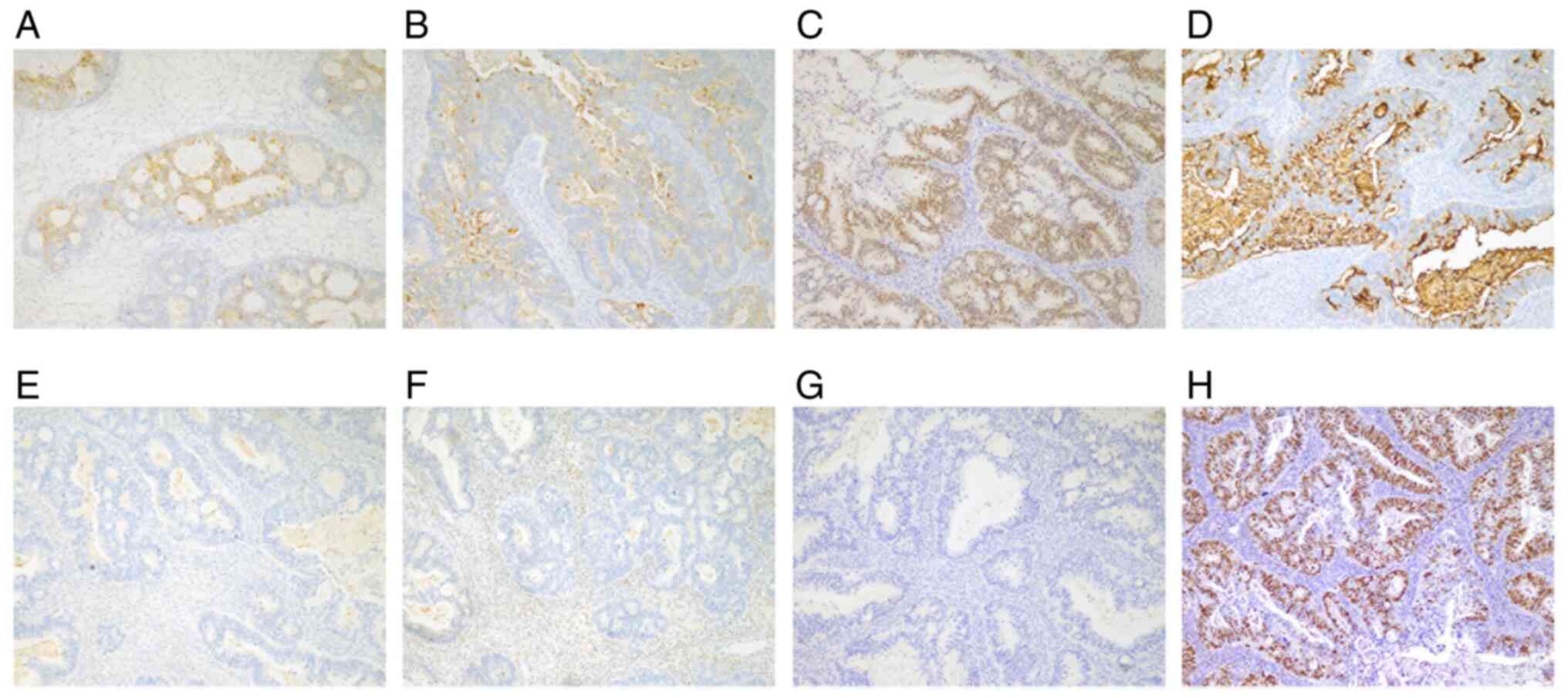
obstruction. Immunohistochemistry (IHC) analysis (Fig. 3) provided the following results:
Ki-67 (60%), p53(75%), CK7(+), VIM(−), ER(−), PR(−), WT-1(+),
HNF-1B(−), CA125(−), p16(+), CK20(+), CDX2(+), SATB2(+), PMS2(+),
MLH1(+), MSH2(+), MSH6(+), PD-L1(−), PAX-2(−), PAX-8(−) and
MUC1(+). Pathological review diagnosed metastatic adenocarcinoma of
pancreatic origin based on IHC of the ovarian mass [CK20(+) and
CA125(−)].
Systemic antineoplastic chemotherapy was suggested
but the patient refused intravenous chemotherapy due to poor
tolerance. The patient accepted four cycles of second-line
combination treatment 10 months after admission, including
immunotherapy, targeted therapy and oral chemotherapy (200 mg
sintilimab on day 1; 50 mg tegafur on days 1–14, two times a day;
and 8 mg anlotinib on days 1–14 every 3 weeks). A follow-up CT scan
after the second and fourth cycles showed that the morphology of
the pancreatic tumor and the metastatic lesions did not change
markedly, suggesting stable disease (SD) (Fig. 4). Nevertheless, the patient
experienced grade 3 diarrhea after the fourth cycle of treatment.
Stool smears and cultures tested negative for fungus,
Staphylococcus, acid-fast bacillus, Salmonella, Shigella,
Staphylococcus aureus, Escherichia coli O-157, Vibrio
cholerae, Vibrio parahaemolyticus and Clostridium
difficile. Colonoscopy revealed multiple ulcers, with
autoimmune enteritis as the pathological diagnosis. Considering
this immune-induced toxicity, vedolizumab (300 mg on day 1) was
prescribed and sintilimab was stopped permanently. However, the
patient exhibited a high fever, with a thermal spike of 40°C.
Therefore, 40 mg methylprednisolone was administered for 6 days for
the immunological fever. Subsequently, 30 mg/day prednisolone was
taken orally, tapered off 5 mg each time and eventually
discontinued in 18 days. According to the recommendation of the
gastroenterologist, 2 g/day mesalazine was also prescribed to
reduce intestinal inflammation. The patient recovered after 1 week,
before the treatment plan was changed to oral chemotherapy and
targeted therapy (50 mg tegafur on days 1–14, two times a day; and
8 mg anlotinib on days 1–14 every 3 weeks). During this period, the
patient suffered a Candida tropicalis infection diagnosed by
stool culture. Therefore, oral fluconazole at 100 mg/day was
administered for 1 week. Then diarrhea and jaundice occurred during
the sixth cycle of therapy. Endoscopic sphinctopapillotomy,
endoscopic papillary balloon dilation and endoscopic retrograde
biliary drainage were completed successfully, where bile drainage
was unobstructed following the surgery. Methylprednisolone was
prescribed at 35 mg/day orally for 1 month. PFS time after this
second-line therapy was 6 months.
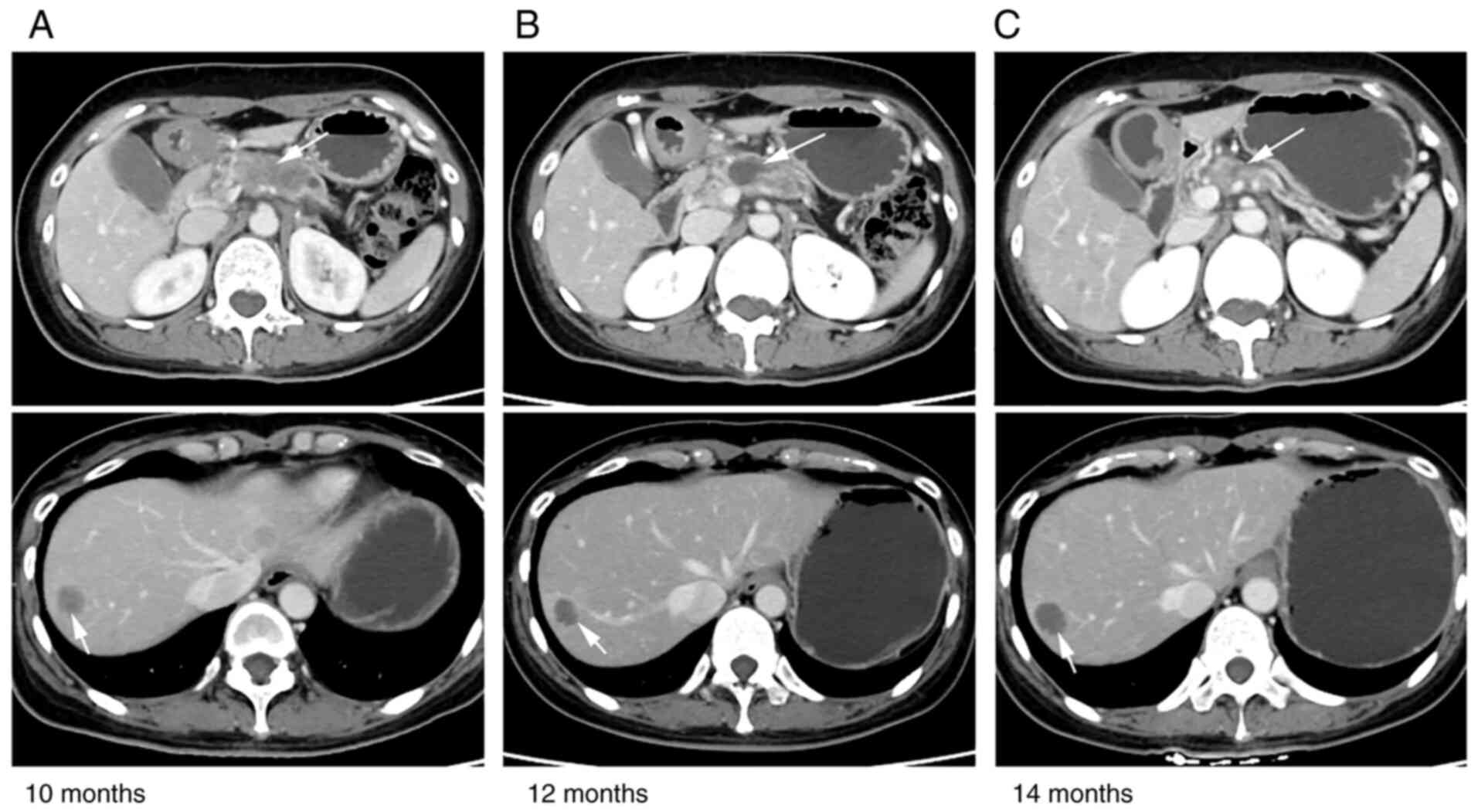
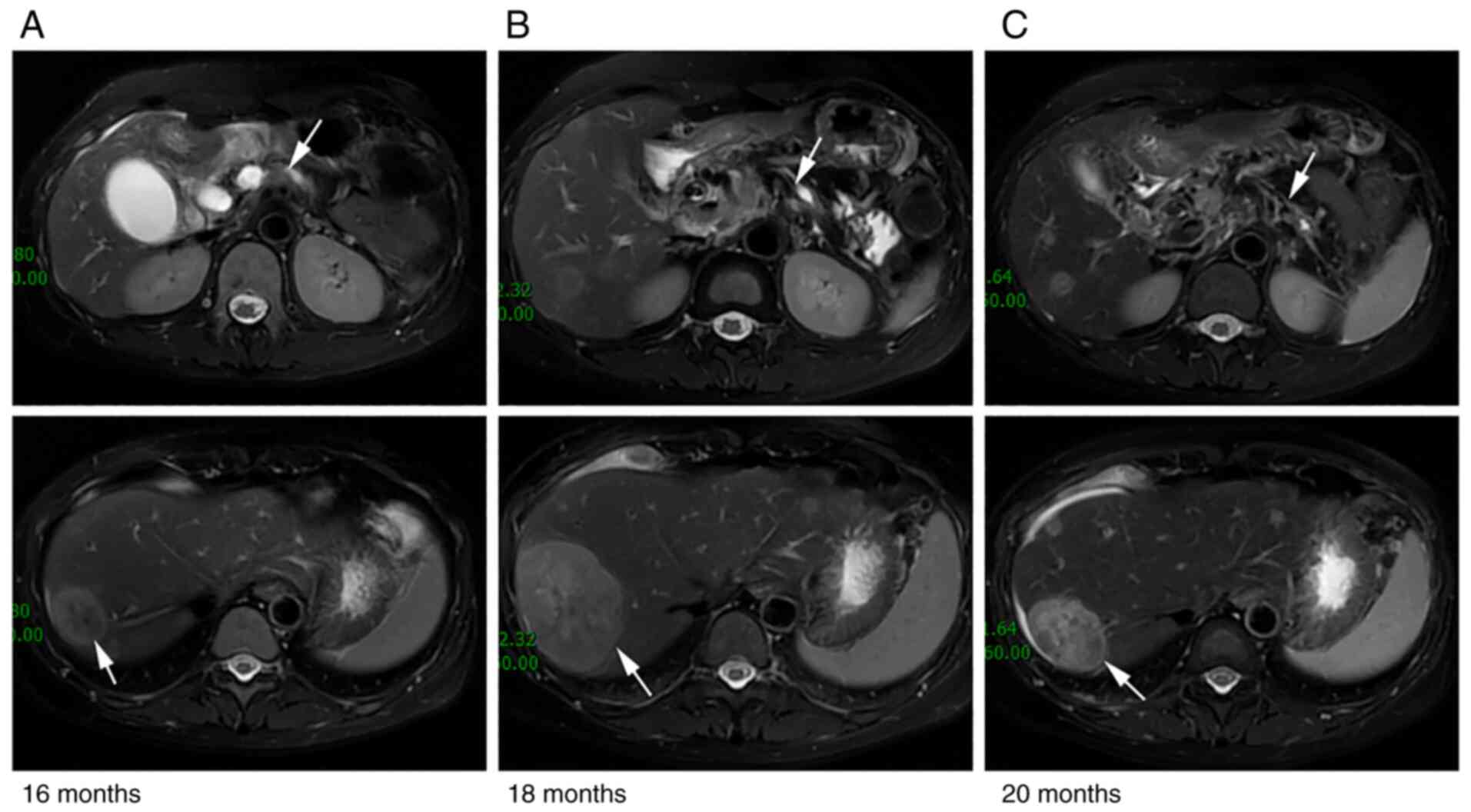
The disease was now considered to be at progressive
disease (PD) due to the increasing lesion size in the liver
(maximum diameter 2.7 cm) and the target mass in the pancreas
(4.3×2.1 cm; Fig. 5A) being
similar to that measured previously (4.3×2.0 cm; Fig. 4C) at month 16 after first
diagnosis. Since the patient had benefited greatly (evaluated as
PR) from the nab-paclitaxel therapy and it had been discontinued
for >6 months, the third-line mono-chemotherapy of
nab-paclitaxel (125 mg/m2; days 1 and 8; every 3 weeks)
was recommended. The patient tolerated this regimen adequately, but
the disease progressed and the size of metastases in the liver grew
bigger rapidly after two cycles of nab-paclitaxel (Fig. 5B).
The fourth-line treatment of irinotecan (240 mg; day
1) and anlotinib (8 mg; days 1–14 every 3 weeks) was well tolerated
and applied to treat the progressive disease (PD) status (Fig. 5B). The patient accepted tomotherapy
palliative radiotherapy (60 Gy over 12 fractions) for the hepatic
metastasis. Repeat imaging assessment after four cycles of
combination treatment showed a significant reduction in the size of
the liver lesions (Fig. 5C). The
tumor size continued to decrease and the disease remained stable
with no clinical evidence of progression. The tumor marker levels
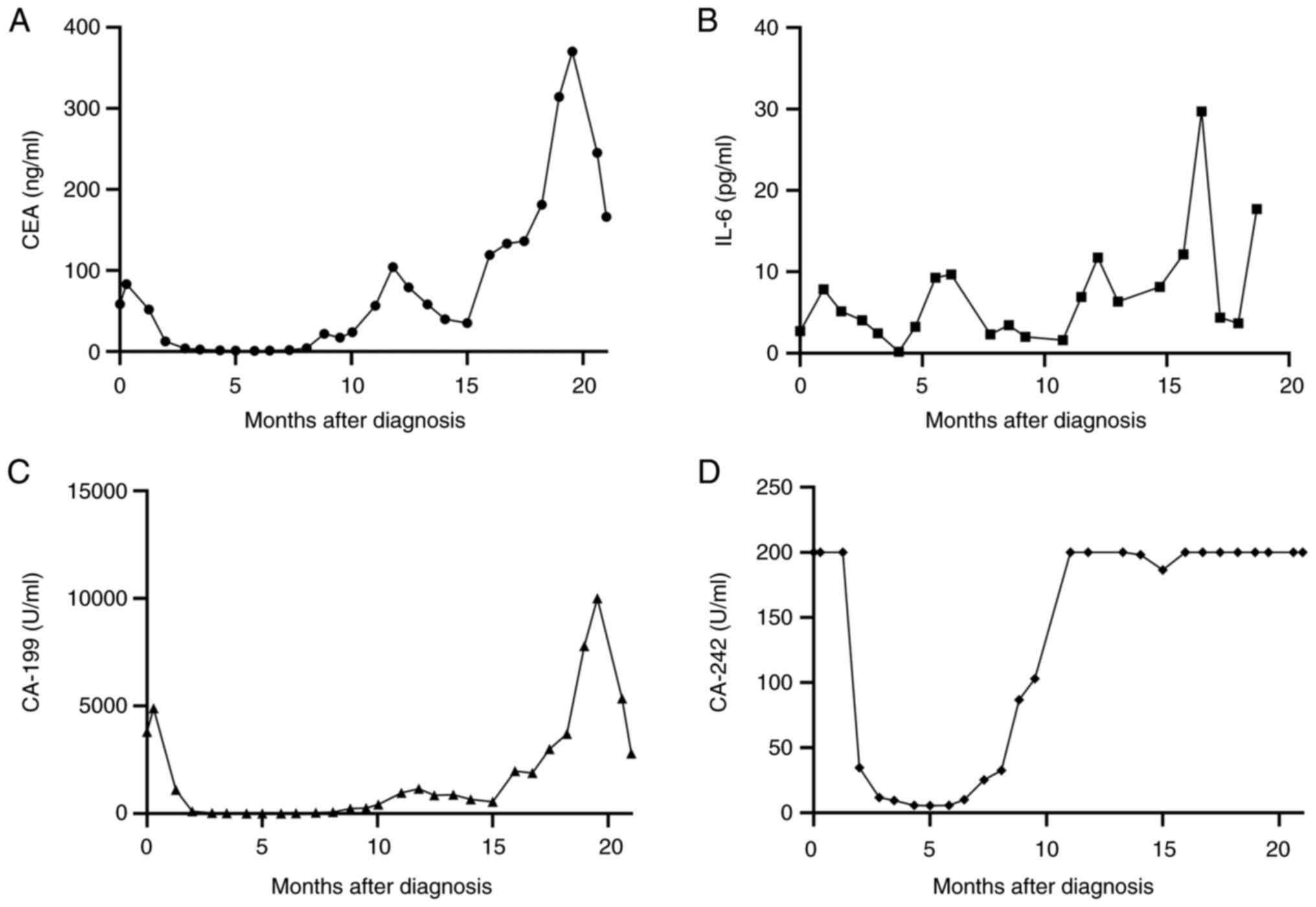
of CA19-9, CA242 and CEA, in addition to those of the inflammatory
marker IL-6, decreased again (Fig.
6A-D) and the overall survival (OS) time was extended to 24
months and counting. The general status of the patient is good in
July 2022 and the patient will be followed up every 45 days.
Discussion
Pancreatic ductal adenocarcinoma (PDAC) is a lethal
disease with a dismal 5-year survival rate of 5–10% and the highest
incidence-to-mortality ratio of any solid tumors (12). This poor outcome is mainly due to
delayed diagnosis and the aggressive nature of the disease, with a
high likelihood of early systemic dissemination (13). Currently, there are two
recommendations for the first-line systemic treatment of metastatic
PC that have demonstrated good performance (i.e., Eastern
Cooperative Oncology Group 0–1) (14). The first recommendation is the
regimen of fluorouracil, leucovorin, irinotecan and oxaliplatin
(FOLFIRINOX) or modified FOLFIRINOX (15), which improved the median OS time
from 6 to 8.7 months compared with gemcitabine monotherapy. The
other recommendation is gemcitabine plus nab-paclitaxel (16), which markedly improved the median
OS time from 6 to 11.1 months compared with gemcitabine
monotherapy. However, the selected patients did not benefit
sufficiently and could not achieve long-term survival outcomes.
Therefore, novel effective systemic treatments remain in urgent
demand.
In recent years, immune checkpoint blocking agents
have been assessed for their efficacy in PDAC (17). Immune checkpoint blockade of the
PD-1 pathway has been proposed to be a potentially viable treatment
strategy due to encouraging phase II trial results for
pembrolizumab (18). Similar
results were reported in the single-arm phase II keynote-158 study,
which included patients with PC. The median PFS time was 4.1 months
and the median OS time was 23.5 months (19). As a result of these promising data,
pembrolizumab was provided with accelerated FDA approval in 2017
for patients with unresectable, metastatic solid tumors harboring
microsatellite instability-high (MSI-H) or deficient mismatch
repair (dMMR) who exhibited progression following initial
treatment, but no longer have any alternative treatment options
(20). In addition, the National
Comprehensive Cancer Network (NCCN) panel recommended this drug as
an option for advanced PDAC with MSI-H or dMMR (21).
Camrelizumab is an anti-PD-1 receptor antibody that
blocks the interaction of the receptor with PD-L1 and PD-L2,
reversing the PD-1-mediated inhibition of the immune response. This
strategy was approved by the National Medical Products
Administration (NMPA) of China in December 2018 for Hodgkin's
lymphoma, locally advanced or metastatic esophageal squamous cell
carcinoma, hepatocellular carcinoma and nasopharyngeal carcinoma
after systemic treatment failure (22). Preclinical and clinical data have
provided supporting rationale for establishing immunotherapy
combined with AG as a first-line treatment for metastatic PDAC
(23,24). Data supporting the current regimens
of an anti-PD-1-based (camrelizumab) combinatory strategy for PC
are summarized in Table I. In
addition, the MDT panel of Renji Hospital speculated that
significant participation in clinical trials is critical for
deepening the understanding into this disease. Therefore,
participation in clinical trials assessing the effects of standard
or accepted therapy would be encouraged for this patient. In the
present case, the original lesions shrank and the liver lesions
almost disappeared after four cycles of treatment. Notably, the
patient also exhibited relatively high levels of tolerance towards
these combined antitumor drugs, especially with only mild PD-1
inhibitor-related immune adverse events.
In the present case, the patient developed new
ovarian lesions, identified by PET-CT scans after 9 months of
treatment. Therefore, it is crucial to distinguish between primary
ovarian diseases and metastatic progression from primary PC for
appropriate treatment. Currently, imaging modalities cannot
distinguish between the two types of lesions, necessitating a
histopathological evaluation. Putative disease recurrence was
determined after careful review of the imaging data by the MDT team
and a confirmatory biopsy following surgery. Subsequently, a
dedicated pathologist conducted a histopathological review of the
ovarian lesions and IHC labeling to deduce that the origin of these
lesions is the PDAC.
Statistically, 10–25% of all ovarian malignancies
are secondary tumors, and the most common primary malignant tumors
causing ovarian metastases include breast, colorectal, endometrial,
gastric and appendiceal cancer (25). At present, three routes of ovarian
metastasis have been identified: Lymphatic, implantation and blood
metastasis. Reticular lymphatic tissues are abundant in ovaries,
and cancer cells can metastasize through the retroperitoneal lymph
nodes to the lumbar lymph nodes before metastasizing to the ovaries
in a retrograde manner, which is considered to be the most likely
mode of metastasis (26,27). Patients with ovarian metastases
tend to be younger compared with those with primary ovarian cancer
(8). Notably, ovarian metastasis
is more frequently observed in non-menopausal women, which may be
attributed to the nutrient-rich and more functionally active
ovaries suitable for metastases. In addition, the secondary
metastasis ovarian cancer tends to be bilateral and often causes
interstitial changes in the ovary, which in turn promotes
hematogenous metastases (28). In
terms of implantation metastases, it has been proposed that when
the tumor invades the serosal membrane, the cancer cells are shed
into the peritoneal cavity and metastasize with the flow of the
peritoneal fluid, spreading to the ovaries to develop metastases
(29). Implantation metastases are
frequently accompanied with extensive peritoneal spread (29). The present patient did not have
ascites or peritoneal metastases. Based on the medical history,
both lymph node and hematogenous metastases may have been involved
in the development of the ovarian metastases.
The accurate diagnosis of secondary ovarian tumors
can be challenging, since they can be easily misdiagnosed as
primary ovarian cancer, especially in cases of mucinous
adenocarcinoma (30). Several
features would indicate metastases, including lesions of a small
size (<10–12 cm), bilateralism, nodular growth patterns and
presence on the surface and/or in the superficial cortex of the
ovary (25). According to the
gross morphology, the present patient fulfilled the aforementioned
features that indicate ovarian metastases. An ovarian tumor with an
irregular glandular tube-like structure and poorly differentiated
cells formed the focal intraglandular cribriform architecture.
Compared with the samples from the fine-needle aspiration biopsy of
the pancreas, there was similar cribriform architecture and cell
morphology. The cells are predominantly columnar with apical
mucinous cytoplasm and show moderate nuclear atypia in the primary
mucinous adenocarcinoma (30).
However, due to diagnostic difficulty, it is necessary to set up a
broad-spectrum IHC investigation to distinguish between secondary
metastatic ovarian tumor and primary ovarian endometrioid or
mucinous adenocarcinomas. PC does not have sensitive markers that
can be used for routine clinical use and immunophenotyping can only
be used to determine an origin from the pancreaticobiliary duct
(31). Positive MUC1 indicates
tumor cells from the pancreaticobiliary duct, whereas positive
CK20, SATB2 and CDX2 would indicate a lower gastrointestinal tract
origin (32–34). In the present report, IHC on the
pathological ovarian sections showed positive MUC1, CK20, SATB2 and
CDX2 staining. By contrast, primary ovarian endometrioid
adenocarcinomas are typically diffusely positive for CK7 and CA125,
but negative for CK20 and CDX2 (26). In the present report, CK7
expression was partially positive on the cell membrane, CA125 was
negative whereas CK20 and CDX2 were positive. Other IHC markers,
such as ER, PR and PAX8, are positive in primary tumors of the
female genital system, while a negative result of these three
markers was shown in the present case, indicating a metastatic
ovarian tumor.
According to the gross morphology, histology and IHC
results, the present study reported a rare case of ovarian
metastasis from PC. However, a definitive diagnosis of metastatic
PC in the ovary could not be established until the pathology of the
ovarian tumor was confirmed as that from the PDAC. The present case
highlights the importance of considering metastases when distinct
masses are detected in a range of organs in order to administer
different treatments.
The present patient was treated with
immunotherapy-targeted therapy and oral chemotherapy (200 mg
sintilimab on day 1; 50 mg tegafur on days 1–14, twice daily; and 8
mg anlotinib on days 1–14, every 3 weeks). The PFS time from this
second-line treatment was 6 months, with severe life-threatening
treatment-related toxicity. The initial benefit of the first-line
immunotherapy was significant for this patient, with a stable
condition recorded after oophorectomy of the ovarian metastases.
The complication of autoimmune enteritis happened in the
second-line treatment and was finally controlled. However, the
disease progressed after the third-line chemotherapy and was
therefore switched to fourth-line targeted therapy and palliative
radiotherapy. Currently, the patient is stable with an improved
quality of life. The trends in inflammatory factor (IL-6) levels
and tumor marker levels (CEA, CA-199 and CA-242) were consistent
with the changes in the condition of the patient.
Due to the rarity, heterogeneity and lack of
evidence, guidelines for the optimal management of patients with
PDAC and ovarian metastasis remain elusive. The findings of the
present case suggested that high-quality imaging is essential for
detecting subclinical disease. This also emphasizes the
significance of a multidisciplinary approach involving gynecology,
oncology, radiology, pathology and surgical oncology for an optimal
work-up and disease management. Although the combination of
resection and immunotherapy-based systemic treatment resulted in
survival benefits for the present patient, there are few such cases
in the literature. Therefore, there is a need for further treatment
options for PC patients with ovarian metastasis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81172522 and 81301858), the Suzhou
Science and Technology Project (grant nos. SYS201508 and SYS201308)
and the Jiangsu Natural Science Foundation Project (grant no.
BK20181186).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, LT and LZ wrote the manuscript, formatted the
figures and table, and analyzed the patient data. YM performed the
surgery, obtained the medical images and advised on the patient
treatment. YX and FZ collected clinical information, assisted with
drafting the manuscript, and they both contributed to the
conceptualization, overall design and quality control of the study.
FZ and YX confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Suzhou Kowloon Hospital (Suzhou, China; approval no.
BL-2022-001).
Patient consent for publication
Written informed consent for the publication of
clinical details and images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Xu R, Wang C, Qiu J, Ren B and You
L: Early screening and diagnosis strategies of pancreatic cancer: A
comprehensive review. Cancer Commun (Lond). 41:1257–1274. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Habib JR, Pasha S, Khan S, Kinny-Köster B,
Shoucair S, Thompson ED, Yu J, Lafaro K, Burkhart RA, Burns WR, et
al: Ovarian metastasis from pancreatic ductal adenocarcinoma. World
J Surg. 45:3157–3164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin T, Dai C and Xu F: Surgical and local
treatment of hepatic metastasis in pancreatic ductal
adenocarcinoma: Recent advances and future prospects. Ther Adv Med
Oncol. Jun 23–2020.(Epub ahead of print). View Article : Google Scholar
|
|
7
|
Shah A, Korrapati P, Siegel J and Kasmin
F: Rare metastasis of primary pancreatic adenocarcinoma to the
bladder. ACG Case Rep J. 5:e272018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Waal YR, Thomas CM, Oei AL, Sweep FC
and Massuger LF: Secondary ovarian malignancies: Frequency, origin,
and characteristics. Int J Gynecol Cancer. 19:1160–1165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chun YS, Pawlik TM and Vauthey JN: 8th
edition of the AJCC cancer staging manual: Pancreas and
hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-standardisation and disease-specific adaptations:
Perspectives from the RECIST working group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
U.S. Department of Health and Human
Services; National Institutes of Health and the National Cancer
Institute, : Commn Terminology Criteria for Adverse Events (CTCAE).
V4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdfMay
17–2010
|
|
12
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gourgou-Bourgade S, Bascoul-Mollevi C,
Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A,
Raoul JL, Boige V, et al: Impact of FOLFIRINOX compared with
gemcitabine on quality of life in patients with metastatic
pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized
trial. J Clin Oncol. 31:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christenson ES, Jaffee E and Azad NS:
Current and emerging therapies for patients with advanced
pancreatic ductal adenocarcinoma: A bright future. Lancet Oncol.
21:e135–e145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patnaik A, Kang SP, Rasco D, Papadopoulos
KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L,
Espino G, et al: Phase I study of pembrolizumab (MK-3475; Anti-PD-1
monoclonal antibody) in patients with advanced solid tumors. Clin
Cancer Res. 21:4286–4293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:31–10. 2020. View Article : Google Scholar
|
|
20
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tempero MA: NCCN guidelines updates:
Pancreatic cancer. J Natl Compr Canc Netw. 17:603–605.
2019.PubMed/NCBI
|
|
22
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Padrón LJ, Maurer DM, O'Hara MH, O'Reilly
EM, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, et
al: Sotigalimab and/or nivolumab with chemotherapy in first-line
metastatic pancreatic cancer: Clinical and immunologic analyses
from the randomized phase 2 PRINCE trial. Nat Med. 28:1167–1177.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tempero M, Oh DY, Tabernero J, Reni M, Van
Cutsem E, Hendifar A, Waldschmidt DT, Starling N, Bachet JB, Chang
HM, et al: Ibrutinib in combination with nab-paclitaxel and
gemcitabine for first-line treatment of patients with metastatic
pancreatic adenocarcinoma: Phase III RESOLVE study. Ann Oncol.
32:600–608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubeček O, Laco J, Špaček J, Petera J,
Kopecký J, Kubečková A and Filip S: The pathogenesis, diagnosis,
and management of metastatic tumors to the ovary: A comprehensive
review. Clin Exp Metastasis. 34:295–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Agha OM and Nicastri AD: An in-depth
look at krukenberg tumor: An overview. Arch Pathol Lab Med.
130:1725–1730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamanishi Y, Koshiyama M, Ohnaka M, Ueda
M, Ukita S, Hishikawa K, Nagura M, Kim T, Hirose M, Ozasa H and
Shirase T: Pathways of metastases from primary organs to the
ovaries. Obstet Gynecol Int. 2011:6128172011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prat J: Ovarian carcinomas, including
secondary tumors: Diagnostically challenging areas. Mod Pathol. 18
(Suppl 2):S99–S111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sodek KL, Murphy KJ, Brown TJ and
Ringuette MJ: Cell-cell and cell-matrix dynamics in intraperitoneal
cancer metastasis. Cancer Metastasis Rev. 31:397–414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taylor EC, Irshaid L and Mathur M:
Multimodality imaging approach to ovarian neoplasms with pathologic
correlation. Radiographics. 41:289–315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ansari D, Rosendahl A, Elebro J and
Andersson R: Systematic review of immunohistochemical biomarkers to
identify prognostic subgroups of patients with pancreatic cancer.
Br J Surg. 98:1041–1055. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nath S, Daneshvar K, Roy LD, Grover P,
Kidiyoor A, Mosley L, Sahraei M and Mukherjee P: MUC1 induces drug
resistance in pancreatic cancer cells via upregulation of multidrug
resistance genes. Oncogenesis. 2:e512013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JH and Kim JH: Pathologic
differential diagnosis of metastatic carcinoma in the liver. Clin
Mol Hepatol. 25:12–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dabir PD, Svanholm H and Christiansen JJ:
SATB2 is a supplementary immunohistochemical marker to CDX2 in the
diagnosis of colorectal carcinoma metastasis in an unknown primary.
APMIS. 126:494–500. 2018. View Article : Google Scholar : PubMed/NCBI
|