Introduction
Pulmonary embolism includes pulmonary
thromboembolism (PTE), fat embolism syndrome, amniotic fluid
embolism, air embolism and tumor embolism (1), among which PTE is the most common
type. Pulmonary arteritis, fibrinous mediastinitis and pulmonary
artery sarcoma are easily misdiagnosed as pulmonary embolism. Up to
2021, ~400 cases of pulmonary artery intimal sarcoma (PAIS) have
been reported in the literature (2–4),
most of which were published as case reports. Despite these
publications, the pathogenesis of the disease remains elusive. The
average age at onset of PAIS is 50 years and the age range of onset
is estimated to be 13–86 years (5–7).
There are no significant differences between male and female
subjects in terms of the prevalence and outcomes of this condition
based on previous evaluations (8).
The clinical symptoms, laboratory findings and imaging features of
PAIS are non-specific, which makes diagnosis difficult. To date,
there is no standard treatment for PAIS. The present study
retrospectively analyzed the symptoms, clinical images,
pathological features (based on morphological analysis,
immunohistochemical and genetic testing), diagnosis, differential
diagnosis and treatment of one patient who was initially diagnosed
with pulmonary embolism at an external hospital and was
subsequently referred to our hospital. The analysis was combined
with a literature review to enhance awareness and prevent early
misdiagnosis and missed diagnosis of pulmonary embolism.
Case report
Clinical course in external
hospital
A 57-year-old male patient had complaints of dyspnea
and wheezing following climbing 3 floors persisting for 6 months.
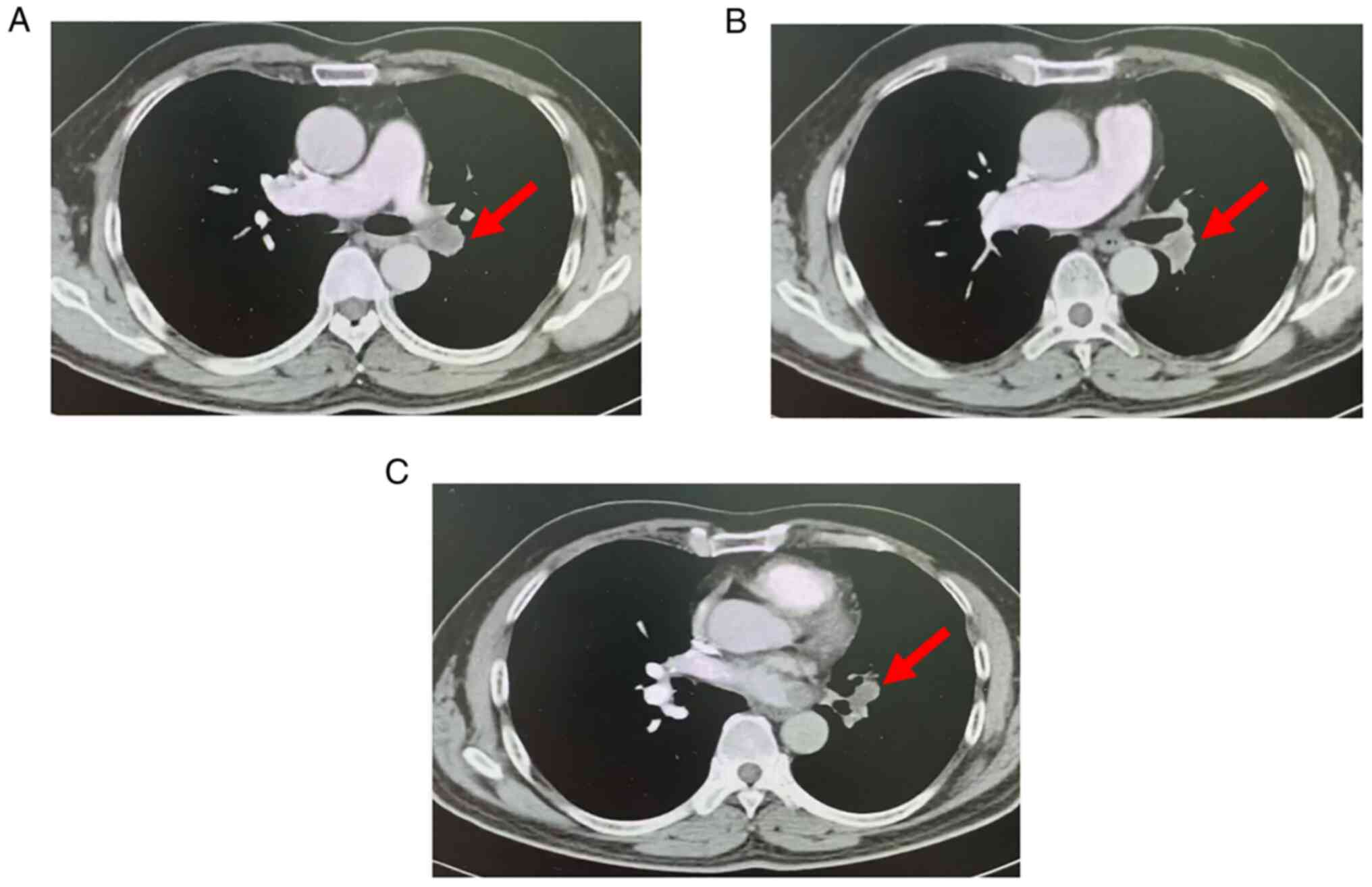
Pulmonary artery computed tomography angiography (CTA) examination
(Fig. 1) performed at an external
hospital indicated that the left lower pulmonary artery and its
branches had multiple pulmonary embolisms. The laboratory
examinations included D-dimer (0.46 mg/l) and the tumor antigen
markers carbohydrate antigen 19–9, carcinoembryonic antigen,
α-fetoprotein and prostate-specific antigen (normal concentration
values). The symptoms worsened following 3 months of irregular
anticoagulation.
Clinical course at the hospital
The patient then consulted our department at the
First Affiliated Hospital of Fujian Medical University (Fuzhou,
China) in May 2021 and received standard anticoagulant therapy for
an additional 3 months. The patient's symptoms improved slightly.
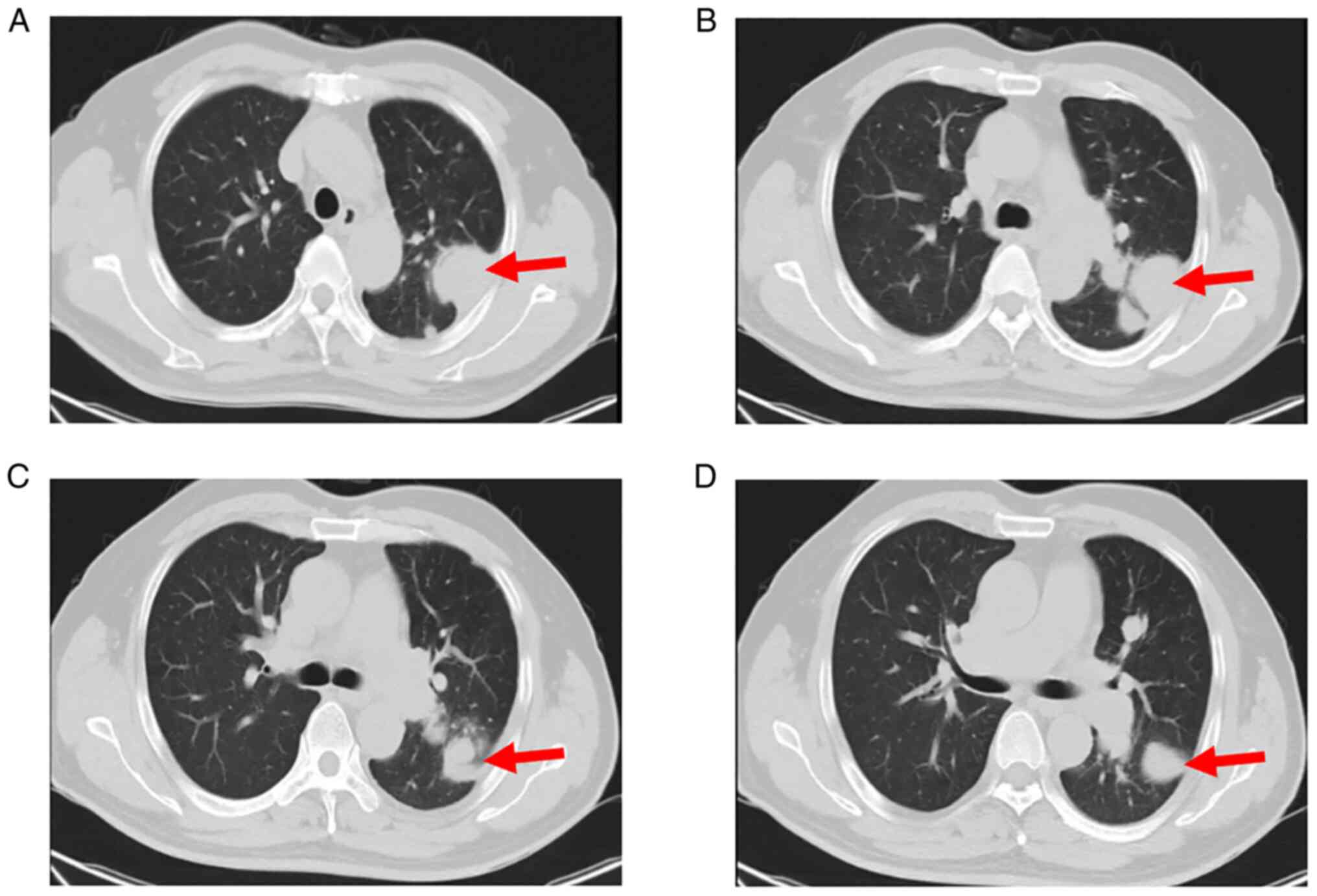
However, chest CT re-examination (Fig.
2) indicated multiple pulmonary nodules and consequently, the
patient was hospitalized. Examination of the patient's family
history indicated a lack of evidence supporting any special history
or family history. The physical examination indicated pulmonary
valve second heart sound > aortic valve second heart sound and
no swelling on both of the lower limbs.
Examinations
During the hospitalization period, right heart
catheterization, which is a possibly helpful test for cases with
similar presentation, e.g. to rule out the possibility of chronic
thromboembolic pulmonary hypertension, was planned. However, the
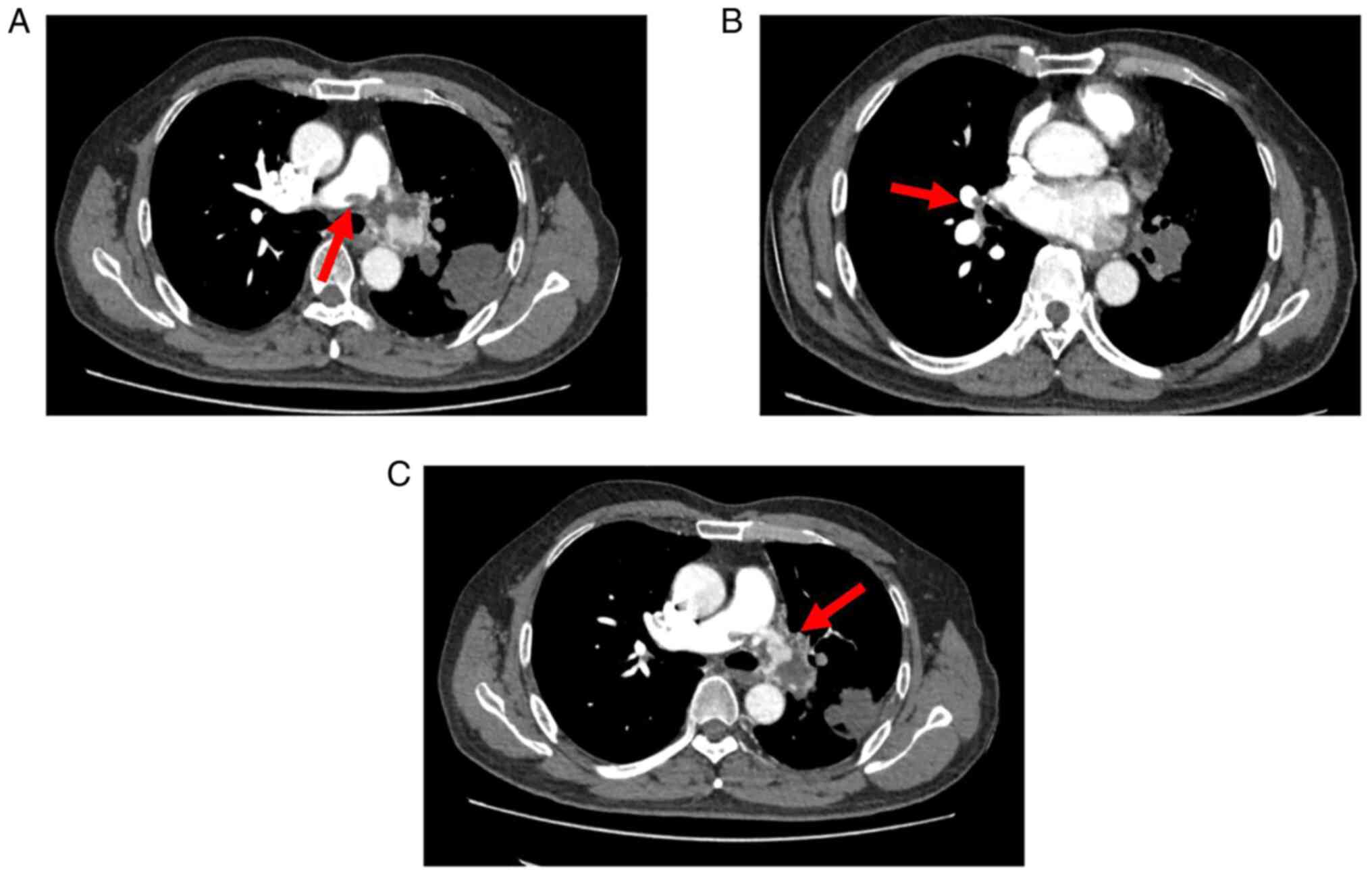
patient did not consent to undergo this examination. Re-examination
of the pulmonary artery CTA (Fig.
3) revealed that the left pulmonary artery was completely
embolized, whereas the right main and middle pulmonary arteries
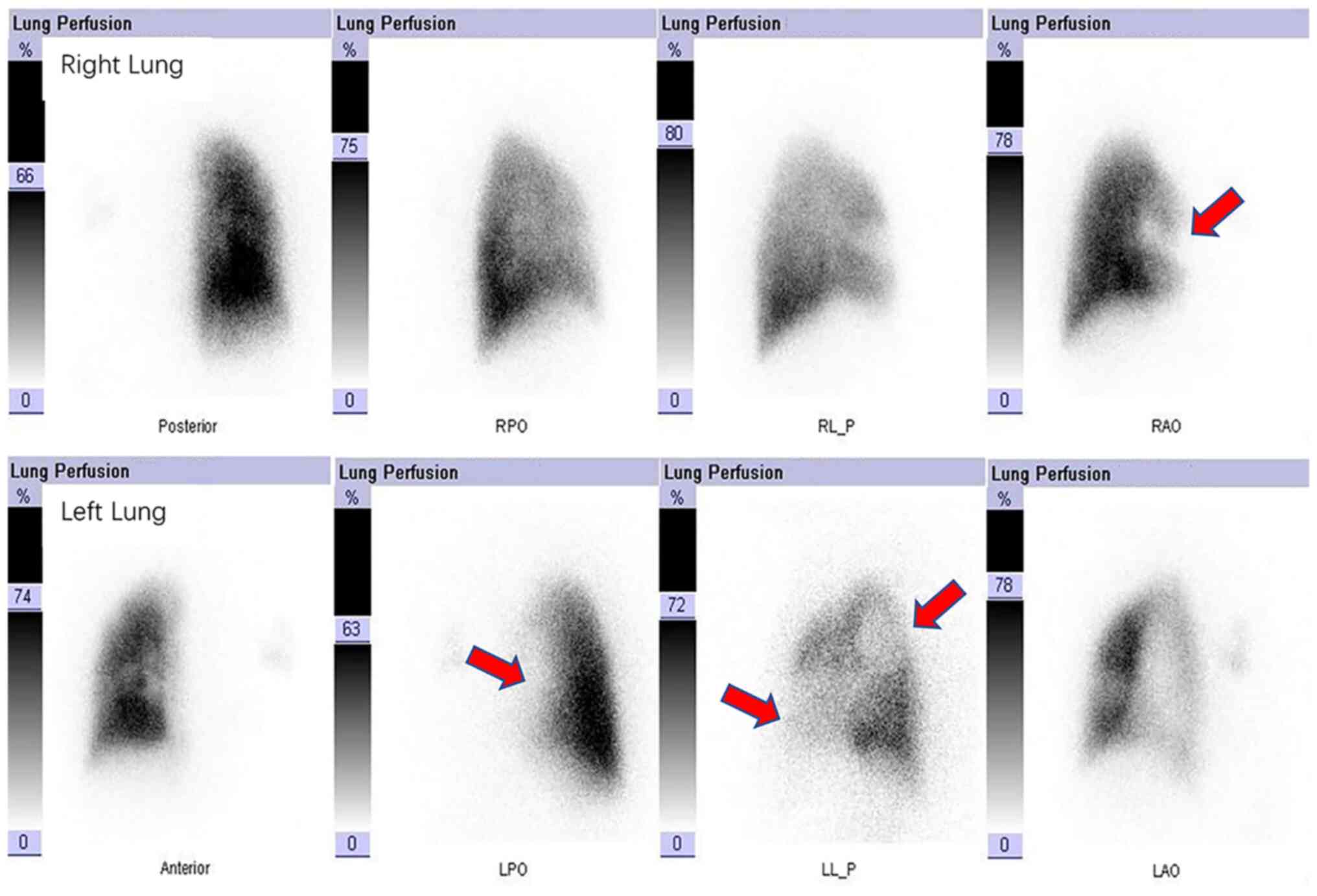
were partially embolized. Lung perfusion imaging (Fig. 4) indicated that blood perfusion was
reduced in the majority of the left lung and in the anterior
segment of the right upper lobe, which was consistent with
pulmonary embolism. The patient underwent CT-guided percutaneous
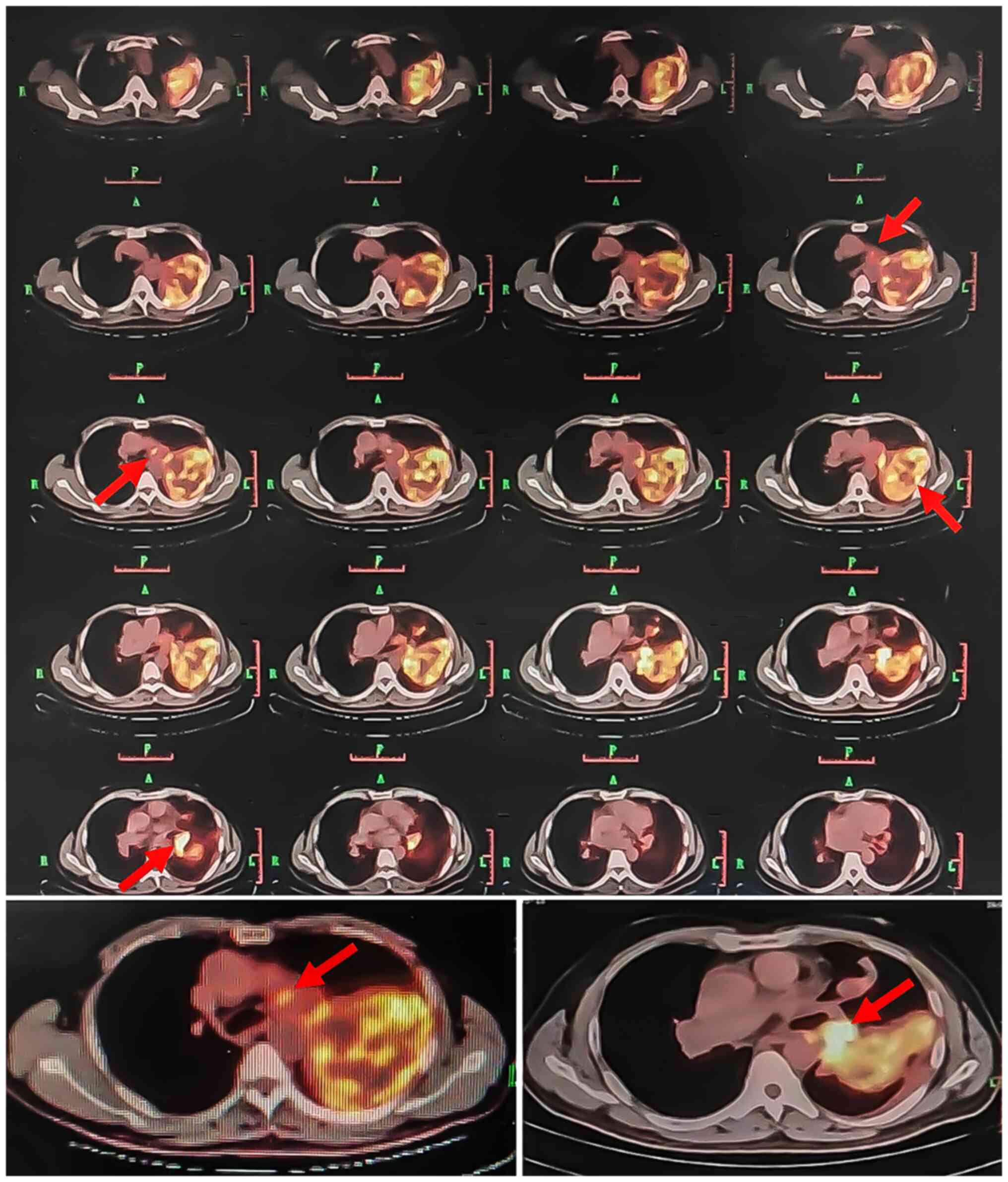
puncture biopsy and positron emission tomography (PET)/CT (Fig. 5), which revealed the following:
Soft tissue mass in the area of the left pulmonary artery and
slightly increased metabolism; space-occupying upper lobe of the
left lung, slightly increased metabolism; nodules in both lungs;
left hilum lymph nodes, increased metabolism; thickened left pleura
with a small amount of effusion in the left pleural cavity.
Pathological test
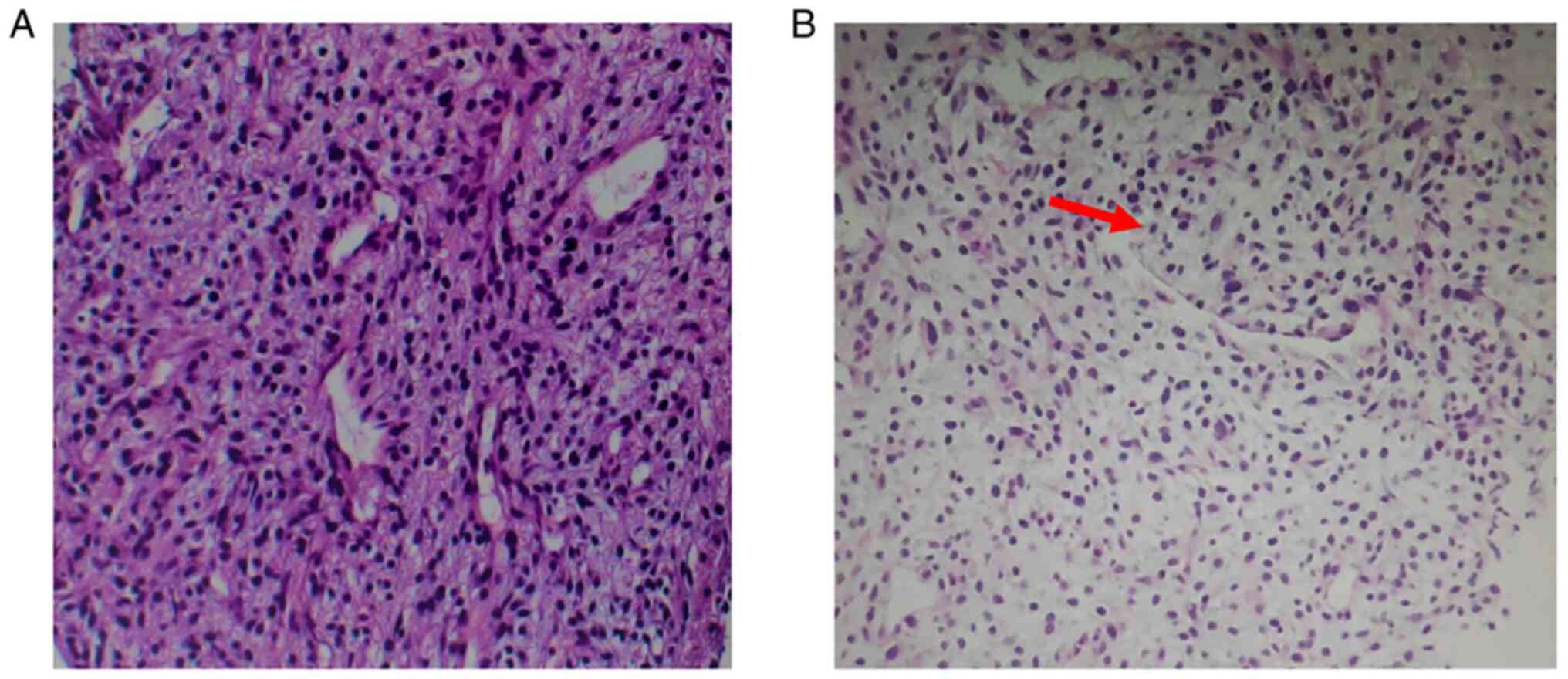
Pulmonary nodule puncture pathological evaluation
(Fig. 6) suggested the presence of
mesenchymal tumors. Special staining indicated the presence of mesh
fibers surrounding the tumor cells. Immunohistochemical evaluation
demonstrated the following staining patterns: Thyroid transcription
factor-1 (alveolar epithelium +), smooth muscle actin (vascular +),
CD31, CD34 (vascular +), vimentin (+) and Ki-67 (85%+). Molecular
detection analysis indicated that the Ewing sarcoma RNA binding
protein 1 (EWSR1) gene isolation test was negative, whereas the
mouse double minute 2 homolog (MDM2) gene amplification test was
positive (further details of the primary and secondary antibodies
used for immunohistochemistry and molecular test information are
provided in Data S1). Based on
the medical history, clinical imaging and immunohistochemical and
molecular test analyses, PAIS was diagnosed.
Treatment results, follow-up and
outcome
The patient had been hospitalized for about 13 days
before being discharged., the patient was transferred to a local
tumor hospital for chemotherapy treatment and received the first
cycle of chemotherapy with the ‘epirubicin 70 mg igvtt’ regimen in
October 2021. The patient responded to the treatment and the
symptoms were slightly improved. Furthermore, the patient was also
prescribed ‘anlotinib one tablet qd’ orally. However, the
chemotherapy was ineffective and the patient succumbed to the
disease in November 2021.
Discussion
The incidence rate of PAIS, which is a rare
malignant mesenchymal tumor, is estimated to be 0.001-0.03%
(2). Its classic characteristic is
the growth and obstruction of the vascular cavity, resulting in
distant organ embolization or implantation of tumor emboli. PAIS
mainly involves the proximal end of the blood vessel, which is
frequently located in the main pulmonary artery (80%) and the left
or right pulmonary artery (50–70%). In addition, ~20% of cases have
demonstrated additional thoracic malignant metastases, involving
the lungs, kidneys, brain, lymph nodes and skin (9). Features of PAIS, such as age of
onset, predisposing area, symptoms and imaging manifestations, as
well as its treatment, are summarized in Table I.
 | Table I.Summary of the characteristics of
PAIS. |
Table I.
Summary of the characteristics of
PAIS.
| Item | Details |
|---|
| Age of onset | The average age of
onset of PAIS is 50 years and the age range of onset is 13–86 years
(5–7). |
| Predisposing
area | It is frequently
located in the main pulmonary artery (80%) or left or right
pulmonary artery (50–70%) (9). |
| Transferring
possibility | Approximately 20% of
cases demonstrate additional thoracic malignant metastases,
involving lungs, kidneys, brain, lymph nodes and skin (9). |
| Symptoms and
signs | The classic symptoms
include dyspnea, cough, chest tightness, hemoptysis, fever, fatigue
and weight loss (10). |
| Microscopy image | The tumor cells
mainly include spindle cells, whereas others are epithelial, and
giant and multinucleated cells present in focal points (2). |
| Radiological
characteristics | It displays the
filling defect in the pulmonary artery lumen with uneven
enhancement, extraluminal tumor invasion and a nodular appearance
(4,7,14). |
| FDG PET/CT | The SUVmax of PAIS
may be higher than that of CPTE. |
| Treatment | Surgical resection is
generally considered to be the best treatment option. Whether
adjuvant chemotherapy and radiotherapy improve the treatment
response remains controversial (10,20). |
It has been indicated that the manifestation of
chronic right heart failure is frequently derived from
PAIS-associated long-term occupation of the lumen in the pulmonary
artery. The most common symptoms are dyspnea, cough, chest
tightness and hemoptysis (10).
The features associated with malignant tumors, such as fever,
fatigue and weight loss, may also be present. In the present case,
the patient had difficulty breathing, which was accompanied by
wheezing following exercise. Pulmonary embolism initially consisted
of anticoagulant therapy, which was administered for a total period
of 6 months. Despite this treatment, the symptoms did not improve
significantly. Subsequently, the patient was hospitalized following
the analysis of the biopsy and CT results, which suggested the
presence of multiple nodules in the left hilum and lung. The final
diagnosis was PAIS.
According to previous reports, PAIS has the
histological features of a poorly differentiated or
undifferentiated mesenchymal tissue-derived malignant tumor. The
majority of the tumor cells are spindle cells, whereas certain
epithelial and certain giant and multinucleated tumor cells are
also visible in the focal points (2). The pathological analysis of the
present case revealed that the tumor cells were arranged in an
epithelioid or spindle shape with concomitant nuclear atypia and
pathological mitosis. It has been reported in the literature that
PAIS is frequently detected based on MDM2 (65%), cyclin-dependent
kinase 4, platelet-derived growth factor receptor α (81%) and EGFR
(76%) gene amplifications (11).
In the present case report, a lack of ectopic expression of the
EWSR1 gene was noted by fluorescence in situ hybridization
(FISH; results not shown). The patient was positive for MDM2 gene
amplification, which was similar to the results of Wang et
al (2).
In addition, comprehensive genomic profiling (CGP)
is the sequencing of DNA and RNA from tumor samples, which enables
the identification of known and novel alterations that may drive
oncogenicity. Therefore, it is not surprising that CGP is
increasingly being used in the evaluation and management of
sarcomas (12). For instance, Wu
et al (13) reported a case
of disseminated primary pulmonary artery sarcoma achieving clinical
tumor response to olaparib based on genetic alterations detected by
CGP, which involved the DNA repair pathway. This provides
supportive evidence that olaparib may be a promising therapeutic
agent for patients with disseminated primary pulmonary artery
sarcoma harboring haploinsufficiency of the DNA damage repair
mechanism (13). Although CGP may
refine the histological tumor diagnosis with its inherent
ramifications for management, little is known regarding its
application in PAIS. Therefore, due to the limitation in the
equipment used, the FISH method was selected as an alternative to
CGP for the early detection of PAIS.
The radiological characteristics of PAIS comprise a
filling defect in the pulmonary artery lumen, extraluminal tumor
invasion and a nodular appearance. Despite its specific
characteristics, previously published studies have indicated that
≥50% of patients with PAIS are initially misdiagnosed as cases of
chronic pulmonary thromboembolism (CPTE) (4,7,14).
According to previous reports, lesion morphology (determined by CT)
and location may help distinguish between PAIS and CPTE. The CT
features of PAIS are expansive growth and a bulging appearance
against the direction of blood flow. The proximal end of the tumor
exhibits a lobulated structure and the distal end of the tumor has
a grape-shaped appearance with uneven enhancement (4,14).
The tumor may extend to the bifurcation of the pulmonary artery,
pulmonary valve and right ventricular outflow tract. In contrast to
these findings, the proximal end of CPTE is a straight cup-shaped
structure, which is caused by the blood flow to the surface of the
blood clot. In addition, pulmonary thromboembolism rarely occurs in
the pulmonary valve or pulmonary trunk due to the increased blood
flow in these areas (15). In the
present case report, the early enhancement CT did not demonstrate
any significant enhancement. The pulmonary artery CTA exhibited a
filling defect of the pulmonary artery trunk and the tumor was
confined to the lumen; therefore, it was misdiagnosed as pulmonary
embolism.
According to previous studies,
18F-fluorodeoxyglucose (FDG) PET/CT may help distinguish
PAIS from CPTE based on the maximum standardized uptake value
(SUVmax). The SUVmax of PAIS is significantly higher than that of
CPTE. When the cutoff value was 3.3, the reported sensitivity,
specificity and accuracy were 98.4, 96.8 and 97.8%, respectively
(16). However, certain
false-negative cases have also been reported. In the reports of
Suto et al (17) and
Takauchi et al (18), the
uptake of FDG in PET/CT used for the diagnosis of patients with
PAIS was poor. The histopathology of these cases indicated highly
malignant cells with low cellularity and a significant type of
interstitial myxoid tissue. In the current case report, the left
pulmonary artery exhibited mild FDG uptake (SUVmax 4.2) and
increased FDG uptake of the left hilar multiple lymph node (SUVmax
8.1), which supported the diagnosis of PAIS.
The clinical and imaging manifestations of PAIS are
frequently similar to those of pulmonary embolism. In addition, the
tumor may easily cause thrombosis, which may be misdiagnosed. This
is the reason why PAIS requires to be distinguished from CPTE. The
common symptom of CPTE is difficulty in breathing following
exercise, which is progressively worsening. In the present case
report, the patient had undergone regular anticoagulation therapy
for ≥3 months; however, the symptoms did not improve significantly.
Furthermore, early laboratory examinations displayed normal D-dimer
levels, which indicated a reduced possibility of the patient having
CPTE. However, imaging examinations (CT pulmonary angiography and
radionuclide pulmonary perfusion imaging) indicated pulmonary
embolism, since CPTE could not be ruled out. Subsequently, the
patient's pulmonary embolism was progressively worsening and the
lumen of the filling defect was not obviously swelling but
demonstrated a ‘worm-eaten’ appearance and infiltrating changes.
Finally, the condition was diagnosed as PAIS based on puncture
pathology and immunohistochemistry, which was further supported by
a high intraluminal uptake rate indicated in PET-CT. The
differential diagnosis of PAIS from pulmonary fungal infection is
not always easy, since the imaging manifestations of the mass-like
or nodular lung fungal infection have various similarities to those
of malignant tumors. However, fungal infections are frequently
secondary to various immune dysfunctions; therefore, the ‘air
crescent sign’ is a typical manifestation of pulmonary mycosis and
may be adopted as the main diagnostic criterion for pulmonary
fungal infection (19).
To date, there is no standard treatment for PAIS.
Surgical resection is generally considered to be the best treatment
option for PAIS at present, including pulmonary endarterectomy,
lobectomy or pneumonectomy. The appropriate surgical approach must
be evaluated according to tumor performance, the existence of
pulmonary hypertension and the patient's clinical condition
(20). The potential of treatment
improvement by adjuvant chemotherapy and radiotherapy remains
controversial. Currently, no consensus exists on the effect of
surgery combined with radiotherapy and chemotherapy on the overall
survival rate of patients (10).
Chemotherapy may be an option for patients with unresectable or
recurring focal sarcoma (21). The
most common adjuvant therapy includes doxorubicin and ifosfamide
chemotherapy either alone or in combination with radiotherapy
(22). In the current case report,
the patient received chemotherapy with the drug ‘epirubicin’.
The prognosis of patients with PAIS is poor and the
survival time of untreated cases is ~1.5-3 months (23). A previous study reported that the
median survival after complete surgical resection may be extended
to 36.5±20.2 months, whereas that of incomplete surgical resection
may be extended to 11±3 months (5).
The present case report demonstrated that in the
case of anticoagulant thrombolytic therapy for pulmonary embolism
being ineffective (notably in middle-aged patients) the possibility
of PAIS should be taken into consideration. It is also suggested
that early diagnosis by means of the multi-faceted observation of
medical history, clinical signs, imaging features and
histopathological examinations may improve the prognosis of the
disease.
Supplementary Material
Supporting Data
Acknowledgements
The present manuscript was edited and the language
was polished by Dr Binmin Chen, an English major teacher at the
School of Arts and Sciences of Fujian Medical University (Fuzhou,
China).
Funding
The present manuscript was supported by Projects of Financial
Foundation on Health in Fujian Province (grant no. BPB-DCS2021) and
the Science and Technology Department of Fujian Province (grant no.
2019Y9126).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NS was the major contributor in writing the
manuscript. NS and CD contributed to the conception and revisions
of the manuscript. NS and CD confirmed the authenticity of all the
raw data. Both authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Fujian Medical
University (Fuzhou, China).
Patient consent for publication
Written informed consent was obtained from the
patient's family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pulmonary Embolism and Pulmonary Vascular
Disease Group of Respiratory Medicine Branch of Chinese Medical
Association, Pulmonary Embolism and Pulmonary Vascular Disease
Working Committee of Respiratory Physician Branch of Chinese
Medical Association, National Cooperative Group for Prevention and
Treatment of Pulmonary Embolism and Pulmonary Vascular Disease, .
Guidelines for Diagnosis, Treatment and Prevention of Pulmonary
Thromboembolism. Natl Med J China. 98:1060–1087. 2018.
|
|
2
|
Wang B, Zhang T, Liu HY, Chen RR, Zhang
XY, Zhang HL, Zhai ZG and Zhong DR: Clinicopathological
characteristics of pulmonary artery intimal sarcoma. Zhonghua Bing
Li Xue Za Zhi. 50:38–43. 2021.(In Chinese). PubMed/NCBI
|
|
3
|
Xu R, Zhao Y, Xu X, Liu S, Hu C, Lv D and
Wu H: Pulmonary intimal sarcoma involving the pulmonary valve and
right ventricular outflow tract: A case report and literature
review. Medicine (Baltimore). 99:e188132020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moguillansky NI, Verma N, Shah P, Knapik J
and Mohammed TL: Pulmonary artery sarcoma: Case report and review
of the literature. Respir Med Case Rep. 27:1008572020.PubMed/NCBI
|
|
5
|
Blackmon SH, Rice DC, Correa AM, Mehran R,
Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, et al:
Management of primary pulmonary artery sarcomas. Ann Thorac Surg.
87:977–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng L, Zhu J, Xu J, Guo S, Liu S and Song
Y: Clinical presentation and surgical treatment of primary
pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg.
26:243–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee Y, Kim HJ, Yoon H, Choi CM, Oh YM, Lee
SD, Lim CM, Kim WS, Koh Y and Lee JS: Clinical characteristics and
treatment outcomes of primary pulmonary artery sarcoma in korea. J
Korean Med Sci. 31:1755–1760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hieu NL, Tu VN, Hoan L, Hai HB, Luu DT,
Cuong NN, My TT, Minh TN and Manh PT: A case report of primary
pulmonary artery intimal sarcoma. Radiol Case Rep. 17:1986–1990.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu P and Yin BB: Misdiagnosis of primary
intimal sarcoma of the pulmonary artery as chronic pulmonary
embolism: A case report. World J Clin Cases. 8:986–994. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YC, Li LY, Tong HC, Xu HT, Ma S, Yang
LH, Zhang WL, Sotolongo G and Wang E: Pulmonary artery intimal
sarcoma mimicking pulmonary thromboembolism: A case report.
Medicine (Baltimore). 100:e246992021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Dievel J, Sciot R, Delcroix M,
Vandeweyer RO, Debiec-Rychter M, Dewaele B, Cornillie J, Van Cann
T, Meyns B and Schöffski P: Single-center experience with intimal
sarcoma, an ultra-orphan, commonly fatal mesenchymal malignancy.
Oncol Res Treat. 40:353–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hay MA, Severson EA, Miller VA, Liebner
DA, Vergilio JA, Millis SZ and Chen JL: Identifying opportunities
and challenges for patients with sarcoma as a result of
comprehensive genomic profiling of sarcoma specimens. JCO Precis
Oncol. Mar 18–2020.(Epub ahead of print). doi: 10.1200/PO.19.00227.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CE, Ng CT and Tan KT: Transient
response of olaparib on pulmonary artery sarcoma harboring multiple
homologous recombinant repair gene alterations. J Pers Med.
11:3572021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pu X, Song M, Huang X, Zhu G, Chen D, Gan
H and Huang L: Clinical and radiological features of pulmonary
artery sarcoma: A report of nine cases. Clin Respir J.
12:1820–1829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim C, Kim MY, Kang JW, Song JS, Lee KY
and Kim SS: Pulmonary artery intimal sarcoma versus pulmonary
artery thromboembolism: CT and clinical findings. Korean J Radiol.
19:792–802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xi XY, Gao W, Gong JN, Guo XJ, Wu JY, Yang
YH and Yang MF: Value of 18F-FDG PET/CT in
differentiating malignancy of pulmonary artery from pulmonary
thromboembolism: A cohort study and literature review. Int J
Cardiovasc Imaging. 35:1395–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suto H, Suto M, Inui Y and Okamura A:
Difficulty in distinguishing pulmonary arterial intimal sarcoma
from pulmonary thromboembolism using FDG PET/CT. In Vivo.
36:1519–1522. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takauchi T, Murai R, Musiake K, Akaike Y,
Hirayama M, Ueda A, Komiya T and Kadota K: Pedunculated pulmonary
artery intimal sarcoma with poor uptake in 18F-FDG PET/CT: A case
report. J Cardiol Cases. 24:110–113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchiori E, Hochhegger B and Zanetti G:
The air crescent sign. J Bras Pneumol. 48:e202200352022.(In
English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Secondino S, Grazioli V, Valentino F, Pin
M, Pagani A, Sciortino A, Klersy C, Callegari MG, Morbini P, Dore
R, et al: Multimodal Approach of Pulmonary Artery Intimal Sarcoma:
A single-institution experience. Sarcoma. 2017:79414322017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goerlich CE, Zehr K, Aziz H and Kilic A:
Repeat resection for recurrence of pulmonary artery intimal
sarcoma. J Card Surg. 36:3889–3891. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allen A, Smith SC, Pillappa R, Boikos S,
Medalion B, Grizzard J, Cassano A and Harris T: Intimal sarcoma of
the pulmonary artery treated with neoadjuvant radiation prior to
pulmonary artery resection and reconstruction. Respir Med Case Rep.
33:1014142021.PubMed/NCBI
|
|
23
|
Li X, Hong L and Huo XY: Undifferentiated
intimal sarcoma of the pulmonary artery: A case report. World J
Clin Cases. 9:3960–3965. 2021. View Article : Google Scholar : PubMed/NCBI
|