Introduction
Primary central nervous system lymphoma (PCNSL) are
highly aggressive extranodal non-Hodgkin's lymphoma affecting the
brain, eyes, leptomeninges or spinal cord (1–3).
PCNSL account for ~3% of diagnosed brain tumors (1). High incidence rates are exhibited in
immunocompromised patients, particularly among those infected with
human immunodeficiency virus (4,5).
PCNSL often exhibits a high chemosensitivity and radiosensitivity;
however, only ~50% of patients demonstrate long-term control
(1,2). Despite recent advances in treatment
options, 5-year survival rates remain low and treatment-associated
neurotoxicity is common (2,6).
Notably, prognosis, age and performance status have been identified
as treatment-independent factors, and have been introduced into
applied clinical scoring systems (7,8).
Low skeletal muscle mass (LSMM) as a proxy parameter
for sarcopenia acts as a non-invasive imaging tool for the
prediction of prognosis in numerous cancers, including gastric,
pancreatic and colorectal cancer (9–12).
By contrast with other tools used to measure sarcopenia, LSMM is
assessed using routine imaging. Frequently applied methods include
detection of the skeletal muscle index (SMI) and the psoas muscle
index (PMI). In addition, muscle density on computed tomography
(CT) scans indicates lipid content, which is indicative of muscle
quality (13). The skeletal muscle
gauge (SMG) integrates both the muscle index and muscle density,
and is associated with outcomes in patients with cancer (14,15).
Sarcopenia is an independent predictor of survival
in hematologic diseases (9). In
Non-Hodgkin's lymphoma, cachectic patients exhibited a shorter
progression free survival (PFS) and overall survival (OS) than
non-cachectic patients (16).
Camus et al (17)
demonstrated that a cachexia score, including adipopenia and
sarcopenia, predicted OS in patients with diffuse large B-cell
lymphoma (17).
The relevance of sarcopenia in PCNSL remains
unknown. Low temporal muscle thickness (TMT) measured using
T1w-magnetic resonance imaging (MRI) was associated with a shorter
OS (18). In another cohort, both
low TMT or SMI predicted reduced PFS and OS scores (19).
The present study aimed to evaluate whether baseline
body composition parameters, such as SMI, muscle density and muscle
gauge [measured using third lumbar vertebra (L3) cross-sectional CT
images] were associated with OS and PFS in patients diagnosed with
PCNSL.
Materials and methods
Study population
A total of 98 patients with PCNSL treated at a
primary care center in Germany from 2013–2019 were retrospectively
studied. Patients with a pre-treatment staging CT scan that
included the L3 region were reviewed for analysis. The inclusion
criteria were as follows: Histologically proven diagnosis of PCNSL
(1), available CT scan, including
the psoas muscle on the L3 level prior to treatment, and available
clinical data regarding PFS and OS. The exclusion criteria were as
follows: Missing pretreatment CT images, strong motion artifacts in
CT scans and missing clinical data.
Patient characteristics, such as age, height and
weight were collected from the internal hospital files. Patients
were followed-up for at least two years or until death. The present
retrospective study was approved by the institutional review board.
Informed patient consent was waived given the retrospective nature
of the study (ethics approval no. 145/21; Ethics Committee,
University of Magdeburg, Magdeburg, Germany).
Overall, 72 patients were included in the present
study. A total of 37 patients were male and 35 were female. The
median age was 68 years (range, 23–81 years), and median OS was 10
months (range, 1–181 months). Notably, 37 patients (51.4%)
presented with sarcopenia. All patients were treated with high
dose-methotrexate (MTX; 8 g/m). In 7 patients, additional whole
brain radiotherapy was performed. OS was defined as survival within
the observation period, and PFS was defined as the time frame until
PCNSL growth occurred, determined using MRI.
Image analysis
All CT scans were obtained on a multidetector CT
scanner (Siemens Somatom Definition AS+, Siemens Healthineers,
Germany; Canon Aquilion Prime, Canon Medical Systems Corporation,
Japan). Patients were placed in the supine position. The CT
protocol was as follows: Acquisition slice thickness, 1 mm with 5
mm reconstructions; tube voltage, 120 kV; automatic tube current
modulation; pitch factor, 1.2; collimation, 0.6 mm and 90 ml i.v.
administration of contrast medium (300 ml/mg; Accupaque).
Staging CT scans were used prior to therapy
initiation. All images were assessed in consensus by two
experienced radiologists who were blinded to the clinical course of
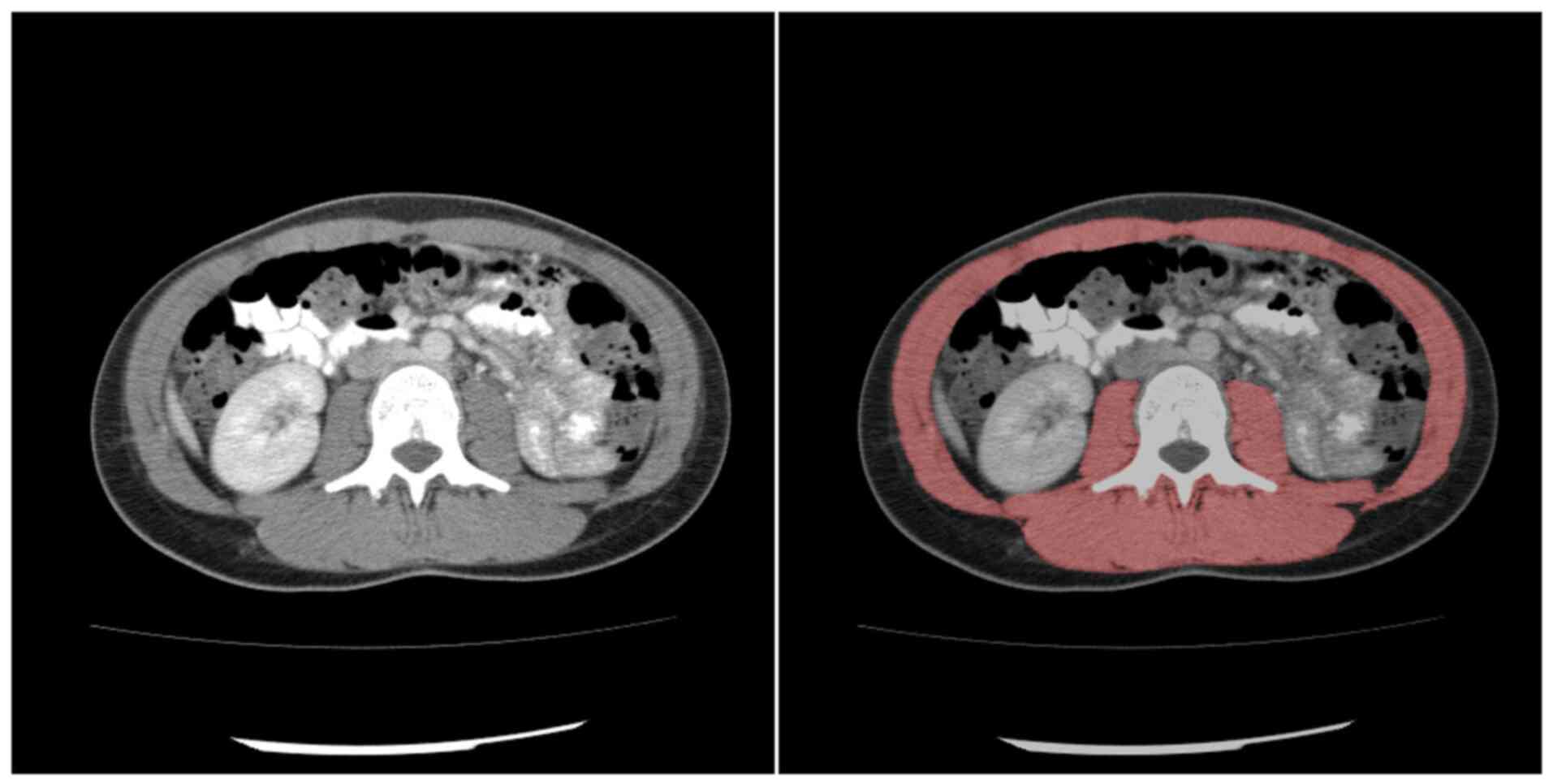
the patients. Measurements of cross-sectional muscle were obtained
semi-automatically on axial images at the L3 level in the soft
tissue window (window, 45–250 HU) using ImageJ software (Fig. 1; version, 1.48v; National
Institutes of Health). The mean muscle density was calculated using
this software. SMI was calculated by dividing the SMA by the height
of the patient. Sarcopenia was defined as an SMI <52.4
cm2/m2 for males and <38.5
cm2/m2 for females (20). SMG was calculated by multiplying
the muscle index and mean muscle density, as reported previously
(18). SMG units are
cm2 × HU/m2 but are reported as arbitrary
units (AU) for simplicity.
Statistical analysis
SPSS (version, 25; IBM Corp.) was used for
statistical analysis. Mean, standard deviation (SD), median and
interquartile range (IQR) were calculated for continuous variables.
Influence of LSMM on OS was assessed using the log-rank test and a
Cox proportional hazards regression. Odds ratios are presented
together with 95% confidence intervals (CI). Multivariate
regression analysis was adjusted for age and sex. P<0.05 was
considered to indicate a statistically significant difference.
Results
OS
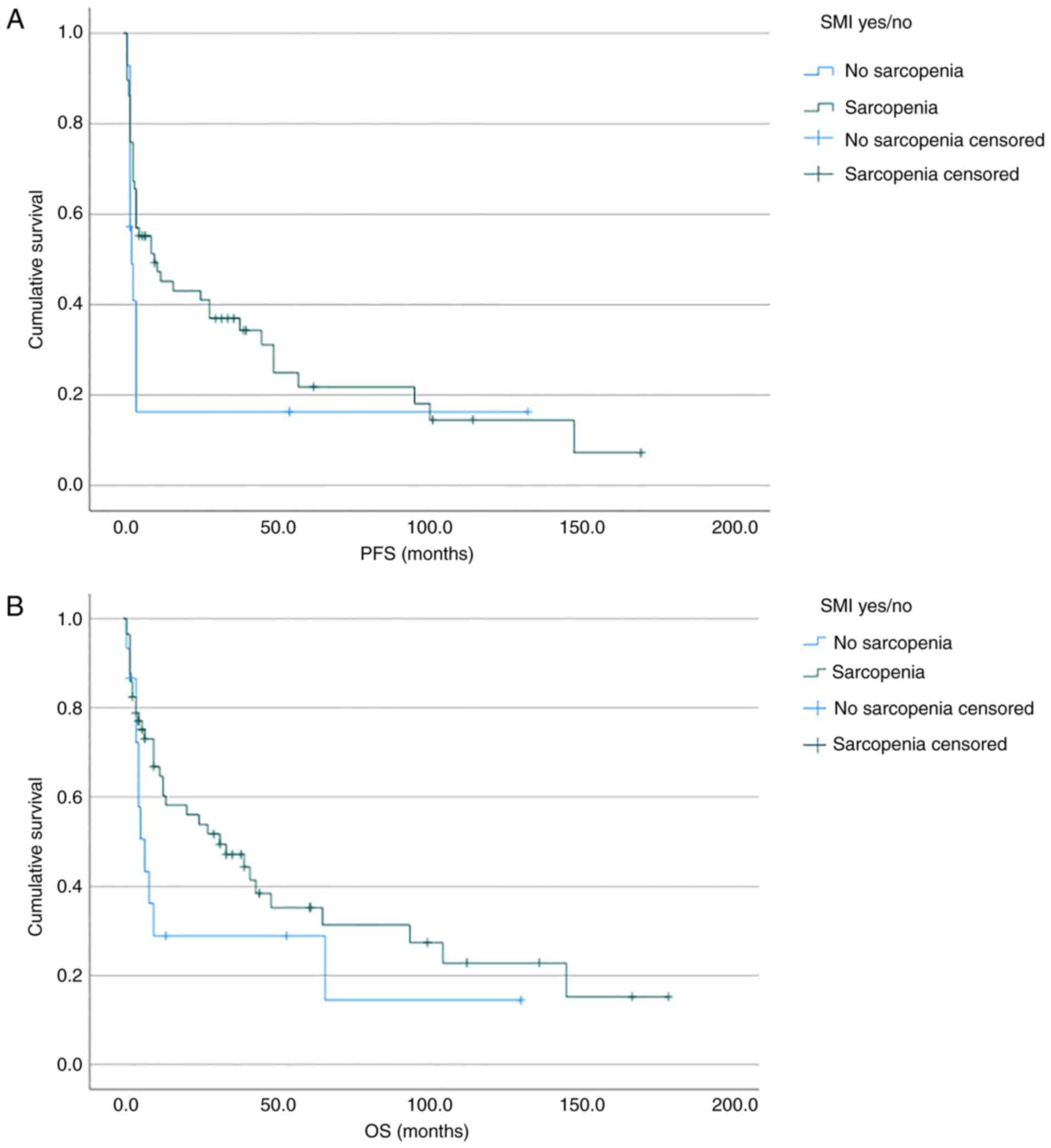
Results of the present study demonstrated that
median OS was 7 months for the sarcopenic group and 32 months for
the non-sarcopenic group (Fig. 2,
Table I). Median SMI was 45.39
cm2/m2 (SD, 7.54
cm2/m2) for survivors and 46.46
cm2/m2 (SD, 9.91
cm2/m2) for non-survivors. There was no
significant influence of sarcopenia on the values for survivors and
non-survivors (Table II). Results
of the present study also demonstrated no major difference in
survival using the log-rank test (P=0.15; Fig. 1), and no influence of sarcopenia
was demonstrated in the univariate analysis (HR, 0.61; 95% CI,
0.31-1.21; P=0.16). There was no influence of SMA (HR, 0.999; 95%
CI, 0.99-1.01; P=0.89) or SMG (HR, 1.00; 95% CI, 0.999-1.00;
P=0.07). The univariate analysis of muscle density demonstrated an
influence on OS (HR, 0.97; 95% CI, 0.94-0.997; P=0.03). However,
when adjusted for age and sex, there was no effect on OS in the
multivariate analysis. (HR, 0.98; 95% CI, 0.94-1.02; P=0.23).
Moreover, there was no significant effect of SMI on OS (Table III).
 | Table I.PFS and OS for sarcopenic vs.
non-sarcopenic patients. |
Table I.
PFS and OS for sarcopenic vs.
non-sarcopenic patients.
| Parameter | Low SMI | Normal SMI | P-value |
|---|
| PFS (months) | 2.5 | 10 | 0.18 |
| OS (months) | 7 | 32 | 0.15 |
 | Table II.Measured values of body composition
parameters for survivors and non-survivors. |
Table II.
Measured values of body composition
parameters for survivors and non-survivors.
| A, Overall
survival |
|---|
|
|---|
| Values | Survivors, M ±
SD | Non-survivors, M ±
SD | P-value |
|---|
| SMA (cm2) | 133.67±31.00 | 137.21±33.66 | 0.69 |
| SMI (cm2/m2) | 45.21±6.35 | 46.62±9.64 | 0.48 |
| Muscle density
(HU) | 33.20±8.65 | 31.61±10.05 | 0.54 |
| Muscle gauge
(AU) |
1,514.97±511.02 |
1,496.56±598.91 | 0.91 |
|
| B, Progression
free survival |
|
| Values | Survivors, M ±
SD | Non-survivors, M
± SD | P-value |
|
| SMA (cm2) | 133.08±26.64 | 135.03±34.87 | 0.79 |
| SMI (cm2/m2) | 45.39±7.54 | 46.46±9.91 | 0.60 |
| Muscle density
(HU) | 33.01±10.92 | 31.23±9.15 | 0.46 |
| Muscle gauge
(AU) |
1,535.99±611.31 |
1,471.13±565.11 | 0.65 |
 | Table III.Regression results for progression
free survival and overall survival. |
Table III.
Regression results for progression
free survival and overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameters | HR | CI 95% | P-value | HR | CI 95% | P-value |
|---|
| Low vs. high
SMI | 0.61 | (0.31, 1.21) | 0.16 |
|
|
|
| SMA | 0.999 | (0.99, 1.01) | 0.89 |
|
|
|
| SMI | 0.997 | (0.96, 1.03) | 0.85 |
|
|
|
| Muscle density | 0.97 | (0.94, 0.997) | 0.03 | 0.98 | (0.94, 1.02) | 0.23 |
| Muscle gauge | 1.00 | (0.999, 1.00) | 0.07 |
|
|
|
PFS
Results of the present study also demonstrated that
the median PFS was 2.5 months for the low SMI group, and 10 months
for the normal SMI group (Fig. 2,
Table I). There were no
significant differences between survivors and non-survivors
(Table II), and there was no
significant difference in PFS between the sarcopenic and
non-sarcopenic groups, demonstrated using a log-rank test (P=0.18).
Results of the present study also demonstrated that sarcopenia did
not exert a significant effect on PFS, demonstrated using the
univariate analysis (HR, 0.65; 95% CI, 0.33-1.27; P=0.20). Notably,
there was no significant effect of SMI on PFS (Table III).
Discussion
The present study investigated whether muscle-based
body composition parameters measured using cross-sectional CT
images act as prognostic factors for PFS or OS in patients
diagnosed with PCNSL. The present study investigated numerous body
composition parameters, such as SMI, muscle density and SMG. To the
best of our knowledge, the present study is the largest study
employing measurements of sarcopenia in PCNSL to date. However,
results of the present study did not demonstrate a significant
association between body composition measurement with PFS or
OS.
While clinical parameters alone may not suffice to
stratify patients according to prognosis and treatment-associated
risks, non-invasive objective imaging markers may be an important
additive tool. Notably, the results of previous studies are
contradictory in detailing sarcopenia and clinical outcomes in
hematologic diseases. Results of a previous meta-analysis
demonstrated that sarcopenia is an independent predictor of OS in
patients with diffuse large b-cell lymphoma (DLBCL) after
chemotherapy (9). Clinical
outcomes, such as complications and hospital stay, were negatively
affected by sarcopenia in patients with lymphoma after autologous
transplant (21). Chu et al
(22) indicated that skeletal
muscle density was associated with complete response and improved
OS in elderly patients (22).
However, Takeoka et al (23) did not find an association between
sarcopenia, measured using SMI, and OS in patients with multiple
myeloma (23). Moreover, results
of the aforementioned meta-analysis demonstrated that sarcopenia
was not associated with OS in the leukemia subgroup (9). Neto et al (24) did not highlight any effects of
sarcopenia on mortality and toxicity in patients with lymphoma
undergoing autologous hematopoietic stem cell transplantation
(24). Results of a multicenter
study by Zilioli et al (25) suggested that there was no
significant association between sarcopenia and either PFS or OS in
patients with Hodgkin's lymphoma (25). Sarcopenia was also not associated
with mortality in patients with hematopoetic malignancies in a
subgroup analysis carried out by Au et al (26). In addition, Besutti et al
(27) demonstrated that decreased
levels of muscle density at the L3 level, but not SMI, were
associated with OS in patients with diffuse large B-cell lymphoma
(27).
Limited research into the potential influence of
body composition parameters on PCNSL in clinical practice is
available at present. Furtner et al (18) assessed the relevance of TMT as a
proxy of sarcopenia for OS, and the results demonstrated that low
levels of TMT were associated with shorter OS (HR 2.504; 95% CI,
1.608-3.911; P<0.001) (18).
Leone et al (19) defined
sarcopenia as either low L3-SMI or low TMT, demonstrating an
association with both lower PFS (HR, 4.40; 95% CI, 1.66-11.61;
P=0.003 and HR, 4.40; 95% CI, 1.68-11.49; P=0.003, respectively)
and shorter OS (HR, 3.16; 95% CI, 1.09-9.11; P=0.034 and HR, 4.93;
95% CI, 1.78-13.65; P=0.002, respectively) (19). By contrast, results of the present
study did not demonstrate a significant association with either OS
or PFS in the present cohort. Compared with other datasets,
differences in patient characteristics in the present cohort may
account for the disparate results. Patients included in the present
study exhibited an increased age. For example, the median age in
the present study was 67.5 years, compared with 61 years in the
study carried out by Leone et al and 62.7 years in the study
carried out by Furtner et al (18,19). Moreover, 35/73 (48.0%) of the
patients involved in the present study were sarcopenic (determined
by SMI), while only 30.2% patients in the cohort presented by Leone
et al (19) demonstrated an
SMI below the threshold. In the cohort presented by Furtner et
al, only 39.3% patients were sarcopenic as defined by TMT
(18). In the present study, the
OS time of 10 months was lower than the OS time of 31.9 months
discussed by Furtner et al (18). In addition, 63.9% patients in the
present study died during the observation period, compared with a
57% survival rate in the study carried out by Leone et al
(19).
Notably, OS time in the present cohort may be too
short to account for influences of sarcopenia on either clinical
outcome. Hacker et al studied patients with gastric and
gastroesophageal junction cancer, and reported that in cohorts with
aggressive tumor characteristics and short survival times, the
effect of sarcopenia may not lead to relevant differences in OS
(28). In tumor entities or
cohorts with an improved overall prognosis, differences in body
composition may translate into relevant differences in outcome. The
present cohort therefore does not prove that there is no influence
of sarcopenia in PCNSL on either clinical parameter. However,
within tumor entities, there will be patient groups that will not
significantly profit from physical exercise in terms of prolonged
survival time. Beyond survival parameters, sarcopenia may exert an
influence on variables not measured in the present study, such as
quality of life or other functional parameters (29).
The present study exhibits numerous limitations.
This was a single center analysis with a retrospective design and a
relatively small sample size. Further prospective studies on the
relationship between sarcopenia and post-operative survival are
required to verify the results obtained. Moreover, the association
between LSMM and patient survival remained the key focus, and
further clinical parameters were not considered. For example,
well-established clinical parameters that influence survival, such
as involvement of deep brain structures, were not analyzed
(30,31). Moreover, parameters for age and sex
were adjusted for in the multivariate analysis, as age has
previously been shown to exert an effect. Patients were excluded
from the present study due to missing staging CT scans or missing
clinical data, potentially leading to selection bias. Notably,
muscle indices were not associated with comorbidities.
In this work SMI was used as a measure of LSMM and
only the cut-off values determined by Prado et al were used
(20). The effects of other
measurements of LSMM, such as PMI, or other cut-off values, were
not evaluated. In our view, the cut-off values presented by Prado
et al (20). are more
practicable when compared to those by Martin et al (32). The definitions by Prado have been
adopted in the international definition of sarcopenia (33). We preferred not to use Martin's
definitions because the cut-off values are discontinuous, leading
to diagnostic inaccuracies (34).
As the SMI has already been normalized by body height, we do not
deem an additional BMI cut-off necessary. Other cut-off values, for
example those based on the psoas muscle area or psoas muscle index,
are not as well validated in oncologic patients (35). We therefore chose not to apply
them. Similarly, muscle measurements on other levels are not well
substantiated. A combination of imaging and clinical tests might
provide a more accurate measurement of low skeletal muscle mass.
However, every clinical test carries with it the downside of
subjectivity, in that they are dependent on patients' answers or
the examiner. The advantage of imaging tests are their
reproducibility and reliability in a routine clinical setting.
SMI does not measure sarcopenia, but low skeletal
muscle mass. It is regarded as a proxy parameter for sarcopenia.
Yet sarcopenia is a complex syndrome, including low muscle
strength, low muscle quality and quantity and low muscle
performance. Imaging parameters can account for muscle quantity and
to a lesser extent for quality. These do not capture the entire
syndrome. Further studies are warranted to see whether a
combination of parameters might be better suited to identify
patients at risk. However, we deem LSMM as measured on routine
imaging a rapid and useful marker to screen for sarcopenia and
potentially initiate adequate treatment.
In conclusion, results of the present study did not
demonstrate a significant association between sarcopenia and
clinical outcomes in patients with PCNSL. However, further studies
are required to determine whether sarcopenia exerts an influence in
other patient subgroups, after receiving certain treatments or when
other measurements of body composition are applied.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VF, MT, JO, ASu performed the study conception and
design, data acquisition, data interpretation, drafting and
revision of manuscript. MP performed data interpretation and
analysis and revision. AW and ASt performed data acquisition, data
interpretation, data analysis and revision. MH, DW, DM and VZ
performed data acquisition and revision. ASu and DW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
institutional review board. Informed patient consent was waived
given the retrospective nature of the study (approval no. 145/21;
Ethics Committee, University of Magdeburg, Magdeburg, Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CR
|
complete response
|
|
LSMM
|
low skeletal muscle mass
|
|
OS
|
overall survival
|
|
PCNSL
|
primary central nervous system
lymphoma
|
|
PFS
|
progression free survival
|
|
PMI
|
psoas muscle index
|
|
SMG
|
skeletal muscle gauge
|
|
SMI
|
low skeletal muscle index
|
|
TMT
|
temporal muscle thickness
|
References
|
1
|
Hoang-Xuan K, Bessell E, Bromberg J,
Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C,
Abacioglu U, et al: Diagnosis and treatment of primary CNS lymphoma
in immunocompetent patients: Guidelines from the European
association for neuro-oncology. Lancet Oncol. 16:e322–e332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grommes C and DeAngelis LM: Primary CNS
lymphoma. J Clin Oncol. 35:2410–2418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Batchelor T and Loeffler JS: Primary CNS
lymphoma. J Clin Oncol. 24:1281–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gopal S, Patel MR, Yanik EL, Cole SR,
Achenbach CJ, Napravnik S, Burkholder GA, Reid EG, Rodriguez B,
Deeks SG, et al: Temporal trends in presentation and survival for
HIV-associated lymphoma in the antiretroviral therapy era. J Natl
Cancer Inst. 105:1221–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han CH and Batchelor TT: Diagnosis and
management of primary central nervous system lymphoma. Cancer.
123:4314–4324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrey LE, Ben-Porat L, Panageas KS,
Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta
M and DeAngelis LM: Primary central nervous system lymphoma: The
memorial sloan-kettering cancer center prognostic model. J Clin
Oncol. 24:5711–5715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreri AJM, Blay JY, Reni M, Pasini F,
Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi
A, et al: Prognostic scoring system for primary CNS lymphomas: The
international extranodal lymphoma study group experience. J Clin
Oncol. 21:266–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Surov A and Wienke A: Sarcopenia predicts
overall survival in patients with malignant hematological diseases:
A meta-analysis. Clin Nutr. 40:1155–1160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamarajah SK, Bundred J and Tan BHL: Body
composition assessment and sarcopenia in patients with gastric
cancer: A systematic review and meta-analysis. Gastric Cancer.
22:10–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mintziras I, Miligkos M, Wächter S,
Manoharan J, Maurer E and Bartsch DK: Sarcopenia and sarcopenic
obesity are significantly associated with poorer overall survival
in patients with pancreatic cancer: Systematic review and
meta-analysis. Int J Surg. 59:19–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X,
Zhang Q and Li Z: Can sarcopenia be a predictor of prognosis for
patients with non-metastatic colorectal cancer? A systematic review
and meta-analysis. Int J Colorectal Dis. 33:1419–1427. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bak SH, Kwon SO, Han SS and Kim WJ:
Computed tomography-derived area and density of pectoralis muscle
associated disease severity and longitudinal changes in chronic
obstructive pulmonary disease: A case control study. Respir Res.
20:2262019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weinberg MS, Shachar SS, Muss HB, Deal AM,
Popuri K, Yu H, Nyrop KA, Alston SM and Williams GR: Beyond
sarcopenia: Characterization and integration of skeletal muscle
quantity and radiodensity in a curable breast cancer population.
Breast J. 24:278–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shachar SS, Deal AM, Weinberg M, Nyrop KA,
Williams GR, Nishijima TF, Benbow JM and Muss HB: Skeletal muscle
measures as predictors of toxicity, hospitalization, and survival
in patients with metastatic breast cancer receiving taxane-based
chemotherapy. Clin Cancer Res. 23:658–665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karmali R, Alrifai T, Fughhi IAM, Ng R,
Chukkapalli V, Shah P, Basu S, Nathan S, Szymanski-Grant K, Gordon
LI, et al: Impact of cachexia on outcomes in aggressive lymphomas.
Ann Hematol. 96:951–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camus V, Lanic H, Kraut J, Modzelewski R,
Clatot F, Picquenot JM, Contentin N, Lenain P, Groza L, Lemasle E,
et al: Prognostic impact of fat tissue loss and cachexia assessed
by computed tomography scan in elderly patients with diffuse large
B-cell lymphoma treated with immunochemotherapy. Eur J Haematol.
93:9–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furtner J, Nenning KH, Roetzer T,
Gesperger J, Seebrecht L, Weber M, Grams A, Leber SL, Marhold F,
Sherif C, et al: Evaluation of the temporal muscle thickness as an
independent prognostic biomarker in patients with primary central
nervous system lymphoma. Cancers (Basel). 13:5662021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leone R, Sferruzza G, Calimeri T,
Steffanoni S, Conte GM, De Cobelli F, Falini A, Ferreri AJM and
Anzalone N: Quantitative muscle mass biomarkers are independent
prognosis factors in primary central nervous system lymphoma: The
role of L3-skeletal muscle index and temporal muscle thickness. Eur
J Radiol. 143:1099452021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumours
of the respiratory and gastrointestinal tracts: A population-based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caram MV, Bellile EL, Englesbe MJ,
Terjimanian M, Wang SC, Griggs JJ and Couriel D: Sarcopenia is
associated with autologous transplant-related outcomes in patients
with lymphoma. Leuk Lymphoma. 56:2855–2862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu MP, Lieffers J, Ghosh S, Belch A, Chua
NS, Fontaine A, Sangha R, Turner RA, Baracos VE and Sawyer MB:
Skeletal muscle density is an independent predictor of diffuse
large B-cell lymphoma outcomes treated with rituximab-based
chemoimmunotherapy. J Cachexia Sarcopenia Muscle. 8:298–304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeoka Y, Sakatoku K, Miura A, Yamamura
R, Araki T, Seura H, Okamura T, Koh H, Nakamae H, Hino M and Ohta
K: Prognostic Effect of low subcutaneous adipose tissue on survival
outcome in patients with multiple myeloma. Clin Lymphoma Myeloma
Leuk. 16:434–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neto AC, Moraes BDGC, Rocha IMG, Bezerra
FA, Medeiros GOC, Alves LBO, Rossetti RAM, Fayh APT, Mariano LCB
and Rocha V: Association of sarcopenia with toxicities and survival
after autologous hematopoietic stem cell transplantation for adults
with lymphomas. Blood. 132 (Suppl 1):S21582018. View Article : Google Scholar
|
|
25
|
Zilioli VR, Albano D, Arcari A, Merli F,
Coppola A, Besutti G, Marcheselli L, Gramegna D, Muzi C, Manicone
M, et al: Clinical and prognostic role of sarcopenia in elderly
patients with classical Hodgkin lymphoma: A multicentre experience.
J Cachexia Sarcopenia Muscle. 12:1042–1055. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Au PC, Li HL, Lee GK, Li GH, Chan M,
Cheung BM, Wong IC, Lee VH, Mok J, Yip BH, et al: Sarcopenia and
mortality in cancer: A meta-analysis. Osteoporos Sarcopenia. 7
(Suppl 1):S28–S33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Besutti G, Massaro F, Bonelli E, Braglia
L, Casali M, Versari A, Ligabue G, Pattacini P, Cavuto S, Merlo DF,
et al: Prognostic impact of muscle quantity and quality and fat
distribution in diffuse large B-cell lymphoma patients. Front Nutr.
8:6206962021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hacker UT, Hasenclever D, Linder N,
Stocker G, Chung HC, Kang YK, Moehler M, Busse H and Lordick F:
Prognostic role of body composition parameters in
gastric/gastroesophageal junction cancer patients from the EXPAND
trial. J Cachexia Sarcopenia Muscle. 11:135–144. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nipp RD, Fuchs G, El-Jawahri A, Mario J,
Troschel FM, Greer JA, Gallagher ER, Jackson VA, Kambadakone A,
Hong TS, et al: Sarcopenia is associated with quality of life and
depression in patients with advanced cancer. Oncologist. 23:97–104.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moulignier A, Lamirel C, Picard H,
Lebrette MG, Amiel C, Hamidi M, Polivka M, Mikol J, Cochereau I and
Pialoux G: Long-term AIDS-related PCNSL outcomes with HD-MTX and
combined antiretroviral therapy. Neurology. 89:796–804. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CH, Yang CF, Yang HC, Fay LY, Yeh CM,
Kuan AS, Wang HY, Gau JP, Hsiao LT, Chiou TJ, et al: Risk
prediction for early mortality in patients with newly diagnosed
primary CNS lymphoma. J Cancer. 10:39582019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin L, Birdsell L, Macdonald N, Reiman
T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB and
Baracos VE: Cancer cachexia in the age of obesity: Skeletal muscle
depletion is a powerful prognostic factor, independent of body mass
index. J Clin Oncol. 31:1539–1547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taguchi S, Nakagawa T and Fukuhara H:
Inconsistencies in currently used definitions of sarcopenia in
oncology. Ann Oncol. 31:318–319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rutten IJG, Ubachs J, Kruitwagen RFPM,
Beets-Tan RGH, Olde Damink SWM and Van Gorp T: Psoas muscle area is
not representative of total skeletal muscle area in the assessment
of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle.
8:630–638. 2017. View Article : Google Scholar : PubMed/NCBI
|