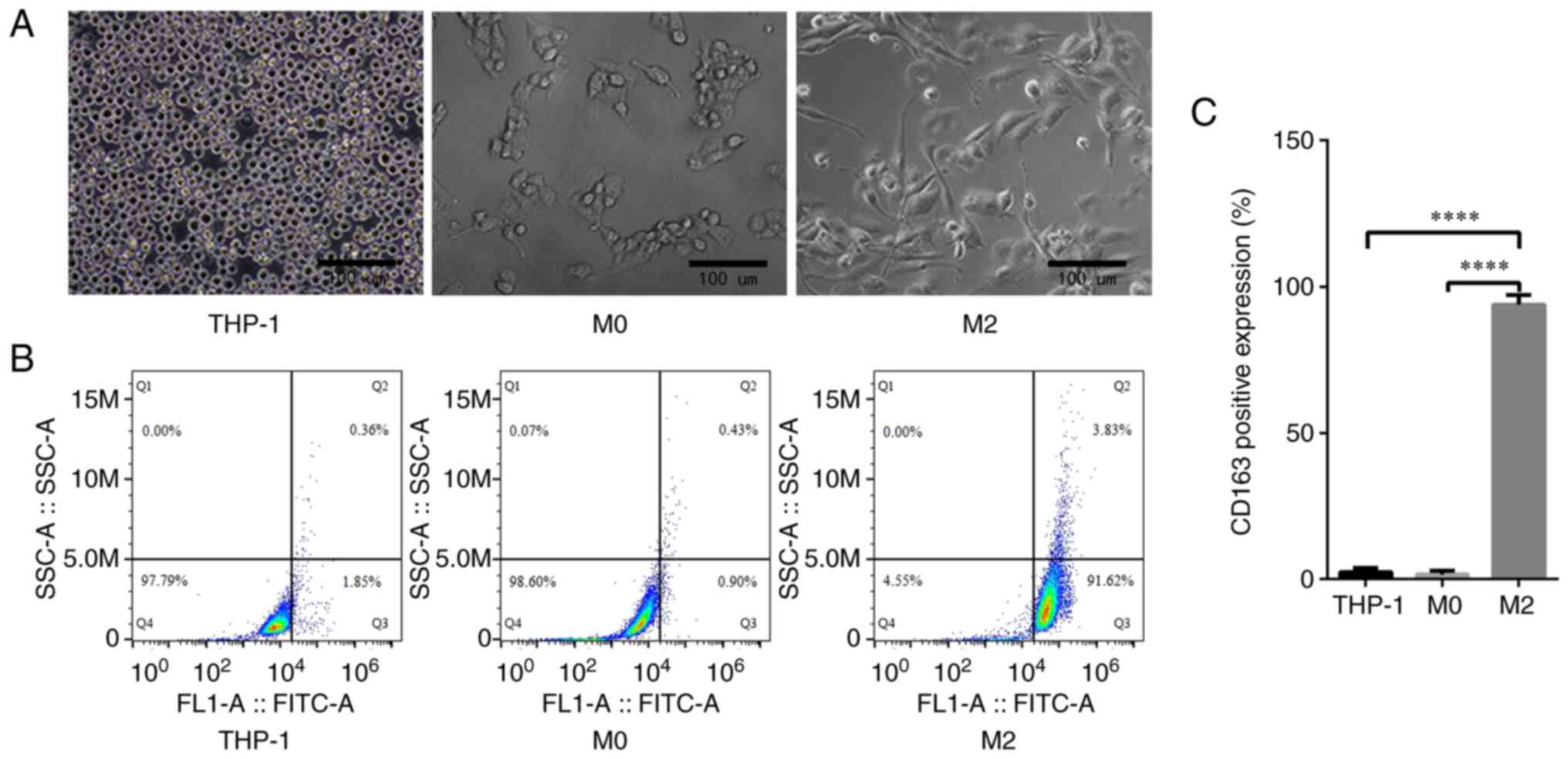
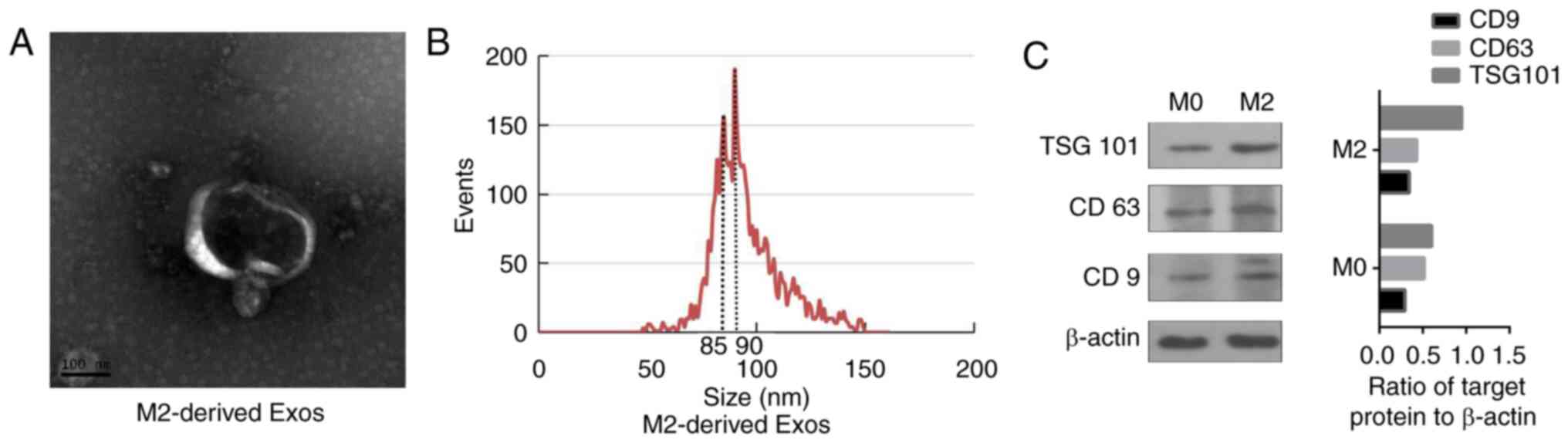
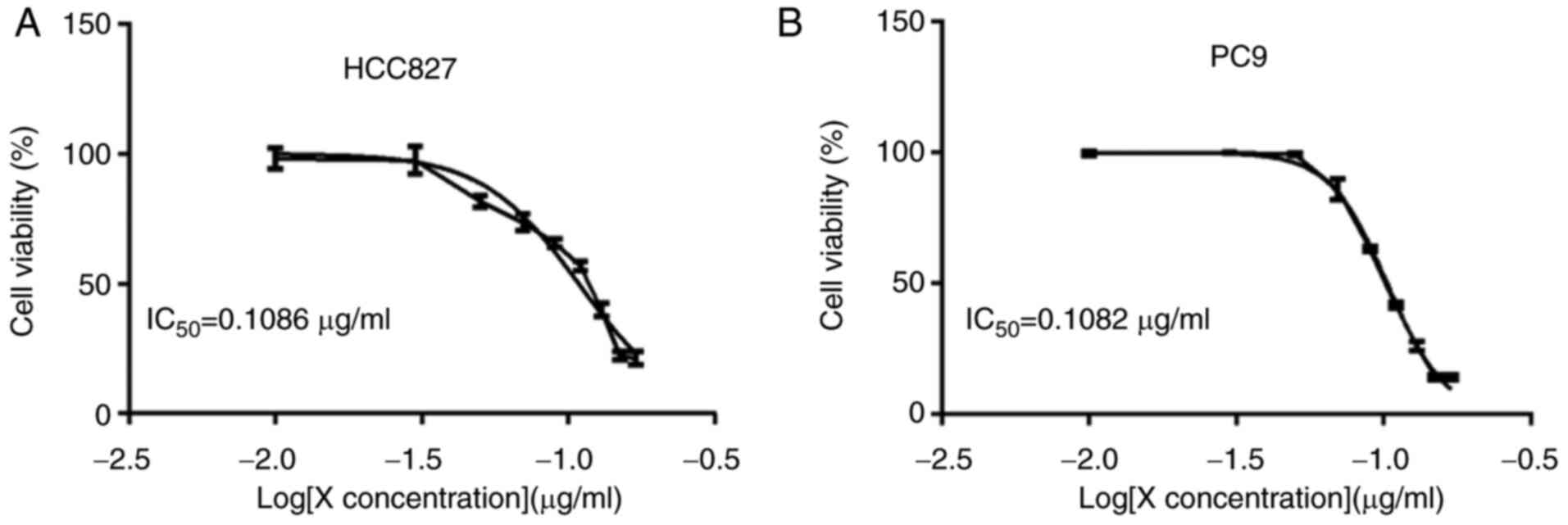
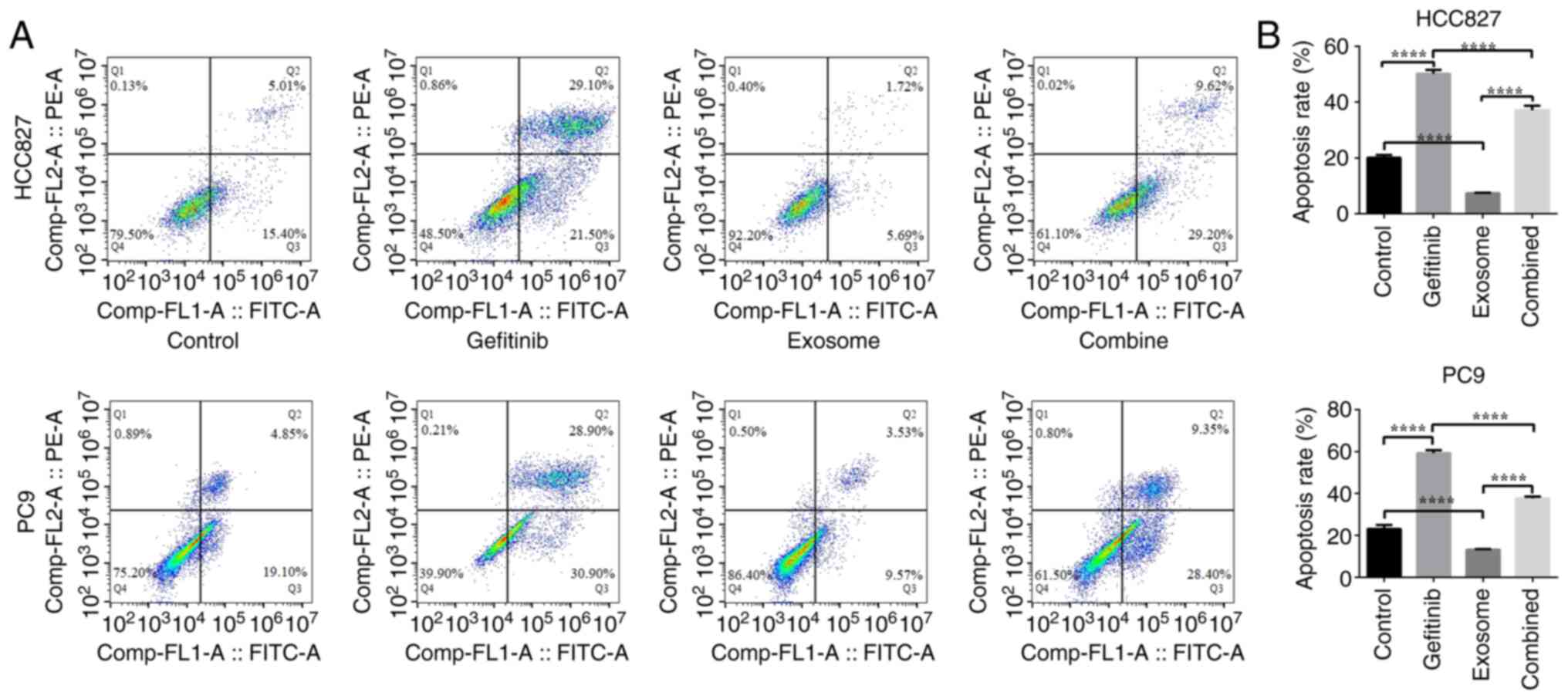
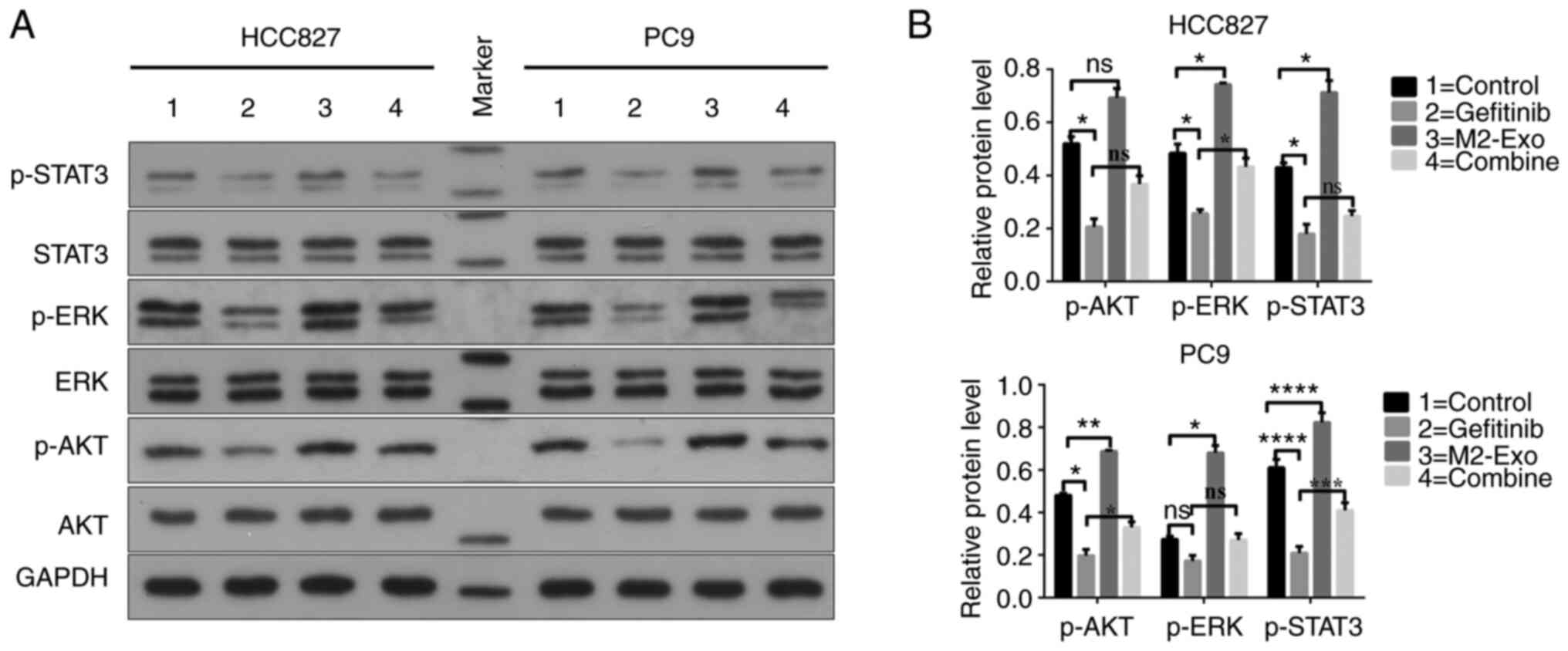
|
1
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daly ME, Singh N, Ismaila N, Antonoff MB,
Arenberg DA, Bradley J, David E, Detterbeck F, Fruh M, Gubens MA,
et al: Management of stage III Non-small-cell lung cancer: ASCO
guideline. J Clin Oncol. 40:1356–1384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JJ, Zhu VW, Schoenfeld AJ, Yeap BY,
Saxena A, Ferris LA, Dagogo-Jack I, Farago AF, Taber A, Traynor A,
et al: Brigatinib in patients with alectinib-refractory
ALK-positive NSCLC. J Thorac Oncol. 13:1530–1538. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peled N, Gillis R, Kilickap S, Froesch P,
Orlov S, Filippova E, Demirci U, Christopoulos P, Cicin I, Basal
FB, et al: GLASS: Global Lorlatinib for ALK(+) and ROS1(+)
retrospective Study: Real world data of 123 NSCLC patients. Lung
Cancer. 148:48–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seto T, Ohashi K, Sugawara S, Nishio M,
Takeda M, Aoe K, Moizumi S, Nomura S, Tajima T and Hida T:
Capmatinib in Japanese patients with MET exon 14 skipping-mutated
or MET-amplified advanced NSCLC: GEOMETRY mono-1 study. Cancer Sci.
112:1556–1566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmid S, Li J and Leighl NB: Mechanisms
of osimertinib resistance and emerging treatment options. Lung
Cancer. 147:123–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaikh M, Shinde Y, Pawara R, Noolvi M,
Surana S, Ahmad I and Patel H: Emerging approaches to overcome
acquired drug resistance obstacles to osimertinib in non-small-cell
lung cancer. J Med Chem. 65:1008–1046. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostrand-Rosenberg S: Tolerance and immune
suppression in the tumor microenvironment. Cell Immunol. 299:23–29.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kerkar SP and Restifo NP: Cellular
constituents of immune escape within the tumor microenvironment.
Cancer Res. 72:3125–3130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung FT, Lee KY, Wang CW, Heh CC, Chan
YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, et al:
Tumor-associated macrophages correlate with response to epidermal
growth factor receptor-tyrosine kinase inhibitors in advanced
non-small cell lung cancer. Int J Cancer. 131:E227–E235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang B, Zhang Y, Zhao J, Wang Z, Wu T, Ou
W, Wang J, Yang B, Zhao Y, Rao Z and Gao J: M2-polarized
macrophages contribute to the decreased sensitivity of EGFR-TKIs
treatment in patients with advanced lung adenocarcinoma. Med Oncol.
31:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan S, Dong Y, Peng L, Yang M, Niu L, Liu
Z and Xie J: Tumor-associated macrophages affect the biological
behavior of lung adenocarcinoma A549 cells through the PI3K/AKT
signaling pathway. Oncol Lett. 18:1840–1846. 2019.PubMed/NCBI
|
|
16
|
Wendler F, Favicchio R, Simon T,
Alifrangis C, Stebbing J and Giamas G: Extracellular vesicles swarm
the cancer microenvironment: From tumor-stroma communication to
drug intervention. Oncogene. 36:877–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robbins PD and Morelli AE: Regulation of
immune responses by extracellular vesicles. Nat Rev Immunol.
14:195–208. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Looze C, Yui D, Leung L, Ingham M, Kaler
M, Yao X, Wu WW, Shen RF, Daniels MP and Levine SJ: Proteomic
profiling of human plasma exosomes identifies PPARgamma as an
exosome-associated protein. Biochem Biophys Res Commun.
378:433–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, Zhou J, Chen F, Yu J, Li H, Li Q and
Li W: 13-Methyl-palmatrubine shows an anti-tumor role in non-small
cell lung cancer via shifting M2 to M1 polarization of tumor
macrophages. Int Immunopharmacol. 104:1084682022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun D, Luo T, Dong P, Zhang N, Chen J and
Zhang S, Dong L, Janssen H and Zhang S: M2-polarized
tumor-associated macrophages promote epithelial-mesenchymal
transition via activation of the AKT3/PRAS40 signaling pathway in
intrahepatic cholangiocarcinoma. J Cell Biochem. 121:2828–2838.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Li D, Cang H and Guo B: Crosstalk
between cancer and immune cells: Role of tumor-associated
macrophages in the tumor microenvironment. Cancer Med. 8:4709–4721.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Goede KE, Driessen A and Van den
Bossche J: Metabolic cancer-macrophage crosstalk in the tumor
microenvironment. Biology (Basel). 9:3802020.PubMed/NCBI
|
|
24
|
Ruivo CF, Adem B, Silva M and Melo SA: The
biology of cancer exosomes: Insights and new perspectives. Cancer
Res. 77:6480–6488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pashazadeh M: The role of tumor-isolated
exosomes on suppression of immune reactions and cancer progression:
A systematic review. Med J Islam Repub Iran. 34:912020.PubMed/NCBI
|
|
26
|
Wei K, Ma Z, Yang F, Zhao X, Jiang W, Pan
C, Li Z, Pan X, He Z, Xu J, et al: M2 macrophage-derived exosomes
promote lung adenocarcinoma progression by delivering miR-942.
Cancer Lett. 526:205–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Chen Z, Ni Y, Bian C, Huang J, Chen
L, Xie X and Wang J: Tumor-associated macrophages secret exosomal
miR-155 and miR-196a-5p to promote metastasis of non-small-cell
lung cancer. Transl Lung Cancer Res. 10:1338–1354. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Wang L, Pan H, Wang Y, Shi M, Yu
H, Wang C, Pan X and Chen Z: Exosomes derived from macrophages
enhance aerobic glycolysis and Chemoresistance in lung cancer by
stabilizing c-Myc via the inhibition of NEDD4L. Front Cell Dev
Biol. 8:6206032020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou D, Xia Z, Xie M, Gao Y, Yu Q and He
B: Exosomal long non-coding RNA SOX2 overlapping transcript
enhances the resistance to EGFR-TKIs in non-small cell lung cancer
cell line H1975. Hum Cell. 34:1478–1489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Li D, Cang H and Guo B: Crosstalk
between cancer and immune cells: Role of tumor-associated
macrophages in the tumor microenvironment. Cancer Med. 8:4709–4721.
2019. View Article : Google Scholar : PubMed/NCBI
|