Introduction
Ovarian carcinoma (OC) is one of the most common
neoplasms of the female reproductive tract (1). With over 240,000 new diagnoses and
152,000 annual deaths worldwide in 2020 according to the Global
Cancer Statistics (2), OC exhibits
the worst prognosis among gynecological malignancies (3). With a median age of 68 years at
diagnosis, OC is a typical cancer of the older generation (4). However, increasing biological age is
independently associated with more aggressive and advanced diseases
(5,6).
At present, radical primary or interval surgical
debulking remains as the preferred first-line treatment option,
possibly with extensive multivisceral cytoreduction to achieve
complete macroscopic tumor reduction with no residual tumor burden
(7–9). Although, excessive surgical
resections have been shown to initiate metastasis via circulating
tumor cells to blood and lymph or activate dormant pre-existing
micrometastases (10–12). Whether the minimal residual tumor
burden results in clinical metastases depends primarily on the
balance between the body's immunity and natural killer (NK) cell
activity and the tumor's ability to proliferate or colonize a new
site (13). The most popular
hypothesis about the immunomodulation effect of surgical stress
explains the adverse impact of the inhibition of the body's innate
tumor defense mechanism (14).
Otherwise, an effective immune response is affected by the
integrated network of different cytokines, including interleukins
and interferons. Certain cytokines are antitumorigenic and permit
cancer growth (e.g. IL-2 and IFN-γ), whereas others are
pro-tumorigenic and promote the immune system's anti-tumor
capability (e.g. IL-6 and IL-8) (15).
Interestingly, the technique of anesthesia may
influence the long-term outcome of solid cancer patients by
modulating the neuroendocrine and cytokine imparted immune
competence and stress response during excessive surgery (16,17).
Compared with general anesthesia using intravenous opioids or
inhaled anesthetics, regional anesthesia can effectively increase
the level of activated NK cells to preserve host immunity, as a
primary immunological defense against cancer cells (18,19).
This possible survival advantage for epidural-supplemented
anesthesia with decreased levels of opioids depends on a reduction
of cancer recurrences and postoperative mortality without
metastasis, mainly in elderly cancer patients (20,21).
Besides, exposure to volatile anesthetics leads to resistance of
cancer cells against apoptosis in a dose-dependent pattern
(22). Moreover, opioids may
suppress the NK cell cytotoxicity, as one component of the human
cellular immune system, as well as promote tumor growth by
activating the µ-opioid receptor (23,24).
As an unavoidable adverse effect of general anesthesia during
partial cell vagotomy, an endocrine stress response followed
(25).
Thereof, this study aimed to evaluate the prognostic
influence of epidural-supplemented anesthesia on cancer survival in
a highly specified cohort of older ovarian cancer patients after
major oncological surgery.
Materials and methods
Inclusion and exclusion criteria
Patients with all stages of OC [based on the 2010
FIGO staging system (26)] older
than 60 years of age, who underwent standardized surgical treatment
at the University Medical Center of the Johannes Gutenberg
University Mainz between January 2008 and December 2019, were
included in the retrospective cohort study. Standardized
oncological surgery required primary or interval tumor debulking
operations to decrease the postoperative residual tumor burden as
much as possible (aim R0 resection) and was defined as a further
inclusion criterion. Exclusion criteria were: 1) surgically
treatment not in the University Medical Center Mainz, 2)
Non-malignant or borderline ovarian tumors, 3) No information about
epidural analgesia available and 4) No complete follow-up
information. Long-term follow-up was performed by evaluation of
patient's clinical records, written inquiries to the patients or
their physicians, and by telephone calls up to February 2021. The
follow-up ended at death, and the longest follow-up period lasted
nearly 11.5 years (June 2008-December 2019 according to 138
months).
Baseline characteristics
We screened the archives and the electronically
patients' records to gather all using general patient information.
Clinical-pathological tumor characteristics were collected
according to the current national guidelines, which may influence
the postoperative prognosis in OC patients. These factors are
summarized within three categories: 1) clinical-pathological
tumor parameters, including the tumor stage [TNM and
International Fédération of Gynecology and Obstetrics (FIGO)
(26)], histological subtype and
grading, 2) anesthesiologic parameters such as comorbidities
[summarized in the Charlson Comorbidity Index (CCI) (27,28)]
and American Society of Anesthesiologists Performance Status (ASA
PS) (29) and 3) oncological
and surgical parameters (e.g. postoperative residual tumor
burden, surgical radicality retrospectively evaluated through
Surgical Complexity Score (SCS) (30), as well as timing and completeness
of chemotherapy).
Anesthetic techniques
Epidural catheter anesthesia was placed during the
anesthesiologic induction before the surgical intervention.
Epidural analgesia was administrated in OC patients expect those
with contraindications like local infections or malformations, as
well as spinal surgery or trauma, bacteremia, that may cause
epidural infection and urinary tract infections, for example.
Furthermore, low coagulation status or insufficient stopping time
anticoagulant and patients who refused the technique were absolute
contraindications for epidural anesthesia. Relative
contraindications arise from preexisting neurological deficits and
failures such as signs of paralysis subsequently slipped disc.
Intraoperative epidural anesthesia was provided by bupivacaine
0,25% or ropivacaine 0,375% with or without addition of epidural
sufentanil according to the attending anesthetist. All patients
that received epidural catheter placement were treated by
postoperative patient-controlled epidural anesthesia consisting of
bupivacaine 0.125%. Epidural fentanyl was added as long as the
patients were in intensive or intermediate care units, whereas
bupivacaine 0,125% alone was applied in the normal ward. Patients
that did not receive epidural catheter placement or showed signs of
insufficient epidural anesthesia received a patient-controlled
intravenous anesthesia-device with piritramide. All patients were
cared for by a physician-based acute pain service affiliated to the
Department of Anesthesiology as long as they received a
patient-controlled anesthesia.
General anesthesia was conducted as balanced or
total intravenous anesthesia according to the standard operating
procedures of the Department of Anesthesiology as amended (31,32).
Statistical analysis
The data were recorded in Microsoft Excel and SPSS
statistical software program, version 23.0 V5 R (SPSS Inc, Chicago,
IL, U.S.A.), as well as StataBE 17 V5 were performed for the data
analyses. Patients' characteristics were expressed as a mean +/-
standard deviation [SD], or as median with their interquartile
range [IQR]. We divided the study cohort into two groups, according
to the epidural supplementing. Normal distribution was explored by
Shapiro-Wilk test. Categorical variables were compared using the
chi-square or Fisher's exact test. Kaplan-Meier estimates were used
to determine the progression-free survival (PFS) and overall
survival (OS) rates after five years for univariate analyses. The
Log-Rank-Test was used to compare the curves. Timepoints in months
were the date of diagnosis which resulted in the date of tumor
debulking surgery up to death (or recurrence) or last follow-up.
PFS included loco-regional lower abdomen recurrences and/or distant
metastasis and death as an event. The Cox proportional hazards
regression model was determined for multivariate analyses of the
survival time after debulking surgery. At first, univariate Cox
regression analyses for all factors that affect oncological
survival after cancer surgery were performed. Secondly, each
hypothesis with a significance level of <0.05 was included in
the multivariate Cox regression analysis. The variable selection
was examined via backward elimination. All tests were two-sided and
a P-value of <0.05 was considered statistically significant.
Because no correction was made for multiple testing due to the
exploratory nature of the study, these are descriptive measures
that should be interpreted with caution.
Results
Clinical features of OC patients
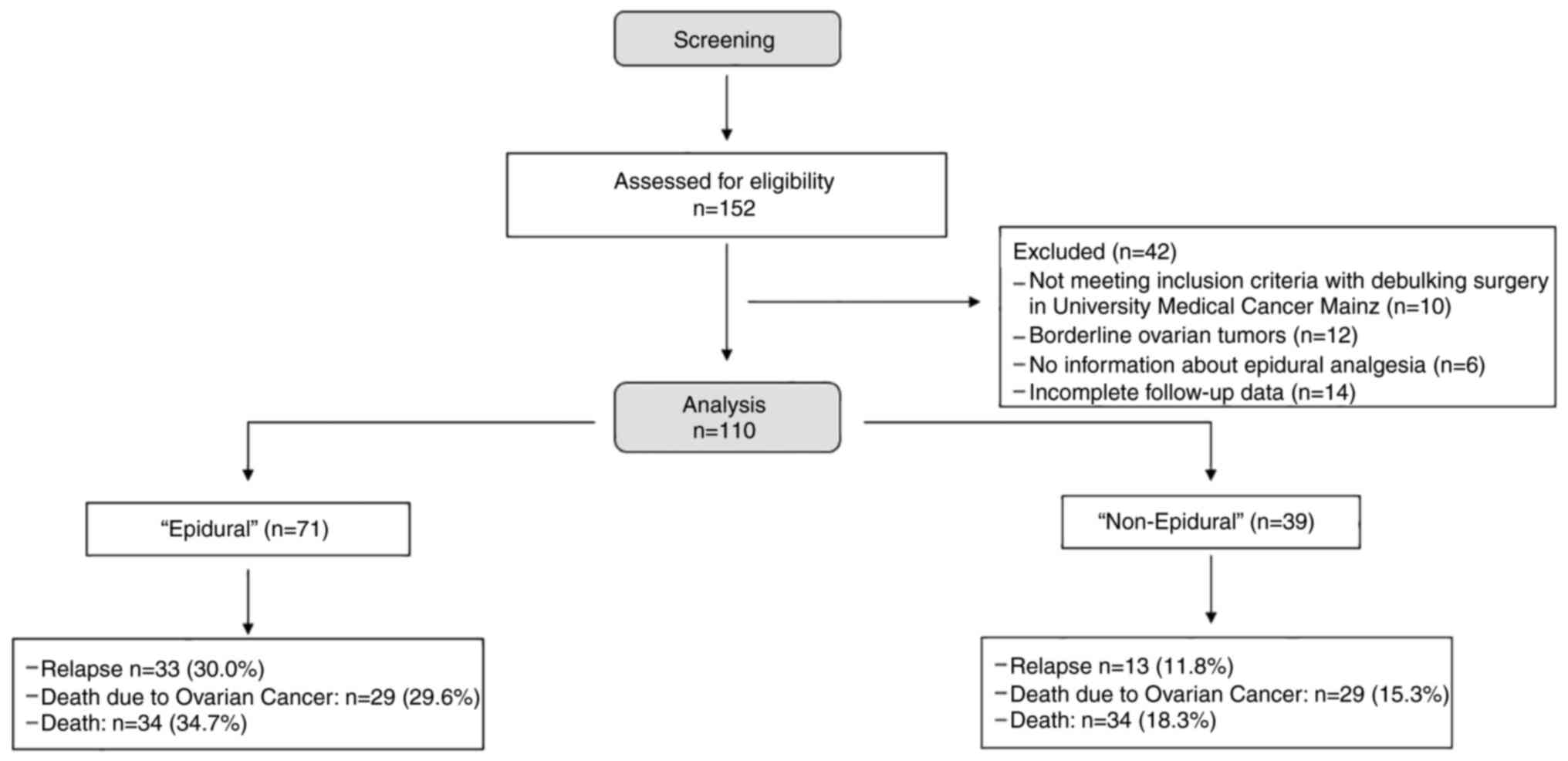
A total of 152 patients, with OC were screened and
recruited from the achieves of the University Medical center Mainz
(Fig. 1). Our final cohort was
composed of 110 women aged 60 years and older (mean 70.9+/-5.9
years). Missing data arise from the inclusion and exclusion
criteria (Fig. 1). All baseline
factors, including various clinical-pathological tumor
characteristics, as well as anesthesiologic, oncological and
surgical parameters, were compared between the ‘Epidural’ and
‘non-Epidural’ group. Demographic and clinical characteristics are
summarized in Table I.
 | Table I.Patient characteristics for the two
types of anesthesia techniques. |
Table I.
Patient characteristics for the two
types of anesthesia techniques.
| Parameter | Total (n=110) | Epidural
(n=71) | Non-epidural
(n=39) | P-value |
|---|
|
Clinical-pathological tumor
characteristics |
|
|
|
|
| Tumor
stage (TNM), n (%) (n=107) |
|
|
| 0.272 |
|
I | 17 (15.9) | 10 (9.3) | 7 (6.5) |
|
|
II | 8 (7.5) | 7 (6.5) | 1 (0.9) |
|
|
III | 81 (75.7) | 52 (48.6) | 29 (27.1) |
|
|
IV | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
Tx | 1 (0.9) | 0 (0.0) | 1 (0.9) |
|
| Tumor
stage (FIGO), n (%) (n=106) |
|
|
| 0.106 |
|
Early ovarian
cancer < FIGO IIa | 15 (14.2) | 7 (6.6) | 8 (7.5) |
|
|
Late ovarian
cancer ≥ FIGO IIa | 91 (85.8) | 62 (58.5) | 29 (27.4) |
|
|
Histological subtype, n (%)
(n=110) |
|
|
| 0.240 |
|
Low grade serous +
others | 33 (30.0) | 24 (21.8) | 9 (8.2) |
|
|
High grade
serous | 77 (70.0) | 47 (42.7) | 30 (27.3) |
|
|
Histological grading, n (%)
(n=108) |
|
|
| 0.854 |
|
G1 | 6 (5.6) | 4 (3.7) | 2 (1.9) |
|
|
G2 | 20 (18.5) | 14 (13.0) | 6 (5.6) |
|
|
G3 | 82 (75.9) | 52 (48.1) | 30 (27.8) |
|
| Anesthesiologic
characteristics |
|
|
|
|
| Mean
age, years (+/-SD) | 71.08
(+/-5.95) | 72.18
(+/-6.17) | 70.55
(+/-5.75) |
|
| CCI, n
(%) (n=110) |
|
|
| 0.020 |
|
CCI 1 | 22 (20.0) | 18 (16.4) | 4 (3.6) |
|
|
CCI 2 | 61 (55.5) | 41 (37.3) | 20 (18.2) |
|
|
CCI 3 | 27 (24.5) | 12 (10.9) | 15 (13.6) |
|
| American Society of
Anesthesiologists |
|
|
|
|
|
Performance Status, n (%)
(n=109) |
|
|
| 0.020 |
|
1+4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
2 | 48 (44.0) | 37 (33.9) | 11 (10.1) |
|
|
3 | 61 (56.0) | 34 (31.2) | 27 (24.8) |
|
| Oncological and
surgical characteristics |
|
|
|
|
|
Postoperative residual tumor
burden, n (%) (n=109) |
|
|
| 0.533 |
|
None | 63 (57.8) | 42 (38.5) | 21 (19.3) |
|
|
Present | 46 (42.2) | 28 (25.7) | 18 (16.5) |
|
| SCS, n
(%) (n=110) |
|
|
| 0.837 |
|
SCS 1 | 37 (33.6) | 23 (20.9) | 14 (12.7) |
|
|
SCS 2 | 53 (48.2) | 34 (30.9) | 19 (17.3) |
|
|
SCS 3 | 20 (18.2) | 14 (12.7) | 6 (5.5) |
|
|
Completeness of chemotherapy,
n (%) | 75 (68.2) | 62 (82.7) | 13 (17.3) | 0.008 |
| Timing
of chemotherapy, n (%) (n=99) |
|
|
| 0.911 |
|
Neoadjuvant
chemotherapy | 22 (22.2) | 14 (14.1) | 8 (8.1) |
|
|
Adjuvant
chemotherapy | 77 (77.8) | 50 (50.5) | 27 (27.3) |
|
|
Clinical events, n (%) |
|
|
|
|
|
Relapse | 46 (41.8) | 33 (30.0) | 13 (11.8) | 0.181 |
|
Death due to
OC | 44 (44.9) | 29 (29.6) | 15 (15.3) | 0.937 |
|
Death | 52 (53.1) | 34 (34.7) | 18 (18.3) | 0.834 |
Comparison of baseline clinical data
between the two groups
Most patients were diagnosed with high-grade serous
histology (n=77, 70.0%) and had a higher histological grading (G3:
n=82, 75.9%). Approximately fifty-eight percent of the patients
(n=63) underwent optimal tumor debulking. We reported no
differences between the ‘Epidural’ and the ‘non-Epidural’ group
concerning the clinical prognostic parameters (all P values
>0.05) with the expectation of the ASA PS and CCI evaluation
(P=0.020) (Table I). Furthermore,
a lower number of relevant comorbidities was associated with more
epidural-supplemented anesthesia. The median survival time for all
OC patients was 26.0 months (11.8–38.0). The ‘Non-Epidural’ cohort
survived with 27.0 months (15.0–39.0) longer than the
‘Epidural’-supplemented group with 19.0 months (8.0–36.5),
respectively. No significant differences were found between the
groups (P=0.355).
Comparison of survival data between
the two groups
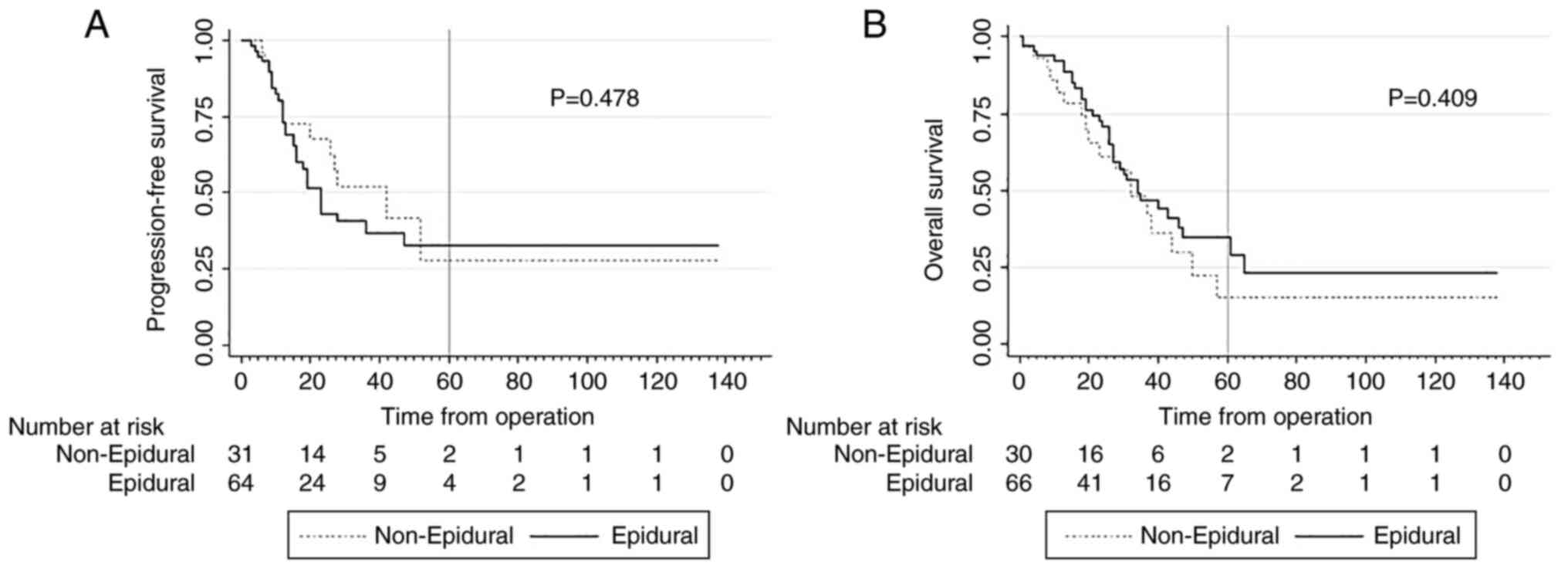
The Kaplan-Meier analyses indicated no association
between epidural-supplemented anesthesia and prolonged
progression-free or overall survival (PFS: 32.8% vs. 27.8%; P=0.478
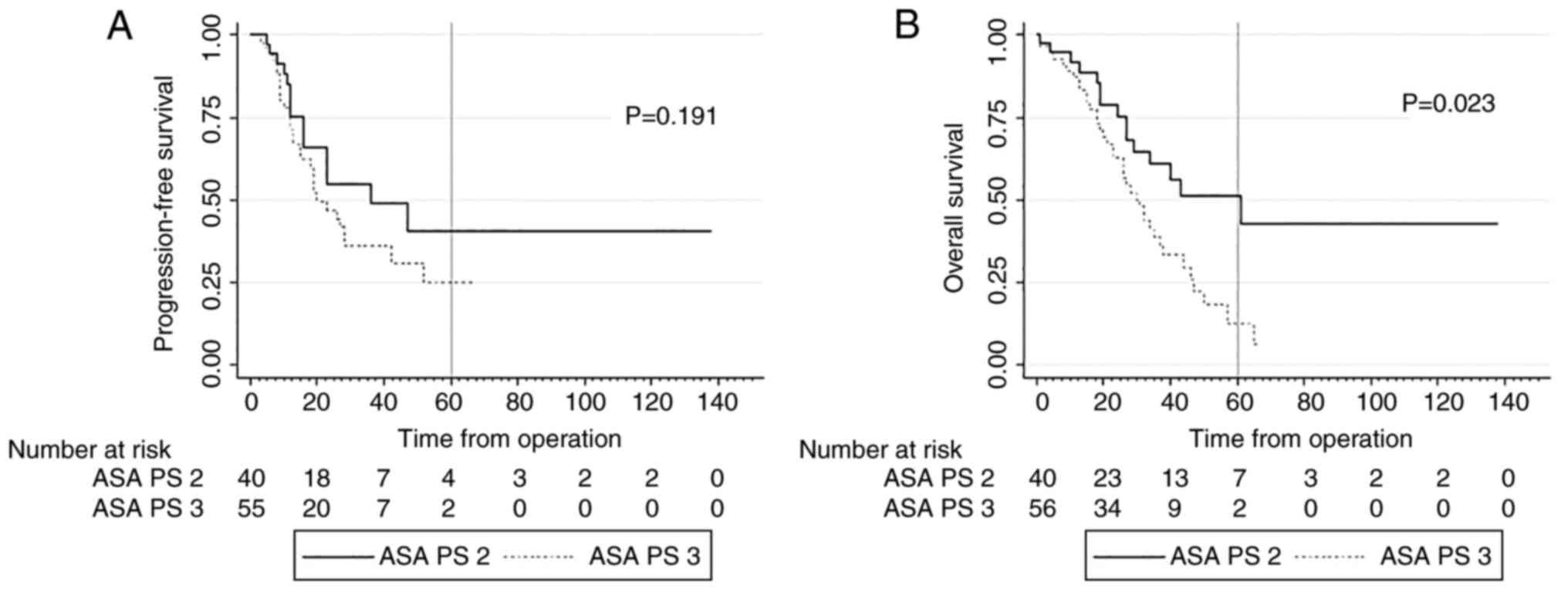
and OS: 29.5% vs. 15.0%; P=0.409; respectively) (Fig. 2). In contrast, the preoperative ASA
classification showed statistically significant differences in
terms of 5-years OS (ASA 2 49.9% vs. ASA 3 12.9%; P=0.023) as also
shown in Fig. 3. In the subgroup
of ASA 2 patients, significantly more patients received combined
anesthesia with epidural anesthesia as solely general anesthesia
(77.1% vs. 22.9%; P=0.020) whereby no differences were found in the
ASA 3 group (‘Epidural’: 55.7% vs. ‘non-Epidural’: 44.3%).
The Cox regression model indicated no significant
association between epidural use and prolonged survival after
cancer debulking surgery (PFS: HR: 1.26; 95%-CI [0.66–2.39] and OS:
HR: 0.79; 95%-CI [0.45-1.40]; respectively) (Table II). In the multivariate Cox model,
only the conventional clinical-pathological tumor parameter
TNM-tumor stage retained its independent significance for PFS and
OS (PFS: HR: 3.09; 95%-CI: [1.72–5.55] and OS: HR: 3.11; 95%-CI:
[1.73–5.58]; respectively).
 | Table II.Cox univariate and multivariate
regression analyses. |
Table II.
Cox univariate and multivariate
regression analyses.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| PFS | OS | PFS | OS |
|---|
|
|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
|
Clinical-pathological tumor
parameters |
|
|
|
|
|
|
|
|
|
|
|
|
| Tumor
stage-TNM | 2.81 | 1.59–4.95 | <0.001 | 3.70 | 2.05–6.68 | <0.001 | 3.09 | 1.72–5.55 | <0.001 | 3.11 | 1.73–5.58 | <0.001 |
| Tumor
stage-FIGO | 6.78 | 2.07–22.26 | 0.002 | 11.47 | 2.73–47.91 | 0.001 | 2.14 | 0.38–12.21 | 0.392 | 2.13 | 0.37–12.17 | 0.394 |
|
Histological subtype | 1.48 | 0.77–2.87 | 0.241 | 1.73 | 0.90–3.29 | 0.098a | - |
|
| - |
|
|
|
Histological grading | 1.68 | 0.94–3.02 | 0.082a | 1.78 | 1.01–3.14 | 0.048 | - |
|
| 1.14 | 0.63–2.08 | 0.667 |
| Anaesthesiologic
parameters |
|
|
|
|
|
|
|
|
|
|
|
|
| Mean
age | 0.79 | 0.44–1.42 | 0.428 | 1.19 | 0.69–2.06 | 0.533 | - |
|
| - |
|
|
|
CCI | 0.95 | 0.63–1.43 | 0.791 | 1.43 | 0.95–2.15 | 0.091a | - |
|
| - |
|
|
| ASA
PS | 1.49 | 0.81–2.73 | 0.202 | 1.97 | 1.08–3.57 | 0.026 | - |
|
| 1.24 | 0.67–2.29 | 0.494 |
|
Epidural analgesia | 1.26 | 0.66–2.39 | 0.487 | 0.79 | 0.45–1.40 | 0.413 | - |
|
| - |
|
|
| Oncological and
surgical parameters |
|
|
|
|
|
|
|
|
|
|
|
|
|
Postoperative residual tumor
burden | 2.26 | 1.25–4.07 | 0.007 | 2.63 | 1.49–4.62 | 0.001 | 1.14 | 0.61–2.12 | 0.680 | 1.13 | 0.60–2.12 | 0.715 |
|
SCS | 1.39 | 0.91–2.13 | 0.129 | 1.44 | 0.96–2.16 | 0.079a | - |
|
| - |
|
|
|
Completeness of CTX | 1.76 | 0.69–4.48 | 0.234 | 0.65 | 0.30–1.40 | 0.267 | - |
|
| - |
|
|
| Timing
of CTX | 0.92 | 0.42–1.98 | 0.825 | 0.93 | 0.43–2.02 | 0.863 |
|
|
| - |
|
|
Discussion
The results of this retrospective study did not
confirm a definite association between epidural-supplemented
anesthesia and cancer progression and OS in elderly patients
following tumor debulking surgery for all stages of ovarian
cancer.
Possible benefits of regional anesthesia techniques
on the postoperative analgetic effect in gynecological
malignancies, especially in OC patients have been examined
(33,34). With regard to survival data of OC
related to the presence of an epidural, controversial data have
been published (35–39). Overall, the positive impact of
neuraxial analgesia seems to be solely detectable in more advanced
disease, the prolonged relapse-free survival seems to depend on the
timing of catheter insertion. In the study by Tseng et al
(35), 435 women with advanced
stages of OC receiving epidural anesthesia during primary debulking
surgery were compared to 213 patients did not. The median PFS and
OS was significantly improved in those who received epidurals (PFS:
20.8 months vs. 13.9 months; P=0.021 and OS: 62.4 months vs. 41.9
months, P<0.001; respectively). Oliveira and colleagues
demonstrated the epidural-related preservation of the immune system
function resulting in an increased time to tumor recurrence after
surgery in 182 patients executively for intraoperative used
epidural catheters (HR: 0.37; 95%-CI [0.19–0.73]) (37).
Other published reports showed similar findings to
those of the current study. In Lacassies's prospective clinical
registry, there were no benefits in survival in patients with
advanced stages of OC after the use of epidural reported (38). They obtained propensity score
matching, adjusting for chemotherapy, besides the multivariate Cox
regression model, without any differences in cancer prognosis. In
2012, Capmas et al (39)
examined 104 advanced-stage OC patients and declared no clear
impact of regional analgesia on cancer recurrence. A systematic
Cochrane Review addressing this topic showed no association between
epidural use with lengthened survival in solid cancer patients
(40).
Physiologic negative stress was highest at the time
of surgery and affected the adaptive immune surveillance (41). Immune disturbance due to surgical
distress may facilitate cancer cell migration (16). Various types of anesthesia showed
different effects on human cancer immunity and carcinogenesis.
Epidural analgesia could attenuate intraoperative suppression of NK
cell function and help preserve effective defense against tumor
progression, by limiting the use of opioids (42). Additionally, regional anesthesia
was linked to earlier recovery times by reducing postoperative
complications such as thromboembolic, cardiac and pulmonary, as
well as gastrointestinal complications and inflammation (43). The role of cytokines in cancer
immunity was either directly influenced by proliferative effects or
indirectly by enhancing proinflammatory and proangiogenic pathways
in host cells.
The results of our trial suggested that survival
prognosis in OC disease might not be primarily determined by
immunomodulatory effects caused by the epidural analgesia,
especially not in elderly cancer patients. Although, increasing age
resulted in changes in body's homeostasis and vulnerability to
external stressors increased the relevant prognostic factors
remain. In advanced cancer diseases the best option of controlling
progression and lengthening survival was optimal surgical tumor
debulking as well as completeness of platinum-based chemotherapy
(44). Anesthesia and analgesia
techniques were important, but they did not appear to have an
independent and significant impact on cancer prognosis in this
population.
Study limitations arise from the biases associated
with the retrospective single-institution nature of the study. The
single institution design allowed the patients in both groups,
‘Epidural’ and ‘non-Epidural’ to receive the same perioperative
care. Our follow-up period of almost 14 years was robust and we
also performed a through multivariate analysis, controlling for
conventional-established prognostic factors in OC. Even though, the
intraoperative epidural technique was approximately homogenous
(bupivacaine 0.25% or ropivacaine 0.375% and postoperative with
bupivacaine 0.125% in combination with fentanyl concentration
varied between 0.75% and 1.0% and was administered with or without
an opioid).
Because of the fact that elderly patients were
underrepresented in the vast majority of existing comparative
reports, prospective studies to investigate the effects of
perioperative epidural anesthesia use in the special cohort of
elderly ovarian cancer women on survival are warranted. Given the
limited number of modifiable prognostic parameters for elderly OC
patients, studies investigating the impact of different anesthetic
techniques potentially influencing the immune function after
debulking surgery will be desirable.
In conclusion, we could not find a survival benefit
in patients with ovarian cancer after the perioperative use of
epidural anesthesia after debulking surgery. Primary tumor stage in
combination with optimal cytoreduction and completeness of
chemotherapy are still the strongest prognostic factors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KA and MJB contributed to the study design. KA, MJB,
MWS, RS, AD, MS, SK, MR, RH, EKH and AH contributed to the data
acquisition. KA and MJB analyzed the data. KA and MJB wrote the
paper. KA and MJB confirm the authenticity of all the raw data. KA
and MJB revised the manuscript for important intellectual content.
All authors critically and substantively revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The retrospective cohort study was conducted in
accordance with the ‘Ethical principles for medical research
involving human subjects’ of the current version of the Declaration
of Helsinki. Data collected for the present study were obtained as
part of routine medical care. Ethical approval for use of these
samples for research purposes was not required for the present
study in accordance with local/national guidelines. Written
informed consent from participants was obtained in accordance with
local/national guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wimberger P, Lehmann N, Kimmig R, Burges
A, Meier W, Hoppenau B and du Bois A: AGO-OVAR: Impact of age on
outcome in patients with advanced ovarian cancer treated within a
prospectively randomized phase III study of the arbeitsgemeinschaft
gynaekologische onkologie ovarian cancer study group (AGO-OVAR).
Gynecol Oncol. 100:300–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martins FC, Couturier DL, Paterson A,
Karnezis AN, Chow C, Nazeran TM, Odunsi A, Gentry-Maharaj A, Vrvilo
A, Hein A, et al: Clinical and pathological associations of PTEN
expression in ovarian cancer: A multicentre study from the ovarian
tumour tissue analysis consortium. Br J Cancer. 123:793–802. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Courtney-Brooks M, Tellawi AR, Scalici J,
Duska LR, Jazaeri AA, Modesitt SC and Cantrell LA: Frailty: An
outcome predictor for elderly gynecologic oncology patients.
Gynecol Oncol. 126:20–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anic K, Birkert S, Schmidt MW, Linz VC,
Heimes AS, Krajnak S, Schwab R, Schmidt M, Westphalen C, Hartmann
EK, et al: G-8 geriatric screening tool independently predicts
progression-free survival in older ovarian cancer patients
irrespective of maximal surgical effort: Results of a retrospective
cohort study. Gerontology. 1–10. 2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibson SJ, Fleming GF, Temkin SM and Chase
DM: The application and outcome of standard of care treatment in
elderly women with ovarian cancer: A literature review over the
last 10 years. Front Oncol. 6:632016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fotopoulou C, Planchamp F, Aytulu T, Chiva
L, Cina A, Ergönül Ö, Fagotti A, Haidopoulos D, Hasenburg A, Hughes
C, et al: European society of gynaecological oncology guidelines
for the peri-operative management of advanced ovarian cancer
patients undergoing debulking surgery. Int J Gynecol Cancer.
31:1199–1206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landrum LM, Java J, Mathews CA, Lanneau GS
Jr, Copeland LJ, Armstrong DK and Walker JL: Prognostic factors for
stage III epithelial ovarian cancer treated with intraperitoneal
chemotherapy: A gynecologic oncology group study. Gynecol Oncol.
130:12–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuchiya Y, Sawada S, Yoshioka I, Ohashi
Y, Matsuo M, Harimaya Y, Tsukada K and Saiki I: Increased surgical
stress promotes tumor metastasis. Surgery. 133:547–555. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eschwège P, Dumas F, Blanchet P, Le Maire
V, Benoit G, Jardin A, Lacour B and Loric S: Haematogenous
dissemination of prostatic epithelial cells during radical
prostatectomy. Lancet. 346:1528–1530. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiller JG, Perry NJ, Poulogiannis G,
Riedel B and Sloan EK: Perioperative events influence cancer
recurrence risk after surgery. Nat Rev Clin Oncol. 15:205–218.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bar-Yosef S, Melamed R, Page GG, Shakhar
G, Shakhar K and Ben-Eliyahu S: Attenuation of the tumor-promoting
effect of surgery by spinal blockade in rats. Anesthesiology.
94:1066–1073. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen XB, Zhang HL and Fang XD:
Surgery-induced immunomodulation in breast cancer. J Surg Oncol.
103:197author reply 196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao VS, Dyer CE, Jameel JK, Drew PJ and
Greenman J: Potential prognostic and therapeutic roles for
cytokines in breast cancer (Review). Oncol Rep. 15:179–185.
2006.PubMed/NCBI
|
|
16
|
Pei L, Tan G, Wang L, Guo W, Xiao B, Gao
X, Wang L, Li H, Xu Z, Zhang X, et al: Comparison of combined
general-epidural anesthesia with general anesthesia effects on
survival and cancer recurrence: A meta-analysis of retrospective
and prospective studies. PLoS One. 9:e1146672014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed N, Balega J, Barwick T, Buckley L,
Burton K, Eminowicz G, Forrest J, Ganesan R, Harrand R, Holland C,
et al: British Gynaecological cancer society (BGCS) cervical cancer
guidelines: Recommendations for practice. Eur J Obstet Gynecol
Reprod Biol. 256:433–465. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brittenden J, Heys SD, Ross J and Eremin
O: Natural killer cells and cancer. Cancer. 77:1226–1243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cummings KC III, Xu F, Cummings LC and
Cooper GS: A comparison of epidural analgesia and traditional pain
management effects on survival and cancer recurrence after
colectomy: A population-based study. Anesthesiology. 116:797–806.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gottschalk A, Ford JG, Regelin CC, You J,
Mascha EJ, Sessler DI, Durieux ME and Nemergut EC: Association
between epidural analgesia and cancer recurrence after colorectal
cancer surgery. Anesthesiology. 113:27–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawaraguchi Y, Horikawa YT, Murphy AN,
Murray F, Miyanohara A, Ali SS, Head BP, Patel PM, Roth DM and
Patel HH: Volatile anesthetics protect cancer cells against tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
via caveolins. Anesthesiology. 115:499–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeager MP, Colacchio TA, Yu CT,
Hildebrandt L, Howell AL, Weiss J and Guyre PM: Morphine inhibits
spontaneous and cytokine-enhanced natural killer cell cytotoxicity
in volunteers. Anesthesiology. 83:500–508. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singleton PA, Moss J, Karp DD, Atkins JT
and Janku F: The mu opioid receptor: A new target for cancer
therapy? Cancer. 121:2681–2688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Guo W, Wu Q, Zhang R and Fang J:
Impact of combination epidural and general anesthesia on the
long-term survival of gastric cancer patients: A retrospective
study. Med Sci Monit. 22:2379–2385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colombo N, Peiretti M, Parma G, Lapresa M,
Mancari R, Carinelli S, Sessa C and Castiglione M; ESMO Guidelines
Working Group, : Newly diagnosed and relapsed epithelial ovarian
carcinoma: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21 (Suppl 5):v23–v30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sundararajan V, Henderson T, Perry C,
Muggivan A, Quan H and Ghali WA: New ICD-10 version of the Charlson
comorbidity index predicted in-hospital mortality. J Clin
Epidemiol. 57:1288–1294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Hoore W, Bouckaert A and Tilquin C:
Practical considerations on the use of the Charlson comorbidity
index with administrative data bases. J Clin Epidemiol.
49:1429–1433. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davenport DL, Bowe EA, Henderson WG, Khuri
SF and Mentzer RM Jr: National surgical quality improvement program
(NSQIP) risk factors can be used to validate American Society of
anesthesiologists physical status classification (ASA PS) levels.
Ann Surg. 243:636–41; discussion 641-4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aletti GD, Dowdy SC, Podratz KC and Cliby
WA: Relationship among surgical complexity, short-term morbidity,
and overall survival in primary surgery for advanced ovarian
cancer. Am J Obstet Gynecol. 197:676e1–e7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eger EI II, Saidman LJ and Brandstater B:
Minimum alveolar anesthetic concentration: A standard of anesthetic
potency. Anesthesiology. 26:756–763. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campagna JA, Miller KW and Forman SA:
Mechanisms of actions of inhaled anesthetics. N Engl J Med.
348:2110–2124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuniyoshi H, Yamamoto Y, Kimura S, Hiroe
T, Terui T and Kase Y: Comparison of the analgesic effects
continuous epidural anesthesia and continuous rectus sheath block
in patients undergoing gynecological cancer surgery: A
non-inferiority randomized control trial. J Anesth. 35:663–670.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong S, Zhong X, Zhong X and Liu Y:
Comparison between the effect of epidural anesthesia combined with
epidural analgesia and general anesthesia combined with intravenous
analgesia on prognosis of ovarian cancer patients. Oncol Lett.
17:5662–5668. 2019.PubMed/NCBI
|
|
35
|
Tseng JH, Cowan RA, Afonso AM, Zhou Q,
Iasonos A, Ali N, Thompson E, Sonoda Y, O'Cearbhaill RE, Chi DS, et
al: Perioperative epidural use and survival outcomes in patients
undergoing primary debulking surgery for advanced ovarian cancer.
Gynecol Oncol. 151:287–293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin L, Liu C, Tan H, Ouyang H, Zhang Y and
Zeng W: Anaesthetic technique may affect prognosis for ovarian
serous adenocarcinoma: A retrospective analysis. Br J Anaesth.
106:814–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Oliveira GS Jr, Ahmad S, Schink JC,
Singh DK, Fitzgerald PC and McCarthy RJ: Intraoperative neuraxial
anesthesia but not postoperative neuraxial analgesia is associated
with increased relapse-free survival in ovarian cancer patients
after primary cytoreductive surgery. Reg Anesth Pain Med.
36:271–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lacassie HJ, Cartagena J, Brañes J, Assel
M and Echevarría GC: The relationship between neuraxial anesthesia
and advanced ovarian cancer-related outcomes in the Chilean
population. Anesth Analg. 117:653–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Capmas P, Billard V, Gouy S, Lhommé C,
Pautier P, Morice P and Uzan C: Impact of epidural analgesia on
survival in patients undergoing complete cytoreductive surgery for
ovarian cancer. Anticancer Res. 32:1537–1542. 2012.PubMed/NCBI
|
|
40
|
Cakmakkaya OS, Kolodzie K, Apfel CC and
Pace NL: Anaesthetic techniques for risk of malignant tumour
recurrence. Cochrane Database Syst Rev. ((11)):
CD0088772014.PubMed/NCBI
|
|
41
|
Bartal I, Melamed R, Greenfeld K, Atzil S,
Glasner A, Domankevich V, Naor R, Beilin B, Yardeni IZ and
Ben-Eliyahu S: Immune perturbations in patients along the
perioperative period: Alterations in cell surface markers and
leukocyte subtypes before and after surgery. Brain Behav Immun.
24:376–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li JM, Shao JL, Zeng WJ and Liang RB:
General/epidural anesthesia in combination preserves NK cell
activity and affects cytokine response in cervical carcinoma
patients undergoing radical resection: A cohort prospective study.
Eur J Gynaecol Oncol. 36:703–707. 2015.PubMed/NCBI
|
|
43
|
Shavit Y, Ben-Eliyahu S, Zeidel A and
Beilin B: Effects of fentanyl on natural killer cell activity and
on resistance to tumor metastasis in rats. Neuroimmunomodulation.
11:255–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953. 2010.
View Article : Google Scholar : PubMed/NCBI
|