Introduction
Lung cancer is one of the most common malignant
tumors in the world. GLOBOCAN 2020 cancer statistics estimate 2.2
million new lung cancer cases and 1.8 million new lung
cancer-associated deaths worldwide (1). With the increase of age, the exposure
to carcinogenic factors, such as smoking, increases, and
carcinogenic mutations accumulate, leading to an increased risk of
suffering from lung cancer. However, according to cancer statistics
in the United States and Japan, 4.5-9.0% of lung cancer patients
are <50 years old at the time of the initial cancer diagnosis
(2–4). Due to the low proportion of AYAs in
lung cancer, there have been few studies (5–7) on
the clinical characteristics, incidence of targetable genomic
mutations and prognosis in this group. In addition, conclusions
from these studies are not completely consistent.
Although previous studies used different ages for
the cut-off value defining AYA patients, the results consistently
suggested that AYAs with lung cancer consisted of cases
predominantly with adenocarcinoma, a higher proportion of females
and patients who tended to present with advanced disease (8–10).
Nevertheless, there were conflicting conclusions on targetable
genomic mutations and the prognosis of AYAs. Some reports have
shown that a significantly higher percentage of young patients
present with metastatic disease, resulting in a shorter OS time
compared with older adults (11,12),
while others showed that overall survival time was significantly
better or similar for younger patients compared with older adults
(2–4,7,13–15).
The inconsistent conclusions on prognosis between AYAs and older
adults with lung cancer may be attributed to ethnic differences,
pathological types, targetable genomic mutations, treatment
patterns and economic factors.
To date, most European and American lung cancer
screening guidelines recommend 55 years as the starting age for
lung cancer screening by low-dose computed tomography, while
China's National Lung Cancer Screening Guideline with Low-dose
Computed Tomography (2015 and 2018 version) recommends starting in
high-risk individuals at 50 years of age (16,17).
Therefore, the present study used 50 years as the age cut-off value
for defining AYA patients. In this study, the incidence and
clinical characteristics of AYAs in patients with lung
adenocarcinomais comprehensively investigated. Additionally, since
targeted therapy and systematic chemotherapy were found to be the
main treatment patterns for lung adenocarcinoma, the study also
analyzed whether age influenced toxicity, efficacy and prognostic
response to treatment.
Materials and methods
Data source
The data for all patients with lung malignancies
treated in YueBei People's Hospital (Shaoguan, China) between
January 2013 and December 2017 were extracted by identifying the
diagnostic code C34 (ICD-10-CM) in the discharged patients
database. This hospital is the biggest tertiary hospital in
Shaoguan. The number of outpatient and inpatient visits is ~1.45
million and ~0.12 million per year, respectively. Patients admitted
to this hospital are mainly residents of this city. Lung
adenocarcinoma had been pathologically diagnosed according to
pathological morphology and positive thyroid transcription
factor-1, NapsinA or Alcian blue, and periodic acid Schiff staining
in tumor cells. Patients included in this study were staged
according to the eighth edition of the Tumor-Node-Metastasis (TNM)
criteria of the American Joint Committee on Cancer in conjunction
with The American College of Radiology Appropriateness Criteria
(18). This study was a
retrospective analysis of patient medical records, and ethical
approval was obtained from the Institutional Ethics Committee of
YueBei People's Hospital and is not considered subject to the
Medical Research Involving Human Subjects Act.
Patients
Inclusion criteria for patients with lung malignancy
or lung adenocarcinoma were as follows: Age of ≥18 years; and
pathological diagnosis of de novo lung cancer between
January 2013 and December 2017. Exclusion criteria: Concomitant
cancer at the time of or within 5 years of the lung cancer
diagnosis (except for cancers in situ). For multiple
hospital visits by the same patient, the first visit that met the
inclusion and exclusion criteria was selected.
A total of 3,218 patients coded C34 were registered
in the discharged patients database. Patients who were diagnosed
and treated at other hospitals before January 2013 (n=297), were
without original pathological data (n=21), were <18 years old
(n=1), had mixed tumors (n=36) or tracheal tumors (n=3), and those
suffering from other malignant tumors in the past 5 years (n=65)
were excluded from the analysis. In total, this study included
2,795 eligible lung malignancies, among which 1,349 cases were of
lung adenocarcinoma. There were 807 males and 542 females, with a
median age of 61 years (range, 52–68 years). Eligible patients with
lung adenocarcinoma were grouped by their age at the time of the
initial cancer diagnosis. Targetable genomic mutation sequencing,
including that for epidermal growth factor receptor (EGFR),
anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 receptor
tyrosine kinase (ROS1) and KRAS proto-oncogene GTPase (KRAS), was
recorded as performed in 637 patients. First-line systemic
treatments were categorized as targeted therapy [EGFR-tyrosine
kinase inhibitors (TKIs) and ALK/ROS1-TKIs] and systematic
chemotherapy with or without radiotherapy. In a subsequent analysis
of toxicity, efficacy and prognostic response to treatments, those
who had received targeted therapy without targetable genomic
mutations sequencing and those who had not completed at least two
cycles of chemotherapy were excluded.
Clinical assessments and
follow-up
Generally, radiographic assessments were performed
at baseline, then every 3 months for patients receiving targeted
therapy, every two or three cycles for chemotherapy, and every 3
months thereafter until the progression of disease (PD). Response
Evaluation Criteria in Solid Tumors v.1.1 guidelines were used for
the assessment of response, progression or stability of disease
resulting from systematic treatments (19). Treatment-related adverse events
(TRAEs) were graded according to the Common Terminology Criteria
for Adverse Events, version 4.0 (20). After PD, survival information was
obtained from the medical records or by telephone interview. The
follow-up data cut-off was set as December 1, 2021.
Statistical analysis
Categorical variables were presented as counts
(percentage) and were compared using Pearson's χ2 or
Fisher's exact test, as appropriate. All continuous variables were
tested with Kolmogorov-Smirnov and the Shapiro-Wilk tests,
continuous variables of normal distribution are expressed as the
mean ± standard deviation and were analyzed using a unpaired
t-test, and partial distribution is expressed as the median
(inter-quartile-range) and was analyzed using the Mann-Whitney U
test. The survival probability was estimated by the Kaplan-Meier
method and compared between the two groups using log-rank tests.
Multivariate Cox regression models were applied to evaluate
predictors of survival. Statistical analyses were performed using
SPSS software (version 26.0; IBM Corp.). The results were based on
two-sided tests and P<0.05 was considered to indicate a
statistically significant difference.
Results
Epidemiological characteristics
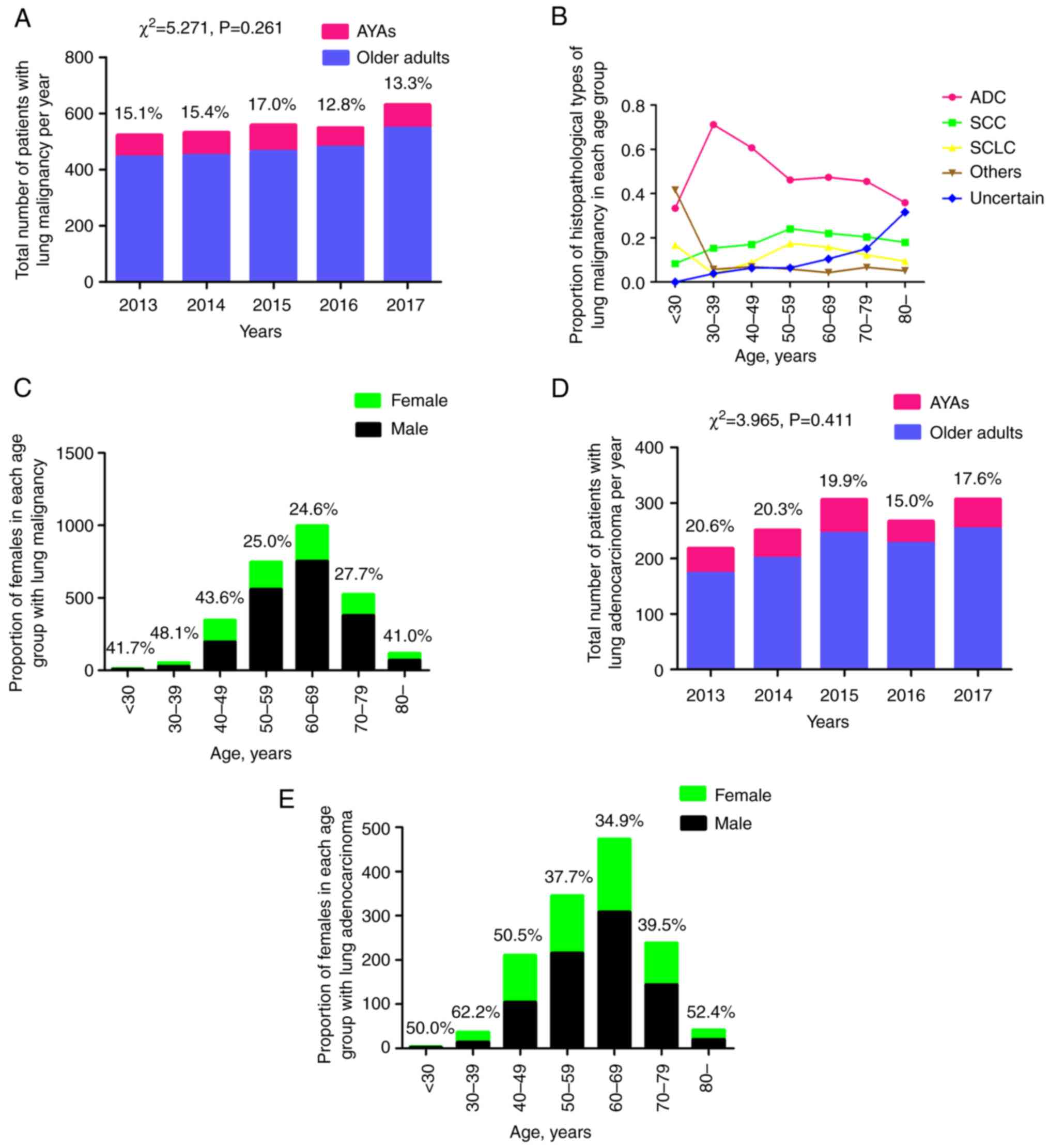
A total of 2,795 patients were pathologically
diagnosed with primary lung malignancy during the 5 years, among
who 410 (14.7%) were aged <50 years at the time of the initial
cancer diagnosis. Notably, overall stability in the ratio of AYAs
was observed from the year 2013 to 2017 (χ2=5.271,
P=0.261) (Fig. 1A). Subsequently,
the percentage of pathological types over different age groups was
analyzed (Fig. 1B). It was shown
that rare pathological types (others), such as carcinoid,
lymphoepithelial carcinoma and sarcoma, were common in patients
<30 years of age (5/12; 41.7%), but the sample size was very
small. The percentage of adenocarcinoma peaked at 30–39 years of
age (71.2%; 37/52), then decreased with increasing age. The
incidence of squamous cell carcinoma increased with age until 60
years old, then slowly decreased with increasing age. The ratio of
small cell lung cancer (SCLC) decreased from 16.7% (2/12) in
patients under 30 years of age to 3.8% (2/52) in the group aged
30–39 years, then increased with age until 60 years old and slowly
decreased again with increasing age. During the study period, 10.5%
(293/2,795) of patients had not been accurately diagnosed by
immunohistochemical staining due to insufficient tumor cells in the
biopsy, bronchoalveolar fluid or hydrothorax, and refused another
invasive examination due to the lack of treatment willingness. The
ratio of females was also analyzed over the five age groups, and it
was shown that the proportion of females dropped markedly in the
groups >50 years of age, with the exception of the group >80
years of age (Fig. 1C).
During the 5 years, 1,349 of the 2,795 lung
malignancies (48.3%) were diagnosed as adenocarcinoma, with AYAs
accounting for 18.6% (251/1,349) of cases. Similarly, the
percentage of AYAs in lung adenocarcinoma remained stable between
2013 and 2017 (χ2=3.965; P=0.411) (Fig. 1D). In each of the three groups aged
<50 years, the proportion of females was ≥50%; the group aged
30–39 years reached the highest percentage of 62.2% (23/37), then
the rate dropped to <40% until the group aged >80 years, in
which the ratio of females was >50% again (Fig. 1E).
Clinical features
Clinical characteristics and treatment patterns of
lung adenocarcinoma were compared between AYAs and older adults
(Table I). Compared with older
adults, AYAs had a significantly higher proportion of females (52.2
vs. 37.4%; P<0.001) and non-smokers (66.9 vs. 41.3%;
P<0.001). More AYAs were treated with first-line chemotherapy
(29.5 vs. 21.4%; P=0.006) and targeted therapy (30.7 vs. 17.6%;
P<0.001), while more older adults did not receive any antitumor
therapy (39.1 vs. 14.7%; P<0.001). There was no difference in
tumor stage between the two age groups. Targetable genomic mutation
sequencing and targeted drugs were not covered by basic medical
insurance until 2017 in China, and cisplatin-containing
chemotherapy was the main available systematic treatment for lung
cancer for 5 years. Our previous study (21) showed that old age was one of the
factors independently associated with patients being untreated, and
fear of the TRAEs of chemotherapy was the top reason.
 | Table I.Clinical characteristics and
treatment patterns of AYAs and older adults with lung
adenocarcinoma. |
Table I.
Clinical characteristics and
treatment patterns of AYAs and older adults with lung
adenocarcinoma.
| Variables | AYAs (n=251) | Older adults
(n=1,098) | P-value |
|---|
| Median age (range),
years | 45 (41–48) | 63 (58–70) |
<0.001a,b |
| Sex, n (%) |
|
|
<0.001a |
|
Male | 120 (47.8) | 687 (62.6) |
|
|
Female | 131 (52.2) | 411 (37.4) |
|
| Smoking status, n
(%) |
|
|
<0.001a |
|
Now/ever | 83 (33.1) | 644 (58.7) |
|
|
Never | 168 (66.9) | 454 (41.3) |
|
| Lobe, n (%) |
|
| 0.192 |
|
Right | 143 (57.0) | 677 (61.7) |
|
|
Left | 105 (41.8) | 398 (36.2) |
|
|
Bilateral | 3 (1.2) | 23 (2.1) |
|
| Location, n
(%) |
|
| 0.087 |
|
Central | 24 (9.6) | 149 (13.6) |
|
|
Peripheral | 227 (90.4) | 949 (86.4) |
|
| Maximal lesion
size, n (%) |
|
| 0.329 |
| ≤3
cm | 86 (34.3) | 322 (29.3) |
|
| ˃3 and
≤5 cm | 116 (46.2) | 575 (52.4) |
|
| ˃5 and
≤7 cm | 41 (16.3) | 172 (15.7) |
|
| ˃7
cm | 8 (3.2) | 29 (2.6) |
|
| TNM stage (8th
AJCC), n (%) |
|
| 0.535 |
| I | 24 (9.6) | 81 (7.4) |
|
| II | 27 (10.8) | 138 (12.6) |
|
|
III | 47 (18.7) | 225 (20.5) |
|
| IV | 153 (60.9) | 654 (59.5) |
|
| First-line
treatment strategies, n (%) |
|
|
|
|
Surgery | 61 (24.3) | 220 (20.0) | 0.133 |
|
Chemotherapy | 74 (29.5) | 235 (21.4) | 0.006a |
|
Targeted therapy | 77 (30.7) | 193 (17.6) |
<0.001a |
| Other
treatments | 2 (0.8) | 21 (1.9) | 0.336 |
|
Untreated | 37 (14.7) | 429 (39.1) |
<0.001a |
In comparing pathological characteristics of the two
age groups that received complete resection (Table SI), AYAs were similar to older
adults in terms of main microscopic pattern, T stage, N stage,
Tumor-Node-Metastasis stage, and the ratio of patients with >5%
of the micropapillary subtype in tumor tissue.
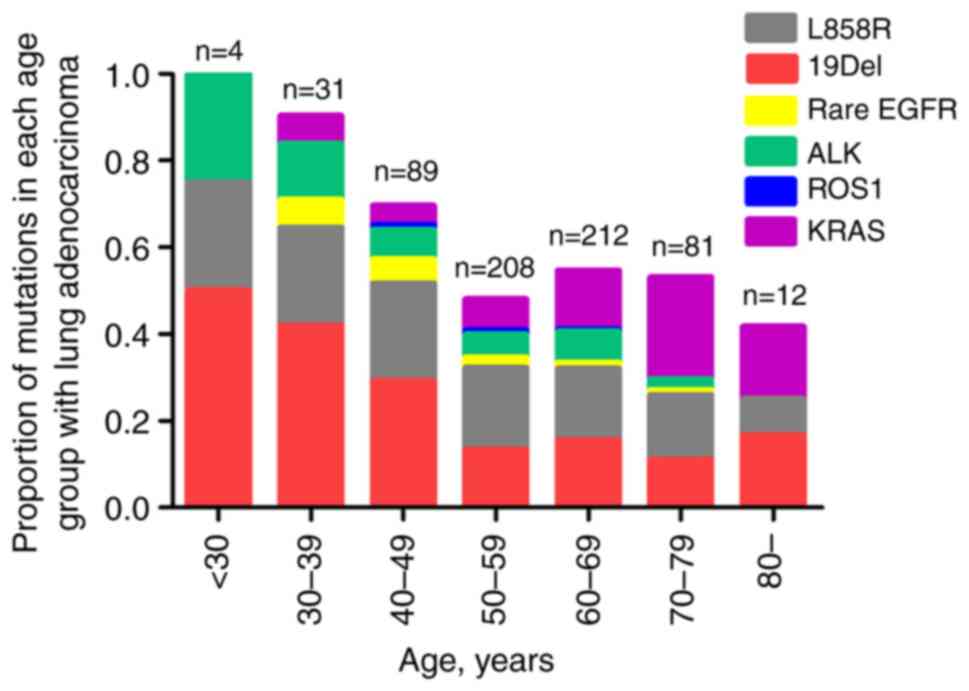
Information on targetable genomic mutations was
obtained in 637 patients (124 AYAs and 513 older adults) (Table II). EGFR mutations (61.3 vs.
32.7%; P<0.001) and ALK rearrangements (11.3 vs. 5.5%; P=0.019)
were more prevalent in AYAs, while KRAS mutations (12.7 vs. 4.8%;
P=0.013) were more prevalent in older adults. Further analysis
showed that EGFR 19Del was more prevalent in AYAs (53.9 vs. 42.9%;
P=0.108), but the difference was not statistically significant,
while EGFR L858R was more common in older adults (51.8 vs. 36.8%;
P=0.030). The analysis revealed that the incidence of targetable
genomic mutations was age-dependent and showed a clear decrease
with increasing age (Fig. 2). In
the youngest AYA patient group between 18 and 29 years of age, all
4 patients harbored targetable genomic mutations, while only 25% of
patients 80 years and older harbored targetable genomic
mutations.
 | Table II.Targetable genomic mutations of lung
adenocarcinoma in AYAs and older adults. |
Table II.
Targetable genomic mutations of lung
adenocarcinoma in AYAs and older adults.
| Variables | AYAs (n=124) | Older adults
(n=513) | P value |
|---|
| Sex, n (%) |
|
|
<0.001a |
|
Male | 49 (39.5) | 295 (57.5) |
|
|
Female | 75 (60.5) | 218 (42.5) |
|
| EGFR, n (%) |
|
|
<0.001a |
|
Mutation | 76 (61.3) | 168 (32.7) | 0.080b |
|
19Del | 41 (53.9) | 72 (42.9) | 0.108 |
|
L858R | 28 (36.8) | 87 (51.8) | 0.030a |
|
Others | 7 (9.2) | 9 (5.4) | 0.260 |
|
Wild-type | 48 (38.7) | 345(67.3) | - |
| ALK, n (%) |
|
| 0.019a |
|
Mutation | 14 (11.3) | 28 (5.5) |
|
|
Wild-type | 110 (88.7) | 485 (94.5) |
|
| ROS1, n (%) |
|
| NA |
|
Mutation | 1 (0.8) | 3 (0.6) |
|
|
Wild-type | 123 (99.2) | 510 (99.4) |
|
| KRAS, n (%) |
|
| 0.013a |
|
Mutation | 6 (4.8) | 65 (12.7) |
|
|
Wild-type | 118 (95.2) | 448 (87.3) |
|
Efficacy and toxicity of systematic
treatment
The objective response rate (ORR) of the two groups
of patients stratified by first-line anti-EGFR, anti-ALK/ROS1
(crizotinib was the only drug approved for first-line use during
the study period) and at least two cycles of cisplatin-containing
chemotherapy was compared, as shown in Table III. There was no significant
difference in ORR between the two groups after first-line anti-EGFR
(70.3 vs. 76.8%; P=0.307) and anti-ALK/ROS1 (84.6 vs. 96.6%;
P=0.222), while there was a higher ORR in AYAs than that of older
adults (35.1 vs. 22.6%; P=0.030) in response to first-line
cisplatin-containing chemotherapy.
 | Table III.Clinical response following systemic
treatment in AYAs and older adults. |
Table III.
Clinical response following systemic
treatment in AYAs and older adults.
| Treatment | AYAs | Older adults | P-value |
|---|
|
Anti-EGFRa |
|
|
|
| CR | 8 (12.5) | 10 (6.1) |
|
| PR | 37 (57.8) | 116 (70.7) |
|
| SD | 15 (23.4) | 17 (10.4) |
|
| PD | 4 (6.3) | 21 (12.8) |
|
|
ORR | 45 (70.3) | 126 (76.8) | 0.307 |
|
Anti-ALK/ROS1b |
|
|
|
| CR | 2 (15.4) | 6 (20.7) |
|
| PR | 9 (69.2) | 22 (75.9) |
|
| SD | 2 (15.4) | 1 (3.4) |
|
| PD | 0 (0.0) | 0 (0.0) |
|
|
ORR | 11 (84.6) | 28 (96.6) | 0.222 |
|
Chemotherapyc,d |
|
|
|
| CR | 2 (2.7) | 4 (1.7) |
|
| PR | 24 (32.4) | 49 (20.9) |
|
| SD | 19 (25.7) | 76 (32.3) |
|
| PD | 26 (35.1) | 84 (35.7) |
|
|
ORR | 26 (35.1) | 53 (22.6) | 0.030e |
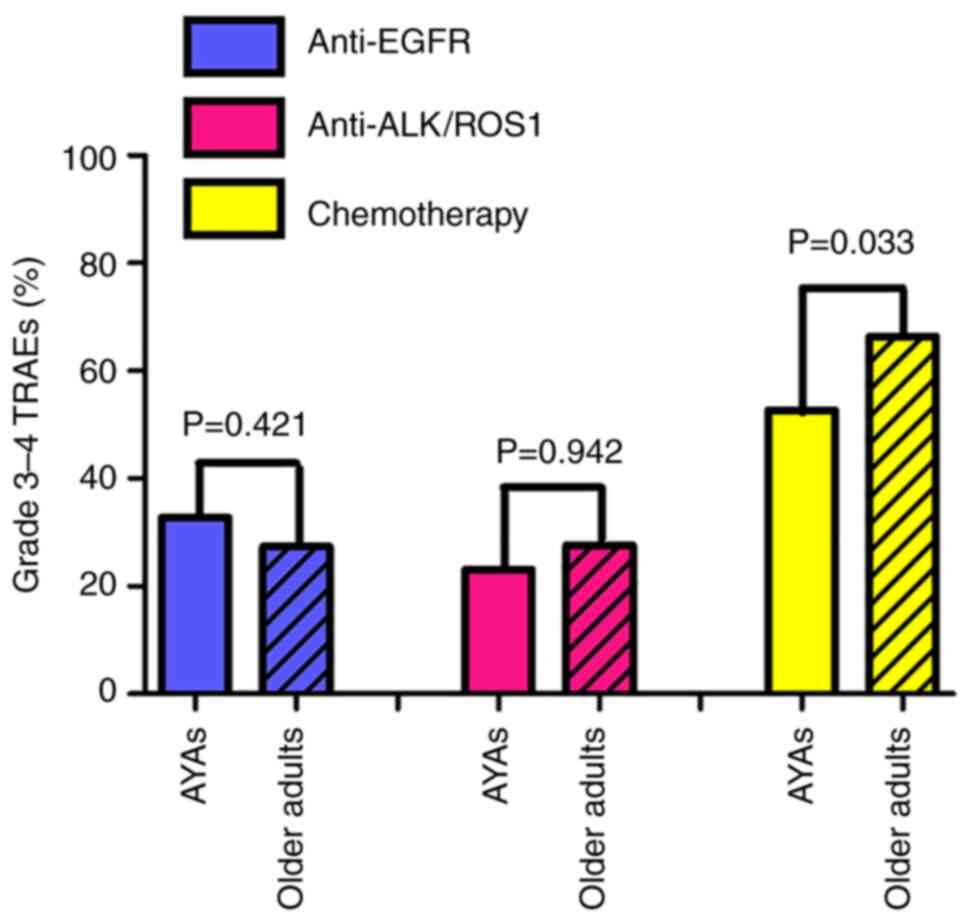
There was no difference in the occurrence of grade
3–4 TRAEs between AYAs and older adults following anti-EGFR (32.8
vs. 27.4%; P=0.421) and anti-ALK/ROS1 (23.1 vs. 27.6%; P=0.942)
treatment. Following treatment with cisplatin-containing
chemotherapy, older adults more frequently developed grade 3–4
TRAEs than AYAs (52.7 vs. 66.4%; P=0.033) (Fig. 3). Furthermore, older adults more
frequently developed grade 3–4 neutropenia than AYAs (14.9 vs.
28.9%; P=0.016), but the difference in the incidence of other grade
3–4 TRAEs between the two groups was not significant (Table SII).
Prognosis and predictors of systematic
treatment
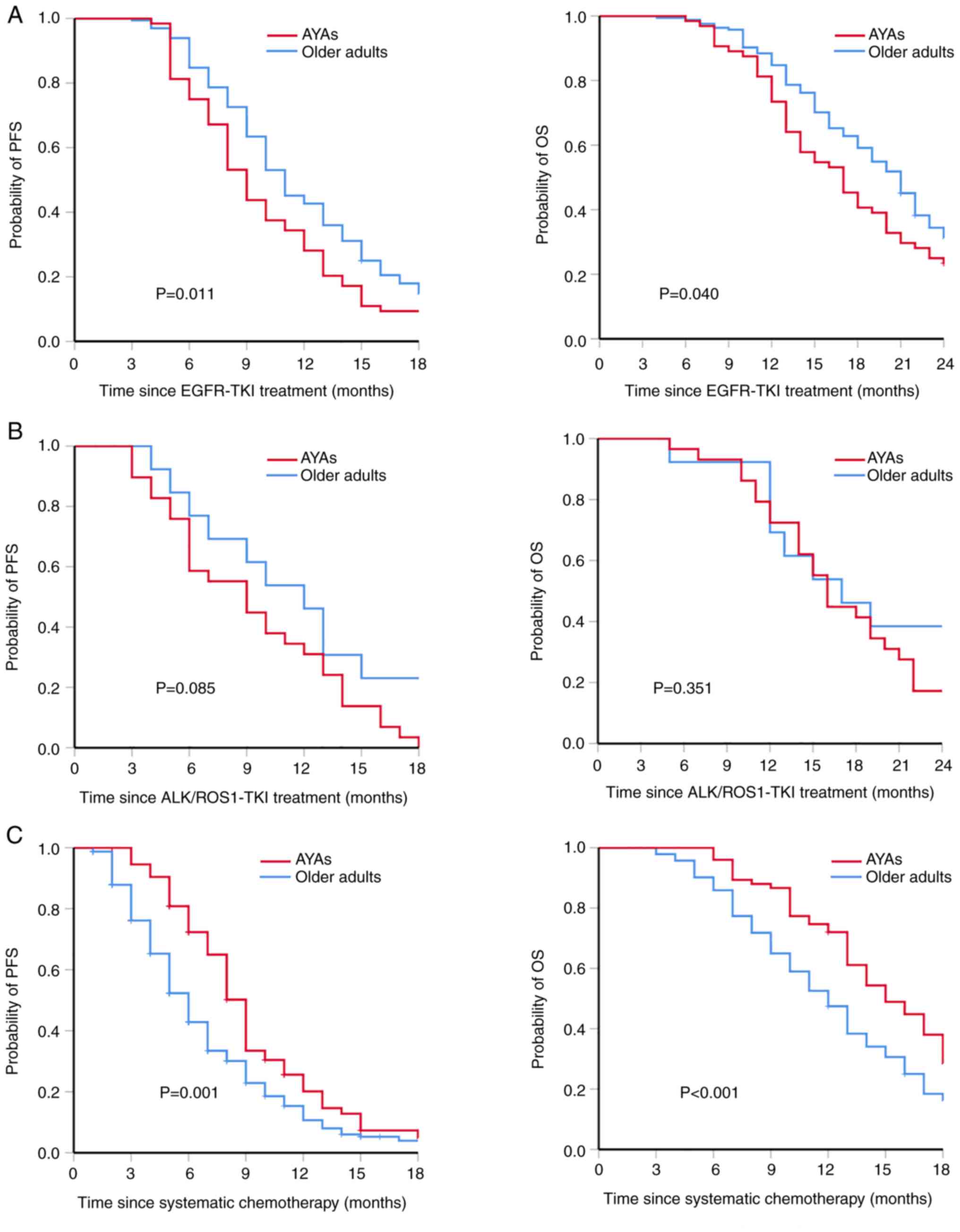
The prognosis of AYAs was significantly worse than
that of older adults treated with first-line anti-EGFR, as shown in
Fig. 4A. The PFS time was
significantly shorter in AYAs than in older adults (median, 9 vs.
11 months; P=0.011). The OS time was also shorter in AYAs than in
older adults (median, 17 vs. 21 months; P=0.040). In the
multivariate survival analysis (Table
IV), bone metastasis (HR, 2.021; 95% CI, 1.471-2.762;
P<0.001), liver metastasis (HR, 5.154; 95% CI, 3.005-8.838;
P<0.001) and the number of metastatic organs (HR, 1.772; 95% CI,
1.482-2.119; P<0.001) were negative predictors of PFS following
first-line EGFR-TKI treatment, rather than age <50 years.
Previous studies have indicated sex-based differences in survival
in lung cancer (22–24). Therefore, sex was added to the
multivariate model, but it was found to have a negligible impact on
the results of the sex-adjusted multivariate model (Table IV). The metastasis patterns of the
two groups were further compared. The results showed that there was
no significant difference in the incidence of bone metastasis (53.1
vs. 45.1%; P=0.277) and liver metastasis (7.8 vs. 7.3%; P=0.898);
however, the number of metastatic organs in AYAs was greater than
that in older adults (P=0.015) (Table
SIII).
 | Table IV.Multivariate Cox regression analysis
for predictors of progression-free survival of patients following
EGFR-tyrosine kinase inhibitor treatment. |
Table IV.
Multivariate Cox regression analysis
for predictors of progression-free survival of patients following
EGFR-tyrosine kinase inhibitor treatment.
|
| Univariate Cox
regression | Multivariate
analysis | Sex-adjusted
multivariate analysis |
|---|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<50 vs. ≥50
years) | 1.385
(1.028-1.866) | 0.032a | 1.276
(0.938-1.737) | 0.121 |
|
|
| Sex (male vs.
female) | 0.939
(0.711-1.240) | 0.657 |
|
|
|
|
| EGFR mutation
site |
|
|
|
|
|
|
|
19del | 1.091
(0.834-1.428) | 0.524 |
|
|
|
|
|
L858R | 0.829
(0.633-1.086) | 0.173 |
|
|
|
|
|
Others | 1.600
(0.944-2.712) | 0.081 |
|
|
|
|
|
Location of tumor (central vs.
peripheral) | 1.491
(0.966-2.303) | 0.071 |
|
|
|
|
| Smoking
behavior (yes vs. no) | 0.865
(0.634-1.181) | 0.361 |
|
|
|
|
| Maximal lesion
size, n (%) |
|
|
|
|
|
|
| ≤3
cm | Reference |
|
|
|
|
|
| ˃3 and
≤5 cm | 0.875
(0.420-1.822) | 0.721 |
|
|
|
|
| ˃5 and
≤7 cm | 0.715
(0.348-1.470) | 0.362 |
|
|
|
|
| ˃7
cm | 0.748
(0.346-1.619) | 0.461 |
|
|
|
|
| Metastatic
organs |
|
|
|
|
|
|
|
Bone | 2.686
(2.017-3.576) |
<0.001a | 2.021
(1.471-2.762) |
<0.001a | 2.074
(1.506-2.858) |
<0.001a |
|
Lung | 1.395
(1.062-1.833) | 0.017a | 1.275
(0.925-1.758) | 0.138 |
|
|
|
Pleura | 1.272
(0.967-1.674) | 0.085 |
|
|
|
|
|
Liver | 3.519
(2.090-5.923) |
<0.001a | 5.154
(3.005-8.838) |
<0.001a | 5.285
(3.075-9.085) |
<0.001a |
|
Brain | 1.103
(0.823-1.479) | 0.511 |
|
|
|
|
|
Renicapsule | 1.694
(1.000-2.872) | 0.050 |
|
|
|
|
| Number
of metastatic organs (reference=0 metastatic organs) | 2.021
(1.721-2.373) |
<0.001a | 1.772
(1.482-2.119) |
<0.001a | 1.764
(1.474-2.112) |
<0.001a |
After first-line anti-ALK/ROS1 treatment, there was
no significant difference in PFS and OS time between the two
groups, as shown in Fig. 4B. The
median PFS time was 9 months (95% CI, 5.9-12.5) in the AYAs and 12
months (95% CI, 8.5-15.5) in older adults (P=0.085). The mOS time
was 16 months (95% CI, 14.3-17.8) in the AYAs and 17 months (95%
CI, 10.0-24.0) in the older adults (P=0.351). Due to the small
sample size, predictors of survival of first-line anti-ALK/ROS1
treatment could not be evaluated.
Compared with older adults, AYAs exhibited PFS and
OS advantages following first-line cisplatin-containing
chemotherapy (Fig. 4C), with a
longer PFS time (median, 9 vs. 6 months; P=0.001) and OS time
(median, 15 vs. 12 months; P<0.001). As patients may receive
different cycles and chemotherapy regimens, multivariate Cox
regression was performed to evaluate predictors of OS rather than
PFS following cisplatin-containing chemotherapy. The results showed
that liver metastasis (HR, 2.635; 95% CI, 1.542-4.504; P<0.001),
brain metastasis (HR, 1.571; 95% CI, 1.058-2.332; P=0.025) and the
number of metastatic organs (HR, 1.723; 95% CI, 1.500-1.979;
P<0.001) were negative predictors of OS, while age <50 years
was a positive predictor (HR, 0.706; 95% CI, 0.539-0.925; P=0.012)
(Table V). The sex-adjusted
results also showed that sex had no impact on the multivariate
analysis of OS in patients who received first-line
cisplatin-containing chemotherapy (Table V).
 | Table V.Multivariate Cox regression analysis
for predictors of overall survival of patients following systematic
chemotherapy. |
Table V.
Multivariate Cox regression analysis
for predictors of overall survival of patients following systematic
chemotherapy.
|
| Univariate Cox
regression | Multivariate
analysis | Sex-adjusted
multivariate analysis |
|---|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<50 vs. ≥50
years) | 0.720
(0.550-0.941) | 0.016a | 0.706
(0.539-0.925) | 0.012a | 0.708
(0.536-0.934) | 0.015a |
| Sex (male vs.
female) | 1.259
(0.958-1.654) | 0.098 |
|
|
|
|
| Location of tumor
(central vs. peripheral) | 1.174
(0.834-1.653) | 0.357 |
|
|
|
|
| Smoking behavior
(yes vs. no) | 1.663
(1.320-2.094) |
<0.001a | 0.960
(0.725-1.272) | 0.778 |
|
|
| Maximal lesion
size, n (%) |
|
|
|
|
|
|
| ≤3
cm | Reference |
|
|
|
|
|
| ˃3 and
≤5 cm | 0.607
(0.299-1.234) | 0.168 |
|
|
|
|
| ˃5 and
≤7 cm | 0.992
(0.505-1.948) | 0.980 |
|
|
|
|
| ˃7
cm | 1.727
(0.867-3.442) | 0.120 |
|
|
|
|
| Metastatic
organs |
|
|
|
|
|
|
|
Bone | 2.177
(1.676-2.829) |
<0.001a | 1.262
(0.817-1.950) | 0.237 |
|
|
|
Lung | 2.104
(1.617-2.737) |
<0.001a | 1.378
(0.906-2.096) | 0.134 |
|
|
|
Pleura | 1.823
(1.352-2.458) |
<0.001a | 0.958
(0.723-1.268) | 0.762 |
|
|
|
Liver | 5.408
(3.238-9.034) |
<0.001a | 2.635
(1.542-4.504) | ˂0.001a | 2.639
(1.542-4.517) |
<0.001a |
|
Brain | 2.914
(2.043-4.157) |
<0.001a | 1.571
(1.058-2.332) | 0.025a | 1.572
(1.058-2.337) | 0.025a |
|
Renicapsule | 1.557
(1.052-2.305) | 0.027a | 1.286
(0.820-2.017) | 0.272 |
|
|
| Number
of metastatic organs (reference=0 metastatic organs) | 1.890
(1.676-2.132) |
<0.001a | 1.723
(1.500-1.979) |
<0.001a | 1.721
(1.496-1.980) |
<0.001a |
Discussion
Adenocarcinoma is the most common pathological type
of lung cancer, accounting for about one-half of all lung cancer
cases (25,26). Together with the other pathological
types of lung cancer, lung adenocarcinoma mainly occurs in older
patients, but it is not uncommon in adolescents or young adults
<50 years old (27,28). The present study observed that AYAs
accounted for 18.6% of lung adenocarcinoma, with a stable tendency
between 2013 and 2017. This study, with its large cohort of
patients with lung adenocarcinoma, explored the incidence, clinical
characteristics, targetable genomic mutations and prognosis of
AYAs. Furthermore, the large number of patients in the cohort
provided statistical power of data analysis on predictors of
survival stratified by first-line treatment patterns. AYAs with
lung adenocarcinoma were more frequently female and non-smokers.
Targetable genomic mutations were more prevalent in AYAs, while
KRAS mutations were more prevalent in older adults. There was no
difference in efficacy and toxicity response to targeted therapy
between AYAs and older adults, but older adults experienced a
higher incidence of grade 3–4 TRAEs and a lower ORR following
chemotherapy. Genomic profiling, efficacy and toxicity response to
chemotherapy translated into distinct first-line treatment
patterns, where more AYAs were treated with targeted therapy and
systematic chemotherapy, while more older adults refused medical
treatment. Patients with lung adenocarcinoma and co-existing bone
metastasis, liver metastasis and a larger number of metastatic
organs, rather than those of a younger age, experienced a shorter
PFS time following first-line EGFR-TKI treatment. By contrast,
there were PFS and OS advantages for AYAs over older adults in
terms of the response to chemotherapy, and an age <50 years was
shown to be a positive predictor of OS.
To date, the historical pattern of a higher
incidence of lung cancer among men has never reversed, which is an
attribute likely to be associated with differences in smoking
behaviors. Consistent with most previous reports (29–31),
the present study found that there was a significantly higher
proportion of females among the AYAs than among the older adults,
and nearly all of the females in this cohort were non-smokers.
Therefore, this sex difference could not be explained by smoking
behavior at all. Some studies have indicated the potential
contribution of second-hand smoke, residential radon gas and
cooking oil fumes to the early onset of lung cancer in females
(30–32), but these hypotheses lack direct
evidence. Some hypotheses have proposed that women may be more
susceptible to the deleterious effects of tobacco carcinogens
(33). However, several
prospective studies have not been able to replicate any results
showing that women were at a higher risk of lung cancer than men at
comparable levels of exposure to cigarette smoking (34,35).
Zhang et al (36) also
found that no smoking-related genomic changes were detected in lung
cancer from passive smoking. Therefore, it is necessary to further
explore other etiologies that could be responsible for the higher
incidence of lung adenocarcinoma in young women compared with young
men.
The present study did not detect a significant
difference in the distribution of histological subtype in
completely resected lung adenocarcinoma between the two age groups
studied. However, the genomic profiling of AYAs was significantly
different from that of older adults. AYAs more frequently harbored
EGFR and ALK mutations compared with older adults, while more older
adults presented with KRAS mutations. Furthermore, the study
revealed that the presence of targetable genomic mutations was
age-dependent and that the incidence decreased with increasing age.
Previous studies have shown conflicting results on the incidence of
EGFR mutation in young patients when compared with the older
population. Some previous Asian studies showed that the incidence
of EGFR mutations in young patients with lung adenocarcinoma was
higher than that of old patients (37,38).
However, a prospective epidemiological survey on EGFR mutations in
Asian patients with lung adenocarcinoma did not find any
significant correlation with age (39). Nevertheless, the study included
patients from seven Asian regions, among which the incidence of
EGFR mutations fluctuates from 22.2% in India to 64.2% in Vietnam.
Therefore, the results of the univariate analysis of age might be
influenced by ethnicity. There were also very few studies that
showed that the incidence of EGFR mutations in young patients was
lower than that in old patients (11,40).
The striking finding of these studies was that the ALK mutation was
the most common targetable genomic mutation in young patients, with
significantly higher levels than those of older patients. In
addition, the present study demonstrated a different distribution
of EGFR mutation genotypes of lung adenocarcinoma between the two
age groups. The study showed that EGFR-19del was comparatively
common in AYAs (but not significantly different), while EGFR-L858R
was more prevalent in older adults, consistent with previous Asian
estimates (11,40,41).
The IPASS and ENSURE studies have shown that a subset of tumors
with EGFR-19del had better clinical outcomes than L858R tumors
following EGFR-TKI treatment (42,43).
Nevertheless, the present study did not find a PFS advantage for
AYAs benefiting from EGFR-19del. The statistically non-significant
difference in the incidence of EGFR-19del between the two age
groups and the fact that AYAs more frequently presented with
uncommon EGFR mutations (9.2 vs. 5.4%) might weaken the advantages
from the genomic subset. The prevalence of ALK rearrangement is as
much as 3–6% in lung adenocarcinoma, ranking only second to EGFR
(44,45). The present findings support those
of most previous studies that found ALK mutation to be more
abundant in AYAs than in older adults. Mutations in EGFR and ALK
may generally be early events during the carcinogenesis of lung
adenocarcinoma, appearing several years before the tumors are
clinically evident (32,46). A possible explanation for
targetable genomic mutations being more prevalent in AYAs than in
older adults may be that mutations of EGFR and ALK are not only the
characteristic of young patients but also the genomic etiologies of
the early onset of cancer.
Due to the lower ratio of patients with targetable
genomic mutations in older adults, cisplatin-containing
chemotherapy is supposed to be a standard treatment according to
clinical practice guidelines. However, in the present study, a
lower proportion of patients receiving chemotherapy and a
significantly higher proportion of untreated patients was observed
in older adults compared with that in AYAs. Stinchcombe et
al (47) previously indicated
that chemotherapy was more toxic to older patients, with shorter OS
time and a higher mortality rate during treatment compared with
that of young patients. The finding of the present study indicated
a similar conclusion that older adults suffered from more grade 3–4
TRAEs resulting from chemotherapy, which may be responsible for a
considerable number of patients remaining untreated. In addition,
the study indeed showed that an age of ≥50 years was one of the
negative predictors of OS response to chemotherapy. A total of
28.9% of older adults suffered from grade 3–4 neutropenia following
chemotherapy, suggesting that older patients receiving chemotherapy
should be cautious of hematotoxicity. Conventional cytotoxic
chemotherapeutic agents suffer from extensive toxicity (48), which in numerous instances has
limited their clinical utilization. In the past decade,
conventional cytotoxic drugs have gradually been supplanted by
chemotherapy-free regimens comprising diverse immunotherapy and/or
targeted agents (49,50). It has been reported that KRAS
signaling may stabilize programmed death-ligand 1 mRNA by
post-transcriptional regulation; therefore, it was identified as a
positive predictive biomarker for tumor response to first-line
immune checkpoint inhibitors, especially when co-existing with
tumor protein p53 mutation (51,52).
Moreover, small molecule antiangiogenic drugs, such as arotinib,
combined with programmed cell death protein 1 blockade, have
achieved encouraging efficacy and manageable toxicity in negative
driver gene mutation non-SCLC (NSCLC) (53). This indicates that
chemotherapy-free regimens should be performed in first-line rather
than second- or later-line treatments of relapsed cancer in old
patients.
Prognosis in young patients with lung adenocarcinoma
compared with that in older patients is controversial. This is
probably influenced by treatment patterns according to the stage
and genomic profiling. In the current study, most of the patients
with lung adenocarcinoma presented with metastatic disease; thus,
systematic treatments, such as targeted therapy and chemotherapy,
were the main first-line treatment patterns. The present study
investigated the predictors and prognosis of lung adenocarcinoma
stratified by treatment patterns. A significantly worse prognosis
was observed for AYAs treated with first-line EGFR-TKIs compared
with older adults. Between 2013 and 2017, only one generation of
EGFR-TKIs, including gefitinib, erlotinib and icotinib, were
approved for first-line treatment in lung cancer with EGFR
mutations. Therefore, the differences in drugs could not be
responsible for the discrepancy in survival. These results were
consistent with previous studies. Although these studies used
different age cut-off values to define AYA patients, their results
showed that young patients with lung cancer have a poor prognosis
even if they harbor EGFR mutations (5,10,12).
The same phenomenon was observed in patients with other tumors.
Panian et al (54) observed
that, although OS time was similar between age groups, younger
individuals with advanced renal cell carcinoma treated with
targeted therapy had a shorter PFS time. The present study showed
that bone metastasis, liver metastasis and the number of metastatic
organs, rather than age, were independent predictors of PFS
following EGFR-TKI treatment. Further analysis revealed that a
higher number of metastatic organs at the time of the initial
cancer diagnosis in AYAs indirectly contributed to a shorter PFS
time. A study by Bryant and Cerfolio (55) not only found that young patients
were more likely to be symptomatic at the time of diagnosis, which
generally indicated an advanced disease stage, but also emphasized
a greater delay in seeking medical treatment in this population. In
addition, Zhang et al (36)
and Durham et al (56)
revealed that cells with activating receptor tyrosine kinase have
an incomparable advantage in terms of growth. Accordingly, the
delay in diagnosis and rapid progress of cancer with EGFR mutations
predisposed patients to a high tumor load, which led to a worse
prognosis. This indicates that more aggressive treatments should be
added to EGFR-TKIs to improve the prognosis of AYAs. Adding the use
of immunotherapy in patients harboring targetable genotypes remains
controversial. However, most published reports found that compared
with EGFR-TKIs alone, EGFR-TKIs combined with platinum plus
pemetrexed improved the PFS and OS time of untreated advanced NSCLC
with EGFR mutations, but possibly increased the toxicity (57–59).
The present study indicated that EGFR-TKIs combined with
chemotherapy should be given higher priority in AYAs for the
following two reasons: i) A worse prognosis of AYAs with EGFR
mutations resulted in a demand for more aggressive treatments; and
ii) the lower incidence of grade 3–4 TRAEs in AYAs receiving
chemotherapy resulted in more favorable tolerability (57,60,61).
There are certain limitations to the present study.
Firstly, there are several limitations inherent to a real-world
retrospective study, including potential misclassification of
diagnostic records, non-standard clinical data on disease staging
and dose reduction in chemotherapy-treated patients to avoid
medical risks. Secondly, this study was conducted at a single
institution, and a single geographic and demographic location
limits the representativeness of the results. Thirdly, in
second-line and subsequent line treatments, the crossover of
targeted therapy and chemotherapy was not taken into account in
this study, which may result in potential unreliability in OS data.
Fourthly, targetable genomic alteration sequencing was not
performed in all patients during the 5 years. Furthermore, some
patients with lung cancer were excluded due to an inaccurate
pathological diagnosis. The proportion of AYAs may have been
overestimated, as older patients were more likely to abandon
treatment.
In conclusion, lung adenocarcinoma in young patients
<50 years of age is a common entity that harbors distinctive
clinical and genomic characteristics. Although targetable genomic
mutations have a higher prevalence in young patients, the AYAs
frequently presented with multi-organ metastasis indirectly
responsible for a worse prognosis following EGFR-TKI treatment. An
age of <50 years is the predominant positive factor that is
associated with OS time in patients receiving first-line
chemotherapy. The present study results support the combination of
EGFR-TKIs and chemotherapy as a potential treatment pattern for
young patients with lung adenocarcinoma and EGFR mutations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and LZ were responsible for study conception and
design. LZ and HL performed the collection, analysis and
interpretation of the data. LZ and SY drafted the manuscript. LZ,
HL and SY confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Institutional
Ethics Committee of YueBei People's Hospital and is not considered
subject to the Medical Research Involving Human Subjects Act.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lara MS, Brunson A, Wun T, Tomlinson B, Qi
L, Cress R, Gandara DR and Kelly K: Predictors of survival for
younger patients less than 50 years of age with non-small cell lung
cancer (NSCLC): A California cancer registry analysis. Lung Cancer.
85:264–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inoue M, Okumura M, Sawabata N, Miyaoka E,
Asamura H, Yoshino I, Tada H, Fujii Y, Nakanishi Y, Eguchi K, et
al: Clinicopathological characteristics and surgical results of
lung cancer patients aged up to 50 years: The Japanese lung cancer
registry study 2004. Lung Cancer. 83:246–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam S, Pawlish K, Gadgeel S, Demers
R and Kalemkerian GP: Lung cancer in young patients: Analysis of a
surveillance, epidemiology, and end results database. J Clin Oncol.
16:651–657. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang S, Song Z and Cheng G: Genomic
alterations and survival in young patients aged under 40 years with
completely resected non-small cell lung cancer. Ann Transl Med.
7:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bratova M, Brat K, Hurdalkova K, Barinova
M, Drosslerova M, Kultan J, Wanke M, Koubkova L, Krejci J and
Svaton M: Lung cancer versus ‘young cancer’: Is non-small cell lung
cancer in young patients a different entity? J Adolesc Young Adult
Oncol. Nov 2–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arnold BN, Thomas DC, Rosen JE, Salazar
MC, Blasberg JD, Boffa DJ, Detterbeck FC and Kim AW: Lung cancer in
the very young: Treatment and survival in the national cancer data
base. J Thorac Oncol. 11:1121–1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subramanian J, Morgensztern D, Goodgame B,
Baggstrom MQ, Gao F, Piccirillo J and Govindan R: Distinctive
characteristics of non-small cell lung cancer (NSCLC) in the young:
A surveillance, epidemiology, and end results (SEER) analysis. J
Thorac Oncol. 5:23–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viñal D, Martínez D, Higuera O and de
Castro J: Genomic profiling in non-small-cell lung cancer in young
patients. A systematic review. ESMO Open. 6:1000452021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garrana SH, Dagogo-Jack I, Cobb R, Kuo AH,
Mendoza DP, Zhang EW, Heeger A, Sequist LV and Digumarthy SR:
Clinical and imaging features of non-small-cell lung cancer in
young patients. Clin Lung Cancer. 22:23–31. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Hu X, Wu H, Liu J, Mu X, Wu H and
Zhao Y: Unique profiles of targetable genomic alterations and
prognosis in young Chinese patients with lung adenocarcinoma.
Pathol Res Pract. 215:1524072019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sacher AG, Dahlberg SE, Heng J, Mach S,
Jänne PA and Oxnard GR: Association between younger age and
targetable genomic alterations and prognosis in non-small-cell lung
cancer. JAMA Oncol. 2:313–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan X, Lv T, Zhang F, Fan H, Liu H and
Song Y: Frequent genomic alterations and better prognosis among
young patients with non-small-cell lung cancer aged 40 years or
younger. Clin Transl Oncol. 20:1168–1174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia W, Wang A, Jin M, Mao Q, Xia W, Dong
G, Chen B, Ma W, Xu L and Jiang F: Young age increases risk for
lymph node positivity but decreases risk for non-small cell lung
cancer death. Cancer Manag Res. 10:41–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu M, Tan J, Liu Z, Li L, Zhang H, Zhao D,
Li B, Gao X, Che N and Zhang T: Comprehensive comparative molecular
characterization of young and old lung cancer patients. Front
Oncol. 11:8068452021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G,
Huang Y, Wang X, Wu N, Zhang G, Zheng X, et al: China national lung
cancer screening guideline with low-dose computed tomography (2018
version). Zhongguo Fei Ai Za Zhi. 21:67–75. 2018.(In Chinese).
PubMed/NCBI
|
|
17
|
Zhou QH, Fan YG, Bu H, Wang Y, Wu N, Huang
YC, Wang G, Wang XY and Qiao YL: China national lung cancer
screening guideline with low-dose computed tomography (2015
version). Thorac Cancer. 6:812–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Expert Panel on Thoracic Imaging, . de
Groot PM, Chung JH, Ackman JB, Berry MF, Carter BW, Colletti PM,
Hobbs SB, McComb BL, Movsas B, et al: ACR appropriateness
criteria® noninvasive clinical staging of primary lung
cancer. J Am Coll Radiol. 16 (5 Suppl):S184–S195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morse B, Jeong D, Ihnat G and Silva AC:
Pearls and pitfalls of response evaluation criteria in solid tumors
(RECIST) v1.1 non-target lesion assessment. Abdom Radiol (NY).
44:766–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Du R, Huang M, Wan R, Li W and
Zhou L: Analysis of sociodemographic and clinical factors
influencing the treatment compliance of patients with lung cancer.
J Pract Med. 36:2714–2719. 2020.(In Chinese).
|
|
22
|
Pinto JA, Vallejos CS, Raez LE, Mas LA,
Ruiz R, Torres-Roman JS, Morante Z, Araujo JM, Gómez HL, Aguilar A,
et al: Gender and outcomes in non-small cell lung cancer: An old
prognostic variable comes back for targeted therapy and
immunotherapy? ESMO Open. 3:e0003442018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ragavan M and Patel MI: The evolving
landscape of sex-based differences in lung cancer: A distinct
disease in women. Eur Respir Rev. 31:2101002022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mederos N, Friedlaender A, Peters S and
Addeo A: Gender-specific aspects of epidemiology, molecular
genetics and outcome: Lung cancer. ESMO Open. 5 (Suppl
4):e0007962020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campbell JD, Alexandrov A, Kim J, Wala J,
Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et
al: Distinct patterns of somatic genome alterations in lung
adenocarcinomas and squamous cell carcinomas. Nat Genet.
48:607–616. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tavernari D, Battistello E, Dheilly E,
Petruzzella AS, Mina M, Sordet-Dessimoz J, Peters S, Krueger T,
Gfeller D, Riggi N, et al: Nongenetic evolution drives lung
adenocarcinoma spatial heterogeneity and progression. Cancer
Discov. 11:1490–1507. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang J, Deng Y, Tin MS, Lok V, Ngai CH,
Zhang L, Lucero-Prisno DE III, Xu W, Zheng ZJ, Elcarte E, et al:
Distribution, risk factors, and temporal trends for lung cancer
incidence and mortality: A global analysis. Chest. 161:1101–1111.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galvez-Nino M, Ruiz R, Pinto JA, Roque K,
Mantilla R, Raez LE and Mas L: Lung cancer in the young. Lung.
198:195–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomas A, Chen Y, Yu T, Jakopovic M and
Giaccone G: Trends and characteristics of young non-small cell lung
cancer patients in the United States. Front Oncol. 5:1132015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fidler-Benaoudia MM, Torre LA, Bray F,
Ferlay J and Jemal A: Lung cancer incidence in young women vs.
young men: A systematic analysis in 40 countries. Int J Cancer.
147:811–819. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruano-Ravina A, Varela Lema L, García
Talavera M, García Gómez M, González Muñoz S, Santiago-Pérez MI,
Rey-Brandariz J, Barros-Dios J and Pérez-Ríos M: Lung cancer
mortality attributable to residential radon exposure in Spain and
its regions. Environ Res. 199:1113722021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Risch HA, Howe GR, Jain M, Burch JD,
Holowaty EJ and Miller AB: Are female smokers at higher risk for
lung cancer than male smokers? A case-control analysis by
histologic type. Am J Epidemiol. 138:281–293. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freedman ND, Leitzmann MF, Hollenbeck AR,
Schatzkin A and Abnet CC: Cigarette smoking and subsequent risk of
lung cancer in men and women: Analysis of a prospective cohort
study. Lancet Oncol. 9:649–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bain C, Feskanich D, Speizer FE, Thun M,
Hertzmark E, Rosner BA and Colditz GA: Lung cancer rates in men and
women with comparable histories of smoking. J Natl Cancer Inst.
96:826–834. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang T, Joubert P, Ansari-Pour N, Zhao W,
Hoang PH, Lokanga R, Moye AL, Rosenbaum J, Gonzalez-Perez A,
Martínez-Jiménez F, et al: Genomic and evolutionary classification
of lung cancer in never smokers. Nat Genet. 53:1348–1359. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou H, Zhang C, Qi X, Zhou L, Liu D, Lv H,
Li T, Sun D and Zhang X: Distinctive targetable genotypes of
younger patients with lung adenocarcinoma: A cBioPortal for cancer
genomics data base analysis. Cancer Biol Ther. 21:26–33. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He CH, Shih JF, Lai SL and Chen YM:
Non-small cell lung cancer in the very young: Higher EGFR/ALK
mutation proportion than the elder. J Chin Med Assoc. 83:461–465.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka K, Hida T, Oya Y, Yoshida T,
Shimizu J, Mizuno T, Kuroda H, Sakakura N, Yoshimura K, Horio Y, et
al: Unique prevalence of oncogenic genetic alterations in young
patients with lung adenocarcinoma. Cancer. 123:1731–1740. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ye T, Pan Y, Wang R, Hu H, Zhang Y, Li H,
Wang L, Sun Y and Chen H: Analysis of the molecular and
clinicopathologic features of surgically resected lung
adenocarcinoma in patients under 40 years old. J Thorac Dis.
6:1396–1402. 2014.PubMed/NCBI
|
|
42
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong
Z, Lu S, Cheng Y, Han B, Chen L, et al: First-line erlotinib versus
gemcitabine/cisplatin in patients with advanced EGFR
mutation-positive non-small-cell lung cancer: Analyses from the
phase III, randomized, open-label, ENSURE study. Ann Oncol.
26:1883–1889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang WF, Fu R, Liang Y, Lin JS, Qiu ZB, Wu
YL and Zhong WZ: Genomic evolution of lung cancer metastasis:
Current status and perspectives. Cancer Commun (Lond).
41:1252–1256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stinchcombe TE, Zhang Y, Vokes EE,
Schiller JH, Bradley JD, Kelly K, Curran WJ Jr, Schild SE, Movsas
B, Clamon G, et al: Pooled analysis of individual patient data on
concurrent chemoradiotherapy for stage III non-small-cell lung
cancer in elderly patients compared with younger patients who
participated in US national cancer institute cooperative group
studies. J Clin Oncol. 35:2885–2892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Y, Zhang Y, Guo G, Cai X, Yu H, Cai
Y, Zhang B, Hong S and Zhang L: Nivolumab plus ipilimumab versus
pembrolizumab as chemotherapy-free, first-line treatment for
PD-L1-positive non-small cell lung cancer. Clin Transl Med.
10:107–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Klener P Jr, Etrych T and Klener P:
Biological therapy of hematologic malignancies: Toward a
chemotherapy-free era. Curr Med Chem. 26:1002–1018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coelho MA, de Carné, Trécesson S, Rana S,
Zecchin D, Moore C, Molina-Arcas M, East P, Spencer-Dene B, Nye E,
Barnouin K, et al: Oncogenic RAS signaling promotes tumor
immunoresistance by stabilizing PD-L1 mRNA. Immunity.
47:1083–1099.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Adderley H, Blackhall FH and Lindsay CR:
KRAS-mutant non-small cell lung cancer: Converging small molecules
and immune checkpoint inhibition. EBioMedicine. 41:711–716. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang X, Zeng L, Li Y, Xu Q, Yang H,
Lizaso A, Mao X, Jin R, Zeng Y, Li Q, et al: Anlotinib combined
with PD-1 blockade for the treatment of lung cancer: A real-world
retrospective study in China. Cancer Immunol Immunother.
70:2517–2528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Panian J, Lin X, Simantov R, Derweesh I,
Choueiri TK and McKay RR: The impact of age and gender on outcomes
of patients with advanced renal cell carcinoma treated with
targeted therapy. Clin Genitourin Cancer. 18:e598–e609. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bryant AS and Cerfolio RJ: Differences in
outcomes between younger and older patients with non-small cell
lung cancer. Ann Thorac Surg. 85:1735–1739. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Durham BH, Lopez Rodrigo E, Picarsic J,
Abramson D, Rotemberg V, De Munck S, Pannecoucke E, Lu SX, Pastore
A, Yoshimi A, et al: Activating mutations in CSF1R and additional
receptor tyrosine kinases in histiocytic neoplasms. Nat Med.
25:1839–1842. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Noronha V, Patil VM, Joshi A, Menon N,
Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, et al:
Gefitinib versus gefitinib plus pemetrexed and carboplatin
chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 38:124–136.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang JC, Cheng Y, Murakami H, Yang PC, He
J, Nakagawa K, Kang JH, Kim JH, Hozak RR, Nguyen TS, et al: A
randomized phase 2 study of gefitinib with or without pemetrexed as
first-line treatment in nonsquamous NSCLC With EGFR mutation: Final
overall survival and biomarker analysis. J Thorac Oncol. 15:91–100.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cheng Y, Murakami H, Yang PC, He J,
Nakagawa K, Kang JH, Kim JH, Wang X, Enatsu S, Puri T, et al:
Randomized phase II trial of gefitinib with and without pemetrexed
as first-line therapy in patients with advanced nonsquamous
non-small-cell lung cancer with activating epidermal growth factor
receptor mutations. J Clin Oncol. 34:3258–3266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hosomi Y, Morita S, Sugawara S, Kato T,
Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et
al: Gefitinib alone versus gefitinib plus chemotherapy for
non-small-cell lung cancer with mutated epidermal growth factor
receptor: NEJ009 study. J Clin Oncol. 38:115–123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J,
Gu A, Zhong H, Wang H, Zhang X, et al: Combination of chemotherapy
and gefitinib as first-line treatment for patients with advanced
lung adenocarcinoma and sensitive EGFR mutations: A randomized
controlled trial. Int J Cancer. 141:1249–1256. 2017. View Article : Google Scholar : PubMed/NCBI
|