Introduction
Oral cancer is one of the 20 most common cancer
types globally, accounting for 377,713 new cases and 177,757 deaths
in 2020 (1). The high mortality
rate and the disfigurement that survivors may suffer account for a
rise in the global public health burden (1). Oral cancer is widespread in South,
Central and Southeast Asia, including Indonesia (2). Global Cancer Observatory data
reported that the prevalence of oral cancer in Indonesia ranked
17th among all cancer types and 15th for deaths due to cancer in
2020 (3). Identifying the
incidence of oral cancer is essential for understanding the pattern
of the disease within populations.
Oral squamous cell carcinoma (OSCC) constitutes
>90% of all oral cancer cases (4). OSCC presents as an abnormal
proliferation of cells in the squamous layer of the epithelium,
with OSCC cells depicting varying grades of resemblance with normal
epithelial cells (5). Evaluation
of histological characteristics serves a vital role in diagnosing
resected tumor specimens, and efforts have been undertaken to
predict clinical outcomes and therapeutic responses using these
(6). Numerous studies have
reported that parameters involved include size and primary site of
the tumor, Tumor-Node-Metastasis (TNM) staging, tumor
differentiation and lymphovascular invasion (LVI) (4,6–8).
Moreover, the increasing frequency of OSCC among young patients in
several regions should attract more attention to this disease
(7). It has been reported that
OSCC biological behavior in young patients differs from that in
patients with advanced age (8).
However, this concept remains controversial and requires further
investigation into prognosis-related factors (9). Two of the most definite
prognosis-related factors to have been reported are advanced stage
and invasion of surgical margins (10,11),
which can be predicted by understanding the role of
clinicopathological characteristics of OSCC. However, these
parameters have not been widely investigated.
Although the incidence of OSCC has been documented
with considerable regional variations (12), Indonesian studies of OSCC
epidemiology are still lacking. The increasing prevalence of OSCC
among Asian countries (13–16)
has demonstrated the value of profiling Indonesian OSCC
epidemiology to give a new perspective on this disease and
contribute to better prognosis and therapy planning. Previous
studies in Indonesia (17–20) did not report a long study period
and did not highlight the role of examining histopathological
features. These studies did not assess contributing factors related
to advanced-stage cancer and invasion of surgical margins in
resected cases. Therefore, the present retrospective study aimed to
identify the demographic, clinical and histopathological
characteristics of patients with OSCC based on 10 years of cancer
registry data in the largest referral hospital in Indonesia, and
investigate distinct clinicopathological characteristics of OSCC
according to sex and age. Furthermore, a comparative analysis was
performed to obtain tumor subsite-specific patterns according to
histological differentiation, and to assess the pivotal role of LVI
in nodal and distant metastasis. A multivariate logistic regression
analysis based on different clinicopathological characteristics of
patients and tumors was performed to determine the predictors of
advanced cancer staging and positive surgical margins in OSCC.
Materials and methods
Study design, patients, specimens and
inclusion/exclusion criteria
A retrospective analysis of 581 cases of OSCC that
underwent a histopathological examination was performed in the
present study. Data on the characteristics of subjects with a
primary oral cancer diagnosis defined as International
Classification of Diseases (ICD) 10th revision (ICD-10) codes
C01-C06 (21) between January 2011
and December 2020 were retrieved from the Dr Cipto Mangunkusumo
Hospital (Jakarta, Indonesia). The data were collected from patient
clinical records, slide archives, and hematoxylin and eosin-stained
tissue blocks. To be included in the present study, patients had to
be diagnosed with OSCC, have undergone primary surgery, and have
had the diagnosis of OSCC confirmed by presurgical and postsurgical
examinations. All data were then reviewed to confirm the inclusion
of data on all of the investigated variables. Specimen slides were
doubly reassessed to confirm the diagnosis independently and the
final agreed diagnosis was used. Patients with recurrent disease on
the initial presentation, those with changed diagnoses after
re-examination and those whose slides were missing or duplicated
due to multiple specimen-taking procedures on the same patient,
were excluded from the study. Fig.
1 presents a flowchart of how samples were recruited and
analyzed. All the included cases were subjected to the analysis of
demography, clinicopathological characteristics and features
associated with prognosis.
Ethical approval
The present study was approved by the Ethics
Committee of the Faculty of Medicine of the University of Indonesia
and Dr Cipto Mangunkusumo Hospital (Jakarta, Indonesia; approval
no., KET-178/UN2.F1/ETIK/PPM.00.02/2021).
Patient demographic and
clinicopathological characteristics
Patient demographic and clinicopathological
characteristics were retrieved from histopathological reports,
including registry year, age, sex, tumor subsites, keratinization
status, World Health Organization (WHO) histological
differentiation (22), Bryne's
(1992) cellular differentiation score (23), clinical TNM staging (24), LVI (22), and invasion of surgical margins
(22). Registry year was used to
group patients per 5-year period and per year. The age of the
patients was used to group patients into eight groups with a
10-year range. When specifically assessing OSCC in young patients,
a cut-off age of ≤45 years was used to determine if a patient was
of young, as reported in previous studies (25,26).
The procedure via which specimens were obtained was
divided into three categories: Resection, biopsy and excision.
Resection was classified as surgery to remove part or all of an
organ, the tumor, adjacent tissues and surrounding lymph nodes
(LNs). Biopsy was classified as the removal of cells or tissues;
this could be an incisional biopsy where a cut was made in the skin
to remove a sample of aberrant tissue or a portion of a lump or
suspicious region, or a needle biopsy where a sample of tissue or
fluid was extracted with a needle. However, the needle biopsy was
not used to obtain a sample in this study. Excision included an
excisional biopsy or wide local incision and was classified as a
surgical procedure that entailed the removal of a whole lump or
suspicious region that had to include some normal-appearing/healthy
tissue around it. In our institution, not every patient was
eligible to undergo optimal resection. Therefore, excision was
occasionally preferable for specific reasons, such as a challenging
and complicated location, advanced-stage cancer when the tumor was
widespread, debulking to make a resection possible or in palliative
care. To be noted, in the biopsy procedure, we could not fully
assess margins and LN involvement.
The tumor subsites and keratinization status were
coded according to ICD-10 and WHO classifications (21). The histological differentiation of
lesions was classified into three categories: i)
Well-differentiated; ii) moderately differentiated; and iii) poorly
differentiated (27). The degree
of cellular differentiation was classified using Bryne's (1992)
system as 4–8 (Grade I), 9–12 (Grade II) and 13–16 (Grade III)
(23). The surgical margins and
LVI were only assessed using the resection specimen and not in the
samples obtained from biopsy or excision. Negative margins were
defined as those with resection margins of ≥5 mm, and positive
margins as those with the tumor still involved (<1 mm) or close
to (1–5 mm) healthy tissue, based on several previous studies
(28–30). Clinical TNM staging of patients who
underwent operative procedures were categorized based on the
criteria published by the 8th American Joint Committee on Cancer
(31). For multivariate analysis,
cases were more simply ranked into early stage (I–II) and
advanced-stage (III–IV) OSCC, using the same grouping method as in
a previous study (32).
Statistical analysis
The data were analyzed using the χ2,
Fisher's exact test, or Kruskal-Wallis test with post hoc
Mann-Whitney U test as appropriate, using SPSS v24.0 software (IBM
Corp.). The demographic and clinicopathological profiles of the
parameters assessed were made into frequencies and percentages for
categorical parameters and mean ± standard deviations for
continuous parameters; they were primarily presented as
cross-tabulations to create descriptive statistics. The findings
were then presented in the form of frequency tables. The
clinicopathological factors were analyzed via bivariate analysis
using χ2 or Fisher's exact tests with Mantel-Haenszel
common odds ratio (OR) estimate. Variables that were significantly
(P≤0.20) associated with the groups of interest (advanced-stage
OSCC and invaded surgical margin status) in the bivariate analysis
were analyzed using a stepwise and backward multiple logistic
regression to produce an OR between the factors that contributed to
the condition of the disease (33,34).
P<0.05 was considered to indicate a statistically significant
difference, with a 95% confidence interval (CI). To evaluate the
performance and externally validate the risk-factor model, the fit
of the data to the model was calibrated using the Hosmer-Lemeshow
test and discrimination values were assessed using receiver
operating characteristic (ROC) and area under the receiver
operating characteristic curve (AUC) (33). The quality of the predictive model
was classified based on the AUC value as excellent (0.9-1.0), very
good (0.8-0.9), good (0.7-0.8), satisfactory (0.6-0.7) or
unsatisfactory (0.5-0.6) (35).
The research methods and results were written and presented
according to the Strengthening the Reporting of Observational
Studies in Epidemiology reporting guidelines for cross-sectional
studies (36).
Results
Characteristics and
clinicopathological features of all included patients
The distribution of patient demographic and
clinicopathological characteristics is presented in Table I. A greater number of OSCC cases
occurred in the second interval of the assessed period (2016–2020),
demonstrating an increase of 5.6% from the previous 5-year period.
A total of 581 subjects with a mean age of 50.77±13.64 years were
included (age range, 19–99 years old. The mean age of males was
49.74±14.18 years and for females the mean age was 50.99±13.75
years. Patients with stage I–II cancer had a mean age of
53.67±15.07 years and the mean age for patients with stage III–IV
cancer was 49.74±13.66 years. Of the total cases, 36.1% were
patients ≤45 years and 52.8% were male patients. The tongue was the
most commonly affected subsite (68.7%), followed by mouth not
otherwise specified (NOS; 14.1%) and the palate (6.7%). Most tumors
demonstrated keratinization (84.7%). Based on histopathological
parameters, the majority of tumors were well-differentiated
(52.0%), with Bryne's score grade I (53.2%) and were resected
(49.7%). The demographic and clinicopathological profiles between
young and old patients were similar, yet a significant difference
between the sexes was demonstrated with regard to tumor subsites
(P=0.002).
 | Table I.Characteristics and
clinicopathological features of all included patients (n=581). |
Table I.
Characteristics and
clinicopathological features of all included patients (n=581).
|
| Sex |
| Age |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| Male (n=307) | Female (n=274) |
| Young, ≤45 years
(n=210) | Old, >45 years
(n=371) |
| Total (n=581) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | n | % | n | % | P-value | n | % | n | % | P-value | n | % |
|---|
| Year of
registration |
|
|
|
| 0.837a |
|
|
|
| 0.598a |
|
|
|
2011 | 33 | 10.7 | 20 | 7.3 |
| 20 | 9.5 | 33 | 8.9 |
| 53 | 9.1 |
|
2012 | 24 | 7.8 | 25 | 9.1 |
| 15 | 7.1 | 34 | 9.2 |
| 49 | 8.4 |
|
2013 | 23 | 7.5 | 21 | 7.7 |
| 11 | 5.2 | 33 | 8.9 |
| 44 | 7.6 |
|
2014 | 28 | 9.1 | 19 | 6.9 |
| 17 | 8.1 | 30 | 8.1 |
| 47 | 8.1 |
|
2015 | 26 | 8.5 | 26 | 9.5 |
| 18 | 8.6 | 34 | 9.2 |
| 52 | 9.0 |
|
2016 | 18 | 5.9 | 21 | 7.7 |
| 12 | 5.7 | 27 | 7.3 |
| 39 | 6.7 |
|
2017 | 44 | 14.3 | 43 | 15.7 |
| 33 | 15.7 | 54 | 14.6 |
| 87 | 15.0 |
|
2018 | 41 | 13.4 | 37 | 13.5 |
| 36 | 17.1 | 42 | 11.3 |
| 78 | 13.4 |
|
2019 | 26 | 8.5 | 28 | 10.2 |
| 21 | 10.0 | 33 | 8.9 |
| 54 | 9.3 |
|
2020 | 44 | 14.3 | 34 | 12.4 |
| 27 | 12.9 | 51 | 13.7 |
| 78 | 13.4 |
| Interval of
registry year |
|
|
|
| 0.445a |
|
|
|
| 0.186a |
|
|
|
2011-2015 | 134 | 43.6 | 111 | 40.5 |
| 81 | 38.6 | 164 | 44.2 |
| 245 | 42.2 |
|
2016-2020 | 173 | 56.4 | 163 | 59.5 |
| 129 | 61.4 | 207 | 55.8 |
| 336 | 57.8 |
| Age, years |
|
|
|
| 0.150a |
|
|
|
|
|
|
|
|
11-20 | 1 | 0.3 | 1 | 0.4 |
|
|
|
|
|
| 2 | 0.3 |
|
21-30 | 22 | 7.2 | 18 | 6.6 |
|
|
|
|
|
| 40 | 6.9 |
|
31-40 | 46 | 15.0 | 49 | 17.9 |
|
|
|
|
|
| 95 | 16.4 |
|
41-50 | 79 | 25.7 | 60 | 21.9 |
|
|
|
|
|
| 139 | 23.9 |
|
51-60 | 76 | 24.8 | 86 | 31.4 |
|
|
|
|
|
| 162 | 27.9 |
|
61-70 | 65 | 21.2 | 37 | 13.5 |
|
|
|
|
|
| 102 | 17.6 |
|
71-80 | 14 | 4.6 | 20 | 7.3 |
|
|
|
|
|
| 34 | 5.9 |
|
>80 | 4 | 1.3 | 3 | 1.1 |
|
|
|
|
|
| 7 | 1.2 |
| Age
classification |
|
|
|
| 0.858a |
|
|
|
|
|
|
|
| Young,
≤45 years | 112 | 36.5 | 98 | 35.8 |
|
|
|
|
|
| 210 | 36.1 |
| Old,
>45 years | 195 | 63.5 | 176 | 64.2 |
|
|
|
|
|
| 371 | 63.9 |
| Tumor subsites |
|
|
|
| 0.002a |
|
|
|
| 0.070a |
|
|
|
Tongue | 203 | 66.1 | 196 | 71.5 |
| 157 | 74.8 | 242 | 65.2 |
| 399 | 68.7 |
| Mouth
NOS | 39 | 12.7 | 43 | 15.7 |
| 19 | 9.0 | 63 | 17.0 |
| 82 | 14.1 |
|
Palate | 31 | 10.1 | 8 | 2.9 |
| 16 | 7.6 | 23 | 6.2 |
| 39 | 6.7 |
|
Gingiva | 20 | 6.5 | 8 | 2.9 |
| 11 | 5.2 | 17 | 4.6 |
| 28 | 4.8 |
|
Lip | 8 | 2.6 | 9 | 3.3 |
| 3 | 1.4 | 14 | 3.8 |
| 17 | 2.9 |
| Buccal
mucosa | 3 | 1.0 | 9 | 3.3 |
| 3 | 1.4 | 9 | 2.4 |
| 12 | 2.1 |
|
FOM | 3 | 1.0 | 1 | 0.4 |
| 1 | 0.5 | 3 | 0.8 |
| 4 | 0.7 |
| Keratinization |
|
|
|
| 0.438a |
|
|
|
| 0.803a |
|
|
|
Yes | 265 | 86.3 | 227 | 82.8 |
| 180 | 85.7 | 312 | 84.1 |
| 492 | 84.7 |
| No | 35 | 11.4 | 37 | 13.5 |
| 25 | 11.9 | 47 | 12.7 |
| 72 | 12.4 |
|
Non-specific | 7 | 2.3 | 10 | 3.6 |
| 5 | 2.4 | 12 | 3.2 |
| 17 | 2.9 |
| WHO histological
grading |
|
|
|
| 0.451a |
|
|
|
| 0.171a |
|
|
|
Well-differentiated | 137 | 53.5 | 117 | 50.4 |
| 91 | 52.0 | 163 | 52.1 |
| 254 | 52.0 |
|
Moderately differentiated | 64 | 25.0 | 63 | 27.2 |
| 42 | 24.0 | 85 | 27.2 |
| 127 | 26.0 |
| Poorly
differentiated | 30 | 11.7 | 21 | 9.1 |
| 25 | 14.3 | 26 | 8.3 |
| 51 | 10.5 |
|
Undifferentiated | 25 | 9.8 | 31 | 13.4 |
| 17 | 9.7 | 39 | 12.5 |
| 56 | 11.5 |
| Missing
data | 51 |
| 42 |
|
| 35 |
| 58 |
|
| 93 |
|
| Bryne Score (1992)
of cellular differentiation |
|
|
|
| 0.513a |
|
|
|
| 0.248a |
|
|
| Grade
I | 149 | 55.0 | 128 | 51.2 |
| 92 | 48.4 | 185 | 55.9 |
| 277 | 53.2 |
| Grade
II | 92 | 33.9 | 97 | 38.8 |
| 75 | 39.5 | 114 | 34.4 |
| 189 | 36.3 |
| Grade
III | 30 | 11.1 | 25 | 10.0 |
| 23 | 12.1 | 32 | 9.7 |
| 55 | 10.6 |
|
Missing | 36 |
|
| 24 |
| 20 |
| 40 |
|
| 60 |
|
| Specimen type |
|
|
|
| 0.351a |
|
|
|
| 0.978a |
|
|
|
Resection | 145 | 47.2 | 144 | 52.6 |
| 105 | 50.0 | 184 | 49.6 |
| 289 | 49.7 |
|
Biopsy | 158 | 51.5 | 125 | 45.6 |
| 101 | 48.1 | 182 | 49.1 |
| 283 | 48.7 |
|
Excision | 4 | 1.3 | 5 | 1.8 |
| 4 | 1.9 | 5 | 1.3 |
| 9 | 1.5 |
Pathological characteristics of OSCC
with regard to prognosis in patients who underwent resection
The clinicopathological characteristics of OSCC
associated with staging and prognosis according to sex and age
among patients who underwent resection are presented in Table II. The tumors diagnosed in the Dr
Cipto Mangunkusumo Hospital tended to be extensive (T4: 62.9%),
without LN involvement (42.2%) and had no distant metastasis
(98.4%). Patients were more likely to present with advanced-stage
disease (83.2%). Surgical results were positive, with 85.8% of
cases demonstrating a primarily tumor-free resection margin;
however, 54.7% of cases were found to have LVI. There was no
significant pattern for these characteristics according to sex;
however, there was a significant difference between young and old
patients with regard to the stage of disease (P=0.023).
 | Table II.Pathological characteristics of oral
squamous cell carcinoma related to prognosis in patients who
underwent resection (n=289). |
Table II.
Pathological characteristics of oral
squamous cell carcinoma related to prognosis in patients who
underwent resection (n=289).
|
|
|
|
|
|
| Age |
|
|
|
|---|
|
| Sex |
|
|
|
|
|
|---|
|
|
|
| Young, ≤45 years
(n=105) | Old, >45 years
(n=184) |
| Total (n=289) |
|---|
|
| Male (n=145) | Female (n=144) |
|
|
|---|
| Pathological
characteristics |
|
|
|
|
|
|
|
|---|
| n | % | n | % | P-value | n | % | n | % | P-value | n | % |
|---|
| Tumor size |
|
|
|
| 0.782a |
|
|
|
| 0.225a |
|
|
| T1 | 6 | 4.5 | 7 | 5.7 |
| 5 | 5.1 | 8 | 5.1 |
| 13 | 5.1 |
| T2 | 22 | 16.5 | 24 | 19.5 |
| 12 | 12.1 | 34 | 21.7 |
| 46 | 18.0 |
| T3 | 21 | 15.8 | 15 | 12.2 |
| 13 | 13.1 | 23 | 14.6 |
| 36 | 14.1 |
| T4 | 84 | 63.2 | 77 | 62.6 |
| 69 | 69.7 | 92 | 58.6 |
| 161 | 62.9 |
| Missing
data | 12 |
| 11 |
|
| 6 |
| 27 |
|
| 33 |
|
| Node
involvement |
|
|
|
| 0.266a |
|
|
|
| 0.630a |
|
|
| N0 | 51 | 38.3 | 57 | 46.3 |
| 40 | 40.4 | 68 | 43.3 |
| 108 | 42.2 |
| N1 | 44 | 33.1 | 30 | 24.4 |
| 27 | 27.3 | 47 | 29.9 |
| 74 | 28.9 |
| N2 | 38 | 28.6 | 36 | 29.3 |
| 32 | 32.3 | 42 | 26.8 |
| 74 | 28.9 |
| Missing
data | 12 |
| 11 |
|
| 6 |
| 27 |
|
| 33 |
|
| Distant
metastasis |
|
|
|
| 0.353b |
|
|
|
| 0.642b |
|
|
| M0 | 132 | 99.2 | 120 | 97.6 |
| 97 | 98.0 | 155 | 98.7 |
| 252 | 98.4 |
| M1 | 1 | 0.8 | 3 | 2.4 |
| 2 | 2.0 | 2 | 1.3 |
| 4 | 1.6 |
| Missing
data | 12 |
| 11 |
|
| 6 |
| 27 |
|
| 33 |
|
| Staging |
|
|
|
| 0.918a |
|
|
|
| 0.195a |
|
|
| I | 5 | 3.8 | 5 | 4.1 |
| 2 | 2.0 | 8 | 5.1 |
| 10 | 3.9 |
| II | 15 | 11.3 | 18 | 14.6 |
| 8 | 8.1 | 25 | 15.9 |
| 33 | 12.9 |
|
III | 22 | 16.5 | 17 | 13.8 |
| 15 | 15.2 | 24 | 15.3 |
| 39 | 15.2 |
|
IVA | 77 | 57.9 | 67 | 54.5 |
| 58 | 58.6 | 86 | 54.8 |
| 144 | 56.3 |
|
IVB | 12 | 9.0 | 13 | 10.6 |
| 13 | 13.1 | 12 | 7.6 |
| 25 | 9.8 |
|
IVC | 2 | 1.5 | 3 | 2.4 |
| 3 | 3.0 | 2 | 1.3 |
| 5 | 2.0 |
| Missing
data | 12 |
| 11 |
|
|
|
|
|
|
| 33 |
|
| Staging group |
|
|
|
| 0.434a |
|
|
|
| 0.023a |
|
|
| I–II
(early stage) | 20 | 15.0 | 23 | 18.7 |
| 10 | 10.1 | 33 | 21.0 |
| 43 | 16.8 |
| III–IV
(advanced stage) | 113 | 85.0 | 100 | 81.3 |
| 89 | 89.9 | 124 | 79.0 |
| 213 | 83.2 |
| Missing
data | 12 |
| 11 |
|
|
|
|
|
|
| 33 |
|
| LVI |
|
|
|
| 0.591a |
|
|
|
| 0.105a |
|
|
|
Negative | 68 | 46.9 | 63 | 43.8 |
| 41 | 39.0 | 90 | 48.9 |
| 131 | 45.3 |
|
Positive | 77 | 53.1 | 81 | 56.3 |
| 64 | 61.0 | 94 | 51.1 |
| 158 | 54.7 |
| Margin of
resection |
|
|
|
| 0.229a |
|
|
|
| 0.669a |
|
|
| Negative | 128 | 88.3 | 120 | 83.3 |
| 89 | 84.8 | 159 | 86.4 |
| 248 | 85.8 |
| Positive | 17 | 11.7 | 24 | 16.7 |
| 16 | 15.2 | 25 | 13.6 |
| 41 | 14.2 |
Comparative analysis of tumor subsites
with regard to WHO histological grading of all OSCC cases
The comparative analysis presented in Table III demonstrated that the specific
histological differentiation patterns in different tumor subsites
were significantly different from each other (P=0.017). In most
anatomical origins, the proportion of well-differentiated cases was
higher than other grades, except in the buccal mucosa, in which
moderately differentiated OSCCs were more prevalent, and the floor
of the mouth (FOM), for which the proportions of
well-differentiated and moderately differentiated cancers were
similar. In more detail, the pos hoc analysis elucidated a
remarkable difference in grading between tongue and palate subsites
(P=004), mouth NOS and palate (P=0.007), and palate and buccal
mucosa (P=0.015), meanwhile other two subsites comparisons in pos
hoc analysis revealed nonsignificant differences (P≥0.05).
 | Table III.Comparative analysis of anatomical
tumor subsites with regard to age and WHO histological grading of
all oral squamous cell carcinoma cases (n=581). |
Table III.
Comparative analysis of anatomical
tumor subsites with regard to age and WHO histological grading of
all oral squamous cell carcinoma cases (n=581).
|
| WHO histological
grading |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
| Well-differentiated
(n=254) | Moderately
differentiated (n=127) | Poorly
differentiated (n=51) | Undifferentiated
(n=56) | Total (n=488) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Tumor subsites | n | % | n | % | n | % | n | % | n | % | P-value |
|---|
| Tongueb | 163 | 50.2 | 84 | 25.8 | 29 | 8.9 | 49 | 15.1 | 325 | 66.6 | 0.017a |
| Mouth
NOSc | 36 | 48.0 | 20 | 26.7 | 16 | 21.3 | 3 | 4.0 | 75 | 15.4 |
|
| Palated | 26 | 72.2 | 4 | 22.2 | 2 | 5.6 | 0 | 0.0 | 36 | 7.4 |
|
| Gingiva | 15 | 65.2 | 8 | 17.4 | 3 | 13.0 | 1 | 4.3 | 23 | 4.7 |
|
| Lip | 9 | 60.0 | 4 | 26.7 | 0 | 0.0 | 2 | 13.3 | 15 | 3.1 |
|
| Buccal mucosa | 4 | 33.3 | 6 | 50.0 | 1 | 8.3 | 1 | 8.3 | 12 | 2.5 |
|
| FOM | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 |
|
Associations between lymphovascular
invasion, lymph node metastasis and distant metastasis for all
patients with OSCC who underwent resection with complete
staging
A significant association between the presence of
LVI and lymph node metastasis (LNM) (OR, 8.96; 95% CI, 5.06-15.88;
P<0.0001) was found (Table
IV). LVI was not significantly associated with distant
metastasis (P=0.142); however, all cases with distant metastasis
were also found to have LVI.
 | Table IV.Associations between LVI and LNM and
distant metastasis of all oral squamous cell carcinoma cases that
underwent resection with complete staging (n=256). |
Table IV.
Associations between LVI and LNM and
distant metastasis of all oral squamous cell carcinoma cases that
underwent resection with complete staging (n=256).
|
| LNM |
|
| Distant
metastasis |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| Negative
(n=108) | Positive
(n=148) | Total (n=256) |
|
| Negative
(n=252) | Positive (n=4) | Total (n=256) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| LVI | n | % | n | % | n | % | OR (95% CI) | P-value | n | % | n | % | n | % | OR (95% CI) | P-value |
|---|
| Negative | 76 | 70.4 | 31 | 20.9 | 107 | 41.8 | 8.96
(5.06-15.88) |
<0.0001a | 107 | 42.5 | 0 | 0.0 | 107 | 41.8 | n/ac | 0.142b |
| Positive | 32 | 29.6 | 117 | 79.1 | 149 | 58.2 |
|
| 145 | 57.5 | 4 | 100.0 | 149 | 58.2 |
|
|
Multivariate logistic regression
analysis of predictors for advanced-stage OSCC among general and
young patients who underwent resection with complete clinical
staging
The possible predictors of advanced-stage OSCC are
presented in Table V. Multivariate
logistic regression analysis elucidated two statistically
significant predictors of advanced-stage cancer in the general
population, such as younger age ≤45 years (OR, 2.26; 95% CI,
1.02-5.04; P=0.046) and the presence of LVI (OR, 8.42; 95% CI;
3.70-19.20; P<0.0001). LVI was also an independent predictor (OR
8.28; 95% CI; 1.65-41.47; P=0.010) in developing advanced-stage
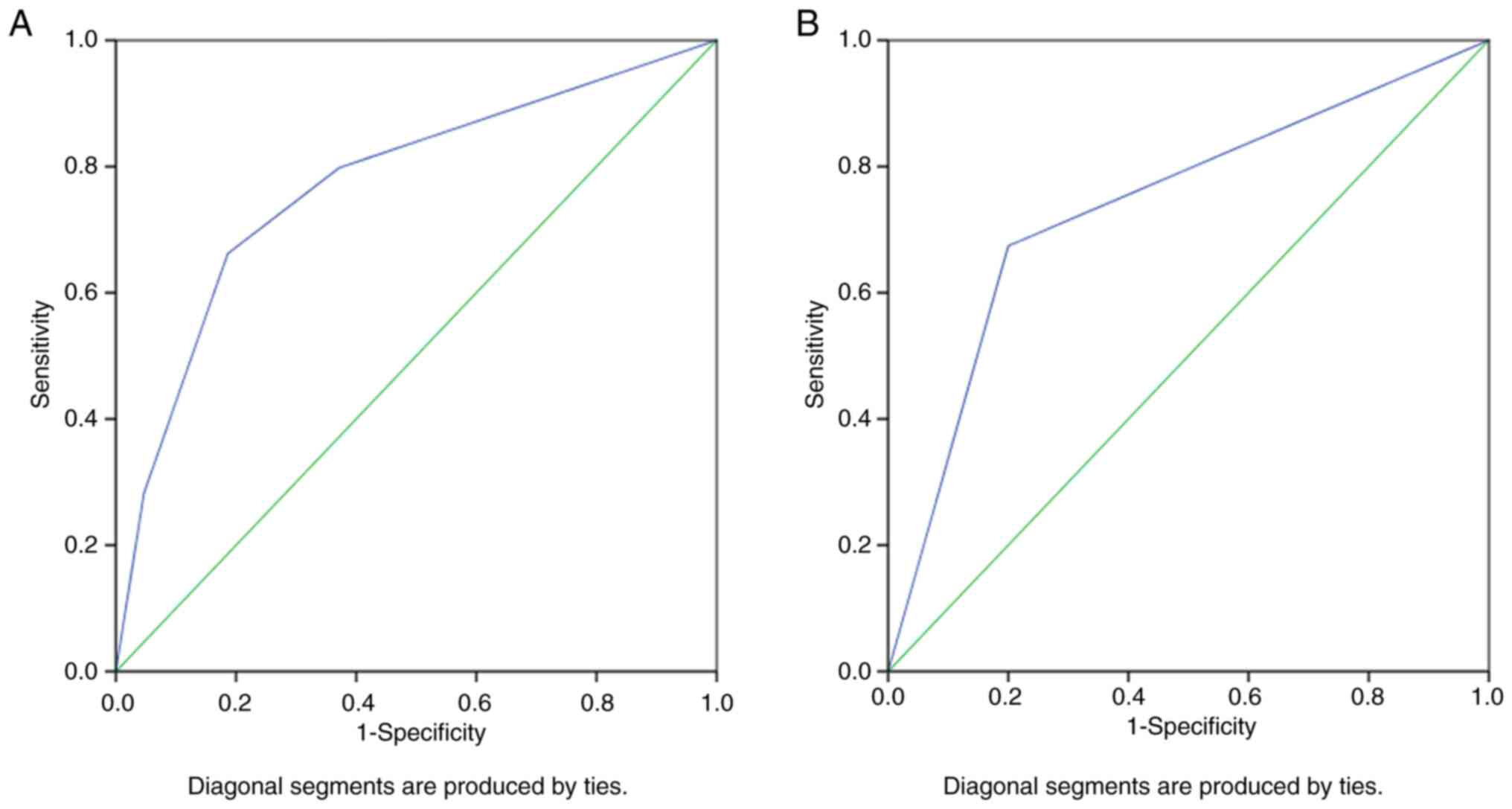
OSCC among young patients. The AUC was 0.773 (95% CI, 0.700-0.846;
P<0.0001) for the predictor model of advanced-stage OSCC among
the general population and was 0.737 (95% CI; 0.581-0.894; P=0.014)
among young patients (Fig. 2).
These AUC values demonstrated good discrimination and high-quality
results, as a minimum value of 70% for AUC was considered
clinically meaningful (33,37).
 | Table V.Multivariate logistic regression
analysis of predictors to advanced-stage oral squamous cell
carcinomas among general (n=256) and young patients (n=99) who
underwent resection with complete clinical staging. |
Table V.
Multivariate logistic regression
analysis of predictors to advanced-stage oral squamous cell
carcinomas among general (n=256) and young patients (n=99) who
underwent resection with complete clinical staging.
| A, General
population (n=256) |
|---|
|
|---|
|
| Staging |
|
|
|
|
|
|---|
|
|
|
| Bivariate
analysis | Multivariate
analysis |
|---|
|
| Early stage | Advanced stage |
|
|
|
|---|
|
|
|
|
| OR unadjusted (95%
CI) |
| OR adjusted (95%
CI) |
|
|---|
| Variables | n | % | n | % | Total, n | P-value | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Female | 23 | 53.5 | 100 | 46.9 | 123 | Ref. |
|
|
|
|
Male | 20 | 46.5 | 113 | 53.1 | 133 | 1.30
(0.67-2.50) | 0.434a |
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
>45 | 33 | 76.7 | 124 | 58.2 | 157 | Ref. |
| Ref. |
|
|
≤45 | 10 | 23.3 | 89 | 41.8 | 99 | 2.37
(1.11-5.06) | 0.023a,b | 2.26
(1.02-5.04) | 0.046c |
| Tumor subsites |
|
|
|
|
|
|
|
|
|
|
Tongue | 32 | 74.4 | 150 | 70.4 | 182 | Ref. |
| Ref. |
|
| Mouth
NOS | 5 | 11.6 | 26 | 12.2 | 31 | 1.11
(0.40-3.11) | 0.843a |
|
|
|
Palate | 1 | 2.3 | 13 | 6.1 | 14 | 2.77
(0.35-21.97) | 0.472d |
|
|
|
Gingiva | 1 | 2.3 | 12 | 5.6 | 13 | 2.56
(0.32-20.40) | 0.700d |
|
|
|
Lip | 4 | 9.3 | 5 | 2.3 | 9 | 0.27
(0.07-1.05) | 0.066d | 0.43
(0.09-1.98) | 0.276c |
| Buccal
mucosa | 0 | 0.0 | 6 | 2.8 | 6 | n/a | 0.592d |
|
|
|
FOM | 0 | 0.0 | 1 | 0.5 | 1 | n/a |
>0.999d |
|
|
| Keratinization |
|
|
|
|
|
|
|
|
|
|
Yes | 39 | 90.7 | 188 | 88.3 | 227 | Ref. |
|
|
|
| No | 4 | 9.3 | 24 | 11.3 | 28 | 1.25
(0.41-3.79) |
>0.999d |
|
|
|
NOS | 0 | 0.0 | 1 | 0.5 | 1 | n/a |
>0.999d |
|
|
| WHO histological
grading |
|
|
|
|
|
|
|
|
|
|
Well-differentiated | 22 | 75.9 | 86 | 62.3 | 108 | Ref. |
|
|
|
|
Moderately differentiated | 1 | 3.4 | 20 | 14.5 | 21 | 1.42
(0.57-3.54) | 0.448a |
|
|
| Poorly
differentiated | 1 | 3.4 | 12 | 8.7 | 13 | 2.02
(0.57-7.24) | 0.271a |
|
|
|
Undifferentiated | 5 | 17.2 | 20 | 14.5 | 25 | 1.34
(0.56-3.19) | 0.515a |
|
|
| Bryne score
(1992) |
|
|
|
|
|
|
|
|
|
| Grade
I | 27 | 62.8 | 105 | 49.3 | 132 | Ref. |
|
|
|
| Grade
II | 14 | 32.6 | 75 | 35.2 | 89 | 1.38
(0.68-2.80) | 0.376a |
|
|
| Grade
III | 2 | 4.7 | 33 | 15.5 | 35 | 4.24
(0.96-18.80) | 0.041a |
|
|
| LVI |
|
|
|
|
|
|
|
|
|
|
Negative | 25 | 86.2 | 48 | 34.8 | 73 | Ref. |
| Ref. |
|
|
Positive | 4 | 13.8 | 90 | 65.2 | 94 | 11.72
(3.85-35.63) |
<0.001a,b | 8.42
(3.70-19.20) |
<0.001c |
|
| B, Young
Patients (n=99) |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Female | 4 | 40.0 | 39 | 43.8 | 43 | Ref. |
|
|
|
|
Male | 6 | 60.0 | 50 | 56.2 | 56 | 0.86
(0.23-3.24) |
>0.999d |
|
|
| Tumor subsites |
|
|
|
|
|
|
|
|
|
|
Tongue | 9 | 90.0 | 66 | 74.2 | 75 | Ref. |
|
|
|
| Mouth
NOS | 0 | 0.0 | 8 | 9.0 | 8 | n/a | 0.589d |
|
|
|
Palate | 0 | 0.0 | 8 | 9.0 | 8 | n/a | 0.589d |
|
|
|
Gingiva | 0 | 0.0 | 5 | 5.6 | 5 | n/a |
>0.999d |
|
|
|
Lip | 1 | 10.0 | 1 | 1.1 | 2 | 0.14
(0.01-2.38) | 0.244d |
|
|
| Buccal
mucosa | 0 | 0.0 | 1 | 1.1 | 1 | n/a |
>0.999d |
|
|
|
FOM | 0 | 0.0 | 0 | 0.0 | 0 | n/a | n/a |
|
|
| Keratinization |
|
|
|
|
|
|
|
|
|
|
Yes | 8 | 80.0 | 82 | 92.1 | 90 | Ref. |
|
|
|
| No | 2 | 20.0 | 7 | 7.9 | 9 | 0.34
(0.06-1.93) | 0.225d |
|
|
|
NOS | 0 | 0.0 | 0 | 0.0 | 99 | n/a |
|
|
|
| WHO histological
grading |
|
|
|
|
|
|
|
|
|
|
Well-differentiated | 5 | 50.0 | 49 | 55.1 | 54 | Ref. |
|
|
|
|
Moderately differentiated | 3 | 20.0 | 15 | 16.9 | 17 | 0.77
(0.13-4.36) | 0.670d |
|
|
| Poorly
differentiated | 3 | 20.0 | 11 | 12.4 | 13 | 0.56
(0.09-3.27) | 0.614d |
|
|
|
Undifferentiated | 2 | 10.0 | 14 | 15.7 | 15 | 1.43
(0.15-13.26) |
>0.999d |
|
|
| Bryne score
(1992) |
|
|
|
|
|
|
|
|
|
| Grade
I | 4 | 40.0 | 45 | 50.6 | 49 | Ref. |
|
|
|
| Grade
II | 4 | 40.0 | 31 | 34.8 | 35 | 0.69
(0.16-2.97) | 0.714d |
|
|
| Grade
III | 2 | 20.0 | 13 | 14.6 | 15 | 0.58
(0.09-3.52) | 0.618d |
|
|
| LVI |
|
|
|
|
|
|
|
|
|
|
Negative | 8 | 80.0 | 29 | 32.6 | 37 | Ref. |
| Ref. |
|
|
Positive | 2 | 20.0 | 60 | 67.4 | 62 | 8.28
(1.65-41.47) | 0.005d,b | 8.28
(1.65-41.47) | 0.010c |
Multivariate logistic regression
analysis of predictors for invaded surgical margin among general
and young patients who underwent resection with complete clinical
staging
The significant predictors for invasion of surgical
margins in the general population are presented in Table VI. Significant predictors included
particular tumor subsites, such as mouth NOS (OR, 3.04; 95% CI,
1.17-7.93; P=0.023) and the palate (OR, 6.13; 95% CI, 1.73-21.74;
P=0.005). However, advanced-stage (stage III–IV) cancer status and
Bryne score grade II were not statistically significant as risk
factors in multivariate analysis. For the young population, the
palate tumor subsite (OR, 38.77; 95% CI, 3.36-447.66; P=0.003) and
positive LVI (OR, 11.61; 95% CI, 1.34-100.61; P=0.026) were
significant predictors for the invasion of surgical margins.
 | Table VI.Multivariate logistic regression
analysis of predictors to invaded surgical margin among general
(n=256) and young patients (n=99) who underwent resection with
complete clinical staging. |
Table VI.
Multivariate logistic regression
analysis of predictors to invaded surgical margin among general
(n=256) and young patients (n=99) who underwent resection with
complete clinical staging.
| A, General
population (n=256) |
|---|
|
|---|
|
| Surgical
margins |
|
|
|
|
|
|---|
|
|
|
| Bivariate
analysis | Multivariate
analysis |
|---|
|
| Negative | Positive |
|
|
|
|---|
|
|
|
|
| OR unadjusted (95%
CI) |
| OR adjusted (95%
CI) |
|
|---|
| Variables | n | % | n | % | Total, n | P-value | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 118 | 53.6 | 15 | 41.7 | 133 | Ref. |
| Ref. |
|
|
Female | 102 | 46.4 | 21 | 58.3 | 123 | 1.62
(0.79-3.31) | 0.183a | 1.95
(0.89-4.30) | 0.097b |
| Age, years |
|
|
|
|
|
|
|
|
|
|
>45 | 137 | 62.3 | 20 | 55.6 | 157 | Ref. |
|
|
|
|
≤45 | 83 | 37.7 | 16 | 44.4 | 99 | 2.37
(1.11-5.06) |
| 0.443a |
|
| Tumor subsites |
|
|
|
|
|
|
|
|
|
|
Tongue | 164 | 74.5 | 18 | 50.0 | 182 | Ref. |
| Ref. |
|
| Mouth
NOS | 23 | 10.5 | 8 | 22.2 | 31 | 3.17
(1.24-8.12) | 0.032c,d | 3.04
(1.17-7.93) | 0.023b |
|
Gingiva | 10 | 4.5 | 3 | 8.3 | 13 | 2.73
(0.68-10.85) | 0.151c,d | 2.88
(0.70-11.82) | 0.142b |
|
Lip | 9 | 4.1 | 0 | 0.0 | 9 | n/a |
|
>0.999c |
|
|
Palate | 9 | 4.1 | 5 | 13.9 | 14 | 5.06
(1.53-16.75) | 0.014c,d | 6.13
(1.73-21.74) | 0.005b |
| Buccal
mucosa | 4 | 1.8 | 2 | 5.6 | 6 | 4.56
(0.78-26.63) | 0.124c,d | 3.24
(0.54-19.41) | 0.199b |
|
FOM | 1 | 0.5 | 0 | 0.0 | 1 | n/a |
>0.999c |
|
|
| Keratinization |
|
|
|
|
|
|
|
|
|
|
Yes | 39 | 90.7 | 188 | 88.3 | 227 | Ref. |
|
|
|
| No | 4 | 9.3 | 24 | 11.3 | 28 | 1.25
(0.41-3.79) |
>0.999c |
|
|
|
NOS | 0 | 0.0 | 1 | 0.5 | 1 | n/a |
>0.999c |
|
|
| WHO histological
grading |
|
|
|
|
|
|
|
|
|
|
Well-differentiated | 110 | 50.0 | 18 | 50.0 | 128 | Ref. |
|
|
|
|
Moderately differentiated | 40 | 18.2 | 8 | 22.2 | 48 | 1.22
(0.49-3.03) | 0.665a |
|
|
| Poorly
differentiated | 22 | 10.0 | 6 | 16.7 | 28 | 1.67
(0.59-4.67) | 0.385c |
|
|
|
Undifferentiated | 48 | 21.8 | 4 | 11.1 | 52 | 0.51
(0.16-1.58) | 0.237a |
|
|
| Bryne score
(1992) |
|
|
|
|
|
|
|
|
|
| Grade
I | 119 | 54.1 | 13 | 36.1 | 132 | Ref. |
| Ref. |
|
| Grade
II | 71 | 32.3 | 18 | 50.0 | 89 | 2.32
(1.07-5.02) | 0.029a,d | 2.17
(0.95-4.95) | 0.066b |
| Grade
III | 30 | 13.6 | 5 | 13.9 | 35 | 1.53
(0.50-4.61) | 0.539c |
|
|
| Staging |
|
|
|
|
|
|
|
|
|
| I–II
(early) | 41 | 18.6 | 2 | 5.6 | 43 | Ref. |
| Ref. |
|
| III–IV
(advanced) | 179 | 81.4 | 34 | 94.4 | 213 | 3.89
(0.90-16.87) | 0.052a,d | 3.29
(0.74-14.64) | 0.119b |
| LVI |
|
|
|
|
|
|
|
|
|
|
Negative | 94 | 42.7 | 13 | 36.1 | 107 | Ref. |
|
|
|
|
Positive | 126 | 57.3 | 23 | 63.9 | 149 | 1.32
(0.64-2.74) | 0.456a |
|
|
|
| B, Young
patients (n=99) |
|
|
| Surgical
margins |
|
|
|
|
|
|
|
|
| Bivariate
analysis | Multivariate
analysis |
|
|
Negative |
Positive |
|
|
|
|
|
|
|
| OR unadjusted
(95% CI) |
| OR adjusted (95%
CI) |
|
|
Variables | n | % | n | % | Total,
n | P-value | P-value |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Female | 37 | 44.6 | 6 | 37.5 | 43 | Ref. |
|
|
|
|
Male | 46 | 55.4 | 10 | 62.5 | 56 | 1.34
(0.45-4.03) | 0.601a |
|
|
| Tumor subsites |
|
|
|
|
|
|
|
|
|
|
Tongue | 67 | 80.7 | 8 | 50.0 | 75 | Ref. |
| Ref. |
|
| Mouth
NOS | 6 | 7.2 | 2 | 12.5 | 8 | 2.79
(0.48-16.23) | 0.246c | 3.28
(0.50-21.41) | 0.214b |
|
Gingiva | 3 | 3.6 | 2 | 12.5 | 5 | 5.58
(0.81-38.60) | 0.115c,d | 8.09
(0.90-73.09) | 0.063b |
|
Lip | 2 | 2.4 | 0 | 0.0 | 2 | n/a |
>0.999c |
|
|
|
Palate | 4 | 4.8 | 4 | 25.0 | 8 | 8.38
(1.75-40.17) | 0.013c,d | 38.77
(3.36-447.66) | 0.003b |
| Buccal
mucosa | 1 | 1.2 | 0 | 0.0 | 1 | n/a |
>0.999c |
|
|
|
FOM | 0 | 0.0 | 0 | 0.0 | 0 | n/a | - |
|
|
| Keratinization |
|
|
|
|
|
|
|
|
|
|
Yes | 8 | 80.0 | 82 | 92.1 | 90 | Ref. |
|
|
|
| No | 2 | 20.0 | 7 | 7.9 | 9 | 0.34
(0.06-1.93) | 0.225c |
|
|
|
NOS | 0 | 0.0 | 0 | 0.0 | 0 | n/a | n/a |
|
|
| WHO histological
grading |
|
|
|
|
|
|
|
|
|
|
Well-differentiated | 44 | 53.0 | 10 | 62.5 | 54 | Ref. |
|
|
|
|
Moderately differentiated | 14 | 16.9 | 3 | 18.8 | 17 | 0.94
(0.23-3.91) |
>0.999c |
|
|
| Poorly
differentiated | 11 | 13.3 | 2 | 12.5 | 13 | 0.80
(0.15-4.19) |
>0.999c |
|
|
|
Undifferentiated | 14 | 16.9 | 1 | 6.3 | 15 | 0.31
(0.04-2.68) | 0.434c |
|
|
| Bryne score
(1992) |
|
|
|
|
|
|
|
|
|
| Grade
I | 42 | 50.6 | 7 | 43.8 | 49 | Ref. |
|
|
|
| Grade
II | 29 | 34.9 | 6 | 37.5 | 35 | 1.24
(0.38-4.08) | 0.721a |
|
|
| Grade
III | 12 | 14.5 | 3 | 18.8 | 15 | 1.50
(0.34-6.70) | 0.687c |
|
|
| Staging |
|
|
|
|
|
|
|
|
|
| I–II
(early) | 10 | 12.0 | 0 | 0.0 | 10 | Ref. |
|
|
|
| III–IV
(advanced) | 73 | 88.0 | 16 | 100.0 | 89 | n/a | 0.359c |
|
|
| LVI |
|
|
|
|
|
|
|
|
|
|
Negative | 34 | 41.0 | 3 | 18.8 | 37 | Ref. |
| Ref. |
|
|
Positive | 49 | 59.0 | 13 | 81.3 | 62 | 3.00
(0.80-11.36) | 0.157c,d | 11.61
(1.34-100.61) | 0.026b |
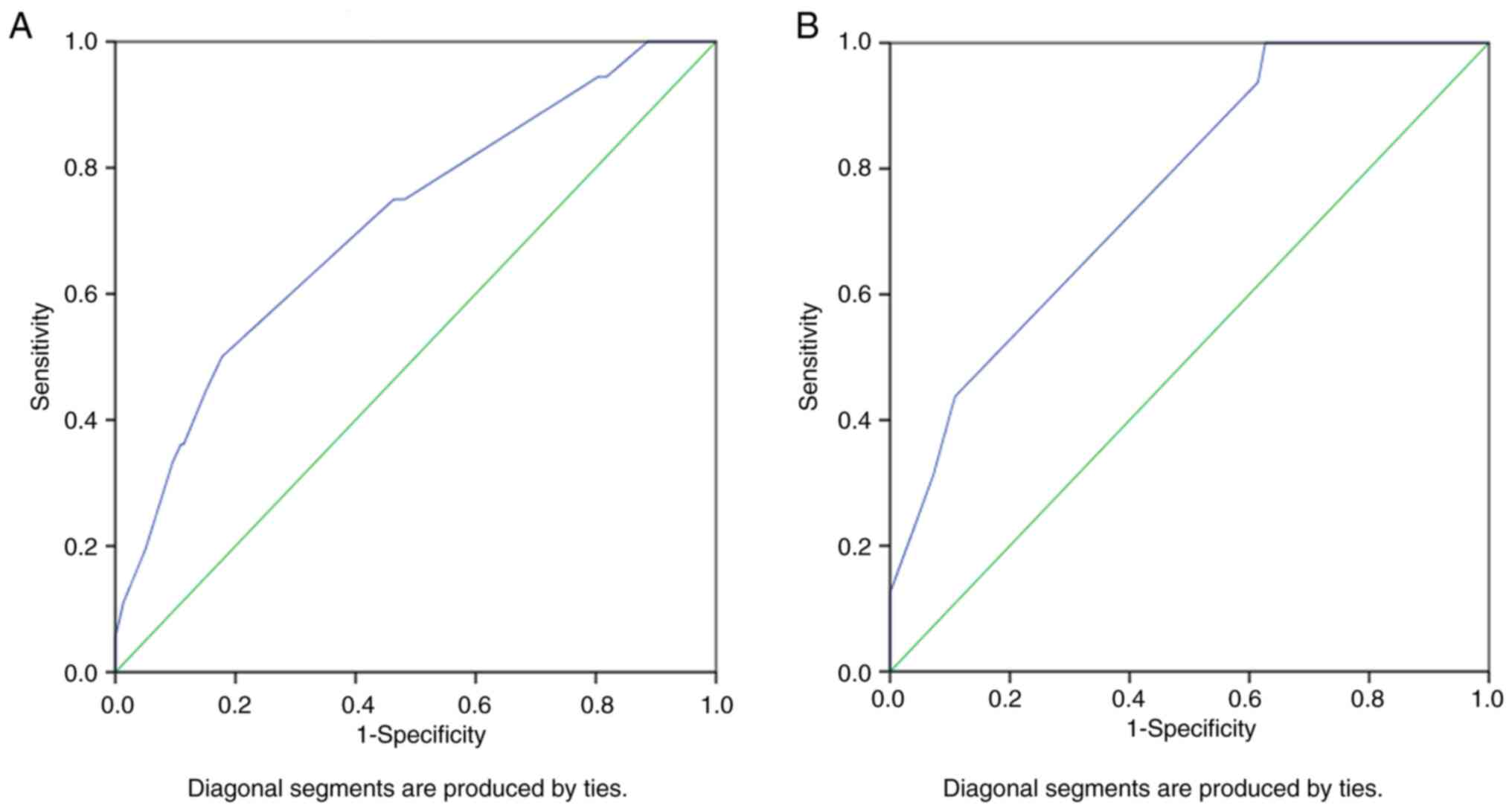
Furthermore, the AUC was 0.711 (95% CI, 0.619-0.804;
P<0.0001) for the predictor model of OSCC with invaded surgical
margins among the general population and 0.762 (95% CI,
0.645-0.880; P=0.001) among young patients (Fig. 3). These AUC values demonstrated
good discrimination and quality for the predictive model results
and showed that the predictive model had a good separability
measure. The AUC was ~0.7 in the present study; which demonstrates
that there was a 70% chance that the model would be able to
distinguish between cases with invaded and clear surgical
margins.
Discussion
Over the 10 years of the present study, an increase
in the proportion of cases between the first 5-year interval
(2001–2015) and the second 5-year interval (2016–2020) was
demonstrated. It was possibly caused by the improvement of
healthcare access and the advancement of healthcare in Indonesia
due to the implementation of the National Health Insurance (Jaminan
Kesehatan Nasional) scheme in 2014 (38) and the achievement of Universal
Health Coverage in 2019 (39). It
has been reported that the National Health Insurance scheme
enhanced the pace of gaining diagnosis and equity in healthcare
access. Nonetheless, it did not improve decrease the time before
treatment was received due to limited expansion of healthcare
facilities (40).
In the present study, males were more commonly
diagnosed with OSCC than females, with a male/female ratio of
0.53:0.47. This result is consistent with earlier studies from
numerous countries, including South Korea (males, 56.4%) (41) and Iran (males, 59.6%) (42), which reported a greater frequency
of OSCC in men (8,43). By contrast, a study in Thailand
reported a greater prevalence of OSCC in women, with a male/female
ratio of 1.00:1.56 (44). Male
patients are prone to habits such as frequent smoking and the
consumption of tobacco products (45), which have long been recognized as
risk factors for OSCC. Tobacco contains ~300 carcinogenic
compounds, which can be converted to reactive metabolites that
interact with DNA, resulting in oxidative stress. Continuous
exposure of these agents to the heat from tobacco combustion
further aggravates the stress placed on the oral mucosa (46). Data from Indonesia (2018)
demonstrated that the percentage of males smoking on a daily basis
was 47.3% compared with 1.2% in females (47).
The present study also demonstrated a sex-specific
pattern in tumor subsites. Females were more commonly affected at
the site of the lip, buccal mucosa, tongue and mouth NOS. However,
the anatomical sites most prevalent in males were the palate,
gingiva and FOM, in agreement with previous studies (48–50).
Kruse et al (51) also
reported sex-specific patterns. The exact reasons for this
differential pattern of common sites for OSCC in males and females
are still unknown; however, disparities in the prevalence of OSCC
by sex may also be influenced by various factors such as genetic
predisposition, altered immune and hormonal modulations, and HPV
infection (52). Lip cancer
affected more females than males in the present study, which was
possibly due to the improper practice of using unsafe and
unstandardized lipstick or other cosmetic products containing
carcinogens, which are more common in adolescent girls (53,54).
The lip epithelium has a thinner keratin covering, less melanin,
less sweat and sebaceous gland secretions, and thus has less
protection than the skin. Therefore, the use of unsafe and
non-standard lipsticks could put users at greater risk of
developing cancer due to chronic local irritation and corrosion
(53,54). Furthermore, the higher incidence of
buccal cancer in the female population could have been linked to
the prevalent practice of chewing betel nuts and consuming
smokeless tobacco products in Asian women (55). Consuming these carcinogenic agents
irritates the buccal mucosa and results in a greater risk of oral
lesions compared with that in males (56,57).
The peak incidence in the populations were
demonstrated in the 41–60 years age group; other studies have
reported an older age range between 50 and 70 years (46,58–62).
The present study demonstrated that OSCC in young patients was more
prevalent in the Indonesian study population than in other parts of
the world (36.1 vs. 4–6% of total cases) (48,63–67).
The large proportion of OSCC cases presenting at a younger age in
Indonesia should be a public health concern. In 2020, the
productive age group (15–49 years) dominated the Indonesian
population, totaling 145,571,000 or ~54% of the population, with a
slight predominance of men compared with women (50.45%). This
demographic bonus (also called as demographic dividend) will be
less meaningful if non-communicable diseases, including cancer,
contribute to the younger generation's morbidity and mortality
(68,69). Moreover, the present study
demonstrated that the mean age for male patients is younger than
that for their female counterparts, similar to the results of
studies in Nigeria (70) and
Thailand (48). This is socially
important, as men have a leading role and are the main source of
income in most families. Moreover, treatment may severely
debilitate young patients, including disfigurement from surgery and
the severe side effects of chemotherapy and radiotherapy. These
effects may degrade a patient's quality of life.
In the present study, most tumors arose in the
tongue, similar to previous studies (71,72).
The population in the present study had fewer OSCCs starting from
the FOM, possibly due to the difficulty of identifying the tumors
originating from that subsite when a patient presents with
extensive tumor growth occupying the entire oral cavity in
advanced-stage disease. Factors affecting the location of OSCC
could be linked to the geographical distribution of certain
habitual risk factors. Tongue cancer is frequently correlated with
the younger age group (73). In
the present study an association between tumor topography and
histological differentiation was demonstrated. Numerous
well-differentiated tumors were identified in the lips, gingiva,
tongue, palate and mouth NOS. However, moderately differentiated
cases were significantly more frequently diagnosed on the buccal
mucosa, and these are known to have less favorable prognoses
(74,75). It was also demonstrated that the
highest proportion of poorly differentiated tumors developed in the
mouth NOS, followed by the gingiva, tongue and buccal mucosa. Pires
et al (50) and Costa et
al (76,77) also reported that histological
differentiation was associated with the site of the tumors;
however, the reason for this is still unclear.
In the present study, the tumors of most patients
demonstrated keratinization and there was no significant difference
between younger and older patients or the sexes. The existence of
surface keratinization reflects the rapid rate of maturation of the
epithelium. In OSCC, this is a genetically based process to
increase the turnover rate by maintaining development or
differentiation, which, enables the tumor to remain
well-differentiated (78).
The present study demonstrated that
well-differentiated OSCC was the most common histological subtype
in both age groups, sexes, and clinical stages, which was in line
with previous studies (79–81).
On the other hand, the least common subtype was diverse depending
on the grouping. The least frequent subtype in both males and young
patients was undifferentiated. Meanwhile, among females, old
patients, and OSCC survivors at an advanced stage, poorly
differentiated became the least prevalent subtype. Additionally,
the joint least common histological subtype in the early stage was
poorly differentiated and moderately differentiated.
Well-differentiated SCCs almost resemble Malpighian cells of the
normal epithelium. However, they disrupt the basal membrane and
invade the underlying corium under various patterns of uncontrolled
growth, with loss of polarity, development of dyskeratosis and the
formation of ‘keratin pearls’ (82).
Histological grading has been used to predict the
clinical behavior of OSCC for numerous decades, but its prognostic
value is still controversial (83). WHO histological grading is well
suited to the grading of tumors resembling the typical appearance
of tissues, but cannot exclusively rate or reflect the
aggressiveness of the tumor, thus leading to an inaccurate
prognosis. The prognostic prediction from WHO histological grading
can be difficult to derive because this characteristic typically
relies on subjective inspection. Additionally, it will be more
complicated if the specimen comes from a biopsy that serves a
relatively small size of tumors but has high intratumor
heterogeneity (75,84). To address the shortcomings of the
WHO histological classification system, the Bryne score (1992) was
introduced. It better reflects the idea of intratumor heterogeneity
and cellular aggressiveness in differentiation (85). This system implied that the more
invasive areas of the tumor, known as the invasive front, may have
a different character compared to different areas of the same
tumor. Hence this system is more relevant for the prognosis of OSCC
(23,78). Based on this scoring system, more
than half of the cases in the current study presented with grade I
tumors. No significant differences between the subgroups of old and
young patients, or between sexes, were demonstrated as being
related to this parameter; however, the results did demonstrate
that the proportion of grade II and III in younger patients was
markedly higher than that in older patients.
Almost half of the patients in the present study
underwent resection of their tumor and the remaining cases had
specimens taken by biopsy or excision. The decision to resect was
made on a case-by-case basis, as recommended by a multidisciplinary
meeting. However, as the Dr Cipto Mangunkusumo Hospital is the
leading referral hospital in Indonesia, the increasing volume and
demand for surgery may have caused queues in operation schedules,
making certain patients seek private healthcare facilities and
therefore undergo resection outside of the data available to the
present study. Moreover, 81.3% of the patients in the present study
were at an advanced stage and so might not have survived the
waiting time for surgery, as the overall survival (OS) rate of
advanced-stage OSCC is poor (86).
A prior study reported that the 1-year OS rate for OSCC in
Indonesia was 60.6%, and that after 2 years it was 12.1%, with a
median survival of only 20 months (95% CI, 9.07-30.9) (87). This scenario could be further
complicated by a lack of health insurance. It has been reported
that patients without medical insurance are more likely to present
with metastatic head and neck cancer, and not receive definitive
treatment (88). Differences in
the type of samples obtained can also impact the result of the
analysis. Biopsy and excisional specimens may not represent all
aspects of the tumor, such as LNM, LVI and surgical margins. Other
important considerations in specimen analysis are the therapeutic
approaches employed and the survival rates of the patients.
Resection is the best choice of treatment for OSCC and was
correlated with the highest survival rate for this malignancy in a
previous study (89). Patients who
underwent surgical procedures demonstrated higher survival rates
(74.4±4.9 months) than those who did not (51.5±3.9 months),
according to the study by de Barros Silva et al (89). Radiotherapy and chemotherapy are
essential components of the management of OSCC. However, it has
been reported that these modalities are not always correlated with
a better prognosis (89).
Most patients were classified as clinical stage T4,
N0 and M0 in the present study. These results were different to the
results of a study by Elaiwy et al (90), which reported that patients with
early T-stage disease made up more than one-half of the patient
population sampled, but that half of the patients had no nodal
metastases. More than 80% of patients in this study were diagnosed
with OSCC at an advanced stage, consistent with the results in
prior studies (91,92). The absence of pain in the early
stages of OSCC may account for the late diagnosis. The late
diagnosis could also be linked with the status of the Dr Cipto
Mangunkusumo Hospital; as a national referral hospital (and thus a
tertiary healthcare center), a large proportion of patients with
the most advanced stage of disease development is expected. This
result was similar to studies performed in referral hospitals in
India (93) and Brazil (71,94),
which reported that most patients also presented with late-stage
disease (86.79 and 65.5% respectively). The late presentation of
OSCC is most likely due to a combination of factors, including a
lack of knowledge about the disease, poverty, the high expense of
therapy, the seeking of alternative non-evidence-based medications
by patients, professional delay in primary care and insufficient
attention to oral health (93,95).
Almost 90% of the younger population in the present study was
diagnosed with advanced-stage disease, which was similar to
findings in a previous study (64). The late diagnosis in younger
patients is frustrating, as the prognosis of OSCC worsens with
progressing TNM staging (96).
The presence of LVI indicates the initial steps in
metastasis, and it can be assumed that the clinical staging of
patients will be more advanced than that in those with no LVI
(97). More than one-half of the
patients were positive for LVI in the present study. This was
similar to a study by Ting et al (98), which reported that most patients
with T3–4 OSCC (44.9%) demonstrated LVI.
Almost 15% of cases in the present study
demonstrated invaded surgical margins, similar to findings in
previous studies (17–44% inadequate surgical margins) (11,99–101); however, one study reported a
lower proportion of invaded margins (7.5%) (28). In OSCC, assessing surgical margins
is a crucial part of determining the therapeutic outcome, while
also considering tumor location, tumor stage, tissue shrinkage and
mucosal elasticity (102).
Clinical TNM staging is the most reliable indicator
of patient survival in OSCC (74,84),
which also dictates the course of treatment (46). However, TNM staging alone is
insufficient for optimal prognostication and needs
histopathological features to maximize the accuracy of the
prediction of outcomes (103).
Due to the effects of staging and the lack of data on the
clinicopathological contribution to prognosis, the present study
focused on identifying several predictors contributing to the
advanced stage of OSCC among general and young populations.
Multivariate analysis demonstrated that young age at
diagnosis (≤45 years) and the presence of LVI significantly
predicted patients having advanced-stage OSCC in the general
population. Moreover, LVI was independently a significant predictor
among young patients. However, other clinicopathological factors
failed to predict advanced-stage OSCC among both the population in
general and young patients. The role of young age as a predictor of
advanced-stage status supported the hypothesis that that young age
OSCCs are prone to be more aggressive because of their biological
behavior and etiology, which differ from OSCC in older age groups
(104). Consequently, younger
patients have poorer survival (105–107). However, an alternative idea could
be that LVI, not age, is a more significant predictor of
advanced-stage disease and that LVI is more prominent at a young
age (108). These findings
reinforce the possibility that the worse prognosis of young
patients, as demonstrated in the present study, is due to LVI.
LVI is a predictor of the progression of
advanced-stage disease, as its presence is attributed to aggressive
tumor behavior in head and neck cancer (109). The presence of LVI indicates that
a significant amount of neoplastic cells have been accessing the
lymphovascular flow to form tumor emboli, consequently increasing
the chance of LNM, distant metastasis and recurrence (16,110,111). The present study demonstrated
that the presence of LVI was significantly associated with LNM (OR,
8.96; P<0.0001). Furthermore, all metastatic OSCC cases in the
present study had a positive LVI status, which is one of the
earliest stages of metastatic development (97). Moreover, LVI significantly affects
tumor size, histological grading, invasive front, prognosis and OS
(109). A meta-analysis by Huang
et al (97) also reported
that LVI predicted poor OS [hazard ratio (HR), 1.55; 95% CI,
1.43-1.69; P<0.00001] and disease-specific survival (HR, 1.76;
95% CI, 1.48-2.09; P<0.00001). In the young patient group, among
all proposed predictors, only LVI resulted in a significant
possibility of patients developing advanced-stage OSCC, with
markedly higher OR than in the general population. Thus, LVI can be
identified as a critical pathological marker of tumor
aggressiveness in OSCC (112).
The present study also revealed that sex did not
significantly determine staging, prognosis or survival for a
patient with OSCC. Even if there is a consensus that oral cancer is
more common in males (113),
whether sex significantly influences outcome has not been
established and results are still conflicting (114–119). The model produced in the present
study did not demonstrate that keratinization had valuable
prognostic value in predicting advanced stage, similar to the
results of a previous study (120). However, other studies reported
that the degree of keratin expression was a predictor of prognosis
(121,122) and that absent or minimal
keratinization in OSCC was significantly associated with LNM
compared with a high degree of keratinization (9,123,124). However, variables related to
keratinization as a classification degree by scoring were not
analyzed in the present study. The association between WHO
histological grading and the Bryne score (1992) cellular
differentiation system as predictors of disease severity in OSCC is
still controversial (75,84). Although Lin et al (6) reported that histologically,
high-grade OSCC had a worse survival rate and a greater probability
of recurrence than other groups, the present study did not
demonstrate statistical significance between these factors to
predict the advanced stage of OSCC cases. Further research on the
role of these characteristics in OSCC is required.
A previous study reported that identified tumor
subsite carried a prognostic value in the TNM clinical
classification (74). However, the
present study did not demonstrate a significant association between
tumor subsites and TNM staging in general or young populations,
confirming findings reported by Oliveira et al (91). In the general population, the
present study demonstrated a tendency for patients with lesions in
mouth NOS, palate, gingiva, buccal mucosa and FOM to be admitted
with advanced-stage disease compared with those with tongue and lip
OSCC, similar to findings in a prior study (74). These subsite patterns might relate
to daily habits; a tongue lesion might be easier to detect as
complaints in day-to-day eating use might be prominent, whereas a
lip lesion is easily detected due to the cosmetic impact it brings
to the appearance of the patient. Advanced-stage OSCC is often
coded as being identified in the mouth NOS, as the extensive nature
of advanced-stage disease means that an originating subsite of
cancer cannot be determined in most cases.
Patients with positive and close margins should
receive additional care (e.g., adjuvant therapy) and close
monitoring since they are at an increased risk of local disease
recurrence (102). Predicting the
risk of positive surgical margins when treating the advanced-stage
group is essential for local disease control (125). However, the effect of positive
margins on the prognosis of OSCC is still debatable. In a prior
study, the relative risk of death for involved and close surgical
margins compared with clean margin status was 11.61 (P=0.0013) and
2.66 (P=0.002), respectively (126). Positive surgical margin status
indicates the aggressiveness and likelihood of OSCC to recur
(99,126). It also has been acknowledged as
enormously impactful on the survival outcomes of patients treated
surgically for oral cancer (127). In previous studies, the ability
to achieve a wide free margin was linked with some clinical
aspects, such as age, sex, the epicenter of the tumor, T and N
status, and treatment modality (11,99,125,126,128,129). However, occasionally, oral
surgeons cannot acquire an adequate surgical margin for OSCC, as
the oral cavity has a complicated anatomy, and wider resection
might cause more significant disfiguration or functional
disability. The present study demonstrated that surgical margin
invasion status was predicted solely by the particular subsite of
the tumor (worse prognosis if the tumor was identified in the mouth
NOS or palate) in the general population; however, in young
patients, the location of the tumor (particularly in the palate)
and the presence of LVI were predictors of invaded surgical margin
status. The results of the present study aligned with those of
several previous studies, which reported that the rate of
inadequate (close or positive) margins was highest in palate
tumors, followed by mouth NOS; moreover, tumors of the lip and FOM
had the lowest proportions of positive surgical margins (28,84).
Tumor subsites are considered to be a related
prognostic factor due to the particular gene expression profile,
which differs according to tumor subsite, and the compact and
complex anatomy of the oral cavity, which leads to variable tissue
composition among distinct subsites, suggesting that these two
explanations cause dissimilar vulnerability to tumor invasion in
every subsite (130). Compared to
the tongue as a reference, the present study demonstrated that
based on the tumor subsite, mouth NOS and palate had a higher
chance to result in poor tumor outcomes due to their likelihood to
have invaded surgical margins. The tongue was used as a reference
as it had the fewest amount of cases with positive surgical
margins, and following other studies, which commonly used the
tongue as a reference in OSCC analysis (11,28,99,102,131,132). The anatomy of the tongue permits
the design and adaptation of a hemiglossectomy adequate for
achieving clear margins (102).
In the present study, the prevalence of positive margins in OSCC of
the mouth NOS was high, linked to cases where the tumor has
extended through the entire mouth area, demonstrating that it is
undoubtedly complicated to free the margins. The palate was the
most common area that resulted in positive margins due to the
difficulty of entirely separating tumors from the superior aspect
of the skull base and its surroundings during surgery, which may
factor in a higher risk of recurrence (133). If cancer has grown into the hard
palate, all or part of the involved bone (maxilla) will need to be
removed (maxillectomy) and a wide local resection is therefore the
preferred treatment (134).
The prediction model in young patients of the
present study demonstrated that besides tumor subsites, LVI
presence also contributed to positive margin status, a finding
reported in other studies, such as those by Abbas et al
(122) and Clark et al
(135). The risk of locoregional
recurrence and distant metastasis related to LVI can also be
connected to margin status; however, to the best of our knowledge,
no study has previously assessed the association between LVI and
margin status in OSCC. However, similar results have been reported
in prostate cancer, in which LVI increases the recurrence risk in
patients with stage T3 tumors related to positive resection margin
status (136,137). Moreover, the present study
demonstrated that LVI was consistently associated as a predictor of
advanced disease and invaded surgical margins for young patients
with OSCC.
The present study has several shortcomings. First,
as this was a retrospective cross-sectional study that relied
heavily on the acquisition of proper documentation by the
investigators, there may be certain missing data and a risk of
bias. Second, the data were collected at a single institution,
limiting external validity, and only a part of the recorded
population had undergone resection due to the limited setting.
However, these challenges have been addressed by performing several
sub-analyses only for the resection specimen data. Third,
evaluation of the histopathological features was performed by
pathologists and individual disagreement is conceivable. To
mitigate this, two independent pathologists were used to minimize
bias. Furthermore, >80% of the patients diagnosed with OSCCs in
the present study were in the late stages of the disease;
therefore, the findings may not be generalizable to patients with
early stage disease. Furthermore, thorough histopathological
assessment to predict staging and surgical outcomes is
required.
An epidemiological study is essential for a
comprehensive understanding of disease in the community. The
present study demonstrated significant differences in
clinicopathological characteristics of patients with OSCC according
to sex with regard to tumor subsites and a significant difference
in clinical staging between young and old patients. A tumor
subsite-specific pattern in histological differentiation was also
demonstrated, as well as a link between LVI and LNM, but not
between LVI and distant metastasis. In developing a model to
predict advanced stage and margin invasion, the presence of LVI and
young age predicted advanced-stage OSCC among the general
population, yet only LVI predicted advanced-stage disease in young
patients. Mouth NOS and palate subsites predicted the invasion of
surgical margins in the general population; however, the palate
subsite and LVI were predictive factors for invaded margins in
young patients. Given the importance of LVI as a predictive factor
for advanced-stage disease and invaded surgical margins,
pathologists should thoroughly examine the LVI status of patients
with early stage OSCC, particularly young patients with lesions in
the palate. Clinicians should also closely follow up with these
patients to prevent morbidity and decline in quality of life.
Acknowledgments
Not applicable.
Funding
The present study was part of a research project supported by
the Ministry of Research and Technology through a Research and
Community Service Information System (SIMLITABMAS) and Top Basic
Research in University (PDUPT) research grant scheme (grant no.
NKB-122; year, 2021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NR conceived the study. NR, DRH, MS and EK
interpreted data. The formal analysis was performed by NR and MH.
NR acquired the funding and was project administrator. NR and MH
performed the investigation. NR and MH were responsible for the
methodology. NR, DRH, MS and EK provided resources. Software was
used by MH. NR and EK supervised the project, NR, DRH, MS and EK
validated the work. NR and MH wrote the original draft, NR, MH,
DRH, MS and EK reviewed and edited the manuscript. NR and MH
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Faculty of Medicine,
Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital approved the
study protocols (approval no.
KET-178/UN2.F1/ETIK/PPM.00.02/2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krishna Rao SV, Mejia G, Roberts-Thomson K
and Logan R: Epidemiology of oral cancer in Asia in the past
decade-an update (2000–2012). Asian Pac J Cancer Prev.
14:5567–5577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
International Agency for Research on
Cancer and World Health Organization, . GLOBOCAN 2020: Global
Cancer Observatory. Indonesia: 2021
|
|
4
|
Siriwardena BSMS, Karunathilaka HDNU,
Kumarasiri PVR and Tilakaratne WM: Impact of histological and
molecular parameters on prognosis of oral squamous cell carcinoma:
Analysis of 290 cases. Biomed Res Int. 2020:20592402020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dantas DD, Ramos CC, Costa AL, Souza LB
and de Pinto LP: Clinical-pathological parameters in squamous cell
carcinoma of the tongue. Braz Dent J. 14:22–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin NC, Hsu JT and Tsai KY: Survival and
clinicopathological characteristics of different histological
grades of oral cavity squamous cell carcinoma: A single-center
retrospective study. PLoS One. 15:e02381032020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Jamaei AAH, van Dijk BAC, Helder MN,
Forouzanfar T, Leemans CR and de Visscher JGAM: A population-based
study of the epidemiology of oral squamous cell carcinoma in the
Netherlands 1989–2018, with emphasis on young adults. Int J Oral
Maxillofac Surg. 51:18–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santos HB, dos Santos TK, Paz AR,
Cavalcanti YW, Nonaka CF, Godoy GP and Alves PM: Clinical findings
and risk factors to oral squamous cell carcinoma in young patients:
A 12-year retrospective analysis. Med Oral Patol Oral Cir Bucal.
21:e151–e156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dissanayaka WL, Pitiyage G, Kumarasiri PV,
Liyanage RL, Dias KD and Tilakaratne WM: Clinical and
histopathologic parameters in survival of oral squamous cell
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 113:518–525.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seoane-Romero JM, Vázquez-Mahía I, Seoane
J, Varela-Centelles P, Tomás I and López-Cedrún JL: Factors related
to late stage diagnosis of oral squamous cell carcinoma. Med Oral
Patol Oral Cir Bucal. 17:e35–e40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakanishi Y, Yamada SI, Nishizawa R,
Shimane T, Kamata T, Koike T, Uehara S and Kurita H: Risk factors
in securing successful surgical resection of oral squamous cell
carcinoma. Oral Sci Int. 15:56–60. 2018. View Article : Google Scholar
|
|
12
|
Salehiniya H and Raei M: Oral cavity and
lip cancer in the world: An epidemiological review. Biomed Res
Ther. 7:3898–3905. 2020. View Article : Google Scholar
|
|
13
|
Braakhuis BJ, Leemans CR and Visser O:
Incidence and survival trends of head and neck squamous cell
carcinoma in the Netherlands between 1989 and 2011. Oral Oncol.
50:670–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho HC, Lee MS, Hsiao SH, Hwang JH, Hung
SK, Chou P and Lee CC: Squamous cell carcinoma of the oral cavity
in young patients: A matched-pair analysis. Eur Arch
Otorhinolaryngol. 265 (Suppl 1):S57–S61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subramaniam N, Balasubramanian D, Low TH,
Vidhyadharan S, Menon A, Murthy S, Thankappan K, Clark JR, Gao K
and Iyer S: Squamous cell carcinoma of the oral tongue in young
patients: outcomes and implications for treatment. Indian J Surg
Oncol. 11:274–280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maulina T, Iskandarsyah A, Hardianto A,
Sjamsudin E, Nandini M, Kasim A and Yusuf HY: The incidence of oral
squamous cell carcinoma (OSCC) and its relationship with orofacial
pain in oral cancer patients in West Java Province, Indonesia. J
Oral Maxillofac Surg Med Pathol. 29:29–32. 2017. View Article : Google Scholar
|
|
18
|
Gracia I, Utoro T, Supriatno Astuti I,
Heriyanto DS and Pramono D: Epidemiologic profile of oral squamous
cell carcinoma in Yogyakarta, Indonesia. Padjadjaran J Dent.
29:32–37. 2017. View Article : Google Scholar
|
|
19
|
Prayitno A, Aznar E, Poernomo P and Putra
ST: Oral squamous cell carcinoma patients which human papilloma
virus infection: A case control study in Muwardi Hospital
Surakarta, Central Java, Indonesia. Nusant Biosci. 3:64–67.
2011.
|
|
20
|
Purwanto DJ, Soedarsono N, Reuwpassa JO,
Adisasmita AC, Ramli M and Djuwita R: The prevalence of oral
high-risk HPV infection in Indonesian oral squamous cell carcinoma
patients. Oral Dis. 26:72–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Health Organization (WHO), .
International Classification of Diseases for Oncology. Fritz A,
Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM and Whelan S:
3rd edition. WHO; Malta: 2013
|
|
22
|
World Health Organization (WHO), . WHO
classification of tumours: Digestive system tumours. 5th edition.
WHO; Geneva: 2019
|
|
23
|
Wagner VP, Webber LP, Curra M, Klein IP,
Meurer L, Carrad VC and Martins MD: Bryne's grading system predicts
poor disease-specific survival of oral squamous cell carcinoma: A
comparative study among different histologic grading systems. Oral
Surg Oral Med Oral Pathol Oral Radiol. 123:688–696. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kowalski LP and Köhler HF: Relevant
changes in the AJCC 8th edition staging manual for oral cavity
cancer and future implications. Chin Clin Oncol. 8 (Suppl
1):S182019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iamaroon A, Pattanaporn K, Pongsiriwet S,
Wanachantararak S, Prapayasatok S, Jittidecharaks S, Chitapanarux I
and Lorvidhaya V: Analysis of 587 cases of oral squamous cell
carcinoma in northern Thailand with a focus on young people. Int J
Oral Maxillofac Surg. 33:84–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
International Labour Organization (ILO), .
Supporting longer working lives: Multistage approaches for decent
and productive work. ILO; Geneva: 2019
|
|
27
|
World Health Organization, . WHO
classification of head and neck tumours. 4th edition. Vol 9.
El-Naggar AK, Chan JKC, Grandis JR, Takata T and Slootweg PJ: WHO;
Geneva, Switzerland: 2017
|
|
28
|
Luryi AL, Chen MM, Mehra S, Roman SA, Sosa
JA and Judson BL: Positive surgical margins in early stage oral
cavity cancer: An analysis of 20,602 cases. Otolaryngol Head Neck
Surg. 151:984–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thake M and Kalantzis A: Excision margins
in squamous cell carcinoma of the tongue: A retrospective audit and
review of the literature. Open J Stomatol. 3:70–74. 2013.
View Article : Google Scholar
|
|
30
|
Tasche KK, Buchakjian MR, Pagedar NA and
Sperry SM: Definition of ‘close margin’ in oral cancer surgery and
association of margin distance with local recurrence rate. JAMA
Otolaryngol Head Neck Surg. 143:1166–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Franceschi L, Santo JM, de Abreu AM,
Kulcsar MAV, Cernea CR, Garcia MRT, Dedivitis RA and Matos LL:
Staging of oral cavity cancer in the 8th edition of the TNM
classification: The role of computed tomography in the assessment
of depth of invasion and extranodal extension. Arch Head Neck Surg.
47:e08692018. View Article : Google Scholar
|
|
32
|
Wang Q, Gao P, Wang X and Duan Y: The
early diagnosis and monitoring of squamous cell carcinoma via
saliva metabolomics. Sci Rep. 4:68022014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dahlan MS: Logistics regression
multivariate analysis. 2nd edition. Epidemiologi Indonesia Jakarta:
2019, (In Indonesian).
|
|
34
|
Ranganathan P, Pramesh CS and Aggarwal R:
Common pitfalls in statistical analysis: Logistic regression.
Perspect Clin Res. 8:148–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trifonova OP, Lokhov PG and Archakov AI:
Metabolic profiling of human blood. Biomed Khim. 60:281–294.
2014.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cuschieri S: The STROBE guidelines. Saudi
J Anaesth. 13 (Suppl 1):S31–S34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mandrekar JN: Receiver operating
characteristic curve in diagnostic test assessment. J Thorac Oncol.
5:1315–1316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kharisma DD: Indonesian health system
performance assessment: The association between health insurance
expansion with health status and health care access. J Perenc
Pembang Indones J Dev Plan. 4:312–326. 2020.
|
|
39
|
Office of the Vice President, The Republic
of Indonesia, . The Road to National Health Insurance (JKN).
National Team for the Acceleration of Poverty Reduction; Jakarta
Pusat: pp. p1082012, PubMed/NCBI
|
|
40
|
Khuzairi G, Hutajulu SH and Kurnianda J:
The impact of national health insurance of Indonesia on delay in
diagnosis, treatment and survival of nasopharyngeal carcinoma
patients [Abstracts Head and Neck Cancer, Exclusing Thyroid]. Ann
Oncol. 28 (Suppl 10):x1052017. View Article : Google Scholar
|
|
41
|
Jeon JH, Kim MG, Park JY, Lee JH, Kim MJ,
Myoung H and Choi SW: Analysis of the outcome of young age tongue
squamous cell carcinoma. Maxillofac Plast Reconstr Surg. 39:412017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghafari R, Jalayer Naderi N and Emami
Razavi A: A retrospective institutional study of histopathologic
pattern of oral squamous cell carcinoma (OSCC) in Tehran, Iran
during 2006–2015. J Res Med Sci. 24:532019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abdulla R, Adyanthaya S, Kini P, Mohanty
V, D'Souza N and Subbannayya Y: Clinicopathological analysis of
oral squamous cell carcinoma among the younger age group in coastal
Karnataka, India: A retrospective study. J Oral Maxillofac Pathol.
22:180–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kruaysawat W, Aekplakorn W and Chapman RS:
Survival time and prognostic factors of oral cancer in Ubon
Ratchathani cancer center. J Med Assoc Thai. 93:278–284.
2010.PubMed/NCBI
|
|
45
|
Fronie A, Bunget A, Afrem E, Preoţescu LL,
Corlan Puşcu D, Streba L, Mogoantă L and Dumitrescu D: Squamous
cell carcinoma of the oral cavity: Clinical and pathological
aspects. Rom J Morphol Embryol. 54:343–348. 2013.PubMed/NCBI
|
|
46
|
Losi-Guembarovski R, Menezes RP, Poliseli
F, Chaves VN, Kuasne H, Leichsenring A, Maciel ME, Guembarovski AL,
Oliveira BW, Ramos G, et al: Oral carcinoma epidemiology in Paraná
State, Southern Brazil. Cad Saude Publica. 25:393–400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
National Institute of Health Research and
Development Indonesia, . Basic Health Research. Jakarta: 2019, (In
Indonesian).
|
|
48
|
Jainkittivong A, Swasdison S,
Thangpisityotin M and Langlais RP: Oral squamous cell carcinoma: A
clinicopathological study of 342 Thai cases. J Contemp Dent Pract.
10:E033–E40. 2009.PubMed/NCBI
|
|
49
|
Barasch A, Gofa A, Krutchkoff DJ and
Eisenberg E: Squamous cell carcinoma of the gingiva. A case series
analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
80:183–187. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pires FR, Ramos AB, Oliveira JB, Tavares
AS, Luz PS and Santos TC: Oral squamous cell carcinoma:
Clinicopathological features from 346 cases from a single oral
pathology service during an 8-year period. J Appl Oral Sci.
21:460–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kruse AL, Bredell M and Grätz KW: Oral
cancer in men and women: Are there differences? Oral Maxillofac
Surg. 15:51–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Falaki F, Dalirsani Z, Pakfetrat A, Falaki
A, Saghravanian N, Nosratzehi T and Pazouki M: Clinical and
histopathological analysis of oral squamous cell carcinoma of young
patients in Mashhad, Iran: A retrospective study and review of
litera. Med Oral Patol Oral Cir Bucal. 16:e473–e477. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Can HOW, Avoid YOU and In C: Carcinogens
in cosmetics. Campaign Safe Cosmet. 2:2014.
|
|
54
|
Liu S, Hammond SK and Rojas-Cheatham A:
Concentrations and potential health risks of metals in lip
products. Environ Health Perspect. 121:705–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang YH, Lien YC, Ho PS, Chen CH, Chang
JSF, Cheng TC and Shieh TY: The effects of chewing areca/betel quid
with and without cigarette smoking on oral submucous fibrosis and
oral mucosal lesions. Oral Dis. 11:88–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Proia NK, Paszkiewicz GM, Nasca MA, Franke
GE and Pauly JL: Smoking and smokeless tobacco-associated human
buccal cell mutations and their association with oral cancer-a
review. Cancer Epidemiol Biomarkers Prev. 15:1061–1077. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Behura SS, Masthan MK and Narayanasamy AB:
Oral mucosal lesions associated with smokers and chewers-a
case-control study in Chennai population. J Clin Diagn Res.
9:ZC17–ZC22. 2015.PubMed/NCBI
|
|
58
|
Al-Rawi NH and Talabani NG: Squamous cell
carcinoma of the oral cavity: A case series analysis of clinical
presentation and histological grading of 1,425 cases from Iraq.
Clin Oral Investig. 12:15–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Andisheh-Tadbir A, Mehrabani D and Heydari
ST: Epidemiology of squamous cell carcinoma of the oral cavity in
Iran. J Craniofac Surg. 19:1699–1702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gervásio OL, Dutra RA, Tartaglia SM,
Vasconcellos WA, Barbosa AA and Aguiar MC: Oral squamous cell
carcinoma: A retrospective study of 740 cases in a Brazilian
population. Braz Dent J. 12:57–61. 2001.PubMed/NCBI
|
|
61
|
Grimm M: Prognostic value of
clinicopathological parameters and outcome in 484 patients with
oral squamous cell carcinoma: Microvascular invasion (V+) is an
independent prognostic factor for OSCC. Clin Transl Oncol.
14:870–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Marocchio LS, Lima J, Sperandio FF, Corrêa
L and de Sousa SOM: Oral squamous cell carcinoma: An analysis of
1,564 cases showing advances in early detection. J Oral Sci.
52:267–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sasaki T, Moles DR, Imai Y and Speight PM:
Clinico-pathological features of squamous cell carcinoma of the
oral cavity in patients <40 years of age. J Oral Pathol Med.
34:129–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ribeiro AC, Silva AR, Simonato LE,
Salzedas LM, Sundefeld ML and Soubhia AM: Clinical and
histopathological analysis of oral squamous cell carcinoma in young
people: A descriptive study in Brazilians. Br J Oral Maxillofac
Surg. 47:95–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Llewellyn CD, Linklater K, Bell J, Johnson
NW and Warnakulasuriya S: An analysis of risk factors for oral
cancer in young people: A case-control study. Oral Oncol.
40:304–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Arotiba GT, Ladeinde AL, Oyeneyin JO,
Nwawolo CC, Banjo AA and Ajayi OF: Malignant orofacial neoplasms in
Lagos, Nigeria. East Afr Med J. 83:62–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Johnson NW, Jayasekara P and Amarasinghe
AA: Squamous cell carcinoma and precursor lesions of the oral
cavity: Epidemiology and aetiology. Periodontol 2000. 57:19–37.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hayes A and Setyonaluri D: Taking
advantage of the demographic dividend in indonesia: A brief
introduction to theory and practice. UNFPA; WHO, Jakarta: 2015
|
|
69
|
Gani A and Budiharsana MP: The
consolidated report on indonesia health sector review 2018. Ali PB,
Siahaan RGM and Ardhiantie A: National Health System Strengthening;
Jakarta: 2019
|
|
70
|
Effiom OA, Adeyemo WL, Omitola OG, Ajayi
OF, Emmanuel MM and Gbotolorun OM: Oral squamous cell carcinoma: A
clinicopathologic review of 233 cases in Lagos, Nigeria. J Oral
Maxillofac Surg. 66:1595–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Toscano de Brito R, França Perazzo M,
Santos Peixoto T, Weege-Nonaka CF, de Melo Brito Costa EM and
Granville-Garcia AF: Profile of patients and factors related to the
clinical staging of oral squamous cell carcinoma. Rev Salud Publica
(Bogota). 20:221–225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Umpiérrez NG and Cortegoso VB: Profile of
oral squamous cell carcinoma at the anatomic pathology laboratory
of the school of dentistry of universidad de la República,
1982–2015 period. Odontoestomatología. 22:34–43. 2020.
|
|
73
|
Smitha T, Mohan CV and Hemavathy S:
Clinicopathological features of oral squamous cell carcinoma: A
hospital-based retrospective study. J Dr NTR Univ Health Sci.
6:29–34. 2017.
|
|
74
|
de Araújo RF Jr, Barboza CA, Clebis NK, de
Moura SA and Lopes Costa Ade L: Prognostic significance of the
anatomical location and TNM clinical classification in oral
squamous cell carcinoma. Med Oral Patol Oral Cir Bucal.
13:E344–E347. 2008.PubMed/NCBI
|
|
75
|
Leite AA, Leonel ACLDS, Castro JFL,
Carvalho EJA, Vargas PA, Kowalski LP and Perez DEDC: Oral squamous
cell carcinoma: A clinicopathological study on 194 cases in
northeastern Brazil. A cross-sectional retrospective study. Sao
Paulo Med J. 136:165–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Costa Ade L, Pereira JC, Nunes AA and
Arruda Mde L: Correlation between TNM classification, histological
grading and anatomical location in oral squamous cell carcinoma.
Pesqui Odontol Bras. 16:216–220. 2002.(In Portuguese). PubMed/NCBI
|
|
77
|
Costa Ade L, Araújo Júnior RF and Ramos
CC: Correlation between TNM classification and malignancy
histological feature of oral squamous cell carcinoma. Braz J
Otorhinolaryngol. 71:181–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sarode SC and Sarode GS: Better grade of
tumor differentiation of oral squamous cell carcinoma arising in
background of oral submucous fibrosis. Med Hypotheses. 81:540–543.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chidzonga MM: Oral malignant neoplasia: A
survey of 428 cases in two Zimbabwean hospitals. Oral Oncol.
42:177–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chidzonga MM and Mahomva L: Squamous cell
carcinoma of the oral cavity, maxillary antrum and lip in a
Zimbabwean population: A descriptive epidemiological study. Oral
Oncol. 42:184–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Padma R, Kalaivani A, Sundaresan S and
Sathish P: The relationship between histological differentiation
and disease recurrence of primary oral squamous cell carcinoma. J
Oral Maxillofac Pathol. 21:4612017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sudhakara M, Reshma V, Khan N and Amulya
SR: Uncommon features in conventional oral squamous cell carcinoma.
J Oral Maxillofac Pathol. 20:316–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Taghavi N and Yazdi I: Prognostic factors
of survival rate in oral squamous cell carcinoma: Clinical,
histologic, genetic and molecular concepts. Arch Iran Med.
18:314–319. 2015.PubMed/NCBI
|
|
84
|
Woolgar JA: Histopathological
prognosticators in oral and oropharyngeal squamous cell carcinoma.
Oral Oncol. 42:229–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xu QS, Wang C, Li B, Li JZ, Mao MH, Qin
LZ, Li H, Huang X, Han Z and Feng Z: Prognostic value of pathologic
grade for patients with oral squamous cell carcinoma. Oral Dis.
24:335–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kartini D, Kurnia A, Putri SR, Thaher TC,
Handjari DR, Khoe LC and Marcevianto KV: Survival rate and
prognostic factors of oral squamous cell carcinoma in Indonesia: A
single-center retrospective study. Forum Clin Oncol. 13:1–8.
2022.
|
|
87
|
Sutandyo N, Ramli R, Sari L and Soeis DS:
Profile and survival of tongue cancer patients in ‘Dharmais’ cancer
hospital, Jakarta. Asian Pac J Cancer Prev. 15:1971–1975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Inverso G, Mahal BA, Aizer AA, Donoff RB
and Chuang SK: Health insurance affects head and neck cancer
treatment patterns and outcomes. J Oral Maxillofac Surg.
74:1241–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
de Barros Silva PG, Mesquita KC, Dantas
TS, Ferreira Junior AEC, Avelar RL, Martins Neto RS, Mota MRL,
Alves APNN and Sousa FB: A ten-year retrospective study of the
clinical, sociodemographic, and survival characteristics of
patients with oral and pharyngeal squamous cell carcinomas. Braz J
Health Rev. 2:5160–5172. 2019. View Article : Google Scholar
|
|
90
|
Elaiwy O, El Ansari W, AlKhalil M and
Ammar A: Epidemiology and pathology of oral squamous cell carcinoma
in a multi-ethnic population: Retrospective study of 154 cases over
7 years in Qatar. Ann Med Surg (Lond). 60:195–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Oliveira ML, Wagner VP, Sant'ana Filho M,
Carrard VC, Hugo FN and Martins MD: A 10-year analysis of the oral
squamous cell carcinoma profile in patients from public health
centers in Uruguay. Braz Oral Res. 29:S1806–83242015000100270.
2015. View Article : Google Scholar
|
|
92
|
Gajurel R, Gautam DK, Pun CB, Dhakal HP,
Petrovski BÉ, Costea DE and Sapkota D: Trends and
clinicopathological characteristics of oral squamous cell
carcinomas reported at a tertiary cancer hospital in Nepal during
1999 to 2009. Clin Exp Dent Res. 6:356–362. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Misra G, Prasad K, Rao RS, Lalitha RM,
Ranganath K, Rajanikanth BR and Mane R: Retrospective analysis of a
6-year cohort of oral squamous cell carcinoma patients. J Adv Clin
Res Insights. 4:129–135. 2017. View Article : Google Scholar
|
|
94
|
Martini G: Clinicopathological parameters
of oral squamous cell carcinoma in Florianópolis: A 10-year study.
J Oral Diag. 3:e201800292018.
|
|
95
|
Shenoi R, Devrukhkar V, Chaudhur Sharma
BK, Sapre SB and Chikhale A: Demographic and clinical profile of
oral squamous cell carcinoma patients: A retrospective study.
Indian J Cancer. 49:21–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kreppel M, Eich HT, Kübler A, Zöller JE
and Scheer M: Prognostic value of the sixth edition of the UICC's
TNM classification and stage grouping for oral cancer. J Surg
Oncol. 102:443–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Huang S, Zhu Y, Cai H, Zhang Y and Hou J:
Impact of lymphovascular invasion in oral squamous cell carcinoma:
A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol.
131:319–328.e1. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ting KC, Lee TL, Li WY, Chang CF, Chu PY,
Wang YF and Tai SK: Perineural invasion/lymphovascular invasion
double positive predicts distant metastasis and poor survival in
T3–4 oral squamous cell carcinoma. Sci Rep. 11:197702021.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nason RW, Binahmed A, Pathak KA, Abdoh AA
and Sándor GK: What is the adequate margin of surgical resection in
oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
107:625–629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sieczka E, Datta R, Singh A, Loree T,
Rigual N, Orner J and Hicks W Jr: Cancer of the buccal mucosa: Are
margins and T-stage accurate predictors of local control? Am J
Otolaryngol. 22:395–399. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jan JC, Hsu WH, Liu SA, Wong YK, Poon CK,
Jiang RS, Jan JS and Chen IF: Prognostic factors in patients with
buccal squamous cell carcinoma: 10-Year experience. J Oral
Maxillofac Surg. 69:396–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kamat M, Rai BD, Puranik RS and Datar UV:
A comprehensive review of surgical margin in oral squamous cell
carcinoma highlighting the significance of tumor-free surgical
margins. J Cancer Res Ther. 15:449–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dissanayake U: Malignancy grading of
invasive fronts of oral squamous cell carcinomas: Correlation with
overall survival. Transl Res Oral Oncol. 2:1–8. 2017.
|
|
104
|
Kuriakose M, Sankaranarayanan M, Nair MK,
Cherian T, Sugar AW, Scully C and Prime SS: Comparison of oral
squamous cell carcinoma in younger and older patients in India. Eur
J Cancer B Oral Oncol. 28B:113–120. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yang YH, Chen CH, Chang JS, Lin CC, Cheng
TC and Shieh TY: Incidence rates of oral cancer and oral
pre-cancerous lesions in a 6-year follow-up study of a Taiwanese
aboriginal community. J Oral Pathol Med. 34:596–601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mignogna MD, Fedele S and Lo Russo L: The
world cancer report and the burden of oral cancer. Eur J Cancer
Prev. 13:139–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liao CT, Chang JT, Wang HM, Ng SH, Hsueh
C, Lee LY, Lin CH, Chen IH, Huang SF, Cheng AJ and Yen TC: Survival
in squamous cell carcinoma of the oral cavity: Differences between
pT4 N0 and other stage IVA categories. Cancer. 110:564–571. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mascitti M, Tempesta A, Togni L,
Capodiferro S, Troiano G, Rubini C, Maiorano E, Santarelli A, Favia
G and Limongelli L: Histological features and survival in young
patients with HPV-negative oral squamous cell carcinoma. Oral Dis.
26:1640–1648. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jerjes W, Upile T, Petrie A, Riskalla A,
Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ,
et al: Clinicopathological parameters, recurrence, locoregional and
distant metastasis in 115 T1-T2 oral squamous cell carcinoma
patients. Head Neck Oncol. 2:92010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sharma M, Deshpandey A, Gupta N and Patel
M: Retrospective analysis of oral cavity squamous cell carcinoma
treated with surgery and adjuvent radiotherapy. Int J Res Med Sci.
4:1000–1004. 2016. View Article : Google Scholar
|
|
112
|
Campbell BR, Netterville JL, Sinard RJ,
Mannion K, Rohde SL, Langerman A, Kim YJ, Lewis JS Jr and Lang Kuhs
KA: Early onset oral tongue cancer in the United States: A
literature review. Oral Oncol. 87:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Garavello W, Spreafico R, Somigliana E,
Gaini L, Pignataro L and Gaini RM: Prognostic influence of gender
in patients with oral tongue cancer. Otolaryngol Head Neck Surg.
138:768–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Pimenta Amaral TM, Da Silva Freire AR,
Carvalho AL, Pinto CA and Kowalski LP: Predictive factors of occult
metastasis and prognosis of clinical stages I and II squamous cell
carcinoma of the tongue and floor of the mouth. Oral Oncol.
40:780–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kowalski LP, Carvalho AL, Martins Priante
AV and Magrin J: Predictive factors for distant metastasis from
oral and oropharyngeal squamous cell carcinoma. Oral Oncol.
41:534–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Monteiro LS, Amaral JB, Vizcaíno JR, Lopes
CA and Torres FO: A clinical-pathological and survival study of
oral squamous cell carcinomas from a population of the North of
Portugal. Med Oral Patol Oral Cir Bucal. 19:e120–e126. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Vargas H, Pitman KT, Johnson JT and Galati
LT: More aggressive behavior of squamous cell carcinoma of the
anterior tongue in young women. Laryngoscope. 110:1623–1626. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen PH, Shieh TY, Ho PS, Tsai CC, Yang
YH, Lin YC, Ko MS, Tsai PC, Chiang SL, Tu HP and Ko YC: Prognostic
factors associated with the survival of oral and pharyngeal
carcinoma in Taiwan. BMC Cancer. 7:1012007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Manuel S, Raghavan SK, Pandey M and
Sebastian P: Survival in patients under 45 years with squamous cell
carcinoma of the oral tongue. Int J Oral Maxillofac Surg.
32:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jamadar S, Narayan TV, Shreedhar B,
Mohanty L and Shenoy S: Comparative study of various grading
systems in oral squamous cell carcinoma and their value in
predicting lymph node metastasis. Indian J Dent Res. 25:357–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Majumdar B, Patil S, Sarode SC, Sarode GS
and Rao RS: Clinico-pathological prognosticators in oral squamous
cell carcinoma: An update. Transl Res Oral Oncol. 2:1–14. 2017.
|
|
122
|
Abbas SA, Saeed J, Tariq MU, Baksh AR and
Hashmi S: Clinicopathological prognostic factors of oral squamous
cell carcinoma: An experience of a tertiary care hospital. J Pak
Med Assoc. 68:1115–1119. 2018.PubMed/NCBI
|
|
123
|
Lopes MA, Nikitakis NG, Reynolds MA, Ord
RA and Sauk J Jr: Biomarkers predictive of lymph node metastases in
oral squamous cell carcinoma. J Oral Maxillofac Surg. 60:142–148.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
de Matos FR, Lima ED, Queiroz LM and da
Silveira EJ: Analysis of inflammatory infiltrate, perineural
invasion, and risk score can indicate concurrent metastasis in
squamous cell carcinoma of the tongue. J Oral Maxillofac Surg.
70:1703–1710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kademani D, Bell RB, Bagheri S, Holmgren
E, Dierks E, Potter B and Homer L: Prognostic factors in intraoral
squamous cell carcinoma: The influence of histologic grade. J Oral
Maxillofac Surg. 63:1599–1605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sutton DN, Brown JS, Rogers SN, Vaughan ED
and Woolgar JA: The prognostic implications of the surgical margin
in oral squamous cell carcinoma. Int J Oral Maxillofac Surg.
32:30–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Solomon J, Hinther A, Matthews TW,
Nakoneshny SC, Hart R, Dort JC and Chandarana SP: The impact of
close surgical margins on recurrence in oral squamous cell
carcinoma. J Otolaryngol Head Neck Surg. 50:92021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Woolgar JA, Scott J, Vaughan ED, Brown JS,
West CR and Rogers S: Survival, metastasis and recurrence of oral
cancer in relation to pathological features. Ann R Coll Surg Engl.
77:325–331. 1995.PubMed/NCBI
|
|
129
|
Binahmed A, Nason RW and Abdoh AA: The
clinical significance of the positive surgical margin in oral
cancer. Oral Oncol. 43:780–784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liu SA, Wang CC, Jiang RS, Lee FY, Lin WJ
and Lin JC: Pathological features and their prognostic impacts on
oral cavity cancer patients among different subsites-a singe
institute's experience in Taiwan. Sci Rep. 7:74512017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Brinkman D, Callanan D, O'Shea R, Jawad H,
Feeley L and Sheahan P: Impact of 3 mm margin on risk of recurrence
and survival in oral cancer. Oral Oncol. 110:1048832020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Campbell DA, Pipkorn P, Divi V, Stadler M,
Massey B, Campbell B, Richmon JD, Graboyes E, Puram S and Zenga J:
The effect of reconstruction on positive margin rates in oral
cancer: Using length of stay as a proxy measure for flap
reconstruction in a national database. Am J Otolaryngol.
42:1030122021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Young A and Okuyemi OT: Malignant tumors
of the palate. StatPearls [Internet]. StatPearls Publishing;
Treasure Island, FL: 2022
|
|
134
|
Hagiwara M, Nusbaum A and Schmidt BL: MR
assessment of oral cavity carcinomas. Magn Reson Imaging Clin N Am.
20:473–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Clark JR, Naranjo N, Franklin JH, de
Almeida J and Gullane PJ: Established prognostic variables in N0
oral carcinoma. Otolaryngol Head Neck Surg. 135:748–753. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kang YJ, Kim HS, Jang WS, Kwon JK, Yoon
CY, Lee JY, Cho KS, Ham WS and Choi YD: Impact of lymphovascular
invasion on lymph node metastasis for patients undergoing radical
prostatectomy with negative resection margin. BMC Cancer.
17:3212017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Galiabovitch E, Hovens CM, Peters JS,
Costello AJ, Battye S, Norden S, Ryan A and Corcoran NM: Routinely
reported ‘equivocal’ lymphovascular invasion in prostatectomy
specimens is associated with adverse outcomes. BJU Int.
119:567–572. 2017. View Article : Google Scholar : PubMed/NCBI
|