Introduction
Small-cell lung cancer (SCLC) is a highly malignant
lung tumor that accounts for 10–15% of all lung cancers (1). It is a poorly differentiated tumor
immunohistochemically expresses neuroendocrine markers and is
characterized by rapid progression, early metastatic spread, and
early response to treatment. In the first-line setting, SCLC is
highly sensitive to chemotherapy with or without radiotherapy;
however, most patients experience relapse within one year after
treatment.
Japanese guidelines recommend platinum-doublet
therapy with etoposide (VP-16) or irinotecan (CPT-11) as the
first-line treatment for extensive stage SCLC and additional immune
checkpoint inhibitors have recently become an option (2,3).
However, little progress has been made in treating patients with
recurrent diseases. In the second-line setting, the time from the
completion of first-line therapy to recurrence is considered a
prognostic factor. In cases of recurrence later than 90 days after
the first-line treatment, we refer to it as ‘sensitive relapse’ and
otherwise as ‘refractory relapse’ (4). For sensitive relapse, the efficacy of
some regimens, including single-agent or combined therapies, has
been reported. As for single agents, nogitecan (NGT) and amrubicin
(AMR) are included (5–11). Combined chemotherapy consists of
cisplatin (CDDP) plus VP-16, CPT-11, or carboplatin (CBDCA) plus
VP-16 (12–14). AMR is preferred for refractory
relapses (9,11,15).
However, there is no recommendation for third-line or later
treatments. Compared with non-small cell carcinoma (NSCLC), fewer
options are available for SCLC. Therefore, we aimed to explore the
efficacy of chemotherapy as a third-line treatment.
Patients and methods
Patients diagnosed with SCLC between January 2015
and August 2019 at Hirosaki University Hospital, Aomori Prefectural
Central Hospital, and Hirosaki Central Hospital were
retrospectively reviewed. All patients were checked for age, sex,
stage (limited or extended), date of last observation and survival,
time to treatment failure (TTF), performance status (PS), the
existence of metastatic brain tumor, hematological and
non-hematological toxicities, and timing of relapse after
first-line therapy (e.g., sensitive or refractory). TTF was defined
as the time from the start of third-line chemotherapy to the date
of treatment discontinuation (all-cause death, disease progression,
or all-causal treatment discontinuation, including toxicity or
aggravation of general condition). In this study, we adopted TTF,
not progression-free survival (PFS) time, because patients who were
assessed as having progressive disease not by the Response
Evaluation Criteria in Solid Tumors (RECIST) but by the definitive
deterioration of clinical symptoms or radiological evaluation were
included. All data were analyzed with a cut-off date of June 30,
2020. All categorical variables were analyzed using Fisher's exact
test. The primary endpoints were overall survival (OS) and TTF. The
association between the type of regimen (platinum-doublet or
single-agent) and either TTF or OS was examined using the Cox
proportional hazards model. Age, sex, stage, PS, the existence of
metastatic brain tumor, and manner of relapse were included as
covariates. Significant factors were assessed in the univariate
analysis. Toxicity was assessed using the National Cancer Institute
Common Toxicity Criteria, version 4.0. P-values are considered to
be significant if less than 0.05. Statistical analyses were
performed using JMP Pro version 15.2. The study was performed
according to the protocol approved by the Ethics Committee of the
Hirosaki University Graduate School of Medicine (approval number;
2020-048). As this was a retrospective cohort study, the
requirement for informed consent was waived. An opt-out option was
conducted on the website of each hospital.
Results
Recruitment of patients
Of the 111 patients diagnosed with SCLC between
January 2015 and August 2019, 37 received third-line treatment.
Fifteen patients received a platinum-doublet regimen, and 22
patients received a single-agent regimen. The patient
characteristics are summarized in Table I. No significant differences in
age, sex, PS at third-line treatment, disease extent at diagnosis,
relapse manner following first-line treatment, the existence of
brain metastases, and proportion of patients who could undergo
subsequent chemotherapy after third-line treatment failure were
found between the two groups. The previous treatments are listed in
Table II. As the first-line
treatment, VP-16 was the preferred complementary agent for platinum
over CPT-11 in our institutions. In the second-line setting, the
most commonly used regimen was amrubicin (AMR), followed by CDBCA +
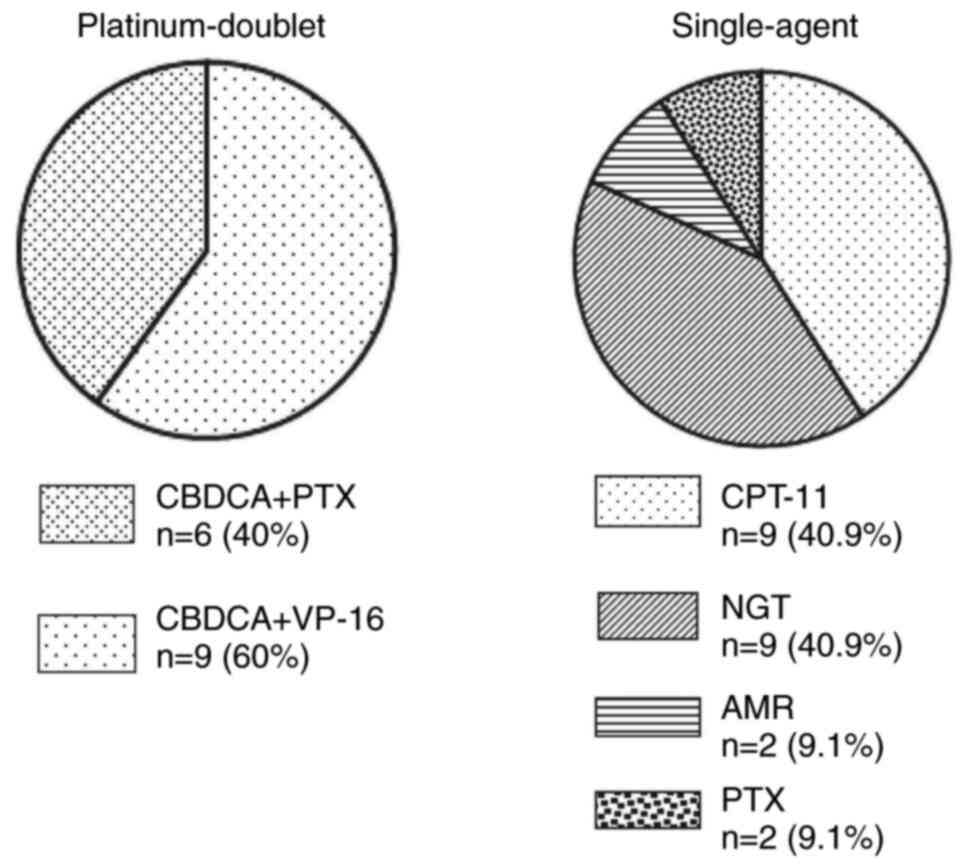
paclitaxel (PTX). The third-line treatment regimen is shown in
Fig. 1. In the platinum doublet
group, CBDCA + VP-16 and CBDCA + PTX accounted for 60 and 40%,
respectively. In the single-agent regimen, CPT-11, NGT, AMR, and
PTX accounted for 40.9, 40.9, 9.1, and 9.1% of cases,
respectively.
 | Table I.Comparison of characteristics between
two groups. |
Table I.
Comparison of characteristics between
two groups.
| Characteristic | Platinum-doublet
(n=15) | Single-agent regimen
(n=22) | P-value |
|---|
| Age, median
(range) | 64 (46–81) | 67 (44–82) | 0.33 |
| Sex
(male/female) | 11/4 | 17/5 | 1.00 |
| PS (0-1/≥2) | 12/3 | 19/3 | 0.67 |
| Manner of
relapse |
|
|
|
|
Sensitive/refractory | 3/12 | 8/14 | 0.47 |
| Disease extent |
|
|
|
| Limited
disease/extensive disease | 3/12 | 5/17 | 1.00 |
| Brain metastases, n
(%) | 8 (53) | 10 (55) | 0.74 |
| Post treatment, n
(%) | 7 (47) | 14 (64) | 0.33 |
 | Table II.Previous treatment regimen in the two
groups. |
Table II.
Previous treatment regimen in the two
groups.
| Regimen | Platinum-doublet
(n=15) | Single-agent
(n=22) |
|---|
| First line |
|
|
| Platinum
+ etoposide | 9 | 19 |
| Platinum
+ irinotecan | 6 | 3 |
| Second line |
|
|
|
Carboplatin + etoposide | 2 | 4 |
|
Carboplatin + paclitaxel | 2 | 1 |
|
Amrubicin | 11 | 17 |
Evaluating the endpoint
We evaluated the impact of the type of regimen on
the endpoint (TTF or OS) using a Cox proportional hazards model,
including covariates. In univariate analysis, only the type of
regimen (platinum-doublet) was significantly associated with TTF
(odds ratio 0.44 (95% confidence interval 0.20-0.95), P=0.03)
(Table III). Thus, we did not
conduct a multivariate analysis of TTF. We evaluated the
association between regimen type and OS. In the univariate
analysis, none of the variables were associated with OS.
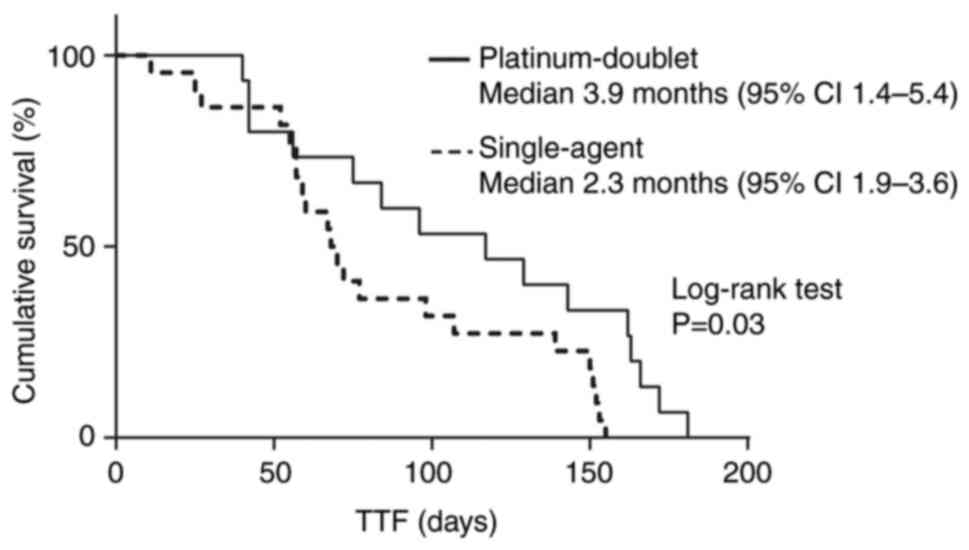
Subsequently, we evaluated the TTF using log-rank tests for the
type of regimen. The median TTF was 2.3 and 3.9 months in
single-agent and platinum-doublet regimens, respectively (P=0.03)
(Fig. 2).
 | Table III.Association of variables with TTF or
OS using Cox proportional hazard model. |
Table III.
Association of variables with TTF or
OS using Cox proportional hazard model.
|
| TTF | OS |
|---|
|
|
|
|
|---|
| Variable | Odds ratio | P-value | Odds ratio | P-value |
|---|
| Sex (male) | 0.74 (0.35-1.60) | 0.46 | 1.28 (0.53-3.05) | 0.56 |
| Age | 1.01 (0.97-1.05) | 0.51 | 1.02 (1.07-0.97) | 0.39 |
| Third-line
regimen |
|
|
|
|
|
Platinum-doublet vs.
single-agent | 0.44 (0.20-0.95) | 0.03 | 1.41 (0.66-3.00) | 0.36 |
| PS at the start of
third-line |
|
|
|
|
| 0–1 vs.
≥2 | 1.09 (0.44-2.70) | 0.83 | 0.73 (0.21-2.55) | 0.64 |
| Disease extent |
|
|
|
|
| LD vs.
ED | 0.94 (0.42-2.07) | 0.87 | 0.83 (0.35-1.97) | 0.66 |
| Manner of
relapse |
|
|
|
|
| Sensitive
vs. refractory | 0.98 (0.48-2.00) | 0.96 | 0.65 (0.26-1.62) | 0.34 |
| Existence of brain
metastases |
|
|
|
|
| Yes vs.
No | 1.45 (0.73-2.85) | 0.28 | 1.29 (0.61-2.71) | 0.49 |
Efficacy
We also evaluated the efficacy in both groups
(Table IV). The platinum-doublet
and single-agent regimens' overall response rates (ORR) were 20.0
and 4.5%, respectively. Disease control rates (DCR) were 73.3 and
36.4% for platinum-doublet and single-agent regimens, respectively.
In addition, we evaluated the efficacy of the platinum doublet
group (Table SI). Six patients
received the CBDCA+VP-16 regimen, and nine patients received the
CBDCA + PTX regimen. The ORR was 0 and 55.5% in the CBDCA + VP-16
and CBDCA + PTX groups, respectively. The DCRs were 66.7 and 44.4%,
respectively.
 | Table IV.Best response following third-line
treatment. |
Table IV.
Best response following third-line
treatment.
| Response | Platinum-doublet
regimen, n | Single-agent
regimen, n |
|---|
| Complete
response | 0 | 0 |
| Partial
response | 3 | 1 |
| Stable disease | 8 | 7 |
| Progressive
disease | 4 | 14 |
| Response rate,
% | 20.0 | 4.5 |
| Disease control
rate, % | 73.3 | 36.4 |
Toxicity
Concerning treatment-related adverse events (TRAEs),
most TRAEs of any grade were more frequent in the platinum-doublet
group, except for anorexia, febrile neutropenia, and fatigue
(Table V). Severe TRAEs, defined
as Common Terminology Criteria for Adverse Events (CTCAE) grade 3
or higher regarding myelosuppression, were more frequent in the
platinum-doublet group.
 | Table V.Adverse events. |
Table V.
Adverse events.
|
| Toxicity, n
(%) |
|---|
|
|
|
|---|
|
| All | Grade 3≤ |
|---|
|
|
|
|
|---|
| Adverse event | Platinum-doublet
regimen | Single-agent
regimen | Platinum-doublet
regimen | Single-agent
regimen |
|---|
| Neutropenia | 11 (73.3) | 13 (59.0) | 4 (26.6) | 3 (13.6) |
| Anemia | 11 (73.3) | 12 (54.5) | 8 (53.3) | 6 (27.2) |
|
Thrombocytopenia | 10 (66.6) | 11 (50.0) | 2 (13.3) | 1 (4.5) |
| Febrile
neutropenia | 0 (0) | 2 (9.0) | 0 (0) | 2 (9.0) |
| Anorexia | 5 (33.3) | 13 (59.0) | 0 (0) | 2 (9.0) |
| Fatigue | 2 (13.3) | 7 (31.8) | 0 (0) | 0 (0) |
| Constipation | 6 (40.0) | 5 (22.7) | 0 (0) | 0 (0) |
| Neuropathy | 5 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Pneumonitis | 1 (6.6) | 1 (4.5) | 1 (6.6) | 1 (4.5) |
Discussion
Most patients with extensive SCLC progress after
first-line therapy. The selection of second-line therapy depends on
the response to first-line chemotherapy, and the treatment strategy
is divided into two types. One is sensitive relapse, and the other
is refractory relapse. Generally, sensitive relapse is defined as
patients who respond to first-line therapy and relapse more than
three months after the completion of first-line chemotherapy.
Refractory relapse is defined as relapse within three months
(4). This distinction is important
because sensitive diseases tend to respond to further systemic
therapies, including agents used for first-line chemotherapy. In
refractory or recurrent disease, we administer drugs other than
those used in first-line therapy (8,16,17).
Moreover, there is little evidence supporting the introduction of
third-line treatment rather than the best supportive care. There is
no recommended drug or combination in the third-line setting or
after that. Given that life expectancy is shorter in patients with
SCLC than in those with NSCLC, we assumed that platinum-doublet
therapy would be admissible in third-line settings. In the
second-line setting, a meta-analysis of the efficacy of
platinum-doublet chemotherapy has been reported (18). Most reports have demonstrated that
platinum-doublet chemotherapy may be superior to single-agent
chemotherapy in terms of OS and PFS. For example, in the
second-line setting of sensitive relapse, a phase 3 study on the
superiority of CBDCA + VP-16 to NGT in terms of PFS has been
recently reported (14). In our
analysis, the number of patients who could move to third-line
therapy was 24% (27/111), which was similar to the number shown in
a previous report (19). In the
analysis of the association between treatment regimens and TTF or
OS, we included the well-known covariates affecting the prognosis
for ED-SCLC, among which only treatment regimen was identified as
the significant factor affecting TTF. In our analysis, the platinum
doublet demonstrated a relatively high efficacy. In a retrospective
analysis of third-line chemotherapy for SCLC, the platinum-doublet
regimen tended to improve OS (hazard ratio: 0.84, 95% confidence
interval: 0.59-1.19) and ORR (P=0.086) (19). In our analysis, platinum-doublet
therapy tended to deteriorate OS in contrast to TTF. OS is likely
to be affected by some factors, including pre-and post-treatment
complications, such as interstitial lung disease. Therefore, we
considered that the platinum-doublet regimen might be the first
choice for appropriate patients. In the platinum doublet group, the
CBDCA + PTX group showed better ORR and DCR. Meanwhile, in the
CBDCA+VP-16 group, all patients except for one received a second
dose as a re-challenge setting. Although none of the patients
responded to CBDCA+VP-16, the DCR was somewhat high. Considering
these results, in third-line settings, the platinum-doublet regimen
might play a role in disease control even in the re-challenge
setting. Notably, the CBDCA + PTX group demonstrated a high ORR and
DCR. The CBDCA + PTX group, which accounted for a large proportion
of the platinum-doublet group, might have contributed to the better
TTF. In the last few years, there have been reports regarding the
efficacy of immune checkpoint inhibitors (nivolumab) for previously
treated SCLC. Ready et al evaluated patients with SCLC who
received nivolumab in the third or later setting in the CheckMate
032 trial. The median PFS and ORR were 1.4 months and 11.9%,
respectively (20). Spigel et
al evaluated the superiority of nivolumab over chemotherapy for
OS in a second-line setting (21).
However, they could not demonstrate the superiority of nivolumab in
terms of OS. PFS and ORR were 1.4 months and 13.7%, respectively.
We assume that the high response rate provided by the
platinum-doublet regimen might be important for better outcomes in
rapidly growing tumors, such as SCLC. The platinum doublet
demonstrated good tolerability in the present study, even in a
third-line setting. In the above meta-analysis, some studies
demonstrated that grade 3 or 4 neutropenia was observed in more
than 70% of patients who received the CBDCA plus VP-16 regimen
(18). In contrast, the phase 3
study stated above demonstrated that the incidence of any grade 3
or 4 adverse events was less than 30% in the CBDCA plus VP-16 group
(14). In our study, grade 3 or 4
neutropenia was relatively less frequent, and grade 3 or 4
thrombocytopenia was more frequent in the platinum-doublet group.
We speculated that neutropenia was less frequent in our study
because pegfilgrastim was administered to most patients who
received a platinum-doublet regimen as primary prevention. However,
in late-line settings, careful attention must be paid to adverse
events, including myelosuppression. We might be able to consider
the platinum-doublet regimen in a third-line setting when the
patients are considered to be in good condition and well
tolerated.
Our study had some limitations. First, those who
could move onto the third-line therapy were very few, which might
have been why well-known covariates affecting the prognosis of SCLC
were not significant even in univariate analysis. Second, since the
present report was a retrospective analysis, it remains unclear
whether a platinum-doublet regimen should be administered to all
patients. Third, most patients with ED-SCLC receive
platinum-doublet chemotherapy plus anti-PD-L1 as the first-line
therapy. Therefore, we must address whether platinum-based
chemotherapy is feasible for relapsed disease following
platinum-based chemotherapy plus anti-PD-L1.
In conclusion, we have demonstrated the feasibility
and safety of platinum-based regimens in patients with SCLC in a
third-line setting. The platinum doublet regimen might favor some
patients who can access third-line treatment. However, this study
was only a small retrospective analysis. Currently, because the
standard first-line therapy is platinum-based chemotherapy plus
anti-PD-L1, we need to explore the feasibility of platinum-based
therapy for patients with relapse following platinum-based
chemotherapy plus anti-PD-L1.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF, TM, and HT conceptualized this study. TF, YT,
KC, MI, SS, YH and KO obtained data. TF, TM and HS prepared figures
and tables. TF, TM, HT, KT and ST designed the study and drafted
the manuscript. KT and ST analyzed the data and provided critical
revisions. KO and YH confirmed the authenticity of the raw data.
All authors contributed to the manuscript revision and have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Ethics Committee of the Hirosaki University Graduate
School of Medicine (approval no. 2020-048). As this was a
retrospective cohort study, the requirement for informed consent
was waived. Opt-out was carried out on the Hirosaki University
Hospital website.
Patient consent for publication
This was a retrospective study. The requirement for
informed consent was waived, and an opt-out option was conducted on
the website of each hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YH, Goto K, Yoh K, Niho S, Ohmatsu H,
Kubota K, Saijo N and Nishiwaki Y: Performance status and
sensitivity to first-line chemotherapy are significant prognostic
factors in patients with recurrent small cell lung cancer receiving
second-line chemotherapy. Cancer. 113:2518–2523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Cuceviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Pawel J, Schiller JH, Shepherd FA,
Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer
MC, Depierre A, et al: Topotecan versus cyclophosphamide,
doxorubicin, and vincristine for the treatment of recurrent
small-cell lung cancer. J Clin Oncol. 17:658–667. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eckardt JR, von Pawel J, Pujol JL, Papai
Z, Quoix E, Ardizzoni A, Poulin R, Preston AJ, Dane G and Ross G:
Phase III study of oral compared with intravenous topotecan as
second-line therapy in small-cell lung cancer. J Clin Oncol.
25:2086–2092. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jotte R, Conkling P, Reynolds C, Galsky
MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF and Oliver JW:
Randomized phase II trial of single-agent amrubicin or topotecan as
second-line treatment in patients with small-cell lung cancer
sensitive to first-line platinum-based chemotherapy. J Clin Oncol.
29:287–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Pawel J, Jotte R, Spigel DR, O'Brien
ME, Socinski MA, Mezger J, Steins M, Bosquée L, Bubis J, Nackaerts
K, et al: Randomized phase III trial of amrubicin versus topotecan
as second-line treatment for patients with small-cell lung cancer.
J Clin Oncol. 32:4012–4019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue A, Sugawara S, Yamazaki K, Maemondo
M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, et
al: Randomized phase II trial comparing amrubicin with topotecan in
patients with previously treated small-cell lung cancer: North
Japan lung cancer study group trial 0402. J Clin Oncol.
26:5401–5406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horita N, Yamamoto M, Sato T, Tsukahara T,
Nagakura H, Tashiro K, Shibata Y, Watanabe H, Nagai K, Nakashima K,
et al: Amrubicin for relapsed small-cell lung cancer: A systematic
review and meta-analysis of 803 patients. Sci Rep. 6:189992016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto K, Ohe Y, Shibata T, Seto T,
Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, et
al: Combined chemotherapy with cisplatin, etoposide, and irinotecan
versus topotecan alone as second-line treatment for patients with
sensitive relapsed small-cell lung cancer (JCOG0605): A
multicentre, open-label, randomised phase 3 trial. Lancet Oncol.
17:1147–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakuda K, Miyawaki T, Miyawaki E, Mamesaya
N, Kawamura T, Kobayashi H, Omori S, Nakashima K, Ono A, Kenmotsu
H, et al: Efficacy of second-line chemotherapy in patients with
sensitive relapsed small-cell lung cancer. In Vivo. 33:2229–2234.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baize N, Monnet I, Greillier L, Geier M,
Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, et
al: Carboplatin plus etoposide versus topotecan as second-line
treatment for patients with sensitive relapsed small-cell lung
cancer: An open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 21:1224–1233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami H, Yamamoto N, Shibata T, Takeda
K, Ichinose Y, Ohe Y, Yamamoto N, Takeda Y, Kudoh S, Atagi S, et
al: A single-arm confirmatory study of amrubicin therapy in
patients with refractory small-cell lung cancer: Japan clinical
oncology group study (JCOG0901). Lung Cancer. 84:67–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardizzoni A, Hansen H, Dombernowsky P,
Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J
and Verweij J: Topotecan, a new active drug in the second-line
treatment of small-cell lung cancer: A phase II study in patients
with refractory and sensitive disease. The European organization
for research and treatment of cancer early clinical studies group
and new drug development office, and the lung cancer cooperative
group. J Clin Oncol. 15:2090–2096. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kondo R, Watanabe S, Shoji S, Ichikawa K,
Abe T, Baba J, Tanaka J, Tsukada H, Terada M, Sato K, et al: A
Phase II study of irinotecan for patients with previously treated
small-cell lung cancer. Oncology. 94:223–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horiuchi K, Sato T, Kuno T, Takagi H,
Hirsch FR, Powell CA and Fukunaga K: Platinum-doublet chemotherapy
as second-line treatment for relapsed patients with small-cell lung
cancer: A systematic review and meta-analysis. Lung Cancer.
156:59–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saruwatari K, Umemura S, Nomura S, Kirita
K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ohe Y and Goto K:
Prognostic factor analysis in patients with small-cell lung cancer
treated with third-line chemotherapy. Clin Lung Cancer. 17:581–587.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ready N, Farago AF, de Braud F, Atmaca A,
Hellmann MD, Schneider JG, Spigel DR, Moreno V, Chau I, Hann CL, et
al: Third-line nivolumab monotherapy in recurrent SCLC: CheckMate
032. J Thorac Oncol. 14:237–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spigel DR, Vicente D, Ciuleanu TE,
Gettinger S, Peters S, Horn L, Audigier-Valette C, Pardo Aranda N,
Juan-Vidal O, Cheng Y, et al: Second-line nivolumab in relapsed
small-cell lung cancer: CheckMate 331. Ann Oncol. 32:631–641. 2021.
View Article : Google Scholar : PubMed/NCBI
|